Abstract
The purpose of this practice recommendation is to specifically identify the critical steps involved in performing and interpreting 123I-β-methyl-iodophenyl-pentadecanoic acid (BMIPP) single-photon emission computed tomography (SPECT) and measurement of washout rate (WR) from the heart. This document will cover backgrounds, patient preparation, testing procedure, visual image interpretation, quantitation methods using planar and SPECT studies, and reporting of WR. The pitfall and some tips for the calculation of 123I-BMIPP WR are also included. The targets of global and regional WR calculation include ischemic heart disease, cardiomyopathy, heart failure, and triglyceride deposit cardiomyovasculopathy, an emerging rare heart disease.
Keywords: Fatty acid metabolism, Imaging procedure, Nuclear medicine imaging, Single-photon emission computed tomography, Washout rate
Background
Scintigraphy using 123I-15-(4-iodophenyl)-3(R, S)-methyl-pentadecanoic acid (123I-BMIPP) is generally regarded as a method of assessing fatty acid images in nuclear medicine. Most basic and research studies of 123I-BMIPP have been conducted in Japan. Scintigraphic images are usually acquired at 20 minutes after 123I-BMIPP injection, and their roles in ischemic memory imaging and perfusion-metabolic mismatch are established. Hence, patients with acute and subacute phases of coronary artery disease and vasospastic angina are often assessed by 123I-BMIPP imaging (1–4). A switch from fatty acid to glucose metabolism has been recognized in hypometabolic areas of 123I-BMIPP (5). A 123I-BMIPP defect is also useful for assessing patients with ischemic heart disease and those on hemodialysis who have end-stage renal failure (6–8). The Japan Circulation Society has summarized practice guidelines for 123I-BMIPP imaging to determine the diagnosis and prognosis of chronic coronary artery disease (CAD) as it has proven effective and useful (9). Planar and single-photon emission computed tomography (SPECT) images acquired soon after an intravenous injection of 123I-BMIPP is nowthe most prevalent procedure. Fatty acid metabolism and left ventricular contractility have also been simultaneously evaluated using gated SPECT imaging (10).
Clinical evidence of 123I-BMIPP washout rates (WRs) determined from early and late images is limited, but, the fundamental kinetics have been explored since the 1990s. The myocardium uptakes 123I-BMIPP dependently on adenosine triphosphate, and subsequent kinetic steps involves alpha and beta oxidation and back diffusion (11–15). Thereafter, 123I-BMIPP is retained mainly in the myocardial triglyceride pool, from which it is slowly cleared. Recent research and clinical studies have investigated WRs using images of patients with ischemic heart diseases and cardiomyopathy. The driving force for this trend is to diagnose triglyceride deposit cardiomyovasculopathy (TGCV) (16–19). A significantly reduced 123I-BMIPP WR is listed as essential in the TGCV diagnostic criteria 2020 of the Research and Development on Intractable Disease by the Japanese Ministry of Labour and Welfare (20), and clinical evidence has accumulated about the value of TGCV images (21–28). However, data acquisition, analysis, and display methods appropriate for quantifying 123I-BMIPP WRs have not been sufficiently investigated (29, 30). Therefore, this practice recommendation aimed to provide standard procedures for data acquisition and analysis for calculating 123I-BMIPP clearance or washout from the heart.
Radiopharmaceuticals and mechanism of accumulation
The clinical indication for 123I-BMIPP scintigraphy is to diagnose cardiac diseases based on fatty acid metabolism. An intravenously injected dose of 74–148 MBq can be adjusted according to age and body weight of patients.
The accumulation of 123I-BMIPP in the heart reflects fatty acid metabolism. Cardiomyocytes uptake 123I-BMIPP in a concentration gradient, then cluster of differentiation (CD) 36 facilitates the transport of long-chain fatty acids, which are moved to the triglyceride pool via BMIPP-CoA. Some 123I-BMIPP is transferred to mitochondria, but most of it is retained in the myocardium due to a methyl group at the beta position. This metabolic feature of retention is convenient for SPECT imaging.
