Abstract
INTRODUCTION
Disease‐modifying therapies (DMTs) for Alzheimer's disease (AD) will increase diagnostic demand. A non‐invasive blood‐based biomarker (BBBM) test for detection of amyloid‐β pathology may reduce diagnostic barriers and facilitate DMT initiation.
OBJECTIVE
To explore heterogeneity in AD care pathways and potential role of BBBM tests.
METHODS
Survey of 213 healthcare professionals/payers in US/China/UK/Germany/Spain/France and two advisory boards (US/Europe).
RESULTS
Current diagnostic pathways are heterogeneous, meaning many AD patients are missed while low‐risk patients undergo unnecessary procedures. Confirmatory amyloid testing (cerebrospinal fluid biomarkers/positron emission tomography) is utilized in few patients, resulting in diagnostic/treatment delays. A high negative‐predictive‐value test could streamline the diagnostic pathway by reducing unnecessary procedures in low‐risk patients; supporting confirmatory testing where needed. Imminent approval of DMTs will increase need for fast and reliable AD diagnostic tests.
DISCUSSION
An easy‐to‐use, accurate, non‐invasive BBBM test for amyloid pathology could guide diagnostic procedures or referral, streamlining early diagnosis and DMT initiation.
Highlights
This study explored AD care pathways and how BBBM may meet diagnostic demands
Current diagnostic pathways are heterogeneous, with country and setting variations
Many AD patients are missed, while low‐risk patients undergo unnecessary procedures
An easy‐to‐use, accurate, non‐invasive BBBM test for amyloid pathology is needed
This test could streamline early diagnosis of amyloid pathology and DMT initiation
Keywords: Alzheimer's disease, amyloid pathology, blood‐based biomarker, clinical practice, diagnosis, qualitative, screening
1. BACKGROUND
Drug development strategies in Alzheimer's disease (AD) aim to identify disease‐modifying therapies (DMTs) that alter the course of disease and slow the rate of cognitive and functional decline. 1 , 2 , 3 , 4 , 5 , 6 Approved DMTs are soon expected to become widely available, 7 , 8 , 9 , 10 transforming clinical practice and the clinical trial landscape, 11 although evidence suggests that timely treatment is likely to achieve a greater degree of disease modification and slow the rate of cognitive and functional decline. 2 , 5 , 12 , 13 Reviews of health systems reported that infrastructure in Europe and the United States (US) lacks capacity for the early and effective detection, diagnosis, and treatment of AD, potentially denying many patients access to transformative care when DMT becomes readily available. 13 , 14 , 15 , 16 Therefore, the care pathway for early AD detection and treatment will need to evolve. 17
One essential requirement to ease patient journeys in the DMT landscape will be development of fully automated, high‐precision, non‐invasive biomarker tests that facilitate rapid identification and treatment of appropriate patients early in the care pathway. 18 , 19 The diagnostic accuracy of biomarkers in cerebrospinal fluid (CSF) is well established in AD. 20 However, CSF testing requires an invasive lumbar puncture, and is not used routinely. Similarly, positron emission tomography (PET) is an effective diagnostic tool, but is invasive and expensive and not routinely used. 21 Lack of available treatments, uncertainty in diagnosis, access issues, and risks to older patients also contribute to low utilization of these tools.
Strategies to prepare management pathways for the widespread implementation of DMTs should aim to develop biomarker assays into an in vitro diagnostic solution, expanding access and enabling routine clinical use. 22 Development of a blood‐based biomarker (BBBM) test may allow early detection of amyloid pathology and optimization of costly and invasive CSF/PET analyses. Initial research has already demonstrated the utility of BBBM tests for detection of amyloid pathology, 23 , 24 , 25 , 26 and such tests may soon become a reality. 27 Appropriately implementing this potential solution would require a thorough understanding of barriers in the current diagnostic pathway and development of strategies to ensure that the solution is accessible and affordable for all.
To identify current barriers to timely AD diagnosis and to investigate a potential role for a BBBM test, we conducted two surveys of healthcare professionals (HCPs) and payers across six countries, and convened two advisory board panels.
2. METHODS
This analysis is based on the outcomes of two qualitative surveys of physicians and payers (an initial survey and a follow‐up survey) and two advisory boards. The surveys were conducted by Hall and Partners, UK, on behalf of Roche Diagnostics International Ltd, and the advisory boards were convened by Roche Diagnostics International Ltd.
2.1. Initial physician and payer survey
A qualitative physician/payer survey including HCPs and payers based in the US, UK, France, Spain, Germany, and China was completed between November 16 and December 3, 2021. Included HCPs were PCPs or specialists (geriatricians, neurologists, or psychiatrists) familiar with aspects of AD and with biomarkers as diagnostic tools. Full inclusion criteria are summarized in Table 1.
TABLE 1.
Inclusion criteria for the qualitative survey (initial survey and follow‐up survey).
| Inclusion criteria | ||
|---|---|---|
| Research phase | HCPs | Payers |
| Initial survey |
|
|
| Follow‐up survey |
|
|
Abbreviations: AD, Alzheimer's disease; CCG, Clinical Commissioning Group; CEPS, Economic Committee for Health Products; CNEDiMTS, Medical Device and Health Technology Evaluation Committee; G‐BA, Federal Joint Committee; HAS, Haute Autorité de Santé; HCP, healthcare professional; HTA, Health Technology Assessment; KOL, key opinion leader; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; PCP, primary care practitioner; UK, United Kingdom; US, United States; VP, Vice President.
