Abstract
Objective
To analyze the oxidative stress status and its association with tissue neutrophilia and oral steroid response in chronic rhinosinusitis with nasal polyps (CRSwNP) patients.
Methods
The levels of total oxidant status (TOS) were detected in the sinonasal tissues by using specific assay kits. Tissue neutrophil was examined by immunohistochemical staining, and oxidant status index (OSI) was evaluated in polyps tissues, and the messenger RNA (mRNA) levels of superoxide dismutase 2 (SOD2), aldehyde dehydrogenase 1 (ALDH1A1), and microsomal glutathione S‐transferase 1 (MGST1) were examined using quantitative real‐time polymerase chain reaction in the sinonasal tissues. The receiver operating characteristics (ROCs) curve of ALDH1A1, MGST1, and SOD2 mRNA levels were evaluated to determine the steroid response of CRSwNP patients.
Results
The levels of TOS and OSI were significantly higher in CRSwNP and CRSsNP than in normal controls, and OSI in polyps tissues was positively associated with tissue neutrophilia and poor steroid response. The ALDH1A1, MGST1, and SOD2 mRNA levels showed comparable accuracy as predictors of poor steroid response indicated by the area under the curve.
Conclusion
These findings provided evidence that the increased level of oxidative stress contributes to enhanced tissue neutrophilia and poor steroid response in CRSwNP patients.
Keywords: chronic rhinosinusitis, nasal polyps, neutrophil, oxidative stress, steroid response
In brief, oxidative stress plays an important role in the pathogenesis of chronic rhinosinusitis. Hu et al. identified enhanced oxidative stress, especially in chronic rhinosinusitis with nasal polyps patients, which could be associated with tissue neutrophilia and poor steroid response. Antioxidative drug rottlerin and curcumin might be an add‐on therapeutic way.
Highlights
Total oxidant status and oxidant status index levels, aldehyde dehydrogenase 1,microsomal glutathione S‐transferase 1, and superoxide dismutase 2 messenger RNA levels are significantly increased in chronic rhinosinusitis with nasal polyps (CRSwNP) patients.
Oxidase stress contributes to neutrophilic mucous inflammation and poor steroid response in Eastern CRSwNP patients.
Rottlerin and curcumin might be used as an add‐on therapy on poor‐steroid‐response (or neutrophilic) CRSwNP patients.
INTRODUCTION
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a heterogeneous disorder with visible polyps in the middle meatus and/or olfactory cleft, which is characterized by distinct pathophysiologic mechanisms. 1 Unlike chronic rhinosinusitis without nasal polyps (CRSsNP) that has been thought to be Th1‐dominant inflammation, CRSwNP can be divided into eosinophilic CRSwNP and noneosinophilic CRSwNP based on the infiltration of eosinophils, eosinophilic CRSwNP is commonly associated with Th2 cytokine polarization and predominantly eosinophilic infiltration in western countries. 2 , 3 Recently, numerous studies demonstrated a large subset of non‐eosinophilic CRSwNP patients in Asian and Eastern populations show distinct endotype. 4 , 5 , 6 , 7 In these CRSwNP patients, an enhanced tissue neutrophilia and mixed Th1/Th17 response were commonly observed. 4 And these several studies have demonstrated increased interleukin (IL)‐8, and IL‐1α expression as well as enhanced infiltration of neutrophils, 8 these neutrophils may play an important role in the nasal epithelial barrier and tissue remodeling in nasal polyps. 3 Importantly, infiltrated neutrophils in nasal polyps have been reported associated with poor steroid response in CRSwNP patients. 4 Currently, the key factors underlying tissue neutrophilia in these CRSwNP patients are not yet completely understood.
The human body has a complex antioxidant defense system that prevents the initiation of numerous free radical chain reactions. It is known that there have three different antioxidant defense systems in the human body. 9 The first representative enzyme is superoxide dismutase (SOD), which contributes to preventing the formation of free radicals and comprises enzymes. The second line contributes to suppressing chain initiation and breaking the chain propagation, such as aldehyde dehydrogenase 1 (ALDH1A1). The third line of antioxidant defense is composed of repair enzymes essential for cell development, such as glutathione peroxidase and microsomal glutathione S‐transferase 1 (MGST1). Generally, total oxidative stress and antioxidant capacity can be parameterized by total oxidant status (TOS), total antioxidant status (TAS), and oxidative stress index (OSI). When the oxidant/antioxidant balance has been disrupted, a lot of inflammatory disorders, including asthma and chronic obstructive pulmonary disease, might be initiated. 10 In CRSwNP patients, previous studies report that the activities of antioxidant enzymes were lower than in controls. 11 , 12 , 13 However, how oxidative stress is involved in CRSwNP has not been fully documented. This study thus aimed to investigate whether and how oxidative stress affects tissue neutrophilia and steroid response in CRSwNP patients.
