Graphical abstract
Keywords: CPAP, Respiration, Pressure, Flow, Venturi, Occlusion, Shutter
Abstract
Respiratory model-based methods require datasets containing enough dynamics to ensure model identifiability for development and validation. Rapid expiratory occlusion has been used to identify elastance and resistance within a single breath. Currently accepted practice for rapid expiratory occlusion involves a 100 ms occlusion of the expiratory pathway. This article presents a low-cost modular rapid shutter attachment to enable identification of passive respiratory mechanics. Shuttering faster than 100 ms creates rapid expiratory occlusion without the added dynamics of muscular response to shutter closure, by eliminating perceived expiratory blockage via high shutter speed. The shutter attachment fits onto a non-invasive venturi-based flow meter with separated inspiratory and expiratory pathways, established using one-way valves. Overall, these elements allow comprehensive collection of respiratory pressure and flow datasets with relatively very rapid expiratory occlusion.
Specifications table
| Hardware name | Respiratory pressure and split flow data collection device with rapid occlusion attachments |
| Subject area |
|
| Hardware type |
|
| Closest commercial analog | Flow sensors are commercially available but expensive (e.g., the TSI4000 which we used to validate this device). Rapid expiratory occlusion devices have been created before, however have had issues with speed and duration of shuttering interrupting normal breathing patterns [1]. |
| Open source license | Creative Commons Attribution 4.0 International license |
| Cost of hardware | ∼ NZD$350 |
| Source file repository | https://doi.org/10.17632/7mshy2kcf3.3 |
| OSHWA certification UID (OPTIONAL) | NZ000004 |
Hardware in context
Subject-specific respiratory modelling with a mechanical and physiological basis is commonly based on pressure and flow measurements [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Thus, respiratory datasets recording pressure and flow are required to develop and validate these model-based methods. However, one key issue with personalising respiratory models is trade-offs associated with the simultaneous identification of respiratory mechanics, such as elastance, resistance, and patient effort, from the measured pressure and flow profiles [2], [3], [4].
Rapid expiratory occlusion (REO), also termed ‘the interrupter technique’, is a method to identify passive mechanics (elastance and resistance) from shutter instances, or shuttering, in expiration, which theoretically allows the simultaneous identification of respiratory mechanics without identifiability trade-off [13], [14]. Prior REO methods have proven these mechanics are identifiable using such occlusion data [1], [13], [14]. However, implementation of REO without excessive expiratory resistance during standard 100 ms duration shutter instances has proven difficult, and they often result in additional patient effort in reaction to this 100 ms expiratory flow limitation, which in turn impacts model identifiability and relevance as these active expiratory terms are not included in the model [1], [13], [14].
This work presents a shuttering device for REO to be used in conjunction with a venturi-based flow meter, which has been adapted from previous designs for similar applications [15], [16]. The REO attachment is developed using a camera shutter to generate measurably shorter shutter instance durations than the prior 100 ms standard [1], [13], [14]. The hypothesis is the profile of a longer occlusion could be predicted or estimated from a shorter occlusions profile uncorrupted by patient effort due to its short duration. Thus, such a rapid occlusion aims to reduce subject-specific muscular reactions to shuttering [1] to improve the identification of passive, model-based respiratory mechanics in resting breathing.
The adapted venturi-based flow meter splits inspiratory and expiratory flow allowing shuttering of exhalation only and easy connection of the inspiratory pathway to non-invasive mechanical ventilation (NIMV), such as continuous positive airway pressure (CPAP). The outlined device can also be used with previously developed dynamic circumference tapes for validation and/or analyses of abdominal and thoracic breathing modes and patterns [15]. The modular nature of the device means it is easily customised and reconfigurable for alternate applications with greater or fewer pressure measurements, inclusion of expiratory shuttering, or additional inputs, such as dynamic circumference or pulse oximetry.
Hardware description
The venturi-based flow meter is adapted from previous designs for similar applications [15], [16]. The new version outlined in this article utilises split inspiratory and expiratory flow pathways, for greater configurability with additional respiratory monitoring or simulation devices, as well for ease of data processing. The venturi is designed to be integrated in series with respiratory circuitry, using standard 22 mm male and female connections. In its current application, the inspiratory input port (22 mm male connection) is connected to a CPAP device using its accompanying circuitry (or is unconnected when CPAP is not required), the expiratory output port vents to atmosphere (22 mm female connection), and the subject port (22 mm male connection) is connected to a mouthpiece, filter, or mask. Differential Pressure Sensors were also upgraded (SSCDRRN001PD2A5) to capture larger flow ranges (seen in maximal breaths and panting).
