Abstract
Genomic imprinting results in parent-of-origin-dependent monoallelic expression of selected genes. Although their importance in development and physiology is recognized, few imprinted genes have been investigated for their effects on brain function. Gnas is a complex imprinted locus whose gene products are involved in early postnatal adaptations and neuroendocrine functions. Gnas encodes the stimulatory G-protein subunit Gsα and two other imprinted protein-coding transcripts. Of these, the Nesp transcript, expressed exclusively from the maternal allele, codes for neuroendocrine secretory protein 55 (Nesp55), a chromogranin-like polypeptide associated with the constitutive secretory pathway but with an unknown function. Nesp is expressed in restricted brain nuclei, suggesting an involvement in specific behaviors. We have generated a knockout of Nesp55 in mice. Nesp55-deficient mice develop normally, excluding a role of this protein in the severe postnatal effects associated with imprinting of the Gnas cluster. Behavioral analysis of adult Nesp55 mutants revealed, in three separate tasks, abnormal reactivity to novel environments independent of general locomotor activity and anxiety. This phenotype may be related to prominent Nesp55 expression in the noradrenergic locus coeruleus. These results indicate a role of maternally expressed Nesp55 in controlling exploratory behavior and are the first demonstration that imprinted genes affect such a fundamental behavior.
Genomic imprinting describes the phenomenon of monoallelic, parental-origin-dependent expression of a gene. DNA methylation and chromatin modifications result in the silencing of either the maternal or paternal allele (12, 38). To date, ∼70 imprinted genes have been identified in the mouse and human (http://www.mgu.har.mrc.ac.uk/research/imprinted/ and http://cancer.otago.ac.nz/IGC/Web/home.html). Functional analysis of a number of imprinted genes by mutagenesis in mice has thus far revealed major effects on pre- and postnatal development and nutrient acquisition by offspring (35, 37, 44). It has also become evident that some imprinted genes have prominent expression patterns in the brain, but only a few have been analyzed for a role in the control of specific types of behavior (21). Furthermore, in the context of the prevailing “parental conflict” or “kinship” theory of genomic imprinting (32, 46), which recognizes (potential) differing interests of the maternal and paternal genomes in acting to maximize their respective inclusive fitness, behavioral substrates sensitive to imprinting effects are a novel and widely unrecognized subject.
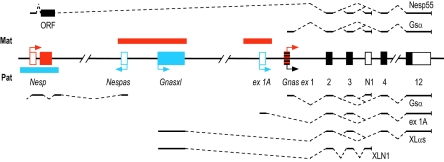
Neuroendocrine secretory protein 55 (Nesp55) was originally described as a chromogranin-like protein in secretory vesicles of adrenal chromaffin cells (18) and also as being expressed in a discrete pattern in the rat brain (2). Subsequent work showed Nesp to be part of the complex imprinted Gnas locus on distal chromosome 2 (Fig. 1) (25, 34). The Nesp transcript, which is expressed exclusively from the maternal allele, initiates from a promoter ∼45 kb upstream of Gnas. The two Nesp-specific exons are spliced onto exons 2 to 12 of Gnas, thus forming an alternatively spliced transcript of the latter, although the Nesp55 open reading frame (ORF) is confined to one upstream exon. Other imprinted transcripts of the locus include Gnas itself, encoding the Gsα subunit of trimeric G proteins; Gnas is expressed biallelically in most tissues but preferentially from the maternal allele in some (17, 51). Paternal-allele-specific transcripts comprise Gnasxl, which also splices onto exon 2 of Gnas and encodes a variant of Gsα (XLαs), and the noncoding RNAs exon 1A and Nespas (Fig. 1) (24, 29, 49).
FIG. 1.
Scheme of the imprinted Gnas locus of the mouse. Maternal (Mat) and paternal (Pat) allele-specific features are indicated in red and blue, respectively. Protein-coding exons are indicated as filled rectangles, and noncoding exons are indicated as open rectangles (for simplicity, exons 5 to 11 are omitted). Initiation of maternal- and paternal-specific transcripts is shown by arrows, and their splice patterns are given above and below. Tissue-specific maternal expression of the Gnas promoter is represented by the striped box (ex 1; Gsα protein). Gnasxl encodes an NH2-terminal variant of Gsα (XLαs) and a neural-specific, truncated protein (XLN1). A single upstream Nesp exon contains the Nesp55 ORF. Nespas and exon 1A (ex 1A) transcripts produce noncoding RNAs. Methylation of differentially methylated regions is indicated by bars above or below exons. (Figure modified from reference 35.)
Previously generated mouse models have revealed an essential role of the Gnas imprinting cluster in postnatal viability, growth, and the regulation of energy homeostasis (7, 8, 50, 51). Moreover, different and partly opposite phenotypes were found after maternal or paternal transmission of these mutations, indicating different functions of maternally and paternally expressed transcripts of the locus. However, since several imprinted transcripts are potentially affected in these models, phenotypic effects could not be unequivocally attributed to deficiency of a specific protein. Recently, the selective disruption of Gnasxl (XLαs) established its contribution to paternally required functions, revealing a major role in postnatal adaptations (35). The two maternally expressed transcripts of the locus, Gnas and Nesp, could both contribute to “lack of maternal function” phenotypes of the existing mouse models.
To reveal a function for Nesp55, we selectively targeted the Nesp coding exon. Nesp55-deficient mice develop without obvious phenotypic effects and are fertile. Given increasing evidence that imprinted genes mediate behavioral functions (21) and the restricted Nesp expression pattern in the mouse brain that we detected, behavioral tests were performed. We found that Nespm−/p+ mice showed, in several independent tests, highly specific changes in their reactivity to novel environments. These behavioral data correlate with high levels of Nesp in brain regions previously implicated in the response to novelty, including the locus coeruleus (39, 45). They also reveal a novel behavioral domain that is sensitive to imprinting effects and suggest that exploration and risk taking in the adult animal may represent important substrates in the evolution of imprinted genes.
MATERIALS AND METHODS
Generation of Nesp55-deficient mice.
