Abstract
Four stress-responsive protein kinases, including GCN2 and PKR, phosphorylate eukaryotic translation initiation factor 2α (eIF2α) on Ser51 to regulate general and gene-specific protein synthesis. Phosphorylated eIF2 is an inhibitor of its guanine nucleotide exchange factor, eIF2B. Mutations that block translational regulation were isolated throughout the N-terminal OB-fold domain in Saccharomyces cerevisiae eIF2α, including those at residues flanking Ser51 and around 20 Å away in the conserved motif K79GYID83. Any mutation at Glu49 or Asp83 blocked translational regulation; however, only a subset of these mutations impaired Ser51 phosphorylation. Substitution of Ala for Asp83 eliminated phosphorylation by GCN2 and PKR both in vivo and in vitro, establishing the critical contributions of remote residues to kinase-substrate recognition. In contrast, mutations that blocked translational regulation but not Ser51 phosphorylation impaired the binding of eIF2B to phosphorylated eIF2α. Thus, two structurally distinct effectors of eIF2 function, eIF2α kinases and eIF2B, have evolved to recognize the same surface and overlapping determinants on eIF2α.
The specificity of signaling pathways requires that protein kinases select their substrate and phosphorylate the appropriate residue from among the myriad of proteins they encounter in a cell. Crystallographic studies have revealed that protein kinase domains fold into a common structure consisting of a smaller N-terminal lobe and a larger C-terminal lobe, with the active site in a cleft between the two lobes (reviewed in references 13 and 18). It is typically thought that kinases recognize their phosphorylation sites at least in part through interactions with the immediately flanking residues, and this notion is supported by the identification of kinase consensus sequences (23, 24). However, sequences remote from the phosphorylation site also have been implicated in substrate recognition. The Jun N-terminal kinases (JNKs) bind to the N terminus of c-Jun (residues 1 to 45) and phosphorylate Ser residues at positions 63 and 73 (15), and a docking site in cyclin A facilitates substrate, and inhibitor, recruitment to the cyclin-dependent kinase cdk2 (1, 35, 39).
Phosphorylation of eukaryotic translation initiation factor 2 (eIF2) on Ser51 of its α subunit is a common means to control protein synthesis. By juxtaposing different regulatory domains with a conserved eIF2α kinase domain, four eIF2α kinases transduce distinct stress signals to regulate protein synthesis (reviewed in reference 7). Binding of double-stranded RNA (dsRNA) to two dsRNA-binding domains in the N terminus of the mammalian antiviral kinase PKR promotes dimerization and activates the kinase both to autophosphorylate and to phosphorylate eIF2α. Dimerization of PKR is necessary for function in vivo and for efficient phosphorylation of eIF2α on Ser51 (4, 40, 43). The kinase HRI is activated under conditions of low heme levels, whereas Perk/PEK senses endoplasmic reticulum stress. Finally, the kinase GCN2, universally conserved in all eukaryotes, responds to amino acid starvation and various other stress conditions (3, 17).
In its active GTP-bound state, eIF2 binds the initiator methionyl-tRNA (Met-tRNAiMet), forming the eIF2-GTP-Met-tRNAiMet ternary complex, and delivers the Met-tRNAiMet to the 40S ribosomal subunit (reviewed in reference 16). Following translation initiation, inactive eIF2-GDP is released from the ribosome. The guanine nucleotide exchange factor (GEF) eIF2B recycles eIF2-GDP to functional eIF2-GTP. Phosphorylation of eIF2α on Ser51 inhibits protein synthesis by converting eIF2 from a substrate to a competitive inhibitor of eIF2B (16). The five subunits of the eIF2B complex can be divided into two groups (reviewed in reference 16). The ɛ subunit, encoded by GCD6 in Saccharomyces cerevisiae, catalyzes nucleotide exchange and forms a complex with the structurally related γ subunit encoded by GCD1 (33). The α, β, and δ subunits, encoded by GCN3, GCD7, and GCD2, respectively, form a regulatory subcomplex in eIF2B. Removal of the α subunit by deletion of GCN3 or specific mutations in any of the three subunits in the regulatory subcomplex desensitizes eIF2B to inhibition by phosphorylated eIF2 (16, 33, 34). In agreement with these in vivo findings, the regulatory subcomplex has been shown to bind directly to eIF2α in a manner dependent on the phosphorylation of Ser51 (27).
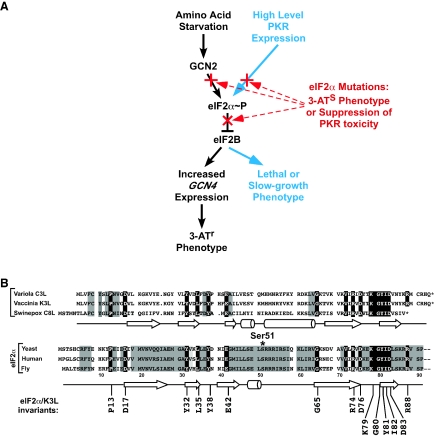
While phosphorylation of eIF2α inhibits general protein synthesis, this modification has been exploited by a few mRNAs to paradoxically increase their translation. The GCN4 protein in yeast is a transcriptional activator of a large number of genes, including most of the genes encoding amino acid biosynthetic enzymes (17). The expression of GCN4 is controlled at the translational level by the eIF2α kinase GCN2 and by regulated reinitiation at four upstream open reading frames in the GCN4 mRNA (reviewed in reference 16). When amino acids are plentiful in the medium, GCN2 is inactive, Ser51 on eIF2α is predominantly nonphosphorylated, and GCN4 is not produced. Under amino acid starvation conditions, GCN2 is activated, and it phosphorylates eIF2α, resulting in inhibition of eIF2B and increased GCN4 expression (Fig. 1A). This gene-specific translational derepression of GCN4 expression is required for the survival of yeast cells under amino acid starvation conditions. Mutations of Ser51 that block phosphorylation, such as replacement by Ala (eIF2α-S51A), impair translational regulation and growth of yeast under starvation conditions.
FIG. 1.
Model of translational control by eIF2α phosphorylation and conservation of eIF2α structure. (A) Model of the role of eIF2α phosphorylation in GCN4 translational control (16) and yeast cell growth. Phosphorylation of eIF2α on Ser51 converts eIF2 from a substrate to an inhibitor of eIF2B, its GEF, and thus lowers the levels of eIF2-GTP-Met-tRNAiMet ternary complexes. (Left pathway) Starvation of yeast for amino acids leads to accumulation of uncharged tRNAs that bind to and activate the eIF2α kinase GCN2. The modest reduction in ternary complex levels causes derepression of GCN4 mRNA translation, and the GCN4 protein promotes transcription of a large regulon including many of the amino acid biosynthetic genes. Increased expression of the histidine biosynthesis genes in cells expressing high levels of GCN4 confers resistance to the drug 3-AT. (Right pathway) High-level expression of PKR causes significant eIF2α phosphorylation, leading to a large decrease in ternary complex levels and a severe slow-growth or lethal phenotype in yeast. Highlighted in red are the ways in which eIF2α mutations can disrupt the pathways by either blocking Ser51 phosphorylation or preventing inhibition of eIF2B by phosphorylated eIF2. (B) Sequence alignment of various eIF2α proteins and poxvirus eIF2α mimics. The amino acid sequences of the N-terminal one-third of eIF2α from yeast, humans, and flies are aligned with the full-length sequences of the vaccinia virus K3L protein and the swinepox virus C8L protein. Secondary structural elements (arrow, β-sheet; cylinder, α-helix) of the vaccinia virus K3L protein (4) and yeast eIF2α (10) are presented below the sequences. The asterisk indicates the Ser51 phosphorylation site in eIF2α (note that Ser51 is actually residue 52 in eIF2α; however, the N-terminal Met is posttranslationally cleaved, at least in mammals, and by convention the Ser is numbered as residue 51). Residues conserved among all of the eIF2α proteins and the K3L and C8L proteins are shown in reverse type and included in the consensus sequence at the bottom of the alignment. Residues conserved only among all of the eIF2α proteins are shaded.
The eIF2α kinases offer a powerful genetic and biochemical system with which to study kinase-substrate recognition, because GCN2 is the sole eIF2α kinase in yeast, GCN2 has only a single identified substrate, the kinase is nonessential, and phosphorylation of eIF2α is required for growth only under certain stress conditions. As detailed in this study, eIF2α is amenable to mutational analysis, because few substitutions impair its essential function in protein synthesis. In addition, recent structural studies have provided 3-dimensional (3D) images of both human and yeast eIF2α, revealing an N-terminal oligonucleotide binding (OB) fold domain containing the Ser51 phosphorylation site followed by an α-helical domain (10, 32).
Insights into the mechanism of substrate recognition by the eIF2α kinases have come from studies of a viral pseudosubstrate inhibitor of PKR. The vaccinia virus K3L protein resembles the OB-fold domain in the N-terminal third of eIF2α (Fig. 1B) and blocks PKR activity both in vivo and in vitro (2, 5, 20). Mutational studies on the K3L protein have identified a conserved KGYID sequence motif, located 30 residues C-terminal of Ser51 in eIF2α (see Fig. 1B), as critical for kinase inhibition (4, 20). The crystal structure of the K3L protein revealed that the KGYID motif is part of a conserved patch on the K3L protein surface located ∼21.5 Å from the phosphorylation site (4). It is anticipated that the corresponding residues in eIF2α may also be important for kinase recognition, as suggested by in vitro binding and kinase assays using an eIF2α variant lacking a portion of this motif (36). Additional support for the notion that residues remote from Ser51 are critical determinants for kinase recognition of eIF2α comes from in vitro kinase assays using peptide substrates versus full-length substrates. A 12-residue peptide centered on Ser51 is a poor substrate for phosphorylation by PKR (Km for the peptide, 1.08 mM; Km for eIF2α, 0.626 μM) (30), whereas the cAMP-dependent protein kinase PKA efficiently phosphorylates a peptide substrate containing its consensus sequence (see reference 21). In addition, heat denaturation of eIF2α significantly reduces its phosphorylation by HRI (26). Thus, kinase recognition of Ser51 is likely to depend on the proper tertiary structure of eIF2α.