Procedures for 123I-BMIPP imaging
Patient preparation
Patients are required to fast for at least 6 hours (6–12 hours) before undergoing image acquisition. Water intake is allowed.
Procedures for imaging
Table 1 shows SPECT or SPECT-CT 123I-BMIPP imaging procedures that can be selected according to the nuclear medicine specialty. Patients are intravenously injected with 74–148 MBq 123I-BMIPP, then planar and SPECT images are respectively acquired at 20 (early) and 180–210 (late) minutes later.
Table 1. Methods for 123I-BMIPP imaging.
| Imaging | General procedures |
|---|---|
| Patient preparation | Fasting at least 6 hours (6–12 hours) except for water intake |
| Scan | Rest scan |
| Administered dose | 74–148 MBq; intravenous injection |
| Time to imaging | Early phase (20 min after injection); SPECT and planar imaging |
| Late phase (180–210 min after injection); SPECT and planar imaging | |
| Imaging | General parameters |
| Field of view | Cardiac or chest |
| Image type | Cardiac or chest SPECT and planar |
| Position | Supine |
| Energy window | 159 keV ± 10% |
| Matrix | Planar, 256 × 256; SPECT, 128 × 128; at least 64 × 64 |
| Pixel size | 4.0–6.0 mm |
| Planar imaging | Specific parameters |
| Views | Anterior |
| Image duration | 300 sec |
| Magnification | × 1 |
| SPECT imaging | Specific parameters |
| Angular range | Recommended, 180°; Optional, 360° |
| Detector configuration | Recommended, 90°; Optional, 180° |
| ECG gating | Non-gated image for WR calculation; 16 gates (optional 8 gates) for ECG gated acquisition |
| Viewangle | 4.0°–6.0°, step and shoot |
| Number of views | 30–120 sec (adjusted depending on projections) |
| Time per stop | 30–60 sec (adjusted depending on projections) |
| Magnification | × 1.5 (adjusted for scinticamera field of view and patients' stature) |
ECG, electrocardiography; WR, washout rate.
Imaging parameters can differ among camera vendors, therefore appropriate adjustments can be made within those recommended above.
Data acquisition
A 123I-specific or low-medium energy collimators are needed because 123I emits 159 keV gamma rays by electron capture. The energy for data acquisition is centered at 159 keV with a 20% (± 10%) or 15% (± 7.5%) window. Despite low-energy collimators, caution is required regarding increased scatter and septal penetration especially from 529 keV gamma rays.
Notes for examinations
-
-
Fasting is required before examinations. No food is allowed for at least 6 hours (6–12 hours) before examination except for water intake to avoid influence of foods on myocardial 123I-BMIPP uptake and washout rate (31).
-
-
Patient motion artifacts should be avoided.
-
-
Radioisotope leakage during intravenous injection should be avoided.
-
-
Patients must be carefully and precisely positioned for early and late image acquisition.
-
-
Data acquisition protocols for early and late image acquisition should be identical.
Electrocardiography (ECG)-gated data acquisition
Non-gated images should be used to calculate WRs because ECG-gated images are influenced by rejected arrhythmias.
SPECT image analysis
Short-, vertical long-, and horizontal long-axis images are generated, and the regional distribution of 123I-BMIPP and WRs can be evaluated from early and late polar maps.
Notes for imaging
-
-
Single-, rather than dual-radionuclide imaging is recommended for 123I-BMIPP WR calculations.
-
-
Dual-radionuclide assessment with 123I-BMIPP and 201Tl might affect the accuracy of WR calculations. Crosstalk between energy peaks of I (159 keV) and Tl (Hg-X 71–80 keV, 167 keV [10%]) can be corrected using various methods recommended by individual camera suppliers. However, when dual-nuclide acquisition is required, the effects of crosstalk should be assessed in advance.
