Abstract
The growth plate is a transitional region of cartilage and highly diversified chondrocytes that controls long bone formation. The composition of growth plate cartilage changes markedly from the epiphysis to the metaphysis, notably with the loss of type II collagen, concomitant with an increase in MMP-13; type X collagen; and the C-propeptide of type II collagen. In contrast, the fate of aggrecan in the growth plate is not clear: there is biosynthesis and loss of aggrecan from hypertrophic cartilage, but the mechanism of loss is unknown. All matrix metalloproteinases (MMPs) cleave aggrecan between amino acids N341 and F342 in the proteinase-sensitive interglobular domain (IGD), and MMPs in the growth plate are thought to have a role in aggrecanolysis. We have generated mice with aggrecan resistant to proteolysis by MMPs in the IGD and found that the mice develop normally with no skeletal deformities. The mutant mice do not accumulate aggrecan, and there is no significant compensatory proteolysis occurring at alternate sites in the IGD. Our studies reveal that MMP cleavage in this key region is not a predominant mechanism for removing aggrecan from growth plate cartilage.
Endochondral ossification is the process by which bone is formed upon a cartilage template. It occurs in skeletal development, in fracture healing, and during bone growth at the growth plates and involves the transition from avascular cartilage, rich in type II collagen and aggrecan, to highly vascular bone, rich in type I collagen and mineral. During endochondral bone formation, prechondrogenic mesenchymal cells differentiate into chondrocytes, which proliferate and then mature into hypertrophic chondrocytes. The hypertrophic cartilage begins to calcify, the cartilage matrix is degraded and is invaded by blood vessels, the terminal hypertrophic chondrocytes apoptose, and bone matrix is deposited on the calcified cartilage trabeculae. This is a busy schedule of events in the growth plate, and their precise timing in relation to each other has not been clearly defined.
The growth plate is a striking remodeling unit both anatomically and biochemically. Anatomically, it is bordered by the secondary center of ossification and epiphysis at one end and the bony metaphysis at the other. It comprises reserve, proliferative, prehypertrophic, and hypertrophic zones. Chondrocytes in the reserve zone are rounded and sparsely distributed, whereas chondrocytes in the proliferative zone are flattened and occupy approximately one-quarter of the tissue volume. Cells of the proliferative zone divide and arrange themselves into ordered columns separated by longitudinal septa. At the same time, they increase their secretion of matrix molecules so that around 75% of the tissue volume is cartilage matrix. In the hypertrophic zones, the cells retain their columnar organization and increase their metabolic activity. They enlarge by as much as 10-fold (28) so that matrix volume is reduced to approximately 40%, with cells occupying 60% of the tissue volume (5). Calcification and chondrocyte apoptosis commence at the bottom of the hypertrophic zone.
Biochemically, there are distinct changes in the content and concentration of structural molecules, transcription factors, growth modulators, and proteinases through the zones of the growth plate. There is a decreasing gradient of type II and type IX collagen from the reserve zone to the lower hypertrophic zone and an increasing gradient of calcium and phosphate (47). Type X collagen is expressed exclusively in the hypertrophic zone, and the concentrations of hyaluronan (50) and chondrocalcin (the C-propeptide of type II collagen) (1) are markedly higher in the hypertrophic zone than in the reserve or proliferative zones.
During endochondral ossification, the aggrecan and type II-collagen-rich cartilage is resorbed. Type II collagen provides shape and tensile strength; aggrecan confers a capacity for weight-bearing and compressive resilience by maintaining tissue hydration. These are the cornerstones of cartilage function after birth. However, aggrecan and type II collagen also have crucial roles in joint formation, and mutations in either gene cause developmental skeletal defects.
A large number of mutations in the human type II collagen gene have been reported, and many cause severe skeletal abnormalities that are lethal before, or soon after, birth. No mutations in the human aggrecan gene have been reported; however, spontaneous mutations in which there is no expression of full-length aggrecan arise in the cmd/cmd mouse (31, 55), the cmd-Bc mouse (33), and the nanomelic chicken (2, 37). These animals have severely disrupted growth plates and skeletal deformities, and the homozygous mutations are lethal. In other mutants, such as the chubby (cby) (80) and brachymorphic (bm/bm) (79) mice and the tibial dyschondroplasia chicken (71), the structure of aggrecan glycosaminoglycan chains is abnormal; these animals also exhibit major growth plate defects.
Most studies of aggrecan turnover have focused on the articular cartilage because of the relationship between aggrecan loss and pathology; in contrast, very little work has been done on aggrecan turnover in the growth plate. In articular cartilage, aggrecan loss is mediated by matrix metalloproteinases (MMPs) and aggrecanases, which are members of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family of enzymes. Neoepitopes representing specific markers for the activities of both these families of enzymes can be detected in humans (3, 14, 34, 66) and mice (62, 72-74). Our previous in vitro studies suggested that MMPs are involved in the baseline turnover of aggrecan, whereas aggrecanases drive accelerated turnover. Other studies in mouse models of arthritis suggest that aggrecanases are involved in initiating early aggrecan loss and cartilage damage but that severe, late-stage disease may be MMP driven (74-76). The major aggrecanase in articular cartilage appears to be ADAMTS-5 (64).
In contrast to the detailed studies in articular cartilage, very little is known about which proteinases are responsible for aggrecanolysis in the growth plate. Neoepitopes specific for the products of MMP and aggrecanase cleavage are present (19, 35, 36, 41), indicating that these enzymes are active in growing cartilage. However, ablation of the major aggrecanases ADAMTS-1 (39), ADAMTS-4, (19, 19a, 64), or ADAMTS-5 (19a, 64) does not produce skeletal defects, suggesting that aggrecanases do not have a role in aggrecan remodeling in the growth plate.
A number of MMPs, including MMP-2, -3, -8, -9, -10, -12, and -13, are also present in the growth plate (18, 23, 43), and null mutations in selected MMPs result in its altered maturation. The MMP-9-null mouse has a phenotype in which vascularization and calcification of the hypertrophic cartilage are delayed, causing elongation and late maturation of the growth plate (77), and the phenotype of the MMP-13-null mouse is very similar (30, 65). Compensation, presumably by other MMPs, resolves the phenotype in the MMP-9- and MMP-13-null mice, and they mature with normal skeletons; however, the findings point to a central role for MMPs in endochondral ossification.
Aggrecan loss from articular cartilage is predominantly a proteolytic process. Multiple cleavage sites are located in the interglobular domain (IGD) near the N terminus and the chondroitin sulfate (CS)-rich region at the C terminus. Proteolysis in the IGD releases the entire glycosaminoglycan-containing portion of aggrecan and is detrimental for articular cartilage function. Proteolysis in the CS-rich region precedes IGD cleavage (70) but appears to be less critical since most aggrecan extracted from adult articular cartilage lacks varying portions of the CS-rich region (9), suggesting that processing from the C terminus is part of normal ageing (29). For this reason, our study is focused on MMP cleavage in the IGD. Aggrecan is cleaved by all MMPs at the sequence DIPEN341↓342FFGVG in the IGD, and cleavage at this site occurs at both the epiphyseal and metaphyseal borders of the growth plate in association with ossification (36). It is not known whether the disruption of normal aggrecan-processing pathways might lead to abnormalities in endochondral ossification.
In the present study, we have generated genetically modified mice with a mutation that renders the aggrecan IGD resistant to cleavage at the major MMP cleavage site. Compared with MMP-null mice in which ablation of any one MMP may be compensated for by another, the mice in this study resisted cleavage by all members of the MMP family at a single key site in the IGD. The mice developed normally with no skeletal abnormalities. This is surprising given the environment of the growth plate with its active MMPs and exceptionally high concentration of aggrecan (substrate). Our results challenge the widely held tenet that loss of aggrecan from the growth plate is mediated by MMPs and lend support to emerging hypotheses implicating nonproteolytic mechanisms of aggrecanolysis in epiphyseal cartilage.
MATERIALS AND METHODS
Generation of mice resistant to MMP cleavage in the aggrecan IGD.
Heterozygous C57BL/6 aggrecan knockin mice with a targeted modification in exon 7 were generated under contract by Ozgene Pty Ltd., Western Australia, Australia, and their genotype was confirmed by Southern blotting. We used sequencing and restriction digests to verify the mutation, which replaced nucleotides TTCTTCGGG with GGTACCCGG in exon 7. The mutation changed amino acids 342FFG to 342GTR, eliminating the MMP cleavage site at the N341↓342F bond (45) and rendering the mice resistant to cleavage at this site by all MMPs. Mice homozygous for the mutation were designated Chloe. In the present study, age-matched wild-type, Chloe, and heterozygous mice were obtained from heterozygote breeding pairs and used to provide tissues for histology, cartilage extracts, and aggrecan analysis. Femoral head cartilage for in vitro culture experiments was obtained from the age-matched offspring of homozygote wild-type or Chloe breeding pairs. All mouse experiments were approved by the Animal Experimentation Ethics Committee of the Royal Children's Hospital, Parkville, Australia.
Genotyping.
