Abstract
Background and purpose
Basilar-artery occlusion (BAO) usually accounts for devastating neurologic sequelae, poor prognosis, and even death. While endovascular thrombectomy (EVT) is the most successful treatment for anterior circulation stroke with large vessel occlusion, its effectiveness in treating acute BAO is still debatable. Our aim is to compare the efficacy and safety between EVT and conservative medical treatment (CMT) in BAO.
Methods
Up until May 2022, relevant literature was gathered using searches in Embase, PubMed, and the Cochrane Library. The primary outcomes were defined as good functional outcome (modified Rankin Scale 0–2) and favorable functional outcome (modified Rankin Scale 0–3) at 3 months between EVT and CMT groups. The secondary outcomes included mortality at 3 months, symptomatic intracerebral hemorrhage (ICH), and any ICH.
Results
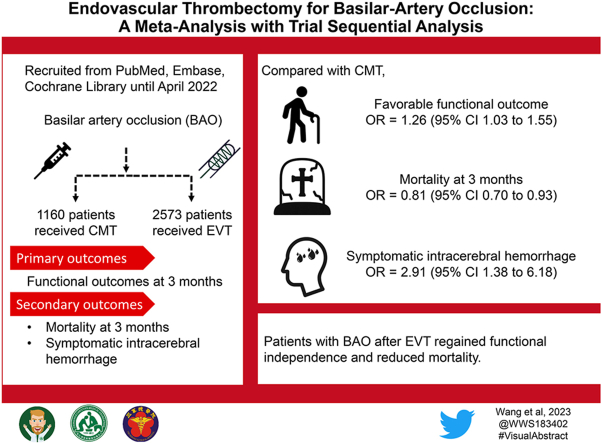
Eight studies involving 3733 patients with BAO were enrolled 2573 individuals underwent EVT, and the remaining 1160 patients received CMT. Compared with CMT, EVT achieved more favorable functional outcome (odds ratio (OR) 1.26, 95% CI 1.03–1.55, I2 = 54%, p = 0.05) in BAO. The good functional outcome showed a similar tendency (OR 1.23, 95% CI 0.97–1.57, I2 = 63%, p = 0.02) as well. EVT decreased mortality at 3 months (OR 0.81, 95% CI 0.70–0.93, I2 = 31 %, p = 0.19), although having a tendency to cause symptomatic ICH (OR 2.91, 95% CI 1.38–6.18, I2 = 22 %, p = 0.27).
Conclusions
EVT in BAO provides superior functional outcomes and less mortality compared with CMT. Even though EVT has the propensity to cause symptomatic ICH, EVT nevertheless improved posterior circulation stroke.
Keywords: Acute ischemic stroke, Endovascular thrombectomy, Basilar-artery occlusion, Meta-analysis
Graphical abstract
1. Introduction
In the era of interventional neurology, the acute management of ischemic stroke strides forward a lot. In 2015, numerous substantial randomized clinical trials have consistently shown that performing endovascular thrombectomy (EVT) as soon as possible improves the severity of stroke-related impairment in patients with large vessel occlusion (LVO) [[1], [2], [3], [4], [5]]. EVT also plays the role on those who fail to meet stringent criteria for receiving systemic thrombolysis, such as anticoagulated patients, overtime strokes, patients suffering from recent trauma or surgery [6]. EVT additionally extends its initial time window from 6 h to 24 h based on stringent patient selection in infarction core and mismatch ratio [7,8].
However, the previous research was mainly limited to anterior circulation. There is no formal advice for physicians to serve as a guide in emergent practice while encountering posterior circulation stroke, even if EVT is thought to be suitable. Additionally, basilar-artery occlusion (BAO), which causes serious neurologic consequences, a poor prognosis, and even mortality, accounts for around 1% of all ischemic strokes and 5%–10% of all proximal intracranial cerebral occlusions. Prodromal symptoms typically manifest in a variety of ways [9]. Therefore, recently, there have been emerging interest in whether EVT could provide similar overwhelming benefits in posterior circulation stroke, especially BAO.
