Abstract
Numb proteins are evolutionarily conserved signaling molecules that make the daughter cells different after asymmetric divisions by segregating to only one daughter. They contain distinct binding motifs for α-adaptin (α-Ada) and proteins with Eps15 homology (EH) domains, which regulate endocytosis, and for E3 ubiquitin ligases, which target proteins for proteasome-mediated degradation. In Drosophila melanogaster, Numb acts by inhibiting Notch activity to cause a bias in Notch-mediated cell-cell communication. These findings have led to the hypothesis that Numb modulates Notch signaling by using endocytosis and proteasomes to directly reduce Notch protein levels at the cell surface. Here we show that two Drosophila EH proteins, Eps15 homologue 1 (EH1) and the dynamin-associated 160-kDa protein (Dap160), negatively regulate Notch signaling. However, neither elimination of the binding motifs for endocytic proteins nor simultaneous reduction of proteasome activity affects the activity of Numb proteins. Our findings indicate that an endocytosis- and proteasome-independent pathway may mediate Numb signaling in asymmetric cell fate specification.
Asymmetric cell division, a process by which a cell divides to produce two different daughter cells, is essential for generating cellular diversity during development. Studies with Drosophila melanogaster show that two signaling pathways, one mediated by Numb (d-Numb) and the other by Notch, play essential but opposing roles in allowing the two daughter cells to adopt distinct fates after asymmetric divisions by a variety of neural precursor cells (3, 12, 30, 46). In the peripheral nervous system (PNS), for example, sensory organ precursor (SOP) cells divide asymmetrically to generate two secondary precursors called IIA and IIB. d-Numb, a membrane-associated protein, becomes asymmetrically localized to one-half of the cell membrane in dividing SOP cells and segregates to the IIB cell only. Loss of d-Numb causes both daughter cells to adopt the IIA fate, whereas its presence in both daughters produces two IIB cells. Loss of Notch, in contrast, produces two IIB cells, whereas activation of Notch signaling in both daughters leads to two IIA cells. d-Numb and Notch play similar antagonistic roles in the subsequent asymmetric divisions of SOP descendants, as well as in those by other neural and nonneural precursor cells. Notch, a transmembrane receptor, mediates cell-cell communication in many developmental pathways (1). Since d-numb Notch double mutants exhibit Notch mutant phenotypes, it has been postulated that d-Numb functions by inhibiting Notch activity in the cell inheriting it to cause a bias in cell-cell communication between the two daughter cells or between each daughter cell and its environment (12, 46).
There are highly conserved vertebrate Numb homologues (34, 48-50, 54, 56). While their precise roles in asymmetric cell division during vertebrate development remain controversial (32, 53), gain- and loss-of-function studies show that they are essential for neurogenesis, particularly in asymmetric divisions that generate a neuron and a daughter progenitor cell (20, 26, 27, 43, 48, 50, 54, 55). For example, elimination of both mouse homologues, m-numb and numblike (Numbl, or Nbl), which are functionally redundant, can cause progenitor cells to be prematurely depleted throughout the embryonic nervous system, pointing to an essential role in maintaining neural progenitor cells (26, 27).
In Drosophila, receptor-mediated endocytosis plays critical roles in modulating Notch signaling, in particular its activation (41, 42). For example, a temperature-sensitive allele of shibire (shits), which encodes a dominant mutation of dynamin, blocks endocytosis at nonpermissive temperatures and consequently causes Notch loss-of-function phenotypes (42). Human Numb and Numbl interact with α-Ada and proteins with Eps15 homology (EH) domains (34, 36), which are regulators of endocytosis (29). These interactions are mediated by two tripeptide motifs, DPF and NPF, which are present in d-Numb and all identified vertebrate homologues (49, 50, 54, 56). Numb proteins also bind to several E3 ubiquitin ligases, which target proteins for degradation by proteasomes (8, 15, 23, 47, 49). In Drosophila, E3 ubiquitin ligases such as Sina and Neuralized also play essential roles in modulating Notch signaling, including during asymmetric divisions by SOP cells (19, 28). These findings have led to the hypothesis that Numb proteins inhibit Notch by reducing its level at the cell surface through receptor-mediated endocytosis and/or proteasome-mediated degradation (5, 23, 36, 44, 47). In support of this hypothesis, d-Numb asymmetrically localizes α-Ada in dividing SOP cells, and Drosophila mutants with a specific α-ada mutation (α-adaear) producing a truncated protein lacking the C-terminal ear domain, which mediates binding to Numb and EH proteins, exhibit cell fate defects consistent with those caused by increases in Notch signaling (5). Reducing proteasome activity can cause similar cell fate defects in the SOP lineage (38).
In the developing nervous system, whereas d-Numb is necessary only in asymmetric divisions by neural precursors such as SOP cells, Notch is also required for their specification. This is accomplished through lateral inhibition, a process that allows only one cell from an equivalent group of epithelial cells to become a neural precursor (1). Loss of Notch function results in the emergence of multiple SOP cells within the group, whereas constitutive activation of Notch in all cells within the group leads to an absence of SOP cells. Interestingly, whereas d-Numb is present during lateral inhibition, changes in its level have no discernible effect on Notch signaling, suggesting that d-Numb modulates only one aspect of Notch signaling, namely, the specification of asymmetric cell fates. Another interesting observation concerns α-Ada. Unlike α-adaear mutants, which show no apparent defects in endocytsis, α-ada null mutants have severe defects in endocytosis but not in cell fate specification (5, 10). These findings raise the possibility that Numb modulates Notch signaling not simply by directly regulating endocytosis.
To examine whether endocytosis and proteasomes mediate Numb signaling, we analyzed two Drosophila EH proteins and also performed a mutagenesis analysis of Numb proteins. Here we report that while EH proteins can modulate Notch signaling, abolishment of the binding motifs for endocytic proteins does not affect the ability of Numb proteins to specify cell fates, even when proteasome activity is simultaneously reduced.
MATERIALS AND METHODS
Fusion protein production and protein-protein interaction assays.
Full-length cDNA clones of EH1 and Dap160 were generated from expressed sequence tag clones (SD07402 and AT13948 for EH1 and AT02290 and LD23696 for Dap160). In studies to be described elsewhere, AT02290 encodes an alternative C terminus of Dap160, and LD23696 contains no EH domains. Production of glutathione S-transferase (GST) fusion proteins and copurification experiments were performed as previously described (45, 54) with minor modifications. Briefly, various EH1 and Dap160 cDNA fragments were fused in frame with GST in pGEX vectors. Crude mouse brain extracts or Drosophila embryonic extracts were precleared with glutathione Sepharose beads (Pharmacia) at 4°C for 1 h in a lysis buffer (50 mM Tris · Cl [pH 8.0], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], and 1% Triton X-100), followed by incubation overnight with a crude bacterial supernatant containing the fusion proteins. After the incubation, the beads with copurified proteins attached were washed three times in phosphate-buffered saline, resuspended in SDS gel sample buffer, and boiled at 100°C for 7 min. The supernatant containing the copurified proteins was subjected to SDS-polyacrylamide gel electrophoresis and immunoblot analysis using antibodies against m-Numb or d-Numb.
