Abstract
The SR family proteins and SR-related polypeptides are important regulators of pre-mRNA splicing. A novel SR-related protein of an apparent molecular mass of 53 kDa was isolated in a gene trap screen that identifies proteins which localize to the nuclear speckles. This novel protein possesses an arginine- and serine-rich domain and was termed SRrp53 (for SR-related protein of 53 kDa). In support for a role of this novel RS-containing protein in pre-mRNA splicing, we identified the mouse ortholog of the Saccharomyces cerevisiae U1 snRNP-specific protein Luc7p and the U2AF65-related factor HCC1 as interacting proteins. In addition, SRrp53 is able to interact with some members of the SR family of proteins and with U2AF35 in a yeast two-hybrid system and in cell extracts. We show that in HeLa nuclear extracts immunodepleted of SRrp53, the second step of pre-mRNA splicing is blocked, and recombinant SRrp53 is able to restore splicing activity. SRrp53 also regulates alternative splicing in a concentration-dependent manner. Taken together, these results suggest that SRrp53 is a novel SR-related protein that has a role both in constitutive and in alternative splicing.
Pre-mRNA splicing takes place in the spliceosome, a large ribonucleoprotein complex that is formed by the small nuclear ribonucleoprotein particles (U1, U2, U4/U6, and U5 snRNPs) and numerous non-snRNP splicing factors (reviewed in reference 36).
The serine- and arginine-rich proteins (SR proteins) are a highly conserved family of structurally and functionally related non-snRNP splicing factors with a dual role in splicing, affecting both constitutive and alternative splicing. They have a modular domain structure consisting of one or two RNA recognition motifs (RRMs) and a C-terminal domain rich in arginine and serine residues, termed the RS domain (20). The RRMs determine RNA binding specificity, whereas the RS domain, which is extensively phosphorylated, promotes protein-protein interactions that are essential for the recruitment of the splicing apparatus and for splice site pairing (61, 67). The RS domains have been shown to directly contact the pre-mRNA branch point; thus, RS domains may not solely function through protein-protein interactions (53). In addition, the RS domain of SR proteins directs subcellular localization and determines the nucleocytoplasmic shuttling of individual SR proteins (10, 27, 39).
The SR family proteins function early in spliceosome formation and are involved in multiple steps of the splicing reaction (59). They facilitate the recruitment of the U1 snRNP particle to the 5′ splice site (18, 33, 35) and also bridge the 5′ and 3′ splice sites via RS-domain-mediated interactions with U1 and U2 snRNP-associated proteins (67). The SR family proteins also participate at later stages of the splicing reaction, when they facilitate the recruitment of the U4/U6 · U5 tri-snRNP complex (48).
A class of related RS-domain-containing proteins that may or may not contain RRMs is also involved in splicing regulation and has been termed the SR-protein-related polypeptides (SRrp) or SR-like proteins (6). The SR-related proteins function in spliceosome assembly and also participate in the recognition of exonic splicing enhancers (ESEs), leading to the activation of otherwise inefficient upstream 3′ splice sites (reviewed in references 5 and 26). For instance, the SR-related nuclear matrix proteins SRm160 and SRm300, which contain RS domains but lack RRMs, mediate interactions between one or more SR family and/or SR-related protein bound to ESEs and basal splicing factors, including U1 and U2 snRNP components (7). Two human SR-related proteins of 65 and 110 kDa are essential for the recruitment of the tri-snRNP to the prespliceosomes but not for the maintenance of tri-snRNP stability (41). Some of the SR-related proteins are structurally very similar to authentic SR proteins, although they are functionally different. For instance, SRrp86, does not complement splicing-defective S100 extracts, but it is able to inhibit the ability of individual SR proteins to activate splicing and also antagonizes SR proteins in alternative splicing regulation (2, 3). Likewise, two SR-protein-like factors, SRrp40 (also known as SRp38) and SRrp35, antagonize authentic SR proteins and regulate alternative splicing (15). Interestingly, specific dephosphorylation of SRp38 converts this protein to a general repressor that inhibits splicing at an early step in M-phase cells and also during heat shock (55, 56). A recent genome-wide survey revealed a large complexity of RS-domain-containing proteins in metazoans with functions not only in pre-mRNA splicing but also in chromatin remodeling, transcription by RNA polymerase II, and cell cycle progression (8). Thus, SR-related proteins may not only be involved in mRNA processing but have a more complex array of functions in mammalian cells.
We have identified and cloned a novel SR-related protein that contains a domain rich in arginines and serines but lacks a recognizable RRM. We show that this novel protein, termed SRrp53 (for SR-related protein of 53 kDa), localizes to the nuclear speckled domain and interacts with other splicing regulators, such as Luc7p, a protein that is part of the U1 snRNP and has a role in 5′ splice site recognition in S. cerevisiae (19), and HCC1, a factor highly homologous to the large subunit of the U2AF splicing factor (32). We also demonstrate that SRrp53 is required for the second step of splicing and also regulates alternative splicing. Taken together, these results suggest that SRrp53 is a novel SR-related protein that has a role both in constitutive and in alternative splicing.
MATERIALS AND METHODS
Bioinformatic analysis of SRrp53 and its related homologues.
Known protein domains and motifs were searched for by using Superfamily (23), InterPro (1), and associated databases. Proteins putatively orthologous to the SRrp53 protein, found in databases as the Q9DBU6 protein, were identified via reciprocal BLAST searches.
Cloning and sequencing of mouse and human SRrp53.
Mouse and human versions of SRrp53 were cloned by reverse transcription (RT)-PCR from total RNA from mouse embryonic stem (ES) cells or human Hep3B cells, respectively. Total RNA was prepared using Total RNA Isolation Reagent (ABgene) according to manufacturer's specifications. Approximately 5 μg of total RNA was used for the synthesis of first-strand cDNA with SuperScriptII RNase H− reverse transcriptase (Invitrogen), and 10% of the cDNA obtained in each case was used for PCR amplification. Fragments corresponding to the full-length coding sequence of mouse and human SRrp53 were amplified using specific primers that introduce SpeI and BamHI restriction sites, ligated into the corresponding sites of pBlueScript SKII−, and fully sequenced.
Expression plasmids.
The mammalian expression vector pCGT7 used to express mouse and human SRrp53 has been previously described (10). Transcription is driven by the cytomegalovirus enhancer/promoter, and the coding sequence begins with an N-terminal epitope tag, MASMTGGQQMG, which corresponds to the first 11 residues of the bacteriophage T7 gene 10-capsid protein and is recognized by the T7 tag monoclonal antibody (MAb) (Novagen). Due to the presence of an internal XbaI site in the SRrp53 cDNA, the amplified fragments were designed with SpeI and BamHI sites and were subcloned into the XbaI-BamHI sites of pCGT7. The plasmid expressing hLuc7a was prepared in a similar way. A fusion of SRrp53 to green fluorescent protein (GFP) was constructed by amplification of mSRrp53 with specific primers, and the resulting PCR product was subcloned as EcoRI-BamHI fragment into pEGFP-C1 (NH2-terminal tag) (Clontech).
Oligonucleotides.
The sequences of the specific primers used for PCR are available upon request.
Expression and purification of recombinant proteins.
Recombinant T7-SRrp53 was expressed in 293T cells and purified as described elsewhere (D. Cazalla and J. F. Cáceres, submitted for publication). Briefly, 293T cells were grown to confluence in T150 flasks and transiently transfected with 60 μg of epitope-tagged, full-length SRrp53 expression plasmid and 120 μl of Lipofectamine 2000 (Invitrogen) per flask. Forty-eight hours after transfection, cells were lysed and recombinant protein was purified by anti-T7-agarose affinity chromatography. The recombinant baculovirus strain expressing His-hnRNP L was kindly provided by A. Bindereif. Recombinant His-hnRNP L was expresed in Sf9 cells and purified as described previously (31).
Production and purification of antibodies against SRrp53.
Polyclonal antibodies were raised in rabbit against two synthetic peptides that are conserved between the human and mouse SRrp53 proteins encompassing amino acids 207 to 221 (207EEEAKRRKEEDQATL221) and the last 15 amino acids of the protein (319LIALRQERLMGSPVA334), which were designated PEP1 and PEP2, respectively. Animals were injected with these peptides coupled to keyhole limpet hemocyanin (KLH) and boosted 4 weeks later. To affinity purify antibodies, rabbit serum from the fourth bleed was passed over peptide affinity columns, which were prepared by coupling 2 mg of peptide to 1 ml of SulfoLink coupling gel (Pierce) following manufacturer's protocol. After binding to the different columns, antibodies were recovered from only the column prepared with PEP2, suggesting that the animal used did not generate antibodies against PEP1. Polyclonal antibodies against HCC1 were raised by injecting a sheep with glutathione S-transferase fused to amino acids 197 to 227 of HCC1. Specific antibodies against HCC1 were obtained by affinity purification from the third-bleed serum over a CNBr-activated Sepharose column (Amersham) covalently bound to an HCC1 peptide encompassing amino acids 197 to 227.
Cell culture and transfections.
