Abstract
Purpose
To compare 3 separate blood flow restriction (BFR) systems in their capacity to reduce repetitions to failure, impact perceptual responses, and cause adverse events during a low-load free-flow exercise.
Methods
The study included healthy subjects aged 18 years or older who presented to an ambulatory-care sports medicine clinic. On day 1, participants’ demographic characteristics and anthropomorphic measurements were recorded. Each participant performed dumbbell biceps curl repetitions to failure using 20% of his or her 1-repetition maximum weight with each arm. Participants were exposed to 3 different tourniquet systems for familiarization. On day 2, each participant’s arm was randomized to a cuff system, and the participant performed 2 sets of biceps curl repetitions to failure with the cuff inflated. Repetitions to failure, rating of perceived effort (RPE), rating of perceived discomfort, and pulse oxygenation levels were recorded after each set. On day 3, participants completed a survey of their perceived delayed-onset muscle soreness.
Results
The final analysis was performed on 42 arms, with 14 limbs per system. The study population had a mean age of 28.7 ± 2.4 years and a mean body mass index of 24.9 ± 4.3. All 3 systems successfully reduced repetitions to failure compared with unrestricted low-load exercise from baseline to BFR set 1 and from baseline to BFR set 2. There were no significant between-group differences among BFR systems regarding the number of repetitions to failure performed at baseline versus BFR set 1 or BFR set 2. The Delfi Personalized Tourniquet System (PTS) cohort had the greatest reductions in repetitions to failure from BFR set 1 to BFR set 2 (P = .002) and reported the highest RPE after set 2 (P = .025).
Conclusions
The Delfi PTS, SmartCuffs Pro, and BStrong BFR systems were each safe and were able to significantly reduce repetitions to failure compared with a low-load free-flow condition when used in a BFR exercise protocol. The Delfi PTS system may produce a higher RPE with prolonged use in comparison to the other systems.
Level of Evidence
Level II, prospective cohort study.
Over the past few decades, blood flow restriction (BFR) training has been investigated as an adjunct to physical therapy and rehabilitation protocols, both within and outside of health care. The technology uses a pneumatic inflation system placed around the proximal aspect of an extremity (upper or lower) to produce venous blood flow occlusion while limiting arterial inflow to the exercising limb. Recent studies have suggested that BFR may offer benefits over traditional rehabilitative therapy, namely through its ability to reduce the repetitions needed to induce a training effect that produces an increase in muscular strength and hypertrophy, thereby potentially mitigating some of the risks incurred by training with heavier weights.1, 2, 3, 4, 5, 6 For this reason, BFR training has shown particular promise for special populations such as elderly patients, physically impaired patients, and presurgical or postsurgical patients who are otherwise unable to tolerate high-load resistance training.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Although the exact mechanisms contributing to the observed increases in muscle mass, muscular strength, and cardiorespiratory function associated with BFR training continue to be investigated, it is thought that the reduction in blood flow produces localized hypoxia and metabolic stress that accelerates fatigue accumulation (e.g., reduced repetitions to failure), resulting in the enhanced recruitment of type II muscle fibers, as well as stimulation of anabolic pathways and hormone secretion.18, 19, 20, 21, 22
The ability of a BFR tourniquet device to determine limb occlusion pressure (LOP) and exercise at a minimum effective percentage of LOP is thought to be an important methodologic consideration for reaping maximum therapeutic benefit from BFR training. Typical inflation pressures used range between 30% and 80% LOP, with recent evidence indicating at least 50% LOP is needed to meaningfully accelerate fatigue compared with unrestricted load-matched exercise.23 However, to date, there is a dearth of studies investigating whether prescribing BFR training pressures at a percentage of LOP accelerates fatigue accumulation in a similar capacity to other approaches (e.g., using an arbitrary applied pressure).
As BFR is increasing in popularity and research interest, various BFR systems have emerged for consumer purchase that have different features thought to affect the safety and/or experience of BFR exercise.24 A proposed BFR cuff classification system labels cuffs as either autoregulated or non-autoregulated devices depending on how pressure is monitored during exercise.25 Autoregulated devices are anchored via an air tube to a computer system that attempts to maintain a uniform pressure throughout both phases of muscular contraction. The computer within an autoregulated BFR cuff system accounts for the increased intramuscular pressures that occur during muscle contraction on the cuff by quickly releasing air from the system during the concentric portion of the exercise while subsequently pumping additional air into the cuff during the eccentric phase.26,27 This serves to keep the total pressure applied to the vasculature relatively constant and may aid in the safety and/or performance of BFR exercise.28 In contrast, non-autoregulated devices do not adjust the pressure over the duration of the muscle contraction cycle and are untethered from the cuff inflation system. In addition, cuffs exist for consumer purchase that are not pneumatic tourniquets per se but are designed to occlude only venous return owing to their multi-chamber bladder designs.24 As such, these cuffs cannot determine LOP and are instead prescribed at manufacturer-recommended pressures29 or an algorithm-based pressure.30 It is interesting to note that there is a paucity of literature investigating whether this bladder type accelerates fatigue during resistance exercise to failure or investigating its impact on perceptual experience and muscle soreness during exercise compared with low-intensity unrestricted exercise or compared with single-chamber bladder systems that have the capacity to autoregulate the applied pressure to the exercising limb.