For yeast two-hybrid assays, cDNA fragments encoding Numb proteins and those encoding EH1 and Dap160 were cloned into pGBT9 and pACT2 vectors (Clontech), respectively. Transformation into the yeast strain PJ69-4A and analysis of protein-protein interactions were performed as described elsewhere (4, 14), with minor modifications. For two proteins to be deemed capable of binding to each other, yeast cells containing both plasmids have to be able to grow without adenine and histidine.
Drosophila mutants and genetic interaction experiments.
P-element insertion lines were obtained from Exelixis (EP2513, EP2543, and EP2468). EH12 was generated by using transposase (Δ2-3)-mediated P-element excision. Southern blot analysis of genomic DNA indicates a deletion covering about 20.5 kb of the promoter region and part of the 5′ untranslated region (5′ UTR), which likely also affects several neighboring genes. Df(2R)DII-MP, H2, and Su(H)1 flies were obtained from the Bloomington Stock Center (BL-1465, BL-517, and BL-417, respectively). EH1 and Su(H)1 were recombined on the second chromosome. Genetic interaction experiments were performed through matings between EH11/Cyo and H2/T(2,3)ap[Xa],ap[Xa] flies or between EH12/Cyo;H2/TM3Sb and EH11/Cyo or EH11,Su(H)1/Cyo flies. Statistic analyses were performed using MicroSoft Excel.
Mutagenesis of Numb proteins, transgenic fly generation, and rescue experiments.
cDNA fragments encoding truncated m-Numb and d-Numb proteins were generated by using restriction enzymes within the coding regions (ApaI for m-numb and XmnI for d-numb). DPF, NPF, and F195V were mutated by using synthetic oligonucleotides and PCR. The cDNA fragment carrying d-numb double-stranded RNA (dsRNA) was generated by using two overlapping 1-kb fragments at the 5′ end (NheI-SacI and NheI-PstI) with a loop of ∼120 nucleotides. All cDNA fragments were cloned into pUAST and injected into embryos to generate transgenic flies (6). d-numb fragments contain the endogenous 5′ UTR and initiation methionine, whereas cDNA fragments encoding various mutant forms of m-Numb proteins were fused in frame with the Xenopus β-globin 5′ UTR and MYC epitope (MEEKLISEED). Overexpression was achieved by mating transgenic flies with ScaGal4 or ScaGal4109-68/Cyo flies (9, 28). Rescue experiments were performed as described previously (54). In these experiments, numb1/Cyo,ftz-lacZ;hairy-Gal4/TM3Sb flies were mated with numb1/Cyo-ftz-lacZ;m-NumbΔC/ΔC flies to generate the rescued mutant embryos. The rescue was efficient, as evidenced by the fact that about half of the numb mutants exhibited the segmented patterns of neuronal rescue as expected.
In situ hybridization and immunostaining.
In situ hybridization and immunostaining of Drosophila embryos were performed as previously described (54, 56), with minor modifications. For notum immunostaining, pupae were dissected from their cases and fixed for 6 h in 4% paraformaldehyde in PBT (phosphate-buffered saline with 0.1% Triton X-100). The dissected nota were then rinsed with PBT and incubated in a blocking solution (2% normal goat serum and 2% bovine serum albumin in PBT) for ∼2 h, followed by an anti-Elav antibody (dilution, 1:20; Developmental Studies Hybridoma Bank [DHSB]) and fluorescein isothiocyanate- or Cy3-conjugated secondary antibodies. Images were collected by using an optical or confocal microscope and were processed by using Adobe Photoshop.
RESULTS
Numb proteins interact with endocytic proteins via multiple domains.
To understand how Numb proteins function, we performed yeast two-hybrid screens to identify proteins that bind directly to m-Numb. Among the proteins identified are the EH protein Resp1 and α-Ada, which bind to the NPF and DPF motifs, respectively, near the C terminus of m-Numb (34, 36, 52) (Fig. 1A). Also identified are two Sina homologues (Siah1 and Siah2) and LNX2, which are E3 ubiquitin ligases (8, 23, 31, 47, 49). The importance of proteasomes and E3 ubiquitin ligases in Notch signaling is known (19, 28, 38). We thus analyzed Drosophila EH1 and Dap160, which encode structurally distinct EH proteins (Fig. 1B), to take advantage of the well-characterized roles of Notch and Numb in flies in order to determine whether EH proteins are also important.
FIG. 1.
Numb proteins interact with endocytic proteins via multiple domains. (A) Schematic diagrams of the mouse and Drosophila Numb proteins. Yellow boxed areas (including the green PTB domain) indicate high levels of conservation (63.3% amino acid identity). Red boxed areas and amino acid sequences indicate the locations of DPF and NPF motifs. The blue boxed area indicates putative SH3 binding sites on m-Numb. (B) Schematic diagrams of the Drosophila EH1 and Dap160 proteins. (C) Immunoblots showing copurification of m-Numb from brain extracts or d-Numb from embryonic extracts by GST fusions of EH1 or Dap160. + and −, presence and absence, respectively, of protein extracts. The regions of EH1 and Dap160 used to copurify Numb proteins correspond to the lines underneath the respective diagrams in panel B. (D) EH1 expression during Drosophila embryogenesis. A digoxigenin-labeled antisense RNA probe of EH1 was used. (a to c) Lateral views; (d) ventral view. The embryo in panel Db is representative of those derived from EH2/Cyo parents that show no expression of EH1.
EH1 was annotated as CG16932 and is likely the Drosophila Eps15 orthologue. Eps15 proteins play important roles in regulating receptor-mediated endocytosis in mammalian cells and Caenorhabditis elegans (11, 35, 37). Like mammalian Eps15, EH1 protein contains three EH domains, a coiled-coil domain, several DPF motifs, and a putative UIM motif at the C terminus (Fig. 1B). UIM is responsible for ubiquitin recognition and monoubiquitination (37). As expected, EH1 could bind to d-Numb and m-Numb in an NPF-dependent manner (Fig. 1C and 3M). Dap160 was identified earlier due to its ability to physically interact with dynamin, another key regulator of endocytosis (33). In addition to two EH domains, which could mediate binding to both d-Numb and m-Numb (data not shown), Dap160 also has four SH3 domains (Fig. 1B). There are putative SH3 binding sites in the C-terminal part of mammalian Numb proteins (49, 54) (Fig. 1A). Indeed, in GST copurification assays, the presence of either Dap160 EH or SH3 domains alone was sufficient to mediate binding to m-Numb (Fig. 1C), although d-Numb appeared to be incapable of binding to SH3 domains (data not shown). These findings suggest that Numb proteins have multiple domains capable of interacting with endocytic proteins.