The mutation in exon 7 introduced a unique KpnI restriction site. Genomic DNA was prepared by proteinase K digestion of tail biopsies, and a 758-bp cDNA fragment was amplified by PCR using forward and reverse primers with sequences 5′-AGGCGGAAATAGGAACTGA and 5′-TCTCCAGTTCTCCTCAGC, respectively. Genotypes were determined by digesting the 758-bp PCR fragment with KpnI.
Aggrecan analysis.
Mouse femoral heads were harvested as described previously (39) and extracted for 48 h at 4°C with buffered 4 M guanidinium hydrochloride and proteinase inhibitors and then dialyzed against ultrapure water for 16 h at 4°C. The tissue residue remaining after extraction was digested with papain. Aggrecan content was determined by the concentration of glycosaminoglycans in the dialyzed extracts and papain digests, measured by the 1,9-dimethylmethylene blue assay (10) using CS C from shark cartilage (Sigma-Aldrich Co.) as a standard.
Extracts of femoral head cartilage were analyzed by Western blotting with neoepitope antibodies for the presence of G1 fragments, produced by MMP or aggrecanase activity. Dialyzed extracts were brought to 0.1 M Tris acetate, pH 6.5, and digested for 2 h at 37°C with 0.01 U of chondroitinase ABC (Seikagaku Corporation, Tokyo, Japan) per 10 μg of glycosaminoglycan. Aliquots of cartilage extracts proportional to equal amounts of tissue weight were electrophoresed on sodium dodecyl sulfate (SDS) gels under reducing conditions and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.; Osmonics) for Western blotting.
Neoepitope antibodies recognize the newly created N or C termini of degradation products generated by proteolysis but fail to recognize the same sequence of amino acids present in the intact protein. Neoepitope antibodies are therefore markers of cleavage by specific families of proteinases. Rabbit polyclonal neoepitope antibodies used in this study were anti-DIPEN (44) and anti-TAFASED that recognize the products of MMP cleavage in the IGD and anti-NITEGE that recognizes the products of aggrecanase cleavage in the IGD (44). The anti-NITEGE antibody was immunoreactive with the mouse sequence containing valine in place of isoleucine-369 (N369VTEGE). A rabbit anti-G1 domain antibody (a gift from Tim Hardingham, University of Manchester, Manchester, United Kingdom) was used to detect all G1-containing fragments. Horseradish peroxidase-conjugated secondary antibodies were from Dako (Glostrup, Denmark), and enhanced chemiluminescence kits were from Amersham (Little Chalfont, United Kingdom).
The glycosaminoglycan composition of aggrecan extracted from wild-type and Chloe mice was analyzed by fluorophore-assisted carbohydrate electrophoresis (FACE) as described previously (52). Briefly, femoral heads harvested from 10-day-old mice were digested overnight with proteinase K at 60°C. After boiling to inactivate the enzyme, tissue debris was removed by centrifugation. Glycosaminoglycans in the supernatants were precipitated at −20°C and recovered by centrifugation. Δ-Disaccharides, generated by overnight digestion of glycosaminoglycans with chondroitinase ABC, were collected in the filtrate of MicroCon 3 filter devices (Millipore), freeze-dried, and resuspended in 5 μl of the fluorophore aminoacridone. Aliquots of standard Δ-disaccharides of CS (Seikagaku Corporation) were prepared at the same time. The samples and standards were incubated at room temperature for 15 min, and then 5 μl of 1 M cyanoborohydride was added and fluorotagging was allowed to proceed for 16 h at 37°C. The samples were cooled to room temperature, mixed with 30 μl of 25% glycerol (BDH, Poole, United Kingdom), and analyzed by electrophoresis on high-percentage polyacrylamide Monogels (Prozyme) or stored at −70°C. Fluorescently labeled Δ-disaccharides were visualized on a Bio-Rad Chemidoc XRS system, and the mass of each fluorotagged species was quantitated using Quantity One software (Bio-Rad).
Cartilage isolation and culture.
Femoral head cartilage was isolated from 12- to 19-day-old wild-type and Chloe mice under sterile conditions by using forceps to induce a capital femoral physeal fracture. The femoral heads were cultured at 37°C in a 5% CO2 humidified incubator for 3 days in HEPES-buffered Dulbecco's modified Eagle medium (DMEM) containing 10% fetal calf serum and then washed (three times for 5 min each) in serum-free DMEM. Individual femoral heads were then cultured for 3 days in 0.4 ml of serum-free DMEM ± 10−5 M retinoic acid (RA; Sigma) or 10 ng of recombinant human interleukin-1α (IL-1α; Sigma)/ml with no medium changes. At the end of the culture period, the media were collected and the femoral heads were blotted dry, weighed, and extracted at 4°C with 4 M GuHCl containing proteinase inhibitors for 48 h, as described above. Sulfated glycosaminoglycans in the dialyzed extracts and culture media were measured by the 1,9-dimethylmethlene blue assay. Differences in the release of sulfated glycosaminoglycan (aggrecan) into the medium (expressed as micrograms of GAG per milligrams [wet weight] of cartilage) associated with genotype and treatment were assessed using a two-factor analysis of variance and Fisher's post hoc analysis. All data were analyzed using the Stat View software package for Macintosh (Acura), with P values of ≤0.05 considered statistically significant.
Growth plate morphology, immunohistology, and calcification.
Knee joints (midtibia to midfemur) harvested from mice were trimmed of surrounding muscle and soft tissue, fixed overnight at 4°C in 10% neutral buffered formalin, and then decalcified for 24 h at 4°C on a rocking platform with 10% formic acid containing 5% formalin. Decalcified limbs were washed in tap water and equilibrated in phosphate-buffered saline containing 0.01 M EDTA for several hours prior to embedding in paraffin. Six-micron-thick sagittal sections were adhered to Superfrost Plus slides (Biolab Scientific, Clayton, Australia) at 60°C overnight, dewaxed, and stained with hematoxylin and eosin or toluidine blue with a fast green counterstain as described previously (38). To visualize G1-DIPEN fragments, the sections were dewaxed, rehydrated in graded ethanol, and incubated in 3% H2O2 in water for 5 min to block endogenous peroxidase activity. Sections were treated with 0.1 U of protease-free chondroitinase ABC (ICN, Irvine, Calif.)/ml for 2 h at 37°C. After blocking with normal goat serum, sections were incubated with primary antibody overnight at 4°C. Immunolocalization was done using the Vector Stain Elite kit (Vector Laboratories) and 3,3′-diaminobenzidine (3 min), and the slides were counterstained with Mayer's hematoxylin. Anti-DIPEN neoepitope specificity was assessed by preabsorption of the antibody with 5 μM immunizing peptide GEDFVDIPEN, a peptide spanning the cleavage site FVDIPENFFG or an irrelevant peptide PLPRNITEGE. Mineralization of skeletal tissues of newborn Chloe and wild-type mice was evaluated in cleared whole mounts stained with alcian blue and alizarin red as described previously (22). Histomorphometry was done according to standard procedures (61) in the proximal tibia by using the Osteomeasure system (Osteometrics Inc., Decatur, Ga.). Growth plate widths were measured across at least 1.2 mm. Statistical analyses were done by analysis of variance and Fisher's post hoc test. Values are expressed as means ± standard errors of the means (SEM).
Generation of the anti-TAFASED neoepitope antibody.
A synthetic peptide with the sequence CGGTAFASED was purchased from Auspep (Melbourne, Australia) and conjugated to ovalbumin by using bromoacetic acid N-hydroxysuccinimide ester (25). Polyclonal antiserum raised against the ovalbumin-conjugated peptide was produced in rabbits at the Institute for Medical and Veterinary Science (Adelaide, South Australia, Australia) and screened against the peptide immunogen conjugated to bovine serum albumin. Truncated and extended 10-mer peptides (Auspep, Parkville, Australia) with the sequences PDSATAFASE and SATAFASEDL, respectively, were used as competitors to demonstrate neoepitope specificity in competition enzyme-linked immunosorbent assays (ELISA) (12) and compared with a 10-mer peptide corresponding to the neoepitope sequence DSATAFASED.
Immunofluorescence.
Knee joints from 3-week-old wild-type and Chloe mice were isolated, embedded in Tissue-Tek, frozen, and stored at −80°C until needed. Frozen sections were cut, fixed, treated with chondroitinase ABC, and stained as described previously (15). Primary antibodies used were either rabbit anti-DIPEN immunoglobulin G (IgG; 0.5 μg/ml) or rabbit anti-TAFASED IgG (0.1 to 0.5 μg/ml) or the preimmune serum from this rabbit (1:6,000). The secondary antibody was a goat anti-rabbit IgG-fluorescein isothiocyanate (Jackson ImmunoResearch Inc., Bar Harbor, Maine). Sections were incubated with primary antibodies diluted in 1% bovine serum albumin-5% normal goat serum in phosphate-buffered saline overnight at 4°C, washed repeatedly, and then incubated with secondary antibody for 1 h at room temperature. The sections were mounted in Vectashield aqueous mountant and viewed by epifluorescence microscopy on an Olympus IX70 inverted microscope fitted with a SPOT RT monochrome camera and software version 3.5 (Diagnostic Instruments Inc.). Fluorescence and phase images of identical fields were captured using the same conditions and then merged using the SPOT software.