The purpose of this study was to compare 3 separate BFR systems in their capacity to reduce repetitions to failure, impact perceptual responses, and cause adverse events during a low-load free-flow exercise. We hypothesized that the autoregulated BFR systems would be more effective at reducing repetitions to failure, would cause participants less discomfort with their use, and would cause fewer adverse events compared with the non-autoregulated BFR system.
Methods
Participants
Institutional review board approval was granted (No. 14126). Participants were enrolled and consented before initiation in the study. All participants presented to an ambulatory-care sports medicine clinic. The inclusion criteria were healthy participants aged 18 years or older. Participants received no compensation for study involvement and were made aware of the potential risk of BFR cuff use during the informed-consent process. The risks presented included but were not limited to the possibility of deep venous thrombosis, pulmonary embolism, and pain from the pressure of the cuff during measurements. The exclusion criteria consisted of a medical history of substantial cardiovascular disease, more than 1 cardiovascular risk factor, blood clots or bleeding disorders, or a diagnosed neurologic condition; pregnancy; any known musculoskeletal disorder; and/or any history of substantial injury or surgery to the extremity.28 All participant data were kept in a password-encrypted digital database over the study duration and were deleted after completion. Data were collected by an orthopaedic surgery resident physician (M.E.D.) and a physical medicine and rehabilitation resident physician (A.S.A.).
BFR Cuff Designs
Three commercially available BFR cuff systems were used for this study: BStrong system (BStrong Training Systems, Park City, UT), SmartCuffs Pro system (Smart Tools Plus, Strongsville, OH), and Personalized Tourniquet System (PTS) for BFR (Delfi Medical Innovations, Vancouver, BC, Canada). The BStrong band is a non-automatic, pneumatic BFR cuff system that purportedly allows for arterial blood flow during BFR resistance training while impeding venous return. The cuff is manually inflated and uses a multi-chamber design to maintain elasticity and promote non-uniform circumferential pressure in the hope of avoiding arterial occlusion, thereby preventing ischemic injury and/or rhabdomyolysis.29 Multiple sizes of cuffs (all 5-cm width) are available for both the upper and lower extremities to accommodate limbs of varying proportions. For our study, all limbs were amenable to size 2, conferring to limbs 10 to 17.5 inches (25.4-44.5 cm) in circumference.
The SmartCuffs Pro system is an automated, pneumatic, single-chamber bladder BFR cuff system (width of 10.16 cm) with a straight cuff design (e.g., same width of the cuff throughout). The inner proportion of the bladder is not constrained during inflation; this design allows the tourniquet to conform to the patient’s extremity during inflation and thereby self-detect LOP without reliance on Doppler or pulse oximetry technology. A recent study by Abbas et al.28 has shown that this technology can accurately and reliably attain LOP measurements comparable to the gold standard of manual Doppler ultrasound. The pneumatic device inflates the tourniquet in 10–mm Hg increments, assessing for pulsatile feedback from the extremity’s arterial pulse, until complete LOP is achieved. After calibration, the user may select the desired percentage of LOP to use for the training protocol. If left attached to the cuff during exercise, the device’s pulse pressure sensor allows for autoregulation of the desired percentage of LOP in response to the muscle contraction-relaxation cycle.
The Delfi PTS for BFR is a Food and Drug Administration–listed class I medical device (low risk) that uses pulse pressure sensor technology within the unit to determine LOP. After calibration, the user may select the percentage of the determined LOP to use for the training protocol. The device likewise autoregulates occlusion pressure in response to the muscle contraction-relaxation cycle during exercise. The Delfi system uses Easi-Fit Variable Contour cuffs (Delfi Medical Innovations), which are available in various sizes to accommodate a wide range of limb circumferences. For this investigation, an 18-inch-long by 4.5-inch-wide 45.7 cm long by 11.5 cm wide) cuff was suitable for all tested participants.
Protocol
The study was performed over 3 separate testing days.
Day 1
On day 1, sex, age, height, and weight were recorded for each participant. Arm length and arm circumference were likewise measured and recorded. Next, each participant’s 1-repetition maximum (1RM) biceps curl was calculated for both arms. The 1RM was calculated using the Epley formula: 1RM = Dumbbell weight × (1 + [Number of biceps curl repetitions/30]). No participants exceeded 10 repetitions when the 1RM was calculated. Next, after 10 minutes of rest, participants performed standing biceps curls with upright posture maintained with each arm at 20% of their estimated 1RM until failure without BFR (no cuff condition). One repetition was measured as progression through a complete flexion-extension range of motion while maintaining a normal cadence (<3 seconds/repetition, monitored by a stopwatch). The number of repetitions to failure completed was recorded. Failure was defined as voluntary cessation of exercise or the inability to complete further repetitions at a normal cadence. No verbal encouragement was given to any participant when exercising to failure, but a verbal cue was given at the first violation of the cadence; the exercise was terminated after the second notice. After a 10-minute break, each participant was briefly exposed to all 3 BFR cuffs at inflated pressures (50% LOP for SmartCuffs Pro and Delfi PTS and 200 mm Hg for BStrong) for familiarization purposes.