The objectives of this phase of the research were to map the pathway of patients with cognitive impairment, including first presentation, referral, diagnostic procedures and treatment, and to uncover existing gaps that a BBBM test could potentially address.
Participants completed a 45‐min Web‐assisted in‐depth interview (based on a pre‐prepared discussion guide and supported by showcards). The research was conducted in three parts, with the first exploring current pathways in AD diagnosis and the second exploring challenges, unmet needs, and opportunities. In the third part, participants were asked how two different putative diagnostic BBBM tests for amyloid positivity could potentially impact referrals. The first was a “rule‐out” test with a high negative predictive value (NPV = 92%), which would exclude patients unlikely to have amyloid pathology, allowing them to be referred for non‐AD diagnostic workup. Patients with a positive test would be referred for confirmatory testing (e.g., PET/CSF), and be given timely access to appropriate care (including DMTs when available) if AD was confirmed. The second test was a “rule‐in” test with a high positive predictive value (PPV = 90%) and an “acceptable” NPV (NPV = 62%). Positive patients would receive appropriate care and treatment for AD, while negative patients would undergo confirmatory testing for amyloid pathology with PET or CSF.
2.2. Follow‐up physician and payer survey
A follow‐up survey, consisting of a 45‐min web‐assisted in‐depth interview, was conducted March 4–18, 2022, recruiting specialists and payers in UK, Spain, France, Germany, and the US. Questions were built on previous learnings regarding patient pathways, while capturing current practices, goals, and unmet needs in AD diagnostics. Participants were also asked questions relating to the putative rule‐out test. Inclusion criteria are summarized in Table 1.
Participants were recruited by international specialist recruitment teams, and were remunerated to reflect time invested. Financial incentives were calculated in line with Fair Market Value guidelines. Recruitment was targeted, using in‐person calls based on online platforms to support the process.
2.3. Moderator training and preparation
The research team conducted a comprehensive moderator training session to promote consistent and coherent methodologies across all countries.
Three UK pilot interviews were conducted at the beginning of the fieldwork process. Audio recordings of these interviews and preliminary learnings from the central research team were shared among international moderators. The central research team also attended a 1‐day virtual interview in a central location in each country.
2.4. Data management and statistical analysis
Data management was conducted according to strictly defined standard operating procedures (Table S1).
RESEARCH IN CONTEXT
Systematic review: Data from a survey of 213 healthcare professionals and payers in the US, China, United Kingdom (UK), Germany, Spain, France, and two advisory boards (US and Europe) were used to explore heterogeneity in AD care pathways and how BBBM tests could address the anticipated increase in diagnostic demands in both primary and secondary care settings.
Interpretation: The consensus amongst survey respondents and advisory board members was that an easy‐to‐use, accurate, non‐invasive BBBM test that detects amyloid pathology could be used with other assessments to guide diagnostic procedures or referral, streamlining early diagnosis of amyloid pathology, and DMT initiation.
Future directions: With DMTs likely to become available in the near future, the need for a fast and reliable AD diagnostic test is expected to grow, and a BBBM test for detection of amyloid pathology is likely to become an integral part of the assessment toolkit.
2.5. Ethical conduct
All necessary consent to data handling and participation was obtained prior to completing the survey. Information provided was fully anonymized and excluded patient names or identifiers, and all data were used in an aggregated format. All research was conducted in line with market research protocols, compliance requirements, data protection/privacy policy, and adverse event reporting (AER) guidelines. Physician/payer surveys were conducted in line with all industry regulations, including Market Research Society, British Healthcare Business Intelligence Association (BHBIA), European Pharmaceutical Market Research Association (EphMRA), and General Data Protection Regulation guidelines. In accordance with BHBIA 28 and EphMRA 29 guidelines, this non‐interventional, market research study does not require specific ethic committee approval in Europe, while the US Food and Drug Administration (FDA) regulations 30 offer exemption from IRB approval in the US, as confirmed in previous similar studies. 31 All professionals involved in the surveys were fully trained in AER by BHBIA and Roche Diagnostics.
2.6. Advisory boards
Two independent virtual advisory boards were convened in December 2021 to understand (1) current patient pathway based on clinical practice; (2) potential impact of the BBBM test on the patient pathway; and (3) patient profiles that may benefit from or receive the BBBM test.
Each 4‐h advisory board meeting included five neurologists, PCPs, or clinical researchers in the US or Europe (Netherlands, Spain, Switzerland, UK) (Table S2), with one member nominated as chairperson. Employees of Roche Diagnostics International Ltd and associated agencies attended as observers.
3. RESULTS
3.1. Sample characteristics
In total, 171 HCPs and 15 payers completed the initial survey. Among the physicians, 71 (41.5%) were PCPs and 100 (58.5%) were specialists, with 21.5% respondents recruited from the US. For the follow‐up study, 32 physicians and 10 payers were recruited March 4‐18, 2022, with 28.6% from the US (Table S3). Seven HCPs and eight payers contributed to both the initial and follow‐up survey, giving a total of 213 unique responders. Representative comments from respondents are included in Table S4.
