Abstract
Emerging data highlights the heightened risk of atherosclerotic cardiovascular diseases (ASCVD) in patients with chronic inflammatory disorders, particularly those afflicted with inflammatory bowel disease (IBD). This review delves into the epidemiological connections between IBD and ASCVD, elucidating potential underlying mechanisms. Furthermore, it discusses the impact of current IBD treatments on cardiovascular risk. Additionally, the cardiovascular adverse effects of novel small molecule drugs used in moderate-to-severe IBD are investigated, drawing parallels with observations in patients with rheumatoid arthritis. This article aims to comprehensively evaluate the existing evidence supporting these associations.
To achieve this, we conducted a meticulous search of PubMed, spanning from inception to August 2023, using a carefully selected set of keywords. The search encompassed topics related to IBD, such as Crohn’s disease and ulcerative colitis, as well as ASCVD, including coronary artery disease, cardiovascular disease, atrial fibrillation, heart failure, conduction abnormalities, heart blocks, and premature coronary artery disease. This review encompasses various types of literature, including retrospective and prospective cohort studies, clinical trials, meta-analyses, and relevant guidelines, with the objective of providing a comprehensive overview of this critical intersection of inflammatory bowel disease and cardiovascular health.
Keywords: Inflammatory bowel diseases, Cardiovascular disorders, Pericarditis, myocarditis, Thromboembolism, Chronic inflammation, Oxidative stress, Endothelial dysfunction
Core Tip: A substantial association has been established between cardiovascular disorders (CVD) and inflammatory bowel diseases (IBD), with a notably higher prevalence of CVD in IBD patients compared to the general population. Potential mechanisms underlying CVD in IBD involve chronic inflammation, oxidative stress, altered platelet function, endothelial dysfunction, hypercoagulability, gut dysbiosis, and drug-related side effects. This review comprehensively synthesizes the latest evidence on the epidemiology, pathophysiological mechanisms, and cardiovascular manifestations in IBD.
INTRODUCTION
Inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory conditions affecting the gastrointestinal tract, characterized by a relapsing-remitting disease course. Extraintestinal symptoms may occur either concomitantly with or independently of luminal symptoms[1-4]. An association has been established between cardiovascular disorders (CVD) and IBD, with a notably higher prevalence of CVD in IBD patients compared to the general population. The cardiovascular manifestations in IBD patients encompass pericarditis, myocarditis, venous and arterial thromboembolism, atherosclerotic CVD, heart failure, arrhythmias and conduction disorders, infective endocarditis, valvulopathy, and rarely, Takayasu arteritis[5-7]. Potential mechanisms underlying CVD in IBD involve chronic inflammation, oxidative stress, altered platelet function, endothelial dysfunction, hypercoagulability, gut dysbiosis, and drug-related side effects[8]. This review comprehensively synthesizes the latest evidence on the epidemiology, pathophysiological mechanisms, and cardiovascular manifestations in IBD.
PATHOPHYSIOLOGY OF CVD IN IBD
The intricate pathogenesis linking IBD and CVD remains an enigma, characterized by a complex interplay of diverse factors. Dysregulated immune responses, endothelial dysfunction, a pro-thrombotic state, accelerated atherosclerosis, and genetic polymorphisms collectively contribute to this intricate web connecting IBD with CVD.
Chronic low-grade inflammation, marked by alterations in both innate and adaptive immunity, plays a pivotal role in the pathogenesis of atherosclerotic CVD[9]. In IBD, the stimulation of inflammatory T cell pathways, mediated by T helper (Th) 17 and Th1 responses fosters a pro-inflammatory milieu, leading to increased production of cytokines, including interleukin (IL)-1b, IL-6, IL-23, tumor necrosis factor (TNF), and interferon-gamma. Elevated expression of Toll-like receptors (TLR)-2 and TLR-4 further contributes to the pro-inflammatory state by amplifying IL-6 and IL-12 production[10,11]. These pro-inflammatory cytokines, via oxidative stress, provoke inflammation, tissue damage, and proliferation of endothelial and mesenchymal cells, synergistically contributing to the pathogenesis of CVD[12]. Furthermore, TNF, IL-1, IL-6, vascular endothelial growth factor (VEGF), and reactive oxidative species promote endothelial dysfunction by increasing the expression of cell adhesion molecules like ICAM-1, MCP-1, E selectin and intensify endothelial cell apoptosis, micro- and macrovascular dysfunction, tissue remodelling, angiogenesis, lymphangiogenesis, and fibrosis[13-15]. C-reactive protein (CRP), a marker of inflammation, is elevated in IBD and contributes to atherogenesis, correlating with increased CVD risk. Elevated CRP levels, especially exceeding 5 mg/L, serve as predictors of cardiovascular events. Notably, CRP levels rise with IBD disease activity, heightening cardiovascular risk during active disease[16,17].
Gut microbial dysbiosis, an important risk factor for IBD development, is also associated with CVD and increased thromboembolic event risk, particularly in younger age groups[12,18,19]. Alterations in the Firmicutes/Bacteroidetes ratio are linked to hypertension, while enrichment in Enterobacteriaceae, including Escherichia coli, is observed in patients with IBD and CVD[20-23]. Streptococcus spp. increase CVD risk, and opportunistic bacteria like Enterobacter and Oscillibacter are associated with ischemic stroke and transient ischemic attacks[24-26].
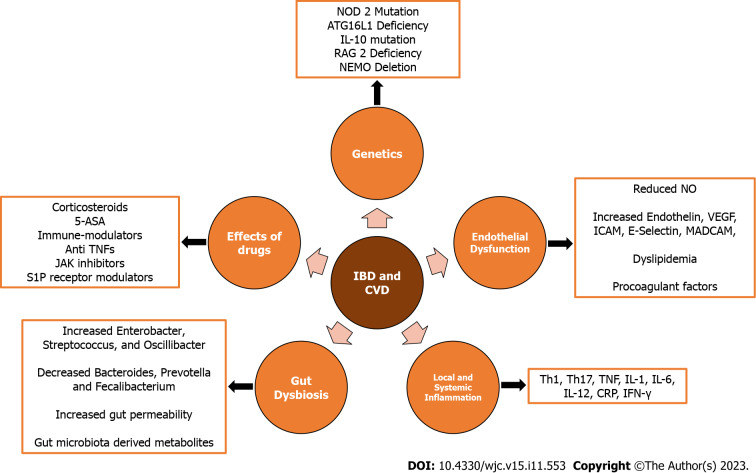
Gut dysbiosis can also increase gut permeability, leading to elevated absorption of lipopolysaccharide (LPS) from the intestines. The LPS, in turn, heightens pro-inflammatory cytokine secretion, exacerbating atherosclerosis, inducing macrophage activation, vascular endothelitis, and increasing CRP[27]. Gut bacterial metabolites, such as indole and phenyl derivatives, also exacerbate atherosclerosis and lead to hypertension[26]. Additionally, the gut bacteria-derived metabolite, Trimethylamine-N-oxide (TMAO), contributes to atherogenesis and hypertension, serving as a predictor of coronary artery disease. TMAO promotes platelet responsiveness, thrombosis, and cardiovascular risk through the expression of pro-inflammatory cytokines, ox-low density lipoprotein (LDL) deposition, and cardiac mitochondrial dysfunction[25,28,29]. The drugs used for treatment in IBD, through various mechanisms, are also associated with cardiovascular side effects and are discussed in the subsequent sections[30]. The pathogenesis of CVD in IBD is outlined in Figure 1.
Figure 1.
Factors implicated in development of cardiovascular disease in patients with inflammatory bowel disease. JAK: Janus kinase; ASA: Amino salicylic acid; TNF: Tumor necrosis factor; CVD: Cardiovascular diseases; IBD: Inflammatory bowel disease; VEGF: Vascular endothelial growth factor; IFN-γ: Interferon-gamma; CRP: C-reactive protein; S1P: Sphingosine-1-phosphate; IL: Interleukin.
CARDIOVASCULAR MANIFESTATIONS AND ITS MANAGEMENT IN IBD
Cardiovascular manifestations in IBD may be infrequent, yet they carry significant clinical implications when left unaddressed. We discuss the common CVD seen in patients with IBD.
Pericarditis and myocarditis
Pericarditis and myocarditis account for 70% and 10% of cardiac extra-intestinal manifestations (EIMs) respectively. Patients with IBD are at a greater risk of developing myopericarditis as compared to the general population[31,32]. Notably, pericarditis displays a higher incidence in males with UC[30-32]. On the other hand, myocarditis, constituting around 10% of cardiovascular EIMs in IBD, is more prevalent in patients diagnosed with CD[33].
Pathogenesis
It is difficult to determine whether the complications are secondary to the systemic disease or therapy related adverse events. Two possible mechanisms that are responsible for pericarditis and myocarditis in patients with IBD include immune mediated, secondary to the exposure of autoantigens, and cardiotoxicity associated with aminosalicylates and its derivatives[34-36].
Experimental models suggest that exposure to autoantigens produced during an acute flare of IBD, via inflammatory cytokines and an activated immune response, can lead to direct cytotoxicity of the cardiac myocytes[37]. This process may involve both the myocardium and pericardium and lead to myopericarditis. Continued inflammation and remodeling may result in chronic myocarditis which may cause valvular abnormalities (via papillary muscle fibroses and dysfunction), chamber dilation resulting in systolic dysfunction and decreased ejection fraction or arrhythmias[38,39].
Pericarditis almost exclusively occurs as a drug induced adverse event, in particular with 5-amino salicylic acid (ASA) derivatives such as sulfasalazine, mesalamine, and balsalazide[40-42]. The underlying mechanisms responsible for pericarditis associated with mesalamine include IgE-mediated allergic reactions, direct cardiac toxicity, cell-mediated hypersensitivity, or a humoral antibody response against 5-ASA derivatives[43].
Clinical features and diagnosis
Patients with 5-ASA induced pericarditis usually develop symptoms within two weeks of initiation of therapy[44]. Myopericarditis may present as acute coronary syndrome, new onset or decompensated heart failure, arrhythmias, cardiogenic shock or sudden death[45]. The electrocardiogram may show ST segment and T wave changes or conduction disorders. Leucocytosis, elevated levels of erythrocyte sedimentation rate, CRP and cardiac biomarkers such as troponin, creatine kinase-MB, B-type natriuretic peptide and N-terminal pro-brain natriuretic peptide may be present[30,46]. Echocardiographic features of myocarditis such as left ventricular dysfunction, anomalies of parietal kinetics, low ejection fraction, or pericardial effusion may be present. The cardiovascular magnetic resonance (CMR) imaging in a patient with myocarditis may reveal myocardial (regional or global) oedema, myocardial hyperaemia, and focal fibrosis or necrosis with non-coronary artery distribution[46]. The endomyocardial biopsy is the gold standard but is seldom performed in view of its invasive nature and availability of non-invasive CMR. The endomyocardial biopsy is however indicated in patients where CMR is not feasible or in life threatening conditions to establish the diagnosis and aetiology of myocarditis[39]. Histologically, two forms of IBD-associated myocarditis are known: The acute/chronic lymphocytic myocarditis and the giant cell myocarditis. Giant cell myocarditis is associated with a poor prognosis[47].