To eliminate Nesp55, we made a deletion of 26 bp at the start codon of the ORF (bp 2362 to 2387 in AJ251761). The targeting construct was generated by homologous recombination in yeast as described earlier (35). Recombinogenic arms extending ∼400 bp upstream and downstream of the sequence to be deleted were amplified by PCR and ligated on either side of the selection marker cassette in plasmid pRAY-Cre (AJ627603) (35). A 9.38-kb mouse genomic DNA fragment (bp 136088 to 145468 in AL593857) was ligated into the yeast-Escherichia coli shuttle vector pRS414 (Stratagene) and cotransformed into yeast with a linear recombinogenic DNA fragment from pRAY-Cre. The recombined shuttle vector was recovered from selected yeast colonies, verified by sequencing, and used as the gene-targeting vector for embryonic stem (ES) cell electroporation.
Gene targeting was performed in IMT11 ES cells (129/SvSLCp; kindly provided by Martin Evans), and colonies were screened by Southern blotting using the AflII and XhoI restriction enzymes combined with 5′ and 3′ external probes, respectively. Correctly targeted ES cell clones were injected into C57BL/6J blastocysts, and germ line-transmitting chimeras were obtained from two independent cell lines. loxP-mediated excision of the selection marker cassette occurred in the germ line of male chimeras through tACE promoter-driven expression of Cre recombinase (6). Genotyping of offspring was performed by PCR using primers NL3 (5′-AGTGGAGGCACCTCTCGGA) and NE-R7 (5′-TCGTGATCAGACTCAGATTCA). The mutant-specific amplification product was cloned and sequenced to confirm correct Cre recombination. Nesp heterozygous offspring from chimeras were crossed onto two different genetic backgrounds (C57BL/6J and 129/Sv) for successive generations.
Mice of strain DS2, which were used in allele-specific gene expression analysis, carry a segment of chromosome 2 including the Gnas locus of Mus spretus origin on a (C57BL/6J × CBA/Ca) F1 background, obtained over five backcrosses.
Expression analysis.
For control reverse transcription (RT)-PCR and Northern blotting on the Gnas imprinting cluster, total RNA, prepared using the RNeasy kit (QIAGEN), was isolated from brain tissues of offspring from DS2 females crossed to Mus musculus males heterozygous for the Nesp mutation. Reverse transcription was carried out using random hexamer primers. Transcript-specific PCR products were obtained using the following primers: XL-F11 (Gnasxl), 5′-GACTACATGTGTACACACCGC; mEx1AF (exon 1A), 5′-CGTGTGAGTGCGTCTCACTCA; mGnasF (Gnas exon 1), 5′-GGAGAAGGCGCAGCGCGAGGCCAA; and mGnas12R (Gnas exon 12), 5′-CTCGTATTGGCGGAGATGCAT. To determine the parental origins of transcripts, PCR products were restricted for the strain-specific Bsh1236I polymorphism, as previously described (35).
Northern blotting and in situ hybridization were performed as previously described (35). For in situ hybridization, 3-month-old wild-type mice were perfused with 4% paraformaldehyde-phosphate-buffered saline (PBS), and the brain tissue was dissected and further fixed in 4% paraformaldehyde-PBS overnight. The tissues were then dehydrated either in 30% sucrose-PBS for cryostat sectioning or in an ethanol series for paraffin embedding. Stainings (see Fig. 3A to D) were obtained from a series of 12-μm-thick paraffin sections from a male, while others (see Fig. 3E to H) were derived from a series of 20-μm-thick cryostat sections of a female tissue sample. Digoxigenin-labeled antisense and sense riboprobes for in situ hybridization were generated by in vitro transcription of a plasmid containing the 762-bp Nesp ORF using digoxigenin RNA-labeling mix (Roche). Western blots and radioimmunoassays were performed using an antibody against the carboxy-terminal octapeptide of Nesp55, as previously described (18). Nesp55 migrates at different sizes in different gel systems, e.g., Lämmli versus Novex gels.
FIG. 3.
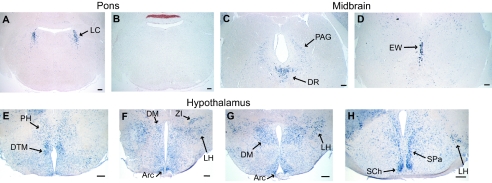
Expression of Nesp in brainstem regions and the hypothalamus. In situ hybridization of coronal sections of adult mouse brain, using a Nesp antisense riboprobe. (A) In the pons, high levels of expression are detected in the locus coeruleus (LC). (B) Control hybridization using the Nesp sense probe. (C and D) In the midbrain, mRNA is primarily detected in the dorsal raphe nucleus (DR) and the periaqueductal gray (PAG). More rostal sections show high Nesp levels in the Edinger-Westphal nucleus (EW). (E to H) Expression in the hypothalamus is shown on sections in caudal-to-rostral order. PH, posterior hypothalamic area; DTM, dorsal tuberomammillary nucleus; DM, dorsomedial hypothalamic nucleus; Arc, arcuate hypothalamic nucleus; LH, lateral hypothalamic area; ZI, zona incerta; SCh, suprachiasmatic nucleus; SPa, subparaventricular zone of the hypothalamus. (Scale bars, 200 μm.)
Behavioral analysis.
Two cohorts of mice with C57BL/6J genetic backgrounds (cohort 1 backcrossed for three generations and cohort 2 backcrossed for six generations and derived from two targeted ES cell lines) and one cohort on a 129/Sv background (backcrossed for five generations) were tested. The tests were performed on males at 4 to 8 months of age. C57BL/6J cohort 1 comprised 11 Nespm−/p+ and 7 Nespm+/p− mice, the second cohort comprised 10 Nespm−/p+ and 13 Nespm+/p− mice, and the 129/Sv cohort comprised 16 Nespm−/p+ and 14 Nespm+/p− mice. Wild-type (Nespm+/p+) littermates were tested alongside these cohorts. Identification of genotypes was done after the behavioral tests.
Locomotor activity (LMA) was measured using a battery of activity cages fitted with infrared beams. Data were collected in 5-min bins over a period of 1 h under red illumination. The subjects of all three cohorts were assessed on three successive days, and data from the 129/Sv cohort and C57BL/6J cohort 1 are presented in Results. C57BL/6J cohort 2 produced similar statistically significant data; however, LMA was generally higher than in cohort 1.