3.2. Current barriers in the AD diagnostic pathway
3.2.1. Referral decisions
Results reveal considerable heterogeneity in the AD diagnostic pathway, with notable differences in HCP behavior regarding patient referral.
Advisory board participants reported that PCPs typically refer patients for confirmatory testing, while themselves performing only blood tests or basic cognitive tests (Table 2). The presence of cognitive symptoms was sufficient for PCPs to make a referral, while specialists required an understanding of the underlying pathophysiology to decide on the best management strategy.
TABLE 2.
Assessments conducted in patients with cognitive complaints: Advisory board insights.
| Europe | US | |
|---|---|---|
| Primary care |
|
|
| Memory clinics |
|
|
Note: Advisory boards convened in December 2021.
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; GPCOG, General Practitioner assessment of Cognition; HIV, human immunodeficiency virus; LP, lumbar puncture; MMSE, mini‐mental state examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; PET, positron emission tomography; US, United States.
A lack of recognition of symptoms (anosognosia) in affected individuals was another major challenge, with advisory board participants reporting that patients early in the diagnostic pathway range from the “worried well” to those who strenuously deny cognitive problems. Some patients refuse referral or a cognitive test, even with evident signs of mild dementia.
The initial survey recorded lack of consistency in reasons for referral or requests for confirmatory tests, with some PCPs preferring to eliminate other causes of cognitive impairment or unable to see value in a diagnosis, given current lack of DMTs (Table 3).
TABLE 3.
Factors considered in referral decision‐making process: Physician/payer survey insights.
| Factors increasing likelihood of referral (decreasing order of importance) | Factors decreasing likelihood of referral (decreasing order of importance) |
|---|---|
|
|
Note: Initial qualitative physician/payer survey (fieldwork: November 16 to December 3, 2021).
Abbreviations: AD, Alzheimer's disease; DMT, disease‐modifying treatment; PCP, primary care practitioner.
The initial survey also revealed wide variability in patients referred to secondary care, with notable differences among countries. For example, a minority of patients were referred in Germany and US, compared with UK where most patients were referred onwards and China which is largely a secondary‐care‐based system (32) (Figure 1).
FIGURE 1.
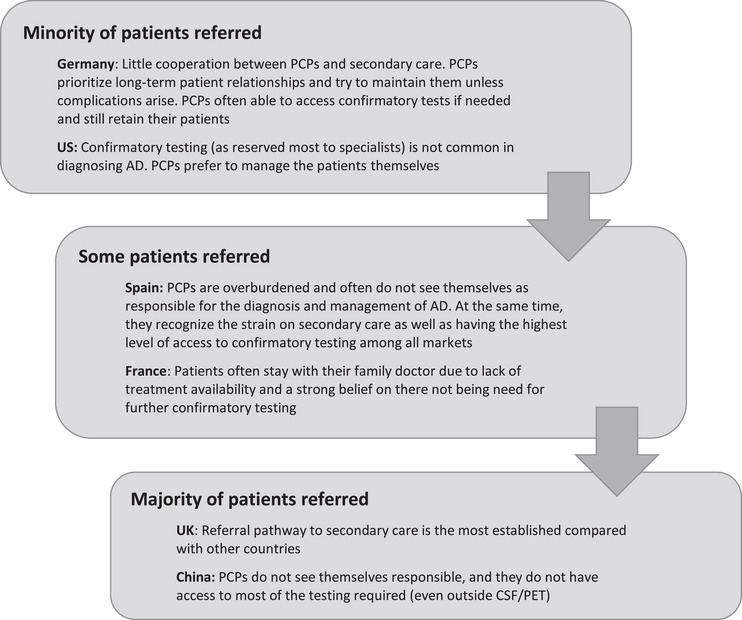
Proportion of patients with cognitive symptoms referred to secondary care by PCPs by country. Source: Initial qualitative physician/payer survey (fieldwork: November 16 to December 3, 2021). AD, Alzheimer's disease; CSF, cerebrospinal fluid; PCP, primary care physician; PET, positron emission tomography.
Specialists in the follow‐up survey confirmed these observations and reported that diagnostic pathways vary at local levels due to limited availabilities of specific tools, insurance constraints (particularly in US), and a lack of diagnostic experience. They regretted the absence of a consistent, streamlined diagnostic pathway, and insufficient innovation in AD diagnostics.
3.2.2. Access to testing
The initial qualitative survey revealed that patients likely to be prioritized for confirmation testing included atypical patients, patients requiring confirmation amyloid testing, and private patients, with barriers to confirmatory testing also explored (Table 4).
TABLE 4.
Factors conducive to and barriers against confirmatory testing for AD, as reported by country: Physician/payer survey insights.
| US | UK | France | Spain | Germany | China | |
|---|---|---|---|---|---|---|
| Patients likely to be prioritized for confirmatory testing | ||||||
| Atypical patient (i.e. young) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Needs confirmation through amyloid specifically (i.e. legal/social care reasons) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Patient willing to pay privately | ✔ | ✔ | ||||
| Required for medication selection | ✔ | |||||
| Barriers to receiving confirmatory testing | ||||||
| Unavailable | ✔ | ✔ | ||||
| Expensive | ✔ | ✔ | ✔ | |||
| Long queues for PET scans; system overburdened | ✔ | |||||
| CSF testing very invasive; burden for old patients particularly | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Confirmatory testing not perceived mandatory for diagnosis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Not part of current diagnosing algorithm | ✔ | |||||
| No treatment available; no need for expensive/invasive diagnosing | ✔ | |||||
| Lack of training in interpreting results | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Lack of time on the HCP part (can diagnose without) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
Note: Initial qualitative physician/payer survey (fieldwork: November 16 to December 3, 2021).