Management
The two major goals of treatment are optimal care of heart failure and arrhythmias, regardless of etiology and disease-specific therapy. Patients with fulminant myocarditis and hemodynamic instability should be shifted to intensive care units (ICU) with facilities of advanced cardiopulmonary support such as mechanical ventilation and extracorporeal membrane oxygenation[36,48].
Discontinuation of the causative drug remains the mainstay of treatment for pericarditis and resolution occurs within 2 wk. For inflammatory myocarditis associated with IBD, immune-suppressive treatment should always be considered especially in the presence of ventricular systolic dysfunction and severe arrhythmias[49-52]. The commonly used immune suppressive agents for treatment of inflammatory myocarditis are corticosteroids, azathioprine, cyclosporine, or immunoglobulins[53]. Interestingly, these agents are also used for treatment of IBD and therefore no specific alteration in therapy may be required in majority of the patients. The current guidelines also discourage patients with myocarditis from participating in competitive and leisure sports[54].
If pericarditis arises as an EIM, steroids are indicated after ruling out sepsis[55-57]. Alternatively, indomethacin, aspirin and colchicine can be used. However, their use can exacerbate underlying IBD and caution is recommended. Pericardial effusion and tamponade can complicate pericarditis which can be managed with pericardiocentesis or pericardiectomy[30,58].
VENOUS THROMBOEMBOLISM (VTE)
IBD patients are at an increased risk for VTE. Systematic reviews and meta-analyses report that patients with IBD are at a two-fold increased risk for VTE as compared to general population (RR = 2.20; 95%CI: 1.83-2.65)[59,60]. The most common reported VTE events include deep vein thrombosis (DVT) and/or pulmonary embolism (PE). The involvement of portal, the superior mesenteric, the splenic, the internal jugular, and the cerebral veins has also been reported[61]. The reported frequency is higher in patients with active IBD and directly proportional to the extent and severity of the disease in the absence of provoking factors[62]. In a retrospective study, VTE was more common in patients with UC (pancolitis more than left sided colitis or proctitis). In CD, VTE is more frequent in patients with ileocolonic or colonic involvement than with ileal disease alone[62].
The risk of in-hospital and post-hospitalisation VTE in patients with IBD is increased with intestinal or non-intestinal surgery when compared to non-IBD patients (OR = 2.03; 95%CI: 1.52-2.70 and OR = 4.45; 95%CI: 1.72-11.49, respectively). The risk factors for VTE include emergency surgery, open procedure, longer operative time, ileostomy formation, anastomotic leak, ileus, diagnosis of UC (higher risk as compared with CD), age > 65 years, and obesity[59,63,64]. Patients with IBD are also at a significantly high risk of recurrent VTE (HR = 2.5; 95%CI: 1.4-4.2; P = 0.001)[65].
Pregnant females with IBD (UC > CD, active disease) are also at two to three times increased risk of VTE during pregnancy and postpartum period (RR = 2.13; 95%CI: 1.66-2.73 and RR = 2.61; 95%CI: 1.84-3.69, respectively)[66,67].
Pathogenesis
The pathogenesis of VTE in IBD patients is multifactorial. The various mechanisms that contribute to thrombosis in IBD include genetic predisposition, inflammation, gut dysbiosis, spontaneous platelet aggregation, vascular thrombotic events secondary to flares, surgery, drug therapy and compounding risk factors such as pregnancy. Altered intestinal microbiota reduces mucus secretion and fibre fermentation that promotes inflammation via endothelial damage[68]. Genetic mutations in NOD2, ATG16L1, recombination activating gene 2, IL-10 receptor deficiency, and nuclear factor kappa beta essential modulator also lead to a pro-inflammatory state[36,69-71]. Inflammatory cytokines such as TNF and IL-1 result in a prothrombotic state due to increased levels of thrombin (which initiates the coagulation cascade through tissue factor) and simultaneous suppression of antithrombotic factors (such as endothelial thrombin and protein C)[72,73]. Another contributory mechanism is hyperhomocysteinemia secondary to inflammation induced malabsorption, and vitamin B and folate deficiency. Increased factor V thromboxane A2, arachidonic acid peroxidation product 8-iso-prostaglandin F2, tissue factor and mRNA synthesis further promote platelet activation and inhibit thromboregulation[74,75]. Estrogen based oral contraceptives and hormone replacement therapy promote production of coagulant factors leading to increased risk of VTE[76,77]. There is increased production of fibrinogen and decreased production of protein S during pregnancy which increases the risk of VTE[78].
IBD drugs and impact on VTE
5-ASA: No VTE or related complications have been reported with 5-ASA. In vitro studies have shown that 5-ASA inhibits platelet activation by thrombin, and therefore could have a role in preventing VTE. However further studies are required to evaluate the beneficial effect of 5-ASA in reducing the VTE risk[79-81].
Corticosteroids: Corticosteroids are potent anti-inflammatory drugs that also exert an independent thrombogenic effect (via increase in the serum levels of the clotting factors and fibrinogen)[82]. Systemic glucocorticoids and endogenous production of cortisol are associated with an increased risk of VTE. The risk of VTE is increased patients who are treated with corticosteroids, more so with higher doses [incidence risk ratio (IRR) = 2.31, 95%CI: 2.18-2.45][83,84].
Immunemodulators: There has been no reported risk of VTE with immunomodulatory drugs[85-87]. Although, it has been hypothesized that thiopurines reduce VTE risk by decreasing platelet aggregation and inhibiting platelet-leucocyte aggregation in vitro, more studies are required to confirm this hypothesis[88].
Biologics: TNF-α directly promotes endothelial dysfunction resulting in increased thrombus formation[89]. Patients treated with anti-TNF-α agents are therefore likely to have a decreased risk of VTE (OR = 0.267; 95%CI: 0.106-0.674, P = 0.005)[83]. It has been demonstrated that clot lysis profile normalises and there is a reversal of clotting abnormalities in patients receiving infliximab, suggesting benefit to reduce the VTE risk[63,72,82,90].
The overall risk of VTE with vedolizumab is low[91,92]. Pooled safety analysis from Phase 2/3 studies on ustekinumab reported no significant difference in VTE risk in patients treated with ustekinumab compared to placebo (0.75/100 person years vs 0.34/100 person years, respectively)[93-95].
Janus kinase (JAK) inhibitors: A safety study done in patients older than 50 year with rheumatoid arthritis and more than one cardiovascular risk factor showed that VTE, DVT and PE was higher for tofacitinib when used in the dose of 10 mg twice daily[95]. Similar to these observations, in the OCTAVE open study, with a follow-up of 7 years, 10 mg tofacitinib group had 0.1% and 0.7% prevalence of DVT and PE, respectively [IR = 0.06 (95%CI: 0.00-0.31) and 0.28 (95%CI: 0.09-0.65)]. There were no cases of DVT or PE in 5 mg tofacitinib group. Overall IR for tofacitinib was 0.06 (95%CI: 0.00-0.31) and 0.28 (95%CI: 0.09-0.65), for DVT and PE, respectively. Majority of the patients with thromboembolic complications had one or more underlying risk factors for DVT, except one patient with no pre-existing risk factors[96]. In IBD, therefore, tofacitinib appears to have an acceptable safety profile from VTE point of view, though The United States Food and Drug Administration has issued a black box warning recommending avoidance of JAK inhibitors in patients at risk of DVT, VTE and PE[97]. These risk factors include history of recent surgery, trauma, stroke or myocardial infarction (MI) in previous 3 mo, age > 50 years, morbid obesity, use of oral contraceptive pills, long flights and previous history of DVT, PE or acute thromboembolic event[98]. In case, when no therapeutic alternatives are available, a close coordination with cardiologist is required. The 10 mg twice daily dose is restricted to a maximum of 3 mo (for induction of remission) with de-escalation to 5 mg twice daily as soon as possible[99]. The randomized controlled trials (RCTs) of upadacitinib and filgotinib did not report a higher rate of VTE[100,101].
Management
All patients with IBD hospitalised for any cause, should receive a prophylactic dose of low-molecular-weight heparin (LMWH) or fondaparinux. LMWH is recommended over unfractionated heparin in critically ill patients[102]. Thromboprophylaxis during hospitalisation reduces the risk of VTE in IBD after discharge by 54% and should be maintained during the inpatient period[103]. Older age, Clostridioides difficile infection in index admission, longer hospital stay (> 7 d), ICU admission, previous VTE, and coronavirus disease are indications of extended prophylaxis (at least 2 mo after discharge) as these conditions are associated with increased the risk of post-discharge VTE[104]. IBD patients treated in the outpatient settings with moderate to severe flare and a high risk profile for VTE may benefit from thromboprophylaxis until resolution[105].
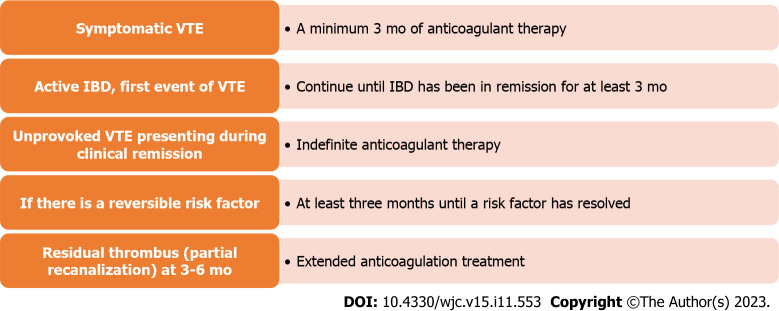
Guidelines recommend that the treatment of VTE should follow the general antithrombotic therapy guidelines. Direct oral anticoagulants are first line drugs and should be used at a therapeutic dose in IBD, LMWH is an alternative. In case of unprovoked VTE, the duration of treatment is indefinite. For provoked VTE secondary to an identifiable risk factor, anticoagulation is continued for 3 mo beyond the resolution of the risk factor. It is essential to know that thromboprophylaxis does not increase the risk of further IBD-related gastrointestinal bleeding in patients with active disease. It is important to remember that controlling the disease activity is most critical to prevent the recurrence of VTE[102,105,106]. The duration of anticoagulation is summarized in Figure 2.
Figure 2.
Duration of anticoagulation in inflammatory bowel disease patients with venous thromboembolism. IBD: Inflammatory bowel disease; VTE: Venous thromboembolism.