In this paper we describe a systematic mutational analysis of eIF2α to identify mutations that block translational regulation. Our data reveal that substrate recognition by the eIF2α kinases requires residues far removed from the phosphorylation site. Interestingly, eIF2α mutations that block Ser51 phosphorylation and mutations that lower the binding of phosphorylated eIF2α to eIF2B map to residues that form a contiguous molecular surface extending more than 20 Å from the Ser51 phosphorylation site. We propose that this conserved surface on eIF2α forms the recognition site for both the eIF2α kinases and eIF2B.
MATERIALS AND METHODS
Yeast strains and plasmids.
Strain H1643 (MATa ura3-52 leu2-3 leu2-112 trp1-Δ63 sui2Δ p[SUI2, URA3] 〈GCN4-lacZ, TRP1〉@TRP1), used for screening eIF2α mutants, has been described previously (9), as has the isogenic gcn2Δ strain H1925 (41). Yeast strain H2767 (MATα leu2 trp1 ura3-52 prb1-1122 pep4-3 gal2 gcd6Δ gcd7Δ p1871[GCD7, GCD2, GCN3, URA3] pTK1.11[GCD1-2×Flag-His, GCD6, LEU2]), overexpressing eIF2B subunits and used for binding assays, has been described previously (27).
The LEU2 low-copy-number plasmids p1097 (9) and pC171, carrying the yeast SUI2 gene encoding eIF2α, were used to screen for mutants. Plasmid pC171 was constructed in three steps. First, the BglII site encoding residues 291 to 292 in yeast eIF2α was destroyed by a silent mutation. Second, a BglII site was inserted at the codons for residues Arg56 and Ser57. Third, the modified SUI2 gene was inserted at the BamHI site of a version of the pRS315 vector (37) in which the polylinker was modified to remove all of the sites from SalI to EcoRV. Thus, in pC171 the AvrII site in the SUI2 promoter, the BglII site at Arg56, the SalI site at Val75, and the HindIII site at Glu142 are all unique. Plasmid pC1655 was constructed by first removing the XbaI site from the multiple cloning site in pC171 and then inserting an XbaI site by a single point mutation 12 bases 5′ of the eIF2α AUG start site.
The URA3 high-copy-number plasmids p1420 and p1246, encoding human PKR and HRI, respectively (8), pTK4, encoding FLAG- and His-tagged PKR (27), and pDH103, encoding FLAG- and His-tagged GCN2 (11), all under the control of a yeast GAL-CYC1 hybrid promoter, have been described previously. The glutathione S-transferase (GST)-eIF2α expression vector p2565, described previously (27), was constructed by inserting the full yeast eIF2α coding region into the pGEX-2T vector at the BamHI site. Point mutations S51A, RRR52-54VKA, K79A, and D83A were introduced into p2565 by subcloning an internal HpaI-HindIII fragment encoding eIF2α residues 22 to 143. DNA fragments encoding full-length and C-terminally truncated forms of eIF2α were generated by PCR and subcloned between the BamHI and XbaI or XhoI sites of vectors pEGKT (31) and pGEX-6P (Amersham Biosciences) for expression in yeast and bacteria, respectively. By using the same strategy, PCR products were generated and subcloned into pGEX-6P to express GST-eIF2α mutants with the L35A, I45N, L50S, K79A, Y81S, or D83E mutation in bacteria.
Plasmids pC2252 and pC2256, encoding eIF2α residues 1 to 200 with a polyhistidine tag [His6-eIF2α(1-200)] and His6-eIF2α(1-200)-D83E, respectively, were generated by subcloning PCR products between the NdeI and BamHI sites of vector pET-15b (Novagen).
Mutagenesis and screening.
The eIF2α codons for the residues flanking Ser51 and those in the KGYID motif were randomly mutated by PCR. For the residues around Ser51, the 5′ oligonucleotide primer included the AvrII site and the 3′ primer included the new BglII site at residues 56 and 57. The 3′ primer was designed to randomly incorporate all four nucleotides at the three positions specifying a particular codon. To mutate the residues in the KGYID motif, the 5′ primer included the SalI site and the mutated codon, and the 3′ primer included the HindIII site. Products of the PCRs were digested with the appropriate restriction enzymes and subcloned into pC171. Separate DNA libraries were generated for each randomly mutated residue and were independently transformed into yeast strain H1643. Approximately 250 independent yeast transformants (4 × 64, the number of possible mutants) were selected and replica printed to 5-fluoroorotic acid (5-FOA) medium in order to select for cells that had lost the URA3 plasmid encoding wild-type (WT) eIF2α. The 5-FOA plate was incubated for 2 days at 30°C and then replica printed to a 3-aminotriazole (3-AT) plate. From the screen of 250 yeast transformants, the number of 3-AT-sensitive (3-ATs) mutants isolated at each residue was as follows: 128 at Glu49, 27 at Leu50, 81 at Lys79, 6 at Gly80, 93 at Tyr81, 110 at Ile82, 215 at Asp83, and 74 at Arg88. Plasmids were isolated from the 3-ATs colonies and retested, and then as many as 40 plasmids from each mutated residue were sequenced. Thus, the screening and sequencing may not have identified all of the 3-ATs mutations at each residue. The sequence of the entire PCR product was determined to confirm that no additional mutations had been inserted during the PCR procedures.
To randomly mutate eIF2α codons 1 to 48, the appropriate region of the SUI2 gene was amplified by error-prone PCR (Stratagene) under low-stringency conditions (1 to 2 mutations per cycle) and the products were subcloned between the XbaI and BglII sites of plasmid pC1655. Similarly, the region of the SUI2 gene encoding amino acids 89 to 288 was amplified by error-prone PCR and subcloned between the SalI and BglII sites of plasmid p1097. Sequencing of randomly selected clones from these two mutagenesis procedures revealed that 40 to 50% of the plasmids contained a mutation. The eIF2α mutant libraries were screened as described above by transformation into yeast strain H1643. Approximately 3,000 independent yeast transformants were picked, replica printed to 5-FOA medium, and subsequently printed to 3-AT medium as described above. Twenty-four plasmids from the residue-1-to-48 screen and six plasmids from the residue-89-to-288 screen conferred a 3-ATs phenotype that was confirmed following isolation and retesting of the plasmids. Sequencing of the plasmids identified the mutations G30R, A31T, L35S, M44K, I45N, and E49G from the residue-1-to-48 screen. Because the sequences of the six plasmids from the residue-89-to-288 screen revealed multiple mutations and Western blot analyses using phosphospecific antibodies against Ser51 indicated that the mutations did not interfere with eIF2α phosphorylation, these mutants were not examined further.
eIF2B binding assays.
GST-eIF2α fusion proteins were expressed in Escherichia coli BL21(DE3) plus cells (Novagen), and the recombinant proteins were purified as previously described (27). Whole-cell extracts (WCEs) were prepared from a saturated culture of yeast strain H2767 that overexpresses all five eIF2B subunits. Cells were suspended in breaking buffer [40 mM Tris-HCl (pH 7.5), 2 μM phenylmethylsulfonyl fluoride (PMSF), 1 mM dithiothreitol, 15 mM disodium EDTA, 45 mM NaF, 25 mM β-glycerophosphate, 120 mM (NH4)2SO4, 10 mM 2-aminopurine] and broken by vortexing with glass beads.
GST-eIF2α fusions were phosphorylated by PKR in vitro with 1 mM ATP in 50 μl of kinase buffer (20 mM Tris-HCl [pH 8.0], 50 mM KCl, 25 mM MgCl2, 1 μM PMSF) at room temperature for 30 min. Phosphorylated eIF2α proteins were immobilized on glutathione-Sepharose beads (Amersham Biosciences; 1 μg of eIF2α per 50 μl of beads [bed volume]) in 950 μl of binding buffer (50 mM Tris-HCl [pH 8.0], 150 mM KCl, 2.5 mM MgCl2, 0.1 mM disodium EDTA, 1 mM dithiothreitol, 0.1% Triton X-100, 5 mM NaF, 0.1 mM ATP, and 1 protease inhibitor tablet [Roche] per 50 ml of buffer) at 4°C for 1 h. The Sepharose beads were washed, resuspended in 900 μl of the same buffer, mixed with 100 μl (1 mg) of yeast WCE from strain H2767, and incubated for 2 h at 4°C with continuous slow shaking. The beads were collected and washed repeatedly with binding buffer; then bound proteins were eluted with glutathione and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to a nitrocellulose membrane and probed with appropriate antisera.
In vitro kinase assay and kinetics.
His6-eIF2α fusion proteins were purified by nickel-silica resin (QIAGEN) using the manufacturer's protocol, and FLAG-tagged PKR was overexpressed and purified from yeast as described previously (27). Various amounts of His-tagged eIF2α proteins (25 nM to 5 μM) were mixed with FLAG-tagged PKR (2.5 nM) in kinase buffer (20 mM Tris-HCl [pH 8.0], 50 mM KCl, 25 mM MgCl2, 1 μM PMSF) with 10 μCi of [γ-33P]ATP. Reactions were stopped after 20 min by addition of 2× SDS dye (Invitrogen), and products were separated by SDS-PAGE. The relative incorporation of radioactive phosphate into eIF2α was determined by PhosphorImager analysis, and the data were analyzed by using KaleidaGraph.