HeLa, 293T, and 3T3 cell lines were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum and incubated at 37°C in the presence of 5% CO2. HeLa and 293T cells were transfected with 3 μg of DNA and 5 μl of Lipofectamine 2000 (Invitrogen) per well in six-well plates or with 15 μg of DNA and 45 μl of Lipofectamine 2000 per 10-cm-diameter dish according to manufacturer's instructions.
Indirect immunofluorescence.
Cells were fixed and permeabilized for immunofluorescence assays at 24 h after transfection. Fixation was with 4% p-formaldehyde in phosphate-buffered saline (PBS) for 15 to 30 min at room temperature, followed by incubation for 10 min in 0.2% Triton X-100 in PBS. Fixed cells were then incubated for 1 h at room temperature with the first antibody, washed with PBS, and incubated for 1 h at room temperature with a secondary antibody, followed by further washes with PBS. The samples were observed on a Zeiss Axioskop microscope, and the images were acquired with a Photometrics CH250 cooled charge-coupled-device camera by using Digital Scientific Smartcapture extensions in software from IP Lab Spectrum. MAbs against SC35 (21) were kindly provided by Xiang-Dong Fu and used in a 1:500 dilution.
Heterokaryon assays.
For transient transfections involving interspecies heterokaryons, due to the need for higher transfection efficiency, transfection was carried out by electroporation using 10 μg of plasmid DNA per 60-mm-diameter dish of 70 to 80% confluent cells in the presence of 20 μg of carrier DNA. Transfected HeLa cells were seeded on coverslips, followed by coincubation with an excess of untransfected mouse NIH 3T3 cells for 3 h in the presence of 50 μg of cycloheximide/ml. The concentration of cycloheximide was then increased to 100 μg/ml, and the cells were incubated for an additional 30 min prior to fusion. Cell fusions were done as described previously (45), and the heterokaryons were further incubated for 2 h in media containing 100 μg of cycloheximide/ml prior to fixation. Immunofluorescence with the anti-T7 MAb was performed as described above, except that DAPI was included at 5 μg/ml.
Western blot analysis.
Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto Hybond-P (Amersham) in transfer buffer (25 mM Tris base, 40 mM glycine-20% methanol) in a Genie Blotter unit (Idea Scientific Company) at 12 V for 1 h. Membranes were blocked with 5% nonfat dry milk in TBST (20 mM Tris [pH 7.5], 137 mM NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4°C. Proteins were detected by subsequent incubation with the following primary antibodies in TBST: anti-SRrp53 at a 1:1,000 dilution, MAb 96 at a 1:500 dilution (for detection of SF2/ASF [25]), MAb 104 for detection of total SR proteins (49), and sheep polyclonal anti-HCC1 antibody at a 1:100 dilution. Incubation with secondary antibodies (horseradish peroxidase [HRP]-conjugated anti-mouse immunoglobulin G [IgG], HRP-conjugated anti-rabbit IgG, and HRP-conjugated anti-sheep IgG at a 1:10,000 dilution) were carried out for 1 h at room temperature in TBST containing 5% nonfat dry milk. Five washes with TBST were done after incubations with each antibody, and immunoreactive protein bands were detected with the SuperSignal system (Pierce) according to the manufacturer's instructions.
Dephosphorylation of SRrp53.
Ten micrograms of HeLa nuclear extract (4C Biotech, Seneffe, Belgium) were dephosphorylated by incubation with 10 U of calf intestinal alkaline phosphatase (CIAP) (Promega) for 30 min at 37°C in the provided phosphatase buffer and analyzed by Western blotting.
Two-hybrid analysis.
The bait construct used for two-hybrid screening, termed pGBKT7-SRrp53, was constructed by amplifying a SRrp53 cDNA with specific primers, and the resulting PCR product was subcloned as EcoRI-BamHI fragment into pGBKT7 (Clontech). pGBKT7-SRrp53 N-term was constructed by amplification of SRrp53 (amino acids 1 through 164) with specific primers, and the resulting PCR product was subcloned as EcoRI-SalI fragment into pGBKT7 (Clontech), whereas pGBKT7-SRrp53 C-term was constructed by amplification of SRrp53 (amino acids 165 to 334) with specific primers, and the resulting PCR product was subcloned as EcoRI-SalI fragment into pGBKT7 (Clontech). The constructs used for direct yeast two-hybrid analysis expressing SR proteins as preys were generated by inserting PCR fragments into plasmid pACT2 (Clontech). In each case, the PCR fragment was purified, digested with the appropriate restriction enzymes, and ligated to the corresponding sites into the pACT2 vector. The constructs expressing U2AF35, U2AF65, SF1, and U1-70K in pACT2 were a gift from Nick Hastie (16). The vectors and strains provided in the Matchmaker Two-Hybrid System 3 (Clontech) were used to screen a pretransformed mouse brain Matchmaker cDNA Library (Clontech) following manufacturer's protocols. The construct expressing full-length SRrp53 in pGBKT7 plasmid was used as the bait in the screen. Interactors were considered true two-hybrid positives if they could activate the three ADE2, HIS3, and MEL1 reporters when cotransformed with pGBKT7-mSRrp53 and were unable to activate them when cotransformed with pGBKT7. To directly test for the ability of two known proteins to interact, the pGBKT7-derived plasmids (expressing the protein of interest as fusion with GAL4 DNA-DB) and the pACT2-derived construct (expressing the protein of interest as a fusion with GAL4 AD) were cotransformed into the AH109 strain. The resulting strains were tested for their ability to activate reporters as described above.
IP.
For immunoprecipitation (IP), 293T cells that were either transfected with a construct expressing the protein of interest or mock transfected were resuspended in 800 μl of IP buffer (50 mM Tris [pH 7.5], 300 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1 mM dithiothreitol, 1× protease inhibitor cocktail) and were then incubated for 30 min at 4°C with continuous mixing on a rotary mixer. The IP extract was centrifuged at 12,000 × g for 20 min at 4°C, after which the pellet was discarded.
To 200 μl of supernatant (IP extract), 1 μl (100 ng) of anti-T7 tag MAb (Novagen) or 10 μl of either immune or preimmune serum and 20 μl of protein A or G (Amersham) were added. The IP extract was then incubated at 4°C for 2 h with continuous rotation. IP reactions were then washed four times with the IP buffer and once with PBS. In some cases, after the last wash, beads were treated with 50 μg of RNase A/ml for 10 min at 4°C. After RNase A treatment, beads were resuspended in 40 μl of loading buffer (50 mM Tris [pH 7.5], 10% glycerol, 0.05% SDS, 2.5% β-mercaptoethanol) and boiled for 10 min. For Western blot analysis of immunoprecipitated proteins, 10 μl of sample was used.
Immunodepletion of HeLa nuclear extracts.
For immunodepletion of HeLa nuclear extracts, 200 μl of the extract (4C Biotech) was first brought to 1 M KCl and then incubated with beads with coupled antibodies for 2 h at 4°C in Mobicolumns (MOBITEC) on a rotating wheel. After two rounds of depletion were completed, the depleted nuclear extract was centrifuged at 13,000 rpm at 4°C for 5 min in a microcentrifuge and then dialyzed against two changes of buffer D+ (20 mM HEPES [pH 8], 20% glycerol, 0.2 mM EDTA, 0.1 M KCl, 1 mM dithiothreitol, 0.015% NP-40). After dialysis, the depleted nuclear extract was aliquoted and stored at −80°C for later use. The extent of depletion was calculated by comparison of the depleted extracts with untreated extract in SDS-PAGE.
In vitro splicing assays.
Labeled transcripts were prepared in the presence of an RNA CAP structure analog [m7G(5′) ppp(5′)G] (New England Biolabs) and [α-32P]GTP (PerkinElmer) as previously described (50). Adenovirus major late (AdML) substrate was linearized with HincII and transcribed with SP6 RNA polymerase (Ambion). The Fushi tarazu (Ftz) pre-mRNA (47) was linearized with XhoI and transcribed with T7 RNA polymerase. Transcripts were gel purified. Standard conditions were used for the splicing reactions (42). In brief, splicing was carried out in 25-μl reactions containing 3.2 mM MgCl2, 1 mM ATP, 20 mM creatine phosphate, 3% polyvinyl alcohol, 30% nuclear extracts, or 45% immunodepleted or mock-depleted nuclear extracts, complemented with buffer D with 0.1 M KCl. Reactions were incubated at 30°C for 1 h, and then RNAs were purified by proteinase K treatment, phenol-chloroform extraction, and precipitation. Spliced products were separated by electrophoresis on 14% denaturing polyacrylamide gels in Tris-borate-EDTA buffer and exposed to Hyperfilm. Immunodepleted extracts were complemented with either buffer D with 0.1 M KCl or T7-SRrp53 and assayed for splicing activity as described above.
In vivo splicing.