ATHEROSCLEROTIC AND ATHEROTHROMBOTIC CARDIOVASCULAR DISEASE (ASCVD)
In addition to VTE, there is a moderate increase in the risk of arterial thrombotic events, such as acute myocardial infarction (MI), mesenteric ischemia, and stroke, in IBD, albeit lower than the VTE risk. Interestingly, this risk is comparable between UC and CD patients[14]. The risk of ischemic heart disease (IHD) is slightly elevated in younger age groups and women, peaking within the first year of IBD diagnosis[12,13,107,108].
Pathogenesis
The pathogenesis involves a multifaceted interplay between inflammatory cytokines, endothelial dysfunction, smooth muscle proliferation mediated by VEGF, ICAM-1, MADCAM-1, E-selectin, reduced vasodilator nitric oxide, and NOD2 polymorphisms[106].
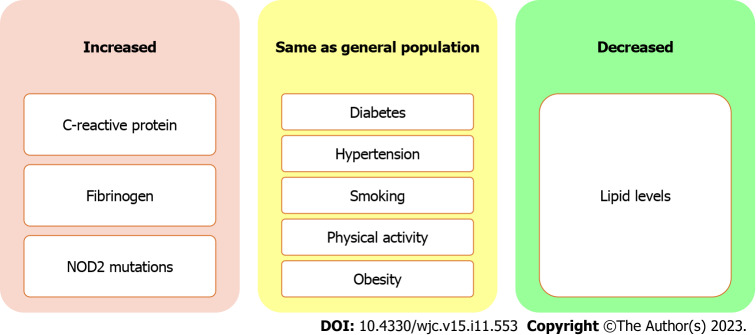
The incidence of CVD and cerebrovascular accidents is higher in females with IBD, and could be due to inherent differences in distribution of risk factors in males and females, including greater immune response and higher levels of CRP in females[109,110]. The role of sex hormones in the development of ASCVD in IBD patients remains inconclusive. Moreover, younger IBD patients exhibit an increased relative risk of ASCVD, possibly stemming from earlier disease onset and a more severe disease course that results in prolonged exposure to chronic inflammation. Notably patients with IBD have similar prevalence traditional risk factors for coronary artery disease such as hypertension, diabetes, smoking, and obesity[111] (Figure 3).
Figure 3.
Prevalence of risk factors for atherosclerotic cardiovascular disease in patients with inflammatory bowel disease.
Despite the lack of conventional risk factors for coronary artery disease, both UC and CD are independently associated with an increased risk of acute MI[112]. The IBD patients with CVD have been reported to have a higher level of high sensitive-CRP and fibrinogen, and greater prevalence of NOD-2 mutations[113]. Paradoxically, IBD patients tend to have lower levels of total cholesterol and LDL-cholesterol, with unaffected high density lipoprotein (HDL)-cholesterol and triglyceride concentrations. In CD patients, ileal resection and ileoanal anastomosis are inversely correlated with plasma total cholesterol and LDL-cholesterol levels[114,115].
The overall level of serum lipids in IBD patients is negatively associated with disease severity[113,116]. On the contrary, disease activity has been reported to be an independent risk factor for development of CVD[117-119]. This quandary is explained on the basis of presence of a more pro-atherogenic lipid profile characterized by small dense LDL-cholesterol particles and dysfunctional HDL-cholesterol in chronic inflammation associated with IBD[120]. The extent and location of inflammation is also associated with CVD risk. Patients with colonic involvement, in both UC and CD, have a threefold higher risk of developing MI[112].
Subclinical atherosclerosis
The occurrence of subclinical atherosclerosis is more frequent in individuals with IBD. To identify subclinical atherosclerosis, various diagnostic measures are employed, including assessing arterial stiffness through pulse-wave velocity between the carotid and femoral arteries (carotid-femoral pulse wave velocity, calculated as Δdistance/Δtime), measuring carotid intima-media thickness, evaluating flow-mediated dilation of arteries, and determining the coronary artery calcium score[106].
IBD drugs and impact on ASCVD
5-ASA: As with nonsteroid anti-inflmmatory drugs such as aspirin, 5-ASA shares anti-inflammatory, anti-platelet and antioxidant properties. Thus 5-ASAs may be associated with a decreased risk of IHD in patients with IBD[121,122]. IBD patients using 5-ASA were reported to have a lower risk of IHD than non-users (IRR = 1.16; 95%CI: 1.06-1.26 and IRR = 1.36; 95%CI: 1.22-1.51 P = 0.02, respectively). In long-term users of 5-ASA, the risk of IHD was even lower (IRR = 1.08; 95%CI: 0.98-1.19)[103,123].
Corticosteroids: Corticosteroid users are at a higher risk of developing IHD compared to non-users[123-125]. Corticosteroids predispose to risk factors such as hypertension, obesity, dyslipidemia and insulin resistance which may exacerbate IHD in IBD[126]. However, a direct causal association cannot be established.
Thiopurines: Thiopurines are not associated with acute arterial events in IBD and the effect of methotrexate on IHD in IBD is unknown. However in a beneficial effect on arterial stiffness has been demonstrated in various other chronic inflammatory disorders[127,128]. Thiopurines also decrease the production of transforming growth factor-beta and IL-10, which are responsible for endothelial dysfunction, and hence may have some protective role, though there is very limited data to make any conclusive recommendations at the moment[129,130].
Biologics: In vitro studies on infliximab have suggested an atheroprotective effect in monocytes by increasing both ABCA1 and LXR gene expression and removing excess cholesterol and preventing foam cell formation[127,129,131]. However, the in vivo biological mechanisms are very complex. TNF-α is proatherogenic. Contradictory results have been reported with regards to the effect of anti TNF-α agents on the lipid profile. While some studies report an increase the levels of HDL-cholesterol and apoprotein-A1, others report an increase in the small dense LDL-cholesterol and total cholesterol. Also, TNF-α inhibition increases abdominal fat, leading to increased risk of ASCVD. On the contrary, the anti TNF-α agents exert beneficial effect by improving insulin sensitivity, endothelial function, arterial stiffness and fibrinolysis[89,132,133]. The anti-TNF-α agents may be associated with reduced risk of new-onset acute arterial events and prevent recurrence when used in patients with previous history of acute arterial events. Vedolizumab and ustekinumab have not reported any augmented risk of ASCVD[134,135].
JAK inhibitors: Small molecules tend to increase the risk of cardiovascular diseases by causing dyslipidaemia. Tofacitinib is associated with reversible changes in the lipid profile specifically total cholesterol, HDL-cholesterol and LDL-cholesterol[136,137]. Clinical trials in rheumatoid arthritis showed that tofacitinib is associated with higher rates of major adverse cardiovascular events (MACE). Older patients aged > 50 years with at least one cardiovascular risk factor had higher risk of MACE (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) as compared with anti TNF agents (HR = 1.33; 95%CI: 0.91-1.94)[138]. However, the same has not been shown in the clinical trials in IBD. In the OCTAVE trials, one patient with several risk factors had acute coronary syndrome, one died of dissecting aortic aneurysm and one patient with a history of cardiovascular disease had congestive heart failure. During the maintenance phase, one subject with several risk factors receiving tofacitinib 5 mg twice daily had an adjudicated MACE (myocardial ischaemia/ myocardial infarction), and one patient, also with multiple risk factors, receiving tofacitinib 10 mg twice daily had an adjudicated MACE (haemorrhagic stroke). The overall incidence rate for MACE in the OCTAVE trials was 0.16 (95%CI: 0.04-0.42). Multiple real life studies of tofacitinib also did not demonstrate increase in the risk for MACE compared to anti TNF agents[96,139,140].
In a meta-analysis assessing safety of JAK inhibitors in IBD and other immune-mediated inflammatory diseases evaluated MACE in 32765 patients on JAK inhibitors (17 tofacitinib; 6 upadacitinib; 4 baricitinib; 3 filgotinib), the incidence rate of MACE was 0.67 per 100 patient-years[141]. Real life safety data on upadacitinib and filgotinib are lacking, however in the registry trials, MACE were infrequent and no difference was reported compared to placebo[142,143]. Though the risk of MACE appears low, JAK inhibitors should be used cautiously in patients over the age of 50 years with concomitant risk factors for CVD.
Management of ASCVD
Current risk assessment tools for predicting CVD, such as the Framingham risk score and the ASCVD risk calculator, lack validation for individuals with chronic inflammatory conditions, potentially leading to an underestimation of their CVD risk. European guidelines suggest incorporating a 1.5-fold multiplier when assessing the 10-year CVD risk in patients with rheumatoid arthritis. However, there remains an information void regarding whether a similar adjustment is warranted for patients with IBD.
Controlling inflammation in IBD is the key to reduce the risk of CVD[105]. Adequate treatment of underlying IBD with the aim to achieve and maintain remission is important. Additionally, the patients should be screened for atherosclerotic risk factors such as obesity, smoking, hypertension, diabetes, dyslipidaemia and positive family history[144]. Definitive role of statins in IBD is controversial, but statins in addition to the lipid lowering function have pleiotropic effects, including modulation of the immune system[145,146]. IBD is not a contraindication to low-dose aspirin for primary and/or secondary prevention.
HEART FAILURE
The risk of heart failure is twice as higher in IBD than non IBD subjects when adjusted for traditional cardiovascular risk factors with the highest risk reported in females with UC[112].
Heart failure in individuals with IBD may manifest as either new-onset (de novo) or as a consequence of deteriorating health in those with pre-existing conditions. These underlying conditions, which predispose individuals to heart failure, encompass IHD, hypertension, dyslipidemia, diabetes, smoking, valvular heart disease, congenital heart disease, etc. The frequent triggers for decompensation leading to heart failure involve infections or inadequate adherence to prescribed treatment regimens.
Pathogenesis
Heart failure in IBD could be a consequence of the chronic inflammation or the drug therapy used. The compromised integrity of the intestinal barrier and ongoing intestinal inflammation are contributing factors to the development of heart failure. This can be attributed to the translocation of bacterial LPS, which triggers the production of TNF-α. Both LPS and TNF-α are implicated in inducing structural changes in the heart that progress to heart failure[147,148]. Additionally, several other proposed mechanisms may contribute to the development of heart failure in these patients. These mechanisms include myocardial fibrosis due to altered collagen metabolism, impaired nitric oxide-mediated vasodilation, deficiencies in essential vitamins and trace elements, heart muscle atrophy resulting from prolonged corticosteroid use, total parenteral nutrition, myocarditis, endocarditis, and valvulopathy[30,149-151].