RESULTS
The N-terminal 180 residues of eIF2α are required for kinase recognition.
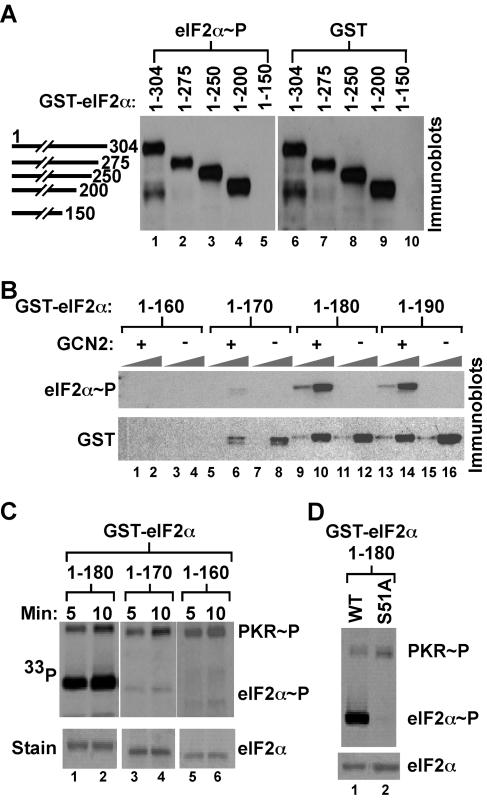
Structural studies revealed that residues 1 to 175 in yeast eIF2α and residues 3 to 182 in human eIF2α (10, 32) form a stable protein consisting of two domains, with an N-terminal OB-fold domain (residues 1 to 89) that resembles the vaccinia virus K3L protein, a pseudosubstrate inhibitor of PKR (4). Previous in vitro studies demonstrated that the two-domain form of eIF2α (residues 1 to 197) was efficiently phosphorylated by PKR (27). To define the minimal kinase recognition domain in yeast eIF2α in vivo, GST-eIF2α fusion proteins truncated at their N or C termini were expressed in a yeast strain in which the chromosomal eIF2α gene (SUI2) was replaced with the phosphorylation site mutant eIF2α-S51A. Cells were treated with 3-AT, an inhibitor of the HIS3 gene product that causes histidine starvation, leading to activation of GCN2. WCEs were subjected to Western blotting using antibodies that recognize eIF2α phosphorylated on Ser51, followed by antibodies that recognize GST (Fig. 2A). C-terminally truncated GST-eIF2α(1-200) was expressed and phosphorylated to a similar extent as a full-length fusion protein, GST-eIF2α(1-304) (Fig. 2A, lanes 1, 4, 6, and 9); however, the larger C-terminal truncations in GST-eIF2α(1-150) and GST-eIF2α(1-160) rendered these proteins unstable in yeast (Fig. 2A, lane 10, and 2B, lanes 1 to 4). GST-eIF2α fusions consisting of residues 1 to 190 or 1 to 180 were readily expressed and phosphorylated in a GCN2-dependent manner in yeast (Fig. 2B, lanes 9 to 16). In contrast, while expression of GST-eIF2α(1-170) was only slightly impaired, the protein was poorly phosphorylated by GCN2 in yeast (Fig. 2B, lanes 5 to 8). Truncation of 10 residues from the N terminus of eIF2α in GST-eIF2α(10-304) reduced Ser51 phosphorylation by GCN2 in vivo (data not shown). However, deletion of the first 10 residues of native (untagged) eIF2α had no effect on yeast cell growth or Ser51 phosphorylation. Truncation of 15 residues from the N terminus of eIF2α was lethal in yeast (data not shown). Taking these findings together, we conclude that both the OB-fold and α-helical domains of eIF2α, residues 10 to 180, are required for protein stability and efficient Ser51 phosphorylation in vivo. Confirming these in vivo results, in vitro kinase assays using PKR revealed efficient phosphorylation of recombinant GST-eIF2α(1-180) but not GST-eIF2α(1-170) or GST-eIF2α(1-160) (Fig. 2C). Finally, replacement of Ser51 by Ala in GST-eIF2α(1-180) blocked phosphorylation by PKR in these in vitro assays, confirming the specificity of the in vitro kinase assays (Fig. 2D). These results support our in vivo finding that GCN2 requires the N-terminal 180 residues of eIF2α for Ser51 phosphorylation.
FIG. 2.
eIF2α residues 1 to 180 are necessary and sufficient for efficient phosphorylation of Ser51 both in vivo and in vitro. (A) The C-terminal half of eIF2α is not required for Ser51 phosphorylation in vivo. GST-eIF2α fusion proteins with the indicated C-terminal residue were expressed under the control of a galactose-inducible promoter in the yeast strain H2653, in which the chromosomal SUI2 gene encoding eIF2α contains the nonphosphorylatable mutation S51A. WCEs were prepared from cells treated with 3-AT to activate GCN2, and proteins were separated by SDS-PAGE and analyzed by Western blotting using antibodies specific for the Ser51-phosphorylated form of eIF2α (left) (Quality Controlled Biochemicals, Inc.), as described previously (43). The membrane was stripped and probed with anti-GST antisera to analyze the expression of the GST-eIF2α fusion proteins (right). (B) Fine mapping of eIF2α residues required for Ser51 phosphorylation in vivo. The indicated GST-eIF2α fusion proteins were expressed under the control of a galactose-inducible promoter in SUI2-S51A yeast strains either containing or lacking GCN2 as indicated. WCEs, in 1× and 10× amounts, were separated by SDS-PAGE and analyzed by Western blotting for Ser51 phosphorylation and total GST-eIF2α expression as described for panel A. (C) eIF2α residues 1 to 180 are required for efficient phosphorylation of Ser51 in vitro. The indicated GST-eIF2α fusion proteins were purified from bacteria and incubated with recombinant human PKR purified from yeast and [γ-33P]ATP for the indicated times. Reaction mixtures were resolved by SDS-PAGE, proteins were detected by Coomassie staining (lower panels), and eIF2α phosphorylation was analyzed by autoradiography (upper panels). (D) PKR phosphorylation of eIF2α in vitro is dependent on Ser51. GST-eIF2α(1-180) and GST-eIF2α-S51A(1-180) fusion proteins were prepared and utilized for in vitro kinase assays with human PKR as described for panel C.
Identification of mutations in eIF2α that impair translational control by GCN2.
Comparison of eIF2α sequences from a variety of organisms revealed near-perfect conservation for the 19 residues flanking the phosphorylation site at Ser51 (Fig. 1B). This sequence is not conserved in the K3L-like pseudosubstrate inhibitors of the eIF2α kinases. A second region of strong sequence conservation among the eIF2α proteins is likewise conserved in the viral pseudosubstrates and includes the pentapeptide KGYID located between residues 79 and 83 in yeast eIF2α (Fig. 1B). Because residues flanking the phosphorylation site have been implicated in substrate recognition by a number of kinases, we set out initially to test the importance of the six residues immediately flanking Ser51.
As detailed in Materials and Methods, the codons for residues 48 to 50 and 52 to 54 in a plasmid-borne SUI2 allele, encoding yeast eIF2α, were independently subjected to random mutagenesis. Pools of the mutated plasmids were introduced into the sui2Δ yeast strain H1643 as the sole source of eIF2α by plasmid shuffling, and the cells were tested for defects in GCN4 expression. Translational derepression of GCN4 is essential for growth of yeast under amino acid starvation conditions. Yeast strains lacking the GCN2 kinase or expressing the nonphosphorylatable eIF2α-S51A mutant are unable to grow on a medium containing 3-AT (Fig. 1A) (9). Thus, the inability to grow on a medium containing 3-AT is a simple screen for identification of eIF2α mutants that impair translational regulation by GCN2.
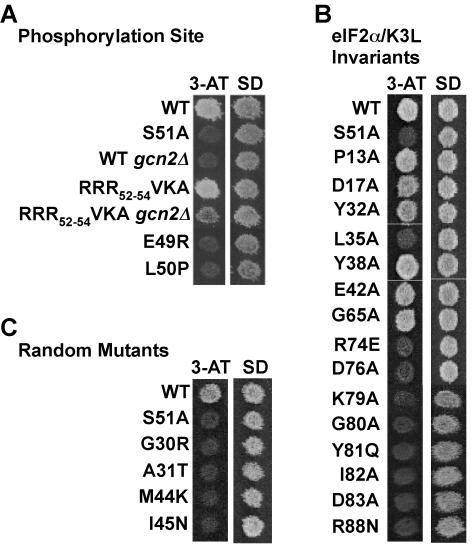
No 3-ATs mutants were isolated among the SUI2 alleles mutated at residues Ser48, Arg52, Arg53, and Arg54. To test the possibility that the three Arg residues play redundant roles in specifying phosphorylation of Ser51 by GCN2, an eIF2α triple mutant with R52V, R53K, and R54A (RRR52-54VKA) was constructed. Each single-site mutant maintained the WT 3-AT-resistant (3-ATr) phenotype, as was also found for the triple mutant (Fig. 3A). Mutations that lower eIF2 activity confer 3-ATr phenotypes independently of GCN2 and eIF2α phosphorylation (14, 42). In cells lacking GCN2, the RRR52-54VKA mutant conferred a weak 3-ATr phenotype; however, growth on 3-AT medium was significantly better in cells containing GCN2 (Fig. 3A). These results, and complementary findings from GCN4-lacZ reporter assays (data not shown), are consistent with the notion that the RRR52-54VKA mutation lowers eIF2 activity but the mutant protein remains a substrate for phosphorylation by GCN2.