In 2009, the BASICS trial provided an observational, prospective registry that showed no appreciable superiority in either intravenous thrombolysis, EVT or conservative antithrombotic treatment [10]. Successor study in 2021, comparing endovascular therapy with standard medical care, remained failed to prove significant differences regarding favorable outcomes [11]. Another randomized clinical trial–BEST had similar results, but the lack of significance was attributed to poor adherence to the study assignment and a smaller sample size because the trial was terminated early [12]. Despite a comparatively higher incidence of symptomatic intracerebral hemorrhage, the BASILAR research, a state-wide nonrandomized cohort, demonstrated that EVT benefited in improving functional outcomes and reduced mortality [13]. The same perspective was reiterated in the two meta-analyses [14,15]. Emerging multicenter randomized clinical trials showed that patients with BAO receiving EVT tend to benefit in functional outcomes and survival as well [16,17]. Overall, it is still debatable whether EVT offers patients with BAO more benefits than conservative medical therapy (CMT), and few studies systematically compare the efficacy and safety of EVT and CMT. Thus, conducting a meta-analysis to update the rising-up evidence regarding EVT in patients with BAO is necessary.
Our study's objective is to compare and contrast EVT and CMT efficacy and safety in patients with BAO. Hoping to bring a dawn to posterior circulation stroke in the endovascular era and make strokologists no more at our wits' end with BAO and its grave prognosis.
2. Methods
To perform systematic reviews and meta-analyses of randomized controlled trials (RCTs) and observation studies, respectively, we followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses [18] (Table S1) and the Meta-analysis of Observational Studies in Epidemiology [19] (Table S2) reporting guidelines. All data necessary for other researchers for reproducing our results are provided in the present work. The protocol for this systematic review was registered at the Open Science Framework (https://osf.io/gn9e2).
2.1. Search strategy
Using the following set of keywords, we carried out a systematic review of the relevant literature in the Embase, Pubmed, and Cochrane Library databases from the beginning to May 2022, “acute ischemic stroke,” “basilar artery occlusion,” “endovascular thrombectomy,” along with possible relevant keywords (Table S3). To find possibly qualifying studies, we manually examined the pertinent journals in the field.
2.2. Study selection
We included studies about the outcomes of EVT compared with CMT in acute ischemic stroke patients with “pure” BAO. The exclusion criteria were as follows: patients with vertebral artery blockage, studies with fewer than 10 patients, children under the age of 18 years, animal studies, studies conducted in languages other than English, and studies with no control group were among the exclusion criteria. If partially overlapping patients were noted, we would use the updated articles to prevent double counting. Using the selection criteria, two reviewers independently screened the studies (WSW and YPC). Disagreements regarding the selection process for each study were resolved by group consensus. As for the CMT, it mainly consisted of tPA administration, use of antiplatelet/anticoagulation drugs, blood pressure management and prophylaxis of deep vein thrombosis according to stroke management guideline and institutional stroke care protocols.
2.3. Outcome measurement
Our main results were excellent functional outcome (a modified Rankin Scale [20] score of 0–1 at 3 months), good functional outcome (a modified Rankin Scale score of 0–2 at 3 months), and favorable functional outcome (a modified Rankin Scale score of 0–3 at 3 months). The safety outcomes were mortality at 3 months, symptomatic ICH, and any ICH. Symptomatic ICH was defined as any intracerebral hemorrhage associated with clinical deterioration by a 4-point increase of the NIHSS score from baseline.
2.4. Data extraction
Two independent authors (WSW and YPC) examined the titles and abstracts before reading the full contents of the remaining articles. The following information were independently gathered by the same investigators: the first author, study design, sample size, participant characteristics, and the outcomes of interest. In the meta-analysis, we employed an intention-to-treat design even if the per-protocol design recommended that larger effect sizes were possible.
2.5. Risk of bias assessment
The Newcastle–Ottawa scale [21] for cohort studies and risk of bias (RoB) for RCTs were used by authors who were not involved in any of the included research to evaluate the RoB. Scores of 0–3, 4–6, and 7–9 at the Newcastle–Ottawa scale were regarded as low, moderate, and high quality, evidence respectively; RoB rated the evidence based on study constraints from high to low quality.
2.6. Statistical analysis
For binary outcomes, the major summary measure was the odds ratio (OR) and the corresponding 95% confidence interval (CI) [22]. We decided a priori to run random-effects models to get pooled estimates because we assumed that the true effect size could vary between trials. The DerSimonian–Laird random-effects model using the inverse-variance method was performed to synthesize data for all analyses. Heterogeneity between trials was assessed via using the Cochran Q test [23] (given as a Cochran Q p-value) and I2 statistics. Heterogeneity was classified as moderate (I2 ≥ 30%), substantial (I2 ≥ 50%), or considerable (I2 ≥ 75%) [24].
Based on the study design and geographical region, subgroup analyses were carried out. To investigate the potential influence of covariates on outcomes, a mixed-effects meta-regression analyses were performed. Egger's test was used to assess the publication bias and look for funnel plot asymmetry [25]. All statistical tests were two-tailed, and significance was set at <0.05. R software version 4.0.1's “metaphor” [26] and “meta” packages were used to conduct all statistical analyses [27].