Enzyme digestions.
Pro-MMP-2 purified from human gingival fibroblast conditioned medium (78) and recombinant human pro-MMP-3 (32) were gifts from Gillian Murphy, Cambridge, United Kingdom. Recombinant human pro-MMP-13 was a gift from Vera Knäuper and Gillian Murphy, Cambridge, United Kingdom. Pro-MMP-2 and -13 were activated with 2 mM 4-aminophenylmercuric acetate for 1 h at 37°C, and pro-MMP-3 was activated with 10 μg of trypsin/ml for 20 min at room temperature. Trypsin was inactivated with a 10-fold molar excess of soybean trypsin inhibitor. Solution digests were done in buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 10 mM CaCl2 at 37°C for specified times. To create the DIPEN and TAFASED neoepitopes in tissue sections, chondroitinase-digested sections were incubated at 37°C with either purified MMP-13 (10 μg/ml for 1 h) or a cocktail containing MMP-2, -3, and -13 (10 μg/ml each for 24 h) prior to immunolocalization. For N-glycosidase F treatment, samples were denatured by boiling in 0.125 M Tris-HCl, pH 6.8, containing 1% SDS and 0.6% (wt/vol) Nonidet P-40. The cooled samples were digested overnight at 37°C with 1 U of N-glycosidase F.
RESULTS
The mutation changing F342FG to G342TR introduced a unique KpnI restriction site in exon 7 that was used for genotyping (Fig. 1). Following PCR amplification of a 758-bp cDNA fragment from genomic DNA, KpnI restriction digestion yielded 530- and 228-bp fragments from Chloe DNA; 530-, 228-, and 758-bp fragments from heterozygote DNA; and the undigested 758-bp fragment from wild-type DNA. Heterozygous breeding pairs produced normal litters (340 pups from 49 litters, 48% female) with the expected frequencies of wild-type, heterozygous, and Chloe offspring (28, 47, and 25%, respectively). Homozygous Chloe breeders were fertile and had litters of normal size and sex distribution.
FIG. 1.
Genotyping by restriction enzyme digestion. (A) The mutation that changes amino acids 341FFG to 341GTR introduces a unique KpnI restriction site in exon 7. (B) A 758-bp PCR fragment amplified from wild-type DNA is resistant to KpnI digestion. KpnI digestion of the PCR fragment amplified from Chloe DNA yields products of 530 and 228 bp. KpnI digestion of the PCR fragment amplified from heterozygote (Het) DNA yields products of 758, 530, and 228 bp.
Chloe aggrecan is resistant to MMP cleavage at the major site in the IGD.
Cartilage from the femoral heads of 4- to 6-week-old mice was extracted and analyzed for aggrecan fragments by Western blotting. The analyses revealed that DIPEN neoepitope was readily detectable in extracts from wild-type mice (Fig. 2A, lanes 1 and 2) but absent from Chloe cartilage, indicating that there was no cleavage at the mutant MMP cleavage site in Chloe mice. There was no difference in the levels of the aggrecanase neoepitope NVTEGE (Fig. 2A, lanes 3 and 4) between wild-type and Chloe mice, indicating that aggrecanases did not compensate for the lack of MMP cleavage in the IGD in Chloe mice. One difference was that the G1-NVTEGE fragment in Chloe extracts migrated more slowly on SDS gels than NVTEGE fragments from wild-type extracts (Fig. 2A, lanes 3 and 4). This was possibly due to additional glycosylation at the N×T consensus sequence for N-linked glycosylation that was created by changing DIPENFFG to DIPENGTR. To test this hypothesis, cartilage extracts were treated with N-glycosidase F and duplicate samples were analyzed for G1-NVTEGE and G1-DIPEN (Fig. 2B). The Western blots showed that N-glycosidase F treatment reduces wild-type and Chloe NVTEGE to bands of the same size (Fig. 2B, lanes 2 and 4), confirming that the increased size of Chloe NVTEGE was due to an additional N-linked sugar, most likely at N341. A duplicate blot was probed with the anti-DIPEN antibody. Treatment with N-glycosidase F failed to rescue DIPEN immunoreactivity in the Chloe extracts (Fig. 2B, lane 8), indicating that the loss of DIPEN immunoreactivity in Chloe mice was not a consequence of N-linked glycosylation at N341GT but rather absence of cleavage at the MMP site.
FIG. 2.
G1 fragments in cartilage extracts from wild-type and Chloe mice. (A) Extracts of cartilage from wild-type (lanes 1, 3, and 5) and Chloe (lanes 2, 4, and 6) mice were treated with chondroitinase ABC, electrophoresed on 7% SDS gels, and analyzed by Western blotting with anti-DIPEN, anti-NITEGE, and anti-G1 domain antibodies. The extracts were loaded based on equal amounts [wet weight] of cartilage, representing 0.75, 0.125, and 0.125 mg for anti-NITEGE, anti-DIPEN, and anti-G1 antibodies, respectively. (B) Dialyzed extracts of wild-type (lanes 1, 2, 5, and 6) or Chloe (lanes 3, 4, 7, and 8) cartilage were incubated with (+) or without (−) N-glycosidase F to liberate N-linked carbohydrates. Duplicate Western blots were probed with anti-NITEGE (lanes 1 through 4) or anti-DIPEN (lanes 5 through 8) antibody. (C) Dialyzed extracts of equal amounts (wet weight) of cartilage from wild-type (lanes 1 and 2) and Chloe (lanes 3 and 4) mice were digested with (+) or without (−) MMP-13 and analyzed by anti-DIPEN Western blotting.
Western blotting with a polyclonal anti-G1 antibody revealed a different ratio of G1 catabolites in Chloe extracts compared with that in wild-type extracts (Fig. 2A, lanes 5 and 6). A G1-positive band migrating at an approximate Mr of 100 to 120 was present in extracts of wild-type mice and was increased in Chloe extracts. The increase in the 100- to 120-Mr band appeared to be at the expense of a band migrating at an approximate Mr of 130 to 150. To further confirm that Chloe aggrecan was resistant to MMP cleavage at the N341↓342G bond, aggrecan extracted from Chloe and wild-type mice was digested with MMP-13 and analyzed for G1-DIPEN. Western blotting revealed that MMP-13 generates a strong G1-DIPEN band in extracts from wild-type mice (Fig. 2C, lane 2) but fails to generate G1-DIPEN in extracts from Chloe homozygotes (Fig. 2C, lane 4). MMP-13 digestion did not generate any new G1-positive bands in Chloe mice compared with that in the wild type (data not shown).
The G1-DIPEN in wild-type animals was immunolocalized to the growth plate, particularly the resorbing border with the metaphysis (Fig. 3A, panel a) but not the articular cartilage (see Fig. 5). In contrast, the G1-NVTEGE epitope was localized to the articular surfaces of femoral heads, with none detected in the growth plate (data not shown). In Chloe joints, no G1-DIPEN was detected by immunolocalization (Fig. 3A, panel b) and the distribution of G1-NVTEGE was the same as that in wild-type animals (data not shown). The neoepitope specificity of the G1-DIPEN antibody was confirmed by competition experiments in which the immunizing peptide (GEDFVDIPEN) blocked immunoreactivity in sections from wild-type mice (Fig. 3A, panel c). A peptide spanning the cleavage site (FVDIPENFFG) and an irrelevant peptide (PLPRNITEGE) were unable to compete for antibody binding to the sections (Fig. 3A, panels d and e, respectively). Digestion of tissue sections with MMP-13 markedly increased G1-DIPEN staining in wild-type mice (Fig. 3B, panel c) but failed to generate staining in Chloe mutants (Fig. 3B, panel d). These results confirm that changing amino acids 342FFG to 342GTR renders aggrecan resistant to MMP cleavage at the major MMP site in the IGD in vivo.
FIG. 3.
Immunohistochemistry of wild-type (Wt) and Chloe growth plate. (A) Paraffin sections of growth plate from the tibiofemoral joint of 3-week-old wild-type (panels a and c through e) and Chloe (panel b) mice were immunolocalized with anti-DIPEN antibody without (panels a and b) or following preabsorption with peptides GEDFVDIPEN (panel c), FVDIPENFFG (panel d), or PLPRNITEGE (panel e). (B) Paraffin sections of growth plate from the tibiofemoral joint of 3-week-old wild-type (panels a and c) or Chloe (panels b and d) mice were stained with anti-DIPEN antibody before (panels a and b) or after (panels c and d) digestion with 10 μg of MMP-13/ml.
FIG. 5.