-
-
As attenuation and scatter correction might affect the accuracy of WR calculation, these should not be corrected at present because methods vary among equipment vendors. Thus, the accuracy of individual methods requires determination.
-
-
Washout rates can be calculated using images with a cardiocentric configuration acquired by cameras with cadmium-zinc-chloride (CZT) detectors with high resolution and high sensitivity. However, the reliability of WR calculations requires further investigation due to limited experience.
Visual interpretation
Cardiac accumulation of 123I-BMIPP is confirmed using anterior planar images. Time-dependent count decay should be corrected because 123I-BMIPP is usually washed out from the heart within 4 hours, which is within the timeframe when early and late images are acquired. Myocardial washout can then be interpreted visually using the same scale that was displayed after decay correction (Figures 1 and 2).
Figure 1 Three short-axis SPECT slices and anterior planar images of patients.
A: Patient with WR 2% and clinical diagnosis of TGCV.
B: Patient with WR 11%. Color scale of late images was corrected for time decay between early and late imaging. This can be achieved either by multiplying counts derived from late images by decay correction factor to the late image or modifying the maximum count in the late image adjusted for time decay. Correction for time decay led to similar heart counts between early and late images in patient A, whereas heart count was decreased in late image in patient B.
TGCV, triglyceride deposit cardiomyovasculopathy; WR, washout rate.
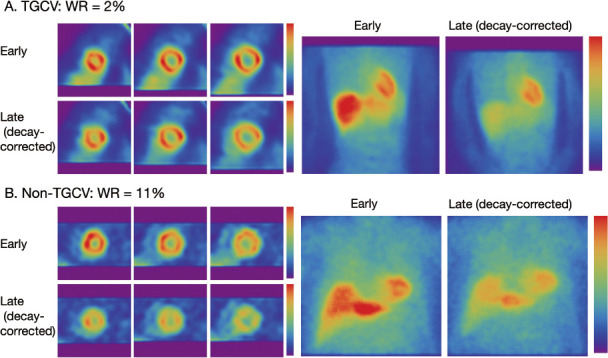
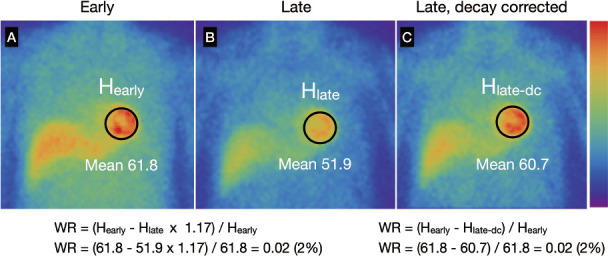
Figure 2 Examples of washout rates calculated using early (A), late (B) and decay-corrected late (C) planar images.
The WR can be calculated from the average heart counts. Background correction is not applied.
Hearly, early heart count; Hlate, late heart count; Hlate-dc, late heart count after decay correction. When interval between early and late images is 3 hours (physical half-life of 123I = 13.2 hours), calculated decay correction factor was 1.17 (Table 2).
WR, washout rate.
Metabolic defects and the homogeneity of 123I-BMIPP distribution can then be evaluated using standard short-, vertical long-, and horizontal long-axis images after standard reconstruction.
Notes for interpretation
-
-
Time-dependent decay can be calculated as 0.5^ (duration between early and late images/13.2 [h]). For example, 3-hour decay can be corrected by multiplying counts derived from late images by 1.17 (Table 2).
-
-
Washout is invisible when early and late SPECT images are each displayed separately on a scale of 0%–100%. However, myocardial washout can be easily interpreted visually when the display range is adjusted for decay correction in the late image. Figures 2 and 3 showlate images for planar and SPECT studies, respectively.