2.7. Quality assessment
To examine the degree of evidence for all outcomes, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) technique [28] was used (GRADEpro, version 20; McMaster University, 2014).
2.8. Trial sequential analysis
Accumulating data in a meta-analysis due to sparse data and repetitive testing may raise the probability of type I and 2 errors [29]. Even if a meta-analysis yields compelling results, the statistical power to quantify the true effects is still insufficient. Trial sequential analysis (TSA), a cumulative MA method, was developed to weigh type I and 2 errors while estimating when the effect is large enough to be unlikely to be impacted by further studies [30], and it can also be used to determine whether the evidence in a meta-analysis is conclusive and reliable [31]. When the cumulative Z-curve crosses the needed information border, the trial sequential monitoring boundary, or enters the futility area, no further additional trials are required. If the Z-curve does not cross any boundary and reaches the required information size, the conclusion is insufficient evidence [32].
A TSA model was created using the O'Brien–Fleming alpha-spending function [33], 5% (two-sided = 0.05) Type 1 error, and 80% statistical power. We set the relative risk reduction of 15% and the amount of heterogeneity correction was determined based on a model variance. The TSA program (version 0.9.5.10 Beta; Copenhagen Trial Unit, Copenhagen, Denmark) and Stata software 16.0 meta cum bounds package were used to conduct the fixed-effect TSA [34].
3. Results
3.1. Search strategy
A total of 3521 articles were identified from three electronic databases and manual supplements. Only 8 studies that met our eligibility were retrieved after duplicates were removed, titles and abstracts were artificially screened, and relevance was assessed based on inclusion and exclusion during the full-text review (Fig. 1). After thorough appraisal and data extraction, in sum, 3733 patients with pure BAO were enrolled for further quantitative synthesis. 2573 individuals underwent EVT and the remaining 1160 patients received CMT. Among the analyzed studies, 1 were RCTs, 5 were prospective cohort studies, and 2 were retrospective cohort studies [11,[35], [36], [37], [38], [39], [40], [41]]. Study characteristics are summarized in Table 1. The patients’ average ages ranged from 65 to 75; their sex ratios were 2:1 and 1:1; the percentage of patients who had tPA administration ranged from 22.1% to 100%; and their mean score of National Institute of Health Stroke Scale, ranging from 4 to 32. The most prevalent comorbidities were atrial fibrillation, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, and diabetes mellitus.
Fig. 1.
Flowchart of the selection process for eligible studies.
Table 1.
Characteristics of included studies.
| Author, year | Country | Study design | No. of patients (EVT/CMT) | Age (year mean) | Female (%) | tPA(EVT/CMT) (%) | NIHSS (mean) | Initial mRS (mean) | HTN (%) | DM (%) | DLP (%) | CAD (%) | AF (%) | HF (%) | Smoking (%) | Previous Stroke (%) | pc-ASPECT (EVT/CMT) | TICI 2b-3 (%) | mRS 0-2 (%) |
mRS 0-3 (%) |
sICH | Mortality at 3 months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tao, 2022 | China | PCS | (1672/462) | 65* | 32.3 | 24.2/18.6 | 21* | N/A | 60.9 | 21.1 | 27.7 | 11.2 | 24.5 | N/A | N/A | 20.2 | 89.5 % ≥ 8/88.5 % ≥ 8 | N/A | N/A | N/A | N/A | N/A |
| Yan, 2022 | China | PCS | (188/286) | 68 | 36.1 | 49.5/31.8 | 16.6* | N/R | 72.4 | 20 | N/R | N/R | 19 | N/R | 32.3 | N/R | 9/10* | 69.5 | 33.8 | 41.8 | 4 | 27.6 |
| Langezaal, 2021 | Western | RCT | (154/146) | 67 | 34.7 | 78.6/79.5 | 22 | 0.35 | 58.3 | 21.7 | 28.3 | 13.3 | 22 | N/R | 40 | 17.3 | 8(nCT); 9(CTA)/8(nCT); 9(CTA)* | 40.9 | 32.7 | 41 | 2.7 | 40.7 |
| Gruder, 2021 | Germany | PCS | (270/133) | 72 | 45.2 | 16.7/33.1 | 12 | N/R | 76.4 | 25.6 | N/R | N/R | N/R | N/R | N/R | 23.1 | N/R | 74.1 | N/R | N/R | N/R | 34.2 |
| Seners, 2021 | France | RCS | (28/29) | 69* | 36.8 | 100/100 | 4* | N/R | 54.4 | 8.8 | N/R | N/R | 15.8 | N/R | 26.3# | N/R | 9/9* | 60.7 | 80.7 | N/R | 0 | N/R |