Cleavage at the minor MMP site is not increased in Chloe aggrecan. Frozen sections of tibiofemoral joints from 3-week-old wild-type (WT) or Chloe mice were analyzed by immunofluorescence with anti-DIPEN (panels a through d), anti-TAFASED (panels e through i and k), or the anti-TAFASED preimmune serum (panels j and l), either undigested (panels a, c, e, and g), digested with 10 μg of MMP-13/ml for 1 h (panels b, d, f, and h; +MMP), or digested with a cocktail of 10-μg/ml concentrations of MMPs-2, -3, and -13 together for 24 h (panels i through l; ++++MMP). Images show merged fluorescence and phase. Arrows point from the joint space toward the layer of flattened cells on the femoral and tibial articular surfaces. The arrowheads point to DIPEN-positive cells at the junction between the articular surface and the secondary center of ossification. The secondary ossification center (S) is marked.
Cleavage at the minor MMP site in the IGD is not compensatory in Chloe mice.
In addition to the preferred site of MMP cleavage at DIPEN341↓342FFGVG, some MMPs cleave at a minor cleavage site in the IGD, located 100 amino acids C terminal to the major site, at the D441↓442L bond (human sequence enumeration; equivalent to D444↓445L in mice) (13, 16). The sequence surrounding this minor MMP cleavage site in the mouse is TAFASED444↓445LVVR. To determine whether blocking cleavage at the major MMP site would force cleavage at the minor site, we generated a new neoepitope antibody against the C-terminal neoepitope sequence TAFASED444. The neoepitope specificity of the antibody was confirmed by competition ELISA (Fig. 4A). The peptide competitor corresponding to the sequence of the immunogen (DSATAFASED) was able to inhibit antibody binding to immunogen coated on ELISA plates in a dose-dependent manner. In contrast, there was no competition with peptides in which the C-terminal amino acid had been removed (PDSATAFASE) or extended by 1 residue (SATAFASEDL). The neoepitope specificity of the anti-TAFASED antibody was further confirmed by Western blot analysis of cartilage extracts (Fig. 4B). The antibody detected a single band of an approximate Mr of 120, consistent with the size predicted for G1 fragments terminating at TAFASED444 (57). The TAFASED fragment comigrated with a fragment that was detected with the anti-G1-antibody and appeared to be more abundant by Western blotting in Chloe mice than in wild-type extracts (Fig. 4B, lane 3, and 2A, lane 6).
FIG. 4.
Anti-TAFASED neoepitope antibody detects the G1-TAFASED fragment after cleavage at the minor MMP site in the IGD. (A) Polyclonal rabbit anti-TAFASED antiserum was analyzed by competition ELISA using DSATAFASED (▪), SATAFASEDL (•), and PDSATAFASE (○) as competing peptides. No competition was seen with the peptides containing one extra, or one less, C-terminal amino acid. (B) Dialyzed extracts of Chloe (lanes 1, 3, and 5) and wild-type (lanes 2, 4, and 6) cartilage were electrophoresed on SDS gels and analyzed by Western blotting with anti-G1, anti-TAFASED, and anti-DIPEN antibodies. The extracts were loaded based on equal amounts [wet weight] of cartilage, representing 0.125 mg for each of anti-TAFASED, anti-DIPEN, and anti-G1.
Immunofluorescence was used to compare MMP cleavage at the minor MMP site in Chloe and wild-type aggrecan. Anti-DIPEN staining of cartilage sections from wild-type mice detected a band of cells at the junction between the articular cartilage and the secondary center of ossification but failed to stain the cartilage matrix (Fig. 5a). MMP-13 digestion (10 μg/ml for 1 h) of an adjacent cartilage section produced uniform, diffuse DIPEN staining in the articular cartilage (Fig. 5b). In contrast, sections of cartilage from Chloe mice showed no DIPEN immunoreactivity, with (Fig. 5d) or without (Fig. 5c) MMP-13 digestion. There was no TAFASED immunostaining evident in wild-type cartilage (Fig. 5e), and, consistent with the D444↓445L bond being a minor cleavage site, no further TAFASED neoepitope was generated following MMP digestion (Fig. 5f). Similarly, sections of cartilage from Chloe mice showed no TAFASED immunoreactivity, with (Fig. 5h) or without (Fig. 5g) MMP-13 digestion. These results suggest that blocking cleavage at the major MMP site in Chloe mice does not force additional cleavage at the minor site.
To confirm that the anti-TAFASED antibody was able to detect epitope in fixed tissue, cartilage sections from Chloe and wild-type mice were digested with a cocktail of MMP-2, -3, and -13 for a longer time (10 μg/ml each for 24 h). In these sections, pericellular TAFASED staining was evident throughout the cartilage (Fig. 5i and k); however, the amount of staining was the same for Chloe and wild-type mice. No staining, other than autofluorescence in the secondary centers of ossification, was seen with the preimmune serum at the equivalent dilution (Fig. 5j and l). These results indicate that blocking MMP cleavage at the major site is not compensated for by MMP cleavage at the minor site in the IGD.
Aggrecan degradation in vitro.
We have shown previously, in porcine explant cultures, that MMPs are involved in the baseline turnover of mature articular aggrecan (15). We therefore reasoned that baseline turnover of aggrecan in Chloe cartilage would be less than that in wild type. Individual femoral heads were cultured without stimulation or with stimulation by RA or IL-1α (Fig. 6). As expected, RA and IL-1α increased the release of aggrecan into the conditioned medium (P < 0.001 for all analyses), and this stimulated increase was the same in Chloe and wild-type cultures (Fig. 6A). However, aggrecan release from unstimulated (control) Chloe cultures was 45% of that in the wild type (P < 0.003). This finding is consistent with the hypothesis that MMP cleavage in the IGD is involved in baseline aggrecanolysis and that this pathway is blocked in Chloe mice. It is also consistent with a lack of compensation by MMP cleavage at the minor site, as shown in Fig. 5. Western blotting of tissue extracts after culture showed first that, in Chloe mice, there was no cleavage at the MMP site, with or without stimulation by RA or IL-1α (Fig. 6B, lanes 4 through 6). Second, there were no novel G1-bearing metabolites and no increase in the 120-kDa TAFASED band in stimulated Chloe cultures (data not shown), consistent with a lack of alternate compensatory cleavage by MMPs. Third, the blots showed that the IL-1α- induced increase in aggrecanase activity (NVTEGE epitope) is the same in Chloe mice as in the wild type (Fig. 6B, lanes 9 and 12). Finally, the results showed that in wild-type cartilage, RA treatment generates more DIPEN neoepitope than has been reported for other species in culture. We have observed this previously with the mouse explant model (39) and shown that the increased epitope is localized to the growth plate.
FIG. 6.
Aggrecan degradation in vitro. Aggrecan loss from 3-day cultures of femoral head cartilage from wild-type (Wt) and Chloe mice. (A) Release of glycosaminoglycans from cartilage from wild-type (grey bars) and Chloe (white bars) mice in the absence (Control) or presence of 10−5 M RA or 10 ng of IL-1α/ml (n = 8 to 10 per treatment). (B) Western blot analysis of wild-type (lanes 1 through 3 and 7 through 9) or Chloe (lanes 4 through 6 and 10 through 12) G1 fragments extracted after culture in the presence of RA (lanes 2, 5, 8, and 11), IL-1α (lanes 3, 6, 9, and 12) or no additives (lanes 1, 4, 7, and 10). Membranes were immunolocalized with antibodies recognizing G1-DIPEN (lanes 1 through 6) or G1-NITEGE (lanes 7 through 12) fragments. The extracts were loaded based on equal amounts (wet weight) of cartilage, representing 0.75 and 0.125 mg for anti-NITEGE and anti-DIPEN, respectively.
Aggrecan does not accumulate in Chloe mice.
We postulated that aggrecan turnover may have been reduced in Chloe cartilage, giving rise to an increase in the concentration of aggrecan (measured as micrograms of sulfated glycosaminoglycan per milligram [wet weight] of tissue). However, the aggrecan concentrations in femoral heads from age- and sex-matched wild-type and Chloe mice were 23.65 ± 1.70 and 23.24 ± 1.55 (mean ± SEM), respectively, with n = 11, and n = 17, respectively. These results showed that blocking MMP cleavage in the IGD does not lead to aggrecan accumulation in cartilage.
Most CS on aggrecan is sulfated on the C6 or C4 moiety of N-acetylgalactosamine; however, CS disaccharides can also be unsulfated or sulfated at the C2 position of glucuronic acid. To eliminate the possibility that an accumulation of aggrecan in Chloe mice might go undetected in the dye binding assay if Chloe aggrecan was significantly undersulfated, we analyzed the Δ-disaccharides of CS by FACE. The results showed that in 10-day mouse cartilage, the 4-sulfated disaccharide, Δdi-4S, is the predominant species, and whereas the Δdi-0S is present in small amounts, Δdi-6S and Δdi-2S are undetectable (Fig. 7A). The masses of Δdi-4S and Δdi-0S per microgram of glycosaminoglycan in Chloe and wild-type cartilage were not significantly different (Fig. 7B). These results showed that there is no difference in the type or extent of sulfation on CS chains in Chloe mice compared with that in the wild type and further demonstrate that there was no accumulation of aggrecan in Chloe mice.
FIG. 7.
