Summary
Human immunodeficiency virus-1 (HIV) infection is a chronic disease under antiretroviral therapy (ART), during which active HIV replication is effectively suppressed. Stable viral reservoirs are established early in infection and cannot be eradicated in people with HIV (PWH) by ART alone, which features residual immune inflammation with disease-associated secondary comorbidities. Mammalian cells are equipped with integrated stress response (ISR) machinery to detect intrinsic and extrinsic stresses such as heme deficiency, nutrient fluctuation, the accumulation of unfolded proteins, and viral infection. ISR is the part of the innate immunity that defends against pathogen infection or environmental alteration, thereby maintaining homeostasis to avoid diseases. Here, we describe how this machinery responds to the off-target effects of ART and persistent HIV infection in both the peripheral compartments and the brain. The latter may be important for us to better understand the mechanisms of stable HIV reservoirs and HIV-associated neurocognitive disorders.
Subject areas: Virology, Cell biology
Graphical abstract

Virology; Cell biology
Introduction
The mammalian immune system consists of multiple checkpoints that survey changes in the environment in the presence of various conditions, including oxidative stress, pathogen infections, and changes in metabolites such as lipids, sugars, and amino acids. The latter are known as the essential building blocks of cells.1,2 To provide the best possible means under the selective pressure of eukaryotes, cells have evolved several mechanisms in response to these changes to correct the imbalance for the pre-stress state of recovery. Otherwise, prolonged or extreme stress can sometimes cause more disorders than a quick recovery, leading to cell death. One of these signaling pathways, the integrated stress response (ISR), deals with such cellular stresses by regulating important translation factors to temporarily terminate protein translation, allowing cells to rapidly reprogram metabolism through the production of specialized stress response proteins.3 Of note, recent studies have shown that viruses can also exploit these stress signaling pathways for their own purposes, such as viral replication and latent infection. One such pathogen is the human immunodeficiency virus-1 (HIV).4,5,6 This review will focus on recent studies of ISR signaling in HIV transcription and its persistent infection. A growing body of evidence supports that latency reversal agents (LRAs) flush out HIV from latently infected CD4+ T cells by activating ISR signaling. Since the downstream signaling of ISR activation includes cell death, this perspective posits that ISR activation may be a possible avenue to reduce HIV reservoirs by activating apoptosis pathways in HIV+ immune cells after latency reversal.
The integrated stress response signaling
ISR is a signaling system that enables cells to recognize environmental abnormalities such as increased levels of unfolded proteins in the endoplasmic reticulum (ER), viral double-stranded RNA, hypoxia, nutrient deprivation, and low levels of glucose and amino acids. These upstream pathways of ISR signaling induce elF2α phosphorylation, which temporarily reduces cap-dependent protein translation.7,8 However, under stressful conditions, cells shift their cellular metabolisms in a more efficient direction to control the translational machinery. For example, when a cell suffers from nutrient deprivation, ISR acts through the disruption of new protein synthesis, degrading unfolded proteins accumulated in the ER that may lead to the formation of potentially toxic aggregates of unfolded proteins and induction of autophagy in some cell organelles. Attempting to redirect limited resources to stay alive, the ISR signaling also triggers a series of processes that prevent the misuse of limited resources such as amino acids and glucose under stressful conditions. When cells are severely impaired by stress, the activation of apoptosis is the last resort to help cells reach equilibrium before sustaining damage to themselves and surrounding cells.9 In the following section, the diverse components of the ISR pathway will be addressed. We will highlight HIV latency and HIV transcription related to ISR-specific subpathways, along with the manipulation of the ISR pathway as a new perspective on the HIV cure (Figure 1).
Figure 1.
The integrated stress response and HIV transcription
ISR relies on a group of eIF2α kinases to sense changes in cell homeostasis and environmental stress. (1) HRI sensor detects heme changes in red cells; (2) GCN2 or EIF2AK4 recognizes nutrient deficiency such as amino acid and glucose; (3) PKR recognizes double-stranded RNA, typical of viral infections; and (4) UPR responds to ER stress. UPR is composed of 3 transmembrane sensors: (a) ATF6, (b) IRE1α, and (c) PERK. Once activated, all the above upstream kinases (5) phosphorylate elF2α at its serine 51 using ATP as a phosphate source, (6) block the regeneration of the GDP-GTP exchange factor elF2B, and prohibit Cap-dependent protein translation initiation. All these upstream signaling pathways converge to induce the protein expression of ATF4 (7). ATF4 triggers cell death signaling by CHOP (8), serves as a transcription factor after recruitment into its target genes, including HIV promotor as an example (9), or induces autophagy by ATG proteins (10).
The ER and the cytoplasm contain proteins that are part of the dual surveillance system of the ISR. The ER, an organelle that synthesizes proteins and lipids and integrates cell membrane traffic between the cytosol and the extracellular environment via the Golgi complex, contains the unfolded protein response (UPR) complex. UPR usually detects the presence of unfolded proteins via BiP, a chaperone that recognizes unfolded proteins and exerts control over the activation of UPR.10 The latter is composed of three major sensors: activating transcription factor 6 (ATF6), PKR-like endoplasmic reticulum kinase (PERK), and IRE1α. ATF6 is a membrane-bound leucine zipper transcription factor that surveils the ER lumen for protein stress such as the accumulation of unfolded proteins. Under stress, ATF6 is transported to the Golgi complex, where it is processed and converted through proteolysis by S1P and S2P to ATF6f, a cytosolic form that controls the ER-associated degradation (ERAD) signature genes. After being processed in its N-terminal, ATF6 enters the cellular nucleus to regulate its downstream gene promoters from the UPR response.11 PERK, another component of the UPR surveillance system, oligomerizes and self-phosphorylates once activated by ER stress or hypoxia, leading to the activation of elF2α and the production of activating transcription factor 4 (ATF4), which controls the transcription of chaperones such as heat shock proteins (HSPs), BiP, GRP94, and the C/EBP homologous protein (CHOP). ATF4 also controls the production of proteins such as the DNA damage-inducible protein (GADD34) to dephosphorylate elF2α, thereby turning off the signaling cascade to avoid severe ISR signaling activation.12 Inositol-requiring protein 1α (IRE1α), a serine/threonine kinase with endonuclease activity, is the third sensor available in the luminal membrane of the ER. Once dimerized and self-phosphorylated in its cytoplasmic tail, it induces the production of the X-box binding protein 1 (XBP-1), which is involved in protein folding responses or leads to the activation of the c-Jun N-terminal kinase pathway via ASK1 that, in turn, binds to the promoters of genes related to the UPR such as CHOP to counterbalance the cellular stress.13 Other elements also compose the ISR system, such as the general control non-depressive 2 (GCN2 or EIF2AK4), which recognizes nutrient imbalances and senses uncharged tRNAs; heme-regulated elF2 (HRI) regulates globin synthesis by recognizing erythrocyte heme variations when reactive oxygen species are present, participating in iron-induced mitochondria stress. HRI activation is dependent on mitochondrial proteins, such as DELE1, that are involved in mitochondria respiratory stress.14 The last important sensor, which is also activated by the interferon response, is the double-stranded RNA-dependent kinase (PKR) that specializes in double-stranded RNA recognition and is responsible for the detection of nutritional fluctuations and viral infections.15
The activation of all the previous upstream kinases converges into the activation of cytoplasmic elF2α kinases via protein phosphorylation. The elF2α subunit from the elF2αβγ heterotrimer is phosphorylated at serine 51, preventing the GDP-GTP exchange molecule elF2B from regenerating GTP that is required for the translation initiation in the TC-elF2α complex. The phosphorylation of elF2α inhibits protein translation but activates only a few genes including the essential ISR downstream transcription factors ATF4, ATG5, and CHOP.16,17 The latter also controls cell growth and cell death signaling during ISR. Once recruited, these factors may use a translation initiation mechanism independent of AUG start codon recognition to bind target genes, including ATF3 (Figure 1). However, when prolonged, ISR induces apoptosis via CHOP rather than cell survival.15
Because viruses exploit the ISR pathway via cap-dependent translation, its role has recently received attention during viral infections, including arbovirus, influenza virus, coronavirus, picornavirus, foot-and-mouth disease, mammalian orthoreovirus, and HIV, in which ISR may facilitate viral replication during acute infection.4,18 Among viral infections, the emerging role of ISR signaling during HIV infection has been reported5,19 (Figure 1). In the following section, we will discuss the unique role of ISR signaling during antiretroviral therapy (ART), HIV latency, viral reservoir elimination, and HIV-associated neurocognitive disorders (HAND).