FIG. 3.
Binding to endocytic proteins is not necessary for Numb function. (A to C and E to L) The notum of a wild-type (WT) adult fly and nota of those overexpressing WT and various mutant forms of Numb proteins. Overexpression was achieved by using ScaGal4 at either 18°C (A to C and E to I), 25°C (L), or 29°C (J and K). (D) Quantitative analysis of the effects of d-Numb and d-NumbΔC overexpression in independently generated transgenic lines. Each bar represents the average number of hairs from five nota, and overexpression was achieved by using ScaGal4109-68 at 25°C. ScaGal4109-68 causes proteins to be expressed at levels lower than those obtained by use of ScaGal4 and consequently causes less-severe defects. (M to O) Yeast two-hybrid assays showing the effects of various mutations on protein-protein interactions between Numb proteins and their binding partners. The control is either an empty vector or lamin. ND, not determined. EH1 and Nak are Drosophila proteins, Siah1 and LNX2 are mouse proteins, and α-Ada is from a human.
EH1 appears to be fairly widely expressed during early stages of embryogenesis, including in neural precursor cells (Fig. 1Da). Later during development, however, its expression becomes most prominent in postmitotic neurons (Fig. 1Dc and d), a pattern consistent with a potential role in the endocytosis of synaptic vesicles. Dap160 has a similar pattern of expression and has recently been shown to regulate vesicle cycling at neural synapses (18, 22).
EH1 negatively regulates Notch signaling.
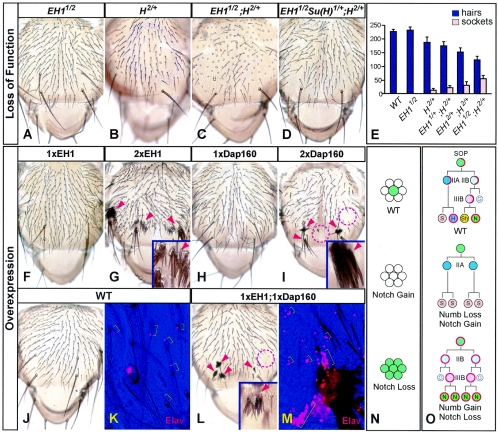
To examine whether EH1 is necessary for cell fate determination, we generated several EH1 mutant alleles. EH11 is a previously identified P-element insertion (EP2513) (21) located within the 5′ UTR of EH1. Flies homozygous for this allele survived to the pupa stage, but ∼40% of them failed to eclose. Those that did eclose exhibited no apparent behavioral abnormalities and were fertile. EH11 mutants exhibited no defects in the SOP lineage on the notum and the scutellum, although some (∼10%) had small balding patches on abdominal segments, with neither hairs nor sockets. Staining using an antibody against Elav, a neuronal marker, revealed that there were also no neurons underneath these patches (data not shown), suggesting that the phenotype is likely due to an absence of SOP cells. Since flies carrying this allele over Df(2R)DII-MP, a deficiency covering the EH1 locus, showed no increases in lethality or balding, EH11 is likely a strong loss-of-function or null allele. We also generated additional EH1 alleles by using transposase-mediated P-element excision. While most of the resultant flies were viable and normal, likely due to precise excision of the P element, about 10% of the Drosophila lines remained semilethal. One line, EH12, has a deletion of more than 20 kb of the upstream promoter region, and as expected, about one-quarter of the embryos from heterozygous parents showed no detectable EH1 expression (Fig. 1Db). EH12 and EH11/2 mutants (Fig. 2A), as well as the offspring of homozygous mutants, showed no increases in lethality or balding.
FIG. 2.
Drosophila EH1 and Dap160 negatively regulate Notch signaling. (A to D) Nota of adult flies carrying various combinations of wild-type (+) and mutant alleles of EH1, H, and Su(H). Each hair in the wild type represents an es organ. (E) Quantitative analysis of genetic interactions between EH1 and H mutant alleles. Blue bars represent numbers of hairs on the notum and scutellum, whereas yellow bars represent numbers of double sockets (without hair). Each bar represents the average from 10 nota, except for those for the wild-type (WT) and EH1/2 alleles, which represent averages from 4 nota. (F to M) Nota of adult WT flies or those overexpressing EH1 and/or Dap160 from 1 or 2 copies of the UAS-EH1 and UAS-Dap160 transgenes. Images in panels K and M show the number of neurons (in red; by Elav antibody staining) underneath the notum surface (in blue). Arrowheads in panels G, I, and L point to clusters of hairs, whereas dashed circles indicate balding areas. Brackets in panel K show that a single neuron accompanies each hair in the WT. Images are shown at ×4, except for those in the insets and panels K and M, which are shown at ×20. EH1 and Dap160 were overexpressed by using ScaGal4 at 25°C (F, H to M) or 29°C (G). (N) Schematic drawings of the effects of changes in Notch signaling on the specification of SOP cells (light green) during lateral inhibition. (O) Schematic drawings of the SOP lineage that produces es organs on the notum and the effects of changes in Numb and Notch activities. In the WT, d-Numb (in red) localizes asymmetrically in dividing SOP cells and segregates to daughter cell IIB to promote its fate. Subsequently, cells IIA and IIIB asymmetrically segregate newly synthesized d-Numb to distinguish their daughter cells. S, socket cell; H, hair cell; Sh, sheath cell; N, neuron. The glial cell (G) is not part of the es organ, and IIB divisions appear to be insensitive to Numb activity.
The mild phenotype exhibited by EH1 mutants on the abdomen, i.e., an absence of external sensory (es) organs, is consistent with a gain of Notch function (Fig. 2N). We therefore generated Drosophila mutants for both EH1 and Hairless (H). H is a potent and specific inhibitor of Notch signaling (2, 3). Flies heterozygous for a loss-of-function H allele, H2, show defects in sensory organ formation. On the notum, for example, ∼17% of the hairs are absent (Fig. 2B). This is due to increased Notch signaling that causes a failure in SOP cell specification (Fig. 2N) or transformation of the hair daughter into an extra socket cell (i.e., double socket) (Fig. 2O). We reasoned that if EH1 modulates Notch signaling, its mutation might affect the severity of the phenotypes caused by H heterozygosity. Indeed, whereas EH1 mutation alone did not cause any defects on the notum, it significantly enhanced the hair loss phenotype in H2 heterozygotes (Fig. 2C). Quantitative analysis shows that this enhancement was gene dosage dependent, and loss of both copies of wild-type EH1 increased such hair loss to ∼46% (Fig. 2E). The latter was accompanied by increases of nearly fourfold in the number of double sockets and nearly twofold in the number of areas with no es organs. We also generated Drosophila lines carrying mutations of EH1, H, and Suppressor of Hairless [Su(H)]. Su(H) is essential for transducing Notch signaling, and its heterozygosity suppresses the defects in H heterozygotes (3, 39). This was also the case in the EH1 mutant background (Fig. 2D). Su(H) heterozygosity could also suppress the lethality caused by EH11 mutation. EH1/1 Su(H)1/+ flies exhibited no apparent morphological or behavioral defects.