Anti-TNFs and heart failure
There have been case reports and studies of anti TNF induced heart failure[152,153]. The biological effects of TNF-α are mediated via two distinct cell surface receptors. TNFFR-1 is cardiotoxic and antagonising its action attenuates ventricular dysfunction and improves post MI survival whereas TNFR2 is cardioprotective and its inhibition upregulates TNFR1 and increases ventricular dysfunction and remodelling[154,155]. The effects of TNF-α are concentration dependent and involve two pathways. In lower concentrations, survivor activating factor enhancement pathway is activated, while higher concentration leads to stimulation of death-promoting pathway functions[156,157]. Chung et al[158] evaluated the effect of infliximab in patients with New York heart association (NYHA) Class III or IV heart failure with ejection fraction ≤ 35% and found that patients in the 10 mg/kg infliximab group were more likely to die or be hospitalized for heart failure than patients in the placebo group or 5 mg/kg infliximab group (HR = 2.84, 95%CI: 1.01-7.97; P = 0.043).
Prevention
Routine screening tests for cardiovascular diseases prior to the administration of biologics is not recommended. However, employment of an echocardiogram prior to initiation of anti TNF therapy to evaluate baseline cardiac function is vital[105,157]. Although there are no specific guidelines for the use of anti TNF in heart failure, it is suggested to avoid anti TNF agents in patients with NYHA class III or IV disease and switching to an alternative non-TNF inhibitor in patients with patients who develop acute heart failure on anti TNFs[105,157,159,160].
ARRHYTHMIAS AND CONDUCTION DISORDERS
As with other chronic inflammatory disorders, IBD carries a risk of major cardiac arrhythmias, which include atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation. The risk of arrhythmias correlates with the disease activity[43,161]. A large population-based cohort study found that risk of atrial fibrillation was increased in patients with IBD with a higher risk in CD and was particularly increased in younger patients with age < 45 years[21].
Pathogenesis
Although the pathogenesis of arrhythmias is incompletely understood, chronic inflammation is hypothesized to predisposes to rhythm disorders and conduction abnormalities in patients with IBD[30,162]. Also, patients on systemic steroids, immunomodulators or biologics had a higher risk, highlighting the role of moderate-to-severe active disease. Atrial electromechanical conduction delay, a predictor of atrial fibrillation, has been shown to be significantly prolonged in patients with IBD, especially those with active disease and longer disease duration.
IBD drugs and arrhythmias
Sphingosine-1-phosphate (S1P) receptor modulators (ozanimod) have been implicated in cardiac arrhythmias. S1PRMs have 5 G protein coupled receptor subtypes S1PR1 to S1PR5. The S1PR1, which is extensively expressed on cardiomyocytes and vascular endothelial cells, is the target of S1P modulators. In phase 3 RCT of ozanimod in UC, five cases of bradycardia were reported during the induction period and none during the maintenance period[163-165]. In the TOUCHSTONE open label long term extension study of ozanimod 1 mg per day in patients with UC, no bradycardia nor evidence of atrioventricular (AV) block was reported at 44 wk[163,166]. In the OASIS trial, a phase 2 induction trial of etrasimod which selectively target S1P1, S1P4, and S1P5, no such cardiac events were reported[167,168]. In the ELEVATE UC study, 5 patients receiving etrasimod reported bradycardia and 1 patient had first degree AV block that resolved without interventional treatment. The real-world studies, though scarce, did not report cardiac conduction abnormalities after 26 wk of treatment exposure of ozanimod.
Recommendations for patients with IBD to mitigate cardiovascular risk
The probability of experiencing cardiovascular events is inherently intertwined with the presence of systemic inflammation and the level of disease activity in individuals with IBD. It is imperative to adopt a proactive approach by conducting regular screenings and monitoring of cardiovascular risk factors for all IBD patients. Those identified as being at risk should adhere to established recommendations applicable to the general population. Collaborative efforts with cardiologists are vital in managing these risks effectively. Considering that these risk factors may evolve over time, especially with advancing age, routine screening and monitoring are indispensable for sustaining optimal cardiovascular well-being. It is of paramount importance to provide counseling and education to patients regarding their specific cardiovascular risks. Encouraging the adoption of healthy lifestyle modifications is crucial in this regard[169]. A concise summary of practical guidance for managing CVD in individuals with IBD is presented in Table 1.
Table 1.
Practical guide to management of cardiovascular diseases in inflammatory bowel disease
|
Cardiovascular disease
|
Risk factors
|
Suggested testing
|
Therapeutic considerations
|
| Pericarditis and myocarditis | Disease related | Onset of symptoms within 2-4 wk of starting 5-ASA | Discontinuation of therapy |
| Disease activity | |||
| Drugs | ECG: ST-T changes | Immunesuppressives for inflammation associated myocarditis | |
| 5-ASA | |||
| 2D Echocardiography: LV dysfunction, pericardial effusion | Pericardiocentesis or pericardial window, if cardiac tamponade | ||
| Cardiac MRI | Control IBD disease activity | ||
| Endo-myocardial biopsy, if cardiac MRI contraindicated or life threatening disease | |||
| Elevated cardiac biomarkers | |||
| Venous Thromboembolism | Patient related | Screening for genetic risk factors in patients with recurrent venous thromboembolic events | Thromboprophylaxis |
| Elderly age | All IBD patients during hospitalization of any cause | ||
| Females | Ambulatory patient with active IBD and known risk factors for VTE | ||
| Obesity | Prophylaxis should be maintained during the inpatient period | ||
| Malnutrition | |||
| Disease related | Treatment | ||
| Disease activity | LMWH | ||
| Colonic disease location | Direct oral anticoagulants | ||
| UC > CD | Cautious use of JAK inhibitors | ||
| Hospitalization | Aim the lowest effective dose to maintain remission | ||
| Emergency surgery | |||
| Longer operative time | |||
| Open surgery | |||
| Drugs | |||
| JAK inhibitors | |||
| Corticosteroids | |||
| Atherosclerotic cardiovascular disease | Patient related | Lipid profile at baseline, end of induction and every 6 mo | Treatment of ASCVD is similar to non IBD patients and should be done in close collaboration with an expert cardiologist |
| Younger age | |||
| Females | |||
| Disease related | Test for subclinical atherosclerosis | Control IBD disease activity | |
| Disease activity | Carotid intima media thickness | ||
| Colonic disease location | Pulse-wave velocity between the carotid and femoral arteries | ||
| Increased hs CRP | Coronary artery calcium | ||
| Increased fibrinogen | |||
| Drugs | 2D echocardiography/stress echocardiography/TMT | Cautious use of JAK inhibitors | |
| Corticosteroids | |||
| JAK inhibitors | |||
| Coronary angiography | Treat JAK inhibitor induced dyslipidemia/hyerlipidemia with statins | ||
| Heart failure | Patient related | 2D Echocardiography | Avoid anti TNF in NYHA Class III or IV heart failure, especially with ejection fraction ≤ 35% |
| Females | Ventricular dysfunction | ||
| Underlying cardiac structural diseases | Structural abnormalities | ||
| Diabetes | |||
| Hypertensive heart disease | |||
| Chagas disease | |||
| Deposit diseases | |||
| Valvular heart disease | |||
| Disease related | |||
| UC > CD | |||
| Drugs | |||
| Anti TNF agents in high dose | |||
| Arrhythmias and conduction abnormalities | Patient related | ECG | Control disease activity |
| Age > 65 yr | Increased P-wave dispersion | Caution with S1P receptor modulators | |
| Previous arrhythmias or cardiac conduction abnormalities | Increased QTc dispersion | Caution in patients with risk factors | |
| Ischemic heart disease | Prolonged QTc interval | ||
| Cardiomyopathy with septal involvement | |||
| Drugs (e.g: beta-blockers, calciumchannel inhibitors, antiarrhythmics) | |||
| Uncontrolled hypertension | |||
| Previous cardiac surgery | |||
| Surgical/percutaneous treatment of valvular disease | |||
| Disease related | |||
| Disease activity | |||
| Drugs | |||
| S1P receptor modulators |
ASA: Amino salicylic acid; ECG: Electrocardiogram; LV: Left ventricular; MRI: Magnetic resonance imaging; IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; VTE: Venous thromboembolism; LMWH: Low-molecular-weight heparin; JAK: Janus kinase; ASCVD: Atherosclerotic cardiovascular diseases; CRP: C-reactive protein; TMT: Treadmill test; TNF: Tumor necrosis factor; NYHA: New York heart association; S1P: Sphingosine-1-phosphate.
Future directions
The pathophysiology of CVD in IBD needs further elaboration. The current knowledge gaps include the following: Immunological mechanisms at play in both the development of IBD and the formation of atherosclerosis, prevalence of cardiometabolic risk factors, risk stratification and identification of IBD patients at the highest risk for cardiovascular complications, allowing for more targeted preventive measures, role of pre-emptive screening for subclinical atherosclerosis and its cost effectiveness, long term outcomes in patients with CVD and IBD, and effective strategies for monitoring cardiovascular risk factors in IBD patients, and how often should such monitoring occur.
CONCLUSION
The prevalence of cardiovascular manifestations in patients with IBD, though rare, is higher when compared to the general population. The CVD in IBD represent a complex and multifaceted relationship between chronic gastrointestinal inflammation and cardiovascular health. The inflammatory cytokines, immune responses and chronic systemic inflammation associated with disease activity contributes to the development and progression of CVD. Individual patient factors, such as age, gender, pre-existing cardiovascular conditions, and genetics, also play a significant role in determining the cardiovascular impact. The spectrum of CVD in IBD is wide. Additionally, the cardiovascular effects of drugs used in IBD are multifaceted and depend on various factors, including the specific medicines involved and individual patient characteristics.
In individuals with IBD who are at an elevated risk of cardiovascular issues, there is a need to shift the focus of care from a reactive approach to a proactive one, emphasizing preventive measures for cardiovascular management. To minimize cardiovascular risk a multidisciplinary approach involving gastroenterologists and cardiologists is often necessary. This will ensure that IBD treatment is optimized while minimizing cardiovascular risk.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 8, 2023
First decision: October 17, 2023
Article in press: November 8, 2023
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mostafavinia A, Iran S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
Contributor Information
Arshia Bhardwaj, Department of Gastroenterology, Dayanand Medical College and Hospital, Punjab, Ludhiana 141001, India.
Arshdeep Singh, Department of Gastroenterology, Dayanand Medical College and Hospital, Punjab, Ludhiana 141001, India.
Vandana Midha, Department of Internal Medicine, Dayanand Medical College and Hospital, Punjab, Ludhiana 141001, India.
Ajit Sood, Department of Gastroenterology, Dayanand Medical College and Hospital, Punjab, Ludhiana 141001, India.
Gurpreet Singh Wander, Department of Cardiology, Dayanand Medical College and Hospital, Punjab, Ludhiana 141001, India.
