Abstract
Increasing evidence suggests that elevated intracellular levels of reactive oxygen species (ROS) play a significant role in the pathogenesis of many diseases. Increased intracellular levels of ROS can lead to the oxidation of lipids, DNA, and proteins, contributing to cellular damage. Hence, the maintenance of redox hemostasis is essential. Naringenin (NAR) is a flavonoid included in the flavanones subcategory. Various pharmacological actions have been ascribable to this phytochemical composition, including antioxidant, anti-inflammatory, antibacterial, antiviral, antitumor, antiadipogenic, neuro-, and cardio-protective activities. This review focused on the underlying mechanism responsible for the antioxidative stress properties of NAR and its' nanoformulations. Several lines of in vitro and in vivo investigations suggest the effects of NAR and its nanoformulation on their target cells via modulating signaling pathways. These nanoformulations include nanoemulsion, nanocarriers, solid lipid nanoparticles (SLN), and nanomicelle. This review also highlights several beneficial health effects of NAR nanoformulations on human diseases including brain disorders, cancer, rheumatoid arthritis, and small intestine injuries. Employing nanoformulation can improve the pharmacokinetic properties of NAR and consequently efficiency by reducing its limitations, such as low bioavailability. The protective effects of NAR and its’ nanoformulations against oxidative stress may be linked to the modulation of Nrf2-heme oxygenase-1, NO/cGMP/potassium channel, COX-2, NF-κB, AMPK/SIRT3, PI3K/Akt/mTOR, BDNF, NOX, and LOX-1 pathways. Understanding the mechanism behind the protective effects of NAR can facilitate drug development for the treatment of oxidative stress-related disorders.
Keywords: Naringenin, Nanoformulation, Nanotechnology, Redox mechanisms, Oxidative stress, Antioxidant
1. Introduction
The oxidative stress state is caused by an imbalance of oxygen reactive species (ROS; also called free radicals) and the intrinsic antioxidant defenses. The main members of the ROS family include free radicals of superoxide radicals (O2•−) and hydroxyl radicals (•OH) as well as non-radicals of singlet oxygen (1O2 and hydrogen peroxide (H2O2)) [1,2]. Several sources of ROS have been identified in the cell including xanthine oxidase (XO), cytochrome P450, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), mitochondrial electron transport chain, and uncoupling of nitric oxide synthase (NOS) [3]. ROS is also produced by arachidonic acid metabolism by lipoxygenases and cyclooxygenases (COX) [4]. Inflammation, ischemia, activation of immune cells, infection, cancer, intense exercise, and aging are the main endogenous sources of ROS. The exogenous sources of ROS include exposures to environmental pollutants, toxic metals (e.g., mercury, arsenic, copper lead, and cadmium), alcohol consumption, ultraviolet radiation, and cigarette smoking [2].
Under physiological states, ROS act as cell signaling molecules for normal cellular processes such as protein phosphorylation, cell differentiation, apoptosis, and activation of multiple transcriptional factors such as nuclear factor kappa B (NF-κB) transcriptional activity [2,5]. However, overproduction of ROS can induce oxidative stress and damage proteins, lipids, and DNA, which if unrepaired, results in cell death [6].
To prevent the deleterious impacts of ROS, cells have developed several defense strategies that include (a) the production of enzymatic antioxidant defense molecules including catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) (b) generation of non-enzymatic reducing molecules such as glutathione, vitamins A, C, and E [7]. Failure of these antioxidant defenses to properly neutralize ROS may lead to disease development.
A large body of evidence indicates that oxidative stress is a leading contributing factor in the development and progression of a variety of diseases including heart disease, neurodegenerative diseases (e.g. Parkinson's disease (PD), and Alzheimer's disease (AD)), cancers [8,9], and gastrointestinal diseases [10].
Interestingly, some epidemiological reports have shown an inverse correlation between flavonoid consumption and oxidation status [[11], [12], [13], [14]]. Flavonoids are naturally found in vegetables, fruits, and nuts, as well as in beverages such as tea, green tea, coffee, and red wine. These phytochemicals have several pharmacological activities, such as antioxidant, anticancer, anti-allergic, antimicrobial, and anti-inflammatory activity [15]. Flavonoids are classified into flavonols, flavones, isoflavones, flavanones, catechins, and anthocyanidins [15].
NAR, chemically named 2,3-dihydro-5,7- dihydroxy-2-(4- hydroxyphenyl)-4H-1-benzopyran-4-one, is part of the flavanones and can be found in large amounts in fruits such as grapefruit and oranges as well as vegetables. NAR is also included in tomatoes in low concentrations. Tomato skin contains NAR chalcone, which is altered to NAR during processing into tomato ketchup [16]. NAR has been used in cosmetic, perfumery, and pharmaceutical products. According to preclinical and clinical studies, NAR possesses a wide range of biological and pharmacological effects such as antioxidant, anti-inflammatory, immunomodulatory, anti-carcinogenic, anti-fibrogenic, nephro- and hepatoprotective properties [[17], [18], [19], [20], [21]]. Previous studies also support the benefits of NAR in the treatment of cancer, osteoporosis, and cardiovascular diseases. As well, NAR possesses lipid-lowering and insulin-like characteristics [15].
During the past decades, the body of evidence suggested that NAR may lower the risk of chronic diseases such as neurodegenerative diseases, cardiovascular diseases, and cancer [22], due to its anti-oxidant potential [[18], [19], [20]]. The flavonoid structure of NAR is responsible for its antioxidant activity. There are two main objectives in this review. First, to highlight the molecular mechanisms related to the antioxidant function of NAR, focusing on the issue of oxidative stress. At the end of this part, it will be determined by what mechanisms NAR plays a role in human health. The second is the study of nanoformulations of NAR. The study of nanoformulations of this compound is important from the point of view that it is known what kind of materials have been evaluated to make this compound nano, it is also known how the effects of each nanoformulation have been in modifying the properties of NAR. It should be noted that this review only presents findings from studies that have investigated the applications of NAR and its nanoformulations in diseases related to oxidative stress in vitro and in vivo.
2. Methodology
A literature search in PubMed, Scopus, ScienceDirect, and Google Scholar based on key words was mostly conducted in title, abstract, and key words to find relevant English language articles. The search was last performed in February 2023. Our search strategy covered terms related to nano-based formulation systems, ‘naringenin’ and ‘oxidative stress’ in one section. In the second section of the study, the terms ‘naringenin’, ‘oxidative stress’, ‘molecular mechanism’, and ‘signaling’ were considered. In the first step, the authors consisted of critically reading titles, keywords, and abstracts. In the second step, the full texts of the selected articles in the first step were assessed. The inclusion criteria were:
-
●
The study must include an in vitro or in vivo tests.
-
●
The study must use naringenin for the treatment (as a nanosystem or free naringenin).
-
●
The article must be in English.
Reviews, systematic reviews, observational studies, case reports, opinion papers, surveys, or letters were excluded.
3. Targeting oxidative stress in diseases
A wide range of evidence indicates that oxidative stress is involved, with varying degrees of importance, in the development and/or progression of multiple pathologies including metabolic disorders, cardiovascular diseases, diabetes, atherosclerosis, and cancer [23].
Free radicals, especially ROS, are well known to induce all forms of DNA damage, including base and sugar damage, base-free sites, and DNA–protein crosslinks. These oxidative DNA damages have been reported to be responsible for cancer onset [[24], [25], [26]]. Chronic inflammatory events, tobacco smoking, and exposure to air pollutants can enhance oxidative DNA damage, which in turn may contribute to an overall enhancement in cancer rates [24,27,28]. Lifestyle factors such as dietary fat intake have also been demonstrated to be associated with oxidative stress by increasing lipid peroxidation and thereby could play a significant role in tumor development [29,30]. In this regard, a significant relationship between dietary fat intake and cancer mortality rate has been reported in previous studies [29,30].
Cardiovascular diseases are a group of clinical phenotypes that are associated with many risk factors including high blood pressure, high cholesterol, unhealthy dietary patterns, and diabetes mellitus [[31], [32], [33]]. Stress oxidative is suggested to be a major cause of many cardiovascular diseases [34]. Oxidative stress in particular has been shown to trigger atherosclerosis, which is considered the principal etiopathogenic mechanism of cardiovascular diseases [35]. Findings from both ex vivo and in vivo studies have also reported the role of oxidative stress in the pathogenesis of congestive heart failure, hypertension, cardiomyopathy, and cardiac hypertrophy [29,[31], [32], [33]].
Oxidative stress has also been implicated in the pathogenesis of multiple neurological disorders including amyotrophic lateral sclerosis, depression, memory deficits, PD, AD, and multiple sclerosis [[36], [37], [38], [39]] Oxidative stress particularly has been demonstrated to induce the production of β-amyloid, the key peptide in the pathogenesis of AD, in the brain and thereby contribute, at least partly, to neurodegeneration seen in AD and the progression to dementia [38,39].
Oxidative stress is also linked to respiratory diseases, presumably in part through increasing inflammatory responses in the lungs [40,41]. A role of free radicals has also been shown in both onset and progression of rheumatoid arthritis and a protective and supportive role of antioxidant supplementation has been reported in patients with rheumatoid arthritis [42].
A relationship between oxidative stress and kidney diseases has been demonstrated in prior studies, mainly due to the role of aberrant ROS generation in inducing inflammatory responses resulting in further renal tissue injury and the development and progression of kidney disease. Moreover, it has been reported that certain drugs including gentamicin, tacrolimus, bleomycin, and cyclosporine have a high potential for nephrotoxicity mostly by enhancing oxidative stress through lipid peroxidation [[43], [44], [45], [46]]. Also, heavy metals (e.g., mercury, arsenic, copper lead, and cadmium), which are strong sources of oxidative stress, have been shown to contribute to nephropathy [47].
Oxidative stress was also found to delay puberty onset in rats and adolescents [48,49], contribute to the pathogenesis of different gastrointestinal diseases (e.g., inflammatory bowel disease, gastrointestinal cancers, and peptic ulcers) [50], and play a role in metabolic syndrome development both in humans [51,52] and in animals [53]. Importantly, chronic liver diseases have proved to be marked by elevated oxidative stress, irrespective of the cause of the liver disease [54].
Overall, these data show that oxidative stress is generally harmful to human health, and especially could be responsible for the development and/or progression of several diseases including cancer, cardiovascular, neurological, respiratory, kidney, metabolic syndrome, and liver diseases. Therefore, the use of natural flavonoid antioxidants including NAR might be beneficial in preventing, managing, or treating these human diseases.
4. Physicochemical and pharmacokinetic properties of NAR
NAR has a molecular weight of 272.26. The chemical formulation of NAR is C15H12O5 (Fig. 1). This bioactive compound is obtained from the hydrolysis of glycone forms of flavanone, such as naringin or narirutin [55]. Naringin is responsible for the bitter taste of grapefruit [55]. The bioavailability of NAR is low due to the broad degradation. Previous works have suggested a great pre-systemic gut flora metabolism, resulting in a wide range of degradation products (i.e., phenolic acids) [56,57]. As well, several researchers have detected NAR in human urine and plasma after oral intakes of grapefruit juice or pure naringin [[58], [59], [60], [61], [62]]. Because of the lipophilic nature of NAR, probably it accumulates within tissues in concentrations more than those detected in the plasma. This accumulation would most probably appear in organs such as the liver and intestine [63]. Its metabolism effect was also examined in male endurance athletes [64]. In this trial, orange juice was fed before and after 7 days of physical activity ending and the renal excretion of phenolic metabolites was determined. The authors found that the bioavailability in endurance athletes was lower in comparison with less-trained persons.
Fig. 1.
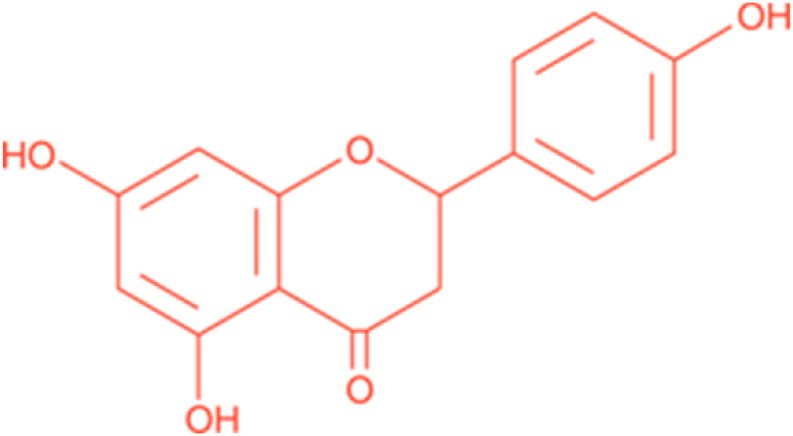
The chemical name of 2,3-dihydro-5,7- dihydroxy-2-(4- hydroxyphenyl)-4H-1-benzopyran-4-one has been known as naringenin.
In addition to body metabolic rate and enzymatic degradation, the pharmacologic benefit of an active compound also relies on the extent of absorption or bioavailability, after consumption. Chemically, NAR is lipophilic in nature and almost insoluble in water, and soluble in organic solvents. In addition to extensive degradation, the major challenge in the clinical development of NAR is the low aqueous solubility and thus, the low permeability and the poor oral bioavailability (approximately 5.81 %) [65]. This drawback has led to several studies trying to develop strategies to increase its bioavailability [[66], [67], [68]].
5. Nanotechnology for delivery of NAR
Nano-formulations are commonly developed for a wide variety of applications in the biomedical area such as specific analyte detection [[69], [70], [71]], specific disorder diagnosis [72], targeted delivery and co-delivery of therapeutic agents [73], efflux pump circumvention [74,75] as well as preventing embedded drugs' enzymatic degradation, adjusted drug release, improving pharmacokinetics, and enhancing the wound-healing efficacy [76,77]. Accordingly, nanotechnology is the vanguard for the improvement of the solubility and bioavailability of aqueous insoluble drugs/compounds. It has been reported that the solubility and bioavailability of NAR nano-formulations are improved versus the normal form of NAR [78]. Consequently, when NAR is inserted in a nano system, it presents more antioxidant activity.
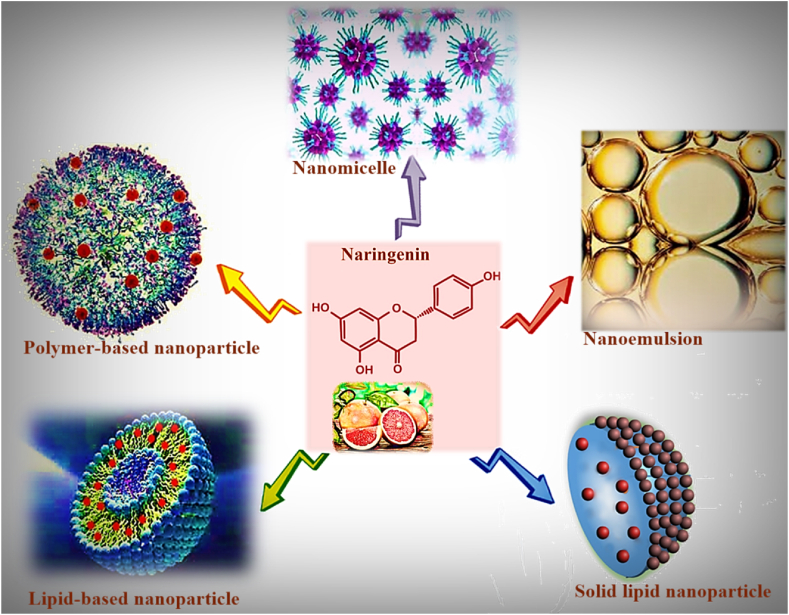
Many nanoformulations have been employed for NAR delivery. Among these, NAR -loaded polymeric nanoparticles [[79], [80], [81]], lipid-based-drug-delivery-systems such as SLNs [67,82] and liposomes [66], micro- and nano-emulsions [83], have been previously prepared to enhance oral bioavailability of NAR. Fig. 2 demonstrates some prepared nanoformulations of NAR. However, the main trouble for nanoparticle applications in the clinic is their safety and risks to health. Hence, it is obligatory to study and search for new nanoparticles to address their toxicity concern before clinical applications. A combination of botanical medicine with nanoization techniques has been widely used because of its being more eco-friendly as well as enhancement of safety and reduction of toxicity and side effects [84].