The integrated stress response and off-target effects of the antiretroviral therapy
The ISR has been studied extensively with antiretroviral drugs (ARDs). The number of treatment options has increased with the development of ART, beginning with zidovudine in 1987 and followed by two-to three-drug combinations based on protease inhibitors, viral fusion inhibitors, and reverse transcriptase inhibitors. ART was designed to control the replication of HIV. It is composed of several classes of drugs targeting HIV-specific enzymes, including (a) integrase strand transfer inhibitors such as raltegravir, dolutegravir, and elvitegravir, to block HIV integration into the host genome20; (b) reverse transcriptase inhibitors, including non-nucleoside reverse transcriptase inhibitors (NNRTI) such as delavirdine, efavirenz, and rilpivirine, and nucleoside reverse transcriptase inhibitors (NRTI) like lamivudine, abacavir, tenofovir, and zidovudine21; (c) protease inhibitors such as nelfinavir, saquinavir, and ritonavir, which interfere with maturation of HIV proteins for the production of infectious viral particles22; (d) viral entry inhibitors such as maraviroc and enfuvirtide that targeting HIV receptors/co-receptors23; and lastly, (e) capsid inhibitors such as lenacapavir that target viral core proteins.24
While ARDs are effective in suppressing active HIV replication, side effects of ARDs have been observed, such as neurotoxicity, anemia, pancreatitis, and hepatotoxicity. Studies have been conducted to better understand how some current ARDs trigger stress signaling that disrupts metabolic pathways and is related to cellular side effects.25 Thus, ARD-induced ISR activation should be considered in the clinic. In lymphocytes isolated from people with HIV (PWH) receiving ART, IRE1α was activated in comparison with HIV-negative donors, being highly expressed in PWH under therapy with or without a protease inhibitor. BiP expression was also increased compared with non-infected subjects. In addition, p-elF2α and cleaved ATF6 expression were observed in HIV-infected donors with or without therapy, compared with HIV-negative donors, which were independent of protease inhibitors.26 Treatment of primary hepatocytes with NNRTI efavirenz augmented XBP1 splicing and the expression of XBP1, CHOP, and BiP/GRP78 genes, suggestive of ISR activation.27 These observations may explain the hepatic toxicity associated with the life-long use of efavirenz in PWH. Additionally, both protease inhibitors atazanavir and ritonavir activate the UPR response in an ATF4/CHOP-dependent manner in primary rat hepatocytes, which was associated with decreased bile acid production and an increased apoptotic death of hepatocytes.28
In trophoblasts, protease inhibitor lopinavir reduced plasma membrane fusion and chromatin condensation, leading to larger mitochondria but a thinner ER. ISR activation is related to lopinavir-induced IRE1α activation and the expression of sXBP1 and GRP78 proteins. These observations point to the in vivo toxicity of protease inhibitors in the placenta, which are known to induce preterm birth.29
In the brain, lopinavir may induce reactive oxygen species, which was associated with the augmentation in heme-oxygenase expression, with a slight increase in BiP protein expression being observed.30 In mice, the NNRTI nevirapine was associated with cognitive impairment and brain lipid peroxidation.31
Taken together, ART usually uses a combination of two NRTI/NNRTI with a PI, which disrupts several cellular pathways that are important for maintaining cellular homeostasis, including growth factors, glucose uptake, and lipid membrane fluidity, thereby activating ISR signaling through induction of elF2 phosphorylation. Some ARDs also activate the ISR pathway in the lung, the liver, and the brain,32 which could be harmful to PWH if ISR activation is prolonged and not properly resolved. Intriguingly, new studies have been considering ISR activation as a new strategy for HIV elimination, which will be discussed in the following section.
HIV transcription, integrated stress response, and HIV latency
After ART initiation, viral replication can be effectively controlled. However, ART alone cannot eliminate latently infected viral reservoirs,33 which exist in diverse tissue compartments including blood, the gut, the brain, lymph nodes, and the reproductive tract. Many cells have been described to harbor latently infected replication-competent HIV, including circulating CD4+ T cells, mainly resting memory ones, and monocytes and dendritic cells, the ones present in organs such as the liver and spleen. It may also exist in tissue-resident macrophages derived from hematopoietic cells, Kupffer cells in the liver, alveolar macrophages in the lungs, and microglia, which are the brain resident macrophages derived from the fetal yolk sac. Macrophages and monocytes may play a role in spreading the viral infection to lymphocytes during reservoir formation and viral blips.34,35,36,37,38,39 In the last decades, many groups have worked to decipher the mechanism behind HIV latency in the aforementioned resident immune cells but mainly focused on peripheral CD4+ T cells. Diverse mechanisms were addressed that may be responsible for HIV latency, such as the epigenetic and non-epigenetic regulation of HIV latency. Some of these mechanisms will be discussed in the following section.