| Yoshimoto, 2020 | Japan | PCS | (129/38) | 75* | 31.1 | 43.4/26.3 | 22.4* | 0* | 62.3 | 20.4 | 28.1 | N/R | 49.1 | 14.4 | 20.4# | 7.2 | 8(mild); 7(severe)/9(mild); 6(severe)* | 98.4 | 46.1 | 55.7 | 1.8 | 13.2 |
| Dias, 2017 | Brazil | RCS | (19/44) | 67 | 34.9 | 36.8/34.1 | 32* | N/R | 77.8 | 30.2 | 28.6 | 14.3 | 14.3 | 14.3 | 33.3# | 34.9$ | 8/6(non-IVT); 8(IVT)* | 78.9 | N/R | 17.5 | 3.2 | 61.9 |
| Broussalis, 2013 | Austria | PCS | (77/22) | 69 | 48.5 | 39.0/22.7 | 22.2 | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | 51 | 23.2 | 32.3 | 17.1 | 46.5 |
Abbreviations: AF: atrial fibrillation, CAD: coronary artery disease, CTA: computed tomography angiography, CMT: conservative medical therapy, DLP: dyslipidemia, DM: diabetes mellitus, EVT: endovascular thrombectomy, HF: heart failure, HTN: hypertension, IVT: intravenous thrombolysis, mRS: Modified Rankin Scale, N/A: not available, N/R: not reported, nCT: non-contrast computed tomography, NIHSS: National Institutes of Health Stroke Scale, RCS: retrospective cohort study, RCT: randomized control trial, PCS: prospective cohort study, sICH: symptomatic intracranial hemorrhage, TICI: Thrombolysis in Cerebral Infarction, tPA: tissue plasminogen activator.
* median or average from median # only current smoking $ including transient ischemic attack.
3.2. Primary outcomes
Data on functional independence at 3 months submitted by a total of 3733 patients were assessed to evaluate efficacy. Our primary outcomes as the functional outcomes were compared in two treatment groups. Between the EVT and CMT groups, excellent functional outcomes were identical (OR 1.17, 95% CI.073–1.87, I2 = 65%, p = 0.04; Fig. S1). Patients receiving EVT had superior good functional outcomes, although the differences were not statistically significant (OR 1.23, 95% CI.097–1.57, I2 = 63%, p = 0.02; Fig. 2A). Patients with BAO who underwent EVT with or without bridging therapy experienced a substantial improvement in favorable functional outcomes compared to those who underwent CMT (OR 1.26, 95% CI 1.03–1.55, I2 = 54%, p = 0.05; Fig. 2B).
Fig. 2.
Meta-analysis of outcomes among patients with basilar-artery occlusion secondary to endovascular therapy. (A) Good functional outcome, (B) favorable functional outcome, (C) mortality at 3 months, (D) symptomatic intracerebral hemorrhage.
3.3. Safety outcomes
Regarding the safety concerns, EVT unquestionably decreased the three-month mortality in BAO patients (OR 0.81, 95% CI 0.70–0.93, I2 = 31%, p = 0.19; Fig. 2C). However, the incidence of events involving symptomatic ICH was substantial higher in the EVT group compared to the CMT group (OR 2.91, 95% CI 1.38–6.18, I2 = 22%, p = 0.27; Fig. 2D); statistics revealed that the EVT group had more ICHs overall than the CMT group (OR 1.92, 95% CI 0.98–3.79, I2 = 0%, p = 0.92; Fig. S1) but the difference was not statistically significant.
3.4. Subgroup analysis and meta-regression
Subgroup analysis was additionally conducted based on study design, and global region. Easterners earned more favorable functional outcomes; moreover, sICH was prone to Westerners (Table S4). Meta-regression yielded that good functional outcomes were more often in the female gender (Table S5). While, none of the other variants, including age, percentage of tPA, initial NIHSS, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, history of old stroke, smoking, TICI 2b/3, showed significant associations with the overall results of our meta-analysis.
3.5. Assessment of study quality
Appraisal of the RoB for all studies included was provided in Table S6 and Table S7. There are five high-quality studies. The funnel plot and Egger's test did not reveal any evidence of publication bias (Fig. S3). Based on the features of the included studies, the strength of the evidence, and other factors, the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) criteria were applied to assess the certainty of the pooled results (Table S8). The certainty of the evidence regarding all outcomes was also very low.