Fig. 2.
Illustration of various nanoformulations of naringenin.
6. NAR nanoformulations with anti-oxidative stress properties and protective effects against metal toxicity
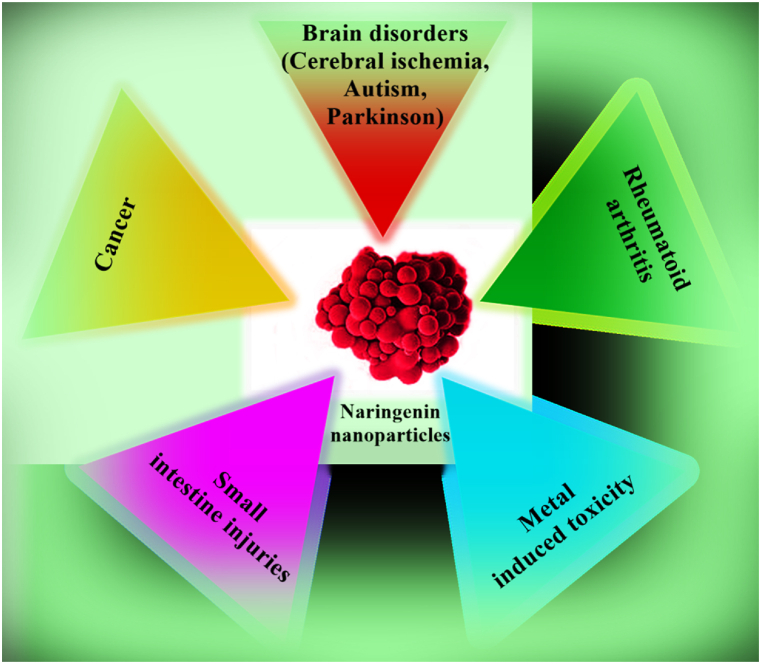
In recent years the combination of herbal medicine and nanotechnology has attracted quickly growing considerations and many researchers have become interested in this area. Based on multiple biological activities of NAR, it has been potentially suggested for reducing the risk of diverse diseases and advancing human health. Hence, different kinds of NAR nanoformulations have been developed (Fig. 3).
Fig. 3.
Some applications of naringenin nanoformulations in alleviating diverse diseases.
A prooxidant environment is involved in the pathologic process of various diseases through sustaining oxidative modifications of cellular molecules including nuclear and mitochondrial DNA, proteins, and lipids. An imbalance between the antioxidant defense system and the production of free radicals leads to increased ROS and consequently tissue damage. Oxidative damage results in diabetes mellitus, coronary heart disease, rheumatoid arthritis, and aging [85]. Changes at the level of the cellular redox system including glutathione S-transferase (GST), glutathione peroxidase (GPx), catalase, and superoxide dismutase (SOD) are considered as remarkable mechanisms of oxidative stress. Finding antioxidants’ mechanism of action has significant importance in the management of these diseases.
A wide variety of research studies evidenced that ROS has a major role in tumor initiation, promotion, and progression [86]. In this context, hydroxyl radicals attack DNA molecules resulting in damage and mutation of DNA, as well as altered signaling pathways due to damage to purine and pyrimidine bases. Moreover, 8-OH deoxyguanosine (8-OHdG) can be produced during ROS generation which directly leads to a rise in mutagenesis [87].
NAR exhibited antioxidant activity previously relying on their radical-scavenging properties and the upregulation of endogenous antioxidant enzymes [88,89]. Also, according to the fact that cells with a higher growth rate are more sensitive to radiation, cancer cells possess diverse degrees of radiosensitization. It has been shown that polyphenolic compounds such as curcumin and NAR induce a moderate radiosensitization effect [[90], [91], [92]]. In addition, magnetic nanoparticles (MNPs) exhibit strong antioxidant activity and free radical-scavenging properties, due to electron transfer from the Fe+2/Fe+3 systems of iron oxide nanoparticles [93]. In the work by Askar et al., in 2021 two natural polyphenols curcumin and NAR were loaded in dextran-coated magnetic nanoparticles (CUR-NAR-D-MNPs) and used as a radiosensitizer and chemotherapeutic agent to improve the effectiveness of curcumin-NAR in treating tumors [94]. Dextran is a water-soluble polysaccharide that can decrease the saturation magnetization of magnetic nanoparticles, thereby decreasing their cytotoxicity and increasing their biocompatibility [95]. In the HeLa cell line, curcumin and NAR co-delivery indicated an inhibitory effect on cell proliferation while increasing apoptosis which may be due to the generation of ROS. This formulation is speculated to improve the cellular uptake and thereby higher accumulation of nanoparticles (NPs) inside the cancer cell. Further experiments revealed that oral administration of CUR-NAR-D-MNPs to breast cancer-bearing rats was associated with reduced tumor volume and cell cycle arrest possibly through modulation of P53high, P21high, TNF-low, CD44low, and ROS-high signaling [94].
It has been reported that heavy metal toxicity such as cadmium (Cd) toxicity is primarily ascribed to the oxidative damage to cellular organelles via ROS production [96]. The current therapeutic strategy in metal toxicities is the use of chelators, antioxidants, and phytocompounds [97]. For example, treatment of a mouse model of AD with naringin (30 and 60 mg/kg/day, 4 weeks) markedly reduced amyloid-beta plaque formation in the cornu ammonis (CA1, CA3), and dentate gyrus areas of the hippocampus in part via its strong iron chelation capacity [98]. Naringin also could chelate iron and protect cells against free radical damage in vitro [99]. The effect of NAR and its nanosized form (n-NAR) on Cd toxicity in Nile tilapia was also studied [100]. Cd-induced oxidative stress was confirmed by a noticeable increase in the hepatic levels of malondialdehyde (MDA) and heat shock protein 70 (HSP70) as well as modulation of antioxidant enzymes including catalase (CAT), and superoxide dismutase (SOD). The results showed that the treatment with n-NAR was successful in the Cd concentration reduction both in the liver and kidneys, while the bulk NAR was efficient only in the kidneys. Flavonoids and particularly NAR are active metal chelators that can reduce the Cd concentration with a displacement of metal cofactors and/or Cd binding with enzymes [13,18]. This study revealed that Cd induces oxidative stress in the fish via enhancing ROS production and enhancing lipid peroxidation as evidenced by a fantastic increase in the hepatic MDA and HSP70 levels in the liver as well as altered activity of the main antioxidant enzymes, CAT and SOD in the liver and kidneys. Due to the presence of the 4-hydroxyl group in the β-ring, NAR possesses the electron-donating property and can effectively scavenge free radicals, thereby inhibiting the peroxidation of membrane lipids [101]. As well, the lipophilic nature of NAR causes its adherence to the cell membrane thus hindering the free radicals from attacking the membrane [18]. Here, the biosynthesis of metallothionein-2 (MT-2) in the liver has been increased in response to Cd accumulation to shield the cells against toxification. Treatment with both bulk NAR and n-NAR significantly reduced the hepatic MT-2 levels. Treating with both bulk and nano NAR decreases the MDA concentration significantly in comparison to the untreated group. Treatment with n-NAR and bulk NAR significantly altered the CAT and SOD activity in comparison to the untreated group in the liver and kidney. A significant reduction in the HSP70 level was observed in the n-NAR-treated group. However, bulk NAR couldn't significantly reduce the HSP70 concentration. This proved that the efficacy of antioxidant activity is remarkably different between bulk and nano forms of NAR. This finding is in agreement with the previous study of the use of bulk NAR to reduce the MDA level [102].
Oxidative stress has been also found to contribute to the progression of non-steroidal anti-inflammatory drug (NSAID)-induced gastrointestinal cell apoptosis. It has been reported that the persistent consumption of NSAIDs such as aspirin and indomethacin can harm the small intestine [103] and oxidative stress is a potential mechanism beyond NSAID-induced small intestine wounds [104,105]. Because of the high potential of NAR for repressing ROS, in a research performed by Wang et al. (2020), the novel self-assembling nanomicelle based on Rebaudioside-A and NAR were prepared (RA-NAR) and orally administered for assessment of the therapeutic efficacy [106]. After the administration of RA-NAR to rats, an increase in the area under the curve and an increase in maximum concentration were observed compared to the bulk NAR. The tissue distribution assessments further demonstrated that RA-NAR could effectively increase the NAR concentration in all tested intestinal segments. These findings prove that the nanomicellizing of NAR improved its bioavailability. As well, this study proved that RA-NAR is suitable for treating intestinal diseases because of the highly concentrated distribution of RA-NAR in the intestines. The oral administration of RA-NAR to mouse model showed that RA-NAR could efficiently protect against small intestine injuries induced by indomethacin by inhibiting pro-inflammatory cytokines and oxidative stress. In this investigation, the level of MDA, as a free-radical-induced lipid peroxidation marker, and SOD and GPx, as markers for the antioxidant defense system, was evaluated. Higher amounts of MDA and much lower amounts of GPx and SOD were observed in indomethacin-treated mice. In contrast, the results show that the treatment with RA-NAR significantly corrected the SOD, GPx, and MDA levels relative to Rebaudioside-A- and bulk NAR-treated small intestine injury groups. Further experiments showed a significant increase in the IL-1β and IL-6 mRNA expression in indomethacin-treated tissue. The treatment rank order for the IL-1β mRNA expression was RA-NAR > bulk NAR > Rebaudioside-A > roxatidine, while for the IL-6 mRNA expression, it was RA-NAR > Rebaudioside-A > bulk NAR ≈ roxatidine. Thus, it has been inferred that RA-NAR protects against indomethacin-induced small intestine injury via the suppression of ROS and inflammation.
The other NAR nanoformulation is a nanohybrid constructed by conjugation of chitosan-cellulose hydrogel with l-Histidine which contains zinc oxide NPs and NAR [107]. The studies on this system revealed the significant antimicrobial activity of the hydrogel against Staphylococcus aureus and Trichophyton rubrum strains. The improvement in cytotoxicity was exhibited by NAR-loaded hydrogel nanohybrid towards A431 cells with IC50 value which was about sixteen-fold lower than that of the bulk NAR. Thus, the present formulation possesses remarkable antibacterial and anticancer potential because of the synergic effect exhibited by l-Histidine, zinc oxide NPs, and NAR as well as sustained drug release behavior.
The chitosan NPs can protect the drug embedded from enzymatic degradation [108]. Accordingly, chitosan NP encapsulated NAR (CSNPs/NAR) was synthesized and its antioxidant and free radical scavenging activity was evaluated [109]. According to the results, CSNPs/NAR demonstrated a greater nitrate, 1,1- diphenyl-2-picrylhydrazyl (DPPH), and hydroxyl radical scavenging relative to bulk NAR. This result is ascribed to the sustained and prolonged release of NAR [110]. Thus, the rate of NAR release is considered an important parameter in developing a nanoformulation for NAR. Furthermore, the release of NAR from CSNPs/NAR in the simulated gastric fluid was found to be about only 15 %. The cell viability assay showed cytotoxic effects of CS-NPs/NAR against A549 lung cancer cells, while it had no significant effect on the viability of fibroblast 3T3 cells.
Stem cell therapy has attracted rapidly growing attention as a potential therapy for ischemic stroke. However, the major obstacle in stem cell therapy is the low survival rate of transplanted cells [[111], [112], [113]]. In the study by Anas Ahmad et al. (2018) [114], the protective effects of NAR-loaded gelatin, and coated with polycaprolactone NPs (NAR@GEL-PCL NPs) were evaluated using ischemia-like conditions created by oxygen glucose-deprivation of human mesenchymal stem cells (hMSCs). Due to its strong anti-inflammatory and antioxidant effects, NAR was suggested as a therapeutic agent in neurological disorders. Neuronal oxidative stress is closely related to nitric oxide (NO) generated by the activation of neuronal nitric oxide synthase (nNOS). NO reacts with O2 to produce peroxynitrite (ONOO), a strong oxidant of proteins and lipids. Also, increasing evidence showed that COX2 expression is associated with the oxidative stress-induced generation of prostaglandins thus, aggravating the ischemic pathogenesis. The aforementioned study revealed that the protein expression of COX2 and iNOS significantly was increased in the oxygen-glucose deprived (OGD)-insulted hMSC group relative to the control group. It was found that treatment with NAR@GEL-PCL NPs significantly decreased the level of COX2 and iNOS as compared to the untreated group. Meanwhile, blank NPs couldn't alter the expression of any of the aforementioned proteins. During the inflammation process, monocytes, macrophages, and neutrophils produce myeloperoxidase which in turn produces hypochlorous acid and generates radicals that exacerbate oxidative stress and tissue injury [115]. Here, treatment with NAR@GEL-PCL NPs significantly decreased the myeloperoxidase activity versus the untreated group, indicating the NAR anti-inflammatory effects associated with the decreased generation of free radicals and oxidative stress. Hence, the results of this study support the idea that NAR@GEL-PCL NPs were able to maintain cellular morphology.
Rheumatoid arthritis is a common inflammatory and autoimmune disease of joints without any permanent cure. Oxidative stress produced within an inflammatory joint can trigger autoimmune phenomena and destruction of connective tissue in rheumatoid synovitis (Free radicals in inflammation: second messengers and mediators of tissue destruction). It has been suggested that ROS generated inside the joints may contribute markedly to the pathogenesis of arthritis since they are capable of degrading matrix components by both direct action and indirect activation of latent collagenases [116]. In the study by Adil Munir, (2021) [81], NAR was loaded in lipid-based nanocarriers including stearic acid, stearic-lauric, and lecithin-chitosan. In this research, oral administration of the nanoparticles (40 mg/kg) to a rat model of arthritis induced by complete Freund's adjuvant was associated with improvement of histopathological changes in live and joint tissues. Meanwhile, the NAR-loaded lipid NPs significantly restored the liver and kidney function parameters including aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), bilirubin, and creatinine compared to the untreated group. These findings may be ascribed to the antioxidant effect of the NAR. Therefore, lipid-based nanoformulation of NAR exerted a hepatoprotective effect as well as an enhanced therapeutic response for a rheumatoid arthritis remedy.
NAR may have the potential for the treatment of brain ischemic disease. The blood-brain barrier plays an important role in brain homeostasis by controlling the diffusion of various ions and molecules. Hence, it is regarded as one of the major barriers to delivering drugs to the brain. Different properties of the NP itself might have a central role in the successful blood-brain barrier crossing, such as size and charge. Hence, to improve the blood-brain barrier crossing, the NPs’ surface needs to be modified [117]. On the other hand, oxidative stress is a characteristic of cerebral ischemia. ROS and reactive nitrogen species (RNS) cause cell death in vascular cells, glial cells, and neurons, as well as neurological recovery disorders after stroke [118].