During latency, the HIV long-terminal repeats (LTR) or HIV promoter is localized between two nucleosomes, which are repressed by histone deacetylation, methylation, and possibly histone decrotonylation,40,41 resulting in a tightly packed chromatin that physically blocks access to the essential transcription factors of HIV. The exchange of methyl groups for acetyl groups or crotonyl groups is necessary to obtain an open conformation of chromatins,40,42 allowing the transcription initiation complex to access the HIV promoter with regulatory and/or enhancing molecules capable of stabilizing complexes such as P-TEFb required for HIV transcription elongation. Some of the improved methods to flush the virus out of infected cells are latency reactivation-based, followed by killing HIV+ immune cells to eradicate HIV reservoirs, i.e., “kick and kill”. Others suggest the “block and lock” approach for a functional cure of HIV43,44 to prevent the production of new viruses after treatment interruption.40,43,45 The “kick and kill” approach has been tested in several animal models of HIV latency and small clinical trials in PWH on suppressive ART.46 By itself, latency reversal has a limited effect on the reduction of HIV reservoirs in infected cells. These include TLR agonists such as MGN1703 and GS-9620, NF-κB/PKC activators such as ingenols, IAP inhibitors such as AZD5582, and histone post-translational modulators such as vorinostat.47 The identification of other pathways to be used in combination with the previously described approaches is underway, as one strategy may not be sufficient to reactivate HIV reservoirs in diverse cell types. To this end, a better understanding of ISR in HIV transcription is needed and could be used to find a cure for HIV if successful.
HIV infection induced BiP expression, phosphorylation of elF2α, phosphorylation of IRE1α, and ATF6 cleavage in CD4+ T cells and/or monocytes isolated from PWH, indicating the activation of ISR signaling in vivo.26 Of note, the ISR signaling is involved in HIV transcription. In the latently infected U1 cell line, transduction of PKR almost completely inhibited TNFα-induced HIV latency reversal, which is associated with phosphorylation of eIF2α.48 HIV infection induces GCN2 protein expression, leading to the inhibition of protein translation and diminished viral integration.49 This host innate immunity may have evolved to prevent active viral replication. Unexpectedly, the transfection of ATF4 in Jurkat cells directly induced HIV transcription even without HIV Tat.50 Furthermore, HIV protease can degrade the essential ISR kinase GCN2 needed for the elimination of ISR signaling.51 Interestingly, the downstream effector protein ATF4 serves as a new transcription factor of HIV. There exist several potential ATF4 binding motifs at the HIV LTR,5,52 which can be methylated during the establishment of HIV latency.53 Others have shown that knocking down ATF4 suppressed HIV production, and the N-terminal domains of ATF4 are critical for HIV LTR-mediated transcriptional activation.54 Thus, HIV hijacks the anti-viral innate ISR signaling for its own replication.5
In the contents of gut lumens collected from non-human primates (NHPs), a dysregulation of amino acid metabolism was observed during the acute SIV infection. Evidence in the literature supports that HIV exploits the ISR pathway and interferes with cellular metabolism to increase its infectious potential; however, the effects during chronic infection may differ from the acute viral infection and have not been adequately explored. In addition, Jiang et al. (2017) observed viral reactivation as a result of ATF4 recruitment to the HIV LTR, indicating the importance of this pathway in inducing HIV transcription and/or latency reversal.5 More recently, the manipulation of the ISR pathway with HA15, which is a specific inhibitor of the retinal endoplasmic chaperone binding immunoglobulin protein, i.e., BiP,55 resulted in the forced activation of ISR and the transient induction of HIV RNA in the primary CD4+ T cell model of HIV latency19 and CD4+ T cells isolated from PWH receiving ART.5 Also, HIV reactivation was reported after the depletion of the essential amino acids, which are dependent on MAPKs but independent of mTOR or GCN2.5,56 Under the aberrant metabolism, ISR activation may be crucial for PWH in areas with low nutrient intake such as African countries. GCN2-ATF4 activation under starvation may support HIV transcription to better spread HIV among starved individuals.5
Interestingly, Jaspart and coworkers (2017) have shown that the HIV integrase (IN) interacts with GCN2 in a non-competitive way, with the S24 and S255 residues at IN appearing as the phosphorylation sites for the HIV protein. IN phosphorylation results in diminished HIV integration, which is counterbalanced by HIV through the GCN2 cleavage via the HIV protease. S255-IN-C-terminal residue mutation, abrogating phosphorylation, or GCN2 depletion results in high infectivity of HIV. Together, these data suggest the ISR is an additional critical regulator of HIV transcription and/or latency reversal.
Exploiting ISR signaling to deplete the HIV reservoir
The stable HIV reservoir may be selected for intrinsic signaling programs that confer resistance to cell death.36,57 The expression of exhaustion surface molecules like LAG-3 and PD-1 may display a non-reactive profile in CD8+ T cells, which is a barrier to the cure. HIV may also down-modulate proapoptotic factors such as caspase-10 and upregulate survival factors like Bcl-2 and Bcl-xL. These are all factors that should be addressed for the persistence of HIV reservoir cells.57 To this end, approaches to directly antagonize resistance to cell death in persistently infected cells may elicit the efficient eradication of HIV reservoirs. Several studies have found that HIV reservoirs could be reduced by directly inducing cell death. Bradley’s group aimed to block Bcl-2 in order to decrease HIV DNA in a Casp8p41-dependent way.58 A recent study by this group showed that strategies using Bcl-2 inhibitors venetoclax plus ixazomib can selectively and efficiently kill HIV+ immune cells; however, it caused undesired toxicity in primary CD4+ T cells.59 Studies with the IAP inhibitor (IAPi) have demonstrated the induction of autophagy-dependent apoptosis.60 However, it is not clear whether IAPi is efficient enough to induce targeted cell death in latently infected resting CD4+ T cells.41 Presumably, it would be ideal if IAPi could not only reactivate latent HIV but also induce apoptosis in these cells. In contrast, suppressing autophagy can induce apoptosis and selectively eliminate host cells that produce HIV in vivo.61 Nevertheless, it is unclear whether PWH can tolerate such a combination therapy, as, in addition to ART, five more compounds were included in this “kick and kill” strategy. Similarly, the inhibition of DDX3 was shown to deplete HIV reservoirs.62 A recent study has shown that CARD8 inflammasome-mediated pyroptosis could be manipulated by the NNRTI rilpivirine to clear persistent HIV infection.63 Studies are underway to understand whether physiological dosages of NNRTI can reduce HIV reservoirs in PWH. Together, these excellent studies support the idea that it is feasible to directly induce cell death of HIV-latently infected immune cells or in combination with other HIV curative strategies.
As shown previously, emerging studies demonstrated that ISR/ATF4 signaling is integrated into the signaling pathways to establish HIV latency. Among several HIV latency models, latent HIV reactivation was observed as a result of the induction of ATF4 and its subsequent recruitment to the HIV LTR, indicating the importance of this pathway in regulating latent infection.5 A follow-up study reported that the activation of ISR signaling through ATF4-impaired autophagy, which disrupted latent HIV after FOXO1 inhibition, a regulator of T lymphocytes. When ISR was suppressed, FOXO1 inhibition-induced HIV latency reversal was also dampened. These findings further support the idea that the suppression of ISR signaling is associated with quiescent HIV infection in CD4+ T cells.5,6
Besides apoptosis, autophagy can be also induced upon mild ISR activation, which serves as a protective pathway to recycle misfolded proteins. Interestingly, while unclear, the induction of HIV latency reversal by ISR/ATF4 activation may not be associated with autophagy activation. Some reports have shown that the suppression of autophagy signaling is required for latency reversal and subsequent reservoir clearance. Specifically, the treatment of HIV-infected CD4+ T cells with LRAs, ingenol-3,20-dibenzoate, in combination with autophagy inhibitors such as chloroquine or SAR405 resulted in latency reversal as well as the killing of HIV-infected cells in the peripheral blood of a humanized mice model.61 Together, these studies indicate that in the context of ISR signaling suppression, persistent HIV infection may be involved in the balanced interplay between latency induction or maintenance, autophagy, and the survival of the reservoir cells. If so, prolonged or severe ISR signaling may break this balance, thereby not only disrupting latent HIV but also inducing cell death to eradicate HIV+ reservoir cells (Figure 2). The concurrent “kick and kill” has been observed in the study of the cure of HTLV-1 infection.64 However, this has not yet been studied in the cure of HIV until recently.