Transfections of HeLa cells and purification of total RNA were done as previously described (12). Briefly, 1 μg of expression plasmid was cotransfected with 2 μg of the adenovirus E1A reporter plasmid pMTE1A (71). Approximately 24 h after transfection, RNA was extracted using the Total RNA isolation reagent (ABgene) following the manufacturer's instructions. Total RNA was analyzed by RT-PCR with Superscript II reverse transcriptase (Invitrogen) and AmpliTaq DNA polymerase (Roche) as previously described (65). E1A mRNA detection was carried out with the 5′-end-labeled exon 1 forward primer 5′-GTTTTCTCCTCCGAGCCGCTCCGA-3′ and the exon 2 reverse primer 5′-CTCAGGCTCAGGTTCAGACACAGG-3′. Amplified products were separated by urea-PAGE, detected by autoradiography, and quantified by PhosphoImage analysis.
RESULTS
Identification and cloning of SRrp53.
We used a gene trap screen approach in mouse cells in order to identify novel nuclear proteins that localize to the nuclear speckles, since this is diagnostic of their involvement in RNA processing. We used a promoterless and ATG-less β-geo construct (pGT) and assayed for the subcellular location of the resultant fusion gene product (62). Integration of the gene trap β-geo construct into the mouse genome ensured that the tagged protein was expressed at endogenous levels and in the correct temporal fashion. The sequence of the trapped gene was identified by 5′ rapid amplification of cDNA ends from the fusion transcript (60).
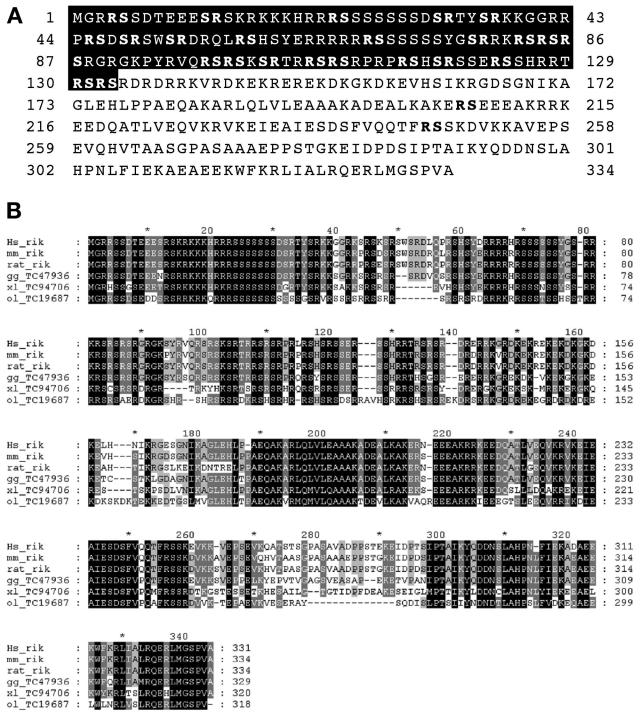
This strategy yielded an insertion mutation in a novel SR-related protein. We cloned and sequenced the full-length mouse gene that matches a RIKEN cDNA clone: 1200013F24 (sequence accession numbers AK004742 and NM_025822). The protein encoded by this gene contains 334 amino acids and includes an amino-terminal domain rich in RS dipeptides and serine-rich regions, followed by a bipartite nuclear targeting sequence between amino acids 214 through 230 that coincides with a helical region predicted to form coiled coil (amino acids 180 through 237), a domain that often mediates protein-protein interactions. No homology to any other known protein motifs was found in the carboxy-terminal half of the protein. Based on experiments described below, we termed this novel SR-related protein SRrp53. Good candidate orthologues for mouse SRrp53 exist in vertebrates such as Homo sapiens and Xenopus laevis (Fig. 1B) but seem to be absent in several other organisms, including fully sequenced organisms such as Drosophila melanogaster, Caenorhabditis elegans, Arabidopsis thaliana, Saccharomyces cerevisiae, and Schizosaccharomyces pombe (data not shown).
FIG. 1.
(A) Amino acid sequence of mouse wild-type SRrp53 is shown. The RS domain is in white with a black background. (B) Human SRrp53 is shown aligned by the ClustalW method with the corresponding proteins from Mus musculus (mm), Rattus norvegicus (rat), Gallus gallus (chicken [gg]), X. laevis (xl), and Oryzias latipes (medaka fish [ol]). Identical residues are shown on a black background, residues conserved in 80% of the analyzed sequences are shown in a dark grey background, and residues conserved in 60% of the analyzed sequences are shown in a light grey background. Gaps are shown by dashed lines.
SRrp53 subcellular localization and nucleocytoplasmic shuttling.
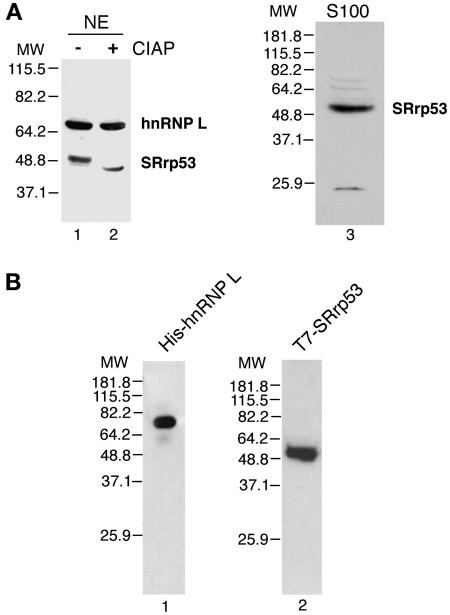
Northern blot analysis showed that SRrp53 is widely yet differentially expressed across a variety of adult mouse tissues (data not shown). We generated antibodies against a C-terminal peptide that is conserved between the human and mouse protein. Western blot analysis with this peptide antibody detected a band that migrates with an apparent molecular mass of 53 kDa in nuclear and S100 extracts (Fig. 2A). A band of higher molecular mass migrating at approximately 66 kDa was also observed. Both bands were immunoprecipitated with the anti-SRrp53 antibody, and mass spectrometry analysis of the 66-kDa band eluted from a gel identified this slower migrating protein as hnRNP L (46). The identity of SRrp53 was also confirmed by mass spectrometry analysis (data not shown). The deduced amino acid sequence of SRrp53 predicted a protein of 334 amino acids with a calculated molecular mass of approximately 38.66 kDa. Presumably, potential phosphorylation of the serine residues in the RS domain of the protein accounts for its anomalous migration at 53 kDa on SDS-PAGE. Similar disparities between predicted molecular mass and observed migration on SDS-PAGE have been reported for other RS domain-containing proteins, including SRrp86 and SRp75 (3, 70). The phosphorylation of SRrp53 was confirmed by treatment of nuclear extracts with CIAP and Western blot analysis of the treated extracts (Fig. 2A). Western blot analysis of recombinant His-hnRNP L purified from baculovirus-infected cells and recombinant T7-SRrp53 purified from 293T cells also showed that the antibodies raised against the synthetic peptide can recognize both proteins (Fig. 2B). Taken together, these results indicate that the α-SRrp53 antibody recognizes SRrp53 and hnRNP L.
FIG. 2.
Characterization of SRrp53 by Western blot analysis. (A) The amounts of 10 μg of nuclear extract (lane 1), 10 μg of CIAP-treated nuclear extract (lane 2), and 50 μg of S100 extract (lane 3) were analyzed by Western blotting with anti-SRrp53-B4 (fourth bleed) antibody. Protein size markers (molecular weights [MW]) are indicated to the left. (B) The amounts of 20 ng of recombinant His-hnRNP L purified form baculovirus-infected Sf9 cells (lane 1) and 20 ng of T7-SRrp53 purified from 293T cells (lane 2) were analyzed as described for panel A. Protein size markers are indicated to the left.
Localization of the β-geo-SRrp53 fusion protein expressed in the gene-trapped mouse ES cells indicated that endogenous SRrp53 could localize to the nuclear speckles (data not shown). We showed that a GFP-tagged version of full-length SRrp53 localized exclusively to the nucleus, with a typical speckled pattern in transiently transfected HeLa cells. Furthermore, GFP-SRrp53 colocalizes with the SR protein SC35 in nuclear speckles (Fig. 3A). Identical results were obtained when HeLa cells were transfected with a construct expressing a T7 epitope-tagged version of wild-type SRrp53 (data not shown). Arrest of transcription by RNA polymerase II inhibitors, such as actinomycin D, causes speckles to become enlarged and rounded up (reviewed by Spector [58]). In the presence of actinomycin D, both the endogenous and transiently transfected SRrp53s relocalized to larger and fewer speckles, which is typical of splicing factors (Fig. 3B and data not shown).
FIG. 3.