Antioxidants are useful organ-protective materials and because of their chemical nature, crossing the blood-brain barrier is a major challenge. For the improvement of NAR bioavailability in the brain for the treatment of cerebral ischemia, a kind of NAR-loaded nanoemulsion was prepared which was then converted into a thermosensitive-mucoadhesive gel using chitosan [83]. The UHPLC-ESI-Q-TOF-MS/MS method was used to measure the pharmacokinetic parameters in the brain as well as plasma and to estimate the cerebral ischemic parameters such as antioxidant activity and the safety/toxicity of the gel after intranasal administration in the cerebral ischemic rats. The area under the Curve (AUC0–24h) of plasma & brain was calculated to be ∼995.60 and ∼5600.99 (ng. min/ml.g). No morphological changes were observed in the microstructure of the nasal mucosa and brain. Due to the lipid nature of the brain membranes, they are susceptible to ROS attacks. Any change in biochemical reactions as a result of oxidative stress can affect ischemic brain damage. During ischemia-reperfusion condition, excess production of free radicals resulted in the reduction in the glutathione (GSH) concentration which is reliant on the enzyme's activity like SOD, GPx, and glutathione reductase (GR) as reported by Khuwaja et al. (2011) [119]. Accordingly, ROS overproduction with subsequent depletion of antioxidant capacity leads to a remarkable increase in lipid peroxidation and threatens neuronal survival. The results of this study showed that it was observed that when NAR-gel formulation had been given prophylactically before the cerebral ischemia, significant SOD activity was exhibited in comparison with the cerebral ischemic group. Therefore, intranasal delivery of NAR-gel formulation can enhance the bioavailability of NAR in the brain of cerebral ischemic rats and then reduce lipid peroxidation and oxidative stress through scavenging the free radicals, leading to improving the neurobehavioral activity.
Although the deep insight into the role of ROS in autism spectrum disorders (ASD) is yet to be uncovered, it is covered that the antioxidant status in patients with ASD is reduced. This indicates that the pathology of ASD is closely related to the over-production of oxidative species and/or wrong antioxidant metabolism. In the blood of persons with ASD, increased levels of several oxidative stress markers were observed. Oxidative stress markers are strongly correlated with cellular injury and severe mitochondrial dysfunction in the pathophysiology of autism [120]. In another study related to ASD, the nano-formulated NAR was used to study its effect on ASD [121]. At first, NAR was coated with GSH or tween 80 (NAR- GSH or NAR-T80) and then was loaded into poly (lactic-co-glycolic acid) NPs (NAR- GSH@PLGA or NAR-T80@PLGA). The uncoated formulation was also prepared (NAR@PLGA). The effect of different NAR formulations on lipid peroxidation (LPO) was evaluated in a rat model of ASD. As a result, LPO was considerably increased in the brains of ASD rats as compared to the untreated group. Treatment with uncoated and coated formulations resulted in a significant decrease in LPO levels as compared to bulk NAR. However, only coated NPs showed a significant reduction in comparison to the group treated with minocycline the standard medication. The levels of GSH, SOD, and CAT were found to be significantly reduced in the brains of ASD rats versus untreated animals. In contrast, treatment with coated formulations promoted a significant increase in the levels of GSH, SOD, and CAT in the brain of treated rats in comparison to those treated with uncoated NPs, bulk NAR, and minocycline. Furthermore, GSH and SOD levels were enhanced more after the treatment with NAR-GSH@PLGA NPs as compared to NAR-T80@PLGA NPs. The uncoated formulation was more effective than bulk NAR and minocycline. It can be concluded that coated formulations are more powerful than other formulations probably due to enhanced brain uptake. The proximal part of Kreb's cycle and electron transport chain was disrupted in ASD rats' brains, as well as a decrease in ATP production [122]. The disruption of metabolic pathways promotes ROS production resulting in the release of pro-inflammatory cytokines, matrix metallopeptidase 9, HSP-70, and activation of the NF-κB pathway. NAR-T80@PLGA NPs exhibited a greater reduction in brain levels of TNF-α in comparison to bulk NAR, the standard medication as well as uncoated and NAR-GSH@PLGA NPs. Furthermore, the coated formulations were more effective in the reduction of matrix metallopeptidase 9 and HSP-70 levels, in comparison to other formulations. Therefore, the administration of the coated NAR attenuated oxidative stress and neuroinflammation.
Studies highlight the role of oxidative stress in the degeneration of dopaminergic neurons in PD. Oxidative and nitrative damage to vital intracellular structures in the PD substantia nigra. PD-causing gene products including PINK1, alpha-synuclein, DJ-1, parkin, and LRRK2 also affect mitochondrial function in complicated ways and lead to worsening of ROS generation and susceptibility to oxidative stress. In addition, oxidative stress affects cellular homeostatic processes including the mitophagy and ubiquitin-proteasome system. It is clear that the interplay between these various mechanisms contributes to neurodegeneration in PD as a feed-forward scenario where primary insults lead to oxidative stress, which damages key cellular pathogenetic proteins that in turn cause more ROS production. Animal models of PD have yielded some mechanisms by which oxidative stress contributes to PD. Currently, available treatment for PD manages clinical symptoms but does not stop disease progression. A study conducted by Mani et al. (2021) [123] investigated the probable protective effects of NAR in a rat model of PD induced by rotenone. To increase NAR bioavailability in the brain, NAR-loaded SLN (NAR-SLN) was used. SLN is one of the nontoxic and efficient drug delivery nonovehicles that can offer sustained drug release as well as an increase in the bioavailability of drugs with poor absorption. A behavioral assessment was performed to evaluate muscle coordination and locomotor ability. Both NAR and NAR-SLN treatment groups indicated significantly good muscular coordination compared to the rotenone group. The locomotor score was significantly reduced in the rotenone-treated rats in comparison with the untreated group which may be linked to attenuating oxidative stress. Similarly, treatment with both NAR and NAR-SLN improved the locomotor score as compared to the rotenone-treated rats. According to the biochemical parameters including SOD, GSH, and CAT in the brain homogenates, the group treated with NAR-SLN formulation exhibited significantly improved redox hemostasis compared to other treatment groups. These findings revealed the antioxidative properties of NAR. A significant reduction in LPO level in the group treated with NAR-SLN obviously indicated that the ROS formed by rotenone is negated by the formulation. The histopathological study conducted on the substantia nigra region showed neurodegeneration as protein aggregation in the rotenone-treated rats. The treatment with NAR-SLN was associated with greater protection as indicated by the lack/no damage to neurons. These findings proved the neuroprotective potential of NAR-SLN nanoformulation across the blood-brain barrier.
Similarly, in a work by Gaba et al., in 2019 [124], vitamin E and NAR were loaded in a nanoemulsion and used for treatment through intranasal delivery in a PD-like rat model. The nanoemulsion was shown to possess greater DPPH scavenging activity and antioxidant potential relative to ascorbic acid (as a standard antioxidant) and the bulk NAR solution. This may be due to the synergistic effect of the NAR and vitamin E. Pharmacokinetic study revealed that NAR concentration in the brain following intranasal administration of NAR nanoemulsion was extensively elevated in comparison to the NAR solution. Also, the AUC of the brain concentration of NAR nanoemulsion was remarkably greater than that of the NAR solution. This better NAR brain delivery of NAR nanoemulsion may be ascribed to the fact that the nasal mucosa provides more permeation for the drug to reach to brain. In addition, the small size and subsequently larger surface area of the nanoemulsion offer better interaction between the drug and the cells followed by more drug uptake via the nasal mucosa. More importantly, the half-life time of NAR via NAR nanoemulsion in both blood and brain was calculated to be considerably longer than the NAR solution. From the behavioral activities point of view, both NAR nanoemulsion and NAR nanoemulsion along with orally administrated levodopa showed marked improvement in the narrow beam maze test, muscular coordination test, forced swimming test, and Akinesia test. This finding is probably owing to the antioxidant effect of NAR which finally resulted in the reversion of PD symptoms. NAR nanoemulsion showed no significant difference in the grip strength test in comparison with the control group (treated with normal saline via the intranasal route). The NAR nanoemulsion-treated group showed a slight increase in the GSH concentration as well as a considerable increase in the levels of SOD when compared to the control group; however, the rats were treated simultaneously with levodopa and NAR nanoemulsion together showed a significantly higher GSH and SOD levels. The group treated with a combination of levodopa and NAR nanoemulsion showed significantly lower MDA content in comparison to the control group. It should be noted that the combination of levodopa and NAR nanoemulsion is more effective than both levodopa and NAR nanoemulsion alone, but the differences are not statistically significant. The potential benefits of NAR nanoformulations in the treatment of different disorders using in vivo experiments have been summarized in Table 1.
Table 1.
In vivo applications of NAR nanoformulations in the treatment of different disorders.
| Dose of administration | Naringenin formulation | Disorder type | Results | Reference |
|---|---|---|---|---|
| 10.0 mg kg-1 (Intranasal) | Poloxamer-Chitosan-based Naringenin nanoemulsion | Cerebral Ischemia | Pre-treatment with NAR decreases the systemic side effects | [83] |
| 25 mg/kg (Orally for 29 days) | uncoated and coated (glutathione & tween 80) naringenin-loaded PLGA nanoparticles | Autism Spectrum Disorders | Post-treatment with NAR restores the behavioral and biochemical deficits | [121] |
| 40 mg/kg (Orally for 28 days) | NAR-loaded lipid NPs including stearic acid, stearic-lauric, and lecithin-chitosan nanocarriers |
Rheumatoid arthritis | Post-treatment with NAR reduces the RAfactor, key inflammatory markers, and joint damage | [81] |
| 50 mg/kg (This DS model predicts blood-brain barrier penetration upon oral Administration) |
NAR-loaded SLN of glycerol monostearate | Parkinson's disease | Pre-treatment with NAR-loaded SLN can exert neuroprotective effects, | [123] |
| 0.72 mg/kg/day (Intranasal) | Naringenin nanoemulsion | Parkinson's disease | Post-treatment with NAR-NE along with levodopa was successful by evaluation of muscle coordination, grip strength, and enhancing swimming activity. | [124] |
| 1 mg/kg of NPs (thrice per week for 8 weeks, gastric intubation) | curcumin-NAR loaded dextran-coated magnetic NPs | Breast cancer | - marked regression of the tumor and- marked necrosis of the neoplastic cells via Post-treatment with NPs | [94] |
| fish were exposed to waterborne nanonaringenin at dose of 3 ppm |
Naringenin NPs | Metal-induced oxidative stress | in comparison to the Cd-exposed group, Post-treatment with NPs cause: - decreased MDA level - reduced catalase activity - decreased SOD activity - reduced metallothionein level - decreased HSP70 level |
[100] |
| 90 mg/kg of nanomicelle (Orally) | Rebaudioside A-based naringenin self-assembling nanomicelle | small intestine injuries | Post-treatment with nanomicelles causes: - decreasing spleen weight to normal levels - improved the small intestine length and alleviated the intestinal lesions to the normal-health mice levels - inhibited the intestinal ulceration and inflammatory cell infiltration and preserved the villi - fully corrected the SOD and MDA levels - partially corrected the GPx levels |
[106] |
7. Safety evaluation of NAR nanoformulations
Gera and coworkers determined the MG-63 cell viability by exposing the cells to different concentrations (0.15–10 μg/mL) of free NAR, NAR nanosuspension, and blank nanosuspension. Results depicted that there was no considerable alteration in the proliferation with the NAR nanosuspension group as compared to the free NAR group except at 10 μg/mL concentration. In this concentration, the viability was more than 75 % in the presence of NAR nanosuspension, while it was less than 50 % in the presence of free NAR [125]. In the other study, the safety of free NAR and polylactic acid/polyvinyl alcohol-NAR NP was evaluated in Caco-2 cell lines by exposing the cells to different concentrations of the samples (5, 10, 20, 40, 60, 80, and 100 μg/mL of NAR). There were no substantial changes in the proliferation of the cells with both free and nanoform of the NAR compared to the control group and cell viability was more than 98 % in all concentrations [126]. These two studies revealed that the safety dose of both free NAR and its nanoformulations is crucially cell-based. The type of cell line determines the safe dose of NAR administration. Polyvinylpyrrolidone is suitable for producing complexes with low water-soluble drugs and increasing their aqueous solubility. This is why the self-assembled nano-complexes of polyvinylpyrrolidone and NAR were prepared (PVP-NAR) and used for developing a kind of ophthalmic solution [127]. An in vitro artificial membrane permeability assay proved that the membrane permeation of PVP-NAR nanocomplexes was 10.3-fold of bulk NAR after 3.5 h. The in vivo topical administration also confirmed the higher concentration of NAR in aqueous humor and iris ciliary body for PVP-NAR nanocomplex rather than bulk NAR. The PVP-NAR nanocomplex exhibited significantly improved antioxidant activity compared to the bulk NAR using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) free radical scavenging assay, although the bulk NAR also demonstrated relatively good ABTS radical scavenging activity. The PVP-NAR nanocomplexes greatly improved the NAR solubility and thereby ocular bioavailability, as well as antioxidative activity. So, all these parameters together could strengthen the pharmacological activities of the NAR nanocomplex. The other study also evaluated the safety of a kind of NAR polymer nanoparticles. Acute and sub-acute toxicity of bulk NAR and the polylactic acid/polyvinyl alcohol-NAR NPs (PLA/PVA-NAR) was evaluated [128]. The histological assay of various organs (brain, liver, kidney, lungs, heart, and intestine) in both studies revealed no dangerous effect arising from NAR and PLA/PVA- NAR. As well, biochemical parameters and the level of hepatic antioxidant enzyme activity such as CAT, SOD, GR, and GPx as well as GSH, and total antioxidant capacity were not affected by the treatment with NAR and PLA/PVA- NAR in comparison to control animals. These results revealed that the NAR and its nanoformulation did not induce toxicity which has negative impacts on our health. It should be noted that this study didn't induce any diseases before or after NAR therapy. It would be better if this study induces a kind of disease after NAR therapy and compares the resistance level of the control and NAR-treated animals against catching the disease, as reported by other studies [83,123], or similar to other works, at first, the disease is induced, and then the NAR therapy is begun [81,121]. However, according to the findings obtained from various studies, it can be concluded that NAR most likely has the ability to potentially reinforce the antioxidant defense system, which subsequently protects the human body so that it is less prone to various diseases. In the other study, a kind of sunscreen cream containing polymeric NPs of NAR was developed for its photo-protective and antioxidant effects [129]. Cytotoxicity evaluation in HaCaT cells indicated no cytotoxicity. UV transmittance through sunscreen creams and calculation of certain parameters were used for the determination of the sun protection factor (SPF) of the NAR cream. The as-prepared cream containing NAR NPs showed the highest SPF value, considerable skin retention, and negligible skin permeation of NAR in comparison with other cream formulations with bulk NAR due to containing NPs rather than the bulk drug. Likely, the sustained release of NAR from NPs causes longer skin retention. It should be noted that great skin retention of the drug is a requirement for sunscreens. In addition, the free radical scavenging activity of the curcumin (as standard), NAR, and NAR NPs was determined using ABTS free radical scavenging. The IC50 value of curcumin, NAR, and NAR NPs was measured as 12.33, 14.78, and 15.44 μg/mL, respectively. Comparatively lower activity of NAR in its nanoparticulate form may be due to the slow release of NAR into the environs because of the polymeric coat. From the free radical scavenging point of view, the different results reported by chitosan NP encapsulated NAR. Table 2 summarizes the safety of NAR nanoformulations.