Bishav Mohan, Department of Cardiology, Dayanand Medical College and Hospital, Punjab, Ludhiana 141001, India.
Akash Batta, Department of Cardiology, Dayanand Medical College and Hospital, Punjab, Ludhiana 141001, India. akashbatta02@gmail.com.
References
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Chen JD. Therapeutic potential and mechanisms of sacral nerve stimulation for gastrointestinal diseases. J Transl Int Med. 2023;11:115–127. doi: 10.2478/jtim-2023-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mengzhu S, Yujie Z, Yafang S, Jing G, Yuhang W, Chen X, Dongmei GU, Jianhua S, Lixia P. Electroacupuncture alleviates water avoidance stress-induced irritable bowel syndrome in mice by improving intestinal barrier functions and suppressing the expression of inflammatory cytokines. J Tradit Chin Med. 2023;43:494–500. doi: 10.19852/j.cnki.jtcm.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkelhammer C, Andrejic J, Mohammed A. Endocarditis in Crohn's disease. Inflamm Bowel Dis. 2009;15:1293–1294. doi: 10.1002/ibd.20822. [DOI] [PubMed] [Google Scholar]
- 6.Kreuzpaintner G, Horstkotte D, Heyll A, Lösse B, Strohmeyer G. Increased risk of bacterial endocarditis in inflammatory bowel disease. Am J Med. 1992;92:391–395. doi: 10.1016/0002-9343(92)90269-h. [DOI] [PubMed] [Google Scholar]
- 7.Kruse MN, Poppele RE. Components of the dynamic response of mammalian muscle spindles that originate in the sensory terminals. Exp Brain Res. 1991;86:359–366. doi: 10.1007/BF00228959. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Hu T, Hao H, Hill MA, Xu C, Liu Z. Inflammatory bowel disease and cardiovascular diseases: a concise review. Eur Heart J Open. 2022;2:oeab029. doi: 10.1093/ehjopen/oeab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song B, Bie Y, Feng H, Xie B, Liu M, Zhao F. Inflammatory Factors Driving Atherosclerotic Plaque Progression New Insights. J Transl Int Med. 2022;10:36–47. doi: 10.2478/jtim-2022-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 11.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 12.Bigeh A, Sanchez A, Maestas C, Gulati M. Inflammatory bowel disease and the risk for cardiovascular disease: Does all inflammation lead to heart disease? Trends Cardiovasc Med. 2020;30:463–469. doi: 10.1016/j.tcm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Łykowska-Szuber L, Rychter AM, Dudek M, Ratajczak AE, Szymczak-Tomczak A, Zawada A, Eder P, Lesiak M, Dobrowolska A, Krela-Kaźmierczak I. What Links an Increased Cardiovascular Risk and Inflammatory Bowel Disease? A Narrative Review. Nutrients. 2021;13 doi: 10.3390/nu13082661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cibor D, Domagala-Rodacka R, Rodacki T, Jurczyszyn A, Mach T, Owczarek D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J Gastroenterol. 2016;22:1067–1077. doi: 10.3748/wjg.v22.i3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkim C, Alkim H, Koksal AR, Boga S, Sen I. Angiogenesis in Inflammatory Bowel Disease. Int J Inflam. 2015;2015:970890. doi: 10.1155/2015/970890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agca R, Smulders Y, Nurmohamed M. Cardiovascular disease risk in immune-mediated inflammatory diseases: recommendations for clinical practice. Heart. 2022;108:73–79. doi: 10.1136/heartjnl-2019-316378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Liu J, Li C, Gao J, Li X, Chen S, Wu S, Ding H, Fan H, Hou S. Cumulative Exposure to High-Sensitivity C-Reactive Protein Predicts the Risk of Cardiovascular Disease. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 19.Mengzhu S, Yujie Z, Yafang S, Jing G, Tingting Z, Yuhang W, Lixia P, Jianhua S. Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor. J Tradit Chin Med. 2022;42:732–740. doi: 10.19852/j.cnki.jtcm.20220719.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D'Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R, Jansson JK. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, Berg D, Schukken Y, Scherl E, Simpson KW. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 24.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen JY, Geng QS, Zhang ZW, Yang H, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L, Pan SY, Zhou HW. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huć T, Nowinski A, Drapala A, Konopelski P, Ufnal M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol Res. 2018;130:172–179. doi: 10.1016/j.phrs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 28.Wilson A, Teft WA, Morse BL, Choi YH, Woolsey S, DeGorter MK, Hegele RA, Tirona RG, Kim RB. Trimethylamine-N-oxide: A Novel Biomarker for the Identification of Inflammatory Bowel Disease. Dig Dis Sci. 2015;60:3620–3630. doi: 10.1007/s10620-015-3797-3. [DOI] [PubMed] [Google Scholar]
- 29.Mutengo KH, Masenga SK, Mweemba A, Mutale W, Kirabo A. Gut microbiota dependant trimethylamine N-oxide and hypertension. Front Physiol. 2023;14:1075641. doi: 10.3389/fphys.2023.1075641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunu DM, Timofte CE, Ciocoiu M, Floria M, Tarniceriu CC, Barboi OB, Tanase DM. Cardiovascular Manifestations of Inflammatory Bowel Disease: Pathogenesis, Diagnosis, and Preventive Strategies. Gastroenterol Res Pract. 2019;2019:3012509. doi: 10.1155/2019/3012509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abid MA, Gitlin N. Pericarditis--an extraintestinal complication of inflammatory bowel disease. West J Med. 1990;153:314–315. [PMC free article] [PubMed] [Google Scholar]
- 32.García-Morán S, Sáez-Royuela F, Pérez-Alvarez JC, Gento E, Téllez J. Myopericarditis and mitral insufficiency associated with ulcerative colitis treated with mesalazine. Inflamm Bowel Dis. 2006;12:334–335. doi: 10.1097/01.MIB.0000209788.19952.b7. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen HT, Fonager KM. Myocarditis and inflammatory bowel disease. A 16-year Danish nationwide cohort study. Dan Med Bull. 1997;44:442–444. [PubMed] [Google Scholar]
- 34.Kiyomatsu H, Kawai K, Tanaka T, Tanaka J, Kiyomatsu T, Nozawa H, Kanazawa T, Kazama S, Ishihara S, Yamaguchi H, Sunami E, Watanabe T. Mesalazine-induced Pleuropericarditis in a Patient with Crohn's Disease. Intern Med. 2015;54:1605–1608. doi: 10.2169/internalmedicine.54.4316. [DOI] [PubMed] [Google Scholar]
- 35.Okoro KU, Roby MD, Bankole AA. Myocarditis Secondary to Mesalamine-Induced Cardiotoxicity in a Patient with Ulcerative Colitis. Case Rep Med. 2018;2018:9813893. doi: 10.1155/2018/9813893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caforio ALP. Myocarditis: endomyocardial biopsy and circulating anti-heart autoantibodies are key to diagnosis and personalized etiology-directed treatment. Eur Heart J. 2021;42:1618–1620. doi: 10.1093/eurheartj/ehab024. [DOI] [PubMed] [Google Scholar]
- 37.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 38.Asadi J, Bhandari SS, Ahmed N. Mesalazine induced myopericarditis in a patient with ulcerative colitis. Echo Res Pract. 2017;5:K1–K5. doi: 10.1530/ERP-17-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominguez F, Kühl U, Pieske B, Garcia-Pavia P, Tschöpe C. Update on Myocarditis and Inflammatory Cardiomyopathy: Reemergence of Endomyocardial Biopsy. Rev Esp Cardiol (Engl Ed) 2016;69:178–187. doi: 10.1016/j.rec.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Breitenstein RA, Salel AF, Watson DW. Letter: Chronic inflammatory bowel disease: acute pericarditis and pericardial tamponade. Ann Intern Med. 1974;81:406. doi: 10.7326/0003-4819-81-3-406_1. [DOI] [PubMed] [Google Scholar]
- 41.Cappell MS, Turkieh A. Chronic pericarditis and pericardial tamponade associated with ulcerative colitis. Dig Dis Sci. 2008;53:149–154. doi: 10.1007/s10620-007-9836-y. [DOI] [PubMed] [Google Scholar]
- 42.Mowat NA, Bennett PN, Finlayson JK, Brunt PW, Lancaster WM. Myopericarditis complicating ulcerative colitis. Br Heart J. 1974;36:724–727. doi: 10.1136/hrt.36.7.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell NE, Harrison N, Junga Z, Singla M. Heart Under Attack: Cardiac Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24:2322–2326. doi: 10.1093/ibd/izy157. [DOI] [PubMed] [Google Scholar]
- 44.Sentongo TA, Piccoli DA. Recurrent pericarditis due to mesalamine hypersensitivity: a pediatric case report and review of the literature. J Pediatr Gastroenterol Nutr. 1998;27:344–347. doi: 10.1097/00005176-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Bracamonte-Baran W, Čiháková D. Cardiac Autoimmunity: Myocarditis. Adv Exp Med Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rroku A, Kottwitz J, Heidecker B. Update on myocarditis - what we know so far and where we may be heading. Eur Heart J Acute Cardiovasc Care. 2020:2048872620910109. doi: 10.1177/2048872620910109. [DOI] [PubMed] [Google Scholar]
- 47.Varnavas VC, Reinsch N, Perrey M, Nensa F, Schlosser T, Baba HA, Gerken G, Erbel R, Janosi RA, Katsounas A. Recurrent lymphocytic myocarditis in a young male with ulcerative colitis. Eur J Med Res. 2014;19:11. doi: 10.1186/2047-783X-19-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tymińska A, Ozierański K, Skwarek A, Kapłon-Cieślicka A, Baritussio A, Grabowski M, Marcolongo R, Caforio AL. Personalized Management of Myocarditis and Inflammatory Cardiomyopathy in Clinical Practice. J Pers Med. 2022;12 doi: 10.3390/jpm12020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcolongo R, Baritussio A, Gianstefani S, Cheng CY, Iliceto S. Clinical Management and Follow-Up of Myocarditis Patients on Immunosuppressive Therapy. Caforio ALP, editor. Myocarditis: Pathogenesis, Diagnosis and Treatment. March 7, 2020. [cited 21 September 2023]. Available from: [DOI]
- 50.Baritussio A, Giordani AS, Rizzo S, Masiero G, Iliceto S, Marcolongo R, Caforio AL. Management of myocarditis in clinical practice. Minerva Cardiol Angiol. 2022;70:273–284. doi: 10.23736/S2724-5683.21.05732-X. [DOI] [PubMed] [Google Scholar]
- 51.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, Zembala M, Polonski L, Rozek MM, Wodniecki J. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- 52.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30:1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 53.Schultheiss HP, Kühl U, Cooper LT. The management of myocarditis. Eur Heart J. 2011;32:2616–2625. doi: 10.1093/eurheartj/ehr165. [DOI] [PubMed] [Google Scholar]
- 54.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 55.Dias T, Santos A, Santos RM, Carvalho A. Recurrent mesalazine-induced myopericarditis in a patient with ulcerative colitis. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-228037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sposato B, Allegri MP, Riccardi MP, Chigiotti S, Nencioni C, Ricciardi B, Carli T, Cresti A, Perari MG, Migliorini MG, Toti M. Mesalazine-induced multi-organ hypersensitivity. Clin Drug Investig. 2010;30:413–417. doi: 10.1007/BF03256911. [DOI] [PubMed] [Google Scholar]