The open field consisted of a white Perspex arena (750 by 750 mm) enclosed by 450-mm-high walls. The floor was divided by nontactile lines into a middle (400 by 400 mm) and outer area, and the whole floor area was further divided into four quadrants. The test was run in low-level white and red light. The animals (C57BL/6J cohort 1) were allowed to explore the arena freely for 10 min. The main measures were time spent in, and number of entries into, the middle section. A measure of locomotor activity was obtained by counting the crossings of quadrant lines.
The elevated plus maze (EPM) comprised a cross-shaped maze (175 by 78 mm) raised 500 mm off the ground with two opposite arms open and the other two arms enclosed by walls (150 mm high). The test was run in low-level white and red light. The animals (C57BL/6J cohort 1) were placed on the maze and allowed to explore freely for 5 min. The main measures were time spent in each location (closed arms, open arms, and middle) and entries into each arm.
The novelty place preference (NPP) arena consisted of two Perspex chambers (300 by 300 by 300 mm), one white and one black, joined by an opening. Sandpaper was fixed to the floor of one or the other chamber to provide additional tactile information. The test was run under red illumination. The animals (C57BL/6J cohort 2) were introduced pseudorandomly into one of the chambers and allowed to habituate for 1 h, with the entrance to the other chamber blocked. After habituation, the animal was removed, the whole arena was cleaned with 2% (vol/vol) acetic acid, and the gate between the two chambers was opened. The animal was replaced in the familiar chamber and allowed to explore the whole arena freely for 14 min. The main measures were time spent exploring, and number of entries made into, the novel environment.
Neurochemical analysis.
Brains for neurochemical analyses were taken from a subset of the animals used in the behavioral experiments, and two regions, defined as the prefrontal cortex (PFC) and the pons, were dissected immediately following removal. Dissected brain tissue was transferred to tubes containing 0.1 M perchloric acid (200 μl for the PFC and 400 μl for the pons) and homogenized at 4°C using a microsonicator. The resulting suspension was centrifuged twice at 4°C (16,060 × g for 5 min), and the supernatant was retained. This supernatant phase was directly assessed for monoamine concentration by high-performance liquid chromatography (HPLC). Samples were run in a pseudorandomized order as soon as possible after they were prepared. If the samples could not be subjected to HPLC analysis immediately, they were snap frozen at −80°C until they were used. Each sample was thawed and frozen a maximum of one time. Dopamine (DA), 5-hydroxytryptamine (5-HT), and norepinephrine (NE) were quantified by microbore HPLC with an electrochemical detection system (BAS LC4B with a Unijet cell and a 6-mm-long glassy carbon electrode at a potential of +0.65 V; Bioanalytical Systems). The mobile phase (10 mM sodium chloride, 85 mM sodium acetate, 150 mg of octan-sulfonic acid/ml, 100 mg of EDTA, and 4% methanol at pH 4.5) was perfused through the system at a flow rate of 210 μl/min. The separation column was a C18 reversed-phase Sperisorb (S3ODS2; 2-mm internal diameter; 150 mm long; Waters, Milford, Mass.). A CMA-200 microinjector (CMA Microdialysis, Stockholm, Sweden) was used to maintain the samples at a constant low temperature and to inject brain homogenate samples onto the column (7-μl injection volume). The system had a detection sensitivity of 100 to 200 pM.
Statistics.
All data were analyzed by analysis of variance with factors GENOTYPE (Nespm−/p+ or Nespm+/p−), POE (parent-of-origin effect; wild-type mice derived from both parental Nesp heterozygote crosses), DAY (day 1, 2, or 3 of LMA testing), and, in the case of behavioral data, TIME (time bin). In some cases, pairwise variability in the behavioral data was shown as the standard error of the difference of means (SED) (see Fig. 4). As the SED can be used as the denominator for post hoc comparisons, it is the most appropriate comparator for the visual evaluation of the difference between two mean values. The relevant formulae are given by Cochran and Cox (9).
FIG. 4.
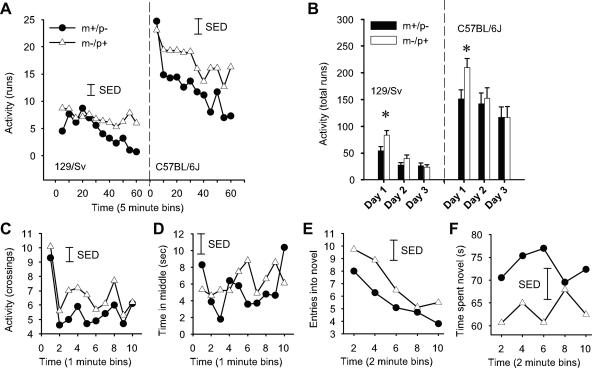
Behavioral analysis of Nesp55-deficient mice. (A) LMA over 1 h (5-min time bins) for Nespm−/p+ and Nespm+/p− mice on 129/Sv and C57BL/6J genetic backgrounds. The results for day 1 of three consecutive days of testing are shown. (B) Total LMA on three consecutive days on both genetic backgrounds. (C) Locomotor activity in the open-field arena as measured by the number of crossings of quadrant boundaries. (D) Time spent in the middle section of the open-field arena. (E) Entries into and (F) time spent in a novel environment in the novelty place preference test. The open-field and NPP data were obtained from cohorts on the C57BL/6J genetic background (see Materials and Methods). The data are mean values, and the vertical error bars represent the SED taken from the error terms for the interaction between the factors GENOTYPE (Nespm−/p+ or Nespm+/p−) and TIME (time bin). As the SED can be used as the denominator for post hoc comparisons, it is the most appropriate comparator for the visual evaluation of the difference between two mean values.
RESULTS
Generation of Nesp55-deficient mice.