(A) HeLa cells were transfected with a plasmid encoding GFP-SRrp53 and fixed 24 h posttransfection. Endogenous SC35 was detected by indirect immunofluorescence with an anti-SC35 MAb and fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Images were superimposed to reveal sites of overlap in yellow (Merge). (B) Effect of transcription inhibition on the subcellular localization of SRrp53. HeLa cells were transfected with a plasmid encoding T7-SRrp53; cells were then incubated with actinomycin D (+AcTD) plus cycloheximide for 3 h and fixed at 24 h posttransfection. The localization of the expressed proteins was determined by indirect immunofluorescence with anti-T7 MAb and FITC-conjugated secondary antibody. (C) Analysis of nucleocytoplasmic shuttling of SRrp53 by transient expression in interspecies heterokaryons. The SRrp53 protein was transiently expressed in HeLa cells, and 24 h posttransfection, the cells were treated with cycloheximide and subsequently fused with mouse NIH 3T3 cells in the presence of polyethylene glycol to form heterokaryons. The cells were further incubated for 2 h in the presence of cycloheximide, followed by fixation. The localization of the epitope-tagged proteins in the heterokaryons was determined by indirect immunofluorescence with anti-T7 MAb and FITC-conjugated secondary antibody (left panel). The cells were simultaneously incubated with DAPI for differential staining of human and mouse nuclei within heterokaryons (center panel). The arrows indicate the mouse nuclei within human-mouse heterokaryons. Phase-contrast images of the same heterokaryons are shown (right panel).
It has been shown that many nuclear proteins involved in RNA metabolism, such as hnRNP proteins (17) and a subset of SR proteins (11), shuttle between the nucleus and the cytoplasm. We used transient transfection in conjunction with interspecies heterokaryons to assay for the nucleocytoplasmic shuttling of SRrp53 (Fig. 3C). HeLa cells were transfected with an epitope-tagged SRrp53 cDNA and then were fused to mouse NIH 3T3 cells to form heterokaryons. Prior to fusion, the cells were treated with cycloheximide so that no further protein synthesis could take place in the heterokaryons. At 2 h postfusion, the cells were fixed and stained to examine the distribution of the tagged proteins by immunofluorescence microscopy with a MAb directed against the epitope tag. To distinguish the human and mouse nuclei, the cells were stained with DAPI (4′,6′-diamidino-2-phenylindole), which gives a characteristic staining of intranuclear bodies in the mouse nuclei. Detection of the tagged protein—originally expressed in the human cells—within the mouse nuclei in the heterokaryons is indicative of shuttling (and therefore of nuclear export). Analysis of heterokaryons formed between human cells expressing T7-tagged SRrp53 and mouse acceptor cells clearly showed that the epitope-tagged SRrp53 was detectable in both nuclei (Fig. 3C). These data indicate that SRrp53 shuttles between the nucleus and the cytoplasm, suggesting that this protein could have a role in postsplicing mRNA processing.
SRrp53 interacts with splicing factors.
In order to identify interacting proteins, yeast two-hybrid screens were carried out with the full-length sequence of SRrp53 as bait. In support for a role of SRrp53 in pre-mRNA splicing, we identified Luc7p and HCC1 as interacting proteins (Fig. 4A and B). An interaction with the DNA binding protein SON, which contains a stretch of RS dipeptides similar to the ones identified in SR proteins (68), was also detected. We confirmed these two-hybrid interactions by testing that SRrp53 used as a bait directly interacted with HCC1 and that Luc7p and SON fused to an activation domain (Fig. 4A).
FIG. 4.
SRrp53 interacts with RS-domain-containing proteins in a yeast two-hybrid system. (A) Reconstitution of two-hybrid interactions found in a yeast two-hybrid screen. The center panel shows growth on selective media without adenine, and the right panel shows activation of the MEL1 reporter. In each panel, yeast cells were cotransformed with each of the prey plasmids (pGAD) isolated from the screen (indicated above the gels) and bait vectors expressing either full-length SRrp53 (first row), the N-terminal half of the protein comprising the RS domain (second row), the C-terminal half of the protein (third row), or the empty vector (fourth row). (B) A diagram illustrating the structure of each interactor. The portions of each protein covered by selected clones are indicated by bars (with the N- and C-terminal amino acid numbers shown). (C) Interactions between wild-type or mutant versions of SRrp53 and different splicing factors. Yeast cells were cotransformed with prey plasmids encoding different splicing factors (indicated above) with bait vectors expressing either full-length SRrp53 (first row), the N-terminal half of the protein comprising the RS domain (second row), the C-terminal half of the protein (third row), or the empty vector (fourth row) and were tested for their ability to grow in the absence of adenine. In all cases, activation of the ADE3 reporter correlated with activation of the MEL1 reporter (data not shown).
The LUC7 gene was identified in yeast in a synthetic lethal screen with the nuclear cap binding complex (CBC) and was shown to be a component of the U1 snRNP and to be involved in 5′site selection (19). The Luc7 gene seems to have undergone duplications several times during evolution, and three Luc7 genes (Luc7A, -B, and -C) were present in the human genome. Interestingly, human Luc7p does not seem to be a stable U1 snRNP component in humans (28). Human Luc7A/CROP (for cisplatin resistance-associated overexpressed protein) was isolated as an overexpressed gene in a cisplatin-resistant cell line (43); it contained arginine-serine and arginine-glutamic acid (RE) repeat sequences characteristic of SR and SR-related proteins and two zinc finger motifs (Zn). HCC1, a 64-kDa nuclear autoantigen that harbors splicing factor motifs, was originally identified with an antibody from a hepatocellular carcinoma patient (32). It comprises two related proteins, most likely generated by alternative splicing, termed HCC1.3 and HCC1.4, which differ by 6 amino acids. HCC1 localizes to the nuclear speckles and contains an N-terminal RS domain and three RRMs. The modular structure of HCC1 and the presence of an octapeptide sequence, the RS-ERK motif, within its RS domain is reminiscent of the large subunit of the U2AF splicing factor U2AF65.
All the identified interacting proteins contained an RS domain, and in every case, the portion of the interacting protein that was expressed as a fusion with the activating domain of the GAL4 transcription factor contained at least part of this domain (Fig. 4). This fact suggested that the interactions observed were mediated directly by these RS domains. We show that, except for HCC1, all the other identified factors showed positive interaction with the RS domain of SRrp53 (Fig. 4A). Surprisingly, the C terminus of SRrp53 seemed to mediate the interaction with HCC1. This is reminiscent of the interaction described between U2AF65 and U2AF35, which is not mediated by their RS domains (72).
A group of RS domain-containing proteins that have a well-characterized role in splicing was tested for their ability to interact with SRrp53. The SR proteins SF2/ASF and SRp40 were able to interact with full-length SRrp53 (Fig. 4C). These interactions seemed to be mediated by their RS domain, since both SF2/ASF and SRp40 interact with the N terminus of SRrp53 but not with its C terminus. In contrast, no interaction was detected with SRp55, SRp20, 9G8, or SRp30c. These data indicate that SRrp53 has specificity for SF2/ASF and SRp40 RS domains and that it does not simply bind to any RS domain. SRrp53 also interacts with the small subunit of the U2AF splicing factor, U2AF35, and this interaction is mediated by its RS domain. In contrast, no interactions were detected with the large subunit, U2AF65, nor with the U1 snRNP-specific U1-70K protein or the splicing factor SF1 (Fig. 4C). Furthermore, the fact that SRrp53 interacts with U2AF35 suggests a possible role for this novel SR-related protein in 3′ splice site selection.
SRrp53 interacts with Luc7p and with HCC1 in cultured cells.
We confirmed the two-hybrid interactions of SRrp53 with Luc7p, SF2/ASF, and HCC1 by coimmunoprecipitation experiments from human 293 cell extracts. An epitope-tagged T7-hLuc7A was transiently expressed in 293T cells, and extracts immunoprecipitated with a MAb against the T7 tag were revealed with an anti-SRrp53 antibody. As shown in Fig. 5A, hLuc7A was able to pull down SRrp53. Furthermore, this interaction is not RNA-mediated, since treatment with RNase decreased, but did not abolish, this interaction. Furthermore, SF2/ASF was detected in an immunoprecipitate from 293T cells with anti-SRrp53 antibodies (Fig. 5B). Again, RNA did not mediate this interaction. Finally, HCC1 was also detected in immunoprecipitate with α-SRrp53 antibodies, even when the immunoprecipitate was treated with RNase, suggesting that this interaction is not mediated by RNA (Fig. 5C). Taken together, these results confirm the interaction data obtained with the yeast two-hybrid system and conclusively demonstrate that this novel SR-related protein functionally interacts with splicing factors.
FIG. 5.