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; HCP, healthcare professional; PET, positron emission tomography; UK, United Kingdom, US, United States.
3.2.3. Time to diagnosis
According to the European advisory board, referral would typically be made after one to six visits to the general practitioner; longer if other health issues required more urgent treatment. Time elapsed between onset of symptoms and referral to a memory clinic also varies considerably. Experts from the US advisory board noted that patients with a cognitive complaint may wait 1–3 years for referral to a specialist center, although younger patients may be referred more rapidly. Although waiting times for a specialist office can be very long, even in memory clinics, patients presenting directly to a specialist typically reach a diagnosis in the shortest time. Patients referred from primary care spend longer in all parts of the diagnostic pathway.
3.2.4. Unmet need
Both advisory boards and the initial survey revealed “missing patients” (AD patients without a timely diagnosis) as a particular unmet need for specialists. Physicians want to manage patients as early as possible and help find holistic solutions (clinical, emotional, social care, as well as personal, financial, and legal planning). In addition, with no standard criteria for selecting the right candidates for PET/CSF confirmatory tests, patients with a low likelihood of AD can undergo unnecessary testing.
Specialists in the follow‐up survey echoed concerns over missing patients, and recorded other aspects of the unmet need (Figure 2).
FIGURE 2.
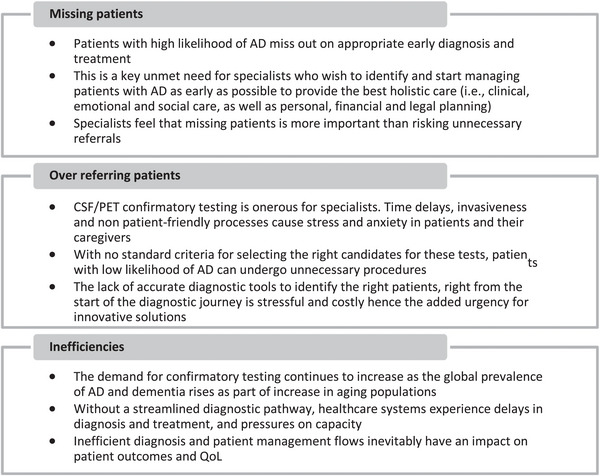
Specialist concerns relating to an unmet need for more and better AD diagnostics. Source: Follow‐up physician/payer survey (fieldwork: March 4‐18, 2022). AD, Alzheimer's disease; CSF, cerebrospinal fluid; PCP, primary care physician; PET, positron emission tomography.
3.3. Potential role and preferred characteristics of a BBBM test
Regarding characteristics of an ideal BBBM test, respondents in the initial survey and advisory boards expressed a preference for a rule‐out test (NPV > 90%) that would allow patients with a low likelihood of amyloid pathology to be referred for non‐AD diagnostic workups. Patients with a positive test would need further diagnostic referral, possibly including confirmatory PET/CSF testing, to access DMT and other appropriate care. The test would also need to be easy‐to‐use and patient‐friendly.
Respondents in the follow‐up survey considered a rule‐out test could save time, money, and resources; streamlining the process and accelerating diagnosis by removing unnecessary tests, simplifying the process flow, enhancing clinical decision‐making, targeting referral, utilizing capacity efficiently, and reducing the number of missing patients. This would help specialists focus on investigating other underlying causes. Furthermore, most specialists would perform the test at the first appointment alongside another test, while a minority would test at a second/follow‐up appointment.
The US advisory board would use a BBBM test with a high NPV and moderate PPV in ‘'worried well'’ patients in combination with other tests to avoid false‐positive results leading to unnecessary patient anxiety. Specialist use of the test together with other clinical information would facilitate an informed discussion with patients.
Factors key to successful implementation of the test highlighted by the surveys and advisory boards included robust performance, appropriate positioning in the patient pathway to impact clinical management (actionability), and evidence of benefit required for reimbursement.
Specialists in the initial survey considered a rule‐in test could be a non‐invasive alternative to existing confirmatory tests that might improve the process flow if its performance equaled confirmatory tests. They felt the high PPV would allow a greater confidence in the test results and improve clarity and certainty regarding AD diagnosis. With the availability of DMTs, such a test could become a gateway to treatment. However, the respondents also noted that the demand for confirmatory tests would continue, potentially leading to inefficiency in the pathway and test redundancy. In addition, concerns around pricing and reimbursement might remain. In the follow‐up survey, payers saw no value in the rule‐in test, as the PPV value would need to match that of PET/CSF to be valuable. Payers in the follow‐up survey also noted that the likelihood of reimbursement would be higher with robust evidence linking it to DMT clinical trials (e.g., if it were co‐developed as a companion diagnostic to a therapeutic).
4. DISCUSSION
These result provide insights regarding current deficiencies in the early AD diagnostic pathway, and the potential of a BBBM test to mitigate some challenges.