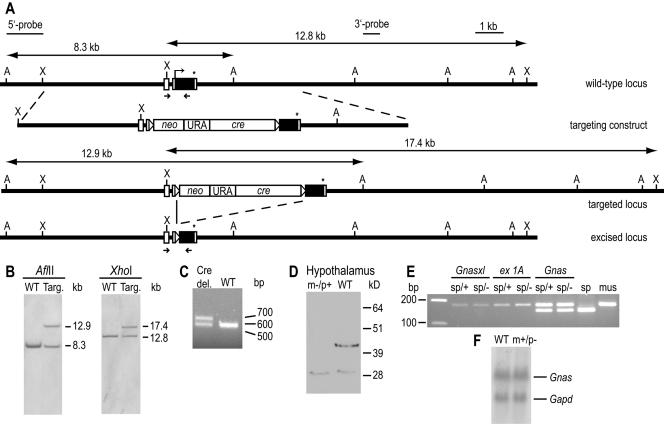
To disrupt the Nesp55-encoding transcript, a small deletion covering the start codon of the ORF was generated via homologous recombination in ES cells (Fig. 2A). Two correctly targeted ES cell lines, identified by Southern blot hybridizations (Fig. 2B), were used to generate chimeric mice. The loxP-flanked targeting cassette was excised through germ line-specific expression of Cre recombinase in male chimeras, resulting in offspring carrying a mutant allele in which one loxP site replaced the Nesp translation initiation sequence (Fig. 2A and C). Maternal transmission of the mutation in the following generation resulted in Nesp55-deficient mice, as no protein was detectable in major sites of expression by Western blotting (brain) (Fig. 2D) or radioimmunoassay (adrenal gland, wild type, 67.2 ± 9.0 fmol/mg [n = 5] and m−/p+, 0.0 fmol/mg [n = 7]; pituitary gland, wild type, 617.9 ± 142.9 fmol/mg [n = 5] and m−/p+, 0.0 fmol/mg [n = 7]).
FIG. 2.
Generation of Nesp55-deficient mice. (A) Nesp gene-targeting strategy. The wild-type locus is depicted on top. Noncoding and coding parts are indicated as open and black rectangles, respectively; the Nesp start and stop codons are shown as an angled arrow and an asterisk. The selection marker cassette (neo-URA-cre) is flanked by loxP sites (open triangles). Restriction sites used for Southern blotting and fragments hybridizing with 5′ and 3′ external probes are indicated (A, AflII; X, XhoI). Small arrows mark the PCR primers NL3 and NE-R7 used for genotyping in panel C. (B) Southern blot analysis of ES cell clones showing wild-type (WT) and targeted (Targ.) alleles. (C) PCR genotyping of mice after germ line transmission of the mutation from chimeras using primers NL3 and NE-R7. Cre-loxP recombination results in a mutant allele, which is increased by 108 bp. del., deletion. (D) Western blot for Nesp55. Maternal transmission of the Nesp mutation (m−/p+) results in Nesp55 deficiency, as shown in hypothalamus samples. The nonspecific low-molecular-weight signal indicates equal loading of the lanes. (E) The paternally inherited Nesp mutation has no effect in cis on downstream Gnas transcripts. RT-PCR was performed on hypothalamus RNA of mice carrying a maternally inherited M. spretus (sp) and a paternally inherited wild-type (sp/+) or mutant (sp/−) M. musculus (mus) allele. Forward primers specific for Gnasxl, exon 1A, and Gnas exon 1, respectively, were combined with a common reverse primer in Gnas exon 12, and amplification products were restricted for the strain-specific Bsh1236I polymorphism (M. spretus, 151 bp; M. musculus, 178 bp). (F) Expression levels of Gnas are unchanged in Nespm+/p− mice. Northern blot hybridization of midbrain-pons RNA for Gnas exon 1 and Gapd as a loading control indicates equal Gnas expression levels.
Since the Nesp-specific exons are located within a differentially methylated region, we checked whether the paternally transmitted mutation had any influence on the imprinted expression of transcripts located downstream in the locus. M. musculus males heterozygous for the Nesp mutation were crossed with DS2 females, which carry a segment of chromosome 2 including the Gnas locus from M. spretus, and offspring that inherited the maternal spretus wild-type allele (sp) and the paternal musculus wild-type (+) or mutant (−) Nesp allele were identified. To assess the parental origin of transcripts, RT-PCRs were performed on hypothalamus RNA and the products were restricted for a strain-specific polymorphic Bsh1236I site. As shown in Fig. 2E, Gnasxl and the noncoding exon 1A transcripts were derived solely from the paternal allele, while Gnas was transcribed biallelically as expected, and no difference could be detected between wild-type and Nespm+/p− samples. Furthermore, quantification of Gnas transcript levels in midbrain and pons tissue by Northern blot hybridization indicated no difference between wild-type and Nespm+/p− samples (Fig. 2F). Thus, while the maternally inherited mutation leads to Nesp55 deficiency, the paternally inherited mutation has no consequences for the Gnas-imprinted locus. Nesp55-deficient mice are born in Mendelian ratios, develop normally without obvious phenotypic abnormalities, and are fertile.
Nesp expression in distinct brain regions.
The detailed analysis of Nesp expression in the rat brain (2) and proposed neural functions of Nesp55 (18) prompted a comparison with the mouse; therefore, we examined an extensive series of coronal sections of adult mouse brain by in situ hybridization. Similar to the rat, we found discrete high-level expression of Nesp in pons and midbrain regions (Fig. 3A). Strong signals were obtained in the noradrenergic locus coeruleus of the pons. Prominent expression was also detected in the dorsal raphe nucleus of the midbrain, while lower levels were found in scattered cells of the periaqueductal gray (Fig. 3C). Very high levels of Nesp were found in cells of the Edinger-Westphal nucleus of the rostral midbrain (Fig. 3D), which constitutes the main source of the neuropeptide urocortin in the brain (41). A more widespread expression pattern was observed in the hypothalamus (Fig. 3E to H). High levels of Nesp were detected in the dorsal tuberomammillary nucleus, the arcuate nucleus, the suprachiasmatic nucleus, and some cells of the lateral hypothalamic area and the subparaventricular zone. Weaker signals appeared in subpopulations of cells of the posterior hypothalamic area, the dorsomedial nucleus, and the zona incerta. Further areas positive for Nesp include the medial amygdaloid nucleus and the lateral globus pallidus, as well as the dorsal motor nucleus of the vagus in the medulla oblongata (data not shown), which were also detected in the rat brain (2). Although the expression pattern was found generally to be similar to that described in the rat, notable differences were observed; for example, there was no detectable expression in the hypoglossal nucleus or the facial nucleus in the mouse. Major brain regions devoid of Nesp include the cerebellum and cortex and forebrain areas, such as the nucleus accumbens.
Behavioral analyses.