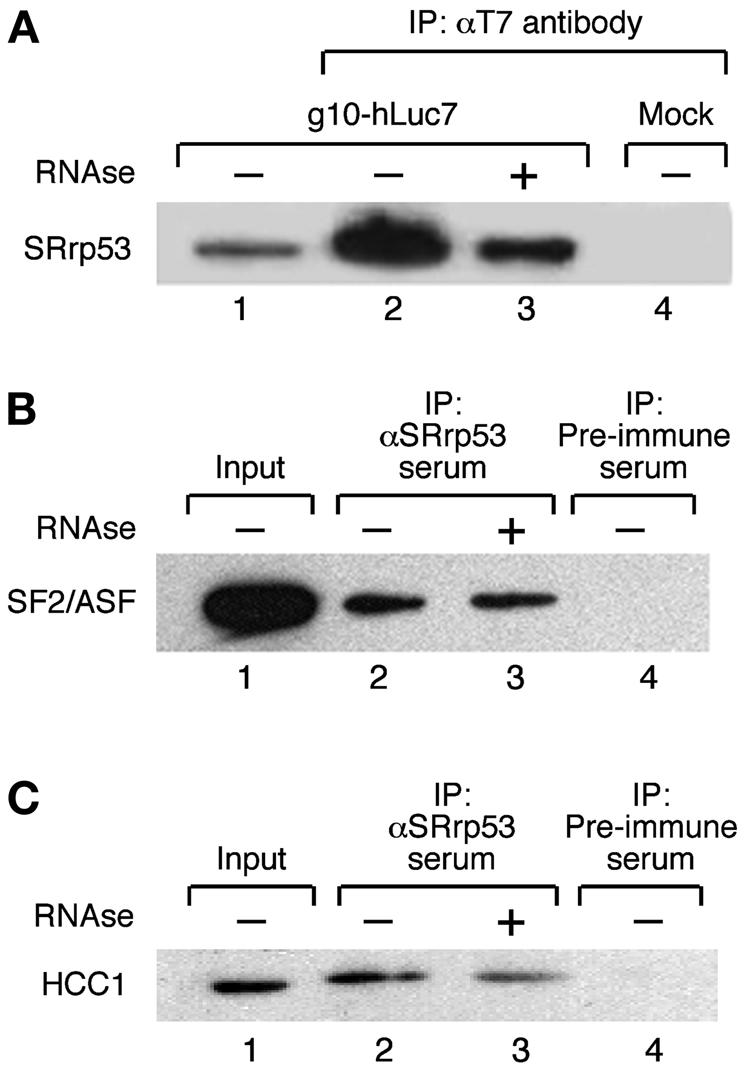
SRrp53 interacts with splicing factors in cultured mammalian cells. (A) IP assays with T7-hLuc7a are shown. Extracts prepared from 293T cells either transiently transfected with a plasmid encoding T7-hLuc7a (lanes 2 and 3) or mock transfected (lane 4) were incubated with anti-T7 antibody bound to Sepharose beads (Novagen). The bound proteins were separated on an SDS-10% polyacrylamide gel and analyzed by Western blotting with anti-SRrp53B4 antibody. Alternatively, the immunoprecipitate was treated with RNase before loading on the gel (lane 3). Lane 1 was loaded with 2% of the amount of extract used for each IP. (B) Extracts prepared from 293T cells were incubated with either anti-SRrp53B4 antibody (lanes 2 and 3) or preimmune serum (lane 4) bound to Sepharose beads and analyzed as described for panel A. The blot was probed with MAb 96 antibody, which recognizes SF2/ASF (25). Alternatively, the immunoprecipitate was treated with RNase before loading on the gel (lane 3). Lane 1 was loaded with 2% of the amount of extract used for each IP. (C) The interaction between SRrp53 and HCC1 was analyzed as described for panel B. The blot was probed with a polyclonal antibody raised in sheep, which specifically recognizes HCC1.
SRrp53 is required for the second catalytic step of pre-mRNA splicing.
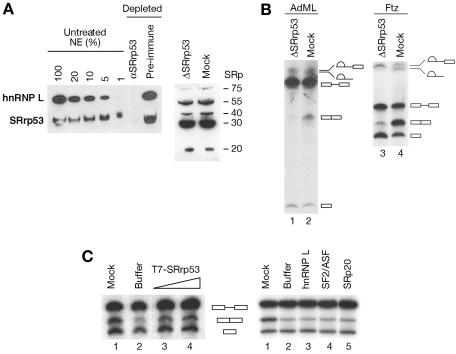
Nuclear extracts were depleted of SRrp53 with specific antibodies and then tested for the ability to splice AdML- and Ftz-derived pre-mRNA substrates. Following two rounds of depletion with a specific antibody at 1 M KCl, the concentration of SRrp53 in the extract was reduced by approximately 99%, whereas it was not significantly depleted with the preimmune antibody (Fig. 6A, left panel). The hnRNP L protein that cross-reacts with anti-SRrp53 antibodies was codepleted with SRrp53 from these extracts. Moreover, the relative amount of individual SR proteins remains unchanged in HeLa extracts immunodepleted of SRrp53, as revealed by Western blot analysis using MAb 104 (Fig. 6A, right panel). We found that treatment with preimmune serum results in a relative accumulation of intermediate products and a lower yield of spliced product compared to the untreated extracts. These effects may, however, be related to nonspecific (e.g., dilution) effects of the depletion procedure. Nevertheless, preimmune depleted extracts were able to carry out splicing, as it was revealed by the accumulation of spliced product. In contrast, no accumulation of spliced product was observed with SRrp53-depleted extracts with both substrates analyzed, indicating that this protein is essential for splicing (Fig. 6B). The second step of the splicing reaction, which generates released intron-lariat and ligated exons, was preferentially affected by SRrp53 depletion under these conditions, indicating that this protein plays a role in this step. More importantly, addition of recombinant SRrp53 to the immunodepleted HeLa extract restored splicing activity (Fig. 6C, left panel). By contrast, complementation of the depleted extracts with recombinant hnRNP L, the cross-reacting band observed on panel A, did not restore splicing activity (Fig. 6C, right panel), in accordance with previous results, indicating that hnRNP L has no activity in constitutive splicing (31). Furthermore, individual SR proteins that interact with SRrp53 (SF2/ASF) or that show no interaction (SRp20) were not able to complement an extract lacking SRrp53 (Fig. 6C, right panel). Taken together, these results show that the block in the second step of splicing is due to the removal of SRrp53, demonstrating that SRrp53 is a second-step splicing factor.
FIG. 6.
Effect of SRrp53 immunodepletion on pre-mRNA splicing in vitro. (A) The extent of SRrp53 depletion from HeLa nuclear extracts (NE) was assayed by Western blot analyses with an anti-SRrp53 antibody in serial dilutions of untreated extracts (lanes 1 through 5), extracts depleted with anti-SRrp53B4 antibody (lane 6), or preimmune-serum-depleted extracts (lane 7) (left panel). The profile of SR proteins was not affected by SRrp53 immunodepletion (right panel). (B) Effect of immunodepletion of SRrp53 on pre-mRNA splicing is shown. AdML pre-mRNA (lanes 1 and 2) or Ftz pre-mRNA (lanes 3 and 4) was incubated in depleted (lanes 1 and 3) or mock-depleted (lanes 2 and 4) nuclear extracts. Positions of splicing intermediates and products are indicated to the right. (C) Ftz pre-mRNA substrate was incubated in mock-depleted (lane 1) or SRrp53-depleted (lanes 2 through 4) nuclear extracts complemented with buffer (lanes 1 and 2) or 100 ng (lane 3) or 300 ng (lane 4) of T7-SRrp53 (left panel). Positions of splicing intermediates and products are indicated to the right. Ftz pre-mRNA substrate was incubated in mock-depleted (lane 1) or SRrp53-depleted (lanes 2 through 5) nuclear extracts complemented with buffer (lanes 1 and 2), hnRNP L (lane 3), SF2/ASF (lane 4), or SRp20 (lane 5) (right panel).
SRrp53 can regulate alternative splicing in vivo.
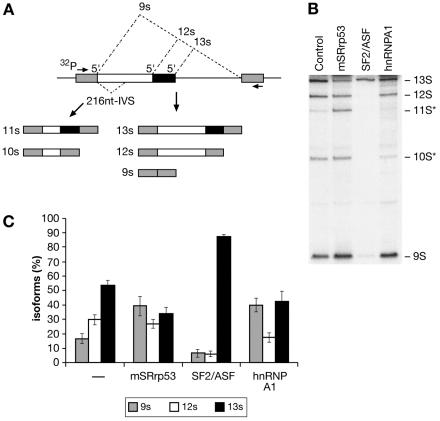
We sought to investigate the role of SRrp53 in the regulation of alternative splicing in vivo. To this end, we overexpressed epitope-tagged SRrp53 and assayed changes in the patterns of alternative splicing of an adenovirus E1A splicing pre-mRNA that undergoes alternative splicing through the use of three alternative 5′ splice sites. In agreement with previous results, wild-type SF2/ASF strongly activated the 13S 5′ splice site (12, 65, 66). In contrast, increased expression of SRrp53 resulted in activation of the most distal 5′ splice site, giving rise to the 9S isoform, as observed for hnRNP A1 (12, 69). However, the strongest effect of SRrp53 overexpression on E1A alternative splicing was in the production of the 11S isoform, which involves selection of the most 5′-proximal 13S splice site and the removal of an additional 216-nucleotide intron located downstream of the 9S splice site (Fig. 7A,B). This splicing event requires the activation of a weak 3′ splice site (22); thus, SRrp53 may be influencing 3′ splice site activation. Interestingly, it was shown that SF2/ASF, and also SC35, binds to a bidirectional purine-rich splicing enhancer located just upstream of the 12S 5′ splice site and activate the 216-nucleotide intron 3′ splice site (9). Quantitation of the relative use of the E1A splice sites upon overexpression of the different proteins is shown in Fig. 7C. As described above, SRrp53 interacts with a subset of SR proteins, such as SF2/ASF and SRp40. Therefore, the selection of the distal 9S 5′ distal splice site by SRrp53 could result from the repression of the activity of SR proteins, such as SF2/ASF, which promotes the use of the proximal alternative 5′ splice site. Furthermore, the appearance of the 11S isoform could reflect the activity of SRrp53 in 3′ splice site selection, in this case, cooperating with the interacting protein SF2/ASF.