Table 2.
Safety and inflammation studies of naringenin nanoformulations.
| Dose and route of administration | Naringenin formulation | Results | Reference |
|---|---|---|---|
| 5 mg/mL NAR (intraocular for ocular abnormality) | polyvinylpyrrolidone K–17PF-NAR nanocomplex | strong anti-inflammatory effect | [127] |
| Acute toxicity doses (5, 50, 300 mg/kg, oral) | PLA/PVA-NAR NPs |
|
[128] |
| Sub-acute doses (10, 50, 100 mg/kg, oral) | |||
| 1 μg of NAR/cm2 area of the back portion of rats (4 h, Topical) | NAR-PLGA-PVA polymeric NPs loaded in sunscreen cream | appreciable skin retention of naringenin after 4 h as a Photo-protective agent | [129] |
8. NAR and oxidative stress in clinical trials
Preclinical studies using mouse models have already shown the different properties of NAR such as anti-inflammatory, antioxidant, anti-cancer, neuroprotective effects, and so on. Despite the large number of experimental animal and in vitro studies [130], there are only a few clinical studies on the biological impacts of NAR [131], mostly due to chemical instability and the presence of limited data on the pharmacokinetic properties of NAR [132]. A protective impact of flavonoids especially NAR on cardiovascular risk factors has been shown in some clinical trials, presumably in part through its antioxidant actions. For example, naringin (a NAR glycoside) supplementation (400 mg/capsule/day for 8 weeks) was shown to reduce plasma total cholesterol and low-density lipoprotein cholesterol levels and augment antioxidant defense, through enhancing the activity of antioxidant enzymes SOD and CAT, in hypercholesterolemic subjects [133]. Conversely, Demonty et al. (2010) showed that naringin (500 mg capsule/day for 4 weeks) did not affect total cholesterol or low-density lipoprotein cholesterol in moderately hypercholesterolemic individuals [134]. Although this divergence should be evaluated more deeply, however, a potential source of this heterogeneity may be the duration of treatment. A clinical trial evaluated the effect of 200 mL of orange juice intake (3 times daily) on mild hypercholesterolemic men for 4 weeks [135]. Results indicated a tendency towards a decrease in endothelial dysfunction, and a small increase in plasma apoA-I level accompanied by a substantial antioxidant effect in male volunteers. In another study, supplementation with orange juice (500 mL/day) for 8 weeks resulted in reductions in the serum concentrations of total cholesterol and LDL-cholesterol, elevated liver enzyme AST, inflammatory responses, and oxidative stress parameters in patients with chronic hepatitis C receiving antiviral therapy [136]. In addition to duration-dependent, the beneficial effect of NAR on cardiovascular risk factors appears to be dose-dependent. For instance, a double-blind, cross-over study compared the antihypertensive effect of naringin between patients with stage I hypertension receiving 500 mL/day of a fruit juice containing 593 μM naringin and patients receiving lower concentration of naringin (143 μM) for 5 weeks. Results showed a substantial reduction in systolic blood pressure in both groups, whereas diastolic blood pressure was more effectively diminished in patients receiving high doses of naringin [137].
The antioxidant effect of NAR also may at least partly contribute to weight management. For example, a double-blinded, randomized, placebo-controlled clinical trial showed that supplementation with polyphenolic citrus dry extract (Sinetrol-XPur), containing nearly 20 % of naringin, for 12 weeks twice daily with meals attenuated waist and hip circumference, abdominal fat, and body weight accompanied by a reduction in inflammatory markers (C-reactive protein and fibrinogen) and oxidative stress parameters (a decrease in MDA content as well as an increase in the antioxidants GSH and SOD) in healthy overweight subjects [138]. Table 3 highlights a summary of clinical studies on the antioxidant effects of NAR/naringin in different diseases.
Table 3.
Summary of clinical studies on antioxidant effects of naringenin/naringin in different diseases.
| Administration route/duration | Naringenin/Naringin dose | Disorder type | Protection | Reference |
|---|---|---|---|---|
| Naringin (consumed as capsule) daily for a period of 8 weeks | 400 mg | Hypercholesterolemic subjects | Diminished plasma total cholesterol by 14 % and low-density lipoprotein cholesterol levels by 17 %. Naringin treatment increased antioxidant defense through enhancing SOD and CAT activities, while GPx activity and plasma TBARS levels remained unaffected. | [133] |
| Orange juice intake, 3 times daily, for 4 weeks. | 200 mL | Mild hypercholesterolemic men | A tendency towards a decrease in endothelial dysfunction, and a small increase in plasma apoA-I level, a substantial antioxidant effect in mild hypercholesterolemic men | [135] |
| Orange juice daily for 8 weeks | 500 mL | Patients with chronic hepatitis C receiving antiviral therapy | Reductions in the serum concentrations of total cholesterol and LDL-cholesterol, elevated liver enzyme AST, inflammation, and oxidative stress parameters in the patients [25]. | [136] |
| Supplementation with the polyphenolic citrus dry extract (Sinetrol-XPur), containing nearly 20 % of naringin, twice daily consumed as a capsule for 12 weeks | Each capsule contains 450 mg of the dry extract. Subjects received 180 Sinetrol-XPur capsules, with 2 capsules per day (900 mg). | Healthy overweight subjects | Reductions in waist and hip circumference, abdominal fat, body weight, inflammatory markers (C-reactive protein and fibrinogen), and oxidative stress parameters (a decrease in MDA content as well as an increase in the antioxidants GSH and SOD) in healthy overweight subjects [100]. | [138] |
9. Role of NAR in the regulation of cellular defense systems against oxidative stress
Although there is an abundance of studies demonstrating that NAR may have antioxidant effects, it is necessary to attempt to fully elucidate the molecular mechanisms via which NAR protects against multiple diseases by attenuating oxidative stress. Table 4 highlights a summary of previous reports on the antioxidant effects of NAR/naringin in different diseases.
Table 4.
Summary of previous studies (in vivo/in vitro) on antioxidant effects of naringenin/naringin in different diseases.
| Administration route/duration | Naringenin/Naringin dose | Disorder type | Protection | Reference |
|---|---|---|---|---|
| Naringenin, gavage | 50 mg/kg | Lead acetate-induced liver injury in male rats | Restored MDA, and antioxidant activity in the liver, but it was not enough against the lead acetate-induced hepatic injury | [102] |
| Naringenin, in vitro | 25–100 μM | Hyperglycemia-induced liver injury | A significant reduction in a hyperglycemia-induced decrease in the liver cell viability (in the cell line change liver) in a dose-dependent manner. | [143] |
| - In vivo, male Wistar rats, Orally for 56 days |
60 mg/kg |
Hyperglycemia-induced liver injury |
Reduction in hepatic oxidative stress and Nrf2 protein expression in liver |
[143] |
| Naringin for a month, intraperitoneally | 30 and 60 mg/kg/day | Iron-overloaded mice | A significant reduction in Aβ plaque numbers in CA1, CA3, and dentate gyrus regions of the hippocampus | [98] |
| Naringenin, orally, for 28 days | 20 or 50 mg/kg/day | Arsenic-induced hepatotoxic and nephrotoxic in rats | Restored the activities of serum biomarkers and antioxidant enzymes in the hepatic and renal tissues in a dose-dependent manner. | [192] |
| Naringenin, in vitro study | 40 μM | A cellular model of senescent myocardial cells (induced by exposing the cells to H2O2) | A reduction in cellular senescence markers via attenuating oxidative damage and improving mitochondrial metabolic activity | [193] |
| Naringenin pre-treatment orally in mice | 50 mg/kg | Superoxide anion-induced inflammatory pain |
|
[142] |
| Oral gavage of naringenin in rat | 100 mg/kg | Benzo[a]pyrene-induced lung toxicity |
|
[153] |
| Naringenin, intraperitoneally | 25, 50, 100 mg/kg | Obesity-associated hypertension in the rat | Treatment with naringenin at doses of 50 and 100 mg/kg ameliorated obesity-associated hypertension by reducing serum MDA and NO and enhancing serum SOD and GSH as well as by regulating lipid disorder | [194] |
| Naringenin orally to rats for 7 days | 50 mg kg | Myocardial ischemia-reperfusion | Reduction in the infarction size and myocardial apoptosis index by suppressing mitochondrial oxidative stress damage and increasing mitochondrial biogenesis | [162] |
| Naringenin, in vitro | 80 μmol L | H9c2 cardiomyoblasts subjected to simulated ischemia-reperfusion treatment | Naringenin caused a profound cytoprotective effect against cell apoptosis by inhibiting mitochondrial oxidative stress damage | [162] |
| Naringenin in combination with vitamins C and E orally for 28 days | 50 mg/kg | Cadmium-induced oxidative hepatotoxicity in rats | Improvement in the altered biochemical and histopathological changes in the liver | [186] |
| Naringenin, Orally, for 21days | 50 mg/kg | Nicotine-induced hepatic oxidative stress in young rats | Recovery of altered TBARS (thiobarbituric acid reactive substances) levels and GPx, GR, and glutathione S-transferase activity | [195] |
| Post-treatment and pre-treatment with naringenin orally for 21 consecutive days | 50 mg/kg | Lambda-cyhalothrin (a synthetic pyrethroid insecticide)-induced | Both post-treatment and pre-treatment significantly improved hepatotoxicity and oxidative stress in rat livers, although pretreatment was found to be more effective in improving liver injury | [190] |
| Post-treatment and pre-treatment with naringin, intraperitoneally | 10 mg/kg | Doxorubicin-induced hepatic oxidative stress in mice | Both post-treatment and pre-treatment increased antioxidants and decreased the doxorubicin-induced lipid peroxidation, but the effect of naringin pretreatment was more effective than its post-treatment. | [191] |
| Naringin, intraperitoneally | 5 mg/kg and 10 mg/kg | Testicular ischemia-reperfusion induced oxidative stress in rat | Protection against testicular ischemic/reperfusion injury by a significant decrease in MDA levels and an increase in the levels of SOD and CAT (at higher doses) | [196] |
| Naringenin pre-treatment, in vitro | H2O2-induced oxidative stress in cultured cardiomyoblast |
|
[144] | |
| Naringenin, oral gavage, for 4 weeks | 50 mg/kg | Phthalates-induced testis damage in rat | Prevented histopathological damage by reducing oxidative stress | [197] |
| Naringin, orally, for 4 weeks | 100 mg/kg | High-Cholesterol Diet-Induced Vascular Dysfunction and Oxidative Stress in Rats | Improvement in endothelium dysfunction in hypercholesterolaemic rats by reducing oxidative stress partially via downregulation of LOX-1 and NOX | [182] |
| Naringin for 16 weeks, injection into the caudal vein | 100 and 200 mg/kg | Streptozoticin-induced diabetic rats |
|
[180] |
| Naringenin, orally | 50 mg/kg | Streptozotocin-induced diabetic rats |
|
[171] |
| Naringenin, in vitro study | 0.4 μmol/L | amyloid beta-induced oxidative injury of adrenal pheochromocytoma cells | Naringenin protected adrenal pheochromocytoma (PC12) cells from amyloid beta-induced oxidative injury, reduced ROS production through activating estrogen receptors and PI3K/Akt signaling pathway | [168] |
| Naringenin, for 4 weeks starting from the 5th week of streptozotocin injection, orally Naringenin in combination with insulin |
25, 50, 100 mg/kg | Streptozotocin-induced diabetic rats | A considerable reduction in neuropathic pain in diabetic rats. It decreased the diabetic condition and alleviated neuropathic pain via modulation of oxidative-nitrosative stress, inflammatory responses, and inhibition of matrix metalloproteinases in diabetic rats |
[141] |
| Naringenin, intraperitoneally | 50 mg/kg, i.p. | Social defeat stress-induced neurobehavioral impairments in mice | Naringenin protected against neurobehavioral derangements induced by social defeat stress in mice reduced oxidative stress (significantly decreased brain level of MDA while increasing antioxidant GSH content) and released pro-inflammatory cytokines | [198] |
| Naringenin, orally | 25, 50, 100 mg/kg | methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced oxidative stress in mice | protected dopaminergic neurons in the substantia nigra and striatal regions against damage caused by MPTP in mice possibly in part via suppressing oxidative stress. | [199] |
Nuclear factor erythroid 2-related factor 2 (Nrf2) is considered a master regulator of neutralizing cellular ROS [139]. A large number of studies demonstrated the antioxidant properties of NAR. In this context, a previous study showed the protective effects of NAR against superoxide anion donor (KO2; a common form of ROS)-induced inflammatory pain in mice, in part, via upregulating Nrf2 and heme oxygenase-1. Heme oxygenase-1 is an enzyme catalyzing oxidative degradation of cellular heme to liberate free iron and up-regulate antioxidant enzymes like SOD [140]. This suggests that modulation of oxidative stress by NR might be a novel strategy to manage chronic pain, supported by the study indicating that treatment with NAR (25, 50, 100 mg/kg) for 4 weeks starting from the 5th week of streptozotocin injection (for the induction of diabetes in rats) substantially and dose-dependently reduced neuropathic pain in diabetic rats. Interestingly, in this study, insulin treatment in combination with NAR diminished the diabetic condition and alleviated neuropathic pain in part via modulation of oxidative-nitrosative stress in diabetic rats [141]. Based on the experimental evidence, up-regulation of Nrf2 mRNA expression was shown to result in the activation of the nitric oxide (NO)−cGMP−PKG−potassium channel signaling, mediating antioxidant effects of NAR [142]. By contrast, in another study, NAR was found to exert protective effects against liver oxidative damage in diabetic rats compared with control rats by preventing an increase in the expression of Nrf2. Given the antioxidant effect of Nrf2, a reduction in the Nrf2 expression following treatment with NAR may reduce activation of the transcription of antioxidant enzyme systems and thereby relieve the oxidative burden caused by diabetes in rats. Instead, NAR possibly exerts antioxidant action directly due to its OH which has a high affinity for ROS. NAR thereby may allow the recovery and restoration of the antioxidant capacity diminished by diabetes [143]. In confirmation of an Nrf2 role in mediating antioxidant actions of NAR, NAR pre-treatment was shown to significantly decrease oxidative stress in cultured cardiomyoblast as indicated by a reduction in NO production and an increase in the synthesis of antioxidant marker enzymes. This protective effect of NAR against oxidative stress in cultured cardiomyoblast cells was exerted via activating Nrf2 signaling [144]. In neurons isolated from the brains of rats exposed to hypoxia, NAR administration (80 μM) could decrease oxidative stress and improve mitochondrial dysfunction by activating the Nrf2/antioxidant response element signaling pathway in neurons [145].
Previous studies have indicated a relationship between Nrf2 and NF-κB under states of oxidative stress [146]. Abrogation of Nrf2 has been shown to enhance NF-κB activity resulting in increased cytokine generation, while NF-κB modulates Nrf2 activity. However, NF-κB has also been reported to antagonize the Nrf2/antioxidant response element pathway [147]. NF-κB thereby may enhance susceptibility to oxidative stress. In addition, it has been suggested that the early phase of oxidative stress can activate the NF-κB pathway but persistent oxidative stress is associated with a reduction in NF-κB activity [146]. In this context, NAR pre-treatment upregulated Nrf2 target genes and protein kinase B (Akt) and downregulated NF-κB accompanied by a marked reduction in oxidative stress in cultured cardiomyoblasts [144].