4.1. Heterogeneity in the diagnostic pathway
One of the first challenges is the heterogeneity observed in current approaches. Respondents in both surveys and the advisory boards noted that differing diagnostic goals lead to discrepancies in the referral process, with PCPs tending to refer based on symptoms while experts require an understanding of underlying pathophysiology. Differences in patient attitudes also drive heterogeneity.
A second survey using quantitative analyses of patient record forms (PRFs) conducted in parallel with the initial survey showed that the setting in which patients typically present (primary or secondary) varies between countries, as does the trigger for initial diagnosis (self‐identification, caregiver‐identification, or opportunistic detection by the patient's long‐term doctor). 32 Other sources of heterogeneity relate to referral behavior; e.g., whether a patient is assessed and diagnosed by the initial HCP or whether a gatekeeper is involved, as well as the timeframe of referral. 32
4.2. Inefficient and complex diagnostic landscape
The initial survey and advisory boards highlight the lack of a streamlined diagnostic pathway in AD, resulting in patients with a high likelihood of AD being missed and patients with a low likelihood undergoing unnecessary procedures. Furthermore, confirmatory amyloid CSF/PET testing is only utilized for a minority of patients, resulting in delays in confirmatory diagnosis and treatment. Similar inefficiencies have also been reported in population‐based studies in other countries including Japan and Norway. 33 , 34
The initial survey also revealed poor consistency in reasons for referral or requests for confirmatory tests, and a reluctance on the part of PCPs to refer patients with cognitive impairment. This is a major barrier to early diagnosis, 35 , 36 , 37 , 38 , 39 and can partly be explained by the lack of standardized screening tools in primary care. 40 Lack of a consistent approach at the PCP level places a high burden on specialists.
We found considerable disparity among countries, settings, and even patient type in the time and number of PCP visits required prior to referral, as noted particularly by the advisory boards. This is supported by the literature, with minority ethnic groups presenting to therapeutic and diagnostic services at a late stage in their illness, 41 with an additional delay of ≤7 years between first symptoms and physician consultation. 42
The parallel qualitative study performed 32 revealed that a higher rate of diagnosis among patients presenting to a specialist versus a PCP (∼60% vs. ∼30%).
4.3. Unmet diagnostic need to facilitate timely detection and treatment initiation
There is no standardized pathway to determine whether patients should be referred to determine a non‐AD cause of cognitive decline or be offered a confirmatory test with PET/CSF.
The initial survey revealed that patients are more likely to be referred if they are considered to have an atypical presentation, if there is particular urgency due to legal, social, or support reasons, or if AD symptoms are unverifiable with tools available in primary care. The same survey indicated that barriers to referral include the perception that no meaningful support can be offered, a belief that AD diagnosis can be ruled out, and the sense that “nothing could be done anyway”. The Clinical Trials on AD Taskforce noted the lack of an effective treatment is one of the main reasons that patients do not seek medical intervention and that PCPs do not refer to specialists, 11 although this will change when DMTs become available.
The advisory boards noted that symptomatic patients with a negative blood test still need to be referred, as AD is not the only cause of cognitive impairment (although the most common 43 ).
Data suggesting that many patients have inconclusive outcomes such as “watch and wait” 32 support the role of a simple BBBM test to detect amyloid pathology and create a consistent and efficient pathway to confirmatory PET/CSF testing.
4.4. Characteristics of an easy‐to‐use, accurate, non‐invasive biomarker test for detection of amyloid pathology
The surveys and advisory boards highlighted several factors key to the successful implementation of a BBBM test: robust performance, appropriate positioning on the patient pathway (actionability), and evidence of benefit required for reimbursement.
The predictive accuracy of a screening tool is determined by its PPV and its NPV, with other important factors including the availability of follow‐up diagnostic resources and therapeutic interventions, and associated costs. 44 The surveys and advisory boards both expressed a strong preference for a rule‐out test that would direct patients with low likelihood of amyloid pathology to non‐AD diagnostic workup. Positive patients would need further diagnostic referral, and could be given timely access to DMT and other care on diagnostic confirmation. All respondents agreed that such a test would also need to be patient‐friendly and easy‐to‐use.
A rule‐out test would also be more effective in a preselected expert/specialist setting where disease prevalence is greater, allowing greater PPV. In a more generalized PCP setting with low disease prevalence, the test would be more likely to achieve a high NPV but a relatively low PPV.
The preference for a rule‐out test with high PPV and moderate NPV is consistent with previous reports suggesting NPV ≥ 90% is required for a BBBM test in AD, with 95%–98% being an ideal target. 45 Reports suggest that PPV should be at least 50%. 44
4.5. Implementation of a blood test in the diagnostic pathway
Our findings suggest that introduction of a BBBM test to streamline the AD patient pathway may provide a systematic route to confirmatory testing and a clear rationale for referral, and reduce time to diagnosis.
Previous reports also suggest the value of BBBM testing in specialized memory clinic settings to distinguish AD from other types of dementia, although challenges remain including clinical use of biomarkers in patients without objective cognitive impairment. 25 , 26 , 27 , 44 The current report adds to the weight of evidence to support the use of a BBBM test to diagnose amyloid pathology and offers some suggestions for its implementation.
Actionability of the BBBM test for triage of patients and supporting referral will be driven by changes to the diagnostic pathway (e.g., the availability of DMTs). Further, payers considered that patients with cognitive impairment and a negative blood test would likely be referred from primary care, as non‐AD causes of cognitive impairment would still need to be investigated.