FIG. 7.
SRrp53 can regulate 5′ splice site selection in vivo. In vivo splicing analyses were performed with HeLa cells transiently cotransfected with an adenovirus E1A reporter plasmid and expression plasmids encoding for either mouse SRrp53, hnRNP A1 or SF2/ASF. (A) Diagram of the E1A reporter gene is shown. The alternative 5′ splice sites and splicing events that generates 13S, 12S, and 9S mRNAs are shown schematically. The location of the exon primers used for RT-PCR analysis is shown. (B) HeLa cells were transiently cotransfected with the adenovirus E1A reporter plasmid and the expression constructs for each of the proteins indicated above the gel or the parental plasmid (Control). RNA was harvested at 24 h posttransfection and analyzed by RT-PCR with a labeled forward primer, denaturing PAGE, and autoradiography as described in Materials and Methods. The positions of the unspliced pre-mRNA and of 13S, 12S, and 9S spliced mRNAs are indicated to the right. The 10S and 11S isoforms (*) did not arise from competition between alternative 5′ splice sites. (C) Quantitation of E1A mRNA isoforms in transfected cells is shown. The relative amounts of 13S, 12S, and 9S E1A mRNAs were calculated from the data given in panel B by using a phosphorimager, and the percentage of each isoform is shown. Each experiment was repeated four times, and the data represent averages, with bars indicating standard errors.
Taken together, these results suggest that SRrp53 is a novel SR-related protein that has a role in both constitutive and alternative splicing.
DISCUSSION
Using a gene trap approach, we have identified a novel RS-containing protein that localizes to the nuclear speckles. Three independent avenues suggest that SRrp53 has a role in pre-mRNA splicing. First, the presence of an RS domain, which is diagnostic of factors involved in pre-mRNA splicing, and more generally in RNA processing, lends support to this conclusion. Second, two-hybrid analysis and IP-Western assays have shown that SRrp53 interacts with different proteins that have either potential or clearly defined functions in splicing. Third, by using in vivo and in vitro splicing assays, SRrp53 was shown to be functionally involved in splicing. Furthermore, nuclear extracts immunodepleted of this protein are unable to perform the second step of splicing in vitro, suggesting that this protein is necessary for this step.
The development of protocols that allowed an improved purification of spliceosomes coupled with advances in mass spectrometry analyses of complex mixtures has allowed the identification of the protein composition of the spliceosome (34 and references therein). Interestingly, SRrp53 is not present in any of these preparations, and together with its loose association with snRNP particles (data not shown), it is suggestive of a transient association of this SR-like protein with the splicing machinery.
Expression and subcellular localization of SRrp53.
The β-geo-SRrp53 fusion protein localized to nuclear speckles in the gene-trapped mouse ES cell (data not shown), and a transiently expressed epitope-tagged version of SRrp53 confirmed this localization and also showed colocalization with SC35 in splicing speckles. This is not surprising, since the RS domain of certain SR proteins has been shown to be necessary and sufficient for targeting these factors to nuclear speckles (10, 27, 39). Several proteins that participate in pre-mRNA splicing in the nucleus have been shown to travel to the cytoplasm with the spliced mRNA. For some of them, cytoplasmic functions have been described. For instance, members of the hnRNP family of proteins have been reported to regulate cytoplasmic events like mRNA localization, translation, and mRNA turnover (reviewed in reference 57). We have previously demonstrated that a subset of SR proteins shuttle continuously from the nucleus to the cytoplasm, an activity which is reminiscent of what is observed with certain hnRNP proteins (11, 17). Thus, shuttling SR proteins may have roles not only in nuclear pre-mRNA splicing but also in mRNA transport and/or in some cytoplasmic events. Indeed, shuttling SR proteins have been shown to facilitate mRNA export (30), to mediate RNA stability (38), and to be involved in translational regulation (51). Moreover, SR proteins have been found to strongly enhance nonsense-mediated decay, and this activity requires the presence of the RS domain but des not correlate with nucleocytoplasmic shuttling (75). The finding that this novel SR-related protein, SRrp53, shuttles continuously from the nucleus to the cytoplasm suggests that it may also be involved in cytoplasmic functions.
The role of SRrp53 in pre-mRNA splicing.
The RS domain is a distinctive feature of SR family proteins and is also present in SR-related proteins. RS domains have been implicated in both protein-protein and protein-RNA interactions (67); reviewed in reference 24). We have shown that the RS domain of SRrp53 mediates protein-protein interactions with other RS-domain-containing proteins in a yeast two-hybrid assay and in cultured mammalian cells. However, this protein-protein interaction profile is different from that reported for other RS-domain-containing proteins. For example, SF2/ASF and SC35 can both interact with U1-70K and U2AF35 but not with U2AF65 (67). In contrast, the SR protein p54 interacts with SF2/ASF and U2AF65 but shows no interaction with either U1-70K or U2AF35 (73). Similarly, Sip1, an SR-like protein initially identified in a two-hybrid screen with SC35, interacts with several SR proteins but also with U1-70K and U2AF65, proteins that are associated with 5′ and 3′ splice sites, respectively (74). Here, we show that SRrp53 interacts with SF2/ASF and U2AF35 but does not interact with U1-70K or U2AF65. This finding suggest that SRrp53 is not directly mediating interactions between components at the 5′ and 3′ splice sites but rather interacts with components of the 3′ splice site, suggesting that it might have a role in 3′ splice site selection. Moreover, these RS-mediated interactions showed a certain degree of specificity, since SRrp53 interacts only with two out of six members of the SR family of proteins that were tested. Interestingly, SRrp53 also interacts with the U2AF65-related factor HCC1 in the yeast two-hybrid system and in cell extracts. Although a role for HCC1 in constitutive splicing is unclear, it can be speculated that its function could be similar to that of U2AF65, since it is structurally related to this factor (32). If this were the case, SRrp53 could facilitate the interaction between U2AF35 and HCC1. Moreover, several factors related to U2AF35 have been characterized in mammalian cells (54, 63), suggesting the existence of multiple U2AF-like complexes.
A role for SRrp53 in the second step of pre-mRNA splicing.
During the second step of pre-mRNA splicing, the first exon and lariat intermediate are joined to form the mRNA. In yeast, four proteins have been shown to function exclusively in the second step of pre-mRNA splicing, namely, Prp16, Prp17, Prp18, and Slu7, whereas Prp8 functions in both steps of the splicing reaction (reviewed by Umen and Guthrie [64]). Although most mechanistic studies dealing with the second step of splicing have been carried out in yeast, human homologs of the second step factors Prp16, Prp17, and Prp18 have been functionally characterized (4, 29, 40, 76). Prp22, a member of the DExH-box family of proteins, is involved in the second step of splicing and also plays a role in the release of spliced mRNA (52). Interestingly, the human homolog of Prp22, designated HRH1 (for human RNA helicase 1), contains an RS domain and was shown to interact with members of the SR protein family through its RS domain (44). Human Slu7 is a second-step splicing factor that functions by restructuring the spliceosome between the catalytic steps of splicing (13, 14).
Here, we show that HeLa nuclear extracts depleted of SRrp53 are unable to perform the second step of splicing. A 66-kDa protein that cross-reacts with anti-SRrp53 antibodies was identified as hnRNP L and was codepleted with SRrp53 from these extracts. However, we were able to restore splicing activity by complementing the depleted extracts with recombinant SRrp53. It had already been shown that extracts immunodepleted of hnRNP L were active in general pre-mRNA splicing (31). This experiment clearly shows that the block in the second step of splicing was due to the removal of SRrp53. Thus, this novel SR-related protein that interacts with factors involved in 3′ splice site selection has a clear role in the second step of pre-mRNA splicing. It remains possible that the transient association of SRrp53 with the spliceosome facilitates the remodeling of the spliceosome between steps I and II. Work from different labs indicates that the 3′ splice site is recognized de novo before the second step of splicing takes place (references 37 and 64 and references therein). Considering the interactions observed between SRp53 and U2AF35 and between SRrp53 and HCC1, a U2AF65-like factor, it is tempting to speculate a role for SRrp53 in the recognition of the 3′ splice site during the second step of splicing. Further experimentation will be required to test this hypothesis.
SRrp53 regulates alternative splicing.