Figure 2.
The activation of ISR eradicates latent HIV reservoirs
(1) Diverse cellular stresses are recognized by stress sensors or ISR agonists, leading to ISR signaling activation (2), elF2α phosphorylation (3), and ATF4 induction. ISR activation leads to ATF4 binding to its ATF/CREB elements at the target genes, including HIV (4). ISR/ATF4 activation may disrupt nucleosomes to open the chromatin (5) with the consequent HIV latency disruption (6) to produce HIV transcripts (7), which may produce viral particles (8). ATF4 can also induce CHOP transcription to activate cellular death (9). Prolonged ISR activation causes the eradication of HIV+ immune cells (10) without significant impact on the autophagy pathway (11).
To further examine the concurrent “kick and kill” hypothesis, the ISR/ATF4 signaling was selectively activated in HIV-latently infected primary CD4+ T cells via the BiP modulator HA15 55. ISR activation transiently disrupted latent HIV.19 Importantly, when ISR was prolonged, HIV RNA+ CD4+ T cells were reduced. Of note, ISR activation-induced HIV reservoir reduction had minimal impact on the cell death of HIV-negative CD4+ T cells or in resting CD4+ T cells derived from PWH, which are known to contain an extremely low number of HIV+ immune cells in the peripheral blood under suppressive ART. Interestingly, the depletion of the HIV reservoir was associated with the activation of ATF4/CHOP and ATF4/IFIT signaling without the modulation of autophagy signaling.19
When resting CD4+ T cells isolated from PWH were tested in a viral outgrowth assay, prolonged ISR activation reduced both cell-associated and cell-free HIV RNA as well as HIV proviral DNA, indicating that prolonged ISR activation reduces the replication-competent HIV by breaking the homeostasis signaling of stable HIV reservoirs in HIV+ resting CD4+ T cells. In one of the resting CD4+ T cell samples, the HIV reservoir (proviral DNA) was reduced >2,000-fold by ISR activation. Unexpectedly, when analyzed with scRNA-seq, a unique cell death pathway—ferroptosis—was downregulated, which could be responsible for the resistance of residual survived HIV+ CD4+ T cells.19 Interestingly, a separate study examined the transcriptional profiling in CD8+ T cells and discovered that mTOR and eIF2, the key kinases regulating cellular growth, proliferation, and metabolism during the activation of ISR, were highly expressed in the Elite controllers who maintained undetectable levels of HIV replication in the absence of ART. Thus, these data suggest that ISR/eIF2α signaling plays a unique role in regulating signaling pathways in both resting CD4+ T and CD8+ T cells, thereby controlling viral reservoirs.65
Together, these studies support the idea that modulating ISR/ATF4 signaling may be useful for the development of strategies that eradicate HIV reservoirs.19 Also, since ISR/ATF4 can be activated by ARDs, ISR/ATF4-induced cell death of infected immune cells in PWH on ART could be partially responsible for the suppression of HIV replication. However, ARD-induced ISR signaling may not be sufficient to further eradicate viral reservoirs, as ARDs are not specific to ISR/ATF4 signaling. They may not be at the optimal dosages to trigger strong ISR/ATF4 signaling for HIV eradication. Lastly, it is unknown whether the activation of ISR signaling is effective in viral eradication in other tissue sites, such as the brain, since exploiting ISR signaling to cure HIV is just emerging. Future animal studies will be beneficial to this.
Brain HIV reservoirs, HIV-associated neurocognitive disorders, and the integrated stress response
Despite successful ART, PWH have a high rate of non-communicable diseases including heart, cancer, stroke, diabetes, kidney disorders, and neurological diseases, which might be due to long-term unresolved immune inflammation.66 HIV DNA/RNA and near intact full-length (FL) HIV were detected in the brain of PWH on ART,67,68,69 suggesting viral persistence in the central nervous system. A recent study demonstrated the evidence of inducible replication-competent HIV reservoirs in brain resident immune cells, i.e., microglia, which could serve as a rebound source of viremia after therapy interruption.39 Residual but persistent HIV transcription was observed despite effective ART, which may trigger and maintain the persistent neuroinflammation in the brain microglia.39 These observations from PWH on ART advanced previous studies that characterized HIV infection and latency using microglial cell lines and in vitro stem cell-differentiated microglia.70 In addition to microglia, other CNS cells including perivascular macrophages, T cells, and astrocytes have been considered as viral reservoirs in the brain.45,71 However, direct evidence remains to be determined in the brains of PWH on long-term ART. Regardless, HIV can cross the blood-brain barrier and cause infection in the brain. There is evidence of HIV-derived proteins in the CNS. To understand how HIV causes neurotoxicity, researchers have proposed that pathogenesis at the cellular level is to be blamed.72
Local inflammation induced by HIV proteins has been associated with damaged brain connections, loss of brain function, and reduced brain white and gray matter.72 In the cerebrospinal fluid of PWH, inflammatory molecules such as IL-6 and IFNγ have been found, as well as chemokines such as MCP-1 and CXCL10, which attract other immune cells to the area of localized inflammation. Thus, neuroinflammation may play a role in the development of dementia.73
HAND occurs among PWH even under suppressive ART,74 which affects learning, speech, and body movements, indicating that both direct and indirect secondary effects of HIV infection contribute to HAND with symptoms ranging from subtle loss of motor control that is difficult to perceive by others in the same social circle, to HIV-related dementia that limits a person’s ability to perform basic daily tasks.75 Dementia was prevalent in PWH and was directly correlated with high blood viremia, low CD4+ T cell counts, and inflammatory markers.76 After ruling out tumors and subsequent infections, Xing and colleagues identified 11 out of 429 postmortem cases of AIDS that were suggestive of encephalitis, leading to dementia. In addition to viremia, characteristic multinucleated giant cells, vacuolation, and microglial nodules were observed in the white matter, as well as local IL-1β produced by microglial cells and TNF-inflammatory cytokine secreted by CD68+ myeloid cells.77 These observations support the idea that neuroinflammation is an important factor in the development of HAND. Fortunately, the worst clinical outcomes, such as dementia and encephalitis, which were commonly seen in the 1980s before the development of ART, have decreased in the presence of modern ART. However, as PWH age with continued ART, low or mild neurocognitive deficits remain common in PWH.74
While it is effective in controlling active HIV replication, the current ARDs still face challenges in effectively overcoming the blood-brain barrier and achieving therapeutic concentrations as present in the plasma. This compromises the control of HIV in this compartment and results in local inflammation. Some of the encountered difficulties rely on drug solubility, size, protein binding, and ionization profile.78 Also, it is unclear if the current LRAs can penetrate the brain with therapeutic potential without inducing toxicity for the CNS cells and whether it is suitable to exploit the “kick and kill” strategy for the cure of HIV in the CNS.