- 57.Jackson JF, Sitaraman SV. Pericarditis as the presenting sign of Crohn's disease. Inflamm Bowel Dis. 2005;11:81–82. doi: 10.1097/00054725-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Farley JD, Thomson AB, Dasgupta MK. Pericarditis and ulcerative colitis. J Clin Gastroenterol. 1986;8:567–568. doi: 10.1097/00004836-198610000-00016. [DOI] [PubMed] [Google Scholar]
- 59.Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis. 2014;8:469–479. doi: 10.1016/j.crohns.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 60.Yuhara H, Steinmaus C, Corley D, Koike J, Igarashi M, Suzuki T, Mine T. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:953–962. doi: 10.1111/apt.12294. [DOI] [PubMed] [Google Scholar]
- 61.Papay P, Miehsler W, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H, Knoflach P, Vogelsang H, Platzer R, Tillinger W, Jaritz B, Schmid A, Blaha B, Dejaco C, Sobala A, Weltermann A, Eichinger S, Novacek G. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis. 2013;7:723–729. doi: 10.1016/j.crohns.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97–101. doi: 10.1046/j.1572-0241.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 63.Schlick CJR, Yuce TK, Yang AD, McGee MF, Bentrem DJ, Bilimoria KY, Merkow RP. A postdischarge venous thromboembolism risk calculator for inflammatory bowel disease surgery. Surgery. 2021;169:240–247. doi: 10.1016/j.surg.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merrill A, Millham F. Increased risk of postoperative deep vein thrombosis and pulmonary embolism in patients with inflammatory bowel disease: a study of National Surgical Quality Improvement Program patients. Arch Surg. 2012;147:120–124. doi: 10.1001/archsurg.2011.297. [DOI] [PubMed] [Google Scholar]
- 65.Novacek G, Weltermann A, Sobala A, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H, Knoflach P, Vogelsang H, Miehsler W, Platzer R, Tillinger W, Jaritz B, Schmid A, Blaha B, Dejaco C, Eichinger S. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779–787, 787.e1. doi: 10.1053/j.gastro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 66.Kim YH, Pfaller B, Marson A, Yim HW, Huang V, Ito S. The risk of venous thromboembolism in women with inflammatory bowel disease during pregnancy and the postpartum period: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e17309. doi: 10.1097/MD.0000000000017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen AT, Erichsen R, Horváth-Puhó E, Sørensen HT. Inflammatory bowel disease and venous thromboembolism during pregnancy and the postpartum period. J Thromb Haemost. 2017;15:702–708. doi: 10.1111/jth.13638. [DOI] [PubMed] [Google Scholar]
- 68.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, Che T, Zhang C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019;8 doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al Nabhani Z, Dietrich G, Hugot JP, Barreau F. Nod2: The intestinal gate keeper. PLoS Pathog. 2017;13:e1006177. doi: 10.1371/journal.ppat.1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, Targan SR, Xavier RJ, Ernst PB, Green DR, McGovern DP, Virgin HW, Mazmanian SK. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chamouard P, Grunebaum L, Wiesel ML, Frey PL, Wittersheim C, Sapin R, Baumann R, Cazenave JP. Prothrombin fragment 1 + 2 and thrombin-antithrombin III complex as markers of activation of blood coagulation in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 1995;7:1183–1188. doi: 10.1097/00042737-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Bisoendial RJ, Kastelein JJ, Levels JH, Zwaginga JJ, van den Bogaard B, Reitsma PH, Meijers JC, Hartman D, Levi M, Stroes ES. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res. 2005;96:714–716. doi: 10.1161/01.RES.0000163015.67711.AB. [DOI] [PubMed] [Google Scholar]
- 74.Danese S, Sgambato A, Papa A, Scaldaferri F, Pola R, Sans M, Lovecchio M, Gasbarrini G, Cittadini A, Gasbarrini A. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol. 2005;100:886–895. doi: 10.1111/j.1572-0241.2005.41469.x. [DOI] [PubMed] [Google Scholar]
- 75.Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 76.Limdi JK, Farraye J, Cannon R, Woodhams E, Farraye FA. Contraception, Venous Thromboembolism, and Inflammatory Bowel Disease: What Clinicians (and Patients) Should Know. Inflamm Bowel Dis. 2019;25:1603–1612. doi: 10.1093/ibd/izz025. [DOI] [PubMed] [Google Scholar]
- 77.Stegeman BH, de Bastos M, Rosendaal FR, van Hylckama Vlieg A, Helmerhorst FM, Stijnen T, Dekkers OM. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298. doi: 10.1136/bmj.f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melis F, Vandenbrouke JP, Büller HR, Colly LP, Bloemenkamp KW. Estimates of risk of venous thrombosis during pregnancy and puerperium are not influenced by diagnostic suspicion and referral basis. Am J Obstet Gynecol. 2004;191:825–829. doi: 10.1016/j.ajog.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 79.Carty E, MacEy M, Rampton DS. Inhibition of platelet activation by 5-aminosalicylic acid in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1169–1179. doi: 10.1046/j.1365-2036.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 80.Winther K, Bondesen S, Hansen SH, Hvidberg EF. Lack of effect of 5-aminosalicylic acid on platelet aggregation and fibrinolytic activity in vivo and in vitro. Eur J Clin Pharmacol. 1987;33:419–422. doi: 10.1007/BF00637641. [DOI] [PubMed] [Google Scholar]
- 81.Sehgal P, Colombel JF, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther. 2018;47:1597–1609. doi: 10.1111/apt.14688. [DOI] [PubMed] [Google Scholar]
- 82.Brotman DJ, Girod JP, Posch A, Jani JT, Patel JV, Gupta M, Lip GY, Reddy S, Kickler TS. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thromb Res. 2006;118:247–252. doi: 10.1016/j.thromres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Sarlos P, Szemes K, Hegyi P, Garami A, Szabo I, Illes A, Solymar M, Petervari E, Vincze A, Par G, Bajor J, Czimmer J, Huszar O, Varju P, Farkas N. Steroid but not Biological Therapy Elevates the risk of Venous Thromboembolic Events in Inflammatory Bowel Disease: A Meta-Analysis. J Crohns Colitis. 2018;12:489–498. doi: 10.1093/ecco-jcc/jjx162. [DOI] [PubMed] [Google Scholar]
- 84.Higgins PD, Skup M, Mulani PM, Lin J, Chao J. Increased risk of venous thromboembolic events with corticosteroid vs biologic therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:316–321. doi: 10.1016/j.cgh.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 85.Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, Gomollón F, García-Planella E, Merino O, Gutiérrez A, Esteve M, Márquez L, Garcia-Sepulcre M, Hinojosa J, Vera I, Muñoz F, Mendoza JL, Cabriada JL, Montoro MA, Barreiro-de Acosta M, Ceña G, Saro C, Aldeguer X, Barrio J, Maté J, Gisbert JP. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–1410. doi: 10.1097/MIB.0b013e318281f28f. [DOI] [PubMed] [Google Scholar]
- 86.Macaluso FS, Renna S, Maida M, Dimarco M, Sapienza C, Affronti M, Orlando E, Rizzuto G, Orlando R, Ventimiglia M, Cottone M, Orlando A. Tolerability profile of thiopurines in inflammatory bowel disease: a prospective experience. Scand J Gastroenterol. 2017;52:981–987. doi: 10.1080/00365521.2017.1333626. [DOI] [PubMed] [Google Scholar]
- 87.Seinen ML, Ponsioen CY, de Boer NK, Oldenburg B, Bouma G, Mulder CJ, van Bodegraven AA. Sustained clinical benefit and tolerability of methotrexate monotherapy after thiopurine therapy in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2013;11:667–672. doi: 10.1016/j.cgh.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 88.Irving PM, Macey MG, Shah U, Webb L, Langmead L, Rampton DS. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:361–372. doi: 10.1097/00054725-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 89.Schinzari F, Armuzzi A, De Pascalis B, Mores N, Tesauro M, Melina D, Cardillo C. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther. 2008;83:70–76. doi: 10.1038/sj.clpt.6100229. [DOI] [PubMed] [Google Scholar]
- 90.deFonseka AM, Tuskey A, Conaway MR, Behm BW. Antitumor Necrosis Factor-α Therapy Is Associated With Reduced Risk of Thromboembolic Events in Hospitalized Patients With Inflammatory Bowel Disease. J Clin Gastroenterol. 2016;50:578–583. doi: 10.1097/MCG.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 91.Cross RK, Chiorean M, Vekeman F, Xiao Y, Wu E, Chao J, Wang AW. Assessment of the real-world safety profile of vedolizumab using the United States Food and Drug Administration adverse event reporting system. PLoS One. 2019;14:e0225572. doi: 10.1371/journal.pone.0225572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loftus EV Jr, Feagan BG, Panaccione R, Colombel JF, Sandborn WJ, Sands BE, Danese S, D'Haens G, Rubin DT, Shafran I, Parfionovas A, Rogers R, Lirio RA, Vermeire S. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:1353–1365. doi: 10.1111/apt.16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liefferinckx C, Verstockt B, Gils A, Noman M, Van Kemseke C, Macken E, De Vos M, Van Moerkercke W, Rahier JF, Bossuyt P, Dutré J, Humblet E, Staessen D, Peeters H, Van Hootegem P, Louis E, Franchimont D, Baert F, Vermeire S Belgian Inflammatory Bowel Disease Research and Development Group [BIRD group] Long-term Clinical Effectiveness of Ustekinumab in Patients with Crohn's Disease Who Failed Biologic Therapies: A National Cohort Study. J Crohns Colitis. 2019;13:1401–1409. doi: 10.1093/ecco-jcc/jjz080. [DOI] [PubMed] [Google Scholar]
- 94.Jones TO. Intramammary antibiotic preparations and cephalosporin resistance in Salmonella typhimurium 204c. Vet Rec. 1987;120:399–400. doi: 10.1136/vr.120.16.399-a. [DOI] [PubMed] [Google Scholar]
- 95.Philippoteaux C, Deprez V, Nottez A, Cailliau E, Houvenagel E, Deprez X, Philippe P, Pascart T, Flipo RM, Goëb V, Letarouilly JG. Characteristics of Patients Treated with JAK Inhibitors in Rheumatoid Arthritis before versus after VTE Risk Warnings. J Clin Med. 2022;12 doi: 10.3390/jcm12010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandborn WJ, Lawendy N, Danese S, Su C, Loftus EV Jr, Hart A, Dotan I, Damião AOMC, Judd DT, Guo X, Modesto I, Wang W, Panés J. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55:464–478. doi: 10.1111/apt.16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate. FDA. Dec 20, 2019. [cited 27 September 2023]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/safety-trial-finds-risk-blood-clots-lungs-and-death-higher-dose-tofacitinib-xeljanz-xeljanz-xr .