We have also shown that SRrp53 regulates alternative splicing in vivo. It promotes selection of the distal 5′ splice site in the adenovirus E1A pre-mRNA, resembling the activity shown by hnRNP A1 but more importantly, it also promotes the appearance of the 11S isoform. It is possible that the effect of SRrp53 in the selection of the distal 5′ splice site in the E1A pre-mRNA results from the titration of interacting SR proteins, such as SF2/ASF and SRp40. Furthermore, the appearance of the 11S isoform could reflect the activity of SRrp53 in 3′ splice site selection, in this case, cooperating with the interacting proteins SF2/ASF, SRp40, and U2AF35.
In summary, we present here the functional characterization of a novel SR-related protein that is required for the second step of pre-mRNA splicing and is also involved in alternative splicing regulation.
Acknowledgments
We thank Colin Semple and Philippe Gautier (MRC HGU) for help with bioinformatic analysis. We are grateful to Rachel Davies and Nick Hastie for the constructs used in two-hybrid analysis. We also thank Albrecht Bindereif for the hnRNP L expression vector. Finally, we are grateful to David S. Horowitz (Bethesda, Md.) for critical reading of the manuscript and Sonia Guil and Jeremy R. Sanford for discussions.
This work was supported by the Medical Research Council.
REFERENCES
- 1.Apweiler, R., T. K. Attwood, A. Bairoch, A. Bateman, E. Birney, M. Biswas, P. Bucher, L. Cerutti, F. Corpet, M. D. Croning, R. Durbin, L. Falquet, W. Fleischmann, J. Gouzy, H. Hermjakob, N. Hulo, I. Jonassen, D. Kahn, A. Kanapin, Y. Karavidopoulou, R. Lopez, B. Marx, N. J. Mulder, T. M. Oinn, M. Pagni, F. Servant, C. J. Sigrist, and E. M. Zdobnov for the InterPro Consortium. 2000. InterPro—an integrated documentation resource for protein families, domains and functional sites. Bioinformatics 16:1145-1150. [DOI] [PubMed] [Google Scholar]
- 2.Barnard, D. C., J. Li, R. Peng, and J. G. Patton. 2002. Regulation of alternative splicing by SRrp86 through coactivation and repression of specific SR proteins. RNA 8:526-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard, D. C., and J. G. Patton. 2000. Identification and characterization of a novel serine-arginine-rich splicing regulatory protein. Mol. Cell. Biol. 20:3049-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben Yehuda, S., I. Dix, C. S. Russell, S. Levy, J. D. Beggs, and M. Kupiec. 1998. Identification and functional analysis of hPRP17, the human homologue of the PRP17/CDC40 yeast gene involved in splicing and cell cycle control. RNA 4:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blencowe, B. J. 2000. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 25:106-110. [DOI] [PubMed] [Google Scholar]
- 6.Blencowe, B. J., J. A. Bowman, S. McCracken, and E. Rosonina. 1999. SR-related proteins and the processing of messenger RNA precursors. Biochem. Cell Biol. 77:277-291. [PubMed] [Google Scholar]
- 7.Blencowe, B. J., R. Issner, J. A. Nickerson, and P. A. Sharp. 1998. A coactivator of pre-mRNA splicing. Genes Dev. 12:996-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher, L., C. A. Ouzounis, A. J. Enright, and B. J. Blencowe. 2001. A genome-wide survey of RS domain proteins. RNA 7:1693-1701. [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgeois, C. F., M. Popielarz, G. Hildwein, and J. Stevenin. 1999. Identification of a bidirectional splicing enhancer: differential involvement of SR proteins in 5′ or 3′ splice site activation. Mol. Cell. Biol. 19:7347-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caceres, J. F., T. Misteli, G. R. Screaton, D. L. Spector, and A. R. Krainer. 1997. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J. Cell Biol. 138:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caceres, J. F., G. R. Screaton, and A. R. Krainer. 1998. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 12:55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caceres, J. F., S. Stamm, D. M. Helfman, and A. R. Krainer. 1994. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science 265:1706-1709. [DOI] [PubMed] [Google Scholar]
- 13.Chua, K., and R. Reed. 1999. Human step II splicing factor hSlu7 functions in restructuring the spliceosome between the catalytic steps of splicing. Genes Dev. 13:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua, K., and R. Reed. 1999. The RNA splicing factor hSlu7 is required for correct 3′ splice-site choice. Nature 402:207-210. [DOI] [PubMed] [Google Scholar]
- 15.Cowper, A. E., J. F. Caceres, A. Mayeda, and G. R. Screaton. 2001. Serine-arginine (SR) protein-like factors that antagonize authentic SR proteins and regulate alternative splicing. J. Biol. Chem. 276:48908-48914. [DOI] [PubMed] [Google Scholar]
- 16.Davies, R. C., C. Calvio, E. Bratt, S. H. Larsson, A. I. Lamond, and N. D. Hastie. 1998. WT1 interacts with the splicing factor U2AF65 in an isoform-dependent manner and can be incorporated into spliceosomes. Genes Dev. 12:3217-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyfuss, G., M. J. Matunis, S. Pinol-Roma, and C. G. Burd. 1993. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 62:289-321. [DOI] [PubMed] [Google Scholar]
- 18.Eperon, I. C., D. C. Ireland, R. A. Smith, A. Mayeda, and A. R. Krainer. 1993. Pathways for selection of 5′ splice sites by U1 snRNPs and SF2/ASF. EMBO J. 12:3607-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortes, P., D. Bilbao-Cortes, M. Fornerod, G. Rigaut, W. Raymond, B. Seraphin, and I. W. Mattaj. 1999. Luc7p, a novel yeast U1 snRNP protein with a role in 5′ splice site recognition. Genes Dev. 13:2425-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu, X. D. 1995. The superfamily of arginine/serine-rich splicing factors. RNA 1:663-680. [PMC free article] [PubMed] [Google Scholar]
- 21.Fu, X. D., and T. Maniatis. 1990. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343:437-441. [DOI] [PubMed] [Google Scholar]
- 22.Gattoni, R., P. Schmitt, and J. Stevenin. 1988. In vitro splicing of adenovirus E1A transcripts: characterization of novel reactions and of multiple branch points abnormally far from the 3′ splice site. Nucleic Acids Res. 16:2389-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gough, J., and C. Chothia. 2002. SUPERFAMILY: HMMs representing all proteins of known structure. SCOP sequence searches, alignments and genome assignments. Nucleic Acids Res. 30:268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graveley, B. R. 2000. Sorting out the complexity of SR protein functions. RNA 6:1197-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanamura, A., J. F. Caceres, A. Mayeda, B. R. Franza, Jr., and A. R. Krainer. 1998. Regulated tissue-specific expression of antagonistic pre-mRNA splicing factors. RNA 4:430-444. [PMC free article] [PubMed] [Google Scholar]
- 26.Hastings, M. L., and A. R. Krainer. 2001. Pre-mRNA splicing in the new millennium. Curr. Opin. Cell Biol. 13:302-309. [DOI] [PubMed] [Google Scholar]
- 27.Hedley, M. L., H. Amrein, and T. Maniatis. 1995. An amino acid sequence motif sufficient for subnuclear localization of an arginine/serine-rich splicing factor. Proc. Natl. Acad. Sci. USA 92:11524-11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochleitner, E. O., B. Kastner, T. Frohlich, A. Schmidt, R. Luhrmann, G. Arnold, and F. Lottspeich. 2005. Protein stoichiometry of a multiprotein complex, the human spliceosomal U1 small nuclear ribonucleoprotein: absolute quantification using isotope-coded tags and mass spectrometry. J. Biol. Chem. 280:2536-2542. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz, D. S., and A. R. Krainer. 1997. A human protein required for the second step of pre-mRNA splicing is functionally related to a yeast splicing factor. Genes Dev. 11:139-151. [DOI] [PubMed] [Google Scholar]
- 30.Huang, Y., R. Gattoni, J. Stevenin, and J. A. Steitz. 2003. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol. Cell 11:837-843. [DOI] [PubMed] [Google Scholar]
- 31.Hui, J., K. Stangl, W. S. Lane, and A. Bindereif. 2003. HnRNP L stimulates splicing of the eNOS gene by binding to variable-length CA repeats. Nat. Struct. Biol. 10:33-37. [DOI] [PubMed] [Google Scholar]
- 32.Imai, H., E. K. Chan, K. Kiyosawa, X. D. Fu, and E. M. Tan. 1993. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J. Clin. Investig. 92:2419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamison, S. F., Z. Pasman, J. Wang, C. Will, R. Luhrmann, J. L. Manley, and M. A. Garcia-Blanco. 1995. U1 snRNP-ASF/SF2 interaction and 5′ splice site recognition: characterization of required elements. Nucleic Acids Res. 23:3260-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jurica, M. S., and M. J. Moore. 2003. Pre-mRNA splicing: awash in a sea of proteins. Mol. Cell 12:5-14. [DOI] [PubMed] [Google Scholar]