These findings indicate that EH1 negatively regulates Notch signaling. To further ascertain this, we generated Drosophila lines expressing EH1 protein under the control of the UAS promoter, which can be activated by the presence of Gal4, a yeast transcription factor (6). We used a Gal4 line driven by the Scabrous promoter (ScaGal4), which is active in SOP cells and their descendants (28). It is also active in proneural clusters from which SOP cells are singled out through Notch-mediated lateral inhibition. EH1 overexpression led to more-numerous es organs in a dosage-dependent manner (Fig. 2F and G). In some areas, large clusters of hairs, with the accompanying sockets, could be observed. This phenotype is similar to that seen in flies in which Notch loss is restricted to the duration of lateral inhibition, which leads to multiple cells within a proneural cluster, instead of just one, choosing the SOP fate (Fig. 2N). Interestingly, EH1 overexpression mostly affected Notch activity in Numb-independent lateral inhibition (Fig. 2G). Asymmetric divisions by SOP cells and their descendants, during which Numb and Notch play opposing roles, were much less affected.
EH protein activities are additive.
We also generated Drosophila lines that overexpress Dap160 by using the UAS-Gal4 system. High levels of Dap160 overexpression also led to more-numerous es organs in a dosage-dependent manner (Fig. 2I). Unlike that of EH1, however, Dap160 overexpression also produced large balding patches (Fig. 2I). Underneath the balding patches, there were clusters of neurons (data not shown), unlike the pattern in the wild type, in which a single neuron accompanies each hair (Fig. 2K). Such phenotypes are consistent with a reduction in Notch activity, both during lateral inhibition and in the subsequent asymmetric divisions by SOP cells and their descendants (Fig. 2N and O). Studies of mammalian Eps15 and Ese1 (intersectin), the mammalian Dap160 homologue, show that they bind to each other (40). This is also the case for Drosophila (data not shown). We therefore took advantage of the mild phenotype exhibited by flies expressing exogenous EH1 or Dap160 from one copy of the transgene (Fig. 2F and H) to investigate whether EH1 and Dap160 could act together. If EH1 and Dap160 functions are additive or synergistic, their coexpression should cause phenotypes resembling those due to higher levels of expression of either protein alone. Indeed, on the nota of flies co-overexpressing EH1 and Dap160, there were clusters of hairs and areas of balding (Fig. 2L), as well as more-numerous neurons underneath the balding patches (Fig. 2M).
We also analyzed Dap160 mutants, as well as EH1 Dap160 double mutants or EH1 Dap160 α-Ada triple mutants, using an α-Ada loss-of-function allele that causes severe defects in endocytosis (10). None of the mutants showed apparent defects in cell fate specification in the SOP lineage (data not shown). As mentioned above, Dap160 loss has been shown recently to severely affect the endocytosis of synaptic vesicles (18, 22).
Binding motifs for endocytic proteins are not necessary for Numb function.
Since there are other EH- and SH3-domain proteins in Drosophila, it is possible that the absence of cell fate defects in EH1, Dap160, and α-ada mutants are due to functional redundancy among such proteins. We therefore mutated the respective binding sites on Numb proteins. If they use endocytic proteins to down-regulate Notch signaling, such mutations should affect their ability to specify cell fates. We first changed the DPF and NPF motifs, respectively, to three alanines (AAA) in m-Numb. We have shown previously that m-Numb, which shares little homology with d-Numb in the C-terminal half except for the two motifs (Fig. 1A), is capable of rescuing d-numb loss-of-function mutant phenotypes (54). m-Numb overexpression in the SOP lineage, which forces its segregation into both daughter cells, causes balding of the notum, a phenotype indistinguishable from that due to d-Numb overexpression (Fig. 3B and E) (30, 49, 54). The balding is due to transformation of the IIA daughter into a second IIB and subsequent transformation of IIIB's sheath daughter into another neuron, resulting in mutant es organs with as many as four neurons (Fig. 2O). The IIB division appears to be insensitive to Numb overexpression. As expected, DPFAAA and NPFAAA mutations completely abolished m-Numb's ability to bind α-Ada and EH1, respectively (Fig. 3M). However, whether alone or in combination, they had no effect on m-Numb's ability to cause cell fate transformation in the SOP lineage (Fig. 3F to H). We also deleted the entire C terminus of m-Numb (amino acids 396 to 593) (m-NumbΔC), which also contains the SH3 binding motifs (Fig. 1A). m-NumbΔC remains fully functional (Fig. 3I). To exclude the possibility that the dispensability of the C terminus is unique to m-Numb, we made a similar deletion on d-Numb (amino acids 336 to 556) (d-NumbΔC). d-NumbΔC was also functional (Fig. 3C).
We point out that in all independently generated lines we have examined (n ≥ 3 each), overexpression of the mutant forms of m-Numb described above at higher levels caused lethality at the pupa stage, just like overexpression of wild-type m-Numb. The nota of such flies exhibited nearly complete baldness, with no or only a few remaining hairs (Fig. 5E, G, and I; also data not shown). Underneath the balding patches, clusters of two to four neurons were observed, as expected (Fig. 5L and M; also data not shown).
FIG. 5.
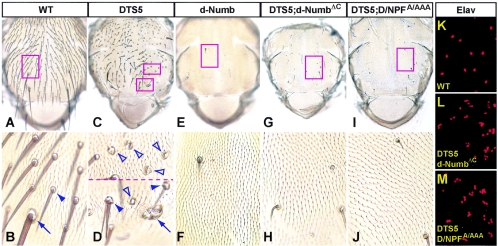
Numb proteins can specify cell fates independently of endocytosis and proteasomes. (A to J) Notum of a wild-type (WT) adult fly and nota of those overexpressing either DTS5 alone, d-Numb alone, or DTS5 together with d-NumbΔC or m-Numb D/NPFAAA. Panels B, D, F, H, and J are higher-magnification images of the respective boxed areas in panels A, C, E, G, and I. Arrows and arrowheads indicate defects in the macrochaete and microchaete lineage, respectively. Closed arrowheads in panel D point to double-hair, double-socket or one-hair, three-socket phenotypes, whereas open arrowheads point to two- or four-socket phenotypes. All these are prototypic defects caused by increases in Notch signaling in the SOP lineage. (K to M) Elav antibody staining (in red) showing the number of neurons underneath the notum surface. Overexpression was achieved by using ScaGal4. Embryos and larvae were raised at 18°C, but within 10 h after pupa formation, the temperature was shifted to 29°C, the nonpermissive temperature for DTS5.