Analysis of CS in wild-type and Chloe cartilage extracts. Femoral heads were harvested from eight wild-type and nine Chloe mice and distributed into four groups per genotype for analysis of CS by FACE. (A) Representative samples of wild-type (open bars) and Chloe (shaded bars) mice with the migrating positions of the Δ-disaccharides of CS and hyaluronan are shown. (B) The sulfated glycosaminoglycan of Δ-di-4S and Δ-di-0S disaccharides in the four pools for each genotype were quantitated (picomoles per micrograms) from the gels and plotted as means ± standard errors.
Chloe mice do not have a growth plate phenotype.
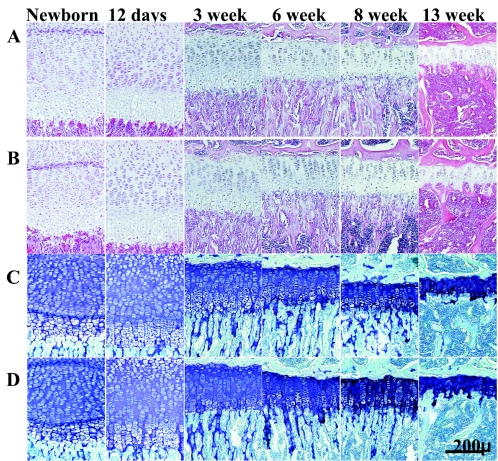
Active MMPs are present in growth plate cartilage during development (36), and the MMP-9- and MMP-13-null mice have prominent growth plate phenotypes in early life. Furthermore, all MMPs cleave at the N341↓342F bond in the aggrecan IGD in vitro. We therefore examined growth plates from knees, hips, and ribs in Chloe and wild-type mice in ages ranging from newborn to 12 weeks for changes in growth plate architecture. Figure 8 shows tibial growth plates from wild-type and Chloe mice examined by staining with hematoxylin and eosin (Fig. 8A and B) or toluidine blue and fast green (Fig. 8C and D) stains. There were no histological differences at any age examined. The widths of the hypertrophic zone, the proliferative zone, and the total growth plate were measured in 8-week-old mice by using standard histomorphometric techniques, and no differences between genotypes were observed (Table 1).
FIG. 8.
Histology of wild-type and Chloe growth plate cartilage during development. Paraffin sections of wild-type (A and C) and Chloe (B and D) proximal tibial growth plates from birth to 13 weeks of age are shown. Sections were stained with hematoxylin and eosin (A and B) or toluidine blue and fast green (C and D) stains.
TABLE 1.
Tibial growth plate widths in 8-week-old Chloe and wild-type mice
| Mice (no. of joints) | Zone width (μm [mean ± SEM])
|
Hypertrophic/proliferative chondrocyte ratio | ||
|---|---|---|---|---|
| Hypertrophic | Proliferative | Total growth plate | ||
| Wild type (4) | 60.298 ± 6.460 | 91.411 ± 4.732 | 151.709 ± 10.108 | 0.395 ± 0.019 |
| Chloe (6) | 58.239 ± 4.216 | 96.985 ± 2.710 | 155.224 ± 4.970 | 0.373 ± 0.022 |
There is evidence in the literature suggesting that aggrecan in high concentration may provide nucleation sites for calcification associated with chondrocyte hypertrophy (26, 27). To determine whether calcification was altered in Chloe compared with wild-type mice, we stained the limbs, paws, spinal columns, and pelvises of newborn mice with alizarin red. There was no detectable difference in calcification of the newborn skeletons (Fig. 9). Finally, we were unable to detect gross or histopathological differences in any tissues examined, including heart, brain, kidney, bladder, spinal cord, eyes, lungs, larynx, and trachea (data not shown).
FIG. 9.
Skeletal calcification in newborns. Alcian blue and alizarin red staining of newborn wild-type (A) and Chloe (B) front paws, forelimbs, and pelvis and lumbar spine.
DISCUSSION
We have characterized genetically modified mice bearing aggrecan that is resistant to proteolysis by MMPs at the major site in the IGD. The mutation in exon 7 was confirmed by Southern blotting, sequencing, and restriction enzyme digestion, and the consequent changes to the amino acid sequence were confirmed by analysis of aggrecan catabolites. The DIPEN neoepitope, used as a marker of MMP cleavage in the IGD, was not detected in direct extracts from Chloe cartilage, nor was it possible to generate this epitope ex vivo by MMP digestion of aggrecan in cartilage extracts or tissue sections. In addition to the MMPs, the cysteine proteinase cathepsin B can generate the DIPEN neoepitope in vitro via exopeptidase activity following cleavage at the G343↓344V bond 3 amino acids C terminal to the major MMP site (46). The lack of G1-DIPEN in Chloe mice indicates that mutant aggrecan is not susceptible to cleavage at DIPEN341↓342GTRVG by either MMPs or cathepsin B in vivo.
To compare MMP-driven aggrecanolysis in Chloe and wild-type mice, we used femoral heads comprising both articular cartilage and growth plate cartilage for characterization by Western blot, FACE, and explant culture. The lack of skeletal phenotype in Chloe mice draws attention not to the articular cartilage but to the growth plate cartilage, since this is the site of extensive matrix remodeling during endochondral bone formation. Within the growth plate, the hypertrophic zone is the most active region of aggrecan resorption. Aggrecan synthesized by hypertrophic chondrocytes has structural features that distinguish it from aggrecan synthesized in the proliferative and reserve zones (7, 59). It has an increased hydrodynamic size due to increased glycosaminoglycan chain length, as well as an increased incidence of the G3 domain, indicative of a newly synthesized, full-length core protein (51). Since new aggrecan is synthesized by hypertrophic chondrocytes, and since the volume of hypertrophic matrix is diminishing as the cells expand to occupy a larger volume, it follows that there must also be loss, or turnover, of aggrecan.
The early literature records some controversy over whether aggrecan was (4, 17, 40, 69) or was not (21, 24, 53, 58) lost from the hypertrophic zone just before cartilage calcifies. More recently, Matsui et al. (42) have shown that although the concentration of aggrecan (aggrecan per extracellular matrix volume) increases in hypertrophic cartilage due to continuing aggrecan synthesis and a reduction in matrix volume (42, 51), there is an overall net loss of aggrecan (aggrecan per total tissue volume) prior to calcification (42). We therefore predicted that the cleavage site mutation would prevent, or at least reduce, the turnover of aggrecan in the growth plate and lead to its accumulation, causing possible disruption to growth plate organization, disrupted joint formation and bone growth, or precocious calcification. Surprisingly, the mice developed normally with no detectable skeletal or growth plate abnormalities. These results indicate that MMP cleavage in the proteinase-sensitive aggrecan IGD is not essential for normal growth and development. At least seven MMPs are expressed by hypertrophic chondrocytes (18, 23, 43), and knockout models show that some of these MMPs are active in vivo (30, 65, 77). Our data show that only a very small amount of DIPEN neoepitope is present in ex vivo growth plate compared with the amount of neoepitope that can be generated experimentally by moderate levels of active MMPs (Fig. 3B and 5b). We interpret this small amount of DIPEN immunoreactivity detected in growth plate by us (Fig. 3A, panel a) and others (36) as “collateral damage” that occurs because MMPs, particularly the collagenases, are active and present in the vicinity of high concentrations of aggrecan substrate. Thus, our data indicate that the primary mechanism by which aggrecan is lost from hypertrophic cartilage is not via the action of MMPs. The aggrecanases ADAMTS-1, ADAMTS-4, and ADAMTS-5 also appear to have no major role in growth plate aggrecanolysis since mice with null mutations in these genes develop normal skeletons.
Cathepsins are expressed by hypertrophic chondrocytes (48, 63) and may therefore have roles in aggrecanolysis. In vitro, cathepsins B and D cleave at G344↓345V (16, 46) and F342↓343F (20), respectively, in the aggrecan IGD. Whether these cleavages occur in vivo is unknown; fragments derived from cathepsin cleavage sites have not been detected. Cathepsin-l-deficient mice have impaired bone development (54); however, the chondrocytic zones of the growth plate are indistinguishable between wild-type and mutant mice and, hence, the deficiency is thought to arise from abnormalities in osteoclast number and function that limit the removal of matrix from the mineralized cartilage.
The calcium-dependent cysteine proteinase m-calpain also cleaves aggrecan in vitro (49, 60), and fragments consistent with m-calpain cleavage are present in extracts of mature bovine cartilage (49). m-Calpain is a cytosolic enzyme thought to have a role in matrix mineralization (81), and it is unclear how this intracellular enzyme is able to degrade extracellular aggrecan. Whether m-calpain is involved in growth plate aggrecanolysis in vivo has not been investigated.