NAR was also suggested to exert antioxidant properties by targeting COX-2. Activation of the COX pathway may result in oxidative stress [148]. Generally, there are two distinct isoforms of COX: COX-1 and COX-2. The enzymes COX-1 and COX-2, also known as prostaglandin-endoperoxide synthase 1 and 2, catalyze the first steps of the synthesis of prostanoids (i.e. PGs, thromboxanes, and prostacyclins) by converting arachidonic acid into prostaglandin H2. COX-1 is expressed in most tissues and considered a housekeeping enzyme, while COX-2 is expressed by inflammatory cells and is responsible for the synthesis of inflammatory mediators [148]. ROS such as superoxide can stimulate inflammatory responses such as the expression of COX-2 [149]. Interestingly, COX-2 has been reported to be an important source of superoxide under certain pathological states such as vascular disease in diabetes and hypertension [[150], [151], [152]]. NAR has been shown to protect against superoxide anion donor KO2-induced inflammatory pain in mice partially by exerting antioxidant effects possibly through downregulating COX-2 [142]. Moreover, NAR protected against benzo[a]pyrene (B[a]P)-induced oxidative stress and lung damage in rats by decreasing the expression of COX-2 and NF-κB and restoring antioxidant enzymes in lung tissue [153].
Sustained oxidative stress can cause protein misfolding and consequently cellular apoptosis [154]. The unfolded protein response is an essential adaptive mechanism in response to protein misfolding in the endoplasmic reticulum to maintain cell survival and function [155]. Heat shock proteins have been shown to induce the unfolded protein response in the endoplasmic reticulum, thereby inhibiting apoptosis, and inflammation [156]. Heat shock proteins assist in the folding of newly synthesized proteins or prohibit protein misfolding and aggregation [157]. NAR, particularly its nanoformulation, was shown to significantly increase HSP70 levels in the gills of Nile tilapia accompanied by a reversal in cadmium-induced oxidative stress in the liver, shown by a reduction in the hepatic MDA levels [100]. HSP70 has been shown to enhance levels of the antioxidant enzyme, GSH, and reduce levels of oxidants (superoxide anion) in cardiac tissue accompanied by improvement of cardiovascular function in rats [158]. In addition to HSP70, NAR was also found to markedly enhance metallothionein in the liver of Nile tilapia and reduce cadmium-induced oxidative stress in the liver [100]. Metallothioneins are cysteine-rich and heavy metal-binding proteins and have been shown to participate in a variety of protective stress responses including cell protection against free radicals [159]. Hence, an increase in hepatic metallothionein and HSP70 following NAR treatment may in part contribute to the protective effect of NAR against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus.
Mitochondria are the main source of cellular ROS and play a key role in apoptosis. Enhancing mitochondrial biogenesis has been shown to protect against mitochondrial apoptotic cell death through attenuating caspase-dependent and caspase-independent signaling pathways [160]. Interestingly, an increase in sirtuin-3 (SIRT3) and AMP-activated protein kinase (AMPK) was found to be associated with mitochondrial biogenesis in cardiomyocytes isolated from neonatal mice [161]. These findings support the idea that the compounds activating the AMPK-SIRT3 signaling pathway may exert protective effects against sepsis-induced cardiomyopathy [161,162]. In this respect, evidence from in vivo and in vitro experiments has shown that NAR (50 mg/kg orally to rats for 7 days in vivo and 80 μmol/L in vitro) ameliorates mitochondrial disruption and subsequent mitochondrial oxidative injury during myocardial ischemia-reperfusion via AMPK-SIRT3 signaling pathway [162]. These data may suggest a possible involvement of the AMPK-SIRT3 signaling pathway in the antioxidant impact of NAR.
In addition to SIRT3, SIRT1 also appears to play an important role in the antioxidant effect of NAR. SIRT1 is a protein that is expressed throughout the body including in the liver, heart, and muscle [163] and its overexpression in mouse cardiac muscle has been reported to protect the heart from oxidative stress and ameliorate age-related cardiac hypertrophy [164]. Moreover, a reduction in the levels of SIRT1 or SIRT3 has been shown to increase ROS production during ischemia/reperfusion stress in an aging heart [165]. SIRT1 is also known to be the cause of modern skin cell aging and its activation has been shown to modulate several cellular proteins such as NF-κB [166]. In the skin, NAR has been shown to regulate the activity of SIRT1, which in turn may reduce oxidative stress-induced skin cellular senescence [167].
Phosphoinositide 3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) pathway has been shown to be frequently dysregulated in lung cancer. Changes in the expression levels of the PI3K/Akt/mTOR pathway were detected in mice with lung cancer compared to normal control mice [167]. NAR was found to significantly inhibit this pathway by suppressing oxidative stress and improving the antioxidant parameters such as SOD and myeloperoxidase [167]. NAR (0.4 μmol/L) was also shown to protect adrenal pheochromocytoma cells from amyloid beta-induced oxidative injury by attenuating ROS production through activating estrogen receptors and PI3K/Akt signaling pathway [168]. This study suggests a relationship between NAR therapeutic effects and estrogen receptors, which are clinically important in patients with breast cancer and are thought to play an important role in the development of breast cancer [169].
Brain-derived neurotrophic factor (BDNF) was suggested as another target mediating the antioxidant effect of NAR, BDNF is an essential molecule involved in neuronal survival and growth [170]. Oxidative stress is thought to be a major contributing factor in the initiation of neuronal damage in the diabetic retina and the development of diabetic retinopathy. Oral administration of NAR (50 mg/kg/day) has been reported to attenuate neuronal damage in the retina of streptozotocin-induced diabetic rats via increasing levels of BDNF and its receptor in the retina accompanied by a reduction in oxidative stress [171]. NAR (10, 25, and 50 mg/kg, intraperitonally) was also found to alleviate depressive- and anxiety-like behaviors in mice exposed to hypoxic stress by reducing oxidative stress-provoked inflammatory responses through modifying NF-κB and BDNF expression [172]. The role of BDNF in reducing oxidative stress could be supported by the study indicating that BDNF-deficient mice were more vulnerable to stress-induced oxidative damage compared to wild-type stress mice [173]. These data suggest a preventive role of NAR antioxidants in treating mood disorders, at least in part, through modulating the levels of BDNF, which has been shown to play a principal role in the pathophysiology of mood disorders [174].
NAR was also suggested to attenuate oxidative stress by targeting chemokine CCL2. Chemokine CCL2 is an important chemokine that is involved in pro-inflammatory responses. It has also been shown to activate a respiratory burst that results in excess ROS production [175]. In this regard, intraperitoneal injection of naringin for 3 consecutive days was shown to ameliorate chemokine CCL2-induced cognitive impairment in rats, at least partially, through attenuating oxidative stress in the hippocampus, an important brain area involved in learning and memory [176].
Another possible molecule mediating the antioxidant effect of NAR may be peroxisome-proliferator activator receptor γ (PPARγ). PPARs (i.e. PPARα, PPARγ, and PPAR β/δ) are a family of ligand-activated nuclear receptor transcription factors that particularly regulate the expression and function of genes implicated in inflammation energy and homeostasis [177]. Increasing evidence indicates that PPARγ is involved in the oxidative stress response. For example, natural endogenous PPARγ ligands such as oxidized lipids and prostaglandin J2 [178] are generated during oxidative stress and inflammation. PPARγ, however, does not act alone and has been suggested to be at the interconnection point of multiple pathways involved in oxidative stress including the Nrf2, Wnt/β-catenin, and forkhead box proteins O (FOXO) pathways [177]. Moreover, neuroinflammation is known to be associated with oxidative stress [179]. In this respect, naringin administration (100 and 200 mg/kg; for 16 weeks) improved cognitive decline (assessed using the Morris water maze test) in streptozoticin (50 mg/kg)-induced diabetic rats accompanied by attenuating oxidative stress and proinflammatory through activating the PPARγ signaling pathway [180].
Other molecules involved in antioxidant actions may be NOX enzymes and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1). NOXs are electron-transporting membrane proteins and their primary function is to produce ROS [181]. NAR (100 mg/kg; orally for 4 weeks) administration was found to alleviate endothelium dysfunction in hypercholesterolaemic rats, in part, via downregulation of NOX [182]. LOX-1 is a receptor for oxidized low-density lipoprotein which plays an essential role in several signal transduction pathways and is implicated in oxidative stress and inflammation [183]. Naringin treatment has also been demonstrated to improve endothelium dysfunction (which has a substantial role in the pathogenesis of cardiovascular disorders [184]) in hypercholesterolaemic rats, at least partly, by attenuating oxidative stress through downregulating LOX-1 [137]. The possible molecular mechanisms involved in the antioxidant properties of NAR are illustrated in Fig. 4.
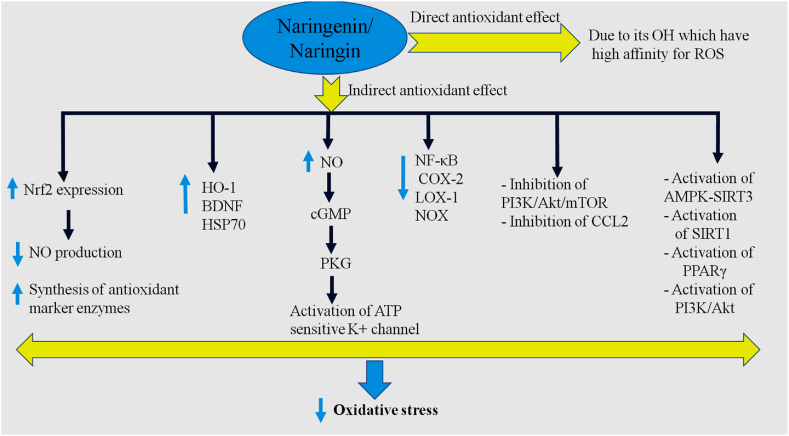
Fig. 4.
The possible molecular mechanisms involved in the antioxidant properties of NAR. HSP70: Heat shock protein 70; NOX: Nicotinamide adenine dinucleotide phosphate oxidase; NO: Nitric oxide; COX-2: Cyclooxygenase 2; NF-κB: Nuclear factor kappa B; HO-1: Heme oxygenase-1; BDNF: Brain-derived neurotrophic factor; LOX-1: Lectin-like oxidized low-density lipoprotein receptor-1; CCL2: Chemokine C–C motif ligand 2; mTOR: Mammalian target of rapamycin; PI3K: Phosphoinositide 3-kinase; PPAR-γ: Peroxisome proliferator-activated receptor gamma; Nrf2: Nuclear factor erythroid 2–related factor 2; PKG: Protein kinase G.
Together these data suggest that NAR administration may be beneficial for treating various diseases in part by modulating oxidative stress via different mechanisms. Nevertheless, the findings from some studies are conflicting. For example, NAR (50 mg/kg by orogastric gavage) was shown to restore MDA level and antioxidant activity in the liver following lead acetate-hepatic damage in male rats, but, it was not enough against the lead acetate-induced hepatic injury [102]. Also, NAR consumption (30, 60, or 120 mg/kg) for 6 weeks increased the activity of NAD(P)H: quinone oxidoreductase-1 (an antioxidant flavoprotein) in the liver of rats under oxidative stress caused by deficient amounts of vitamin E and selenium, but this impact was not strong enough to protect against oxidative stress [185]. In contrast, NAR supplementation (50 mg/kg, orally) with a combination of vitamins C and E for 28 days more substantially improved cadmium-induced oxidative stress and hepatic injury in rats than the NAR or vitamins C and E treatment alone [186]. In this respect, the antioxidant activity of vitamins C and E may potentiate the NAR effect in the detoxification of cadmium in rat liver. However, prooxidant impacts of NAR have also been reported [187]. This divergence could arise from the limited information on the role of NAR in vivo, where both its antioxidant and prooxidant properties may be influenced by metabolism and conjugative enzymes [188,189].
Some studies have assessed the efficiency of pre-treatment and post-treatment with NAR against the toxic effects of different agents and found more efficiency of NAR pre-treatment in these studies. For instance, both post-treatment and pre-treatment with NAR (50 mg/kg) significantly improved Lambda-cyhalothrin (a synthetic pyrethroid insecticide)-induced liver damage and oxidative stress in ratʼs liver, although pre-treatment was found to be more effective in alleviating Lambda-cyhalothrin-induced hepatotoxicity [190]. Also, Treatment with naringin (10 mg/kg) before or after doxorubicin treatment significantly decreased doxorubicin-induced oxidative stress in the liver of mice, with naringin pre-treatment that was more effective in attenuating the oxidative stress caused by doxorubicin [191]. Overall, these data suggest that the protective effect of NAR antioxidant supplements depends on different factors including the dose of NAR used, duration of NAR administration, singly or in combination, and disease stage of NAR administration.
10. Conclusions and future perspectives
During the last decade, NAR has been suggested as a potential therapeutic agent in a variety of diseases due to its antioxidant, anti-inflammatory, immunomodulatory, and anti-cancer activities that provided a material basis for its application in health promotion. NAR possesses direct or indirect antioxidant activity. The indirect antioxidant activity involves the removal of ROS by SOD, CAT, and GSH, whereas the direct antioxidant activity of NAR is attributed to its iron chelation activity. This direct antioxidant activity of NAR, under certain pathological conditions, may relieve the cellular oxidative burden and recover antioxidant capacity decreased by the disease by preventing the transcription of genes involved in the antioxidant defense system.
One limitation we faced in writing this review article was the lack of sufficient clinical evidence, which can substantially restrict the therapeutic uses of NAR. Thus, there is a need to perform larger and sufficiently strong clinical trials in this direction to demonstrate the adequate efficacy and safety of this flavonoid for future therapeutic uses. From a long-term safety point of view, there is a need for long-term safety studies to ensure the broader acceptance and use of NAR, especially in different populations or demographic groups. As well, encouraging interdisciplinary research to explore NAR's impact across a broader spectrum of health conditions and understand its interaction with other substances or treatments is necessary. In addition, the potential global health implications of NAR, especially in low-resource settings or in addressing widespread health issues like oxidative stress-related diseases should be expanded. Another limitation was that many studies included in this review article lack molecular studies including evaluation of the gene and protein expression of the antioxidant enzymes following NAR supplementation. Thus, further research is required to validate the preliminary findings acquired from these studies using molecular techniques. The molecular signaling pathways Nrf2, antioxidant response element, heme oxygenase-1, NO−cGMP−PKG−potassium channel, COX-2, NF-κB, heat shock protein 70, SIRT1, AMPK-SIRT3, PI3K/Akt/mTOR, BDNF, chemokine CCL2, and NOX, LOX-1 pathways might be implicated in NAR-mediated protection against oxidative stress. Furthermore, the safety evaluations are regarded as key steps to accelerating the development of NAR as a functional food. Future treatment approaches for diseases in which oxidative stress plays an important role lie in a deeper understanding of the free radical biology of these diseases including altered signaling induced by aberrant ROS generation.