To more accurately determine the effects of these mutations on Numb activity, we also expressed various mutant forms of Numb proteins by using a weaker ScaGal4 driver (ScaGal4109-68) (9), which causes less-severe defects on the notum when used to express wild-type d-Numb and m-Numb. We tested at least two independently generated lines for each mutant form and did not observe major differences in the severity of balding phenotypes on the notum. For example, while there is variation among the four d-NumbΔC lines we examined (Fig. 3D), quantitative analysis revealed no marked differences in the average effect compared to the phenotype of wild-type d-Numb (177.2 ± 46.2 and 154.7 ± 23.3 hairs/notum, respectively; the number in the wild type is 227.5 ± 7.5). The same was true for m-NumbΔC and m-Numb (51.9 ± 21.8 and 68.4 ± 20.6 hairs/notum, respectively). More importantly, lines with lesser effects invariably had lower levels of protein expression (data not shown). These findings further confirm that the binding motifs for endocytic proteins are not essential for Numb in specifying cell fates.
To examine whether the mutant forms of Numb proteins could function without endogenous d-Numb, we first used a hairpin-loop approach (16) to generate Drosophila lines (numbRNAi) expressing dsRNA of d-numb. Targeting such dsRNA to the SOP lineage phenocopied d-numb loss-of-function mutants as a consequence of RNA interference (RNAi), resulting in adult flies in which 10 to 20% of the notum es organs contained only four sockets (Fig. 4A and B). The nucleotide sequences of m-numb and d-numb are divergent, even in regions with high levels of amino acid conservation. Therefore, d-numb dsRNA should not affect m-numb mRNA. Like wild-type m-Numb, D/NPFA/AAA and m-NumbΔC could completely suppress the four-socket phenotype caused by the absence, or significantly reduced levels, of d-Numb. The nota of these flies contained large balding areas and a few remaining wild-type es organs, a phenotype identical to that caused by Numb overexpression alone (Fig. 4C and data not shown).
FIG. 4.
Rescue of the Drosophila numb mutant phenotypes by Numb proteins lacking the endocytic protein-binding motifs. (A to C) Drosophila notum overexpressing d-numb dsRNA either alone (A and B) or together with the D/NPFA/AAA mutant form of m-Numb (C). Images in panel B are the boxed areas of panel A at a higher magnification. Open and closed arrowheads point to defects in the microchaete and macrochaete lineage, respectively. Each arrowhead points to a mutant socket cell. Overexpression was achieved by using ScaGal4 at 18°C. (D to I) Neuronal rescue. Elav staining (in green) of wild-type (WT) (D and E), d-numb mutant (F and G), and m-NumbΔC-rescued (H and I) embryos. All are stage 15 embryos. Arrowheads point to the two neurons in the two dorsal-most es organs, and brackets show the lateral chordotonal organs. (J to L) Cell fate transformation in the two dorsal-most es organs. Elav staining (in green) shows the presence of two neurons (arrowheads) among the eight Cut-positive cells (in red) in WT (J) and m-NumbΔC-rescued (L) embryos but not in the d-numb mutant (K). Elav is present in both the nucleus and the cytoplasm. Thus, Elav-stained cells appear bigger than those stained with Cut, which is present only in the nucleus. (M and N) Sheath cell rescue. Prospero staining (in red) shows that sheath cells, which accompany neurons (in green) in the lateral chordotonal organs (brackets) in the WT (M), are also rescued by m-NumbΔC (N). Images in panels E, G, I, and J to N are shown at higher magnifications.
To further verify that the C-terminal motifs are dispensable for Numb function in Drosophila, we used a method that strongly promotes exogenous protein expression in alternate parasegments (6) (see Fig. S1 in the supplemental material) to introduce m-NumbΔC into embryos homozygous for a null allele of d-numb. d-numb mutant embryos are marked by a severe loss of neurons in the PNS, as revealed by staining with the neuronal marker Elav (Fig. 4F and G). We showed previously that expressing wild-type m-Numb or d-Numb in this way, which causes no overexpression phenotypes, rescues the mutant phenotypes in the corresponding embryonic segments (54). Indeed, this also holds true for m-NumbΔC. In d-numb mutant embryos that also expressed m-NumbΔC, the abdominal segments, in particular a1, a3, a5, and a7, no longer exhibited the neuronal loss seen in d-numb mutants (Fig. 4H and I).
To confirm that the reappearance of neurons indeed represents a rescue of cell fate defects caused by the loss of endogenous d-Numb, we analyzed the two dorsal-most es organs in the abdominal hemisegments. As detailed above (Fig. 2O), each wild-type es organ has four cells (a neuron and three different support cells), which are generated by a single SOP cell through three rounds of Numb-dependent asymmetric divisions. All four cells express the protein Cut. Thus, in the two wild-type es organs, there are eight Cut-positive cells, two of which are doubly positive for Elav (Fig. 4E and J) (54). The latter are absent in d-numb mutants (Fig. 4G and K). In m-NumbΔC-rescued embryos, however, the two Elav-positive neurons reappeared (Fig. 4I and L), as did the Prospero-expressing sheath cells that are also absent in d-numb mutants (data not shown). This rescue of neuron and sheath cells could also be observed in other regions of the PNS, including the lateral chordotonal organs (Fig. 4H, I, and N). It is worth mentioning that this rescue by m-NumbΔC was comparable to those by wild-type m-Numb and d-Numb (54).
Endocytic protein binding domains do not act in a dominant-negative manner.
While the findings discussed above demonstrate that the binding motifs for endocytic proteins in the C-terminal part of Numb proteins are not necessary for their activity, a network of EH proteins and α-Ada could still be necessary for transducing Numb signaling, if Numb proteins bind to them indirectly via proteins that interact with the N-terminal part of Numb proteins. We reasoned that if this were so, overexpressing only the binding domains for endocytic proteins should either be sufficient for Numb signaling or have a dominant-negative effect. We thus generated Drosophila lines overexpressing only the C-terminal part of m-Numb (amino acids 396 to 593) (m-NumbΔN). Similar C-terminal fragments of m-Numb have been shown to interfere with receptor-mediated endocytosis in cultured cells (36). We verified the production of m-NumbΔN by using an antibody against the C terminus of m-Numb (see Fig. S1A and B in the supplemental material). However, its overexpression, even at extremely high levels by using ScaGal4 to drive multiple copies of the m-NumbΔN transgene at 29°C, neither caused any Numb gain-of-function phenotypes on the notum nor interfered with the endogenous d-Numb activity (Fig. 3J). This result is consistent with findings from studies using d-Numb proteins that lack the N-terminal phosphotyrosine binding (PTB) domain. Even though such proteins remain capable of membrane association and asymmetric localization, they exert no dominant-negative effect on the SOP lineage (17, 51). One such study used a d-Numb mutant (F195V) in which only a single amino acid, phenylananine (F) at position 195, was changed to a valine (V) (51). This mutation abolishes d-Numb activity but not the ability to bind to EH1 and Dap160 (Fig. 3N and data not shown). Like that of m-NumbΔN, however, overexpression of F195V had no effect on cell fate specification in the SOP lineage (Fig. 3L).