3.6. Trial sequential analysis
The cumulative number of patients did not reach necessary information size of 4776, and the Z-curves did not exceed any significance boundary either, indicating inconclusive evidence for good functional outcomes and the possibility that additional research may be necessary for convincing statistical evidence (Fig. 3A). In the TSA for favorable functional outcome, the cumulative number of patients did not arrive at the required information size of 4776. The Z-curves were above the conventional threshold and fell short of crossing the significance boundary in favor of EVT, indicating that there is insufficient evidence to conclude that functional results will be positive (Fig. 3B). The total number of patients in the TSA for 3 months mortality did not exceed the minimum information size requirement of 4776, but the Z-curves had reached the significant limit, indicating strong evidence and a precise at 3 months mortality outcome. (Fig. 3C). In the TSA for symptomatic ICH, the cumulative number of patients did not exceed the required information size of 4776, but the Z-curves have surpassed the significance boundary. As a result, our discovery of symptomatic ICH in patients with BAO related to EVT provides clear proof and a precise outcome (Fig. 3D). TSA for excellent functional outcomes and any intracerebral hemorrhage are listed in supplementary materials (Fig. S2).
Fig. 3.
Trial sequential analysis of outcomes. (A) Good functional outcome, (B) favorable functional outcome, (C) mortality at 3 months, (D) symptomatic intracerebral hemorrhage. We take the number of patients on the x-axis and the cumulative Z-score. The conventional boundaries are indicated using the horizontal green line. The trial sequential monitoring boundaries are shown using the sloping red line at the top left-hand corners and represent the TSA threshold for statistical significance. The required information size (RIS) is shown using the vertical red full line. The cumulative Z-curve is shown using the solid blue line. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
This systemic review enrolled eight studies, including a total of 3733 patients with pure BAO. As far as we are aware, the study was the largest meta-analysis comparing EVT with CMT regarding effectiveness, and safety for BAO. According to the BASILAR trial [13], despite its propensity to cause more symptomatic cerebral bleeding events, EVT given promptly within 24 hours resulted in superior functional outcomes and lower death at 90 days. Our study acted following the culmination. Even though there was an increase in symptomatic ICH when compared to CMT alone, EVT made patients with the fatal BAO more likely to be alive and live independently at 3 months. Besides, we also discovered that females tended to achieve good function outcomes at 3-month in further meta-regression analysis. In terms of the subgroup analysis, we found that Eastern nations had a greater impact on the efficacy of timely EVT for BAO. These findings were valuable, as they assisted the clinicians to establish the position of EVT in the posterior circulation stroke.
According to the American Heart Association/American Stroke Association's amended 2019 Guidelines for the Early Management of Patients With Acute Ischemic Stroke [6], EVT should be conducted on all eligible patients with acute ischemic stroke who have LVO. Nevertheless, the guidance and authentication were restricted in the anterior circulation. Regarding posterior circulation LVO, good functional outcome had been documented in BAO in delayed recanalization, which was originally a potentially deadly stroke [42]. However, the serial retrospective study revealed that EVT in BAO was linked to increased procedural complications, a higher risk of unsuccessful recanalization, and ultimately mortality [43,44]. All indicated that EVT in the posterior circulation, especially in BAO, would be a different thing from the anterior circulation. To increase the likelihood of improved functional outcomes, the Society of NeuroInterventional Surgery Standards and Guidelines Committee advised that EVT is suitable for posterior circulation LVO [45,46]. Concerned about the insufficiency of high-class evidence, serial retrospective cohorts, and RCTs sprinted up in recent years. The goal of our work was to define a more succinct method of determining EVT in BAO.
In our analysis, favorable functional outcome achieved a significant difference; being nonsignificant, good functional outcome was likely to be reached with EVT; nevertheless, an excellent functional outcome did not reveal a significant difference. In the past, around one decade ago, when intraarterial therapy remained green and immature, intraarterial therapy failed to reveal superiority in the BASICS registry [10]. Significant advantages gradually became apparent in recent RCTs–BEST and BASICS [11,12], with more sophisticated thrombectomy equipment [47], more skilled interventionists, and more thorough protocols [48]. After ameliorating the high crossover rate, poor recruitment, and loose patient selection, the efficacy of EVT in improving functional outcomes in BAO patients started to spring up in the BASILAR registry [13]. Similar results were also noted in subsequent additional research, including the two alluring RCTs–ATTENTION [49] and BAOCHE [50], as well as meta-analysis [14,15] and observative cohorts [44,51]. By coincidence, the majority of strokologist also share the cutting-edge interventional neurology perspective that EVT is useful in BAO with tight selection criteria employing imaging modalities [52]. Touching the safety concern, although EVT put BAO patients at higher risk of symptomatic ICH, EVT, in contrast, reduced the mortality rates. Although it may be understood that the symptomatic ICH following EVT was not deadly, it is important to remember that ICH, despite being asymptomatic, was linked to a poor functional result at three months [53]. However, several factors could be taken into consideration to reduce such hemorrhagic complications following reperfusion by EVT, including strict tissue-based patient selection using perfusion imaging in posterior circulation [54,55], direct EVT strategy [56], and optimal blood pressure control [57].