Here, we evaluated the different applications of NAR nanoformulations with a focus on antioxidant properties and related molecular pathways. The novel formulations and improving pharmacokinetic properties may enhance the therapeutic efficacy of NAR. However, there are still many other nanoformulations that can be developed to circumvent the disadvantages of NAR, such as nanofibers, nano-spray, nanovesicles, liposomes, hydrogel, and nanogel, etc. It is worth mentioning that it is still unclear which concentration would be enough to prevent damage by oxidative stress in different diseases. Hence, further pharmacokinetic studies are required for the determination of the adequate dose. The findings of the literature review showed that the kind of nanoformulation affects the rate of NAR release, and the NAR release also has a direct effect on the free radical scavenging activity of NAR nanoformulations. We hope that more research will be done to develop the NAR nanoformulations using FDA-approved materials to reach the optimal drug loading and release profile. From the point of view of potential new applications, it should be noted that the researchers should explore other applications for NAR according to its antioxidant properties. For example, its potential can be explored in stem cell survival and differentiation, tissue engineering, wound healing, etc. This review article may help the researcher to disclose the area of using flavonoid antioxidants to overcome the adverse effects of diseases in which oxidative stress plays a role in their pathogenesis.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Nasrin Mehranfard: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Maedeh Ghasemi: Writing – review & editing, Validation. Arezoo Rajabian: Writing – review & editing, Validation. Legha Ansari: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- NAR
Naringenin
- NP
Nanoparticle
- PLGA
Poly (lactic-co-glycolic acid)
- MDA
Malondialdehyde
- PLA
Polylactic acid
- PVA
Polyvinyl alcohol
- SOD
Superoxide dismutase
- HSP70
Heat shock protein 70
- SLN
Solid lipid nanoparticle
- MNP
Magnetic nanoparticle
- ROS
Reactive oxygen species
- Cd
Cadmium
- CAT
Catalase
- MT-2
Metallothionein-2
- NSAID
Nonsteroidal anti-inflammatory drugs
- GPx
Glutathione peroxidase
- GSH
Glutathione
- GR
Glutathione reductase
- ASD
Autism spectrum disorders
- LPO
Lipid peroxidation
- AST
Aspartate aminotransferase
- ALT
Alanine transaminase
- ALP
Alkaline phosphatase
- PD
Parkinson's disease
- AD
Alzheimer's disease
- H2O2
Hydrogen peroxide
- NOX
Nicotinamide adenine dinucleotide phosphate oxidase
- NOS
Nitric oxide synthase
- COX
Cyclooxygenase
- NF-κB
Nuclear factor kappa B
- AMPK
AMP-activated protein kinase
- NO
Nitric oxide
- SIRT
Sirtuin
- PI3K
Phosphoinositide 3-kinase
- mTOR
Mammalian target of rapamycin
- BDNF
Brain-derived neurotrophic factor
- LOX-1
Lectin-like oxidized low-density lipoprotein receptor-1
References
- 1.Abedi N., Sajadi-Javan Z.S., Kouhi M., Ansari L., Khademi A., Ramakrishna S. Antioxidant materials in oral and maxillofacial tissue regeneration: a narrative review of the literature. Antioxidants. 2023;12:594. doi: 10.3390/antiox12030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X., Fang P., Mai J., Choi E.T., Wang H., Yang X.-f. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho K.-J., Seo J.-M., Kim J.-H. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol Cells. 2011;32:1–5. doi: 10.1007/s10059-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn J.D., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmoud A.M., Wilkinson F.L., Sandhu M.A., Lightfoot A.P. The interplay of oxidative stress and inflammation: mechanistic insights and therapeutic potential of antioxidants. Hindawi. 2021;2021 doi: 10.1155/2021/9851914. [DOI] [Google Scholar]
- 7.Sies H. Strategies of antioxidant defense. Eur. J. Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 8.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 9.Solleiro-Villavicencio H., Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+ T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018;12:114. doi: 10.3389/fncel.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehranfard N., Yazdi A., Sardooi A.R., Shakerin Z., Ghasemi M. Honey protects against chronic unpredictable mild stress induced-intestinal barrier disintegration and hepatic inflammation. Mol. Biol. Rep. 2020;47:8475–8484. doi: 10.1007/s11033-020-05888-4. [DOI] [PubMed] [Google Scholar]
- 11.Jahromi M.F., Ray A.B., Chansouria J. Antihyperlipidemic effect of flavonoids from Pterocarpus marsupium. J Nat Prod. 1993;56:989–994. doi: 10.1021/np50097a001. [DOI] [PubMed] [Google Scholar]
- 12.Ameer B., Weintraub R.A., Johnson J.V., Yost R.A., Rouseff R.L. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin. Pharmacol. Ther. 1996;60:34–40. doi: 10.1016/S0009-9236(96)90164-2. [DOI] [PubMed] [Google Scholar]
- 13.Cavia‐Saiz M., Busto M.D., Pilar‐Izquierdo M.C., Ortega N., Perez‐Mateos M., Muñiz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J. Sci. Food Agric. 2010;90:1238–1244. doi: 10.1002/jsfa.3959. [DOI] [PubMed] [Google Scholar]
- 14.Dou W., Zhang J., Sun A., Zhang E., Ding L., Mukherjee S., Wei X., et al. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013;110:599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel K., Singh G.K., Patel D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018;24:551–560. doi: 10.1007/s11655-014-1960-x. [DOI] [PubMed] [Google Scholar]
- 16.Krause M., Galensa R. Determination of naringenin and naringenin-chalcone in tomato skins by reversed phase HPLC after solid-phase extraction. Zeitschrift fur Lebensmittel-Untersuchung und-Forschung. 1992;194:29–32. doi: 10.1007/BF01191036. [DOI] [Google Scholar]
- 17.Salehi B., Fokou P.V.T., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., Sharifi-Rad J. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renugadevi J., Prabu S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256:128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Renugadevi J., Prabu S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010;62:171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Koyuncu I., Kocyigit A., Gonel A., Arslan E., Durgun M. The protective effect of naringenin-oxime on cisplatin-induced toxicity in rats. Biochem Res Int. 2017 doi: 10.1155/2017/9478958. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Wang W., Hu H., Tang N., Zhang C., Liang W., Wang M. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-β1 in cultured rat hepatic stellate cells. Pharm. Res. (N. Y.) 2006;23:82–89. doi: 10.1007/s11095-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 22.Rashmi R., Magesh S.B., Ramkumar K.M., Suryanarayanan S., SubbaRao M.V. Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep Biochem Mol Biol. 2018;7:76. [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/8416763. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valko M., Izakovic M., Mazur M., Rhodes C.J., Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 25.Valko M., Leibfritz D., Moncola J., Cronin M.D., Mazur M. Telser J, Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Willcox J.K., Ash S.L., Catignani G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 28.Pizzino G., Bitto A., Interdonato M., et al. Oxidative stress and DNA repair and detoxification gene expression in adolescents exposed to heavy metals living in the Milazzo-Valle del Mela area (Sicily, Italy) Redox Biol. 2014;2:686–693. doi: 10.1016/j.redox.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 30.Young I., Woodside J. Antioxidants in health and disease. J. Clin. Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahorun T., Soobrattee M.A., Luximon-Ramma V., Aruoma O.I. Free radicals and antioxidants in cardiovascular health and disease. Internet J. Med. Update. 2006;1:1–17. doi: 10.4314/ijmu.v1i2.39839. [DOI] [Google Scholar]
- 32.Chatterjee M., Saluja R., Kanneganti S., Chinta S., Dikshit M. Biochemical and molecular evaluation of neutrophil NOS in spontaneously hypertensive rats. Cell Mol Biol (Noisy-le-grand) 2007;53:84–93. [PubMed] [Google Scholar]
- 33.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31:S181. doi: 10.2337/dc08-s245. –S184. [DOI] [PubMed] [Google Scholar]
- 34.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halliwell B. Role of free radicals in neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 37.Singh R.P., Sharad S., Kapur S. Free radicals and oxidative stress in neurodegenerative diseases: relevance of dietary antioxidants. J. Indian Acad. Clin. Med. 2004;5:218–225. [Google Scholar]
- 38.Christen Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 39.Butterfield D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic. Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 40.Hoshino Y., Mishima M. Antioxidants & redox signaling redox-based therapeutics for lung diseases. Antioxidants Redox Signal. 2008;10:701–704. doi: 10.1089/ars.2007.1961. [DOI] [PubMed] [Google Scholar]
- 41.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 42.Mahajan A., Tandon V.R. Antioxidants and rheumatoid arthritis. J. Indian Rheumatol. Assoc. 2004;12:139–142. [Google Scholar]
- 43.Galle J. Oxidative stress in chronic renal failure. Nephrol. Dial. Transplant. 2001;16:2135–2142. doi: 10.1093/ndt/16.11.2135. [DOI] [PubMed] [Google Scholar]
- 44.Sadeg N., Pham-Huy C., Martin C., Warnet J.M., Claude J.R. Effect of cyclosporin A and its metabolites and analogs on lipid peroxidation in rabbit renal microsomes. Drug Chem. Toxicol. 1993;16:165–174. doi: 10.3109/01480549309031994. [DOI] [PubMed] [Google Scholar]
- 45.Massicot F., Martin C., Dutertre-Catella H., Ellouk-Achard S., Pham-Huy C., Thevenin M., Rucay P., et al. Modulation of energy status and cytotoxicity induced by FK506 and cyclosporin A in a renal epithelial cell line. Arch. Toxicol. 1997;71:529–531. doi: 10.1007/s002040050423. [DOI] [PubMed] [Google Scholar]
- 46.Massicot F., Lamouri A., Martin C., Pham-Huy C., Heymans F., Warnet J.M., Godfroid J.J., et al. Preventive effects of two PAF-antagonists, PMS 536 and PMS 549, on cyclosporin-induced LLC-PK1 oxidative injury. J. Lipid Mediat. Cell Signal. 1997;15:203–214. doi: 10.1016/s0929-7855(96)00555-x. [DOI] [PubMed] [Google Scholar]
- 47.Valko M., Morris H., D Cronin M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 48.Samuel J.B., Stanley J.A., Princess R.A., Shanthi P., Sebastian M.S. Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. J. Med. Toxicol. 2011;7:195–204. doi: 10.1007/s13181-011-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Interdonato M., Pizzino G., Bitto A., Galfo F., Irrera N., Mecchio A., Pallio G., et al. Cadmium delays puberty onset and testis growth in adolescents. Clin. Endocrinol. 2015;83:357–362. doi: 10.1111/cen.12704. [DOI] [PubMed] [Google Scholar]
- 50.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruce K.D., Hanson M.A. The developmental origins, mechanisms, and implications of metabolic syndrome. J. Nutr. 2010;140:648–652. doi: 10.3945/jn.109.111179. [DOI] [PubMed] [Google Scholar]
- 52.Després J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z.H., Miyahara H., Takeo J., Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab Syndr. 2012;4:32. doi: 10.1186/1758-5996-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madrigal-Santillán E., Madrigal-Bujaidar E., Álvarez-González I., Sumaya-Martínez M.T., Gutiérrez-Salinas J., Bautista M., Morales-González Á., et al. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanaze F., Bounartzi M., Georgarakis M., Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007;61 doi: 10.1038/sj.ejcn.1602543. 472-47. [DOI] [PubMed] [Google Scholar]
- 57.Pereira-Caro G., Borges G., Van Der Hooft J., Clifford M.N., Del Rio D., Lean M.E., Roberts S.A., et al. Orange juice (poly) phenols are highly bioavailable in humans. Am. J. Clin. Nutr. 2014;100:1378–1384. doi: 10.3945/ajcn.114.090282. [DOI] [PubMed] [Google Scholar]
- 58.Hertog M.G., Kromhout D., Aravanis C., Blackburn H., Buzina R., Fidanza F., Giampaoli S., et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 59.Keli S.O., Hertog M.G., Feskens E.J., Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch. Intern. Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 60.Ishii K., Furuta T., Kasuya Y. Determination of naringin and naringenin in human urine by high-performance liquid chromatography utilizing solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 1997;704:299–305. doi: 10.1016/s0378-4347(97)00474-x. [DOI] [PubMed] [Google Scholar]
- 61.Park J.-H., Lee J.-W., Paik H.-D., Cho S.G., Nah S.-Y., Park Y.-S., Han Y.S. Cytotoxic effects of 7-O-butyl naringenin on human breast cancer MCF-7 cells. Food Sci. Biotechnol. 2010;19:717–724. doi: 10.1007/s10068-010-0101-3. [DOI] [Google Scholar]
- 62.Johnson A.R., Justin Milner J., Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiran S., Rohini P., Bhagyasree P. Flavonoid: a review on naringenin. J. Pharmacogn. Phytochem. 2017;6:2778–2783. [Google Scholar]
- 64.Pereira-Caro G., Polyviou T., Ludwig I.A., Nastase A.-M., Moreno-Rojas J.M., Garcia A.L., Malkova D., et al. Bioavailability of orange juice (poly) phenols: the impact of short-term cessation of training by male endurance athletes. Am. J. Clin. Nutr. 2017;106:791–800. doi: 10.3945/ajcn.116.149898. [DOI] [PubMed] [Google Scholar]
- 65.Shulman M., Cohen M., Soto-Gutierrez A., Yagi H., Wang H., Goldwasser J., Lee-Parsons C.W., et al. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Wang S., Firempong C.K., Zhang H., Wang M., Zhang Y., Zhu Y., et al. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: preparation and in vitro and in vivo evaluations. AAPS PharmSciTech. 2017;18:586–594. doi: 10.1208/s12249-016-0537-8. [DOI] [PubMed] [Google Scholar]
- 67.Yang F., Hu S., Sheng X., Liu Y. Naringenin loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in hepatic fibrosis. Biomed. Microdevices. 2020;22:1–9. doi: 10.1007/s10544-020-00524-1. [DOI] [PubMed] [Google Scholar]
- 68.Smruthi M., Nallamuthu I., Anand T. A comparative study of optimized naringenin nanoformulations using nano-carriers (PLA/PVA and zein/pectin) for improvement of bioavailability. Food Chem. 2022;369 doi: 10.1016/j.foodchem.2021.130950. [DOI] [PubMed] [Google Scholar]
- 69.Bagheri E., Ansari L., Abnous K., Taghdisi S.M., Naserifar M., Ramezani M., Alibolandi M. Silica-magnetic inorganic hybrid nanomaterials as versatile sensing platform. Nanomedicine Journal. 2020;7 doi: 10.22038/nmj.2020.07.0002. 183-19. [DOI] [Google Scholar]
- 70.Bagheri E., Ansari L., Sameiyan E., Abnous K., Taghdisi S.M., Ramezani M., Alibolandi M. Sensors design based on hybrid gold-silica nanostructures. Biosens. Bioelectron. 2020;153 doi: 10.1016/j.bios.2020.112054. [DOI] [PubMed] [Google Scholar]
- 71.Bagheri E., Ansari L., Abnous K., Taghdisi S.M., Ramezani P., Ramezani M., Alibolandi M. Silica–quantum dot nanomaterials as a versatile sensing platform. Crit. Rev. Anal. Chem. 2021;51:687–708. doi: 10.1080/10408347.2020.1768358. [DOI] [PubMed] [Google Scholar]