The ISR signaling is activated in the brain. ATF6 protein levels increased in the central frontal cortex in the HAND brain, which may especially occur in neurons and astrocytes and is correlated with high levels of phosphorylated elF2α, compared to healthy donors.79 Furthermore, oligodendrocytes, the cells responsible for myelin synthesis, exhibited an impaired maturation that is associated with elF2 phosphorylation by PERK, leading to activation of the ISR pathway, suggesting that ISR activation may impair neural crest cell replacement.80 It has been found that despite suppressive ART, HIV remains latent in the brain microglia.39 Ryan and colleagues (2020) reported HIV infection of microglia and the production of inflammatory cytokines such as TNF and IL-8 in iPSCs-induced microglia. The team also showed a pro-inflammatory profile using a tri-culture model with microglia, astrocytes, and forebrain-like excitatory neurons. Of note, ISR was activated in microglia, astrocytes, and neurons; however, ATF4 RNA induction was only observed in microglial cells.81 As an HIV-related process observed during the chronic stages of the disease, ISR appears activated and may cause brain damage in PWH with HAND. Lastly, there was a correlation between type I interferon neuroinflammation and increased ISR activation. There was also a correlation between decreased energy metabolism and oligodendrocyte myelin production, which may cause cognitive impairment due to white matter dysfunction.82
Together, ISR signaling activation may be involved in two distinct aspects of HIV pathogenesis in HAND: spontaneous viral reactivation in resident brain immune cells and persistent HIV infection-associated neuroinflammation. It is necessary to thoroughly investigate if viral awakening episodes during blips activate ISR signaling and cause dysfunction of the brain. While ART controls active viral replication, both protease inhibitors and persistent immune activation directly or indirectly activate the ISR pathway, contributing to overall behavioral dysfunction including neurological changes of white and gray matter in the brain of PWH on suppressive ART.83 Regardless, it remains unclear whether the manipulation of the ISR signaling will be beneficial for other tissue sites such as the gut, the reproductive tract, the lungs, and the liver. Future research is necessary to better understand how ISR activation affects HIV clearance throughout the body.
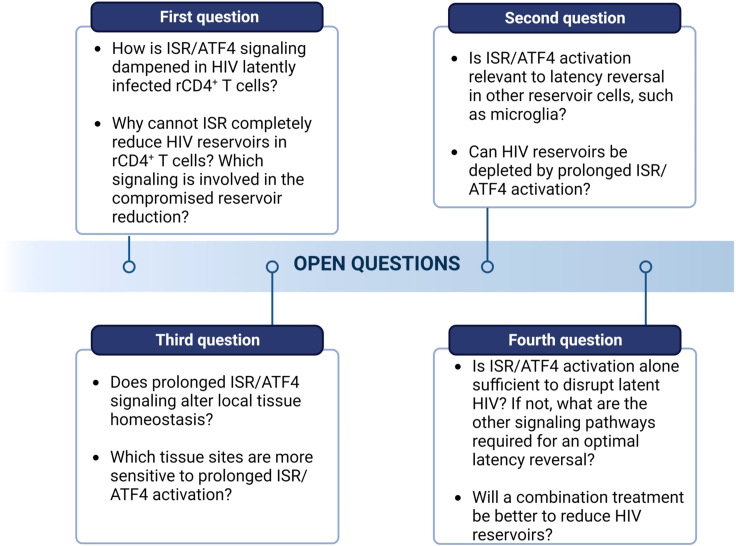
Outstanding questions
Emerging evidence indicates that the suppression of ISR/ATF4 signaling is involved in persistent HIV infection. In contrast, when ISR/ATF4 is activated, latent HIV can be disrupted. Intriguingly, when the signaling is prolonged, the latently infected CD4+ T cells can be depleted with minimal impact on the HIV-negative immune cells.19 However, several outstanding questions remain unanswered (Figure 3). First, how is the ISR/ATF4 signaling dampened in the HIV-latently infected resting CD4+ T cells? Better elucidating this stigma will be essential to not only understand the mechanism of latent HIV infection but also how the reservoir cells maintain their stable infection for HIV persistence. Why can’t ISR activation completely reduce the latent HIV reservoir in the resting CD4+ T cells? Are there any pathways involved in resisting further eradication during ISR induction? Is suppression of ferroptosis involved in it? Second, is ISR signaling similarly suppressed in brain immune cells as in HIV-latently infected T cells? This could be tested with the HIV latency model in human microglia, iPS-induced microglia, organoid model of HIV infection and latency, and patient brain-derived microglial cells.70 Third, do ISR modulators affect the tissue-specific homeostasis of ISR after prolonged activation? Lastly, only activating the ISR pathway may be insufficient to disrupt all latently infected HIV. If so, which combinations will be better and necessary for robust viral reactivation? Does prolonged ISR-induced latency reversal result in reservoir reduction in vivo? When these questions are addressed, future HIV reservoir depletion strategies may be more efficient by manipulating the ISR pathway for HIV reservoir clearance, which will not only eradicate latent HIV reservoirs in the peripheral blood but also the CNS and possibly other tissue sites.
Figure 3.
Questions remain to be answered regarding the feasibility of ISR/ATF4 manipulation for the reduction of HIV reservoirs, including the impact of ISR activation in latency reversal in the myeloid cells, the toxicity of ISR modulators, and the effectiveness of HIV reservoir reduction
Acknowledgments
We apologize for not being able to include all the excellent studies due to citation limits. G.J. is supported by NIMH (R21MH128034) and NIAID (R21AI167709).
Author contributions
We thank Ms. Nan Jiang for critically editing the manuscript. E.A.M. and G.J. wrote the initial version of the review. Y.T. reviewed and revised the HAND section. All authors read and revised multiple versions of the draft and approved the manuscript for submission.
Declaration of interests
The authors declare no conflict of interest.