Table 2. Physical decay of 123I and decay correction factors.
| Time (h) | Decay | Decay correction factor |
|---|---|---|
| 2.5 | 0.88 | 1.14 |
| 2.75 | 0.87 | 1.16 |
| 3.0 | 0.85 | 1.17 |
| 3.25 | 0.84 | 1.19 |
| 3.5 | 0.83 | 1.20 |
| 3.75 | 0.82 | 1.22 |
| 4.0 | 0.81 | 1.23 |
The half-life of 123I is 13.2 hours.
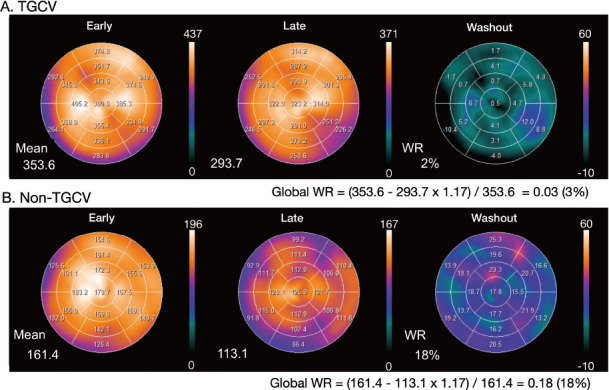
Figure 3 Examples of WRs in SPECT polar maps.
A: Clinical diagnosis of TGCV with WR 3%.
B: Coronary artery disease with WR 18%. Late image is shown with color scale in which maximum count is multiplied by a time-decay correction factor of 3 h (1.17). Global WR can be calculated by defining WR (Eq. 1) after decay correction. Compared with average of pixel-based WR (left lower corner of WR polar map, 2%), global WR (3%) was in agreement when regional WR values were homogeneous. However, averaged pixel-based WR might be influenced by misaligned early and late slices or defective regions.
WRs, washout rates.
Quantitation of washout rates
Washout rates (%) can be calculated for planar and SPECT images using polar maps as:
where late heart counts are corrected for 123I decay with a half-life of 13.2 hours.
Calculating WRs using a planar image: A circular (elliptical or heart-shaped) region of interest (ROI) is set on the heart, and the WR is calculated as average heart counts on early and late images (Figure 2). The cardiac ROI should be placed on the heart and should not extend outwards. A background ROI is not required.
Calculating WRs using a SPECT polar maps: Washout rates are calculated as average counts according to Eq. 1. after early and late counts are averaged (Figure 3). Appropriate selections of slice ranges at the base and apex are important to create a polar map. Early and late images that can be misaligned when basal slices near the valve plane are selected could result in inaccurate WR results. This method can be used for TGCV, ischemic heart diseases, cardiomyopathy, and heart failure.
Regional and segmental WR calculation using SPECT polar maps: Commercial software can calculate WRs using three, five, or 17 segments, or three regions. These algorithms can be applied for example, to compare WRs in normal regions with those that have reduced metabolic activity between normal and ischemic myocardia, and among three coronary artery territories (Figure 4).
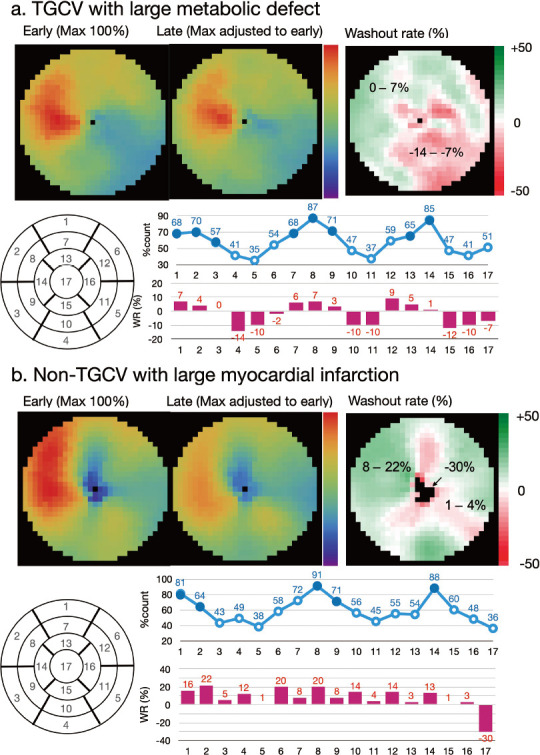
Figure 4 Examples of washout rates calculated from images of patients with metabolic defects.