FIG. 3.
eIF2α mutations near and remote from Ser51 impair GCN4 translational control. Effects of eIF2α mutations either in residues flanking Ser51 (A), in residues conserved among all eIF2α and K3L protein homologs (B), or isolated in random screens (C) on amino acid analog sensitivity as a measure of GCN4 translational regulation. Yeast cells expressing the indicated eIF2α alleles were grown to confluence on a synthetic dextrose (SD) plate and replica plated to a 3-AT plate (30 mM) and an SD plate, as indicated. Plates were incubated for 3 days at 30°C. Where indicated, the yeast strain lacked the GCN2 kinase (gcn2Δ).
Screening of mutants with random mutations of the codon for Glu49 or Leu50 in eIF2α uncovered many mutants with a 3-ATs phenotype. As shown in Fig. 3A, yeast expressing the eIF2α-E49R or -L50P mutant was unable to grow on 3-AT medium and showed defective derepression of GCN4-lacZ when treated with 3-AT (data not shown). Sequence analysis of the 3-ATs alleles identified 10 different mutations at Leu50 and all 19 possible substitutions at Glu-49 (Fig. 4). Thus, Glu49 and Leu50 in eIF2α are essential for normal translational regulation of GCN4 in yeast.
FIG. 4.
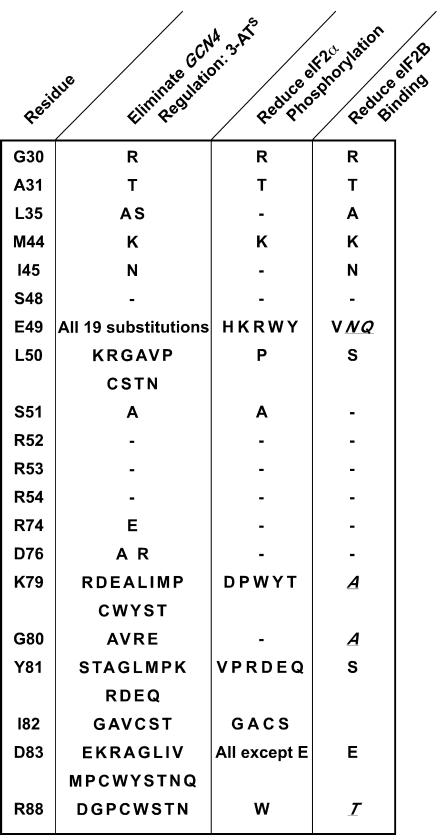
Summary of screen to identify eIF2α mutants with defects in GCN4 translational control, eIF2α phosphorylation, or eIF2B binding. The indicated eIF2α residues were subjected to site-directed or random mutagenesis, and the mutated alleles were screened in yeast to identify mutations that blocked growth on 3-AT medium and thus eliminated GCN4 regulation (second column). Yeast strains expressing the eIF2α alleles that blocked growth on 3-AT medium were also tested for phosphorylation of Ser51 (see Fig. 5), and mutations that eliminated or reduced Ser51 phosphorylation in vivo are listed in the third column. Mutations that conferred a 3-ATs phenotype but did not impair Ser51 phosphorylation were tested for eIF2B binding (see Fig. 7), and mutations that eliminated or reduced eIF2B binding are listed in the last column. Mutations previously reported (27) to impair the binding of eIF2B to phosphorylated eIF2α are also listed (italicized and underlined) in the last column.
The KGYID sequence located at residues 79 to 83 in yeast eIF2α and residues Pro13, Asp17, Tyr32, Leu35, Glu42, Gly65, Arg74, Asp76, and Arg88 are conserved among all eIF2α proteins as well as the pseudosubstrate inhibitors (Fig. 1B). Screening of random mutants with mutations of the KGYID residues and Arg88 identified many 3-ATs mutants, and sequence analysis revealed a variety of substitutions that impaired translational regulation (Fig. 4). As shown in Fig. 3B, the K79A, G80A, Y81Q, I82A, and D83A mutations conferred 3-ATs phenotypes and eliminated the high-level GCN4-lacZ expression (data not shown) typically observed in cells grown under starvation conditions. Perhaps of greatest interest, 17 different substitutions at Asp83 prevented growth on 3-AT medium. The remaining eIF2α and K3L invariant residues (Fig. 1B) were replaced by either Ala or an oppositely charged amino acid. Four of the mutations, L35A, R74E, D76A, and D76R, impaired the growth of yeast on 3-AT medium (Fig. 3B).
In addition to these site-specific mutagenesis protocols, two regions of the SUI2 gene, encoding eIF2α residues 1 to 48 and 89 to 288, were independently mutated by error-prone PCR as described in Materials and Methods. Pools of the mutated plasmids were introduced into the sui2Δ yeast strain H1643 by plasmid shuffling, and the resulting strains were tested for defects in GCN4 expression. Five eIF2α mutations, G30R, A31T, L35S, M44K, and I45N, were found to confer a 3-ATs phenotype (Fig. 3C and 4).
A subset of the eIF2α mutations impairs GCN2 phosphorylation of Ser51.
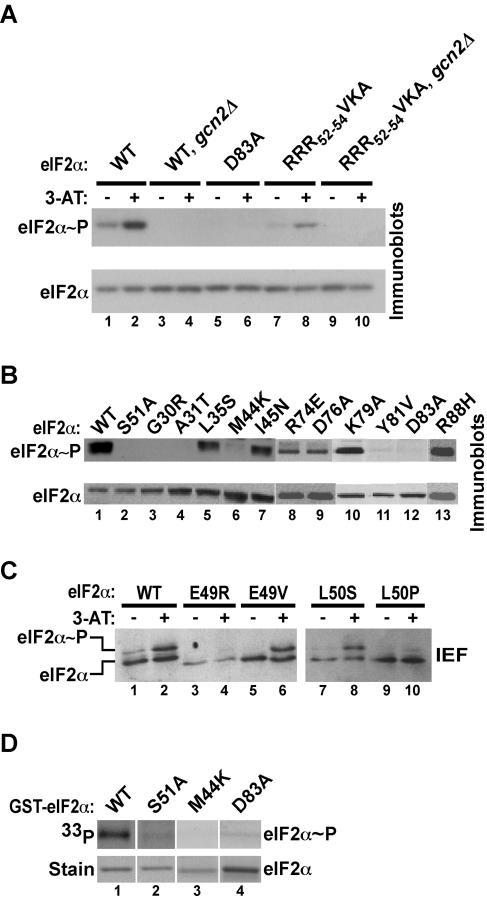
Mutations in eIF2α that disrupt translational control of GCN4 could affect either the ability of GCN2 to phosphorylate Ser51 or the ability of phosphorylated eIF2 to inhibit eIF2B (Fig. 1A). Thus, all of the eIF2α mutants with 3-ATs phenotypes identified in Fig. 4 were screened for their effects on Ser51 phosphorylation. Western blot analyses of WCEs were performed by using phosphospecific antibodies against eIF2α phosphorylated on Ser51 as well as polyclonal eIF2α antibodies. As shown in Fig. 5, Ser51 phosphorylation in cells expressing WT eIF2α increased when cells were treated with 3-AT (Fig. 5A, lane 2 versus lane 1) and was fully dependent on GCN2 (Fig. 5A, lane 4 versus lane 2) and Ser51 (Fig. 5B, lane 2). The eIF2α mutations G30R, A31T, M44K, Y81V, and D83A nearly abolished Ser51 phosphorylation (Fig. 4 and 5B, lanes 3, 4, 6, 11, and 12). Consistent with the 3-ATr phenotype in cells expressing the RRR52-54VKA triple mutant, Ser51 phosphorylation was stimulated in a GCN2-dependent manner in cells treated with 3-AT (Fig. 5A, lanes 7 to 10). We observed that mutations at residues Glu49 and Leu50 completely eliminated detection of eIF2α by the Ser51 phosphospecific antibodies, including the nonspecific detection of unphosphorylated eIF2α in gcn2Δ strains (data not shown), indicating that the primary epitope for the antibodies includes the residues N-terminal to the phosphorylation site. Thus, a second assay, isoelectric focusing (IEF) gel electrophoresis (9), was used to monitor the effects of the Glu49 and Leu50 mutations on Ser51 phosphorylation. As shown in Fig. 5C, the abundance of the Ser51-phosphorylated isoform of eIF2α increased in cells treated with 3-AT (compare lanes 1 and 2). The E49R and L50P mutations greatly reduced and almost completely eliminated phosphorylation of Ser51, respectively (Fig. 5C, lanes 4 and 10). In contrast, the E49V and L50S mutations did not impair Ser51 phosphorylation (Fig. 5C, lanes 6 and 8). It is noteworthy that all of the 3-ATs mutants in eIF2α had a recessive phenotype (data not shown), indicating that the eIF2α mutants did not function as dominant inhibitors of GCN2.
FIG. 5.