- 98.Spiewak TA, Patel A. User's guide to JAK inhibitors in inflammatory bowel disease. Curr Res Pharmacol Drug Discov. 2022;3:100096. doi: 10.1016/j.crphar.2022.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deepak P, Alayo QA, Khatiwada A, Lin B, Fenster M, Dimopoulos C, Bader G, Weisshof R, Jacobs M, Gutierrez A, Ciorba MA, Christophi GP, Patel A, Hirten RP, Colombel JF, Rubin DT, Ha C, Beniwal-Patel P, Ungaro RC, Syal G, Pekow J, Cohen BL, Yarur A. Safety of Tofacitinib in a Real-World Cohort of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2021;19:1592–1601.e3. doi: 10.1016/j.cgh.2020.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Colombel JF, Panaccione R, Nakase H, Burmester G, Cohen SB, Mease P, Guttman-Yassky E, Liu J, Zhou W, Ilo D, Higgins P. P573 The safety profile of upadacitinib maintenance therapy in ulcerative colitis in the Phase 3 U-ACHIEVE study is consistent with that in approved indications. Journal of Crohn’s and Colitis. 2022;16 Suppl 1:i514. [Google Scholar]
- 101.Mannucci A, D'Amico F, El Saadi A, Peyrin-Biroulet L, Danese S. Filgotinib for moderately to severely active ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2022;16:927–940. doi: 10.1080/17474124.2022.2138857. [DOI] [PubMed] [Google Scholar]
- 102.Schünemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer-Westendorf J, Spencer FA, Rezende SM, Zakai NA, Bauer KA, Dentali F, Lansing J, Balduzzi S, Darzi A, Morgano GP, Neumann I, Nieuwlaat R, Yepes-Nuñez JJ, Zhang Y, Wiercioch W. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olivera PA, Zuily S, Kotze PG, Regnault V, Al Awadhi S, Bossuyt P, Gearry RB, Ghosh S, Kobayashi T, Lacolley P, Louis E, Magro F, Ng SC, Papa A, Raine T, Teixeira FV, Rubin DT, Danese S, Peyrin-Biroulet L. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:857–873. doi: 10.1038/s41575-021-00492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Faye AS, Hung KW, Cheng K, Blackett JW, Mckenney AS, Pont AR, Li J, Lawlor G, Lebwohl B, Freedberg DE. Minor Hematochezia Decreases Use of Venous Thromboembolism Prophylaxis in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:1394–1400. doi: 10.1093/ibd/izz269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gordon H, Burisch J, Ellul P, Karmiris K, Katsanos K, Allocca M, Bamias G, Barreiro-de Acosta M, Braithwaite T, Greuter T, Harwood C, Juillerat P, Lobaton T, Müller-Ladner U, Noor N, Pellino G, Savarino E, Schramm C, Soriano A, Stein JM, Uzzan M, van Rheenen PF, Vavricka SR, Vecchi M, Zuily S, Kucharzik T. ECCO Guidelines on Extraintestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2023 doi: 10.1093/ecco-jcc/jjad108. [DOI] [PubMed] [Google Scholar]
- 106.Sleutjes JAM, van Lennep JER, van der Woude CJ, de Vries AC. Thromboembolic and atherosclerotic cardiovascular events in inflammatory bowel disease: epidemiology, pathogenesis and clinical management. Therap Adv Gastroenterol. 2021;14:17562848211032126. doi: 10.1177/17562848211032126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.D'Ascenzo F, Bruno F, Iannaccone M, Testa G, De Filippo O, Giannino G, Caviglia GP, Bernstein CN, De Ferrari GM, Bugianesi E, Armandi A, Ribaldone DG. Patients with inflammatory bowel disease are at increased risk of atherothrombotic disease: A systematic review with meta-analysis. Int J Cardiol. 2023;378:96–104. doi: 10.1016/j.ijcard.2023.02.042. [DOI] [PubMed] [Google Scholar]
- 108.Bernstein CN, Wajda A, Blanchard JF. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:41–45. doi: 10.1016/j.cgh.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 109.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 111.Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. doi: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 112.Aniwan S, Pardi DS, Tremaine WJ, Loftus EV Jr. Increased Risk of Acute Myocardial Infarction and Heart Failure in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2018;16:1607–1615.e1. doi: 10.1016/j.cgh.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aarestrup J, Jess T, Kobylecki CJ, Nordestgaard BG, Allin KH. Cardiovascular Risk Profile Among Patients With Inflammatory Bowel Disease: A Population-based Study of More Than 100 000 Individuals. J Crohns Colitis. 2019;13:319–323. doi: 10.1093/ecco-jcc/jjy164. [DOI] [PubMed] [Google Scholar]
- 114.Omdahl JL. Control of kidney 25-hydroxyvitamin D3 metabolism. Strontium and the involvement of parathyroid hormone. Arch Biochem Biophys. 1977;184:172–178. doi: 10.1016/0003-9861(77)90339-3. [DOI] [PubMed] [Google Scholar]
- 115.Hakala K, Vuoristo M, Luukkonen P, Järvinen HJ, Miettinen TA. Impaired absorption of cholesterol and bile acids in patients with an ileoanal anastomosis. Gut. 1997;41:771–777. doi: 10.1136/gut.41.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen H, Li W, Hu J, Xu F, Lu Y, Zhu L, Shen H. Association of serum lipids with inflammatory bowel disease: a systematic review and meta-analysis. Front Med (Lausanne) 2023;10:1198988. doi: 10.3389/fmed.2023.1198988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kristensen MS, Kjærulff TM, Ersbøll AK, Green A, Hallas J, Thygesen LC. The Influence of Antidepressants on the Disease Course Among Patients With Crohn's Disease and Ulcerative Colitis-A Danish Nationwide Register-Based Cohort Study. Inflamm Bowel Dis. 2019;25:886–893. doi: 10.1093/ibd/izy367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Le Gall G, Kirchgesner J, Bejaoui M, Landman C, Nion-Larmurier I, Bourrier A, Sokol H, Seksik P, Beaugerie L. Clinical activity is an independent risk factor of ischemic heart and cerebrovascular arterial disease in patients with inflammatory bowel disease. PLoS One. 2018;13:e0201991. doi: 10.1371/journal.pone.0201991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Card TR, Zittan E, Nguyen GC, Grainge MJ. Disease Activity in Inflammatory Bowel Disease Is Associated With Arterial Vascular Disease. Inflamm Bowel Dis. 2021;27:629–638. doi: 10.1093/ibd/izaa156. [DOI] [PubMed] [Google Scholar]
- 120.Li S, Gao Y, Ma K, Li Y, Liu C, Yan Y, Liu W, Liu H, Li Z, Song B, Xu Y, Xia Z. Lipid-related protein NECTIN2 is an important marker in the progression of carotid atherosclerosis: An intersection of clinical and basic studies. J Transl Int Med. 2021;9:294–306. doi: 10.2478/jtim-2021-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Desreumaux P, Ghosh S. Review article: mode of action and delivery of 5-aminosalicylic acid - new evidence. Aliment Pharmacol Ther. 2006;24 Suppl 1:2–9. doi: 10.1111/j.1365-2036.2006.03069.x. [DOI] [PubMed] [Google Scholar]
- 122.Britzen-Laurent N, Weidinger C, Stürzl M. Contribution of Blood Vessel Activation, Remodeling and Barrier Function to Inflammatory Bowel Diseases. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24065517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62:689–694. doi: 10.1136/gutjnl-2012-303285. [DOI] [PubMed] [Google Scholar]
- 124.Andersohn F, Waring M, Garbe E. Risk of ischemic stroke in patients with Crohn's disease: a population-based nested case-control study. Inflamm Bowel Dis. 2010;16:1387–1392. doi: 10.1002/ibd.21187. [DOI] [PubMed] [Google Scholar]
- 125.Lewis JD, Scott FI, Brensinger CM, Roy JA, Osterman MT, Mamtani R, Bewtra M, Chen L, Yun H, Xie F, Curtis JR. Increased Mortality Rates With Prolonged Corticosteroid Therapy When Compared With Antitumor Necrosis Factor-α-Directed Therapy for Inflammatory Bowel Disease. Am J Gastroenterol. 2018;113:405–417. doi: 10.1038/ajg.2017.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Massironi S, Mulinacci G, Gallo C, Viganò C, Fichera M, Villatore A, Peretto G, Danese S. The oft-overlooked cardiovascular complications of inflammatory bowel disease. Expert Rev Clin Immunol. 2023;19:375–391. doi: 10.1080/1744666X.2023.2174971. [DOI] [PubMed] [Google Scholar]
- 127.Kirchgesner J, Nyboe Andersen N, Carrat F, Jess T, Beaugerie L BERENICE study group. Risk of acute arterial events associated with treatment of inflammatory bowel diseases: nationwide French cohort study. Gut. 2020;69:852–858. doi: 10.1136/gutjnl-2019-318932. [DOI] [PubMed] [Google Scholar]
- 128.Woodman RJ, Baghdadi LR, Shanahan ME, Mangoni AA. The Temporal Relationship between Arterial Stiffening and Blood Pressure Is Modified by Methotrexate Treatment in Patients with Rheumatoid Arthritis. Front Physiol. 2017;8:593. doi: 10.3389/fphys.2017.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dheyriat L, Ward D, Beaugerie L, Jess T, Kirchgesner J. Risk of Recurrent Acute Arterial Events Associated With Thiopurines and Anti-Tumor Necrosis Factor in Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2023;21:164–172.e11. doi: 10.1016/j.cgh.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 130.dos Santos LC, Costa AV, Lopes LG, Leonel AJ, Aguilar EC, Noviello Mde L, Ferrari Mde L, Alvarez-Leite JI. Combination of Azathioprine and Aminosalicylate Treatment Prevent Risk of Cardiovascular Disease in Women with Ulcerative Colitis by Reducing Inflammation. Med Sci Monit. 2015;21:2305–2315. doi: 10.12659/MSM.893865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Voloshyna I, Seshadri S, Anwar K, Littlefield MJ, Belilos E, Carsons SE, Reiss AB. Infliximab reverses suppression of cholesterol efflux proteins by TNF-α: a possible mechanism for modulation of atherogenesis. Biomed Res Int. 2014;2014:312647. doi: 10.1155/2014/312647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miranda-Bautista J, de Gracia-Fernández C, López-Ibáñez M, Barrientos M, Gallo-Moltó A, González-Arias M, González-Gil C, Díaz-Redondo A, Marín-Jiménez I, Menchén L. Lipid Profile in Inflammatory Bowel Disease Patients on Anti-TNFα Therapy. Dig Dis Sci. 2015;60:2130–2135. doi: 10.1007/s10620-015-3577-0. [DOI] [PubMed] [Google Scholar]