Since it is possible that F195 itself mediates binding (directly or indirectly) to endocytic proteins, which could at least partly explain the lack of dominant-negative effects by F195V, we generated a different form of PTB-defective m-Numb, m-NumbCKO, in which 72 amino acids of the PTB domain (amino acids 63 to 138) were deleted. The F195 equivalent on m-Numb is F151 (Fig. 1A). These 72 amino acids are deleted in a mutant allele of m-numb that we generated, and mice homozygous for this allele die during early embryogenesis (55). Unlike F195V, m-NumbCKO remained capable of binding to proteins such as Nak and LNX2 (7, 8, 31), as well as other known endocytic proteins and E3 ubiquitin ligases (Fig. 3N and O). However, even though m-NumbCKO could be produced at high levels (see Fig. S1C and D in the supplemental material), its overexpression did not cause any defects in the SOP lineage (Fig. 3K). We also examined whether m-NumbCKO could suppress the four-socket phenotypes caused by RNAi-induced d-numb mutation. As expected, the nota of these flies continued to exhibit the multisocket phenotypes (see Fig. S1E and F in the supplemental material).
Reducing proteasome activity does not block Numb signaling.
F195 in the PTB domain and other regions of Numb proteins also bind to E3 ubiquitin ligases such as Sina and the LNX family of proteins (8, 23, 24, 47). Furthermore, inhibition of proteasome activity by using DTS5, a dominant temperature-sensitive mutation of the β6 subunit, causes cell fate changes in the SOP lineage that are consistent with a gain of Notch function, namely, mutant es organs with two to four sockets, or two hairs and two sockets, accompanied by an absence of neurons (38) (Fig. 5C and D). We reasoned that if Numb proteins down-regulate Notch signaling by targeting Notch for degradation by proteasomes, DTS5 co-overexpression should block or significantly reduce the ability of Numb proteins to specify cell fates. This is not the case; their co-overexpression only caused the Numb overexpression phenotype (data not shown). To examine the possibility that endocytosis and proteasomes provide redundant pathways for reduction of Notch levels by Numb proteins, we co-overexpressed DTS5 and m-Numb D/NPFA/AAA or d-NumbΔC. Like their wild-type counterparts, D/NPFA/AAA and d-NumbΔC could also completely suppress the multisocket phenotypes, and the resultant flies exhibited only the Numb overexpression phenotype, namely, balding of the notum (Fig. 5E to J). We used an anti-Elav antibody to further confirm that the balding was due to transformation of SOP daughter cells into additional neurons (Fig. 2O). Indeed, underneath the balding patches, there were clusters of as many as four neurons (Fig. 5L and M), unlike the pattern on the wild-type notum, where a single Elav-positive neuron was present in each es organ (Fig. 5K). It is noteworthy that the balding phenotypes in Fig. 5G and I are more severe than those in Fig. 3H and C, respectively, because the experiments were performed at 29°C, the nonpermissive temperature for DTS5, which causes much higher levels of Numb protein overexpression. Overexpression of Numb alone (wild type or the D/NPF or ΔC mutant) at such levels similarly affected nearly 100% of the es organs (Fig. 5E and data not shown).
DISCUSSION
The findings reported here show that the Drosophila Eps15 homologue EH1 and the dynamin-associated protein Dap160 are negative regulators of Notch signaling. However, neither eliminating the binding motifs for EH proteins and α-Ada nor simultaneously reducing proteasome activity affects the ability of Numb proteins to specify cell fates. These findings support a view that Numb proteins do not modulate Notch signaling by simply removing Notch receptors from the cell membrane through endocytosis and/or degradation by proteasomes.
Three lines of evidence further support this view. First, in wild-type Drosophila, no differences in Notch protein levels could be observed between the two SOP daughter cells or between daughters of other neural precursor cells that divide asymmetrically (12, 46). Second, analysis of Shi mutants, in which endocytosis is completely blocked, shows that endocytosis is essential for the activation, not inactivation, of Notch signaling (42). Moreover, EH proteins also modulate Notch signaling during lateral inhibition (Fig. 2G, I, and L), an aspect not at all affected by Numb proteins. Third, flies homozygous for the α-adaear mutation (adaear/ear), which exhibit defects in asymmetric cell division by the SOP cells, have no apparent defects in endocytosis (5). In such mutants, there are also no changes in Notch protein levels. On the other hand, α-ada-null mutants, which show severe defects in endocytosis, exhibit no defects in cell fate specification (5, 10). More importantly, neither cell fate defects nor apparent perturbations in endocytosis can be observed in ear and null transheterozygotes (5). This suggests that α-adaear is not a loss-of-function allele (otherwise, transheterozygotes and ear homozygous mutants should exhibit the same cell fate defects). In other words, α-ada mutants show that endocytosis and Numb-mediated cell fate specification can be separated, a conclusion consistent with our finding that binding motifs for endocytic proteins are neither necessary nor sufficient for Numb function in cell fate specification.
We emphasize, however, that binding to endocytic proteins and E3 ubiquitin ligases may still play important roles in other aspects of Numb function, particularly in differentiating and mature neurons, where Numb proteins are expressed at high levels. For example, recent studies with mice show that loss of m-Numb and Numbl in sensory neurons causes defects in axon arborization, and Numb activity in this regard is dependent on the α-Ada-interacting domain (13). A similar role for Numb in Drosophila neurons may explain why the binding motifs for endocytic proteins are conserved evolutionarily even though they are not necessary for cell fate specification. It is also possible that endocytosis and proteasomes are important in down-regulating Numb activity itself. In cultured cells, at least, overexpression of LNX, an E3 ubiquitin ligase that binds to the PTB domain of m-Numb, can cause proteasome-dependent degradation of m-Numb (24). Similar findings have also been reported from studies using Siah1 and MDM2, both E3 ubiquitin ligases that bind to other regions of m-Numb (15, 47).
Taken together, our findings strongly suggest that Numb proteins specify asymmetric cell fates via an endocytosis- and proteasome-independent pathway. It is also unlikely that this pathway mediates Numb function redundantly with endocytosis and proteasomes. Numb proteins with various deletions encompassing the PTB domain, in particular F195V and m-NumbCKO, are incapable of specifying cell fates even when expressed at high levels, nor do they exert any dominant-negative effects (Fig. 3K and L) (51). Yet they remain fully capable of binding to endocytic proteins such as EH proteins and E3 ubiquitin ligases such as Sina, all of which are negative regulators of Notch signaling (Fig. 2). It is also worth mentioning that a lack of dominant-negative effects by PTB-defective forms of Numb proteins is not due to an inability to associate with the membrane, which could be essential for proteins to directly affect receptor-mediated endocytosis. In Drosophila, at least, membrane association and asymmetric localization of Numb are not dependent on the PTB domain. m-NumbΔN, for example, is associated with the membrane (see Fig. S1B in the supplemental material). Moreover, simply disrupting the ability of Numb proteins to associate with the cell membrane, or to localize asymmetrically, does not abolish their ability to specify cell fates (17, 56). However, it remains formally possible that motifs in the essential N-terminal region of Numb proteins may link to other endocytic proteins to transduce Numb signaling.