References
- 1.Zhang G., Wang X., Rothermel B.A., Lavandero S., Wang Z.V. The integrated stress response in ischemic diseases. Cell Death Differ. 2022;29:750–757. doi: 10.1038/s41418-021-00889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demirci-Çekiç S., Özkan G., Avan A.N., Uzunboy S., Çapanoğlu E., Apak R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022;209 doi: 10.1016/j.jpba.2021.114477. [DOI] [PubMed] [Google Scholar]
- 3.Costa-Mattioli M., Walter P. The integrated stress response: From mechanism to disease. Science. 2020;368 doi: 10.1126/science.aat5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrbod P., Ande S.R., Alizadeh J., Rahimizadeh S., Shariati A., Malek H., Hashemi M., Glover K.K.M., Sher A.A., Coombs K.M., Ghavami S. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019;10:376–413. doi: 10.1080/21505594.2019.1605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang G., Santos Rocha C., Hirao L.A., Mendes E.A., Tang Y., Thompson G.R., 3rd, Wong J.K., Dandekar S. HIV Exploits Antiviral Host Innate GCN2-ATF4 Signaling for Establishing Viral Replication Early in Infection. mBio. 2017;8 doi: 10.1128/mBio.01518-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallejo-Gracia A., Chen I.P., Perrone R., Besnard E., Boehm D., Battivelli E., Tezil T., Krey K., Raymond K.A., Hull P.A., et al. FOXO1 promotes HIV latency by suppressing ER stress in T cells. Nat. Microbiol. 2020;5:1144–1157. doi: 10.1038/s41564-020-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 8.Dabo S., Meurs E.F. dsRNA-dependent protein kinase PKR and its role in stress, signaling and HCV infection. Viruses. 2012;4:2598–2635. doi: 10.3390/v4112598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J., Back S.H., Hur J., Lin Y.H., Gildersleeve R., Shan J., Yuan C.L., Krokowski D., Wang S., Hatzoglou M., et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 11.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 12.Gambardella G., Staiano L., Moretti M.N., De Cegli R., Fagnocchi L., Di Tullio G., Polletti S., Braccia C., Armirotti A., Zippo A., et al. GADD34 is a modulator of autophagy during starvation. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H., Tian M., Ding C., Yu S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018;9:3083. doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fessler E., Eckl E.M., Schmitt S., Mancilla I.A., Meyer-Bender M.F., Hanf M., Philippou-Massier J., Krebs S., Zischka H., Jae L.T. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature. 2020;579:433–437. doi: 10.1038/s41586-020-2076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y., Zhang Z., Li Y., Li Y. The Regulation of Integrated Stress Response Signaling Pathway on Viral Infection and Viral Antagonism. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.814635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roll-Mecak A., Alone P., Cao C., Dever T.E., Burley S.K. X-ray structure of translation initiation factor eIF2gamma: implications for tRNA and eIF2alpha binding. J. Biol. Chem. 2004;279:10634–10642. doi: 10.1074/jbc.M310418200. [DOI] [PubMed] [Google Scholar]
- 17.Hanson F.M., Hodgson R.E., de Oliveira M.I.R., Allen K.E., Campbell S.G. Regulation and function of elF2B in neurological and metabolic disorders. Biosci. Rep. 2022;42 doi: 10.1042/BSR20211699. BSR20211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloise C., Schipper J.G., de Groot R.J., van Kuppeveld F.J. Move and countermove: the integrated stress response in picorna- and coronavirus-infected cells. Curr. Opin. Immunol. 2022;79 doi: 10.1016/j.coi.2022.102254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D., Wong L.M., Tang Y., Allard B., James K.S., Thompson G.R., Dandekar S., Browne E.P., Li Q., Simon J.M., et al. Depletion of HIV reservoir by activation of ISR signaling in resting CD4(+)T cells. iScience. 2023;26 doi: 10.1016/j.isci.2022.105743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mbhele N., Chimukangara B., Gordon M. HIV-1 integrase strand transfer inhibitors: a review of current drugs, recent advances and drug resistance. Int. J. Antimicrob. Agents. 2021;57 doi: 10.1016/j.ijantimicag.2021.106343. [DOI] [PubMed] [Google Scholar]
- 21.Vanangamudi M., Kurup S., Namasivayam V. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): a brief overview of clinically approved drugs and combination regimens. Curr. Opin. Pharmacol. 2020;54:179–187. doi: 10.1016/j.coph.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh A.K., Osswald H.L., Prato G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016;59:5172–5208. doi: 10.1021/acs.jmedchem.5b01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao T., Cai Y., Chen B. HIV-1 Entry and Membrane Fusion Inhibitors. Viruses. 2021;13:735. doi: 10.3390/v13050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nastri B.M., Pagliano P., Zannella C., Folliero V., Masullo A., Rinaldi L., Galdiero M., Franci G. HIV and Drug-Resistant Subtypes. Microorganisms. 2023;11 doi: 10.3390/microorganisms11010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piacenti F.J. An update and review of antiretroviral therapy. Pharmacotherapy. 2006;26:1111–1133. doi: 10.1592/phco.26.8.1111. [DOI] [PubMed] [Google Scholar]
- 26.Borsa M., Ferreira P.L.C., Petry A., Ferreira L.G.E., Camargo M.M., Bou-Habib D.C., Pinto A.R. HIV infection and antiretroviral therapy lead to unfolded protein response activation. Virol. J. 2015;12:77. doi: 10.1186/s12985-015-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heck C.J.S., Hamlin A.N., Bumpus N.N. Efavirenz and Efavirenz-like Compounds Activate Human, Murine, and Macaque Hepatic IRE1alpha-XBP1. Mol. Pharmacol. 2019;95:183–195. doi: 10.1124/mol.118.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou H., Gurley E.C., Jarujaron S., Ding H., Fang Y., Xu Z., Pandak W.M., Jr., Hylemon P.B. HIV protease inhibitors activate the unfolded protein response and disrupt lipid metabolism in primary hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G1071–G1080. doi: 10.1152/ajpgi.00182.2006. [DOI] [PubMed] [Google Scholar]
- 29.Fraichard C., Bonnet-Serrano F., Laguillier-Morizot C., Hebert-Schuster M., Lai-Kuen R., Sibiude J., Fournier T., Cohen M., Guibourdenche J. Protease Inhibitor Anti-HIV, Lopinavir, Impairs Placental Endocrine Function. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akay C., Cooper M., Odeleye A., Jensen B.K., White M.G., Vassoler F., Gannon P.J., Mankowski J., Dorsey J.L., Buch A.M., et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J. Neurovirol. 2014;20:39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zulu S.S., Abboussi O., Simola N., Mabandla M.V., Daniels W.M.U. Anti-HIV drugs promote beta-amyloid deposition and impair learning and memory in BALB/c mice. Acta Neuropsychiatr. 2020;32:257–264. doi: 10.1017/neu.2020.19. [DOI] [PubMed] [Google Scholar]