Day 2
The second day of testing occurred after a washout period of at least 5 days from the day-1 evaluation. With use of the RAND, CHOOSE, and RANK functions in Microsoft Excel (Microsoft, Redmond, WA), a formula was created to randomize each participant’s arm to one BFR cuff system (either SmartCuffs Pro, BStrong, or Delfi PTS BFR system), such that each device was assigned to 14 arms with no preference for hand dominance.
The SmartCuffs Pro and Delfi PTS BFR systems were set to 50% LOP, whereas the BStrong system was set to 200 mm Hg, which is consistent with the manufacturer’s quick-start default recommendation. All LOPs were determined in the standing position. In BFR set 1, the designated BFR cuff system was inflated on each participant’s arm, and the participant was instructed to perform biceps curl repetitions at 20% of his or her estimated 1RM (20%1RM) until failure. A 1-minute rest period was then provided while the cuff remained inflated. After the rest period, the participant performed BFR set 2, consisting of another set of biceps curl repetitions at 20%1RM until failure. This process was repeated for the contralateral arm with its assigned BFR cuff system, providing a 5-minute rest interval between the tests of the 2 arms. For each arm, baseline pulse oxygen levels were recorded before exercises and after BFR set 1 and BFR set 2. Measurements of the participant-reported 10-point rating of perceived effort (RPE) and rating of perceived discomfort (RPD) were also recorded immediately after both BFR set 1 and BFR set 2 while the cuffs remained inflated.
Day 3
Twenty-four hours after the day-2 session, participants filled out a virtual survey of their perception of muscle soreness and discomfort in each arm using a visual analog scale (VAS) and the McGill Pain Questionnaire.
Outcome Measures
The primary outcome measures were performance, perceptual response, muscle oxygenation levels, and adverse events. Performance was evaluated by recording the number of full-range biceps curl repetitions that participants performed during each of the 2 BFR sets until reaching volitional failure. Repetitions to failure were recorded for each arm separately. Perceptual response was assessed by recording each participant’s reported RPE and RPD after each BFR set. Oxygen saturation levels were monitored for each extremity prior to exercise, after BFR set 1, and after BFR set 2 using a digital pulse oximetry device. Furthermore, the participants were monitored for adverse events immediately and after 24 hours. Adverse events were defined as detrimental effects from the treatment during or after the exercise that rendered a participant unable or unwilling to continue with the exercise and/or causing substantial bodily harm. The secondary outcome measure was delayed-onset muscle soreness (DOMS), which was assessed qualitatively via the completion of the VAS and McGill Pain Questionnaire 24 hours after exercise day 2.
Statistical Analysis
A power analysis was performed based on previous literature with similar methodology and a primary outcome of repetitions to failure.22 To detect a difference of 7 repetitions (standard deviation, 6.3 repetitions) between any of the 3 BFR systems with 80% power, a sample size of 14 arms in each group would be required. The final sample size was set at 42 arms, with 14 arms allocated to each of the 3 tourniquet systems. Continuous variables were described using counts and percentages, whereas categorical variables were described using means and standard deviations. Normality of the data set was evaluated using skewness. Two-group comparisons of categorical data were evaluated using the χ2 test, whereas 2-group comparisons between continuous variables were examined using independent 2-sample t tests for normal distributions and the Wilcoxon rank sum test for non-normal distributions. Three-group comparisons of categorical data and continuous variables were performed using 1-way analysis of variance. The level of statistical significance was set at P < .05. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Participant Demographic Characteristics
All 21 participants completed the protocol, with none lost to follow-up. No individuals declined to participate, and no individuals were excluded. The final analysis was performed on 42 limbs (21 left and 21 right arms), with 14 arms being tested with each tourniquet system. The study population had a mean age of 28.7 ± 2.4 years, with a mean body mass index of 24.94. Men comprised 57% of the study population. There were no significant differences in the percentage of right-handedness or 20%1RM between the BFR system cohorts. Overall, there were no significant differences in baseline demographic characteristics between participants (P > .05 for all comparisons). All demographic characteristics are summarized in Table 1.
Table 1.