Another consideration in the wide implementation of a BBBM test is the need for counseling. Currently 30% of 69 centers in the European Alzheimer's Disease Consortium offer no routine counseling or discussion on the implications of biomarker testing. 46
Specialists are keen to embrace any new tools that could improve their diagnostic capabilities. An additional test is not expected to create a burden or complicate the diagnostic pathway, while any new tools improving diagnostic accuracy are likely to be welcomed by specialists.
A BBBM test could be used to decide whether more complex investigations should be performed, which would streamline the pathway and reduce unnecessary confirmatory CSF or PET testing. This approach is also consistent with the Alzheimer's Association's recent appropriate use recommendations for blood biomarkers in AD, which encourage the cautious introduction of BBBMs in clinical practice, provided AD status is confirmed whenever possible using CSF or PET. 25 Further, a recent EU/US Task Force report notes that as BBBM tests may be less invasive, costly, infrastructure‐dependent, and time‐ and resource‐intensive than CSF and PET biomarkers, they could improve detection, diagnostic accuracy, patient‐centered autonomy and empowerment, and improve overall care. 47
By ruling out patients early and reducing the number of unnecessary PET/CSF investigations, a BBBM test could preserve resources for those who have higher likelihood of being amyloid positive. This is particularly key since access to CSF or PET is limited and not reimbursed in many countries. 48 , 49 , 50 , 51 , 52 , 53
5. STRENGTHS AND LIMITATIONS
While we believe this research provides valuable insights into a potential role for an easy‐to‐use, non‐invasive biomarker test to detect amyloid pathology and facilitate recognition of AD early in the disease continuum, there are a number of limitations that must be acknowledged. First, the findings reported here are qualitative, and preclude any quantitative analysis. Regardless, we have tried to draw fair and balanced conclusions by grouping and summarizing responses to reflect the overall position of respondents on the areas of interest, although we acknowledge some degree of subjectivity in this approach. We have also tried to accurately and objectively represent the opinions of the participants by providing verbatim quotes where appropriate. The total sample size of 213 healthcare professionals is also relatively limited, and does not allow opposing or non‐conforming views to be adequately explored. In addition, the spread of the relatively small sample over six countries necessarily means that there were limited numbers of each type of specialist in each individual country. However, the tightly controlled sample means that it was possible to investigate the attitudes and experiences of individual practitioners much more closely than would be possible with a larger sample, with each participant committing to an in‐depth 45‐min interview for each of the surveys or participating in a 3‐h advisory board session. We believe that this approach allowed us to delve deeply into the real‐world experience of participants, and provide more nuanced and detailed insights than would otherwise be possible. Qualitative findings from the current research have previously been published elsewhere, 33 and may also be evaluated in parallel with the current findings to provide a more comprehensive overview of the insights explored in this manuscript.
6. CONCLUSIONS
Results from a comprehensive physician/payer survey and in‐depth expert panel discussions highlight considerable barriers in the current pathway to a diagnosis of AD in multiple countries. Introduction of an easy‐to‐use, non‐invasive biomarker test to detect amyloid pathology and facilitate recognition of AD early in the disease continuum would accelerate the diagnostic pathway, allow early initiation of DMT, and potentially improve patient outcomes. Such a test could function as a first‐line screening test or triaging tool, informing the decision to perform PET/CSF. With the imminent availability of DMTs, 10 the need for fast and reliable AD diagnostic testing will grow, and a BBBM test for amyloid pathology is likely to become an integral part of the assessment toolkit.
AUTHOR CONTRIBUTIONS
Ivonne Suridjan and Margherita Carboni were responsible for the conception and planning of this manuscript. All authors contributed to the development of each draft. All authors reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
W.M.vd.F., A.U.M., N.B., R.B., M.S., J.V., D.C., and J.J.L. received honoraria to participate in the advisory boards described in this manuscript organized by Roche Diagnostics. J.J.L. serves as a consultant for Roche Diagnostics. WMF serves as a consultant to Roche Diagnostics. I.S. and M.C. are full‐time employees of Roche Diagnostics International Ltd. I.S. also a shareholder of Roche Diagnostics International Ltd.
CONSENT STATEMENT
Consent was provided by all participating HCPs. Information provided by HCPs was fully anonymized and excluded patient names or identifiers. As in other similar surveys, no institutional review board/ethics committee approvals were deemed necessary. All research was conducted in line with market research protocols, compliance requirements, data protection/privacy policy, and AER guidelines. The physician/payer surveys were conducted in line with all industry regulations, including Market Research Society, BHBIA, EphMRA, and General Data Protection Regulation guidelines. All professionals involved in the physician/payer surveys were fully trained in AER by BHBIA and Roche Diagnostics.
MEDICAL WRITING, EDITORIAL, AND OTHER ASSISTANCE
Sophie Roth (Roche Diagnostics International Ltd) contributed to reviewing the manuscript. Medical writing support was provided by Rachel Danks of Bridge Medical Consulting Ltd, London, UK.
AUTHOR STATEMENT
All authors have contributed to the work, agree with the presented findings, and agree that the work has not been published before nor is being considered for publication in another journal.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by Roche Diagnostics International Ltd (Rotkreuz, Switzerland), who initiated and sponsored the advisory board meetings where discussions on this topic were held.