In large-animal-cartilage explant cultures, stimulated aggrecan loss is mediated by aggrecanases whereas aggrecan loss from unstimulated cultures is thought to be mediated by MMPs (15). This appears to be true also in explant cultures of mouse cartilage, since the turnover of aggrecan in unstimulated Chloe mice was significantly less than that in the wild type (indicative of an MMP-driven process), yet the stimulated aggrecan release was the same in both genotypes. This important result also reveals that there is no compensatory cleavage at another site in the IGD, including the aggrecanase site or a minor MMP cleavage site. In vitro cleavage at the minor MMP site occurs only with prolonged and high-dose digestion by MMPs (13, 16). This suggests that cleavage occurs slowly and may be of little physiological relevance in acute MMP-driven aggrecan proteolysis, even in mutant animals. The TAFASED Western blots of direct extracts showed an increase in TAFASED immunoreactivity in Chloe cartilage compared with that in the wild type (Fig. 4B). However, because the DIPEN and TAFASED antibodies have different affinities for their antigens, it is not possible to directly correlate changes in the amount of epitope with changes in Western blot signal. The results do not allow us to assess whether the increase in TAFASED epitope in Chloe mice is large, equivalent, or very small compared with the complete loss of DIPEN epitope in Chloe mice. Instead, the explant culture experiment (Fig. 6) and the immunofluorescence experiment (Fig. 5) give a better readout of potential compensation, and both experiments show that there is no compensatory increase in proteolysis in the IGD. Together, the results suggest that although there may be a slow accumulation of TAFASED epitope in Chloe mice, the inefficiency of cleavage at this site makes its impact on aggrecan resorption insignificant. Overall, the results suggest that cleavage at D444↓445L is minimal in wild-type and Chloe mice and that MMP cleavage at this site in Chloe mice does not compensate for lack of cleavage at the major site.
The results from the present study are consistent with recent, novel studies suggesting that there may be nonproteolytic mechanisms for aggrecanolysis in cartilage (68) and, in particular, in epiphyseal cartilages (51, 67) and that this mechanism may involve the depolymerization of hyaluronan. Depolymerization of hyaluronan is synonymous with depolymerization of the aggrecan aggregate and is consistent with early electron microscopic studies reporting that aggregate size was reduced in calcifying cartilage (6, 8). It is also consistent with recent studies that have identified the release of intact, high-molecular-weight aggrecan from fetal cartilage, with no evidence of IGD cleavage (67). Sztrolovics et al. have shown that hyaluronan released from bovine fetal cartilage explants following stimulation with IL-1β or RA is smaller than the hyaluronan released from unstimulated cultures (68). These results suggest that when fetal chondrocytes are stimulated to degrade their matrix, they do so partly by depolymerizing hyaluronan. The precise mechanisms within the growth plate are not known, but hyaluronan can be degraded by extracellular hyaluronidases (11) and by reactive oxygen radicals (56).
In summary, the phenotype of the unchallenged Chloe mouse strongly suggests that MMPs are not involved in aggrecan turnover in the growth plate. Our results suggest that MMP cleavage in the IGD is not essential for growth plate aggrecanolysis in vivo and that MMP cleavage in this region represents collateral damage rather than a mandatory event in endochondral ossification. We therefore postulate that MMP cleavage of aggrecan in the mouse growth plate is inconsequential and occurs on a minor scale. The normal skeletal phenotype of the ADAMTS-1-, -4-, and -5-deficient mice, together with the lack of a skeletal phenotype in another mouse, engineered to resist aggrecanase cleavage in the IGD (C. B. Little, C. T. Meeker, and A. J. Fosang, unpublished data), suggests that aggrecanases are also unlikely to have a role in growth plate remodeling in development. Collectively, the data lend support to emerging hypotheses concerning nonproteolytic mechanisms of aggrecanolysis in epiphyseal cartilage.
Acknowledgments
This work was supported by the National Health and Medical Research Council (Australia), the Arthritis Foundation of Australia, and Pfizer Inc. R. M. Hembry's contribution to the work was funded by The University of Melbourne Collaborative Research Program.
We gratefully acknowledge the mouse husbandry support provided by staff at the Murdoch Childrens Research Institute.
REFERENCES
- 1.Alini, M., Y. Matsui, G. R. Dodge, and A. R. Poole. 1992. The extracellular matrix of cartilage in the growth plate before and during calcification: changes in composition and degradation of type II collagen. Calcif. Tissue Int. 50:327-335. [DOI] [PubMed] [Google Scholar]
- 2.Argraves, W. S., P. J. McKeown-Longo, and P. F. Goetinck. 1981. Absence of proteoglycan core protein in the cartilage mutant nanomelia. FEBS Lett. 131:265-268. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss, M. T., S. Hutton, J. Hayward, and R. A. Maciewicz. 2001. Distribution of aggrecanase (ADAMts 4/5) cleavage products in normal and osteoarthritic human articular cartilage: the influence of age, topography and zone of tissue. Osteoarthritis Cartilage 9:553-560. [DOI] [PubMed] [Google Scholar]
- 4.Boyde, A., and I. M. Shapiro. 1980. Energy dispersive X-ray elemental analysis of isolated epiphyseal growth plate chondrocyte fragments. Histochemistry 69:85-94. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter, J. A. 1983. Proteoglycan structure in calcifying cartilage. Clin. Orthop. 172:207-232. [PubMed] [Google Scholar]
- 6.Buckwalter, J. A., L. C. Rosenberg, and R. Ungar. 1987. Changes in proteoglycan aggregates during cartilage mineralization. Calcif. Tissue Int. 41:228-236. [DOI] [PubMed] [Google Scholar]
- 7.Byers, S., B. Caterson, J. J. Hopwood, and B. K. Foster. 1992. Immunolocation analysis of glycosaminoglycans in the human growth plate. J. Histochem. Cytochem. 40:275-282. [DOI] [PubMed] [Google Scholar]
- 8.Campo, R. D., and J. E. Romano. 1986. Changes in cartilage proteoglycans associated with calcification. Calcif. Tissue Int. 39:175-184. [DOI] [PubMed] [Google Scholar]
- 9.Dennis, J. E., D. A. Carrino, N. B. Schwartz, and A. I. Caplan. 1990. Ultrastructural characterization of embryonic chick cartilage proteoglycan core protein and the mapping of a monoclonal antibody epitope. J. Biol. Chem. 265:12098-12103. [PubMed] [Google Scholar]
- 10.Farndale, R. W., C. A. Sayers, and A. J. Barrett. 1982. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 9:247-248. [DOI] [PubMed] [Google Scholar]
- 11.Flannery, C. R., C. B. Little, C. E. Hughes, and B. Caterson. 1998. Expression and activity of articular cartilage hyaluronidases. Biochem. Biophys. Res. Commun. 251:824-829. [DOI] [PubMed] [Google Scholar]
- 12.Fosang, A. J., K. Last, P. Gardiner, D. C. Jackson, and L. Brown. 1995. Development of a cleavage-site-specific monoclonal antibody for detecting metalloproteinase-derived aggrecan fragments: detection of fragments in human synovial fluids. Biochem. J. 310:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fosang, A. J., K. Last, V. Knäuper, P. J. Neame, G. Murphy, T. E. Hardingham, H. Tschesche, and J. A. Hamilton. 1993. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem. J. 295:273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosang, A. J., K. Last, and R. A. Maciewicz. 1996. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J. Clin. Investig. 98:2292-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fosang, A. J., K. Last, H. Stanton, D. B. Weeks, I. K. Campbell, T. E. Hardingham, and R. M. Hembry. 2000. Generation and novel distribution of matrix metalloproteinase-derived aggrecan fragments in porcine cartilage explants. J. Biol. Chem. 275:33027-33037. [DOI] [PubMed] [Google Scholar]
- 16.Fosang, A. J., P. J. Neame, K. Last, T. E. Hardingham, G. Murphy, and J. A. Hamilton. 1992. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J. Biol. Chem. 267:19470-19474. [PubMed] [Google Scholar]
- 17.Franzen, A., D. Heinegard, S. Reiland, and S. E. Olsson. 1982. Proteoglycans and calcification of cartilage in the femoral head epiphysis of the immature rat. J. Bone Joint Surg. Am. 64:558-566. [PubMed] [Google Scholar]
- 18.Gack, S., R. Vallon, J. Schmidt, A. Grigoriadis, J. Tuckermann, J. Schenkel, H. Weiher, E. F. Wagner, and P. Angel. 1995. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 6:759-767. [PubMed] [Google Scholar]
- 19.Glasson, S. S., R. Askew, B. Sheppard, B. A. Carito, T. Blanchet, H. L. Ma, C. R. Flannery, K. Kanki, E. Wang, D. Peluso, Z. Yang, M. K. Majumdar, and E. A. Morris. 2004. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 50:2547-2558. [DOI] [PubMed] [Google Scholar]