A subset of the eIF2α mutations impairs Ser51 phosphorylation. (A and B) Immunoblot analysis using eIF2α Ser51 phosphospecific antibodies. WCEs were prepared from strains expressing the indicated eIF2α alleles. The strains were grown in SD minimal medium or in SD medium supplemented with 10 mM 3-AT (lanes marked with plus signs in panel A; all samples in panel B), where the GCN2 kinase is activated. Total proteins were subjected to immunoblot analysis with antibodies against Ser51-phosphorylated eIF2α (upper panels) as described for Fig. 2A. The blot was stripped and reprobed with a polyclonal antiserum against total yeast eIF2α (lower panels). This polyclonal antiserum was raised against the C-terminal half of yeast eIF2α, far from the Ser51 phosphorylation site, and thus should detect unphosphorylated and Ser51-phosphorylated forms of yeast eIF2α equally well. (C) IEF analysis of eIF2α phosphorylation. WCEs were prepared from yeast expressing the indicated eIF2α alleles and were grown as described for panel A. Total proteins were separated by IEF on a vertical slab gel, and eIF2α was detected by immunoblot analysis using the polyclonal anti-yeast eIF2α antiserum, as described previously (9). As indicated, the eIF2α isoform phosphorylated on Ser51 focuses higher in the gel than the basal form. (D) eIF2α mutations impair Ser51 phosphorylation by GCN2 in vitro. GCN2 bearing N-terminal FLAG and polyhistidine tags was overexpressed and purified from yeast as described previously (11). The GCN2 (0.25 μg) was mixed with [γ-33P]ATP and 0.5 μg of the indicated recombinant GST-eIF2α fusion protein purified from bacteria. The kinasebuffer and reaction conditions were identical to those described previously (11). Reaction mixtures were resolved by SDS-PAGE, proteins were detected by Coomassie staining (lower panel), and eIF2α phosphorylation was analyzed by autoradiography (upper panel).
To confirm that the reduced eIF2α phosphorylation observed in cells expressing the mutant proteins was due to impaired kinase recognition, several of the eIF2α mutants were tested for phosphorylation by GCN2 by using in vitro kinase assays. Full-length GST-eIF2α fusion proteins were purified from E. coli and mixed with purified yeast GCN2 and [γ-33P]ATP. Products of the kinase reactions were separated by SDS-PAGE and visualized by autoradiography. Substitution of Ala for Ser51 completely blocked GCN2 phosphorylation of eIF2α (Fig. 5D, lane 1 versus lane 2), confirming the specificity of GCN2 for Ser51 in vitro. The M44K and D83A mutations severely impaired eIF2α phosphorylation in vitro (Fig. 5D, lanes 3 and 4), consistent with the lack of phosphorylation of these mutants in vivo (Fig. 5B, lanes 6 and 12). Thus, the D83A mutation impaired Ser51 phosphorylation both in vivo and in vitro, indicating that residues remote from Ser51 are key determinants for GCN2 recognition of eIF2α.
The results of the comprehensive analysis of Ser51 phosphorylation for all of the eIF2α mutants are summarized in Fig. 4 (third column). Interestingly, only 5 of the 19 3-ATs mutations at Glu49 and only 1 of 10 mutations at Leu50 impaired Ser51 phosphorylation. Similarly, only a subset of the mutations at Lys79, Tyr81, Ile82, and Arg88 reduced Ser51 phosphorylation (Fig. 4). Asp83, 32 residues from the Ser51 phosphorylation site, appeared to be the most critical determinant for phosphorylation. Nearly all mutations at Asp83 impaired GCN4 expression and Ser51 phosphorylation. Only the conservative substitution of Glu for Asp83 did not block phosphorylation, suggesting that the eIF2α kinases require an acidic charge at this position for efficient substrate recognition.
Translational regulation and phosphorylation of Ser51 by the four eIF2α kinases are dependent on the same determinants in eIF2α.
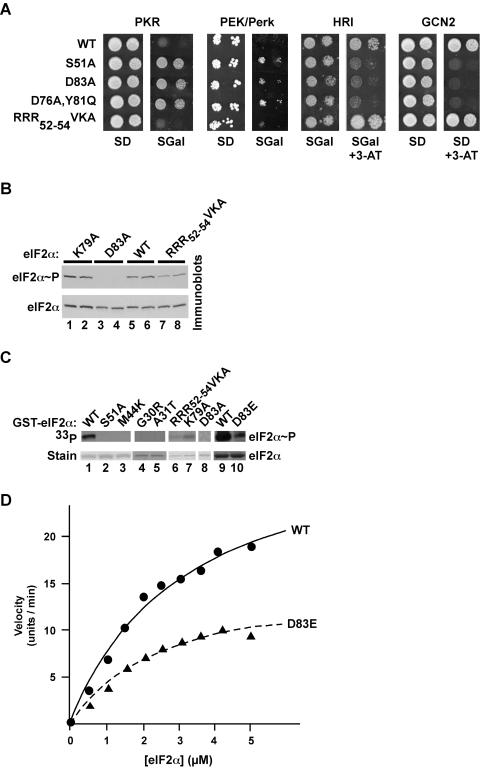
To determine whether the other three eIF2α kinases utilized the same eIF2α determinants as GCN2 for substrate recognition, we expressed human PKR or HRI or Caenorhabditis elegans Perk/PEK under the control of a galactose-inducible promoter in a gcn2Δ yeast strain expressing various forms of eIF2α. High-level expression of PKR or Perk/PEK on galactose medium was lethal, while high-level expression of HRI conferred a 3-ATr phenotype in cells expressing WT eIF2α (Fig. 6A). The PKR and Perk/PEK toxicity, and the HRI phenotype, were suppressed in cells expressing nonphosphorylatable eIF2α-S51A, eIF2α-D83A, or eIF2α-D76A,Y81Q (Fig. 6A). In contrast, the growth of yeast expressing the eIF2α-RRR52-54VKA mutant was severely inhibited by PKR or Perk/PEK (Fig. 6A), consistent with the ability of GCN2 to phosphorylate this mutant. The correlation between the impact of these eIF2α mutations on GCN2 phosphorylation and PKR, Perk/PEK, and HRI suppression suggests that all four kinases recognize the same determinants on eIF2α.
FIG. 6.
The kinases PKR, Perk/PEK, HRI, and GCN2 rely on the same eIF2α determinants for substrate recognition. (A) The eIF2α D83A mutation suppresses PKR, Perk/PEK, HRI, and GCN2 phenotypes in yeast. Plasmid p722, bearing GCN2, and plasmids p1420, p1246, and p551 (38), expressing human PKR, human HRI, and C. elegans PEK/Perk (residues 26 to 1077), respectively, under the control of a yeast GAL-CYC1 hybrid promoter, were introduced into derivatives of the gcn2Δ yeast strain H1925 expressing the indicated eIF2α alleles. Transformants were grown to saturation in minimal SD (2% glucose) medium, and 4 μl of serial dilutions (optical density at 600 nm, 1.0 and 0.1) was spotted onto minimal SD or SGal (10% galactose) medium or the same medium containing 10 mM 3-AT, as indicated. Plates were incubated at 30°C for 6 days. (B) Immunoblot analysis of eIF2α phosphorylation in yeast expressing PKR. The yeast strain H1925 expressing the indicated eIF2α alleles was transformed with the PKR expression vector p1420, and two independent transformants of each strain were grown to exponential phase in SDmedium and then shifted to SGR medium (containing 10% galactose plus 2% raffinose) for ∼18 h to induce PKR expression. WCEs were prepared, and total proteins were subjected to immunoblot analysis with antibodies against Ser51-phosphorylated eIF2α (upper panel) as described for Fig. 2A. The blot was stripped and reprobed with a polyclonal antiserum against total yeast eIF2α (lower panel). (C) In vitro kinase assay of PKR phosphorylation of eIF2α. Purified recombinant PKR was mixed with [γ-33P]ATP and the indicated recombinant GST-eIF2α fusion proteins purified from bacteria. Kinase reactions were resolved by SDS-PAGE, and gels were stained with Coomassie blue to detect GST-eIF2α (bottom panel), followed by autoradiography to visualize phosphorylated eIF2α (upper panel). (D) Kinetic analysis of PKR phosphorylation of eIF2α and eIF2α D83E. His6-eIF2α(1-200) and His6-eIF2α-D83E fusion proteins were purified from bacteria and examined for phosphorylation by purified PKR as described in Materials and Methods. Reaction products were resolved by SDS-PAGE, and the relative incorporation of phosphate into eIF2α was determined by using a PhosphorImager. The data are expressed in arbitrary units. Results are representative of at least three independent experiments.
To test whether the D83A mutation impaired the ability of PKR to phosphorylate Ser51, immunoblot analyses were used to monitor Ser51 phosphorylation. High-level expression of PKR in yeast resulted in substantial phosphorylation of eIF2α on Ser51 (Fig. 6B, lanes 5 and 6), and this phosphorylation was completely prevented by the D83A mutation (Fig. 6B, lanes 3 and 4). Thus, Asp83 is a critical determinant for eIF2α recognition by PKR and GCN2. As was found for GCN2 phosphorylation of eIF2α, neither the K79A nor the RRR52-54VKA substitutions prevented Ser51 phosphorylation by PKR in vivo (Fig. 6B, lanes 1, 2, 7, and 8). Consistent with these in vivo results, recombinant human PKR readily phosphorylated the GST-eIF2α fusion protein in vitro, in a completely Ser51 dependent manner (Fig. 6C, lane 2 versus lane 1). As was found for GCN2, eIF2α phosphorylation by PKR in vitro was severely impaired by the G30R, A31T, M44K, and D83A mutations (Fig. 6C, lanes 3 to 5 and 8), whereas the RRR52-54VKA, K79A, and D83E mutations reduced Ser51 phosphorylation. To quantify the impact of the remote D83E mutation on Ser51 phosphorylation, we characterized the kinetics of PKR phosphorylation of WT eIF2α and eIF2α-D83E. Fitting the results of in vitro kinase assays to the Michaelis-Menten equation revealed similar Km values for WT eIF2α (3.2 μM) and eIF2α-D83E (2.6 μM); however, the kcat for the WT eIF2α reaction (13.5 U/min/nmol) was approximately twofold greater than that for eIF2α-D83E (6.5 U/min/nmol) (Fig. 6D). This twofold difference in kcat, and the related twofold difference in Keff (kcat/Km), indicates that the remote residue contributes to effective binding and phosphorylation of eIF2α by PKR. However, the similar Km values for WT eIF2α and eIF2α-D83E suggest that the remote residues do not contribute to initial substrate binding (KD) but instead are more critical for the phosphorylation reaction and perhaps for substrate binding in the transition state. To conclude, the correlation of the results from the growth and kinase assays demonstrates that substrate recognition by the homologous kinase domains in the four eIF2α kinases is governed by a common set of residues remote from the Ser51 phosphorylation site.