- 72.Fallahnia N., Ansari L., Mohammadkhani H., Mousazadeh F., Khah M., Ketabchi G. Oral cancer: nanoparticles as a new horizon in the diagnosis and phototherapy-based therapies. Nanomedicine Research Journal. 2022;7:124–139. doi: 10.22034/nmrj.2022.02.002. [DOI] [Google Scholar]
- 73.Hanafi-Bojd M.Y., Ansari L., Malaekeh-Nikouei B. Codelivery of anticancer drugs and siRNA by mesoporous silica nanoparticles. Ther. Deliv. 2016;7:649–655. doi: 10.4155/tde-2016-0045. [DOI] [PubMed] [Google Scholar]
- 74.Nasirizadeh S., Jaafari M.R., Iranshahi M., Golmohammadzadeh S., Mahmoudi A., Ansari L., Mosallaei N., et al. The effect of efflux pump inhibitors on in vitro and in vivo efficacy of solid lipid nanoparticles containing SN38. J. Drug Deliv. Sci. Technol. 2020;60 doi: 10.1016/j.jddst.2020.101969. [DOI] [Google Scholar]
- 75.Hanafi-Bojd M.Y., Ansari L., Mosaffa F., Malaekeh-Nikouei B. The effect of mesoporous silica nanoparticles loaded with epirubicin on drug-resistant cancer cells. Nanomedicine Journal. 2017;4 doi: 10.22038/NMJ.2017.8954. 135-14. [DOI] [Google Scholar]
- 76.Ansari L., Mashayekhi‐Sardoo H., Baradaran Rahimi V., Yahyazadeh R., Ghayour‐Mobarhan M., Askari V.R. Curcumin‐based nanoformulations alleviate wounds and related disorders: a comprehensive review. Biofactors. 2023;49:736–781. doi: 10.1002/biof.1945. [DOI] [PubMed] [Google Scholar]
- 77.Derakhshi M., Naseri M., Vafaeipour Z., Malaekeh-Nikouei B., Jafarian A.H., Ansari L. Enhanced wound-healing efficacy of electrospun mesoporous hydroxyapatite nanoparticle-loaded chitosan nanofiber developed using pluronic F127. Int. J. Biol. Macromol. 2023;240 doi: 10.1016/j.ijbiomac.2023.124427. [DOI] [PubMed] [Google Scholar]
- 78.Zobeiri M., Belwal T., Parvizi F., Naseri R., Farzaei M.H., Nabavi S.F., Sureda A., et al. Naringenin and its nano-formulations for fatty liver: cellular modes of action and clinical perspective. Curr Pharm Biotechnol. 2018;19:196–205. doi: 10.2174/1389201019666180514170122. [DOI] [PubMed] [Google Scholar]
- 79.Basir K.S., Mufida H., Ismail N.I. Synthesis and physicochemical characterization of naringenin-and gallic acid-loaded polymeric micelles for cancer drug delivery. Malaysian Journal of Medicine and Health Sciences. 2022 doi: 10.47836/mjmhs.18.s6.7. [DOI] [Google Scholar]
- 80.Maity S., Mukhopadhyay P., Kundu P.P., Chakraborti A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—an in vitro and in vivo approach. Carbohydr. Polym. 2017;170:124–132. doi: 10.1016/j.carbpol.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 81.Munir A., Muhammad F., Zaheer Y., Ali A., Iqbal M., Rehman M., Munir M.U., et al. Synthesis of naringenin loaded lipid based nanocarriers and their in-vivo therapeutic potential in a rheumatoid arthritis model. J. Drug Deliv. Sci. Technol. 2021;66 doi: 10.1016/j.jddst.2021.102854. [DOI] [Google Scholar]
- 82.Wang L., Wang X., Shen L., Alrobaian M., Panda S.K., Almasmoum H.A., Ghaith M.M., et al. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. Biomed. Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111461. [DOI] [PubMed] [Google Scholar]
- 83.Ahmad N., Ahmad R., Ahmad F.J., Ahmad W., Alam M.A., Amir M., Ali A. Poloxamer-chitosan-based Naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J. Biol. Sci. 2020;27:500–551. doi: 10.1016/j.sjbs.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonifacio B.V., da Silva P.B., dos Santos Ramos M.A., Negri K.M.S., Bauab T.M., Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int. J. Nanomed. 2014;9:1. doi: 10.2147/IJN.S52634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Klaunig J.E. Oxidative stress and cancer. Curr. Pharmaceut. Des. 2018;24:4771–4778. doi: 10.2174/1381612825666190215121712. [DOI] [PubMed] [Google Scholar]
- 87.Forcados G.E., James D.B., Sallau A.B., Muhammad A., Mabeta P. Oxidative stress and carcinogenesis: potential of phytochemicals in breast cancer therapy. Nutr. Cancer. 2017;69:365–374. doi: 10.1080/01635581.2017.1267777. [DOI] [PubMed] [Google Scholar]
- 88.Munir D., Maria A., Bashiruddin J. The antioxidant effect of curcumin on cochlear fibroblasts in rat models of diabetes mellitus. Iran J Otorhinolaryngol. 2017;29:197. [PMC free article] [PubMed] [Google Scholar]
- 89.Hong Y., Yin Y., Tan Y., Hong K., Zhou H. The flavanone, naringenin, modifies antioxidant and steroidogenic enzyme activity in a rat model of letrozole-induced polycystic ovary syndrome. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019;25:395. doi: 10.12659/MSM.912341. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Ebrahimi Fard A., Tavakoli M., Salehi H., Emami H. Synergetic effects of docetaxel and ionizing radiation reduced cell viability on MCF-7 breast cancer cell. Applied Cancer Research. 2017;37:1–12. doi: 10.1186/s41241-017-0035-7. [DOI] [Google Scholar]
- 91.Minafra L., Porcino N., Bravatà V., Gaglio D., Bonanomi M., Amore E., Cammarata F.P., et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-47553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hermawan A., Ikawati M., Jenie R.I., Khumaira A., Putri H., Nurhayati I.P., Angraini S.M., et al. Identification of potential therapeutic target of naringenin in breast cancer stem cells inhibition by bioinformatics and in vitro studies. Saudi Pharm J. 2021;29:12–26. doi: 10.1016/j.jsps.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neupane B.P., Chaudhary D., Paudel S., Timsina S., Chapagain B., Jamarkattel N., Tiwari B.R. Himalayan honey loaded iron oxide nanoparticles: synthesis, characterization and study of antioxidant and antimicrobial activities. Int. J. Nanomed. 2019;14:3533. doi: 10.2147/IJN.S196671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Askar M.A., El Shawi O.E., Mansour N.A., Hanafy A.M. Breast cancer suppression by curcumin-naringenin-magnetic-nano-particles: in vitro and in vivo studies. Tumour Biol. 2021;43:225–247. doi: 10.3233/TUB-211506. [DOI] [PubMed] [Google Scholar]
- 95.Shaterabadi Z., Nabiyouni G., Soleymani M. High impact of in situ dextran coating on biocompatibility, stability and magnetic properties of iron oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2017;75:947–956. doi: 10.1016/j.msec.2017.02.143. [DOI] [PubMed] [Google Scholar]
- 96.Unsal V., Dalkıran T., Çiçek M., Kölükçü E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Adv Pharm Bull. 2020;10:184. doi: 10.34172/apb.2020.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flora S.J., Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7:2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jahanshahi M., Khalili M., Margedari A. Naringin chelates excessive iron and prevents the formation of amyloid-beta plaques in the hippocampus of iron-overloaded mice. Front. Pharmacol. 2021;12:1518. doi: 10.3389/fphar.2021.651156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jagetia G.C., Reddy T.K., Venkatesha V., Kedlaya R. Influence of naringin on ferric iron induced oxidative damage in vitro. Clin. Chim. Acta. 2004;347:189–197. doi: 10.1016/j.cccn.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 100.Al-Ghamdi N.A.M., Virk P., Hendi A., Awad M., Elobeid M. Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia. Oreochromis niloticus, Green Processing and Synthesis. 2021;10:392–402. doi: 10.1515/gps-2021-0037. [DOI] [Google Scholar]
- 101.Amić D., Davidović-Amić D., Bešlo D., Trinajstić N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta. 2003;76:55–61. [Google Scholar]
- 102.Ozkaya A., Sahin Z., Dag U., Ozkaraca M. Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J. Biochem. Mol. Toxicol. 2016;30:243–248. doi: 10.1002/jbt.21785. [DOI] [PubMed] [Google Scholar]
- 103.Ma Y., Li P., Chen D., Fang T., Li H., Su W. LC/MS/MS quantitation assay for pharmacokinetics of naringenin and double peaks phenomenon in rats plasma. Int J Pharm. 2006;307:292–299. doi: 10.1016/j.ijpharm.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 104.Corsi L. Effects of the novel non-peptidyl low molecular weight radical scavenger IAC in different models of inflammation: a new perspective in anti-inflammatory therapy. Curr. Med. Chem. 2010;17:3918–3924. doi: 10.2174/092986710793205390. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto A., Itoh T., Nasu R., Nishida R. Sodium alginate ameliorates indomethacin-induced gastrointestinal mucosal injury via inhibiting translocation in rats. World J. Gastroenterol. 2014;20:2641. doi: 10.3748/wjg.v20.i10.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang H., He Y., Hou Y., Geng Y., Wu X. Novel self-nanomicellizing formulation based on Rebaudioside A: a potential nanoplatform for oral delivery of naringenin. Mater. Sci. Eng. C. 2020;112 doi: 10.1016/j.msec.2020.110926. [DOI] [PubMed] [Google Scholar]
- 107.George D., Maheswari P.U., Begum K.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: kinetics and in-vitro biological studies. Carbohydr. Polym. 2020;236 doi: 10.1016/j.carbpol.2020.116101. [DOI] [PubMed] [Google Scholar]
- 108.Sandri G., Bonferoni M., Ferrari F., Rossi S., Mori M., Caramella C. Opportunities offered by chitosan-based nanotechnology in mucosal/skin drug delivery. Curr. Top. Med. Chem. 2015;15:401–412. doi: 10.2174/1568026615666150108124122. [DOI] [PubMed] [Google Scholar]
- 109.Kumar S.P., Birundha K., Kaveri K., Devi K.R. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015;78:87–95. doi: 10.1016/j.ijbiomac.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 110.Khan N., Bharali D.J., Adhami V.M., Siddiqui I.A., Cui H., Shabana S.M., Mousa S.A., et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014;35:415–423. doi: 10.1093/carcin/bgt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chopp M., Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 112.Parr A.M., Kulbatski I., Tator C.H. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J. Neurotrauma. 2007;24:835–845. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- 113.Amini N., Vousooghi N., Alizade A., Ramezani S., Joghataei M.T., Milan P.B., Mehrabi S., et al. Transplantation of adipose tissue-derived stem cells into brain through cerebrospinal fluid in rat models: protocol development and initial outcome data. Curr. Stem Cell Res. Ther. 2019;14:191–195. doi: 10.2174/1574888X13666180720112322. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad A., Fauzia E., Kumar M., Mishra R.K., Kumar A., Khan M.A., Raza S.S., et al. Gelatin-coated polycaprolactone nanoparticle-mediated naringenin delivery rescue human mesenchymal stem cells from oxygen glucose deprivation-induced inflammatory stress. ACS Biomater. Sci. Eng. 2018;5:683–695. doi: 10.1021/acsbiomaterials.8b01081. [DOI] [PubMed] [Google Scholar]
- 115.Slungaard A., Mahoney J., Jr. Thiocyanate is the major substrate for eosinophil peroxidase in physiologic fluids. Implications for cytotoxicity. J. Biol. Chem. 1991;266:4903–4910. [PubMed] [Google Scholar]
- 116.Shingu M., Isayama T., Yasutake C., Naono T., Nobunaga M., Tomari K., Horie K., et al. Role of oxygen radicals and IL-6 in IL-1-dependent cartilage matrix degradation. Inflammation. 1994;18:613–623. doi: 10.1007/BF01535259. [DOI] [PubMed] [Google Scholar]
- 117.Ceña V., Játiva P. Nanoparticle crossing of blood–brain barrier: a road to new therapeutic approaches to central nervous system diseases. Nanomedicine (Lond). 2018;13:1513–1516. doi: 10.2217/nnm-2018-0139. [DOI] [PubMed] [Google Scholar]
- 118.Li P., Stetler R.A., Leak R.K., Shi Y., Li Y., Yu W., Bennett M.V., et al. Oxidative stress and DNA damage after cerebral ischemia: potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134:208–217. doi: 10.1016/j.neuropharm.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khuwaja G., Khan M.M., Ishrat T., Ahmad A., Raza S.S., Ashafaq M., Javed H., et al. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats: behavioral, neurochemical and immunohistochemical studies. Brain Res. 2011;1368:254–263. doi: 10.1016/j.brainres.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 120.Bjørklund G., Meguid N.A., El-Bana M.A., Tinkov A.A., Saad K., Dadar M., Hemimi M., et al. Oxidative stress in autism spectrum disorder. Mol. Neurobiol. 2020;57:2314–2332. doi: 10.1007/s12035-019-01742-2. [DOI] [PubMed] [Google Scholar]
- 121.Bhandari R., Paliwal J.K., Kuhad A. Naringenin and its nanocarriers as potential phytotherapy for autism spectrum disorders. J. Funct.Foods. 2018;47:361–375. doi: 10.1016/j.jff.2018.05.065. [DOI] [Google Scholar]
- 122.Frye R.E., Melnyk S., MacFabe D.F. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl. Psychiatry. 2013;3 doi: 10.1038/tp.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mani M., Balasubramanian S., Manikandan K.R., Kulandaivel B. Neuroprotective potential of Naringenin-loaded solid-lipid nanoparticles against rotenone-induced Parkinson's disease model. J App Pharm Sci. 2021;11:19–28. doi: 10.7324/JAPS.2021.110203. [DOI] [Google Scholar]
- 124.Gaba B., Khan T., Haider M.F., Alam T., Baboota S., Parvez S., Ali J. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson's disease model. BioMed Res. Int. 2019 doi: 10.1155/2019/2382563. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gera S., Sampathi S., Maddukuri S., Dodoala S., Junnuthula V., Dyawanapelly S. Therapeutic potential of naringenin nanosuspension: in vitro and in vivo anti-osteoporotic studies. Pharmaceutics. 2022;7:1449. doi: 10.3390/pharmaceutics14071449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smruthi M.R., Nallamuthu I., Singsit D., Anand T. Toxicological evaluation of PLA/PVA-naringenin nanoparticles: in vitro and in vivo studies. Opennano. 2022;7:100061–100075. doi: 10.1016/j.onano.2022.100061. [DOI] [Google Scholar]
- 127.Wang H., Li X., Yang H., Wang J., Li Q., Qu R., Wu X. Nanocomplexes based polyvinylpyrrolidone K-17PF for ocular drug delivery of naringenin. Int J Pharm. 2020;578 doi: 10.1016/j.ijpharm.2020.119133. [DOI] [PubMed] [Google Scholar]
- 128.Smruthi M., Nallamuthu I., Singsit D., Anand T. Toxicological evaluation of PLA/PVA-naringenin nanoparticles: in vitro and in vivo studies. OpenNano. 2022;7 doi: 10.1016/j.onano.2022.100061. [DOI] [Google Scholar]
- 129.Joshi H., Hegde A.R., Shetty P.K., Gollavilli H., Managuli R.S., Kalthur G., Mutalik S. Sunscreen creams containing naringenin nanoparticles: formulation development and in vitro and in vivo evaluations. Photodermatol. Photoimmunol. Photomed. 2018;34:69–81. doi: 10.1111/phpp.12335. [DOI] [PubMed] [Google Scholar]
- 130.Zaidun N.H., Thent Z.C., Latiff A.A. Combating oxidative stress disorders with citrus flavonoid: naringenin. Life Sci. 2018;208:111–122. doi: 10.1016/j.lfs.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 131.Karim N., Jia Z., Zheng X., Cui S., Chen W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Technol. 2018;79:35–54. doi: 10.1016/j.tifs.2018.06.012. [DOI] [Google Scholar]