- 32.Zeng M., Sang W., Chen S., Chen R., Zhang H., Xue F., Li Z., Liu Y., Gong Y., Zhang H., Kong X. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017;271:26–37. doi: 10.1016/j.toxlet.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Chen J., Zhou T., Zhang Y., Luo S., Chen H., Chen D., Li C., Li W. The reservoir of latent HIV. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.945956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calado M., Pires D., Conceicao C., Ferreira R., Santos-Costa Q., Anes E., Azevedo-Pereira J.M. Cell-to-Cell Transmission of HIV-1 and HIV-2 from Infected Macrophages and Dendritic Cells to CD4+ T Lymphocytes. Viruses. 2023;15:1030. doi: 10.3390/v15051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurga A.M., Paleczna M., Kuter K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020;14:198. doi: 10.3389/fncel.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balestra E., Perno C.F., Aquaro S., Panti S., Bertoli A., Piacentini M., Forbici F., D'Arrigo R., Calió R., Garaci E. Macrophages: a crucial reservoir for human immunodeficiency virus in the body. J. Biol. Regul. Homeost. Agents. 2001;15:272–276. [PubMed] [Google Scholar]
- 37.Bertram K.M., Botting R.A., Baharlou H., Rhodes J.W., Rana H., Graham J.D., Patrick E., Fletcher J., Plasto T.M., Truong N.R., et al. Identification of HIV transmitting CD11c(+) human epidermal dendritic cells. Nat. Commun. 2019;10:2759. doi: 10.1038/s41467-019-10697-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellery P.J., Tippett E., Chiu Y.L., Paukovics G., Cameron P.U., Solomon A., Lewin S.R., Gorry P.R., Jaworowski A., Greene W.C., et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 39.Tang Y., Chaillon A., Gianella S., Wong L.M., Li D., Simermeyer T.L., Porrachia M., Ignacio C., Woodworth B., Zhong D., et al. Brain microglia serve as a persistent HIV reservoir despite durable antiretroviral therapy. J. Clin. Invest. 2023;133 doi: 10.1172/JCI167417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang G., Nguyen D., Archin N.M., Yukl S.A., Méndez-Lagares G., Tang Y., Elsheikh M.M., Thompson G.R., 3rd, Hartigan-O'Connor D.J., Margolis D.M., et al. HIV latency is reversed by ACSS2-driven histone crotonylation. J. Clin. Invest. 2018;128:1190–1198. doi: 10.1172/JCI98071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li D., Dewey M.G., Wang L., Falcinelli S.D., Wong L.M., Tang Y., Browne E.P., Chen X., Archin N.M., Margolis D.M., Jiang G. Crotonylation sensitizes IAPi-induced disruption of latent HIV by enhancing p100 cleavage into p52. iScience. 2022;25 doi: 10.1016/j.isci.2021.103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M., Chen Z., Zhang Y. CBP/p300 and HDAC activities regulate H3K27 acetylation dynamics and zygotic genome activation in mouse preimplantation embryos. EMBO J. 2022;41 doi: 10.15252/embj.2022112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moranguinho I., Valente S.T. Block-And-Lock: New Horizons for a Cure for HIV-1. Viruses. 2020;12:1443. doi: 10.3390/v12121443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori L., Valente S.T. Cure and Long-Term Remission Strategies. Methods Mol. Biol. 2022;2407:391–428. doi: 10.1007/978-1-0716-1871-4_26. [DOI] [PubMed] [Google Scholar]
- 45.Margolis D.M., Archin N.M., Cohen M.S., Eron J.J., Ferrari G., Garcia J.V., Gay C.L., Goonetilleke N., Joseph S.B., Swanstrom R., et al. Curing HIV: Seeking to Target and Clear Persistent Infection. Cell. 2020;181:189–206. doi: 10.1016/j.cell.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abner E., Jordan A. HIV "shock and kill" therapy: In need of revision. Antiviral Res. 2019;166:19–34. doi: 10.1016/j.antiviral.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Dahabieh M.S., Battivelli E., Verdin E. Understanding HIV latency: the road to an HIV cure. Annu. Rev. Med. 2015;66:407–421. doi: 10.1146/annurev-med-092112-152941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muto N.F., Martinand-Mari C., Adelson M.E., Suhadolnik R.J. Inhibition of replication of reactivated human immunodeficiency virus type 1 (HIV-1) in latently infected U1 cells transduced with an HIV-1 long terminal repeat-driven PKR cDNA construct. J. Virol. 1999;73:9021–9028. doi: 10.1128/jvi.73.11.9021-9028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaspart A., Calmels C., Cosnefroy O., Bellecave P., Pinson P., Claverol S., Guyonnet-Dupérat V., Dartigues B., Benleulmi M.S., Mauro E., et al. GCN2 phosphorylates HIV-1 integrase and decreases HIV-1 replication by limiting viral integration. Sci. Rep. 2017;7:2283. doi: 10.1038/s41598-017-02276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caselli E., Benedetti S., Gentili V., Grigolato J., Di Luca D. Short communication: activating transcription factor 4 (ATF4) promotes HIV type 1 activation. AIDS Res. Hum. Retroviruses. 2012;28:907–912. doi: 10.1089/AID.2011.0252. [DOI] [PubMed] [Google Scholar]
- 51.del Pino J., Jiménez J.L., Ventoso I., Castelló A., Muñoz-Fernández M.Á., de Haro C., Berlanga J.J. GCN2 has inhibitory effect on human immunodeficiency virus-1 protein synthesis and is cleaved upon viral infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quiterio S., Grant C., Hogan T.H., Krebs F.C., Wigdahl B. C/EBP- and Tat-mediated activation of the HIV-1 LTR in CD34+ hematopoietic progenitor cells. Biomed. Pharmacother. 2003;57:49–56. doi: 10.1016/s0753-3322(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka J., Ishida T., Choi B.I., Yasuda J., Watanabe T., Iwakura Y. Latent HIV-1 reactivation in transgenic mice requires cell cycle -dependent demethylation of CREB/ATF sites in the LTR. AIDS. 2003;17:167–175. doi: 10.1097/00002030-200301240-00005. [DOI] [PubMed] [Google Scholar]
- 54.Lee S.D., Yu K.L., Park S.H., Jung Y.M., Kim M.J., You J.C. Understanding of the functional role(s) of the Activating Transcription Factor 4(ATF4) in HIV regulation and production. BMB Rep. 2018;51:388–393. doi: 10.5483/BMBRep.2018.51.8.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cerezo M., Lehraiki A., Millet A., Rouaud F., Plaisant M., Jaune E., Botton T., Ronco C., Abbe P., Amdouni H., et al. Compounds Triggering ER Stress Exert Anti-Melanoma Effects and Overcome BRAF Inhibitor Resistance. Cancer Cell. 2016;29:805–819. doi: 10.1016/j.ccell.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 56.De Vito A., Lazzaro M., Palmisano I., Cittaro D., Riba M., Lazarevic D., Bannai M., Gabellini D., Schiaffino M.V. Amino acid deprivation triggers a novel GCN2-independent response leading to the transcriptional reactivation of non-native DNA sequences. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández Larrosa P.N., Croci D.O., Riva D.A., Bibini M., Luzzi R., Saracco M., Mersich S.E., Rabinovich G.A., Martínez Peralta L. Apoptosis resistance in HIV-1 persistently-infected cells is independent of active viral replication and involves modulation of the apoptotic mitochondrial pathway. Retrovirology. 2008;5:19. doi: 10.1186/1742-4690-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sampath R., Cummins N.W., Badley A.D. Casp8p41: The Protean Mediator of Death in CD4 T-cells that Replicate HIV. J. Cell Death. 