During the endovascular era, sex differences remain important issues. During the early stages of endovascular therapy; women had less access to EVT than men did; this gender inequality-related disparity in EVT use might be criticized [58]. The same as other cardiovascular diseases, female also tends to present atypical stroke symptoms. Although there was no difference in the effectiveness of EVT treatment between the sexes, female patients had worse poststroke functional status, which may be related to their older age at stroke onset, more severe symptoms, cardioembolic mechanism, lack of social or financial support, and lack of rehabilitation motivation [59]. In a recently published retrospective analysis about sex difference and EVT utilization, the treatment rates of EVT in both genders got rised dramatically in the neurointerventional era after 2015 (men: 6.6%–18.5%; women: 5.3%–16.7%) [58]. Despite still less likely to receive EVT, no sex-based difference existed after further adjustment. Moreover, with EVT, women were found more likely to survive, even though less likely to ambulate without assistance at discharge. The disparity gap got a big stride in reduction. While, in our meta-regression analysis, compared with male patients with BAO, the female had a tendency to attain good function outcomes at 3 months. Since our study was not intended to account for sex differences and the materials or information referenced were mostly centered on anterior circulation, selection bias may exist.
The results of our study should be interpreted in light of both its strengths and limitations. Random-effect models, subgroup analyses, and meta-regression were used to remove any potential confounding to overcome the clinical heterogeneity. Despite the efforts, residual bias from unmeasured variables could not be completely excluded, for instance, there is still heterogeneity in the definition of safety outcomes regarding hemorrhagic complications among the studies included; thus, the results should be carefully interpreted. Additionally, TSA was carried out to evaluate statistical dependability and offered ample and convincing data. Given the nature of systematic reviews, we lacked various possible predictors, including posterior circulation acute stroke prognosis early computed tomography score, the initial severity of the stroke, stroke etiology, and treatment time window, regarding the efficacy and safety of EVT for further exploration. There remained the need for future RCTs to clarify the mystery of BAO.
5. Conclusion
In conclusion, EVT benefits BAO patients more than CMT alone because they have a larger likelihood of functional independence at 3 months and reduced risks of mortality. Despite the propensity for symptomatic ICH to occur after reperfusion, posterior circulation stroke was nevertheless given hope by EVT.
Funding
This study was supported by the Tri-Service General Hospital/National Defense Medical Center (No. TSGH-D-112157).
Data availability statement
The data of this study will be made available on request from the corresponding author.
Informed consent
Written informed consent was not required, because this work is a systematic review and meta-analysis.
Ethical approval
Institutional Review Board approval was not required, because this work is a systematic review and meta-analysis.
CRediT authorship contribution statement
Wei-Sheng Wang: Data curation, Investigation, Validation, Visualization, Writing - original draft. Yu-Ping Chiu: Data curation, Investigation, Validation, Visualization, Writing - original draft. Po-Huang Chen: Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Writing - review & editing. Hong-Jie Jhou: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This study was supported by the Tri-Service General Hospital/National Defense Medical Center (No. TSGH-D-112157).
Acknowledgements
All authors gratefully credit Enago (www.enago.tw) for assistance with English language review and evaluation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22953.
Contributor Information
Po-Huang Chen, Email: 400010811@mail.ndmctsgh.edu.tw.