In addition, a modular expiratory shutter attachment is outlined for identification of passive respiratory mechanics. Specifically, rapid expiratory shuttering allows breath-wise identification of passive lung elastance and resistance during shutter instances by eliminating model identifiability issues in the simultaneous identification of elastance and resistance during expiration.
Ultimately, the device provides researchers with:
-
-
Rapid expiratory occlusion for identification of passive lung mechanics
-
-
A low-cost alternative to commercial sensor systems
-
-
Individual inspiratory and expiratory flow pathways
-
-
Non-invasive data collection
-
-
A modular design with high customisability
Design files
The included device design files are outlined in Table 1 and the type of files are indicated.
Table 1.
Design Files Summary.
| Design file name | File type | Open source license | Location of the file |
|---|---|---|---|
| VenturiSplitFlow_Inhale | CAD (SLDPRT) and STL files | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 3D Printed Hardware |
| VenturiSplitFlow_Exhale | CAD (SLDPRT) and STL files | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 3D Printed Hardware |
| PressureSensorBracket | CAD (SLDPRT) and STL files | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 3D Printed Hardware |
| ShutterHousing | CAD (SLDPRT) and STL files | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 3D Printed Hardware |
| ControlBoard | KICAD files and PDF schematic | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 PCBs |
| PressureSensorBreakoutBoard | KICAD files and PDF schematic | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 PCBs |
| NanoCode | Arduino files | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 Code |
| DataCollectionCode | Arduino files | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 Code |
| DataProcessingCode | Arduino files | CC BY 4.0 |
https://doi.org/10.17632/7mshy2kcf3.3 Code |
The 3D printed components of this project were designed in SolidWorks (SolidWorks 2020, SolidWorks Corp, Waltham, MA, United States) and have been provided in these.SLDPRT as well as.STL files. The.SLT files were imported to PrusaSlicer (PrusaSlicer 2.5.0, Prusa Research, Prague, Czech Republic), which was used to generate GCODE to print the files on the Prusa Mini (Prusa Mini+, Prusa Research, Prague, Czech Republic). The venturi was printed in two halves, the inhalation (VenturiSplitFlow_Inhale), and exhalation (VenturiSplitFlow_Exhale) pathways. The differential pressure sensors for the venturi tubes were located on a printed bracket (PressureSensorBracket). The shutter was also located in a 3D printed housing (ShutterHousing) connected to the expiratory pathway and has an O-ring recess to create a seal during shutter instances.
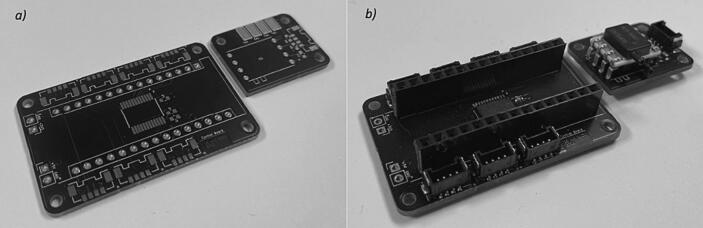
Custom control (ControlBoard) and pressure sensor breakout (PressureSensorBreakoutBoard) PCBs were designed for this project in KiCad (KiCad 5.1.9, KiCad Services Corporation, Long Beach, CA, United States). These were manufactured by JLCPCB and populated in the SMT lab at the University of Canterbury.
Code for this project is provided in three files. Firstly, the microcontroller code (NanoCode) is provided to be uploaded to the Arduino Nano using the Arduino IDE (Arduino IDE 1.8.16, Arduino, Somerville, MA, USA). Secondly, the data collection code (DataCollectionCode) is provided to sample data from the microcontroller using MATLAB (Matlab 2021b, The Mathworks Inc, Natick, MA, USA). Lastly, the data processing code DataProcessingCode is also written in MATLAB and generates files of pressure, flow, tidal volume, and chest and abdominal expansion from the generated sampled data files.
Bill of materials
The bill of materials is attached as an excel file (https://doi.org/10.17632/7mshy2kcf3.3).
Build instructions
-
1.