Demographic Characteristic Comparison Between BFR System Cohorts
| Delfi | BStrong | SmartCuffs Pro | P Value | |
|---|---|---|---|---|
| Age, yr | 28.7 ± 2.4 | 29.9 ± 1.3 | 28.2 ± 1.9 | .064 |
| Height, in | 70.4 ± 3.4 | 68.5 ± 3.3 | 69.4 ± 3.9 | .367 |
| Weight, lb | 180.9 ± 36.5 | 164.6 ± 33.3 | 168.1 ± 31.2 | .416 |
| Dominant arm, n (%) | ||||
| Right | 12 (85.7) | 13 (92.8) | 13 (92.8) | .772 |
| Left | 2 (14.3) | 1 (7.2) | 1 (7.2) | |
| 20%1RM, lb | ||||
| Right | 7.5 ± 2.2 | 6.2 ± 1.9 | 7.0 ± 2.6 | .321 |
| Left | 7.4 ± 2.3 | 6.2 ± 2.0 | 6.9 ± 2.6 | .386 |
NOTE. Data are presented as mean ± standard deviation unless otherwise indicated.
There were no significant differences between the BFR system cohorts regarding the average number of repetitions to failure performed under the no cuff (baseline) condition (P > .05 for all comparisons) (Table 2). All 3 BFR systems successfully accelerated repetitions to failure compared with unrestricted exercise (baseline), in that there were statistically significant within-group decreases in repetitions to failure performed at 20%1RM from baseline to BFR set 1 and from baseline to BFR set 2 (Fig 1). However, there were no significant between-group differences among BFR systems regarding the number of repetitions to failure performed at baseline versus BFR set 1 or at baseline versus BFR set 2. There were no differences among BFR system cohorts in reported RPE or RPD after BFR set 1 or RPD after BFR set 2. The Delfi PTS cohort did report a higher RPE after BFR set 2 compared with the BStrong and SmartCuffs Pro cohorts (8.7 vs 7.6 and 7.0, respectively; P = .025) (Table 2).
Table 2.
Average Number of Repetitions, RPE, and RPD During Each Set Between Cohorts
| Delfi | BStrong | SmartCuffs Pro | P Value | |
|---|---|---|---|---|
| No cuff | ||||
| Repetitions | 84.5 ± 19.1 | 85.2 ± 19.1 | 86.6 ± 17.8 | .956 |
| Set 1 | ||||
| Repetitions | 50.6 ± 7.7 | 43.5 ± 11.3 | 42.3 ± 13.7 | .114 |
| P value for set 1 vs no cuff | <.01∗ | <.01∗ | <.01∗ | |
| RPE | 7.4 ± 1.3 | 6.7 ± 1.5 | 6.3 ± 1.5 | .119 |
| RPD | 4.7 ± 3.1 | 5.4 ± 2.1 | 6.1 ± 1.8 | .331 |
| Set 2 | ||||
| Repetitions | 20.3 ± 5.9 | 23.2 ± 8.2 | 20.8 ± 8.1 | .563 |
| P value for set 2 vs no cuff | <.01∗ | <.01∗ | <.01∗ | |
| RPE | 8.7 ± 0.7 | 7.6 ± 2.1 | 7.0 ± 1.8 | .025∗ |
| RPD | 5.4 ± 3.2 | 6.7 ± 2.2 | 7.1 ± 1.7 | .165 |
NOTE. Data are presented as mean ± standard deviation.
RPE, rating of perceived effort; RPD, rating of perceived discomfort.
Statistically significant (P < .05).
Fig 1.
All 3 blood flow restriction systems successfully accelerated repetitions to failure compared with unrestricted exercise (baseline).
On analysis of the mean differences in number of repetitions, RPE, and RPD between BFR set 1 and BFR set 2, the Delfi PTS cohort produced a significant reduction in repetitions to failure between BFR sets 1 and 2 compared with the BStrong and SmartCuffs cohorts (30.3 vs 20.3 and 21.4, respectively; P = .002) (Table 3). Otherwise, there were no significant between-group relations regarding the mean difference in RPE or RPD between sets.
Table 3.
Mean Differences in Repetitions, RPE, and RPD
| Delfi | BStrong | SmartCuffs Pro | P Value | |
|---|---|---|---|---|
| No cuff vs set 1 | ||||
| Repetitions | 33.1 ± 20.6 | 41.4 ± 21.3 | 43.7 ± 15.9 | .328 |
| No cuff vs set 2 | ||||
| Repetitions | 64.1 ± 20.4 | 62.0 ± 19.3 | 65.7 ± 15.0 | .866 |
| Set 1 vs set 2 | ||||
| Repetitions | 30.3 ± 6.7 | 20.3 ± 7.8 | 21.4 ± 7.6 | .002∗ |
| RPE | –1.3 ± 1.4 | –0.9 ± 1.3 | –0.7 ± 0.9 | .462 |
| RPD | –0.7 ± 1.1 | –1.3 ± 0.9 | –1.0 ± 1.2 | .303 |
NOTE. Data are presented as mean ± standard deviation. Negative mean differences in RPE and RPD signify an increase in BFR set 2 versus BFR set 1.