Hong-Jie Jhou, Email: 182902@cch.org.tw.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Berkhemer O.A., et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M., et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Campbell B.C., et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Saver J.L., et al. Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) trial: protocol for a randomized, controlled, multicenter study comparing the Solitaire revascularization device with IV tPA with IV tPA alone in acute ischemic stroke. Int. J. Stroke. 2015;10(3):439–448. doi: 10.1111/ijs.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jovin T.G., et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015;372(24):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 6.Powers W.J., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira R.G., et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 8.Albers G.W., et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N. Engl. J. Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattle H.P., et al. Basilar artery occlusion. Lancet Neurol. 2011;10(11):1002–1014. doi: 10.1016/S1474-4422(11)70229-0. [DOI] [PubMed] [Google Scholar]
- 10.Schonewille W.J., et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8(8):724–730. doi: 10.1016/S1474-4422(09)70173-5. [DOI] [PubMed] [Google Scholar]
- 11.Langezaal L.C.M., et al. Endovascular therapy for stroke due to basilar-artery occlusion. N. Engl. J. Med. 2021;384(20):1910–1920. doi: 10.1056/NEJMoa2030297. [DOI] [PubMed] [Google Scholar]
- 12.Liu X., et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19(2):115–122. doi: 10.1016/S1474-4422(19)30395-3. [DOI] [PubMed] [Google Scholar]
- 13.Writing Group for the B.G., et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. 2020;77(5):561–573. doi: 10.1001/jamaneurol.2020.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsanos A.H., et al. Endovascular treatment for basilar artery occlusion: a systematic review and meta-analysis. Eur. J. Neurol. 2021;28(6):2106–2110. doi: 10.1111/ene.14751. [DOI] [PubMed] [Google Scholar]
- 15.Adusumilli G., et al. Endovascular therapy versus medical therapy alone for basilar artery stroke: a systematic review and meta‐analysis through nested knowledge. Stroke: Vascular and Interventional Neurology. 2022;2(3) [Google Scholar]
- 16.Tao C., et al. Endovascular treatment for acute basilar artery occlusion: a multicenter randomized controlled trial (ATTENTION) Int. J. Stroke. 2022;17(7):815–819. doi: 10.1177/17474930221077164. [DOI] [PubMed] [Google Scholar]
- 17.Li C., et al. Basilar artery occlusion Chinese endovascular trial: protocol for a prospective randomized controlled study. Int. J. Stroke. 2022;17(6):694–697. doi: 10.1177/17474930211040923. [DOI] [PubMed] [Google Scholar]
- 18.Page M.J., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup D.F., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Zaidat O.O., et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Hartung J., Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat. Med. 2001;20(24):3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 23.Pereira T.V., et al. Critical interpretation of Cochran's Q test depends on power and prior assumptions about heterogeneity. Res. Synth. Methods. 2010;1(2):149–161. doi: 10.1002/jrsm.13. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L., Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–794. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viechtbauer W. Conducting meta-analyses inRwith themetaforPackage. J. Stat. Software. 2010;36(3) [Google Scholar]
- 27.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid. Base Ment. Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt G., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Shah A., Smith A. Wiley Online Library; 2020. Trial Sequential Analysis: Adding a New Dimension to Meta‐analysis; pp. 15–20. [DOI] [PubMed] [Google Scholar]
- 30.Wetterslev J., et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J. Clin. Epidemiol. 2008;61(1):64–75. doi: 10.1016/j.jclinepi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 31.De Cassai A., et al. Trial sequential analysis: plain and simple. Korean J Anesthesiol. 2021;74(4):363–365. doi: 10.4097/kja.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang H. Trial sequential analysis: novel approach for meta-analysis. Anesthesiol. Pain Med. 2021;16(2):138–150. doi: 10.17085/apm.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien P.C., Fleming T.R. Biometrics; 1979. A Multiple Testing Procedure for Clinical Trials; pp. 549–556. [PubMed] [Google Scholar]
- 34.Miladinovic B., Hozo I., Djulbegovic B. Trial sequential boundaries for cumulative meta-analyses. STATA J.: Promoting communications on statistics and Stata. 2013;13(1):77–91. [Google Scholar]
- 35.Tao C., et al. Endovascular treatment versus best medical management in acute basilar artery occlusion strokes: results from the ATTENTION multicenter registry. Circulation. 2022;146(1):6–17. doi: 10.1161/CIRCULATIONAHA.121.058544. [DOI] [PubMed] [Google Scholar]