Suridjan I, van der Flier WM, Monsch AU, et al. Blood‐based biomarkers in Alzheimer's disease: Future directions for implementation. Alzheimer's Dement. 2023;15:e12508. 10.1002/dad2.12508
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Aisen PS, Cummings J, Jack CR Jr, et al. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. 2017;9:60. 10.1186/s13195-017-0283-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parums DV. Editorial: targets for disease‐modifying therapies in Alzheimer's disease, including Amyloid β and Tau protein. Med Sci Monit. 2021;27:e934077. 10.12659/MSM.934077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaz M, Silvestre S. Alzheimer's disease: recent treatment strategies. Eur J Pharmacol. 2020;887:173554. 10.1016/j.ejphar.2020.173554 [DOI] [PubMed] [Google Scholar]
- 5. Monteiro AR, Barbosa DJ, Remião F, Silva R. Alzheimer's disease: insights and new prospects in disease pathophysiology, biomarkers and disease‐modifying drugs. Biochem Pharmacol. 2023;211:115522. 10.1016/j.bcp.2023.115522 [DOI] [PubMed] [Google Scholar]
- 6. Doroszkiewicz J, Mroczko B. New possibilities in the therapeutic approach to Alzheimer's Disease. Int J Mol Sci. 2022;23(16):8902. 10.3390/ijms23168902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration [FDA] . FDA News Release: FDA Grants Accelerated Approval for Alzheimer's Disease Treatment. 2023. Accessed August 15, 2023. https://www.fda.gov/news‐events/press‐announcements/fda‐grants‐accelerated‐approval‐alzheimers‐disease‐treatment
- 8. Cummings JJ, Rabinovici GD, Atri A, et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimers Dis. 2022;9:221‐230. 10.14283/jpad.2022.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA. 2023:e2313239. 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10:362‐377. 10.14283/jpad.2023.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delrieu J, Bateman RJ, Touchon J, Sabbagh M, Cummings J. The future of AD clinical trials with the advent of anti‐amyloid therapies: an CTAD task force report. J Prev Alzheimers Dis. 2022;9:393‐399. 10.14283/jpad.2022.48 [DOI] [PubMed] [Google Scholar]
- 12. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292‐323. 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Dyck CH CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 14. (EFPIA) EFoPIaA . Alzheimer's disease health system readiness – The time to act is now. 14 September 2020. Accessed August 15, 2023. www.efpia.eu/media/554727/efpia‐ad‐platform‐health‐system‐readiness.pdf
- 15. Hlávka JP, Liu JL. Assessing the preparedness of the health care system infrastructure in six European countries for an Alzheimer's treatment. Santa Monica, Calif. RAND Corporation, RR‐2503‐BIOG. 2018. Accessed August 15, 2023. www.rand.org/pubs/research_reports/RR2503.html [PMC free article] [PubMed]
- 16. Mattke S, Hanson M. Expected wait times for access to a disease‐modifying Alzheimer's treatment in the United States. Alzheimer's Dement. 2022;18:1071‐1074. 10.1002/alz.12470 [DOI] [PubMed] [Google Scholar]
- 17. Stefanacci RG, Pakizegee M, Hodgson JW. Proactive steps to ensure appropriate utilization of the first disease‐modifying therapy for Alzheimer disease. J Clin Pathways. 2020;6:34‐36. [Google Scholar]
- 18. Varesi A, Carrara A, Pires VG, et al. Blood‐Based biomarkers for Alzheimer's disease diagnosis and progression: an overview. Cells. 2022;11:1367. 10.3390/cells11081367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu WL, Lin HW, Lin MR, et al. Emerging blood exosome‐based biomarkers for preclinical and clinical Alzheimer's disease: a meta‐analysis and systematic review. Neural Regen Res. 2022;17:2381‐2390. 10.4103/1673-5374.335832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med. 2018;284:643‐663. 10.1111/joim.12816 [DOI] [PubMed] [Google Scholar]
- 21. Ausó E, Gómez‐Vicente V, Esquiva G. Biomarkers for Alzheimer's disease early diagnosis. J Pers Med. 2020;10(3). 10.3390/jpm10030114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mankhong S, Kim S, Lee S, et al. Development of Alzheimer's disease biomarkers: from CSF‐ to blood‐based biomarkers. Biomedicines. 2022;10(4). 10.3390/biomedicines10040850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirmess KM, Meyer MR, Holubasch MS, et al. The PrecivityAD™ test: accurate and reliable LC‐MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin Chim Acta. 2021;519:267‐275. 10.1016/j.cca.2021.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. West T, Kirmess KM, Meyer MR, et al. A blood‐based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021;16:30. 10.1186/s13024-021-00451-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18:2669‐2686. 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Festari C, Massa F, Cotta Ramusino M, et al. European consensus for the diagnosis of MCI and mild dementia: preparatory phase. Alzheimers Dement. 2023;19(5):1729‐1741. 10.1002/alz.12798 [DOI] [PubMed] [Google Scholar]
- 27. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21:66‐77. 10.1016/S1474-4422(21)00361-6 [DOI] [PubMed] [Google Scholar]
- 28. BHBIA . Definition of market research (2). Accessed August 15, 2023. https://www.bhbia.org.uk/online‐training/course/guidance‐for‐reviewing‐approving‐market‐research‐materials/slide/10