A subset of eIF2α regulatory mutations impairs binding of eIF2B to phosphorylated eIF2α.
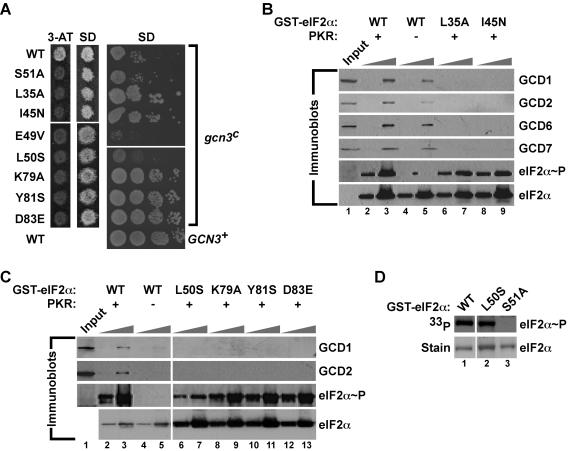
As shown in Fig. 5 and summarized in Fig. 4, several eIF2α mutations eliminated GCN4 translational regulation but retained Ser51 phosphorylation. We hypothesized that these mutations prevented phosphorylated eIF2 inhibition of eIF2B (Fig. 1A). The gcn3c-R104K mutation in the α (regulatory) subunit of yeast eIF2B inhibits eIF2B function in the absence of eIF2α phosphorylation and is thought to mimic the impact of phosphorylated eIF2 on eIF2B activity. Previously identified eIF2α mutations (e.g., L84F) that impaired translational regulation but not Ser51 phosphorylation were found to suppress the toxic effects of the gcn3c-R104K mutation on yeast cell growth (41). The eIF2α mutations L35A, I45N, K79A, Y81S, and D83E, identified in this study as blocking GCN4 translational control but not Ser51 phosphorylation, were found to suppress the slow-growth phenotype associated with the gcn3c-R104K mutation (Fig. 7A), consistent with the idea that these mutations alter the inhibition of eIF2B by phosphorylated eIF2. In contrast, the eIF2α-S51A mutation, which prevents Ser51 phosphorylation, was unable to suppress the gcn3c-R104K mutation (Fig. 7A). Notably, the E49V and L50S mutations, immediately adjacent to the phosphorylation site, failed to suppress the gcn3c-R104K mutation, suggesting that the residues adjacent to Ser51 may not interact directly with eIF2Bα (GCN3). To directly examine the binding of eIF2α to eIF2B, we introduced the L35A, I45N, L50S, K79A, Y81A, and D83E mutations into a GST-eIF2α(1-304) construct. Previously, it was shown that GST-eIF2α bound native or overexpressed eIF2B in yeast WCEs in a manner dependent on Ser51 phosphorylation and sensitive to mutations in eIF2α (27). The GST-eIF2α WT and mutant proteins indicated in Fig. 7 were purified from bacteria, phosphorylated by PKR in vitro, immobilized on glutathione-Sepharose beads, and then incubated with WCEs from yeast overexpressing all five eIF2B subunits. Western blot analysis of the bound proteins demonstrated the PKR (Ser51 phosphorylation)-enhanced binding of eIF2B subunits GCD1, GCD2, GCD6, and GCD7 to WT GST-eIF2α (Fig. 7B and C; compare lanes 3 and 5). In contrast, the eIF2α mutations L35A, I45N, L50S, K79A, Y81S, and D83E impaired the binding of eIF2B subunits to GST-eIF2α (Fig. 7B, lanes 6 to 9, and 7C, lanes 6 to 13). Western blot analyses using phospho-Ser51-specific antibodies confirmed that the eIF2α mutations did not interfere with phosphorylation (Fig. 7B and C). Since the L50S mutation disrupted the epitope for the phospho-Ser51 antibodies, kinase assays using [γ-33P]ATP demonstrated efficient phosphorylation of this mutant (Fig. 7D). We conclude that the eIF2α mutations that abolish GCN4 regulation but not Ser51 phosphorylation weaken the interaction of phosphorylated eIF2α with eIF2B. Consistent with this conclusion, we propose that the mutations prevent the inhibition of eIF2B by phosphorylated eIF2.
FIG. 7.
eIF2α mutations near and remote from Ser51 impair eIF2B binding to phosphorylated eIF2α. (A) Effects of selected eIF2α mutations on amino acid analog sensitivity and growth rate in yeast expressing WT GCN3 (left panels) or the gcn3c-R104K mutant (right panel). (Left panels) Transformants of yeast strain H1643 expressing the indicated eIF2α alleles were grown to confluence on an SD plate and replica plated to a 3-AT plate (30 mM) and an SD plate. Plates were incubated for 3 days at 30°C. (Right panel) Derivatives of yeast strain H1658 (MATα ura3-52 leu2-3 leu2-112 ino− trp1-Δ63 sui2Δ gcn3c-R104K p[SUI2, URA3] 〈HIS4-lacZ, ura3-52〉) expressing the indicated eIF2α mutants were generated by plasmid shuffling, and the resulting strains were tested for growth on SD medium by spotting 10-fold serial dilutions of a saturated culture and incubating for 3 days at 30°C. As a control, WT GCN3 on a low-copy-number plasmid (GCN3+) was introduced into the strain bearing WT eIF2α to complement the recessive gcn3c-R104K mutation. (B and C) Mutations of eIF2α residues near and remote from Ser51 impair binding of eIF2B subunits to phosphorylated eIF2α. WT GST-eIF2α and the indicated mutant derivatives were purified from bacteria and incubated with (+) or without (−) PKR in kinase buffer. Two different amounts of GST-eIF2α fusion proteins (0.1 and 1.0 μg) were immobilized on glutathione-Sepharose beads and then incubated with WCEs from yeast overexpressing all five eIF2B subunits. After extensive washing, bound proteins were resolved by SDS-PAGE and analyzed by Western blotting using antibodies against GCD1, GCD2, GCD6, GCD7, and Ser51-phosphorylated eIF2α, as indicated. The eIF2α blot was stripped and reprobed with polyclonal antisera against total yeast eIF2α. Input lanes contained 10% of the WCEs used in each reaction. (D) Efficient phosphorylation of eIF2α-L50S by PKR in vitro. The indicated WT and mutant GST-eIF2α fusion proteins were purified from bacteria and incubated with recombinant PKR and [γ-33P]ATP. Reaction mixtures were resolved by SDS-PAGE, proteins were detected by Coomassie staining (lower panels), and eIF2α phosphorylation was analyzed by autoradiography (upper panels).
DISCUSSION
In this report we have provided the first detailed analysis of substrate recognition by the family of stress-responsive eIF2α kinases. Any eIF2α mutation at residue 49 and at least 10 mutations at residue 50, immediately N-terminal to the phosphorylation site, destroyed translational regulation, and a subset of these mutations impaired Ser51 phosphorylation in vivo. Likewise, many substitutions of residues in the conserved KGYID83 motif impaired both translational regulation and Ser51 phosphorylation. Importantly, any substitution at residue Asp83 (except for the conservative Glu substitution) prevented phosphorylation of Ser51, located 32 residues away. This finding reveals the importance for kinase-substrate recognition of residues remote from the phosphorylation site. Finally, the identification of mutations that specifically blocked translational regulation, but not Ser51 phosphorylation, in residues preceding Ser51 or in the remote KGYID83 motif led to the discovery that these regions of eIF2α are critical for binding of eIF2B and inhibition of eIF2B by phosphorylated eIF2. Thus, we propose that the eIF2α kinases and eIF2B recognize the same or overlapping determinants on eIF2α.
The eIF2α kinases recognize a large contiguous surface composed of residues near and remote from the Ser51 phosphorylation site.
Biochemical studies revealed that PKA readily phosphorylated denatured substrates, proteolytic fragments derived from substrates, and model peptides derived from pyruvate kinase (Kemptide) with nearly equal efficiencies (see references 21, 23, and 24), indicating that the residues flanking the phosphorylation site, and not the context of a larger structural fold, are the principal determinants for substrate selection by PKA. In contrast to these findings, the eIF2α kinases PKR and HRI function inefficiently with peptide substrates compared with intact eIF2 (30), and our data demonstrate that residues remote from Ser51 are critical for kinase recognition. Previously, we found that both PKR and HRI phosphorylated Tyr in place of Ser51 in eIF2α (28). We propose that PKR and HRI interact with high affinity and stringency with the remote KGYID motif, which then orients eIF2α residue 51 into the kinase active site. A flexible active site enables any phosphoaccepting amino acid at residue 51 to be phosphorylated by the eIF2α kinases. In contrast to PKA, where denaturation of proteins enhanced phosphorylation (24), perhaps by exposing previously buried consensus sequence motifs, denaturation of eIF2α severely impaired phosphorylation (26). These results are consistent with the notion that the 3D structural integrity of eIF2α is important for kinase recognition.