To identify potential functions of Nesp55 in the central nervous system, we conducted a series of behavioral tests. Given the strain differences in performance and the potential confounds regarding C57BL/6J and 129/Sv genetic background contributions in behavioral analysis of transgenic mice (1, 14, 20, 48), we established the Nesp targeted mutation in both strains. Loci closely linked to a targeted mutation cause special concern, since they most likely retain the original 129 ES cell alleles, even after several generations of backcrossing to a different strain. For analysis of mice carrying a homozygous mutation of a biallelically expressed gene, this may imply comparing an associated homozygous 129/Sv genetic background with wild-type C57BL/6J counterparts. However, the knockout analysis of monoallelically expressed genes allows a more direct comparison of heterozygous groups. In the case of maternally expressed Nesp, heterozygous deficient mice (m−129/Sv/p+C57BL/6J) can be compared with heterozygous Nesp-expressing mice (m+C57BL/6J/p−129/Sv) obtained from reciprocal crosses to C57BL/6J. In both of these groups, the loci surrounding the targeted mutation will comprise compound C57BL/6J and 129/Sv alleles, and thus, any potential strain variations should manifest equally in both groups. In contrast, the wild-type littermates of the two heterozygous groups are homozygous C57BL/6J for these genomic regions, which may confound the interpretation of behavioral assays, as it is well established that C57BL/6J mice are more active than 129/Sv mice (14, 20). Thus, we considered it most appropriate to compare Nespm−/p+ with Nespm+/p− mice as controls. Some tasks were also examined using a cohort on the 129/Sv background, in which both Nespm−/p+ and Nespm+/p− mice are homozygous for 129/Sv alleles at loci linked to the Nesp region.
For an initial assessment of behavioral performance, Nesp55-deficient mice were tested for locomotor activity and open-field exploration. LMA and habituation were measured in automated activity cages. Nespm−/p+ mice displayed increased activity relative to Nespm+/p− mice on day 1 of LMA testing (Fig. 4A) (129/Sv, main effect of GENOTYPE, F1,27 = 6.07, P < 0.02). This difference was also present on a C57BL/6J background (Fig. 4A) (C57BL/6J, main effect of GENOTYPE, F1,16 = 5.71, P < 0.04), despite overall increased levels of LMA. Analysis of total activity over three consecutive days demonstrated that both Nespm−/p+ and Nespm+/p− mice habituated over time (Fig. 4B) (main effect of DAY, F2,79 = 27.67, P < 0.01 [129Sv]; F2,48 = 6.60, P < 0.01 [C57BL6/J]). Post hoc tests (Tukey's) revealed that the only significant difference in LMA between Nespm−/p+ and Nespm+/p− mice occurred on day 1 upon first encountering the novel test box (P < 0.05 for both 129/Sv and C57BL/6J cohorts) and that by days 2 and 3 there was no difference between the two groups (Fig. 4B). In further tests, the C57BL/6J background was used, as the inherently higher activity levels provided a more sensitive measure.
Analysis of locomotor activity behavior (quadrant crossings) when placed into the novel surroundings of the open-field arena again revealed that Nespm−/p+ mice were more active than Nespm+/p− mice over the duration of the 10-min test (Fig. 4C) (main effect of GENOTYPE, F1,150 = 5.02, P < 0.03). However, performance analysis in the open-field test revealed no difference in anxiety-related behavior (time spent in the middle of the arena) (Fig. 4D) (main effect of GENOTYPE, F1,150 = 0.82, not significant [NS]). Thus, increased locomotor activity was dissociated from reduced anxiety. To further assess anxiety-related behavior, independent evidence was collected from the elevated-plus-maze test. In the EPM, there was no significant difference in percentage of time spent on the open arms between Nespm−/p+ (13.5 ± 1.6 standard error of the mean [SEM]) and Nespm+/p− (15.8 ± 1.8 SEM) (main effect of GENOTYPE, F1,16 = 0.81, NS), confirming unchanged anxiety-related behavior in Nesp55-deficient mice.
These data indicate differential reactivity to a novel environment between Nespm−/p+ and Nespm+/p− mice. We examined this further using the novelty place preference task, which explicitly tests an animal's propensity to explore a novel environment. Consistent with the above-mentioned results, Nespm−/p+ mice made more entries into (Fig. 4E) (main effect of GENOTYPE, F1,20 = 5.02, P < 0.04), but spent less time in (Fig. 4F) (main effect of GENOTYPE, F1,20 = 4.47, P < 0.05), the novel environment than Nespm+/p− mice.
As Nespm−/p+ and Nespm+/p− mice were generated by different crosses, it was important to establish that the differences described above were not due to differences in maternal care or other effects during the raising of the cohorts. Thus, we compared the wild-type mice generated in each cross. There were no differences between wild-type mice from either cross in LMA (on day 1, main effect of ORIGIN, F1,35 = 0.16, NS; across three consecutive days of testing, main effect of ORIGIN, F1,100 = 0.33, NS), open-field behavior (quadrant crossings; main effect of ORIGIN, F1,64 = 0.05, NS), behavior on the EPM (percentage of time on open arms; main effect of ORIGIN, F1,11 = 1.68, NS), or in the NPP arena (time in novel environment; main effect of ORIGIN, F1,64 = 0.30, NS). There was, however, a suggestion that the wild-type mice may display higher locomotor activity levels than the Nespm+/p− control group (data not shown). However, this locomotor activity was maintained over time (and therefore was independent of novelty) and was more evident in C57BL/6J cohort 2, i.e., after further generations of backcrossing. As mentioned above, C57BL/6J mice have generally higher locomotor activity than 129/Sv mice (14, 20), and our observations confirm earlier reports about genetic background confounds (1, 48). This underlines the importance of directly comparing Nespm−/p+ with Nespm+/p− mice.
Whole-tissue neurochemical analyses.
Since monoaminergic neural pathways are known to influence behavioral reactivity to novelty (16, 39), we assessed the extent to which the Nespm−/p+ and Nespm+/p− mice from the C57BL/6J cohorts differed in terms of whole-tissue levels of NE, DA, and 5-HT. This was carried out on brain regions that are major sources or target areas, e.g., pons and PFC, respectively, of monoaminergic projections, whereby the ascending noradrenergic projection from the pontine locus coeruleus to the PFC, especially, has been recognized as a modulator of novelty behavior (10, 36). HPLC analysis of tissue homogenates of these two brain regions revealed no differences between Nespm−/p+ and Nespm+/p− mice, however, in levels of the three monoamines in either the PFC (NE, F1,19 = 2.8, NS; DA, F1,19 = 0.04, NS; 5-HT, F1,19 = 2.0, NS) or the pons (NE, F1,19 = 0.01, NS; DA, F1,19 = 0.01, NS; 5-HT, F1,19 = 1.6, NS) (Table 1), excluding such gross neurochemical changes as a cause for the behavioral phenotype observed.