Our findings, unfortunately, do not point to the nature of this endocytosis- and proteasome-independent pathway that enables Numb to modulate Notch signaling. Recently, Numb has been shown to physically associate with Sanpodo (Spdo), a four-pass transmembrane protein that also physically interacts with Notch, and to prevent Spdo from localizing to the cell membrane in Drosophila (25). Like Numb, Spdo is only necessary for Notch signaling in asymmetric cell divisions. While there are no apparent vertebrate Spdo homologues, elucidating how Numb inhibits Spdo membrane localization may nevertheless yield interesting clues as to how Numb proteins specify asymmetric cell fates from invertebrates to vertebrates.
Supplementary Material
Acknowledgments
We thank Zhong lab members for discussions, Pietro De Camilli for comments, Sinead Stewart and Christopher Robertson for technical assistance, DHSB for the anti-Elav antibody, Francois Schweisguth for DTS5 flies, and Lauren Yaich and Rolf Bodmer for UAS-d-Numb flies.
This work was supported by grants from the March of Dimes and the National Institutes of Health (National Institute of Neurological Disorders and Stroke) to W.Z.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Artavanis-Tsakonas, S. 1999. Notch signaling: cell fate control and signal integration in development. Science 284:770-776. [DOI] [PubMed] [Google Scholar]
- 2.Bang, A. G., V. Hartenstein, and J. W. Posakony. 1991. Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development 111:89-104. [DOI] [PubMed] [Google Scholar]
- 3.Barolo, S., T. Stone, A. G. Bang, and J. W. Posakony. 2002. Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 16:1964-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel, P. L., C. T. Chien, R. Sternglanz, and S. Fields (ed.). 1993. Using the two-hybrid system to detect protein-protein interactions. Oxford University Press, Oxford, United Kingdom.
- 5.Berdnik, D., T. Torok, M. Gonzalez-Gaitan, and J. A. Knoblich. 2002. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell 3:221-231. [DOI] [PubMed] [Google Scholar]
- 6.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. [DOI] [PubMed] [Google Scholar]
- 7.Chien, C.-T., S. Wang, M. Rothenberg, L. Y. Jan, and Y. N. Jan. 1998. Numb-associated kinase interacts with the phosphotyrosine binding domain of Numb and antagonizes the function of Numb in vivo. Mol. Cell. Biol. 18:598-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dho, S. E., S. Jacob, C. D. Wolting, M. B. French, L. R. Rohrschneider, and C. J. McGlades. 1998. The mammalian numb phosphotyrosine-binding domain. Characterization of binding specificity and identification of a novel PDZ domain-containing numb binding protein, LNX. J. Biol. Chem. 273:9179-9187. [DOI] [PubMed] [Google Scholar]
- 9.Frise, E., J. A. Knoblich, S. Younger-Shepherd, L. Y. Jan, and Y. N. Jan. 1996. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interactions in sensory organ lineage. Proc. Natl. Acad. Sci. USA 93:11925-11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Gaitan, M., and H. Jackle. 1997. Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell 88:767-776. [DOI] [PubMed] [Google Scholar]
- 11.Grant, B., Y. Zhang, M. C. Paupard, S. X. Lin, D. H. Hall, and D. Hirsh. 2001. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat. Cell Biol. 3:573-579. [DOI] [PubMed] [Google Scholar]
- 12.Guo, M., L. Y. Jan, and Y. N. Jan. 1996. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron 17:27-41. [DOI] [PubMed] [Google Scholar]
- 13.Huang, E. J., H. Li, A. A. Tang, A. K. Wiggins, R. L. Neve, W. Zhong, L. Y. Jan, and Y. N. Jan. 2005. Targeted deletion of numb and numblike in sensory neurons reveals their essential functions in axon arborization. Genes Dev. 19:138-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juven-Gershon, T., O. Shifman, T. Unger, A. Elkeles, Y. Haupt, and M. Oren. 1998. The Mdm2 oncoprotein interacts with the cell fate regulator Numb. Mol. Cell. Biol. 18:3974-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennerdell, J. R., and R. W. Carthew. 2000. Heritable gene silencing in Drosophila using double-stranded RNA. Nat. Biotechnol. 17:896-898. [DOI] [PubMed] [Google Scholar]
- 17.Knoblich, J. A., L. Y. Jan, and Y. N. Jan. 1997. The N-terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization. Proc. Natl. Acad. Sci. USA 94:13005-13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh, T. W., P. Verstreken, and H. J. Bellen. 2004. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron 43:193-205. [DOI] [PubMed] [Google Scholar]
- 19.Le Borgne, R., and F. Schweisguth. 2003. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell 5:139-148. [DOI] [PubMed] [Google Scholar]
- 20.Li, H.-S., D. Wang, Q. Shen, M. D. Schonemann, J. A. Gorski, K. R. Jones, S. Temple, L. Y. Jan, and Y. N. Jan. 2003. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron 40:1105-1118. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd, T. E., P. Verstreken, E. J. Ostrin, A. Phillippi, O. Lichtarge, and H. J. Bellen. 2000. A genome-wide search for synaptic vesicle cycle proteins in Drosophila. Neuron 26:45-50. [DOI] [PubMed] [Google Scholar]
- 22.Marie, B., S. T. Sweeney, K. E. Poskanzer, J. Roos, R. B. Kelly, and G. W. Davis. 2004. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron 43:207-219. [DOI] [PubMed] [Google Scholar]
- 23.McGill, M. A., and C. J. McGlade. 2003. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J. Biol. Chem. 278:23196-23203. [DOI] [PubMed] [Google Scholar]
- 24.Nie, J., M. A. McGill, M. Dermer, S. E. Dho, C. D. Wolting, and C. J. McGlade. 2002. LNX functions as a RING type E3 ubiquitin ligase that targets the cell fate determinant Numb for ubiquitin-dependent degradation. EMBO J. 21:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connor-Giles, K. M., and J. B. Skeath. 2003. Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev. Cell 5:231-243. [DOI] [PubMed] [Google Scholar]
- 26.Petersen, P. H., K. Zou, J. K. Hwang, Y. N. Jan, and W. Zhong. 2002. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature 419:929-934. [DOI] [PubMed] [Google Scholar]