The Venturi tubes (‘VenturiSplitFlow_Inhale’, and ‘VenturiSplitFlow_Exhale’), PCB bracket (‘PressureSensorBracket’), and expiratory shutter housing (‘ShutterHousing’) components were 3D printed (Fig. 1) using a Prusa Mini (Prusa Mini+, Prusa Research, Prague, Czech Republic). Supporting Material was removed and stringing on the interior of the venturi tubes were removed using a pipe cleaner.
-
2.
The pressure sensor port guide holes (Fig. 2a) were drilled out using a 1.6 mm drill bit. The Pressure sensor port tube (KS1176) was cut into 8 mm lengths using a Stanley knife. These were inserted into the venturi port holes (Fig. 2b) and glued in place (Fig. 2c). It was ensured that the tubes terminated flush with the interior surface of the venturi tubes.
-
3.
The Venturi components were then connected using one-way valves (IPN913948) to construct a y-split tube device with an inhalation, exhalation, and patient interface pathways (Fig. 3).
-
4.
The control (ControlBoard) and pressure sensor breakout (PressureSensorBreakoutBoard) PCBs were ordered from JLCPCB (Fig. 4a). These were populated, with resistors (R-4 k7 and R-10 k), capacitors (C-1uF and C-100nF), bus splitter (PCA9548ADB), and connector headers (5055680471), in the SMT Laboratory at the University of Canterbury using a pick and place machine (ProtoPlace S, LPKF Laser and Electronics, Naklo, Slovenia) and reflow soldered in a reflow oven (LPKF ProtoFlow, LPKF Laser and Electronics, Naklo, Slovenia). Pressure sensors (SSCDRRN001PD2A5) and pin headers (Headers Female) were then hand-soldered on (Fig. 4b).
-
5.
The microcontroller (Ardunio Nano v3) was then connected, and the microcontroller sampling and shuttering control code (NanoCode) was written and uploaded using Arduino IDE (Arduino IDE 1.8.16, Arduino, Somerville, MA, USA). This code can also be used to sample dynamic abdominal and thoracic circumference (design files openly available) [15].
-
6.
Next, the control and pressure sensor PCBs were attached to pressure sensor bracket using M2 fasteners (M2 x 12 mm Cap Screws and M2 Nylon Nuts) (Fig. 5a). The pressure sensor bracket can be zip-tied, or otherwise attached to the venturi device for testing (Fig. 5b). Flexible silicone tubing (X002O35WBT) connects the pressure sensor ports to the port tubes (Fig. 5c).
-
7.
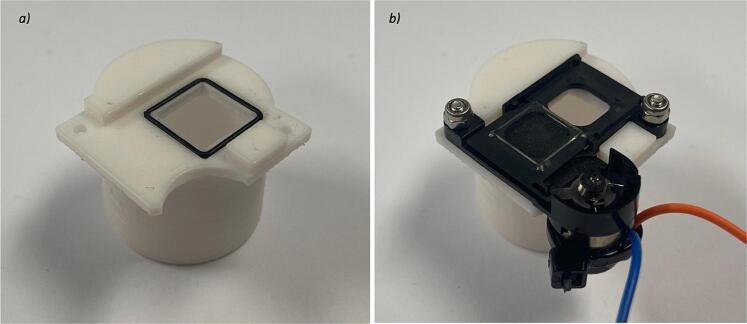
A camera shutter (Camera Shutter) was dismantled as illustrated in Fig. 6a. The IR filter was then removed (Fig. 6b) to create an air pathway when the shutter is not powered, and conversely an obstruction when the shutter is powered. Leads were subsequently soldered onto the shutter solenoid and zip-tied in place (Fig. 6c).
-
8.
An O-Ring (MR12X1) was inserted into the groove on the shutter housing (Fig. 7a) and the camera shutter was secured in the housing using M2 fasteners (M2 x 8 mm Cap Screws and M2 Nyloc Nuts) (Fig. 7b).
-
9.
A connector (451110406) was cut in half and used in combination with jumper cables (Female jumper cables) to construct an adapter from control board to driver board (HG7881) (Fig. 8).
-
10.
The shutter attachment was connected to expiratory pathway and the driver board secured to the venturi tube for testing (Fig. 9a). The board was then connected to the shutter leads (Fig. 9b). Connections from the control board to pressure sensor breakout boards were make using connector cables (451110400 & 451110402), and to the shutter driver board using the adapted 451,110,406 connector (Fig. 9b).
-
11.