- 29. ephmra . Code of conduct. September 2022. Accessed August 15, 2023. https://www.ephmra.org/sites/default/files/2022‐08/EPHMRA%202022%20Code%20of%20Conduct.pdf
- 30. Office for Protection of Research Subjects . Does my study need IRB approval? Accessed August 15, 2023. https://oprs.research.illinois.edu/research‐topics/does‐my‐study‐need‐irb‐approval
- 31. Podhorna J, Winter N, Zoebelein H, Perkins T, Walda S. Alzheimer's diagnosis: real‐world physician behavior across countries. Adv Ther. 2020;37:883‐893. 10.1007/s12325-019-01212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roth S, Burnie N, Suridjan I, Yan J, Carboni M. Current diagnostic pathways for Alzheimer's disease: a cross‐sectional real‐world study across six countries. J Alzheimer's Dis Rep. 2023:1‐16. 10.3233/ADR230007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Black CM, Ambegaonkar BM, Pike J, Jones E, Husbands J, Khandker RK. The diagnostic pathway from cognitive impairment to dementia in Japan: quantification using real‐world data. Alzheimer Dis Assoc Disord. 2019;33(4):346‐353. 10.1097/WAD.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 34. Kvello‐Alme M, Bråthen G, White LR, Sando SB. Time to diagnosis in young onset Alzheimer's disease: a population‐based study from central Norway. J Alzheimers Dis. 2021;82(3):965‐974. 10.3233/JAD-210090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33:1131‐1138. 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chodosh J, Petitti DB, Elliott M, et al. Physician recognition of cognitive impairment: evaluating the need for improvement. J Am Geriatr Soc. 2004;52:1051‐1059. 10.1111/j.1532-5415.2004.52301.x [DOI] [PubMed] [Google Scholar]
- 37. Connolly A, Gaehl E, Martin H, Morris J, Purandare N. Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health. 2011;15:978‐984. 10.1080/13607863.2011.596805 [DOI] [PubMed] [Google Scholar]
- 38. Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta‐analysis. BMJ Open. 2017;7:e011146. 10.1136/bmjopen-2016-011146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitchell AJ, Meader N, Pentzek M. Clinical recognition of dementia and cognitive impairment in primary care: a meta‐analysis of physician accuracy. Acta Psychiatr Scand. 2011;124:165‐183. 10.1111/j.1600-0447.2011.01730.x [DOI] [PubMed] [Google Scholar]
- 40. Bernstein A, Rogers KM, Possin KL, et al. Dementia assessment and management in primary care settings: a survey of current provider practices in the United States. BMC Health Serv Res. 2019;19:919. 10.1186/s12913-019-4603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mukadam N, Cooper C, Livingston G. A systematic review of ethnicity and pathways to care in dementia. Int J Geriatr Psychiatry. 2011;26:12‐20. 10.1002/gps.2484 [DOI] [PubMed] [Google Scholar]
- 42. Clark PC, Kutner NG, Goldstein FC, et al. Impediments to timely diagnosis of Alzheimer's disease in African Americans. J Am Geriatr Soc. 2005;53:2012‐2017. 10.1111/j.1532-5415.2005.53569.x [DOI] [PubMed] [Google Scholar]
- 43. Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7:33. 10.1038/s41572-021-00269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hampel H, O'Bryant SE, Molinuevo JL, et al. Blood‐based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14:639‐652. 10.1038/s41582-018-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Bryant SE, Edwards M, Johnson L, et al. A blood screening test for Alzheimer's disease. Alzheimers Dement. 2016;3:83‐90. 10.1016/j.dadm.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Psychiatry & Neurology Resource Center . Guidance needed on biomarker counseling. 2020. Accessed June 14, 2023. progress.im/en/content/guidance‐needed‐biomarker‐counseling
- 47. Angioni D, Delrieu J, Hansson O, et al. Blood Biomarkers from research use to clinical practice: what must be done? A report from the EU/US CTAD task force. J Prev Alzheimers Dis. 2022;9:569‐579. 10.14283/jpad.2022.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Centers for Medicare & Medicaid Services (CMS) . Decision Memo for Beta Amyloid Positron Emission Tomography in Dementia and Neurodegenerative Disease (CAG‐00431N). 2013. Accessed 15 August 2023. https://www.cms.gov/medicare‐coverage‐database/details/nca‐decision‐memo.aspx?NCAId=265
- 49. Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol. 2017;16:661‐676. 10.1016/S1474-4422(17)30159-X [DOI] [PubMed] [Google Scholar]
- 50. Garnier‐Crussard A, Flaus A. Positive opinion of the French National Authority for Health on the reimbursement of amyloid tracer (Flutemetamol). Eur J Nucl Med Mol Imaging. 2023;50:253‐254. 10.1007/s00259-022-06025-y [DOI] [PubMed] [Google Scholar]
- 51. Liu J, Wang LN, Tan JP. Dementia in China: current status. Neurology. 2013;81(12):1077‐1078. 10.1212/WNL.0b013e3182a4a3cb [DOI] [PubMed] [Google Scholar]
- 52. Shaw LM, Arias J, Blennow K, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2018;14:1505‐1521. 10.1016/j.jalz.2018.07.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stein DP, Meier U, Bless HH. Pathway and reimbursement of mild cognitive impairment diagnosis in Germany (Abstract PND82). Value Health. 2020;23:S637‐S638. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