RPE, rating of perceived effort; RPD, rating of perceived discomfort.
Statistically significant (P < .05).
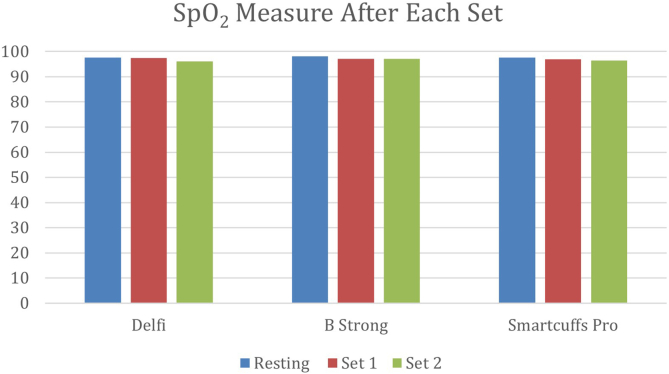
None of the BFR systems had an impact on SpO2 (oxygen saturation as measured by pulse oximetry) over time or between sets (P > .05 for all comparisons) (Table 4, Fig 2). There were no adverse events. At 24 hours of follow-up, the average VAS score for all extremities irrespective of cohort was 0.8, the average McGill score for all extremities irrespective of cohort was 1.1 ± 1.3, and aching pain was the most reported symptom (n = 15, 36% of arms); there were no significant between-group differences among BFR system cohorts (P > .05 for all comparisons).
Table 4.
SpO2 at Baseline and After Each Set
| Delfi | BStrong | SmartCuffs Pro | Between-Group P Value | |
|---|---|---|---|---|
| At baseline | 97.6 ± 1.9 | 98.1 ± 1.6 | 97.7 ± 1.9 | .372 |
| After set 1 | 97.4 ± 3.5 | 97.1 ± 5.7 | 97.0 ± 6.7 | .842 |
| After set 2 | 96.1 ± 5.9 | 97.1 ± 2.7 | 96.5 ± 8.6 | .516 |
| Within-group P value | .081 | .287 | .429 |
NOTE. Data are presented as mean ± standard deviation.
SpO2, oxygen saturation as measured by pulse oximetry.
Fig 2.
None of the blood flow restriction systems had an impact on SpO2 (oxygen saturation as measured by pulse oximetry) over time or between sets.
Discussion
This study found no significant differences between the Delfi PTS, SmartCuffs Pro, and BStrong BFR cuff systems in accelerating fatigue, as measured by repetitions to failure under blood flow–restricted conditions versus baseline free-flow exercise. Delfi PTS participants reported a greater RPE after BFR set 2 compared with participants using the other devices, but otherwise, there were no significant differences in RPD, post-exercise pulse oximetry readings, or VAS or McGill pain scores between the systems. There were no adverse events reported with any BFR system, although aching arm pain was the most common side effect of BFR exercise.
Although the therapeutic benefits of BFR training have been researched in numerous prior studies, there exists great heterogeneity in its implementation, from both an investigational viewpoint and a clinical viewpoint. Specifically, various BFR system designs have been used in practice and research, but these have rarely been compared in the literature in terms of their performance and safety when used in typical BFR training protocols. A 2018 investigation by Hughes et al.27 compared autoregulated BFR tourniquet systems with non-autoregulated BFR systems using an exercise protocol that entailed the completion of 4 sets of unilateral leg press exercises at 30%1RM. The investigators subsequently found that the automatic BFR systems appeared to regulate occlusion pressure more effectively and that the participants who used such systems reported less pain and exertion than those who used non-autoregulated devices. Of note, both the autoregulated and non-autoregulated BFR tourniquet systems used in the study possessed cuffs with a single–inflatable bladder design. More recently, in a study published in 2023, Jacobs et al.26 investigated the effects of autoregulated versus non-autoregulated BFR system application on performance, perceptual response, and adverse effects during lower-body resistance exercise. The SmartCuffs Pro cuff system was used for both the autoregulated and non-autoregulated conditions in the study; this cuff likewise possesses a single–inflatable bladder design. Ultimately, the authors found that autoregulation enabled participants to perform significantly more repetitions when exercising until volitional failure, with lower levels of perceived exertion and discomfort, compared with the same exercise using non-autoregulation of applied pressure. The findings of our investigation are in contrast to the results of the aforementioned studies, in that no significant differences were observed between the non-autoregulated BStrong system and the autoregulated SmartCuffs Pro and Delfi PTS systems in terms of both accelerated fatigue (via performed repetitions to failure) and perceptual responses (via reported RPE and RPD after each set) when comparing performance under baseline conditions with that under BFR conditions. Whereas the Delfi PTS system did show a statistically significantly larger decrease in repetitions to failure from BFR set 1 to BFR set 2 relative to the other systems, it also showed a nonsignificantly lower reduction in repetitions to failure from the baseline condition to BFR set 1 compared with the other systems; therefore, this finding likely represents only a calculatory effect rather than a meaningful result. The Delfi PTS system did produce a greater change in RPE with prolonged use (from BFR set 1 to BFR set 2) compared with the other systems, potentially impacting longitudinal perceptual responses. An interesting finding was that, of the 3 systems, the Delfi PTS system also possessed the greatest cuff width (11.5 cm vs 10.16 cm for SmartCuffs Pro and 5 cm for BStrong). Although cuff width has been found to affect the relative pressure that a BFR system must apply to achieve LOP31—and perhaps the perceived discomfort among subjects32—future investigations would be useful to explore how cuff width specifically affects perceptual response and fatigability when applied during a BFR training protocol.