2016;9:9–17. doi: 10.4137/JCD.S39872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alto A., Natesampillai S., Chandrasekar A.P., Krogman A., Misra A., Shweta F., VanLith C., Yao J.D., Cummins N.W., Badley A.D. The Combination of Venetoclax and Ixazomib Selectively and Efficiently Kills HIV-Infected Cell Lines but Has Unacceptable Toxicity in Primary Cell Models. J. Virol. 2021;95 doi: 10.1128/JVI.00138-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell G.R., Bruckman R.S., Chu Y.L., Trout R.N., Spector S.A. SMAC Mimetics Induce Autophagy-Dependent Apoptosis of HIV-1-Infected Resting Memory CD4+ T Cells. Cell Host Microbe. 2018;24:689–702.e7. doi: 10.1016/j.chom.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M., Liu W., Bauch T., Graviss E.A., Arduino R.C., Kimata J.T., Chen M., Wang J. Clearance of HIV infection by selective elimination of host cells capable of producing HIV. Nat. Commun. 2020;11:4051. doi: 10.1038/s41467-020-17753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao S., Lungu C., Crespo R., Steijaert T.H., Gorska A., Palstra R.J., Prins H.A.B., van Ijcken W., Mueller Y.M., van Kampen J.J.A., et al. Selective cell death in HIV-1-infected cells by DDX3 inhibitors leads to depletion of the inducible reservoir. Nat. Commun. 2021;12:2475. doi: 10.1038/s41467-021-22608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q., Gao H., Clark K.M., Mugisha C.S., Davis K., Tang J.P., Harlan G.H., DeSelm C.J., Presti R.M., Kutluay S.B., Shan L. CARD8 is an inflammasome sensor for HIV-1 protease activity. Science. 2021;371 doi: 10.1126/science.abe1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikebe E., Matsuoka S., Tezuka K., Kuramitsu M., Okuma K., Nakashima M., Kobayashi S., Makiyama J., Yamagishi M., Oyadomari S., et al. Activation of PERK-ATF4-CHOP pathway as a novel therapeutic approach for efficient elimination of HTLV-1-infected cells. Blood Adv. 2020;4:1845–1858. doi: 10.1182/bloodadvances.2019001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chowdhury F.Z., Ouyang Z., Buzon M., Walker B.D., Lichterfeld M., Yu X.G. Metabolic pathway activation distinguishes transcriptional signatures of CD8+ T cells from HIV-1 elite controllers. AIDS. 2018;32:2669–2677. doi: 10.1097/QAD.0000000000002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunt P.W. HIV and inflammation: mechanisms and consequences. Curr. HIV AIDS Rep. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 67.Trillo-Pazos G., Diamanturos A., Rislove L., Menza T., Chao W., Belem P., Sadiq S., Morgello S., Sharer L., Volsky D.J. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cochrane C.R., Angelovich T.A., Byrnes S.J., Waring E., Guanizo A.C., Trollope G.S., Zhou J., Vue J., Senior L., Wanicek E., et al. Intact HIV Proviruses Persist in the Brain Despite Viral Suppression with ART. Ann. Neurol. 2022;92:532–544. doi: 10.1002/ana.26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angelovich T.A., Cochrane C.R., Zhou J., Tumpach C., Byrnes S.J., Jamal Eddine J., Waring E., Busman-Sahay K., Deleage C., Jenkins T.A., et al. Regional Analysis of Intact and Defective HIV Proviruses in the Brain of Viremic and Virally Suppressed People with Hiv. Ann. Neurol. 2023;94:798–802. doi: 10.1002/ana.26750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang Y., Jiang G. Eradication of human immunodeficiency virus-1 reservoir in the brain microglia. Neural Regen. Res. 2023;18:552–553. doi: 10.4103/1673-5374.350198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wahl A., Al-Harthi L. HIV infection of non-classical cells in the brain. Retrovirology. 2023;20:1. doi: 10.1186/s12977-023-00616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Valle L., Croul S., Morgello S., Amini S., Rappaport J., Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J. Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- 73.Mehla R., Bivalkar-Mehla S., Nagarkatti M., Chauhan A. Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J. Neuroinflammation. 2012;9:239. doi: 10.1186/1742-2094-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vastag Z., Fira-Mladinescu O., Rosca E.C. HIV-Associated Neurocognitive Disorder (HAND): Obstacles to Early Neuropsychological Diagnosis. Int. J. Gen. Med. 2022;15:4079–4090. doi: 10.2147/IJGM.S295859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong S., Banks W.A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav. Immun. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y., Liu M., Lu Q., Farrell M., Lappin J.M., Shi J., Lu L., Bao Y. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology. 2020;95:e2610–e2621. doi: 10.1212/WNL.0000000000010752. [DOI] [PubMed] [Google Scholar]
- 77.Xing H.Q., Hayakawa H., Izumo K., Kubota R., Gelpi E., Budka H., Izumo S. In vivo expression of proinflammatory cytokines in HIV encephalitis: an analysis of 11 autopsy cases. Neuropathology. 2009;29:433–442. doi: 10.1111/j.1440-1789.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 78.Ene L., Duiculescu D., Ruta S.M. How much do antiretroviral drugs penetrate into the central nervous system? J. Med. Life. 2011;4:432–439. [PMC free article] [PubMed] [Google Scholar]
- 79.Akay C., Lindl K.A., Shyam N., Nabet B., Goenaga-Vazquez Y., Ruzbarsky J., Wang Y., Kolson D.L., Jordan-Sciutto K.L. Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex. Neuropathol. Appl. Neurobiol. 2012;38:175–200. doi: 10.1111/j.1365-2990.2011.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roth L.M., Zidane B., Festa L., Putatunda R., Romer M., Monnerie H., Jordan-Sciutto K.L., Grinspan J.B. Differential effects of integrase strand transfer inhibitors, elvitegravir and raltegravir, on oligodendrocyte maturation: A role for the integrated stress response. Glia. 2021;69:362–376. doi: 10.1002/glia.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryan S.K., Gonzalez M.V., Garifallou J.P., Bennett F.C., Williams K.S., Sotuyo N.P., Mironets E., Cook K., Hakonarson H., Anderson S.A., Jordan-Sciutto K.L. Neuroinflammation and EIF2 Signaling Persist despite Antiretroviral Treatment in an hiPSC Tri-culture Model of HIV Infection. Stem Cell Rep. 2020;14:991. doi: 10.1016/j.stemcr.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solomon I.H., Chettimada S., Misra V., Lorenz D.R., Gorelick R.J., Gelman B.B., Morgello S., Gabuzda D. White Matter Abnormalities Linked to Interferon, Stress Response, and Energy Metabolism Gene Expression Changes in Older HIV-Positive Patients on Antiretroviral Therapy. Mol. Neurobiol. 2020;57:1115–1130. doi: 10.1007/s12035-019-01795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soontornniyomkij V., Umlauf A., Soontornniyomkij B., Gouaux B., Ellis R.J., Levine A.J., Moore D.J., Letendre S.L. Association of antiretroviral therapy with brain aging changes among HIV-infected adults. AIDS. 2018;32:2005–2015. doi: 10.1097/QAD.0000000000001927. [DOI] [PMC free article] [PubMed] [Google Scholar]