Line graph (blue), percent-counts (%) in early myocardial count using a 17-segment model. Solid circles indicate segments with preserved accumulation. Bar graph (red) shows regional WR per segment.
Patient A (a) was clinically diagnosed with TGCV. Average anteroseptal WR in metabolically preserved segments was 4.1 %. WR: (mean early count - mean late count)/mean early count was 1%; averaged pixel based, −3%; defective region (inferolateral∔apex) −14% to −7%; Anteroseptal ( ), 0% to 7%.
), 0% to 7%.
Patient B (b) had old myocardial infarction and three-vessel disease. WR: (mean early count - mean late count)/mean early count was 9%; averaged pixel based, 0%; infarction (inferolateral), l%–4% and apex, −30%; anteroseptal ( ), 8% to 22%. Average anteroseptal WR in metabolically preserved segments was 15.8% (13%–22%) Adapted from reference (29).
), 8% to 22%. Average anteroseptal WR in metabolically preserved segments was 15.8% (13%–22%) Adapted from reference (29).
TGCV, triglyceride deposit cardiomyovasculopathy; WR, washout rate.
Notes for WR calculation
-
-
The processing range of basal and apical slices must be carefully determined when the WR is calculated using 123I-BMIPP SPECT. A long-axis image can be used as a reference for the selection of slice ranges, and a polar map display of WR is convenient for checking outlier WR values. One algorithm calculates WRs using average counts in early and late images, and another averages pixel-based WRs on polar maps (29, 30). Although the results of the two methods generally agree when patients are defect-free, large metabolic defects and misaligned settings of the slice range could affect pixel-based average WRs.
-
-
Background subtraction using a mediastinal ROI could cause fluctuations in WR and is not recommended for calculating WRs from planar images (29).
-
-
The scale of the polar map can be count-based after decay is corrected on late polar maps. This is preferable to using a percentage scale (0%–100%) to confirm differences in counts between early and late images. Washout rates can be calculated using counts averaged from early and late polar maps and Eq. 1 (Figure 3).
-
-
When patients have large metabolic defects due to previous myocardial infarction or severe fibrosis, defective segments can decrease regional WRs, and misaligned defect segments could cause fluctuations in regional WRs. Outlier WRs that are calculated regionally from defect segments should be excluded (Figure 4) (29). Global WRs should not be misinterpreted by regionally deranged metabolic activity since a decreased WR is critical for a diagnosis of TGCV. In addition to global WRs, regional distribution should be carefully interpreted when calculating WRs.
-
-
Washout rates can be calculated from averaged early and late counts derived from summed short-axis images of base to apical slices (29).
-
-Time decay correction factors can be calculated as (Table 2):
- 1 / 0.5^(elapsed time between early and late images/13.2 [h])
Radiation exposure
The respective absorbed radiation doses (mGy/MBq) according to the Committee on Medical Internal Radiation Dose (MIRD) for the heart, ovaries, testes, and whole body are 0.057, 0.011, 0.0076, and 0.010. The effective dose can be calculated using 0. 016 mSv/MBq for adult (International Commission on Radiological Protection [ICRP] Publication 128, Annals of the ICRP 2015; 44, No. 2S, available at https://www.icrp.org/publication.asp?id=ICRP%20Publication%20128).