- 19a.Glasson, S. S., R. Askew, B. Sheppard, B. Carito, T. Blanchet, H. Ma, C. R. Flannery, D. Peluso, K. Kanki, Z. Yang, M. K. Majumdar, and E. A. Morris. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature, in press. [DOI] [PubMed]
- 20.Handley, C. J., M. Tuck Mok, M. Z. Ilic, C. Adcocks, D. J. Buttle, and H. C. Robinson. 2001. Cathepsin D cleaves aggrecan at unique sites within the interglobular domain and chondroitin sulfate attachment regions that are also cleaved when cartilage is maintained at acid pH. Matrix Biol. 20:543-553. [DOI] [PubMed] [Google Scholar]
- 21.Hargest, T. E., C. V. Gay, H. Schraer, and A. J. Wasserman. 1985. Vertical distribution of elements in cells and matrix of epiphyseal growth plate cartilage determined by quantitative electron probe analysis. J. Histochem. Cytochem. 33:275-286. [DOI] [PubMed] [Google Scholar]
- 22.Hogan, B. L., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Hou, P., T. Troen, M. C. Ovejero, T. Kirkegaard, T. L. Andersen, I. Byrjalsen, M. Ferreras, T. Sato, S. D. Shapiro, N. T. Foged, and J. M. Delaisse. 2004. Matrix metalloproteinase-12 (MMP-12) in osteoclasts: new lesson on the involvement of MMPs in bone resorption. Bone 34:37-47. [DOI] [PubMed] [Google Scholar]
- 24.Howell, D. S., and L. Carlson. 1968. Alterations in the composition of growth cartilage septa during calcification studied by microscopic x-ray elemental analysis. Exp. Cell Res. 51:185-195. [DOI] [PubMed] [Google Scholar]
- 25.Hughes, C., B. Caterson, R. J. White, P. J. Roughley, and J. S. Mort. 1992. Monoclonal antibodies recognizing protease-generated neoepitopes from cartilage proteoglycan degradation. J. Biol. Chem. 267:16011-16014. [PubMed] [Google Scholar]
- 26.Hunter, G. K. 1991. Role of proteoglycan in the provisional calcification of cartilage. A review and reinterpretation. Clin. Orthop. 262:256-280. [PubMed] [Google Scholar]
- 27.Hunter, G. K., and C. A. Weinert. 1996. Inhibition of proteoglycan biosynthesis decreases the calcification of chondrocyte cultures. Connect. Tissue Res. 35:379-384. [DOI] [PubMed] [Google Scholar]
- 28.Hunziker, E. B., R. K. Schenk, and L. M. Cruz-Orive. 1987. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J. Bone Joint Surg. Am. 69:162-173. [PubMed] [Google Scholar]
- 29.Ilic, M. Z., H. C. Robinson, and C. J. Handley. 1998. Characterization of aggrecan retained and lost from the extracellular matrix of articular cartilage. Involvement of carboxyl-terminal processing in the catabolism of aggrecan. J. Biol. Chem. 273:17451-17458. [DOI] [PubMed] [Google Scholar]
- 30.Inada, M., Y. Wang, M. H. Byrne, M. U. Rahman, C. Miyaura, C. Lopez-Otin, and S. M. Krane. 2004. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 101:17192-17197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimata, K., H.-J. Barrach, K. S. Brown, and J. P. Pennypacker. 1981. Absence of proteoglycan core protein in cartilage from the cmd/cmd (cartilage matrix deficiency) mouse. J. Biol. Chem. 256:6961-6968. [PubMed] [Google Scholar]
- 32.Koklitis, P. A., G. Murphy, C. Sutton, and S. Angal. 1991. Purification of recombinant human prostromelysin. Studies on heat activation to give high-Mr and low Mr active forms, and a comparison of recombinant with natural stromelysin activities. Biochem. J. 276:217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krueger, R. C., Jr., K. Kurima, and N. B. Schwartz. 1999. Completion of the mouse aggrecan gene structure and identification of the defect in the cmd-Bc mouse as a near complete deletion of the murine aggrecan gene. Mamm. Genome 10:1119-1125. [DOI] [PubMed] [Google Scholar]
- 34.Lark, M. W., E. K. Bayne, J. Flanagan, C. F. Harper, L. A. Hoerrner, N. I. Hutchinson, I. I. Singer, S. A. Donatelli, J. R. Weidner, H. R. Williams, R. A. Mumford, and L. S. Lohmander. 1997. Aggrecan degradation in human cartilage. Evidence for both metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J. Clin. Investig. 100:93-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, E. R., L. Lamplugh, M. A. Davoli, A. Beauchemin, K. Chan, J. S. Mort, and C. P. Leblond. 2001. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats ages 0-21 days: I. Two groups of proteinases cleave the core protein of aggrecan. Dev. Dyn. 222:52-70. [DOI] [PubMed] [Google Scholar]
- 36.Lee, E. R., L. Lamplugh, C. P. Leblond, S. Mordier, M. C. Magny, and J. S. Mort. 1998. Immunolocalization of the cleavage of the aggrecan core protein at the Asn341-Phe342 bond, as an indicator of the location of the metalloproteinases active in the lysis of the rat growth plate. Anat. Rec. 252:117-132. [DOI] [PubMed] [Google Scholar]
- 37.Li, H., N. B. Schwartz, and B. M. Vertel. 1993. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J. Biol. Chem. 268:23504-23511. [PubMed] [Google Scholar]
- 38.Little, C., S. Smith, P. Ghosh, and C. Bellenger. 1997. Histomorphological and immunohistochemical evaluation of joint changes in a model of osteoarthritis induced by lateral meniscectomy in sheep. J. Rheumatol. 24:2199-2209. [PubMed] [Google Scholar]
- 39.Little, C. B., L. Mittaz, D. Belluoccio, F. M. Rogerson, I. K. Campbell, C. T. Meeker, J. F. Bateman, M. A. Pritchard, and A. J. Fosang. ADAMTS-1 knockout mice do not exhibit abnormalities in aggrecan turnover in vitro or in vivo. Arthritis Rheum., in press. [DOI] [PubMed]
- 40.Lohmander, S., and A. Hjerpe. 1975. Proteoglycans of mineralizing rib and epiphyseal cartilage. Biochim. Biophys. Acta 404:93-109. [DOI] [PubMed] [Google Scholar]
- 41.Makihira, S., W. Yan, H. Murakami, M. Furukawa, T. Kawai, H. Nikawa, E. Yoshida, T. Hamada, Y. Okada, and Y. Kato. 2003. Thyroid hormone enhances aggrecanase-2/ADAM-TS5 expression and proteoglycan degradation in growth plate cartilage. Endocrinology 144:2480-2488. [DOI] [PubMed] [Google Scholar]
- 42.Matsui, Y., M. Alini, C. Webber, and A. R. Poole. 1991. Characterization of aggregating proteoglycans from the proliferative, maturing, hypertrophic, and calcifying zones of the cartilaginous physis. J. Bone Joint Surg. Am. 73:1064-1074. [PubMed] [Google Scholar]
- 43.Mattot, V., M. B. Raes, P. Henriet, Y. Eeckhout, D. Stehelin, B. Vandenbunder, and X. Desbiens. 1995. Expression of interstitial collagenase is restricted to skeletal tissue during mouse embryogenesis. J. Cell Sci. 108:529-535. [DOI] [PubMed] [Google Scholar]
- 44.Mercuri, F. A., K. J. Doege, E. C. Arner, M. A. Pratta, K. Last, and A. J. Fosang. 1999. Recombinant human aggrecan G1-G2 exhibits native binding properties and substrate specificity for matrix metalloproteinases and aggrecanase. J. Biol. Chem. 274:32387-32395. [DOI] [PubMed] [Google Scholar]
- 45.Mercuri, F. A., R. A. Maciewicz, J. Tart, K. Last, and A. J. Fosang. 2000. Mutations in the interglobular domain of aggrecan alter matrix metalloproteinase and aggrecanase cleavage patterns. Evidence that matrix metalloproteinase cleavage interferes with aggrecanase activity. J. Biol. Chem. 275:33038-33045. [DOI] [PubMed] [Google Scholar]
- 46.Mort, J. S., M. C. Magny, and E. R. Lee. 1998. Cathepsin B: an alternative protease for the generation of an aggrecan 'metalloproteinase' cleavage neoepitope. Biochem. J. 335:491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mwale, F., E. Tchetina, C. W. Wu, and A. R. Poole. 2002. The assembly and remodeling of the extracellular matrix in the growth plate in relationship to mineral deposition and cellular hypertrophy: an in situ study of collagens II and IX and proteoglycan. J. Bone Miner. Res. 17:275-283. [DOI] [PubMed] [Google Scholar]
- 48.Nakase, T., M. Kaneko, T. Tomita, A. Myoui, K. Ariga, K. Sugamoto, Y. Uchiyama, T. Ochi, and H. Yoshikawa. 2000. Immunohistochemical detection of cathepsin D, K, and L in the process of endochondral ossification in the human. Histochem. Cell Biol. 114:21-27. [DOI] [PubMed] [Google Scholar]
- 49.Oshita, H., J. D. Sandy, K. Suzuki, A. Akaike, Y. Bai, T. Sasaki, and K. Shimizu. 2004. Mature bovine articular cartilage contains abundant aggrecan that is C-terminally truncated at Ala719-Ala720, a site which is readily cleaved by m-calpain. Biochem. J. 382:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavasant, P., T. Shizari, and C. B. Underhill. 1996. Hyaluronan contributes to the enlargement of hypertrophic lacunae in the growth plate. J. Cell Sci. 109:327-334. [DOI] [PubMed] [Google Scholar]
- 51.Plaas, A. H. K., and J. D. Sandy. 1993. A cartilage explant system for studies on aggrecan structure, biosynthesis and catabolism in discrete zones of the mammalian growth plate. Matrix 13:135-147. [DOI] [PubMed] [Google Scholar]
- 52.Plaas, A. H. K., L. West, R. J. Midura, and V. C. Hascall. 2001. Disaccharide composition of hyaluronan and chondroitin/dermatan sulfate, p. 117-128. In R. V. Iozzo (ed.), Proteoglycan protocols. Humana Press, Totowa, N.J.