- 36.Yan S., et al. Effect of imaging markers on reperfusion therapy in basilar artery occlusion. Ann. Neurol. 2022;92(1):97–106. doi: 10.1002/ana.26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruber K., et al. Evaluation of endovascular treatment for acute basilar occlusion in a state-wide prospective stroke registry. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.678505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seners P., et al. Intended bridging therapy or intravenous thrombolysis alone in minor stroke with basilar artery occlusion. Stroke. 2021;52(2):699–702. doi: 10.1161/STROKEAHA.120.030992. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimoto T., et al. Treatment outcomes by initial neurological deficits in acute stroke patients with basilar artery occlusion: the RESCUE Japan registry 2. J. Stroke Cerebrovasc. Dis. 2020;29(11) doi: 10.1016/j.jstrokecerebrovasdis.2020.105256. [DOI] [PubMed] [Google Scholar]
- 40.Dias F.A., et al. Clinical outcomes of patients with acute basilar artery occlusion in Brazil: an observational study. J. Stroke Cerebrovasc. Dis. 2017;26(10):2191–2198. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 41.Broussalis E., et al. Comparison of endovascular treatment versus conservative medical treatment in patients with acute basilar artery occlusion. Vasc. Endovasc. Surg. 2013;47(6):429–437. doi: 10.1177/1538574413488458. [DOI] [PubMed] [Google Scholar]
- 42.Grigoriadis S., et al. Clinically successful late recanalization of basilar artery occlusion in childhood: what are the odds? Case report and review of the literature. J. Neurol. Sci. 2007;260(1–2):256–260. doi: 10.1016/j.jns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Alonso de Lecinana M., et al. Mechanical thrombectomy for basilar artery thrombosis: a comparison of outcomes with anterior circulation occlusions. J. Neurointerventional Surg. 2017;9(12):1173–1178. doi: 10.1136/neurintsurg-2016-012797. [DOI] [PubMed] [Google Scholar]
- 44.Gory B., et al. Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first-line strategy (ADAPT vs stent retriever) J. Neurosurg. 2018;129(6):1482–1491. doi: 10.3171/2017.7.JNS171043. [DOI] [PubMed] [Google Scholar]
- 45.Kayan Y., et al. Current endovascular strategies for posterior circulation large vessel occlusion stroke: report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. J. Neurointerventional Surg. 2019;11(10):1055–1062. doi: 10.1136/neurintsurg-2019-014873. [DOI] [PubMed] [Google Scholar]
- 46.Wyszomirski A., et al. Treatment of acute basilar artery occlusion: systematic review and meta-analysis. Neurol. Neurochir. Pol. 2017;51(6):486–496. doi: 10.1016/j.pjnns.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Saver J.L., et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 48.Baik S.H., et al. Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology. 2019;291(3):730–737. doi: 10.1148/radiol.2019181924. [DOI] [PubMed] [Google Scholar]
- 49.Tao C., et al. Trial of endovascular treatment of acute basilar-artery occlusion. N. Engl. J. Med. 2022;387(15):1361–1372. doi: 10.1056/NEJMoa2206317. [DOI] [PubMed] [Google Scholar]
- 50.Jovin T.G., et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N. Engl. J. Med. 2022;387(15):1373–1384. doi: 10.1056/NEJMoa2207576. [DOI] [PubMed] [Google Scholar]
- 51.Kang D.H., et al. Endovascular thrombectomy for acute basilar artery occlusion: a multicenter retrospective observational study. J. Am. Heart Assoc. 2018;7(14) doi: 10.1161/JAHA.118.009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huo X., et al. Perceptions on basilar artery occlusion management in China versus other countries: analysis of the after the BEST of BASICS (ABBA) survey. J. Stroke Cerebrovasc. Dis. 2022;31(11) doi: 10.1016/j.jstrokecerebrovasdis.2022.106804. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki K., et al. Asymptomatic intracranial hemorrhage is associated with poor outcomes after mechanical thrombectomy for large vessel occlusion. Stroke: Vascular and Interventional Neurology. 2022 [Google Scholar]
- 54.Kim J.G., et al. DWI-pc-ASPECT score in basilar artery occlusion: is 6 points or less always indicative of a bad outcome? Intervent Neuroradiol. 2019;25(4):371–379. doi: 10.1177/1591019919827505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouyang K., et al. Posterior circulation ASPECTS on CT angiography predicts futile recanalization of endovascular thrombectomy for acute basilar artery occlusion. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.831386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nie X., et al. Endovascular treatment with or without intravenous alteplase for acute ischaemic stroke due to basilar artery occlusion. Stroke Vasc Neurol. 2022;7(3):190–199. doi: 10.1136/svn-2021-001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazighi M., et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2021;20(4):265–274. doi: 10.1016/S1474-4422(20)30483-X. [DOI] [PubMed] [Google Scholar]
- 58.Demel S.L., et al. Sex differences in endovascular therapy for ischemic stroke: results from the get with the guidelines-stroke registry. Stroke. 2022;53(10):3099–3106. doi: 10.1161/STROKEAHA.122.038491. [DOI] [PubMed] [Google Scholar]
- 59.Ospel J.M., et al. Toward a better understanding of sex- and gender-related differences in endovascular stroke treatment: a scientific statement from the American heart association/American stroke association. Stroke. 2022;53(8):e396–e406. doi: 10.1161/STR.0000000000000411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study will be made available on request from the corresponding author.