Prior studies have reported DOMS as a very common side effect of BFR training, particularly when performed by individuals not accustomed to this intervention and when performing exercise until failure.33,34 In the investigation of Jacobs et al.,26 the cohort that exercised to volitional failure under non-autoregulated BFR conditions did experience an increased rate of DOMS compared with the auto-regulated BFR cohort (32.2% vs 42.6%, P < .001). In light of this finding, the authors postulated that a possible reason for the difference in DOMS observed between non-autoregulated and autoregulated training conditions is that auto-regulated devices are better able to maintain and/or regulate stable occlusive pressures throughout the muscle contraction-relaxation cycle, thereby preventing the development of excessive intramuscular pressures during contraction and producing less hypoxia and metabolic byproduct accumulation, compared with non-autoregulated BFR devices.21,35 In our investigation, despite variety in cuff system designs, no differences were found among the 3 cuff systems regarding VAS or McGill pain scores (used as a surrogate for DOMS experienced by participants after the completion of their BFR training protocols). When considering potential explanations for the equivalent rates of DOMS among the autoregulated systems and the non-autoregulated BStrong system, it is possible that despite the lack of occlusion pressure adjustment during the muscle contraction-relaxation cycle, the application of non-uniform circumferential pressure and avoidance of arterial occlusion may mitigate the development of excessive intramuscular pressures and metabolic byproduct accumulation, thereby producing rates of DOMS similar to those of autoregulated systems. Alternatively, our investigation had participants perform only 2 sets to failure; hence, it is possible that longer and/or more rigorous exercise protocols could result in a higher incidence of DOMS and thereby elucidate differences between cuff systems. Furthermore, more invasive measures of metabolic stress (e.g., lactate sample) and varied exercise protocols are certainly warranted to support or refute these possibilities in future investigations.
Finally, previous literature on BFR training using non-autoregulated devices reported adverse events occurring in participants at rates between 10% and 15%.36, 37, 38, 39 More recently, Jacobs et al.26 reported an adverse event rate of just 3.6% with their BFR training protocol using an auto-regulated device. Fortunately, our investigation reports no adverse events. These findings suggest that, currently, BFR devices may be well tolerated under both autoregulated and non-autoregulated conditions and that the decreased adverse event rate observed in our investigation may potentially be due to the ongoing innovation and technological advancement of these systems. Further investigation is necessary to apply this protocol to larger, more demographically diverse study populations before definitive conclusions may be drawn, however.
Limitations
The results of this investigation must be considered within the context of its limitations. Namely, although sample size determination was performed by a power analysis conducted before initiation of the investigation, it must be noted that owing to the nature of this type of investigation and sample size, representative sampling of the entire patient population is not feasible. Furthermore, the study was performed in a relatively small cohort composed of healthy (average body mass index, 24.9), young (aged between 26 and 40 years) adults, warranting cautious extrapolation of the results to clinical populations. Moreover, each participant exercised with 2 of 3 possible cuffs, potentially creating unforeseen bias in the results. Finally, owing to the equipment available within the sports medicine clinical setting, we were unable to determine an exact 1RM for many participants; therefore, we used the Epley formula to produce 1RM estimates based on submaximal biceps curl exercise.
Conclusions
The Delfi PTS, SmartCuffs Pro, and BStrong BFR systems were each safe and were able to significantly reduce repetitions to failure compared with a low-load free-flow condition when used in a BFR exercise protocol. The Delfi PTS system may produce a higher RPE with prolonged use in comparison to the other systems.