- 53.Poole, A. R., I. Pidoux, and L. Rosenberg. 1982. Role of proteoglycans in endochondral ossification: immunofluorescent localization of link protein and proteoglycan monomer in bovine fetal epiphyseal growth plate. J. Cell Biol. 92:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Potts, W., J. Bowyer, H. Jones, D. Tucker, A. J. Freemont, A. Millest, C. Martin, W. Vernon, D. Neerunjun, G. Slynn, F. Harper, and R. Maciewicz. 2004. Cathepsin L-deficient mice exhibit abnormal skin and bone development and show increased resistance to osteoporosis following ovariectomy. Int. J. Exp. Pathol. 85:85-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rittenhouse, E., L. C. Dunn, J. Cookingham, C. Calo, M. Spiegelman, G. B. Dooher, and D. Bennett. 1978. Cartilage matrix deficiency (cmd): a new autosomal recessive lethal mutation in the mouse. J. Embryol. Exp. Morphol. 43:71-84. [PubMed] [Google Scholar]
- 56.Roberts, C. R., P. J. Roughley, and J. S. Mort. 1989. Degradation of human proteoglycan aggregate induced by hydrogen peroxide. Protein fragmentation, amino acid modification and hyaluronic acid cleavage. Biochem. J. 259:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandy, J. D., and C. Verscharen. 2001. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem. J. 358:615-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scherft, J. P., and S. Moskalewski. 1984. The amount of proteoglycans in cartilage matrix and the onset of mineralization. Metab. Bone Dis. Relat. Res. 5:195-203. [DOI] [PubMed] [Google Scholar]
- 59.Shaklee, P. N., and H. E. Conrad. 1985. Structural changes in the large proteoglycan in differentiating chondrocytes from the chick embryo tibiotarsus. J. Biol. Chem. 260:16064-16067. [PubMed] [Google Scholar]
- 60.Shimizu, K., T. Hamamoto, T. Hamakubo, W. J. Lee, K. Suzuki, Y. Nakagawa, T. Murachi, and T. Yamamuro. 1991. Immunohistochemical and biochemical demonstration of calcium-dependent cysteine proteinase (calpain) in calcifying cartilage of rats. J. Orthop. Res. 9:26-36. [DOI] [PubMed] [Google Scholar]
- 61.Sims, N. A., P. Clement-Lacroix, F. Da Ponte, Y. Bouali, N. Binart, R. Moriggl, V. Goffin, K. Coschigano, M. Gaillard-Kelly, J. Kopchick, R. Baron, and P. A. Kelly. 2000. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J. Clin. Investig. 106:1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singer, I. I., S. Scott, D. W. Kawka, E. K. Bayne, J. R. Weidner, H. R. Williams, R. A. Mumford, M. W. Lark, J. McDonnell, A. J. Christen, V. L. Moore, J. S. Mudgett, and D. M. Visco. 1997. Aggrecanase and metalloproteinase-specific aggrecan neo-epitopes are induced in the articular cartilage of mice with collagen II- induced arthritis. Osteoarthritis Cartilage 5:407-418. [DOI] [PubMed] [Google Scholar]
- 63.Soderstrom, M., H. Salminen, V. Glumoff, H. Kirschke, H. Aro, and E. Vuorio. 1999. Cathepsin expression during skeletal development. Biochim. Biophys. Acta 1446:35-46. [DOI] [PubMed] [Google Scholar]
- 64.Stanton, H., F. M. Rogerson, C. East, S. B. Golub, K. M. Lawlor, C. T. Meeker, C. B. Little, K. Last, P. J. Farmer, I. K. Campbell, A. M. Fourie, and A. J. Fosang. ADAMTS-5 is the major aggrecanase in mouse cartilage, in vivo and in vitro. Nature, in press. [DOI] [PubMed]
- 65.Stickens, D., D. J. Behonick, N. Ortega, B. Heyer, B. Hartenstein, Y. Yu, A. J. Fosang, M. Schorpp-Kistner, P. Angel, and Z. Werb. 2004. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 131:5883-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sztrolovics, R., M. Alini, P. J. Roughley, and J. S. Mort. 1997. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem. J. 326:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sztrolovics, R., R. J. White, P. J. Roughley, and J. S. Mort. 2002. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem. J. 362:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sztrolovics, R., A. D. Recklies, P. J. Roughley, and J. S. Mort. 2002. Hyaluronate degradation as an alternative mechanism for proteoglycan release from cartilage during interleukin-1beta-stimulated catabolism. Biochem. J. 362:473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thyberg, J., S. Lohmander, and U. Friberg. 1973. Electron microscopic demonstration of proteoglycans in guinea pig epiphyseal cartilage. J. Ultrastruct. Res. 45:407-427. [DOI] [PubMed] [Google Scholar]
- 70.Tortorella, M. D., M. Pratta, R. Q. Liu, J. Austin, O. H. Ross, I. Abbaszade, T. Burn, and E. Arner. 2000. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4). J. Biol. Chem. 275:18566-18573. [DOI] [PubMed] [Google Scholar]
- 71.Tselepis, C., A. P. Kwan, D. Thornton, and J. Sheehan. 2000. The biochemical characterization of aggrecan from normal and tibial-dyschondroplastic chicken growth-plate cartilage. Biochem. J. 351:517-525. [PMC free article] [PubMed] [Google Scholar]
- 72.van Meurs, J., P. van Lent, A. Holthuysen, D. Lambrou, E. Bayne, I. Singer, and W. van den Berg. 1999. Active matrix metalloproteinases are present in cartilage during immune complex-mediated arthritis: a pivotal role for stromelysin-1 in cartilage destruction. J. Immunol. 163:5633-5639. [PubMed] [Google Scholar]
- 73.van Meurs, J., P. van Lent, R. Stoop, A. Holthuysen, I. Singer, E. Bayne, J. Mudgett, R. Poole, C. Billinghurst, P. van der Kraan, P. Buma, and W. van den Berg. 1999. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: a pivotal role for stromelysin 1 in matrix metalloproteinase activity. Arthritis Rheum. 42:2074-2084. [DOI] [PubMed] [Google Scholar]
- 74.van Meurs, J. B., P. L. van Lent, A. E. Holthuysen, I. I. Singer, E. K. Bayne, and W. B. van den Berg. 1999. Kinetics of aggrecanase- and metalloproteinase-induced neoepitopes in various stages of cartilage destruction in murine arthritis. Arthritis Rheum. 42:1128-1139. [DOI] [PubMed] [Google Scholar]
- 75.van Meurs, J. B., P. L. van Lent, I. I. Singer, E. K. Bayne, F. A. van de Loo, and W. B. van den Berg. 1998. Interleukin-1 receptor antagonist prevents expression of the metalloproteinase-generated neoepitope VDIPEN in antigen-induced arthritis. Arthritis Rheum. 41:647-656. [DOI] [PubMed] [Google Scholar]
- 76.van Meurs, J. B., P. L. van Lent, A. A. van de Loo, A. E. Holthuysen, E. K. Bayne, I. I. Singer, and W. B. van den Berg. 1999. Increased vulnerability of postarthritic cartilage to a second arthritic insult: accelerated MMP activity in a flare up of arthritis. Ann. Rheum. Dis. 58:350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vu, T. H., J. M. Shipley, G. Bergers, J. E. Berger, J. A. Helms, D. Hanahan, S. D. Shapiro, R. M. Senior, and Z. Werb. 1998. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ward, R. V., R. M. Hembry, J. J. Reynolds, and G. Murphy. 1991. The purification of tissue inhibitor of metalloproteinases-2 from its 72 kDa progelatinase complex. Demonstration of the biochemical similarities of tissue inhibitor of metalloproteinases-2 and tissue inhibitor of metalloproteinases-1. Biochem. J. 278:179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wikstrom, B., B. Engfeldt, D. Heinegard, and A. Hjerpe. 1985. Proteoglycans and glycosaminoglycans in cartilage from the brachymorphic (bm/bm) mouse. Coll. Relat. Res. 5:193-204. [DOI] [PubMed] [Google Scholar]
- 80.Wikstrom, B., M. E. Wallace, A. Hjerpe, and B. Engfeldt. 1987. Chubby: a new autosomal recessive skeletal mutation producing dwarfism in the mouse. J. Hered. 78:8-14. [DOI] [PubMed] [Google Scholar]
- 81.Yasuda, T., K. Shimizu, Y. Nakagawa, S. Yamamoto, H. Niibayashi, and T. Yamamuro. 1995. m-calpain in rat growth plate chondrocyte cultures: its involvement in the matrix mineralization process. Dev. Biol. 170:159-168. [DOI] [PubMed] [Google Scholar]