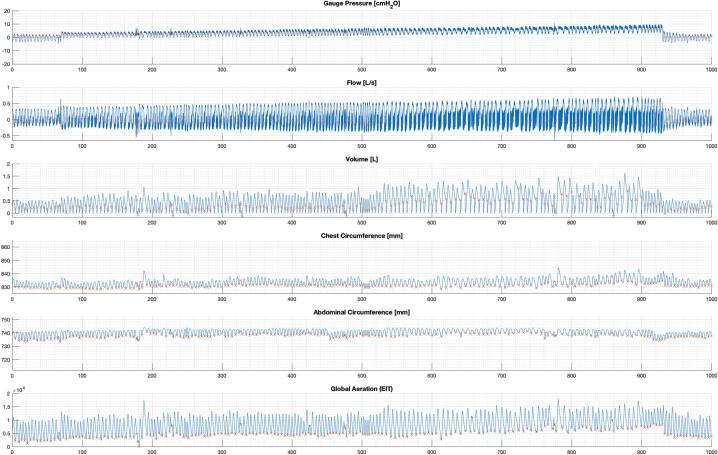
The data collection code (DataCollectionCode), and processing code (DataProcessingCode) was written in MATLAB (Matlab 2021b, The Mathworks Inc, Natick, MA, USA). The included code samples gauge pressure, inspiratory and expiratory different pressures (to calculate flow and volume), and dynamic circumference values (https://doi.org/10.17632/wfw7nyctcy.2). The code can be easily adapted to exclude dynamic circumference measures or include additional sensor data. Fig. 10 illustrates example processed data output using the current configuration, and Fig. 11 illustrates a singular breath from this data (in which the expiratory shuttering can be seen).
Fig. 1.
3D printed components (with support material).
Fig. 2.
Inspiratory and expiratory Venturi with a) drilled out port holes, b) barbs inserted, and c) barbs glued in place.
Fig. 3.
Venturi device connected with one-way valves.
Fig. 4.
Custom PCBs: a) unpopulated and b) populated.
Fig. 5.
Assembly of a) the PCBs to the bracket, b) the the bracket to Venturi device, and c) the connection of pressure sensors to venturi ports.
Fig. 6.
Camera shutter a) dismantled, b) with IR filter removed, and c) with solenoid leads.
Fig. 7.
Camera shutter a) O-ring seal, and b) secured in housing.
Fig. 8.
Control to shutter driver board adapter.
Fig. 9.
Shutter driver board a) fixation to venturi, b) connection to solenoid leads, and c) connection of all breakout boards to control board.
Fig. 10.
Example processed dataset.
Fig. 11.
Example processed breath.
Operation instructions
-
1.
Connect laptop with the data collection code in MATLAB to the Arduino nano microcontroller. If using the auxiliary dynamic and thoracic circumference monitoring tapes, follow the instructions referenced [15].
-
2.
Connect the respiratory circuitry for the desired trial. For example, a filter and mask, (Fig. 12a) or a filter and mouthpiece (Fig. 12b) with CPAP connected to the inspiratory port if required (Fig. 12).
-
3.
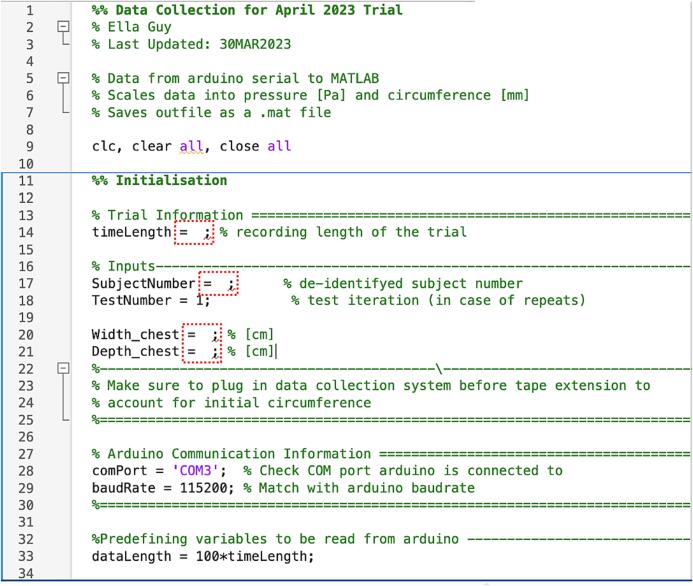
Specify the subject number and recording length in the data collection code and then the sampling code to collect data (Fig. 13). In the provided code subject initial chest width and depth were also manually input from measurements (Fig. 13). The data collection code generates a file of differential pressures (gauge, inhalation venturi, and exhalation venturi), and encoder counts (chest and abdominal) over time.