Disclosures
The authors report the following potential conflicts of interest or sources of funding: K.R.O. receives compensation for services other than consulting from Smith & Nephew; receives education payments from Gemini Medical; receives travel and lodging payments from Arthrex, Stryker, Smith & Nephew, and Gemini Medical; and receives food and beverage payments from Arthrex, Stryker, Smith & Nephew, and Gemini Medical. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Dos Santos L.P., do Espirito Santo R.C., Ramis T.R., Portes J.K.S., da Silva Chakr R.M., Xavier R.M. The effects of resistance training with blood flow restriction on muscle strength, muscle hypertrophy and functionality in patients with osteoarthritis and rheumatoid arthritis: A systematic review with meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q., He X.J., Li Y.D., et al. Dose-response relationship of blood flow restriction training on isometric muscle strength, maximum strength and lower limb extensor strength: A meta-analysis. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.1046625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong J, Li Z, Zhu L, Li L, Chen S. Comparison of blood flow restriction training and conventional resistance training for the improvement of sarcopenia in the older adults: A systematic review and meta-analysis [published online December 17, 2022]. Sports Med Health Sci. https://doi.org/10.1016/j.smhs.2022.12.002. [DOI] [PMC free article] [PubMed]
- 4.Fabero-Garrido R., Gragera-Vela M., Del Corral T., Izquierdo-García J., Plaza-Manzano G., López-de-Uralde-Villanueva I. Effects of low-load blood flow restriction resistance training on muscle strength and hypertrophy compared with traditional resistance training in healthy adults older than 60 years: Systematic review and meta-analysis. J Clin Med Res. 2022;11:7389. doi: 10.3390/jcm11247389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May A.K., Russell A.P., Della Gatta P.A., Warmington S.A. Muscle adaptations to heavy-load and blood flow restriction resistance training methods. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.837697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Queiros V.S., Rolnick N., Dos Santos Í.K., et al. Acute effect of resistance training with blood flow restriction on perceptual responses: A systematic review and meta-analysis. Sports Health. 2023;15:673–688. doi: 10.1177/19417381221131533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson I., Lazarides M., Butler C.R. Blood flow restriction therapy for use after extremity fracture: A critically appraised topic. J Sport Rehabil. 2023;32:102–106. doi: 10.1123/jsr.2022-0166. [DOI] [PubMed] [Google Scholar]
- 8.Takarada Y., Takazawa H., Sato Y., Takebayashi S., Tanaka Y., Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88:2097–2106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda T., Fujita S., Ogasawara R., Sato Y., Abe T. Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: A pilot study. Clin Physiol Funct Imaging. 2010;30:338–343. doi: 10.1111/j.1475-097X.2010.00949.x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes L., Paton B., Rosenblatt B., Gissane C., Patterson S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br J Sports Med. 2017;51:1003–1011. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 11.Whiteley R. Blood flow restriction training in rehabilitation: A useful adjunct or Lucy’s latest trick? J Orthop Sports Phys Ther. 2019;49:294–298. doi: 10.2519/jospt.2019.0608. [DOI] [PubMed] [Google Scholar]
- 12.Watson R., Sullivan B., Stone A., et al. Blood flow restriction therapy: An evidence-based approach to postoperative rehabilitation. JBJS Rev. 2022:10. doi: 10.2106/JBJS.RVW.22.00062. [DOI] [PubMed] [Google Scholar]
- 13.Perera E., Zhu X.M., Horner N.S., Bedi A., Ayeni O.R., Khan M. Effects of blood flow restriction therapy for muscular strength, hypertrophy, and endurance in healthy and special populations: A systematic review and meta-analysis. Clin J Sport Med. 2022;32:531–545. doi: 10.1097/JSM.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 14.Labata-Lezaun N., Llurda-Almuzara L., González-Rueda V., et al. Effectiveness of blood flow restriction training on muscle strength and physical performance in older adults: A systematic review and meta-analysis. Arch Phys Med Rehabil. 2022;103:1848–1857. doi: 10.1016/j.apmr.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Geng Y.U., Zhang L., Wu X. Effects of blood flow restriction training on blood perfusion and work ability of muscles in elite para-alpine skiers. Med Sci Sports Exerc. 2022;54:489–496. doi: 10.1249/MSS.0000000000002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T., Tian G., Wang X. Effects of low-load blood flow restriction training on hemodynamic responses and vascular function in older adults: A meta-analysis. Int J Environ Res Public Health. 2022;19:6750. doi: 10.3390/ijerph19116750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spada J.M., Paul R.W., Tucker B.S. Blood flow restriction training preserves knee flexion and extension torque following anterior cruciate ligament reconstruction: A systematic review. J Orthop. 2022;34:233–239. doi: 10.1016/j.jor.2022.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q., Li D.Y., He J.X., et al. Influence of blood flow restriction training on the aerobic capacity: A systematic review and meta-analysis. J Mens Health. 2022;18:062. [Google Scholar]