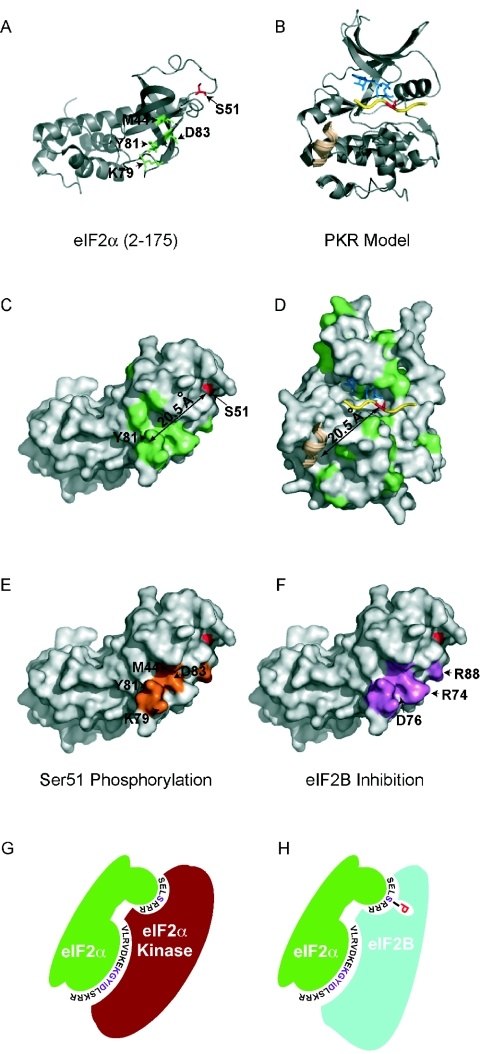
Further support for the importance of the eIF2α tertiary structure for kinase recognition comes from comparison of the structures of eIF2α and the vaccinia virus K3L protein (Fig. 1B). The crystallographic structure of the K3L protein revealed an OB-fold composed of a five-stranded β-barrel (4). The structure of the N-terminal two-thirds of yeast eIF2α revealed a similar OB-fold for residues 1 to 89, followed by a helical domain consisting of residues 90 to 175 (Fig. 8A) (10). Residues 47 to 63 in eIF2α, including the phosphorylation site at Ser51, are in a large loop connecting strands β3 and β4. In agreement with our finding that mutations in the KGYID motif impaired Ser51 phosphorylation, mutations in the corresponding residues of the K3L protein, as well as the homologous C8L protein of swinepox virus, impaired PKR binding and kinase inhibition (4, 19, 20). These results indicate that the K3L protein and eIF2α bind in a similar manner to PKR with multiple contacts, and they support the idea that the 3D structure of these proteins is an important determinant for kinase recognition.
FIG. 8.
Locations of the mutations affecting Ser51 phosphorylation on the eIF2α structure, and model for eIF2α kinases and eIF2B recognizing overlapping surfaces on eIF2α. (A) Ribbon diagram of yeast eIF2α (PDB code 1Q46; residues 2 to 175). Ser51 is shown in red; residues M44, K79, Y81, and D83, mutations of which impair Ser51 phosphorylation, are shown in green. (B) Ribbon diagram of a PKR protein kinase domain model. The PKR protein kinase domain homology model was generated with SWISS-MODEL (12) and is based on structures of the cyclic AMP-dependent protein kinase (PDB codes 2CPK, 1BKX, 1FMO, 1ATP, and 1BX6). The model coordinates (human PKR residues 268 to 330 and 360 to 524) were generated without refinement. Yellow, peptide substrate; blue, ATP; beige, helical residues of the PKA inhibitor PKI. (C) Surface representation of eIF2α. Red, the Ser51 phosphorylation site; green, residues conserved amongeIF2α and K3L-like proteins. The arrow indicates the distance between the Ser51 phosphorylation site and Tyr81, at the center of the conserved surface region. (D) Surface representation of the PKR model. Residues in green are conserved among eIF2α kinases. A 20.5-Å vector projecting from the phosphoacceptor residue (red) within the peptide substrate (yellow) is shown. Blue, ATP; beige, PKI helical residues. (E) Surface representation of eIF2α highlighting residues (orange) that are critical for kinase recognition and Ser51 phosphorylation. Ser51 is shown in red. (F) Surface representation of eIF2α highlighting residues (purple) important for binding and inhibition of eIF2B by phosphorylated eIF2α. All ribbon diagrams and surface models were generated with PyMOL, version 0.9 (6). (G and H) Model depicting eIF2α kinase (red) and eIF2B (blue) recognition of eIF2α (green). The eIF2α Ser51 and KGYID residues are labeled in magenta, with the flanking residues in black; phosphorylated Ser51 is indicated by the letter P, in red.
Structural elements distinct from the phosphorylation site play important roles in substrate recognition for a few other protein kinases. The JNKs bind to a docking site, also known as the delta (δ) domain, located between residues 31 and 59 of c-Jun and then phosphorylate residues Ser63 and Ser73 (15, 29). Substrate recognition by cyclin A-cdk2 and cyclin E-cdk2 is likewise dependent on the residues flanking the phosphorylation site as well as a remote RXL or cyclin-binding (Cy) motif (1, 35, 39).
In analogy with the δ domain in c-Jun and the Cy motif in cyclin A-cdk2 substrates, the KGYID motif in eIF2α may function as an eIF2α kinase docking site. Two possibilities can be considered: either (i) the eIF2α kinases first bind to the KGYID motif and then translocate to Ser51 or (ii) the KGYID motif and Ser51 are part of a contiguous kinase recognition surface on eIF2α. Several findings favor the latter model. First, the remarkable structural similarity between the K3L protein and eIF2α is consistent with recognition by the kinases of a particular 3D structure encompassing both Ser51 and the KGYID motif. If the KGYID motif were a docking site, then conservation of just this motif in the K3L protein might be expected to be sufficient for PKR inhibition. However, mutations in the region of the K3L protein corresponding to Ser51 in eIF2α have been shown to alter PKR binding and inhibition (4, 20). Second, the Ser51 region, the KGYID motif, and kinase determinants Gly30 and Ala31 in strand β-2 and Met44 in strand β-3 form a contiguous surface on one face of eIF2α (Fig. 8A and E), consistent with the idea that the kinases contact this entire face of eIF2α. Third, in the K3L protein and eIF2α structures, the KGYID motif is located ∼21.5 Å from Ser51 (or its equivalent in the K3L protein) (4, 10). As described below, this spacing is compatible with the possibility that the kinases contact both regions simultaneously.
Structures of PKA (Fig. 8B and D), phosphorylase kinase (PhK), and the insulin receptor kinase (IRK) bound to a peptide substrate or pseudosubstrate inhibitor revealed that the substrate residues flanking the phosphorylation site interacted with the activation segment located between subdomains VII and VIII (between helices αE and αF) in the C-terminal lobe of each kinase (see references 18 and 22) (Fig. 8B). This may represent a general mode of substrate recognition by protein kinases, wherein the residues flanking the phosphorylation site are recognized by specific interactions with the residues in the activation loop of the kinase. The structure of PKA bound to its inhibitor peptide, PKI (25, 44), revealed additional interactions between an α-helical region in PKI and a binding groove in the C-terminal lobe of the kinase (Fig. 8B) (see reference 22). Interestingly, the distance between the α-helix in PKI and the phosphorylation site is about the same as the distance between the KGYID motif and Ser51 in eIF2α (Fig. 8C and D), suggesting a potential binding site for the KGYID motif on PKR. The OB-fold of eIF2α can be easily slid into the position of PKI in the PKA-PKI structure, and in this superposition the KGYID motif of eIF2α overlies the α-helix of PKI and Ser51 is positioned in the active site of the kinase. Thus, we propose that PKR and the other eIF2α kinases recognize a contiguous surface on eIF2α extending from the KGYID motif to the vicinity of the Ser51 phosphorylation site (Fig. 8E and G).
Overlapping surfaces on eIF2α mediate eIF2α kinase recognition and eIF2B inhibition.
The identification of eIF2α mutations that block translational regulation but do not impair Ser51 phosphorylation indicates that these mutations likely prevent inhibition of the GEF eIF2B by phosphorylated eIF2. The regulatory subcomplex of eIF2B composed of the α, β, and δ subunits (GCN3, GCD7, and GCD2 in yeast) binds to intact eIF2 as well as the isolated eIF2α subunit (27, 33). Binding of the regulatory subcomplex to eIF2α is enhanced by phosphorylation of Ser51 (27). The eIF2α mutations I58M, L84F, R88C, and V89I alleviate the slow-growth phenotype associated with hyperactive GCN2c alleles in yeast (41). The GCN2c kinases phosphorylate a large fraction of the eIF2α in the cell, resulting in elevated GCN4 expression and impaired cell growth due to inhibition of general protein synthesis. Whereas the R88C mutation appeared to impair Ser51 phosphorylation (41), the other eIF2α mutations apparently blocked the ability of phosphorylated eIF2 to inhibit eIF2B. In agreement with the latter model, Krishnamoorthy et al. (27) showed that SUI2 mutations (including the E49N, E49Q, K79A, G80A, and R88T mutations described in this report) that suppress the inhibition of eIF2B by phosphorylated eIF2 weaken the binding of GST-eIF2α∼P (GST-eIF2α phosphorylated on Ser51) to the eIF2Bαβδ regulatory subcomplex. In this report we extend these findings and demonstrate that the eIF2α mutations L35A, I45N, L50S, Y81A, and D83E impair eIF2B binding to phosphorylated eIF2α (Fig. 7B and C). We propose that the eIF2α mutations weaken the interaction between phosphorylated eIF2α and the eIF2Bαβδ regulatory subcomplex, enabling a productive interaction between eIF2βγ and the eIF2Bγɛ catalytic subcomplex that triggers GTP-GDP exchange.