-
4.
Following the trial, the processing code can be run generate a file of pressure, flow, tidal volume, and chest and abdominal expansion. Flow is computed using Bernoulli’s principle and conservation of flow, based in venturi parameters:
| (1) |
Fig. 12.
Respiratory circuitry configurations with a) mouthpiece, and b) full face mask.
Fig. 13.
Data collection code inputs.
Tidal volume is computed as the cumulative integration of flow over each identified breath, where indices were identified as local minimums in volume (integrated from flow over the entire dataset, normalized by leakage associated drift). Dynamic expansions were computed from encoder counts based on barrel dimensions and processed to remove instances of slippage defined as an over 1 mm jump in 0.01 s.
Validation and characterization
The device has the following basic operating parameters:
-
-
The device operates with a 5 V supply.
-
-
The pressure sensor operating range limits gauge pressure measurements to ± 70 cmH2O.
-
-
With the existing venturi design (15 to 10 mm constriction), these sensors can measure flows up to 9 L/s (by Equation (1), given discharge coefficient = 0.97, and air density of = 1.225 kg/m3).
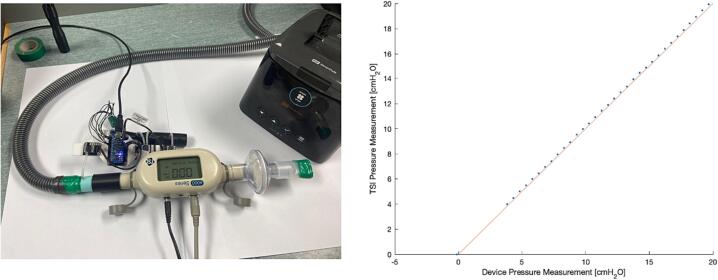
The device was validated against a TSI4000 Flow meter (4000 Series Analog and Digital Flow Meter, TSI, Shoreview, MN, USA) against pressure and flows generated by a Fisher and Paykel Healthcare SleepStyle CPAP Machine (SleepStyle SPSCAA, Fisher and Paykel Healthcare, East Tamaki, Auckland, NZ). Firstly, a gauge pressure port connection was connected in series with the TSI flowmeter and a filter and mouthpiece which was sealed off to maintain pressure (Fig. 14). The CPAP pressure was then increased from 4 to 20 cmH2O on 0.5 cmH2O increments the device was compared to the TSI measured pressure using mean values over simultaneous 5 s recordings (Fig. 14). Fig. 14 shows the gauge pressure measurement correlated well with the TSI measured pressure, with a mean −1.69 % (-0.77 to −3.86 %) percentage error in device mean measured pressure compared to the flow meter.
Fig. 14.
Validation of device gauge pressure against TSI flowmeter using CPAP at incremented PEEP through sealed test setup (left).
The inhalation venturi was then connected in series in the place of the gauge pressure port and the mouthpiece was vented to atmosphere (Fig. 15). This test yielded a mean 8.95 % (5.95 to 10.20 %) percentage error in device mean measured flow compared to the flow meter. The offset could be a result of resistive losses over the venturi attachment or leakage, which explains why the device is measuring a higher flow than the TSI flowmeter (Fig. 15).
Fig. 15.
Validation of device gauge flow against TSI flowmeter using CPAP at incremented PEEP through test setup vented to atmosphere (left).
The full device was then connected in series with the TSI flowmeter at the patient interface connection, followed by a filter and mouthpiece (Fig. 16). The mouthpiece was then breathed through, and the measured pressure and flow compared between the device and the TSI flowmeter (Fig. 16). Fig. 16 illustrated the high pressure sensor accuracy and the relative accuracy of the flow, given the resistive losses associated with the 3D printed circuitry. The higher flow offset in inspiration (periods of negative gauge pressure) (Fig. 16) provides evidence of these resistive losses, as the reversal of the flow direction removes the venturi resistive loss component from the TSI recording.
Fig. 16.