- 133.Paschou SA, Kothonas F, Lafkas A, Myroforidis A, Loi V, Terzi T, Karagianni O, Poulou A, Goumas K, Vryonidou A. Favorable Effect of Anti-TNF Therapy on Insulin Sensitivity in Nonobese, Nondiabetic Patients with Inflammatory Bowel Disease. Int J Endocrinol. 2018;2018:6712901. doi: 10.1155/2018/6712901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sandborn WJ, Rebuck R, Wang Y, Zou B, Adedokun OJ, Gasink C, Sands BE, Hanauer SB, Targan S, Ghosh S, de Villiers WJS, Colombel JF, Feagan BG, Lynch JP. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn's Disease: The IM-UNITI Trial. Clin Gastroenterol Hepatol. 2022;20:578–590.e4. doi: 10.1016/j.cgh.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singh S, Iversen AT, Allin KH, Jess T. Comparative Outcomes and Safety of Vedolizumab vs Tumor Necrosis Factor Antagonists for Older Adults With Inflammatory Bowel Diseases. JAMA Netw Open. 2022;5:e2234200. doi: 10.1001/jamanetworkopen.2022.34200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sands BE, Taub PR, Armuzzi A, Friedman GS, Moscariello M, Lawendy N, Pedersen RD, Chan G, Nduaka CI, Quirk D, Salese L, Su C, Feagan BG. Tofacitinib Treatment Is Associated With Modest and Reversible Increases in Serum Lipids in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:123–132.e3. doi: 10.1016/j.cgh.2019.04.059. [DOI] [PubMed] [Google Scholar]
- 137.Sands BE, Colombel JF, Ha C, Farnier M, Armuzzi A, Quirk D, Friedman GS, Kwok K, Salese L, Su C, Taub PR. Lipid Profiles in Patients With Ulcerative Colitis Receiving Tofacitinib-Implications for Cardiovascular Risk and Patient Management. Inflamm Bowel Dis. 2021;27:797–808. doi: 10.1093/ibd/izaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang WH, Ge J, Gao RL, Yang SM, Li SZ, Mao WS. Nd:YAG laser treatment complications of after-cataract. Yan Ke Xue Bao. 1987;3:81–84. [PubMed] [Google Scholar]
- 139.Kochar BD, Cheng D, Cai T, Ananthakrishnan AN. Comparative Risk of Thrombotic and Cardiovascular Events with Tofacitinib and Anti-TNF Agents in Patients with Inflammatory Bowel Diseases. Dig Dis Sci. 2022;67:5206–5212. doi: 10.1007/s10620-022-07404-z. [DOI] [PubMed] [Google Scholar]
- 140.Seo GH, Jung SH. The Comparative Risk of Serious Adverse Events With Tofacitinib and TNF Inhibitors in Patients With Ulcerative Colitis: The Korean Experience as Revealed by a National Database. J Korean Med Sci. 2022;37:e123. doi: 10.3346/jkms.2022.37.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus Kinase Inhibitors in Patients With Inflammatory Bowel Diseases or Other Immune-mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1554–1573.e12. doi: 10.1053/j.gastro.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 142.Feagan BG, Danese S, Loftus EV Jr, Vermeire S, Schreiber S, Ritter T, Fogel R, Mehta R, Nijhawan S, Kempiński R, Filip R, Hospodarskyy I, Seidler U, Seibold F, Beales ILP, Kim HJ, McNally J, Yun C, Zhao S, Liu X, Hsueh CH, Tasset C, Besuyen R, Watanabe M, Sandborn WJ, Rogler G, Hibi T, Peyrin-Biroulet L. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397:2372–2384. doi: 10.1016/S0140-6736(21)00666-8. [DOI] [PubMed] [Google Scholar]
- 143.Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, D'Haens G, Nakase H, Panés J, Higgins PDR, Juillerat P, Lindsay JO, Loftus EV Jr, Sandborn WJ, Reinisch W, Chen MH, Sanchez Gonzalez Y, Huang B, Xie W, Liu J, Weinreich MA, Panaccione R. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113–2128. doi: 10.1016/S0140-6736(22)00581-5. [DOI] [PubMed] [Google Scholar]
- 144.Gabbiadini R, Dal Buono A, Mastrorocco E, Solitano V, Repici A, Spinelli A, Condorelli G, Armuzzi A. Atherosclerotic cardiovascular diseases in inflammatory bowel diseases: to the heart of the issue. Front Cardiovasc Med. 2023;10:1143293. doi: 10.3389/fcvm.2023.1143293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Crockett SD, Hansen RA, Stürmer T, Schectman R, Darter J, Sandler RS, Kappelman MD. Statins are associated with reduced use of steroids in inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. 2012;18:1048–1056. doi: 10.1002/ibd.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dhamija P, Hota D, Kochhar R, Sachdev A, Chakrabarti A. Randomized clinical trial: atorvastatin versus placebo in patients with acute exacerbation of mild to moderate ulcerative colitis. Indian J Gastroenterol. 2014;33:151–156. doi: 10.1007/s12664-013-0420-4. [DOI] [PubMed] [Google Scholar]
- 147.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90:464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rogler G, Rosano G. The heart and the gut. Eur Heart J. 2014;35:426–430. doi: 10.1093/eurheartj/eht271. [DOI] [PubMed] [Google Scholar]
- 149.Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS. Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol. 2011;17:578–593. doi: 10.3748/wjg.v17.i5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jarmakiewicz-Czaja S, Piątek D, Filip R. The Influence of Nutrients on Inflammatory Bowel Diseases. J Nutr Metab. 2020;2020:2894169. doi: 10.1155/2020/2894169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Katsanos KH, Christodoulou DK, Pappas K, Pappas C, Tsianos EV. Electrocardiograph abnormalities in patients with active inflammatory bowel disease. Annals of Gastroenterology. 2007:275–281. [Google Scholar]
- 152.Keating E, Kelleher TB, Lahiff C. De novo Anti-TNF-α-induced Congestive Heart Failure in a Patient With Turner Syndrome and Crohn's Disease. Inflamm Bowel Dis. 2020;26:e161–e162. doi: 10.1093/ibd/izaa176. [DOI] [PubMed] [Google Scholar]
- 153.Kwon HJ, Coté TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138:807–811. doi: 10.7326/0003-4819-138-10-200305200-00008. [DOI] [PubMed] [Google Scholar]
- 154.Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J Clin Invest. 2000;106:589–597. doi: 10.1172/JCI9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Monden Y, Kubota T, Inoue T, Tsutsumi T, Kawano S, Ide T, Tsutsui H, Sunagawa K. Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H743–H753. doi: 10.1152/ajpheart.00166.2007. [DOI] [PubMed] [Google Scholar]
- 156.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 157.Grillo TG, Silveira CFDSMP, Quaglio AEV, Dutra RM, Baima JP, Bazan SGZ, Sassaki LY. Acute heart failure as an adverse event of tumor necrosis factor inhibitor therapy in inflammatory bowel disease: A review of the literature. World J Cardiol. 2023;15:217–228. doi: 10.4330/wjc.v15.i5.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 159.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 160.Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zuin M, Zuliani G, Rigatelli G, Favero GD, Roncon L. Atrial fibrillation in patients with inflammatory bowel disease: A systematic review and meta-analysis. Eur J Intern Med. 2020;76:120–122. doi: 10.1016/j.ejim.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 162.Mubasher M, Syed T, Hanafi A, Yu Z, Yusuf I, Abdullah AS, Mohamed MF, Alweis R, Rao M, Hoefen R, Danjuma MI. An Investigation into the Association Between Inflammatory Bowel Disease and Cardiac Arrhythmias: An Examination of the United States National Inpatient Sample Database. Clin Med Insights Cardiol. 2020;14:1179546820955179. doi: 10.1177/1179546820955179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sandborn WJ, Feagan BG, D'Haens G, Wolf DC, Jovanovic I, Hanauer SB, Ghosh S, Petersen A, Hua SY, Lee JH, Charles L, Chitkara D, Usiskin K, Colombel JF, Laine L, Danese S True North Study Group. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2021;385:1280–1291. doi: 10.1056/NEJMoa2033617. [DOI] [PubMed] [Google Scholar]
- 164.Subei AM, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs. 2015;29:565–575. doi: 10.1007/s40263-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kappos L, Cohen J, Collins W, de Vera A, Zhang-Auberson L, Ritter S, von Rosenstiel P, Francis G. Fingolimod in relapsing multiple sclerosis: An integrated analysis of safety findings. Mult Scler Relat Disord. 2014;3:494–504. doi: 10.1016/j.msard.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 166.Sandborn WJ, Feagan BG, Hanauer S, Vermeire S, Ghosh S, Liu WJ, Petersen A, Charles L, Huang V, Usiskin K, Wolf DC, D'Haens G. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J Crohns Colitis. 2021;15:1120–1129. doi: 10.1093/ecco-jcc/jjab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sandborn WJ, Peyrin-Biroulet L, Zhang J, Chiorean M, Vermeire S, Lee SD, Kühbacher T, Yacyshyn B, Cabell CH, Naik SU, Klassen P, Panés J. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology. 2020;158:550–561. doi: 10.1053/j.gastro.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 168.Vermeire S, Chiorean M, Panés J, Peyrin-Biroulet L, Zhang J, Sands BE, Lazin K, Klassen P, Naik SU, Cabell CH, Sandborn WJ. Long-term Safety and Efficacy of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study. J Crohns Colitis. 2021;15:950–959. doi: 10.1093/ecco-jcc/jjab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xue J, Li J, Sun D, Sheng L, Gong Y, Wang D, Zhang S, Zou Y, Shi J, Xu W, An M, Dai C, Li W, Zheng L, Vinograd A, Liu G, Kong Y, Li Y. Functional Evaluation of Intermediate Coronary Lesions with Integrated Computed Tomography Angiography and Invasive Angiography in Patients with Stable Coronary Artery Disease. J Transl Int Med. 2022;10:255–263. doi: 10.2478/jtim-2022-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]