Acknowledgments
This practice recommendation summarizes the activity of the Japanese Society of Nuclear Cardiology (JSNC) Working Group 2021 and 2023. The JSNC and Japanese Society of Nuclear Medicine (JSNM) approved its co-publication as a, official JSNC-JSNM report. The authors appreciate the editorial assistance of Norma Foster (English Express, Vancouver, BC, Canada).
Sources of funding
None.
Conflicts of interest
K. Nakajima collaborates with Siemens Medical Solutions USA, Inc. (Princeton, NJ, USA), Spectrum Dynamics Medical (Caesarea, Israel), and PDRadiopharma, Inc. (Tokyo, Japan), and conducts research in a department supported by Siemens Healthcare Japan (Tokyo, Japan), PDRadiopharma, Inc. (Tokyo, Japan), and Nihon MediPhysics (Tokyo, Japan). K. Hirano conducts research in collaboration with TOAEIYO (Tokyo, Japan)
References
- 1.Tamaki N, Morita K, Tsukamoto E, Kawai Y. Future aspects of BMIPP. Int J Card Imaging 1999; 15: 79–89. [DOI] [PubMed] [Google Scholar]
- 2.Inaba Y, Bergmann SR. Diagnostic accuracy of β-methyl-p-[123I] -iodophenyl-pentadecanoic acid (BMIPP) imaging: A meta-analysis. J Nucl Cardiol 2008; 15: 345–52. [DOI] [PubMed] [Google Scholar]
- 3.Tamaki N, Yoshinaga K. Novel iodinated tracers, MIBG and BMIPP, for nuclear cardiology. J Nucl Cardiol 2011; 18: 135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto N, Hirayama A. Current Japanese ministry of health, labor, and welfare approval of cardiac single photon emission computed tomography. Ann Nucl Cardiol 2015; 1: 108–9. [Google Scholar]
- 5.Kudoh T, Tamaki N, Magata Y, et al. Metabolism substrate with negative myocardial uptake of iodine-123-BMIPP. J Nucl Med 1997; 38: 548–53. [PubMed] [Google Scholar]
- 6.Tamaki N, Tadamura E, Kudoh T, et al. Prognostic value of iodine-123 labelled BMIPP fatty acid analogue imaging in patients with myocardial infarction. Eur J Nucl Med 1996; 23: 272–9. [DOI] [PubMed] [Google Scholar]
- 7.Moroi M, Tamaki N, Nishimura M, et al. Association between abnormal myocardial fatty acid metabolism and cardiac-derived death among patients undergoing hemodialysis: Results from a cohort study in Japan. Am J Kidney Dis 2013; 61: 466–75. [DOI] [PubMed] [Google Scholar]
- 8.Inaba Y, Bergmann SR. Prognostic value of myocardial metabolic imaging with BMIPP in the spectrum of coronary artery disease: A systematic review. J Nucl Cardiol 2010; 17: 61–70. [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi M, Tamaki N, Akasaka T, et al. JCS 2018 guideline on diagnosis of chronic coronary heart diseases. Circ J 2021; 85: 402–572. [DOI] [PubMed] [Google Scholar]
- 10.Inubushi M, Tadamura E, Kudoh T, et al. Simultaneous assessment of myocardial free fatty acid utilization and left ventricular function using 123I-BMIPP-gated SPECT. J Nucl Med 1999; 40: 1840–7. [PubMed] [Google Scholar]
- 11.Nohara R, Okuda K, Ogino M, et al. Evaluation of myocardial viability with iodine-123-BMIPP in a canine model. J Nucl Med 1996; 37: 1403–7. [PubMed] [Google Scholar]
- 12.Nohara R, Hosokawa R, Hirai T, et al. Basic kinetics of 15-(p-iodophenyl) -3-R, S-methylpentadecanoic acid (BMIPP) in canine myocardium. Int J Card Imaging 1999; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 13.Hosokawa R, Nohara R, Fujibayashi Y, et al. Myocardial metabolism of I-BMIPP in a canine model with ischemia: implications of perfusion-metabolism mismatch on SPECT images in patients with ischemic heart disease. J Nucl Med 1999; 40: 471–8. [PubMed] [Google Scholar]
- 14.Hosokawa R, Nohara R, Fujibayashi Y, et al. Myocardial kinetics of iodine-123-BMIPP in canine myocardium after regional ischemia and reperfusion: Implications for clinical SPECT. J Nucl Med. 1997; 38: 1857–63. [PubMed] [Google Scholar]