- 19.Wong V., Song J.S., Bell Z.W., et al. Blood flow restriction training on resting blood pressure and heart rate: A meta-analysis of the available literature. J Hum Hypertens. 2022;36:738–743. doi: 10.1038/s41371-021-00561-0. [DOI] [PubMed] [Google Scholar]
- 20.Pearson S.J., Hussain S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187–200. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 21.Takarada Y., Nakamura Y., Aruga S., Onda T., Miyazaki S., Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol. 2000;88:61–65. doi: 10.1152/jappl.2000.88.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Loenneke J.P., Thiebaud R.S., Fahs C.A., Rossow L.M., Abe T., Bemben M.G. Blood flow restriction: Effects of cuff type on fatigue and perceptual responses to resistance exercise. Acta Physiol Hung. 2014;101:158–166. doi: 10.1556/APhysiol.101.2014.2.4. [DOI] [PubMed] [Google Scholar]
- 23.Cerqueira MS, Lira M, Mendonça Barboza JA, et al. Repetition failure occurs earlier during low-load resistance exercise with high but not low blood flow restriction pressures: A systematic review and meta-analysis [published online July 26, 2021]. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000004093. [DOI] [PubMed]
- 24.Rolnick N., Kimbrell K., de Queiros V. Beneath the cuff: Often overlooked and under-reported blood flow restriction device features and their potential impact on practice—A review of the current state of the research. Front Physiol. 2023;14 doi: 10.3389/fphys.2023.1089065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolnick N., Kimbrell K., Cerqueira M.S., Weatherford B., Brandner C. Perceived barriers to blood flow restriction training. Front Rehabil Sci. 2021;2 doi: 10.3389/fresc.2021.697082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs E., Rolnick N., Wezenbeek E., et al. Investigating the autoregulation of applied blood flow restriction training pressures in healthy, physically active adults: An intervention study evaluating acute training responses and safety. Br J Sports Med. 2023;57:914–920. doi: 10.1136/bjsports-2022-106069. [DOI] [PubMed] [Google Scholar]
- 27.Hughes L., Rosenblatt B., Gissane C., Paton B., Patterson S.D. Interface pressure, perceptual, and mean arterial pressure responses to different blood flow restriction systems. Scand J Med Sci Sports. 2018;28:1757–1765. doi: 10.1111/sms.13092. [DOI] [PubMed] [Google Scholar]
- 28.Abbas M.J., Dancy M.E., Marigi E.M., et al. An automated technique for the measurement of limb occlusion pressure during blood flow restriction therapy is equivalent to previous gold standard. Sports Med Arthrosc Rehabil Ther Technol. 2022;4:e1127–e1132. doi: 10.1016/j.asmr.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Early K.S., Rockhill M., Bryan A., Tyo B., Buuck D., McGinty J. Effect of blood flow restriction training on muscular performance, pain and vascular function. Int J Sports Phys Ther. 2020;15:892–900. doi: 10.26603/ijspt20200892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordessa J.M., Hearn M.C., Reinfeldt A.E., et al. Comparison of blood flow restriction devices and their effect on quadriceps muscle activation. Phys Ther Sport. 2021;49:90–97. doi: 10.1016/j.ptsp.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Weatherholt A.M., Vanwye W.R., Lohmann J., et al. The effect of cuff width for determining limb occlusion pressure: A comparison of blood flow restriction devices. Int J Exerc Sci. 2019;12:136–143. doi: 10.70252/RWVU7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitz R.W., Chatakondi R.N., Bell Z.W., et al. Blood flow restriction exercise: Effects of sex, cuff width, and cuff pressure on perceived lower body discomfort. Percept Mot Skills. 2021;128:353–374. doi: 10.1177/0031512520948295. [DOI] [PubMed] [Google Scholar]
- 33.Sieljacks P., Matzon A., Wernbom M., Ringgaard S., Vissing K., Overgaard K. Muscle damage and repeated bout effect following blood flow restricted exercise. Eur J Appl Physiol. 2016;116:513–525. doi: 10.1007/s00421-015-3304-8. [DOI] [PubMed] [Google Scholar]
- 34.de Queiros V.S., Dantas M., Neto G.R., et al. Application and side effects of blood flow restriction technique: A cross-sectional questionnaire survey of professionals. Medicine. 2021;100 doi: 10.1097/MD.0000000000025794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suga T., Okita K., Morita N., et al. Dose effect on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. J Appl Physiol. 2010;108:1563–1567. doi: 10.1152/japplphysiol.00504.2009. [DOI] [PubMed] [Google Scholar]
- 36.Patterson S.D., Brandner C.R. The role of blood flow restriction training for applied practitioners: A questionnaire-based survey. J Sports Sci. 2018;36:123–130. doi: 10.1080/02640414.2017.1284341. [DOI] [PubMed] [Google Scholar]
- 37.Martín-Hernández J., Santos-Lozano A., Foster C., Lucia A. Syncope episodes and blood flow restriction training. Clin J Sport Med. 2018;28:e89–e91. doi: 10.1097/JSM.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima T., Iida H., Kurano M., et al. Hemodynamic responses to simulated weightlessness of 24-h head-down bed rest and KAATSU blood flow restriction. Eur J Appl Physiol. 2008;104:727–737. doi: 10.1007/s00421-008-0834-3. [DOI] [PubMed] [Google Scholar]
- 39.Prue J., Roman D.P., Giampetruzzi N.G., et al. Side effects and patient tolerance with the use of blood flow restriction training after ACL reconstruction in adolescents: A pilot study. Int J Sports Phys Ther. 2022;17:347–354. doi: 10.26603/001c.32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.