Of particular note regarding the two classes of eIF2α mutations identified in this report is the finding that both types of mutations have been found at the same residue in eIF2α. For example, the D83A mutation impaired Ser51 phosphorylation (Fig. 5 and 6), whereas the D83E mutation did not prevent Ser51 phosphorylation but instead blocked eIF2B binding (Fig. 5 to 7). Thus, the same residues in eIF2α contribute to both eIF2α kinase recognition and eIF2B inhibition. As described above, the Ser51 region and the KGYID motif are located on the same face of eIF2α, and as illustrated in Fig. 8E to H, we propose that this face forms the docking site for both the kinases and eIF2B. Future experiments will be aimed at defining the residues in the eIF2α kinases responsible for eIF2α recognition and Ser51 phosphorylation, as well as the residues in the eIF2Bαβδ regulatory subcomplex that recognize phospho-Ser51 on eIF2α.
Acknowledgments
We thank Weimin Yang for the GST-eIF2α plasmid. We also thank present and past members of the Dever and Hinnebusch labs for assistance and helpful discussions, and Alan Hinnebusch for comments on the manuscript.
REFERENCES
- 1.Adams, P. D., W. R. Sellers, S. K. Sharma, A. D. Wu, C. M. Nalin, and W. G. Kaelin, Jr. 1996. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 16:6623-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll, K., O. Elroy-Stein, B. Moss, and R. Jagus. 1993. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2α-specific protein kinase. J. Biol. Chem. 268:12837-12842. [PubMed] [Google Scholar]
- 3.Cherkasova, V. A., and A. G. Hinnebusch. 2003. Translational control by TOR and TAP42 through dephosphorylation of eIF2α kinase GCN2. Genes Dev. 17:859-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dar, A. C., and F. Sicheri. 2002. X-ray crystal structure and functional analysis of vaccinia virus K3L reveals molecular determinants for PKR subversion and substrate recognition. Mol. Cell 10:295-305. [DOI] [PubMed] [Google Scholar]
- 5.Davies, M. V., O. Elroy-Stein, R. Jagus, B. Moss, and R. J. Kaufman. 1992. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 66:1943-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLano, W. L. 2002. The PyMOL Molecular Graphics System. [Online.] DeLano Scientific, South San Francisco, Calif. http://pymol.sourceforge.net
- 7.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 8.Dever, T. E., J. J. Chen, G. N. Barber, A. M. Cigan, L. Feng, T. F. Donahue, I. M. London, M. G. Katze, and A. G. Hinnebusch. 1993. Mammalian eukaryotic initiation factor 2α kinases functionally substitute for GCN2 in the GCN4 translational control mechanism of yeast. Proc. Natl. Acad. Sci. USA 90:4616-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dever, T. E., L. Feng, R. C. Wek, A. M. Cigan, T. D. Donahue, and A. G. Hinnebusch. 1992. Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68:585-596. [DOI] [PubMed] [Google Scholar]
- 10.Dhaliwal, S., and D. W. Hoffman. 2003. The crystal structure of the N-terminal region of the alpha subunit of translation initiation factor 2 (eIF2α) from Saccharomyces cerevisiae provides a view of the loop containing serine 51, the target of the eIF2α-specific kinases. J. Mol. Biol. 334:187-195. [DOI] [PubMed] [Google Scholar]
- 11.Dong, J., H. Qiu, M. Garcia-Barrio, J. Anderson, and A. G. Hinnebusch. 2000. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 6:269-279. [DOI] [PubMed] [Google Scholar]
- 12.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PDBViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 13.Hanks, S. K., and T. Hunter. 1995. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 14.Hannig, E. M., A. M. Cigan, B. A. Freeman, and T. G. Kinzy. 1992. GCD11, a negative regulator of GCN4 expression, encodes the γ subunit of eIF-2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:506-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7:2135-2148. [DOI] [PubMed] [Google Scholar]
- 16.Hinnebusch, A. G. 2000. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes, p. 185-243. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Hinnebusch, A. G., and K. Natarajan. 2002. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot. Cell 1:22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, L., E. Lowe, M. Noble, and D. Owen. 1998. The structural basis for substrate recognition and control by protein kinases. FEBS Lett. 430:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Kawagishi-Kobayashi, M., C. Cao, J. Lu, K. Ozato, and T. E. Dever. 2000. Pseudosubstrate inhibition of protein kinase PKR by swine pox virus C8L gene product. Virology 276:424-434. [DOI] [PubMed] [Google Scholar]
- 20.Kawagishi-Kobayashi, M., J. B. Silverman, T. L. Ung, and T. E. Dever. 1997. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2α. Mol. Cell. Biol. 17:4146-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp, B. (ed.). 1990. Peptides and protein phosphorylation. CRC Press, Inc., Boca Raton, Fla.
- 22.Kemp, B. E., M. W. Parker, S. Hu, T. Tiganis, and C. House. 1994. Substrate and pseudosubstrate interactions with protein kinases: determinants of specificity. Trends Biochem. Sci. 19:440-444. [DOI] [PubMed] [Google Scholar]
- 23.Kemp, B. E., and R. B. Pearson. 1990. Protein kinase recognition sequence motifs. Trends Biochem. Sci. 15:342-346. [DOI] [PubMed] [Google Scholar]
- 24.Kennelly, P. J., and E. G. Krebs. 1991. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 266:15555-15558. [PubMed] [Google Scholar]
- 25.Knighton, D. R., J. Zheng, L. F. T. Eyck, N. H. Xuong, S. S. Taylor, and J. M. Sowadski. 1991. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253:414-420. [DOI] [PubMed] [Google Scholar]
- 26.Kramer, G., and B. Hardesty. 1981. Phosphorylation reactions that influence the activity of eIF-2. Curr. Top. Cell Regul. 20:185-203. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamoorthy, T., G. D. Pavitt, F. Zhang, T. E. Dever, and A. G. Hinnebusch. 2001. Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell. Biol. 21:5018-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, J., E. B. O'Hara, B. A. Trieselmann, P. R. Romano, and T. E. Dever. 1999. The interferon-induced double-stranded RNA-activated protein kinase PKR will phosphorylate serine, threonine, or tyrosine at residue 51 in eukaryotic initiation factor 2α. J. Biol. Chem. 274:32198-32203. [DOI] [PubMed] [Google Scholar]
- 29.May, G., K. Allen, W. Clark, M. Funk, and D. Gillespie. 1998. Analysis of the interaction between c-Jun and c-Jun N-terminal kinase in vivo. J. Biol. Chem. 273:33429-33435. [DOI] [PubMed] [Google Scholar]
- 30.Mellor, H., and C. G. Proud. 1991. A synthetic peptide substrate for initiation factor-2 kinases. Biochem. Biophys. Res. Commun. 178:430-437. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell, D. A., T. K. Marshall, and R. J. Deschenes. 1993. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast 9:715-722. [DOI] [PubMed] [Google Scholar]
- 32.Nonato, M. C., J. Widom, and J. Clardy. 2002. Crystal structure of the N-terminal segment of human eukaryotic translation initiation factor 2α. J. Biol. Chem. 277:17057-17061. [DOI] [PubMed] [Google Scholar]
- 33.Pavitt, G. D., K. V. A. Ramaiah, S. R. Kimball, and A. G. Hinnebusch. 1998. eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev. 12:514-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavitt, G. D., W. Yang, and A. G. Hinnebusch. 1997. Homologous segments in three subunits of the guanine nucleotide exchange factor eIF2B mediate translational regulation by phosphorylation of eIF2. Mol. Cell. Biol. 17:1298-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulman, B. A., D. L. Lindstrom, and E. Harlow. 1998. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc. Natl. Acad. Sci. USA 95:10453-10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharp, T. V., J. E. Witzel, and R. Jagus. 1997. Homologous regions of the α subunit of eukaryotic translational initiation factor 2 (eIF2α) and the vaccinia virus K3L gene product interact with the same domain within the dsRNA-activated protein kinase (PKR). Eur. J. Biochem. 250:85-91. [DOI] [PubMed] [Google Scholar]
- 37.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood, R., A. C. Porter, K. Ma, L. A. Quilliam, and R. C. Wek. 2000. Pancreatic eukaryotic initiation factor-2α kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346:281-293. [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda, D. Y., J. A. Wohlschlegel, and A. Dutta. 2001. A bipartite substrate recognition motif for cyclin-dependent kinases. J. Biol. Chem. 276:1993-1997. [DOI] [PubMed] [Google Scholar]
- 40.Ung, T. L., C. Cao, J. Lu, K. Ozato, and T. E. Dever. 2001. Heterologous dimerization domains functionally substitute for the double-stranded RNA binding domains of the kinase PKR. EMBO J. 20:3728-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez de Aldana, C. R., T. E. Dever, and A. G. Hinnebusch. 1993. Mutations in the α subunit of eukaryotic translation initiation factor 2 (eIF-2α) that overcome the inhibitory effects of eIF-2α phosphorylation on translation initiation. Proc. Natl. Acad. Sci. USA 90:7215-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams, N. P., A. G. Hinnebusch, and T. F. Donahue. 1989. Mutations in the structural genes for eukaryotic initiation factors 2α and 2β of Saccharomyces cerevisiae disrupt translational control of GCN4 mRNA. Proc. Natl. Acad. Sci. USA 86:7515-7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, F., P. Romano, T. Nagamura-Inoue, B. Tian, T. E. Dever, M. B. Mathews, K. Ozato, and A. G. Hinnebusch. 2001. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem. 276:24946-24958. [DOI] [PubMed] [Google Scholar]
- 44.Zheng, J., D. Knighton, L. ten Eyck, R. Karlsson, N. Xuong, S. Taylor, and J. Sowadski. 1993. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochemistry 32:2154-2161. [DOI] [PubMed] [Google Scholar]