TABLE 1.
Monoamine concentrations in PFC and pons as measured by HPLC of tissue homogenates
| Monoamine | Concn (ng/mg of tissue wet wt) (mean ± SEM)
|
|||
|---|---|---|---|---|
| PFC
|
Pons
|
|||
| Nespm−/p+a | Nespm+/p−b | Nespm−/p+a | Nespm+/p−b | |
| Norepinephrine | 2.93 (0.10) | 3.22 (0.14) | 3.79 (0.36) | 3.82 (0.05) |
| Dopamine | 0.88 (0.22) | 0.87 (0.27) | 1.53 (0.09) | 1.52 (0.07) |
| 5-HT | 11.06 (0.45) | 10.21 (0.39) | 13.96 (0.95) | 15.19 (0.37) |
Nespm−/p+ (n = 10) mice.
Nespm+/p− (n = 11) mice.
DISCUSSION
The complexity of the Gnas-imprinted locus—its variously imprinted alternative transcripts—complicated the interpretation of earlier mouse models. Mice carrying paternal uniparental duplication of distal chromosome 2 (PatDp.dist2), which results in a lack of maternally expressed transcripts and a double dose of paternally expressed transcripts of this locus, die shortly after birth with severe edema, tremor, imbalance, and hyperactivity (7). A similar phenotype is found in mice heterozygous for a targeted mutation of Gnas exon 2 after maternal transmission (51). In both cases, two maternally expressed transcripts of the locus, Nesp and Gnas, are potentially affected (34, 51). The generation of mice specifically deficient for Nesp55 now allows us to assign these severe early postnatal phenotypes to tissue-specific lack of maternal-allele-derived Gsα function (47, 51), since Nesp55 deficiency causes no such effects.
Nevertheless, deletion of Nesp55 revealed a role for this imprinted protein in the control of specific types of behavior in adult mice, which can be associated with its prominent expression in the central nervous system. Two separate behavioral tasks (LMA and open field) revealed an increased reactivity to novelty in mice lacking Nesp55. These activity differences were robust, as they were present in two different genetic backgrounds and in cohorts derived from two targeted ES cell clones. Furthermore, increased locomotor activity per se could be excluded as a potentially confounding factor, since all animals habituated to the activity cages during consecutive days of testing, with no discernible difference between genotypes on the final day. An additional explicit test of novelty investigation (NPP) indicated that although Nesp55-deficient animals showed increased excitement toward the novel environment (more entries), they actually spent less time there to explore it. Changes in anxiety can be excluded as an influencing factor, based on the results of tests specifically designed to measure this trait (EPM and open field). Similar changes in reactivity to novelty, dissociated from changes in anxiety-related behavior, were also described in mice lacking the 5-HT5A receptor (16). This is of interest given our expression data showing Nesp-positive cells in the dorsal raphe nucleus. This region provides widespread 5-HT innervation to forebrain structures (22), and Nesp55 could exert its influence on reactivity to novelty via the serotonergic system. Against this is evidence suggesting that the median raphe nucleus, rather than the dorsal raphe nucleus, is implicated in exploratory behavior (42).
An alternative explanation is that Nesp55 impacts on reactivity to novelty via a function in the locus coeruleus and its dorsal ascending noradrenergic bundle innervating the prefrontal cortex, hippocampus, and other forebrain structures (4, 10). The locus coeruleus has a well-established role in the neurobiology of novelty, responding, for example, with increased firing activity upon a first encounter with a novel environment or object (40, 45). Furthermore, exposure to novelty causes elevated levels of extracellular norepinephrine in the locus coeruleus and prefrontal cortex (36), and pharmacological manipulation of adrenergic receptors confirms the regulatory role of the locus coeruleus noradrenergic system on behavioral reactivity to novelty (39, 40).
The function of Nesp55 on a molecular level is currently unresolved. The protein is highly conserved among mammals (80% amino acid identity between mouse and human) and shares features with the chromogranin family of proteins in its hydrophilicity, acidity, and association with secretory vesicles of neuroendocrine cells (3, 18). Proteolytic processing occurs to different extents in various tissues and results in the release of the carboxy-terminal octapeptide (30). Recent results show that Nesp55 is associated with fast anterograde axonal transport in the peripheral nervous system and is considered a marker for the constitutive secretory pathway (13, 27). However, it is unknown whether Nesp55 influences the transport or release of neurotransmitter vesicles. We tested whether Nesp55-null mice had altered monoamine levels in brain tissue taken from the PFC and pons. There were no observable differences, indicating that the role of Nesp55 in novelty reactivity does not involve changes in this gross index of brain function. It remains a high priority to assess the effects of Nesp55 deficiency on neurobiological indices more closely related to synaptic function, such as extracellular levels of transmitters and changes in pre- and postsynaptic receptor moities.
Why imprinting of a gene influencing a behavior as important as novelty reactivity should have evolved is not obvious at first sight. The most robust evolutionary theory of genomic imprinting, the parental conflict or kinship hypothesis, is based on intragenomic conflict between parental alleles whenever asymmetries of relatedness occur (32, 46). This parental conflict is most evident with regard to resource distribution between mother and offspring (35, 37, 44). Among the small number of imprinted genes known to affect adult behavior (5, 11, 15, 23, 26, 28, 31, 33), some indeed have pleiotropic effects pre- and postweaning, which is compatible with the kinship theory (46). However, the work presented here is an example of an imprinted gene that has no role in prenatal or early postnatal development but clearly mediates an adult behavior. In this respect, it has been theorized that reactivity to novelty and exploratory behavior may be substrates for the action of imprinted genes, e.g., in the context of dispersal from the natal area and inbreeding control (19, 43). It is tempting to assume a role for Nesp55 in this context, but reactivity to novelty may be only one aspect among other types of behavior potentially under the influence of Nesp55, given its distribution throughout the brain. Nevertheless, our data thus far raise the intriguing possibility that exploratory and associated risk-taking behaviors may be subject to differential selective pressures on the maternal and paternal genomes.