- 27.Petersen, P. H., K. Zou, S. Krauss, and W. Zhong. 2004. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat. Neurosci. 7:803-811. [DOI] [PubMed] [Google Scholar]
- 28.Pi, H., H. J. Wu, and C. T. Chien. 2001. A dual function of phyllopod in Drosophila external sensory organ development: cell fate specification of sensory organ precursor and its progeny. Development 128:2699-2710. [DOI] [PubMed] [Google Scholar]
- 29.Polo, S., S. Confalonieri, A. E. Salcini, and P. P. Di Fiore. 2003. EH and UIM: endocytosis and more. Sci. STKE 2003:re17. [DOI] [PubMed]
- 30.Rhyu, M. S., L. Y. Jan, and Y. N. Jan. 1994. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76:477-491. [DOI] [PubMed] [Google Scholar]
- 31.Rice, D. S., G. M. Northcutt, and C. Kurschner. 2001. The Lnx family proteins function as molecular scaffolds for Numb family proteins. Mol. Cell. Neurosci. 18:525-540. [DOI] [PubMed] [Google Scholar]
- 32.Roegiers, F., and Y. N. Jan. 2004. Asymmetric cell division. Curr. Opin. Cell Biol. 16:1-11. [DOI] [PubMed] [Google Scholar]
- 33.Roos, J., and R. B. Kelly. 1998. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. J. Biol. Chem. 273:19108-19119. [DOI] [PubMed] [Google Scholar]
- 34.Salcini, A. E., S. Confalonieri, M. Doria, E. Santolini, E. Tassi, O. Minenkova, G. Cesareni, P. G. Pelicci, and P. P. Di Fiore. 1997. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 11:2239-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salcini, A. E., M. A. Hilliard, A. Croce, S. Arbucci, P. Luzzi, C. Tacchetti, L. Daniell, P. De Camilli, P. G. Pelicci, P. P. Di Fiore, and P. Bazzicalupo. 2001. The Eps15 C. elegans homologue EHS-1 is implicated in synaptic vesicle recycling. Nat. Cell Biol. 3:755-760. [DOI] [PubMed] [Google Scholar]
- 36.Santolini, E., C. Puri, A. E. Salcini, M. C. Gagliani, P. G. Pelicci, C. Tacchetti, and P. P. Di Fiore. 2000. Numb is an endocytic protein. J. Cell Biol. 151:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santolini, E., A. E. Salcini, B. K. Kay, M. Yamabhai, and P. P. Di Fiore. 1999. The EH network. Exp. Cell Res. 253:186-209. [DOI] [PubMed] [Google Scholar]
- 38.Schweisguth, F. 1999. Dominant-negative mutation in the β2 and β6 proteasome subunit genes affect alternative cell fate decisions in the Drosophila sense organ lineage. Proc. Natl. Acad. Sci. USA 96:11382-11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweisguth, F., and J. W. Posakony. 1992. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell 69:1199-1212. [DOI] [PubMed] [Google Scholar]
- 40.Sengar, A. S., W. Wang, J. Bishay, S. Cohen, and S. E. Egan. 1999. The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J. 18:1159-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seto, E. S., H. J. Bellen, and T. E. Lloyd. 2002. When cell biology meets development: endocytic regulation of signaling pathways. Genes Dev. 16:1314-1336. [DOI] [PubMed] [Google Scholar]
- 42.Seugnet, L., P. Simpson, and M. Haenlin. 1997. Requirement for Dynamin during Notch signaling in Drosophila neurogenesis. Dev. Biol. 192:585-598. [DOI] [PubMed] [Google Scholar]
- 43.Shen, Q., W. Zhong, Y. N. Jan, and S. Temple. 2002. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development 129:4843-4853. [DOI] [PubMed] [Google Scholar]
- 44.Smith, C. A., S. E. Dho, J. Donaldson, U. Tepass, and C. J. McGlade. 2004. The cell fate determinant numb interacts with EHD/Rme-1 family proteins and has a role in endocytic recycling. Mol. Biol. Cell 15:3698-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 46.Spana, E. P., and C. Q. Doe. 1996. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17:21-26. [DOI] [PubMed] [Google Scholar]
- 47.Susini, L., B. J. Passer, N. Amzallag-Elbaz, T. Juven-Gershon, S. Prieur, N. Privat, M. Tuynder, M. C. Gendron, A. Israel, R. Amson, M. Oren, and A. Telerman. 2001. Siah-1 binds and regulates the function of Numb. Proc. Natl. Acad. Sci. USA 98:15067-15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdi, J. M., A. Bashirullah, D. E. Goldhawk, C. J. Kubu, M. Mamali, S. O. Meakin, and H. D. Lipshitz. 1999. Distinct human NUMB isoforms regulate differentiation vs. proliferation in the neuronal lineage. Proc. Natl. Acad. Sci. USA 96:10472-10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verdi, J. M., R. Schmandt, A. Bashirullah, S. Jacob, R. Salvino, C. G. Craig, A. E. Program, H. D. Lipshitz, and C. J. McGlade. 1996. Mammalian NUMB is an evolutionarily conserved signaling adapter protein that specifies cell fate. Curr. Biol. 6:113-145. [DOI] [PubMed] [Google Scholar]
- 50.Wakamatsu, Y., T. M. Maynard, S. U. Jones, and J. A. Weston. 1999. NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron 23:71-81. [DOI] [PubMed] [Google Scholar]
- 51.Yaich, L., J. Ooi, M. Park, J. P. Borg, C. Landry, R. Bodmer, and B. Margolis. 1998. Functional analysis of the Numb phosphotyrosine-binding domain using site-directed mutagenesis. J. Biol. Chem. 273:10381-10388. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi, A., T. Urano, T. Goi, and L. A. Feig. 1997. An Eps homology (EH) domain protein that binds to the Ral-GTPase target, RalBP1. J. Biol. Chem. 272:31230-31234. [DOI] [PubMed] [Google Scholar]
- 53.Zhong, W. 2003. Diversifying neural cells through order of birth and asymmetry of division. Neuron 37:11-14. [DOI] [PubMed] [Google Scholar]
- 54.Zhong, W., J. N. Feder, M.-M. Jiang, L. Y. Jan, and Y. N. Jan. 1996. Asymmetric localization of a mammalian Numb homolog during mouse cortical neurogenesis. Neuron 17:43-53. [DOI] [PubMed] [Google Scholar]
- 55.Zhong, W., M.-M. Jiang, M. D. Schonemann, J. J. Meneses, R. A. Pedersen, L. Y. Jan, and Y. N. Jan. 2000. Mouse numb is an essential gene involved in cortical neurogenesis. Proc. Natl. Acad. Sci. USA 97:6844-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong, W., M.-M. Jiang, G. Weinmaster, L. Y. Jan, and Y. N. Jan. 1997. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development 124:1887-1897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.