Validation of combined pressure and flow of device (red) against TSI flowmeter (blue) using breathing data over time [s] through in series test setup (right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Ethics statements
Ethical consent for the trials using this hardware was granted by the Human Research Ethics Committee at the University of Canterbury (Refs: HREC 2023/04/LR-PS, HREC 2023/30/LR-PS, and HREC 2022/26/LR).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by a University of Canterbury Doctoral Scholarship and the EU H2020 R&I programme (MSCA-RISE-2019 call) under grant agreement #872488 – DCPM.
Biography

Ella F.S. Guy PhD Candidate at the Centre for Bioengineering in the Mechanical Engineering Department at the University of Canterbury in New Zealand.
References
- 1.Howe S.L., et al. Measuring lung mechanics of expiratory tidal breathing with non-invasive breath occlusion. BioMedical Eng. OnLine. 2020;19(1):32. doi: 10.1186/s12938-020-00777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guy E.F., Chase J.G., Knopp J.L., Shaw G.M. Quantifying ventilator unloading in CPAP ventilation. Comput. Biol. Med. 2022;142 doi: 10.1016/j.compbiomed.2022.105225. [DOI] [PubMed] [Google Scholar]
- 3.Guy E.F., Knopp J.L., Chase J.G. Pilot study of model-based estimation of inspiratory driving pressure in CPAP ventilation. IFAC-PapersOnLine. 2021;54(15):109–114. [Google Scholar]
- 4.Knopp J.L., Guy E., Kim K.T., Shaw G.M., Chase J.G. B-spline modelling of inspiratory drive in NAVA-ventilated patients. IFAC-PapersOnLine. 2021;54(15):103–108. [Google Scholar]
- 5.Bates J.H.T. Cambridge University Press; Cambridge, UK; New York: 2009. Lung mechanics: an inverse modeling approach (no. Book, Whole) [Google Scholar]
- 6.Chiew Y.S., Chase J.G., Shaw G.M., Sundaresan A., Desaive T. Model-based PEEP optimisation in mechanical ventilation. Biomed. Eng. 2011;10(1):111. doi: 10.1186/1475-925X-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiew Y.S., et al. Time-varying respiratory system elastance: a physiological model for patients who are spontaneously breathing. PLoS One. 2015;10(1):e0114847–e. doi: 10.1371/journal.pone.0114847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton S.E., et al. Optimising mechanical ventilation through model-based methods and automation. Annu. Rev. Control. 2019;48:369–382. doi: 10.1016/j.arcontrol.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase J.G., et al. Next-generation, personalised, model-based critical care medicine: a state-of-the art review of in silico virtual patient models, methods, and cohorts, and how to validation them. Biomed. Eng. 2018;17(1):24. doi: 10.1186/s12938-018-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees S.E. The Intelligent Ventilator (INVENT) project: the role of mathematical models in translating physiological knowledge into clinical practice. Comput. Methods Programs Biomed. 2011;104(Suppl 1):S1–S. doi: 10.1016/s0169-2607(11)00307-5. [DOI] [PubMed] [Google Scholar]
- 11.Rees S.E., et al. Using Physiological Models and Decision Theory for Selecting Appropriate Ventilator Settings. J. Clin. Monit. Comput. 2006;20(6):421–429. doi: 10.1007/s10877-006-9049-5. [DOI] [PubMed] [Google Scholar]
- 12.C. Zhou et al., “Virtual patients for mechanical ventilation in the intensive care unit,” Computer Methods and Programs in Biomedicine, vol. 199, pp. Paper #105912, 24-pages, 2021/02/01/ 2021, doi: https://doi.org/10.1016/j.cmpb.2020.105912. [DOI] [PubMed]
- 13.Panagou P., Kottakis I., Tzouvelekis A., Anevlavis S., Bouros D. Use of interrupter technique in assessment of bronchial responsiveness in normal subjects. BMC Pulm. Med. 2004;4(1):1–6. doi: 10.1186/1471-2466-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan E.-Y.-T. Use of the interrupter technique in assessment of lung function. J. Paediatr. Respirol. Crit. Care. 2007;3(4):6–8. [Google Scholar]
- 15.Guy E.F., Chase J.G., Holder-Pearson L.R. Respiratory bi-directional pressure and flow data collection device with thoracic and abdominal circumferential monitoring. HardwareX. 2022;12:e00354. doi: 10.1016/j.ohx.2022.e00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holder-Pearson L., Chase J.G. Physiologic-range flow and pressure sensor for respiratory systems. HardwareX. 2021;10:e00227. doi: 10.1016/j.ohx.2021.e00227. [DOI] [PMC free article] [PubMed] [Google Scholar]