- 15.Fujibayashi Y, Nohara R, Hosokawa R, et al. Metabolism and kinetics of iodine-123-BMIPP in canine myocardium. J Nucl Med 1996; 37: 757–61. [PubMed] [Google Scholar]
- 16.Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N Engl J Med 2008; 359: 2396–8. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima K. Triglyceride deposit cardiomyovasculopathy: Howto recognise a newdisease entity. Heart 2021; 107: 93–5. [DOI] [PubMed] [Google Scholar]
- 18.Hirano K, Ikeda Y, Sugimura K, Sakata Y. Cardiomyocyte steatosis and defective washout of iodine-123-β-methyl iodophenyl-pentadecanoic acid in genetic deficiency of adipose triglyceride lipase. Eur Heart J 2015; 36: 580. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Hirano K, Ikeda Y, et al. Triglyceride deposit cardiomyovasculopathy: A rare cardiovascular disorder. Orphanet J Rare Dis 2019; 14: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi K, Sakata Y, Miyauchi H, et al. The diagnostic criteria 2020 for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2020; 6: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyauchi H, Hashimoto C, Ikeda Y, et al. Diagnostic criteria and severity score for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2018; 4: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyauchi H, Iimori T, Hoshi K, Ohyama M, Hirano K, Kobayashi Y. Correlation perspectives for the diagnosis of idiopathic triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2020; 6: 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano Y, Suzuki M, Hirano K, et al. Association of triglyceride deposit cardiomyovasculopathy with drug-eluting stent restenosis among patients with diabetes. JAMA Netw Open 2020; 3: e2012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onishi T, Nakano Y, Hirano K, et al. Prevalence and clinical outcomes of triglyceride deposit cardiomyovasculopathy among haemodialysis patients. Heart 2021; 107: 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda Y, Zaima N, Hirano K, et al. Coronary triglyceride deposition in contemporary advanced diabetics. Pathol Int 2014; 64: 325–35. [DOI] [PubMed] [Google Scholar]
- 26.Miyauchi H, Hirano K, Nakano Y, et al. 123I-BMIPP scintigraphy shows that CNT-01 (Tricaprin) improves myocardial lipolysis in patients with idiopathic triglyceride deposit cardiomyovasculopathy: First randomized controlled, exploratory trial for TGCV. Ann Nucl Cardiol 2022; 8: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano K, Miyauchi H, Nakano Y, et al. Overall survival rate of patients with triglyceride deposit cardiomyovasculopathy. JACC: Advances 2023; 2: 100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano K, Higashi M, Nakajima K. Remarkable regression of diffuse coronary atherosclerosis in patients with triglyceride deposit cardiomyovasculopathy. Eur Heart J 2023; 44: 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Nakajima K, Hirano K, et al. Methods of calculating 123I-β-methyl-P-iodophenyl-pentadecanoic acid washout rates in triglyceride deposit cardiomyovasculopathy. Ann Nucl Med 2022; 36: 986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyauchi H, Ono R, Iimori T, et al. Modified algorithm using total count for calculating myocardial washout rate in singlephoton emission computerized tomography. Ann Nucl Cardiol 2022; Epub ahead of print. DOI: 10.17996/anc.22-00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Geeter F, Caveliers V, Pansar I, Bossuyt A, Franken PR. Effect of oral glucose loading on the biodistribution of BMIPP in normal volunteers. J Nucl Med 1998; 39: 1850–6. [PubMed] [Google Scholar]