Acknowledgments
G.K. and L.S.W. are supported by the Biotechnology and Biological Sciences Research Council and by a Synergy Initiative award from the Babraham Institute; G.K. is a Senior Fellow of the Medical Research Council. R.F.-C. was supported by grant P16389-B05 from the Austrian Science Foundation.
We thank Jo Peters for critical reading of the manuscript, Martin Evans for IMT-11 ES cells, and staff at the Babraham Institute animal facilities for dedicated husbandry.
REFERENCES
- 1.Banbury Conference on Genetic Background in Mice. 1997. Mutant mice and neuroscience: recommendations concerning genetic background. Neuron 19:755-759. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, R., R. Ischia, J. Marksteiner, I. Kapeller, and R. Fischer-Colbrie. 1999. Localization of neuroendocrine secretory protein 55 messenger RNA in the rat brain. Neuroscience 91:685-694. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, R., C. Weiss, J. Marksteiner, A. Doblinger, R. Fischer-Colbrie, and A. Laslop. 1999. The new chromogranin-like protein NESP55 is preferentially localized in adrenaline-synthesizing cells of the bovine and rat adrenal medulla. Neurosci. Lett. 263:13-16. [DOI] [PubMed] [Google Scholar]
- 4.Berridge, C. W., and B. D. Waterhouse. 2003. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42:33-84. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla, R., N. Gnesutta, L. Minichiello, G. White, A. J. Roylance, C. E. Herron, M. Ramsey, D. P. Wolfer, V. Cestari, C. Rossi-Arnaud, S. G. Grant, P. F. Chapman, H. P. Lipp, E. Sturani, and R. Klein. 1997. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature 390:281-286. [DOI] [PubMed] [Google Scholar]
- 6.Bunting, M., K. E. Bernstein, J. M. Greer, M. R. Capecchi, and K. R. Thomas. 1999. Targeting genes for self-excision in the germ line. Genes Dev. 13:1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattanach, B. M., and M. Kirk. 1985. Differential activity of maternally and paternally derived chromosome regions in mice. Nature 315:496-498. [DOI] [PubMed] [Google Scholar]
- 8.Cattanach, B. M., J. Peters, S. Ball, and C. Rasberry. 2000. Two imprinted gene mutations: three phenotypes. Hum. Mol. Genet. 9:2263-2273. [DOI] [PubMed] [Google Scholar]
- 9.Cochran, W., and G. Cox. 1957. Experimental designs, second ed. Wiley, New York, N.Y.
- 10.Cole, B. J., T. W. Robbins, and B. J. Everitt. 1988. Lesions of the dorsal noradrenergic bundle simultaneously enhance and reduce responsivity to novelty in a food preference test. Brain Res. 472:325-349. [DOI] [PubMed] [Google Scholar]
- 11.Curley, J. P., S. Barton, A. Surani, and E. B. Keverne. 2004. Coadaptation in mother and infant regulated by a paternally expressed imprinted gene. Proc. R. Soc. Lond. B 271:1303-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaval, K., and R. Feil. 2004. Epigenetic regulation of mammalian genomic imprinting. Curr. Opin. Genet. Dev. 14:188-195. [DOI] [PubMed] [Google Scholar]
- 13.Fischer-Colbrie, R., S. Eder, P. Lovisetti-Scamihorn, A. Becker, and A. Laslop. 2002. Neuroendocrine secretory protein 55: a novel marker for the constitutive secretory pathway. Ann. N. Y. Acad. Sci. 971:317-322. [DOI] [PubMed] [Google Scholar]
- 14.Gerlai, R. 1996. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 19:177-181. [DOI] [PubMed] [Google Scholar]
- 15.Giese, K. P., E. Friedman, J. B. Telliez, N. B. Fedorov, M. Wines, L. A. Feig, and A. J. Silva. 2001. Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1). Neuropharmacology 41:791-800. [DOI] [PubMed] [Google Scholar]
- 16.Grailhe, R., C. Waeber, S. C. Dulawa, J. P. Hornung, X. Zhuang, D. Brunner, M. A. Geyer, and R. Hen. 1999. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron 22:581-591. [DOI] [PubMed] [Google Scholar]
- 17.Hayward, B. E., A. Barlier, M. Korbonits, A. B. Grossman, P. Jacquet, A. Enjalbert, and D. T. Bonthron. 2001. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J. Clin. Investig. 107:R31-R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ischia, R., P. Lovisetti-Scamihorn, R. Hogue-Angeletti, M. Wolkersdorfer, H. Winkler, and R. Fischer-Colbrie. 1997. Molecular cloning and characterization of NESP55, a novel chromogranin-like precursor of a peptide with 5-HT1B receptor antagonist activity. J. Biol. Chem. 272:11657-11662. [DOI] [PubMed] [Google Scholar]
- 19.Isles, A. R., M. J. Baum, D. Ma, A. Szeto, E. B. Keverne, and N. D. Allen. 2002. A possible role for imprinted genes in inbreeding avoidance and dispersal from the natal area in mice. Proc. R. Soc. Lond. B 269:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isles, A. R., T. Humby, E. Walters, and L. S. Wilkinson. 2004. Common genetic effects on variation in impulsivity and activity in mice. J. Neurosci. 24:6733-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isles, A. R., and L. S. Wilkinson. 2000. Imprinted genes, cognition and behaviour. Trends Cogn. Sci. 4:309-318. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs, B. L., and E. C. Azmitia. 1992. Structure and function of the brain serotonin system. Physiol. Rev. 72:165-229. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, Y. H., D. Armstrong, U. Albrecht, C. M. Atkins, J. L. Noebels, G. Eichele, J. D. Sweatt, and A. L. Beaudet. 1998. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21:799-811. [DOI] [PubMed] [Google Scholar]
- 24.Kehlenbach, R. H., J. Matthey, and W. B. Huttner. 1994. XL alpha s is a new type of G protein. Nature 372:804-809. [DOI] [PubMed] [Google Scholar]