- 35.Kohtz, J. D., S. F. Jamison, C. L. Will, P. Zuo, R. Luhrmann, M. A. Garcia-Blanco, and J. L. Manley. 1994. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature 368:119-124. [DOI] [PubMed] [Google Scholar]
- 36.Kramer, A. 1996. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu. Rev. Biochem. 65:367-409. [DOI] [PubMed] [Google Scholar]
- 37.Lallena, M. J., K. J. Chalmers, S. Llamazares, A. I. Lamond, and J. Valcarcel. 2002. Splicing regulation at the second catalytic step by Sex-lethal involves 3′ splice site recognition by SPF45. Cell 109:285-296. [DOI] [PubMed] [Google Scholar]
- 38.Lemaire, R., J. Prasad, T. Kashima, J. Gustafson, J. L. Manley, and R. Lafyatis. 2002. Stability of a PKCI-1-related mRNA is controlled by the splicing factor ASF/SF2: a novel function for SR proteins. Genes Dev. 16:594-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, H., and P. M. Bingham. 1991. Arginine/serine-rich domains of the su(wa) and tra RNA processing regulators target proteins to a subnuclear compartment implicated in splicing. Cell 67:335-342. [DOI] [PubMed] [Google Scholar]
- 40.Lindsey, L. A., and M. A. Garcia-Blanco. 1998. Functional conservation of the human homolog of the yeast pre-mRNA splicing factor Prp17p. J. Biol. Chem. 273:32771-32775. [DOI] [PubMed] [Google Scholar]
- 41.Makarova, O. V., E. M. Makarov, and R. Luhrmann. 2001. The 65 and 110 kDa SR-related proteins of the U4/U6 · U5 tri-snRNP are essential for the assembly of mature spliceosomes. EMBO J. 20:2553-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayeda, A., and A. R. Krainer. 1999. Mammalian in vitro splicing assays. Methods Mol. Biol. 118:315-321. [DOI] [PubMed] [Google Scholar]
- 43.Nishii, Y., M. Morishima, Y. Kakehi, K. Umehara, N. Kioka, Y. Terano, T. Amachi, and K. Ueda. 2000. CROP/Luc7A, a novel serine/arginine-rich nuclear protein, isolated from cisplatin-resistant cell line. FEBS Lett. 465:153-156. [DOI] [PubMed] [Google Scholar]
- 44.Ono, Y., M. Ohno, and Y. Shimura. 1994. Identification of a putative RNA helicase (HRH1), a human homolog of yeast Prp22. Mol. Cell. Biol. 14:7611-7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinol-Roma, S., and G. Dreyfuss. 1992. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature 355:730-732. [DOI] [PubMed] [Google Scholar]
- 46.Pinol-Roma, S., M. S. Swanson, J. G. Gall, and G. Dreyfuss. 1989. A novel heterogeneous nuclear RNP protein with a unique distribution on nascent transcripts. J. Cell Biol. 109:2575-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rio, D. C. 1988. Accurate and efficient pre-mRNA splicing in Drosophila cell-free extracts. Proc. Natl. Acad. Sci. USA 85:2904-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roscigno, R. F., and M. A. Garcia-Blanco. 1995. SR proteins escort the U4/U6. U5 tri-snRNP to the spliceosome. RNA 1:692-706. [PMC free article] [PubMed] [Google Scholar]
- 49.Roth, M. B., C. Murphy, and J. G. Gall. 1990. A monoclonal antibody that recognizes a phosphorylated epitope stains lampbrush chromosome loops and small granules in the amphibian germinal vesicle. J. Cell Biol. 111:2217-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanford, J. R., and J. P. Bruzik. 1999. SR proteins are required for nematode trans-splicing in vitro. RNA 5:918-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanford, J. R., N. K. Gray, K. Beckmann, and J. F. Caceres. 2004. A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 18:755-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwer, B., and C. H. Gross. 1998. Prp22, a DExH-box RNA helicase, plays two distinct roles in yeast pre-mRNA splicing. EMBO J. 17:2086-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen, H., J. L. Kan, and M. R. Green. 2004. Arginine-serine-rich domains bound at splicing enhancers contact the branchpoint to promote prespliceosome assembly. Mol. Cell 13:367-376. [DOI] [PubMed] [Google Scholar]
- 54.Shepard, J., M. Reick, S. Olson, and B. R. Graveley. 2002. Characterization of U2AF6, a splicing factor related to U2AF35. Mol. Cell. Biol. 22:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin, C., Y. Feng, and J. L. Manley. 2004. Dephosphorylated SRp38 acts as a splicing repressor in response to heat shock. Nature 427:553-558. [DOI] [PubMed] [Google Scholar]
- 56.Shin, C., and J. L. Manley. 2002. The SR protein SRp38 represses splicing in M phase cells. Cell 111:407-417. [DOI] [PubMed] [Google Scholar]
- 57.Shyu, A. B., and M. F. Wilkinson. 2000. The double lives of shuttling mRNA binding proteins. Cell 102:135-138. [DOI] [PubMed] [Google Scholar]
- 58.Spector, D. L. 1993. Nuclear organization of pre-mRNA processing. Curr. Opin. Cell Biol. 5:442-447. [DOI] [PubMed] [Google Scholar]
- 59.Staknis, D., and R. Reed. 1994. SR proteins promote the first specific recognition of Pre-mRNA and are present together with the U1 small nuclear ribonucleoprotein particle in a general splicing enhancer complex. Mol. Cell. Biol. 14:7670-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutherland, H. G., G. K. Mumford, K. Newton, L. V. Ford, R. Farrall, G. Dellaire, J. F. Caceres, and W. A. Bickmore. 2001. Large-scale identification of mammalian proteins localized to nuclear sub-compartments. Hum. Mol. Genet. 10:1995-2011. [DOI] [PubMed] [Google Scholar]
- 61.Tacke, R., and J. L. Manley. 1999. Determinants of SR protein specificity. Curr. Opin. Cell Biol. 11:358-362. [DOI] [PubMed] [Google Scholar]
- 62.Tate, P., M. Lee, S. Tweedie, W. C. Skarnes, and W. A. Bickmore. 1998. Capturing novel mouse genes encoding chromosomal and other nuclear proteins. J. Cell Sci. 111:2575-2585. [DOI] [PubMed] [Google Scholar]
- 63.Tronchere, H., J. Wang, and X. D. Fu. 1997. A protein related to splicing factor U2AF35 that interacts with U2AF65 and SR proteins in splicing of pre-mRNA. Nature 388:397-400. [DOI] [PubMed] [Google Scholar]
- 64.Umen, J. G., and C. Guthrie. 1995. The second catalytic step of pre-mRNA splicing. RNA 1:869-885. [PMC free article] [PubMed] [Google Scholar]
- 65.van der Houven van Oordt, W., K. Newton, G. R. Screaton, and J. F. Caceres. 2000. Role of SR protein modular domains in alternative splicing specificity in vivo. Nucleic Acids Res. 28:4822-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, J., and J. L. Manley. 1995. Overexpression of the SR proteins ASF/SF2 and SC35 influences alternative splicing in vivo in diverse ways. RNA 1:335-346. [PMC free article] [PubMed] [Google Scholar]
- 67.Wu, J. Y., and T. Maniatis. 1993. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75:1061-1070. [DOI] [PubMed] [Google Scholar]
- 68.Wynn, S. L., R. A. Fisher, C. Pagel, M. Price, Q. Y. Liu, I. M. Khan, P. Zammit, K. Dadrah, W. Mazrani, A. Kessling, J. S. Lee, and L. Buluwela. 2000. Organization and conservation of the GART/SON/DONSON locus in mouse and human genomes. Genomics 68:57-62. [DOI] [PubMed] [Google Scholar]
- 69.Yang, X., M. R. Bani, S. J. Lu, S. Rowan, Y. Ben-David, and B. Chabot. 1994. The A1 and A1B proteins of heterogeneous nuclear ribonucleoparticles modulate 5′ splice site selection in vivo. Proc. Natl. Acad. Sci. USA 91:6924-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zahler, A. M., K. M. Neugebauer, J. A. Stolk, and M. B. Roth. 1993. Human SR proteins and isolation of a cDNA encoding SRp75. Mol. Cell. Biol. 13:4023-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zerler, B., B. Moran, K. Maruyama, J. Moomaw, T. Grodzicker, and H. E. Ruley. 1986. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol. Cell Biol. 6:887-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, M., P. D. Zamore, M. Carmo-Fonseca, A. I. Lamond, and M. R. Green. 1992. Cloning and intracellular localization of the U2 small nuclear ribonucleoprotein auxiliary factor small subunit. Proc. Natl. Acad. Sci. USA 89:8769-8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, W.-J., and J. Y. Wu. 1996. Functional properties of p54, a novel SR protein active in constitutive and alternative splicing. Mol. Cell. Biol. 16:5400-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, W.-J., and J. Y. Wu. 1998. Sip1, a novel RS domain-containing protein essential for pre-mRNA splicing. Mol. Cell. Biol. 18:676-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, Z., and A. R. Krainer. 2004. Involvement of SR proteins in mRNA surveillance. Mol. Cell 16:597-607. [DOI] [PubMed] [Google Scholar]
- 76.Zhou, Z., and R. Reed. 1998. Human homologs of yeast prp16 and prp17 reveal conservation of the mechanism for catalytic step II of pre-mRNA splicing. EMBO J. 17:2095-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]