- 132.Amawi H., Ashby C.R., Jr., Tiwari A.K. Cancer chemoprevention through dietary flavonoids: what's limiting? Chin. J. Cancer. 2017;36:50. doi: 10.1186/s40880-017-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jung U.J., Kim H.J., Lee J.S., Lee M.K., Kim H.O., Park E.J., Kim H.K., et al. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003;22:561–568. doi: 10.1016/S0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 134.Demonty I., Lin Y., Zebregs Y.E., Vermeer M.A., van der Knaap H.C., Jakel M., Trautwein E.A. The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J. Nutr. 2010;140:1615–1620. doi: 10.3945/jn.110.124735. [DOI] [PubMed] [Google Scholar]
- 135.Constans J., Bennetau-Pelissero C., Martin J.F., Rock E., Mazur A., Bedel A., Morand C., et al. Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men. Clin. Nutr. 2015;34:1093–1100. doi: 10.1016/j.clnu.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 136.Goncalves D., Lima C., Ferreira P., Costa P., Costa A., Figueiredo W., Cesar T. Orange juice as dietary source of antioxidants for patients with hepatitis c under antiviral therapy. Food Nutr. Res. 2017;61 doi: 10.1080/16546628.2017.1296675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reshef N., Hayari Y., Goren C., Boaz M., Madar Z., Knobler H. Antihypertensive effect of sweetie fruit in patients with stage i hypertension. Am. J. Hypertens. 2005;18:1360–1363. doi: 10.1016/j.amjhyper.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 138.Dallas C., Gerbi A., Elbez Y., Caillard P., Zamaria N., Cloarec M. Clinical study to assess the efficacy and safety of a citrus polyphenolic extract of red orange, grapefruit, and orange (Sinetrol-XPur) on weight management and metabolic parameters in healthy overweight individuals. Phytother Res. 2014;28:212–218. doi: 10.1002/ptr.4981. [DOI] [PubMed] [Google Scholar]
- 139.Barrera G., Cucci M.A., Grattarola M., Dianzani C., Muzio G., Pizzimenti S. Control of oxidative stress in cancer chemoresistance: spotlight on Nrf2 role. Antioxidants. 2021;10:510. doi: 10.3390/antiox10040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Araujo J.A., Zhang M., Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singh P., Bansal S., Kuhad A., Kumar A., Chopra K. Naringenin ameliorates diabetic neuropathic pain by modulation of oxidative-nitrosative stress, cytokines and MMP-9 levels. Food Funct. 2020;11:4548–4560. doi: 10.1039/C9FO00881K. [DOI] [PubMed] [Google Scholar]
- 142.Manchope M.F., Calixto-Campos C., Coelho-Silva L., Zarpelon A.C., Pinho-Ribeiro F.A., Georgetti S.R., Baracat M.M., et al. Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO− cGMP− PKG− KATPChannel signaling pathway. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kometsi L., Govender K., Mofo Mato E.P., Hurchund R., Owira P.M. By reducing oxidative stress, naringenin mitigates hyperglycaemia-induced upregulation of hepatic nuclear factor erythroid 2-related factor 2 protein. J. Pharm. Pharmacol. 2020;72:1394–1404. doi: 10.1111/jphp.13319. [DOI] [PubMed] [Google Scholar]
- 144.Ramprasath T., Senthamizharasi M., Vasudevan V., Sasikumar S., Yuvaraj S., Selvam G.S. Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J. Physiol. Biochem. 2014;70:407–415. doi: 10.1007/s13105-014-0318-3. [DOI] [PubMed] [Google Scholar]
- 145.Wang K., Chen Z., Huang L., Meng B., Zhou X., Wen X., Ren D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int. J. Mol. Med. 2017;40:1582–1590. doi: 10.3892/ijmm.2017.3134. [DOI] [PubMed] [Google Scholar]
- 146.Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu G.-H., Qu J., Shen X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 148.Madrigal J.L., Moro M.A., Lizasoain I., Lorenzo P., Fernández A.P., Rodrigo J., Boscá L., et al. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28:1579–1588. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- 149.Wong W.T., Tian X.Y., Chen Y., Leung F.P., Liu L., Lee H.K., Ng C.F., et al. Bone morphogenic Protein-4 impairs endothelial function through oxidative stress–dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ. Res. 2010;107:984–991. doi: 10.1161/CIRCRESAHA.110.222794. [DOI] [PubMed] [Google Scholar]
- 150.Shi Y., Vanhoutte P. Oxidative stress and COX cause hyper‐responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br. J. Pharmacol. 2008;154:639–651. doi: 10.1038/bjp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martínez-Revelles S., Avendaño M.S., García-Redondo A.B., Alvarez Y., Aguado A., Pérez-Girón J.V., García-Redondo L., et al. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxidants Redox Signal. 2013;18:51–65. doi: 10.1089/ars.2011.4335. [DOI] [PubMed] [Google Scholar]
- 152.Muñoz M., Sánchez A., Martínez M.P., Benedito S., López-Oliva M.-E., García-Sacristán A., Hernández M., et al. COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radic. Biol. Med. 2015;84:77–90. doi: 10.1016/j.freeradbiomed.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 153.Ali R., Shahid A., Ali N., Hasan S., Majed F., Sultana S. Amelioration of benzo [a] pyrene-induced oxidative stress and pulmonary toxicity by naringenin in Wistar rats: a plausible role of COX-2 and NF-κB. Hum. Exp. Toxicol. 2017;36:349–364. doi: 10.1177/0960327116650009. [DOI] [PubMed] [Google Scholar]
- 154.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? ntioxid Redox Signal. 2007;9:2277–2294. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 155.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Terrab L., Wipf P. Hsp70 and the unfolded protein response as a challenging drug target and an inspiration for probe molecule development. ACS Publications. 2020;11:232–236. doi: 10.1021/acsmedchemlett.9b00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vabulas R.M., Raychaudhuri S., Hayer-Hartl M., Hartl F.U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol. 2010;2:a004390. doi: 10.1101/cshperspect.a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y.-L., Shen H.-H., Cheng P.-Y., Chu Y.-J., Hwang H.-R., Lam K.-K., Lee Y.-M. 17-DMAG, an HSP90 inhibitor, ameliorates multiple organ dysfunction syndrome via induction of HSP70 in endotoxemic rats. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ruttkay-Nedecky B., Nejdl L., Gumulec J., Zitka O., Masarik M., Eckschlager T., Stiborova M., et al. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013;14:6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dam A.D., Mitchell A.S., Quadrilatero J. Induction of mitochondrial biogenesis protects against caspase-dependent and caspase-independent apoptosis in L6 myoblasts. Biochim. Biophys. Acta. 2013;1833:3426–3435. doi: 10.1016/j.bbamcr.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 161.Xin T., Lu C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY) 2020;12 doi: 10.18632/aging.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu L.-M., Dong X., Xue X.-D., Zhang J., Li Z., Wu H.-J., Yang Z.-L., et al. Naringenin improves mitochondrial function and reduces cardiac damage following ischemia-reperfusion injury: the role of the AMPK-SIRT3 signaling pathway. Food Funct. 2019;10:2752–2765. doi: 10.1039/C9FO00001A. [DOI] [PubMed] [Google Scholar]
- 163.Guarente L. vol. 72. Cold Spring Harb Symp Quant Biol; 2007. Sirtuins in aging and disease; pp. 483–488. (Cold Spring Harbor Symposia on Quantitative Biology). [DOI] [PubMed] [Google Scholar]
- 164.Alcendor R.R., Gao S., Zhai P., Zablocki D., Holle E., Yu X., Tian B., et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 165.Zhang J., Ren D., Fedorova J., He Z., Li J. SIRT1/SIRT3 modulates redox homeostasis during ischemia/reperfusion in the aging heart. Antioxidants. 2020;9:858. doi: 10.3390/antiox9090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Elibol B., Kilic U. High levels of SIRT1 expression as a protective mechanism against disease-related conditions. Front. Endocrinol. 2018;9:614. doi: 10.3389/fendo.2018.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lim K.H., Kim G.R. Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. Biomedical Dermatology. 2018;2:1–9. doi: 10.1186/s41702-018-0035-6. [DOI] [Google Scholar]
- 168.Qiu Q., Hu Z.-h., Xu H.-d., Xing H.-y., Sun H.-f., Zhang N., Lei X. Effect of naringenin on oxidative stress and tau protein phosphorylation of aβ25-35-induced PC12 cell injury. Chin. J. Exp. Tradit. Med. Formulae. 2020:92–99. [Google Scholar]
- 169.Clusan L., Ferrière F., Flouriot G., Pakdel F. A basic review on estrogen receptor signaling pathways in breast cancer. Int. J. Mol. Sci. 2023;24:6834. doi: 10.3390/ijms24076834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bathina S., Das U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Al-Dosari D.I., Ahmed M.M., Al-Rejaie S.S., Alhomida A.S., Ola M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients. 2017;9:1161. doi: 10.3390/nu9101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Olugbemide A.S., Ben-Azu B., Bakre A.G., Ajayi A.M., Femi-Akinlosotu O., Umukoro S. Naringenin improves depressive-and anxiety-like behaviors in mice exposed to repeated hypoxic stress through modulation of oxido-inflammatory mediators and NF-kB/BDNF expressions. Brain Res. Bull. 2021;169:214–227. doi: 10.1016/j.brainresbull.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 173.Hacioglu G., Senturk A., Ince I., Alver A. Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran J Basic Med Sci. 2016;19:388–393. [PMC free article] [PubMed] [Google Scholar]
- 174.Hashimoto K., Shimizu E., Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 175.Gibson D., Greaves E., Critchley H., Saunders P. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum. Reprod. 2015;30:1290–1301. doi: 10.1093/humrep/dev067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Long J.-y., Chen J.-m., Liao Y.-j., Zhou Y.-j., Liang B.-y., Zhou Y. Naringin provides neuroprotection in CCL2-induced cognition impairment by attenuating neuronal apoptosis in the hippocampus. Behav. Brain Funct. 2020;16:1–13. doi: 10.1186/s12993-020-00166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Polvani S., Tarocchi M., Galli A. PPARγ and oxidative stress: con (β) catenating NRF2 and FOXO. PPAR Res. 2012;2012 doi: 10.1155/2012/641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Forman B.M., Tontonoz P., Chen J., Brun R.P., Spiegelman B.M., Evans R.M. 15-deoxy-Δ12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 179.Fabisiak T., Patel M. Crosstalk between neuroinflammation and oxidative stress in epilepsy. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.976953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Qi Z., Xu Y., Liang Z., Li S., Wang J., Wei Y., Dong B. Naringin ameliorates cognitive deficits via oxidative stress, proinflammatory factors and the PPARγ signaling pathway in a type 2 diabetic rat model. Mol. Med. Rep. 2015;12:7093–7101. doi: 10.3892/mmr.2015.4232. [DOI] [PubMed] [Google Scholar]
- 181.Tarafdar A., Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018;19:3824. doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pengnet S., Prommaouan S., Sumarithum P., Malakul W. Naringin reverses high-cholesterol diet-induced vascular dysfunction and oxidative stress in rats via regulating LOX-1 and NADPH oxidase subunit expression. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/3708497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yan M., Mehta J.L., Zhang W., Hu C. LOX-1, oxidative stress and inflammation: a novel mechanism for diabetic cardiovascular complications. Cardiovasc. Drugs Ther. 2011;25:451–459. doi: 10.1007/s10557-011-6342-4. [DOI] [PubMed] [Google Scholar]
- 184.Sun H.J., Wu Z.Y., Nie X.W., Bian J.S. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front. Pharmacol. 2020;10:1568. doi: 10.3389/fphar.2019.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Andrade J.E., Burgess J.R. Effect of the citrus flavanone naringenin on oxidative stress in rats. J. Agric. Food Chem. 2007;55:2142–2148. doi: 10.1021/jf061714h. [DOI] [PubMed] [Google Scholar]
- 186.Prabu S.M., Shagirtha K., Renugadevi J. Naringenin in combination with vitamins C and E potentially protects oxidative stress-mediated hepatic injury in cadmium-intoxicated rats. J. Nutr. Sci. Vitaminol. 2011;57:177–185. doi: 10.3177/jnsv.57.177. [DOI] [PubMed] [Google Scholar]
- 187.Galati G., Sabzevari O., Wilson J.X., O'Brien P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 2002;177:91–104. doi: 10.1016/s0300-483x(02)00198-1. [DOI] [PubMed] [Google Scholar]
- 188.Rice-Evans C. Flavonoids and isoflavones: absorption, metabolism, and bioactivity. Free Radical Biol. Med. 2004;36:827–828. doi: 10.1016/j.freeradbiomed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 189.Felgines C., Texier O., Morand C., Manach C., Scalbert A., Regerat F., Remesy C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G1148–G1154. doi: 10.1152/ajpgi.2000.279.6.G1148. [DOI] [PubMed] [Google Scholar]
- 190.El-Saad A.M.A., Abdel-Wahab W.M. Naringenin attenuates toxicity and oxidative stress induced by lambda-cyhalothrin in liver of male rats. Pak J Biol Sci. 2020;23:510–517. doi: 10.3923/pjbs.2020.510.517. [DOI] [PubMed] [Google Scholar]
- 191.Jagetia G., Lalrinengi C. Treatment of mice with naringin alleviates the doxorubicin-induced oxidative stress in the liver of swiss albino mice. MOJ Anat. Physiol. 2017;4 doi: 10.15406/mojap.2017.04.00130. [DOI] [Google Scholar]
- 192.Mershiba S.D., Dassprakash M.V., Saraswathy S.D. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol. Biol. Rep. 2013;40:3681–3691. doi: 10.1007/s11033-012-2444-8. [DOI] [PubMed] [Google Scholar]
- 193.Da Pozzo E., Costa B., Cavallini C., Testai L., Martelli A., Calderone V., Martini C. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/9536148. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu H., Zhao H., Che J., Yao W. Naringenin protects against hypertension by regulating lipid disorder and oxidative stress in a rat model. Kidney Blood Press. Res. 2022;47:423–443. doi: 10.1159/000524172. [DOI] [PubMed] [Google Scholar]
- 195.Jain A., Dwivedi N., Bhargava R., Flora S.J. Silymarin and naringenin protects nicotine induced oxidative stress in young rats. Oxidants and Antioxidants in Medical Science. 2012;1:41–49. doi: 10.5455/oams.130412.or.004. [DOI] [Google Scholar]
- 196.Akondi R.B., Annapurna A. Protective effects of rutin and naringin on gentamycin induced testicular oxidative stress. J Reprod Infertil. 2011;8:57–64. doi: 10.29333/ejgm/82698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Taşlıdere A., Türkmen N., Ciftci O., Aydın M. Investigation into the protective effects of Naringenin in phthalates-induced reproductive damage. Eur. Rev. Med. Pharmacol. Sci. 2022;26:3419–3429. doi: 10.26355/eurrev_202205_28835. [DOI] [PubMed] [Google Scholar]
- 198.Umukoro S., Kalejaye H.A., Ben-Azu B., Ajayi A.M. Naringenin attenuates behavioral derangements induced by social defeat stress in mice via inhibition of acetylcholinesterase activity, oxidative stress and release of pro-inflammatory cytokines. Biomed. Pharmacother. 2018;105:714–723. doi: 10.1016/j.biopha.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 199.Sugumar M., Sevanan M., Sekar S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2019;129:534–539. doi: 10.1080/00207454.2018.1545772. [DOI] [PubMed] [Google Scholar]