- 25.Kelsey, G., D. Bodle, H. J. Miller, C. V. Beechey, C. Coombes, J. Peters, and C. M. Williamson. 1999. Identification of imprinted loci by methylation-sensitive representational difference analysis: application to mouse distal chromosome 2. Genomics 62:129-138. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre, L., S. Viville, S. C. Barton, F. Ishino, E. B. Keverne, and M. A. Surani. 1998. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat. Genet. 20:163-169. [DOI] [PubMed] [Google Scholar]
- 27.Li, J. Y., P. Lovisetti-Scamihorn, R. Fischer-Colbrie, H. Winkler, and A. Dahlstrom. 2002. Distribution and intraneuronal trafficking of a novel member of the chromogranin family, NESP55, in the rat peripheral nervous system. Neuroscience 110:731-745. [DOI] [PubMed] [Google Scholar]
- 28.Li, L., E. B. Keverne, S. A. Aparicio, F. Ishino, S. C. Barton, and M. A. Surani. 1999. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science 284:330-333. [DOI] [PubMed] [Google Scholar]
- 29.Liu, J., S. Yu, D. Litman, W. Chen, and L. S. Weinstein. 2000. Identification of a methylation imprint mark within the mouse Gnas locus. Mol. Cell. Biol. 20:5808-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovisetti-Scamihorn, P., R. Fischer-Colbrie, B. Leitner, G. Scherzer, and H. Winkler. 1999. Relative amounts and molecular forms of NESP55 in various bovine tissues. Brain Res. 829:99-106. [DOI] [PubMed] [Google Scholar]
- 31.Miura, K., T. Kishino, E. Li, H. Webber, P. Dikkes, G. L. Holmes, and J. Wagstaff. 2002. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol. Dis. 9:149-159. [DOI] [PubMed] [Google Scholar]
- 32.Moore, T., and D. Haig. 1991. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 7:45-49. [DOI] [PubMed] [Google Scholar]
- 33.Muscatelli, F., D. N. Abrous, A. Massacrier, I. Boccaccio, M. Le Moal, P. Cau, and H. Cremer. 2000. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum. Mol. Genet. 9:3101-3110. [DOI] [PubMed] [Google Scholar]
- 34.Peters, J., S. F. Wroe, C. A. Wells, H. J. Miller, D. Bodle, C. V. Beechey, C. M. Williamson, and G. Kelsey. 1999. A cluster of oppositely imprinted transcripts at the Gnas locus in the distal imprinting region of mouse chromosome 2. Proc. Natl. Acad. Sci. USA 96:3830-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plagge, A., E. Gordon, W. Dean, R. Boiani, S. Cinti, J. Peters, and G. Kelsey. 2004. The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat. Genet. 36:818-826. [DOI] [PubMed] [Google Scholar]
- 36.Pudovkina, O. L., Y. Kawahara, J. de Vries, and B. H. Westerink. 2001. The release of noradrenaline in the locus coeruleus and prefrontal cortex studied with dual-probe microdialysis. Brain Res. 906:38-45. [DOI] [PubMed] [Google Scholar]
- 37.Reik, W., M. Constancia, A. Fowden, N. Anderson, W. Dean, A. Ferguson-Smith, B. Tycko, and C. Sibley. 2003. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J. Physiol. 547:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reik, W., and J. Walter. 2001. Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2:21-32. [DOI] [PubMed] [Google Scholar]
- 39.Sara, S. J., C. Dyon-Laurent, and A. Herve. 1995. Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Brain Res. Cogn. Brain Res. 2:181-187. [DOI] [PubMed] [Google Scholar]
- 40.Sara, S. J., A. Vankov, and A. Herve. 1994. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res. Bull. 35:457-465. [DOI] [PubMed] [Google Scholar]
- 41.Skelton, K. H., M. J. Owens, and C. B. Nemeroff. 2000. The neurobiology of urocortin. Regul. Pept. 93:85-92. [DOI] [PubMed] [Google Scholar]
- 42.Srebro, B., and S. A. Lorens. 1975. Behavioral effects of selective midbrain raphe lesions in the rat. Brain Res. 89:303-325. [DOI] [PubMed] [Google Scholar]
- 43.Trivers, R., and A. Burt. 1999. Kinship and genomic imprinting, p. 1-21. In R. Ohlsson (ed.), Genomic imprinting—an interdisciplinary approach. Springer, Berlin, Germany.
- 44.Tycko, B., and I. M. Morison. 2002. Physiological functions of imprinted genes. J. Cell Physiol. 192:245-258. [DOI] [PubMed] [Google Scholar]
- 45.Vankov, A., A. Herve-Minvielle, and S. J. Sara. 1995. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur. J. Neurosci. 7:1180-1187. [DOI] [PubMed] [Google Scholar]
- 46.Wilkins, J. F., and D. Haig. 2003. What good is genomic imprinting: the function of parent-specific gene expression. Nat. Rev. Genet. 4:359-368. [DOI] [PubMed] [Google Scholar]
- 47.Williamson, C. M., S. T. Ball, W. T. Nottingham, J. A. Skinner, A. Plagge, M. D. Turner, N. Powles, T. Hough, D. Papworth, W. D. Fraser, M. Maconochie, and J. Peters. 2004. A cis-acting control region is required exclusively for the tissue-specific imprinting of Gnas. Nat. Genet. 36:894-899. [DOI] [PubMed] [Google Scholar]
- 48.Wolfer, D. P., W. E. Crusio, and H. P. Lipp. 2002. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 25:336-340. [DOI] [PubMed] [Google Scholar]
- 49.Wroe, S. F., G. Kelsey, J. A. Skinner, D. Bodle, S. T. Ball, C. V. Beechey, J. Peters, and C. M. Williamson. 2000. An imprinted transcript, antisense to Nesp, adds complexity to the cluster of imprinted genes at the mouse Gnas locus. Proc. Natl. Acad. Sci. USA 97:3342-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, S., O. Gavrilova, H. Chen, R. Lee, J. Liu, K. Pacak, A. F. Parlow, M. J. Quon, M. L. Reitman, and L. S. Weinstein. 2000. Paternal versus maternal transmission of a stimulatory G-protein alpha subunit knockout produces opposite effects on energy metabolism. J. Clin. Investig. 105:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu, S., D. Yu, E. Lee, M. Eckhaus, R. Lee, Z. Corria, D. Accili, H. Westphal, and L. S. Weinstein. 1998. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the gsα gene. Proc. Natl. Acad. Sci. USA 95:8715-8720. [DOI] [PMC free article] [PubMed] [Google Scholar]