MATERIALS AND METHODS
Subjects
This study was approved by the ethical committee of Eye and ENT Hospital, Fudan University, and written informed consent was obtained from all the subjects. Adult CRSwNP and CRSsNP patients (n = 22 and 13) were recruited from the Department of Otolaryngology, Eye and ENT Hospital. The diagnosis of CRSwNP and CRSsNP was made according to the international european position paper on rhinosinusitis and nasal polyps consensus. 1 As normal controls, 10 nonatopic patients undergoing transnasal skull surgery because of anterior skull base tumors were enrolled. The atopic status of the patients and normal controls were evaluated by allergen skin prick tests or serum‐specific immunoglobulin E assay. Polyp size was scored on a scale of 0–3 system: 0, no visible polyp in the middle meatus; 1, polyps in the middle meatus but not reaching below the inferior border of the middle turbinate; 2, polyps reaching below the inferior border of the middle turbinate but not to the inferior border of the inferior turbinate; and 3, extensive large polyps congesting the inferior meatus, and the sum of the bilateral polyp score was recorded as the nasal polyps score (maximum score 6). From this scale system, we considered CRSsNP patients as score 0 and CRSwNP patients as score 1–3. Tissue neutrophil was identified by positive immunostaining of human neutrophil elastase (HNE) as described elsewhere. 4 Patients with exacerbations of acute rhinosinusitis, a history of functional endoscopic sinus surgery (FESS), pregnant or lactating women, former or current alcohol and drug users, clear history of smoking, malignancy, positive HIV test, immune deficiency, and treatment of corticosteroids, antihistamines, and Immunosuppressive drugs within 2 weeks were excluded.
The demographic data from the CRSwNP and CRSsNP patients and normal controls enrolled in this study were listed in Table 1. The polyp tissues in CRSwNP patients and sinus tissues in normal controls were sampled during an endoscopic inspection. Tissue samples were divided into several portions for RNA and protein isolation, and immunohistochemical (IHC) staining. Steroid response for these enrolled CRSwNP patients was evaluated by endoscopic inspection again after 7 days of oral predinisone (20 mg daily), and poor steroid response was defined when patients are unable to reduce more than 1 point by the polyp scoring system in this study.
Table 1.
Subjects' characteristics
| Characteristics | CRSwNP | CRSsNP | Control |
|---|---|---|---|
| Subjects (n) | 20 | 13 | 10 |
| Age (y) | 46.2 (26–57) | 45.2 (24–59) | 49.7 (33–61) |
| Sex (M/F) | 9/11 | 6/7 | 4/6 |
| Asthma | 1/20 | 1/13 | 0/10 |
| Skin prick test (P/N) | 11/20 | 5/13 | 0/10 |
Abbreviations: AERD, aspirin‐exacerbated respiratory disease; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; F, female; M, male.
Tissue TOS and TAS
Fresh sinonasal tissues were weighed and dissected directly after surgery, every 20 mg tissue was dissected on ice and homogenized by sonication in 100 μl cold PBS for 1 min, the homogenates were then centrifuged at 12,000 rpm, 4°C for 5 min and supernatants were collected for further use. The protein concentrations in the supernatants were determined by the bicinchoninic acid assay method. The levels of TOS and TAS were detected by a Fully Automated Third Generation assay kit (Relassay) according to the manufacturer's protocols. For convenient analysis, all values lower than the detectable limit were considered zero. To reduce errors, the measured levels were normalized to the total protein levels. All samples were tested in triplicates.
Quantitative real‐time polymerase chain reaction (qPCR)
The messenger RNA (mRNA) expression levels of SOD2, ALDH1A1, and MGST1 in sinonasal tissues were evaluated using qPCR analysis as we described elsewhere. 5 Briefly, total RNA was extracted with RNAprep pure Tissue Kit (Tiangen) according to the manufacturer's instructions. Reverse transcription was performed, in which cDNA for quantitative PCR was synthesized from 2 μg of total RNA using an oligo (dT) 18 primer and molonney murine leukemia virus reverse transcriptase (Takara). RNA integrity and the success of the reverse transcription reaction were monitored by PCR amplification of β‐actin transcripts. mRNA expression was determined using the ABI PRISM 7500 Detection System (Applied Biosystems) with SYBR Premix Taq (Takara). Primers were chosen based on the published data. The primer sequences were listed as follows: SOD forward: 5′‐CTG ATT TGG ACA AGC AGC AA‐3′; SOD reverse: 5′‐CTG GAC AAA CCT CAG CCC TA‐3′; ALDH1A1 forward: 5′‐TAC TCA CCG ATT TGA AGA TT‐3′, ALDH1A1 reverse: 5′‐TTG TCA ACA TCC TCC TTA TC‐3′; MGST1 forward: 5′‐ATT GGC CTC CTG TAT TCC TTG‐3′, MGST1 reverse: 5′‐TAA TCC CTC TGC TCC CCT CC‐3′; β‐actin forward: 5’‐TGT GTT GGC GTA CAG GTC TTT G‐3’, β‐actin Reverse: 5′‐GGG AAA TCG TGC GTG ACA TTA AG‐3′. The qRT‐PCR amplification protocol consisted of 40 cycles of denaturation at 95°C for 15 s and annealing and extension cycles at 60°C for 45 s each. Melting curve analysis was used to control for amplification specificity. The mean cycle threshold (C t) values were normalized to those of β‐actin, and the relative mRNA levels of the target genes were analyzed using the method. Experiments were performed in triplicate for each data point. All samples were tested in triplicate.
IHC staining
A Paraffin section (4 μm) of polyp tissues was prepared, and IHC staining was performed using the strept avidin‐biotin complex method as we described elsewhere. 5 The sections were incubated overnight at 4°C in the presence of anti‐HNE (1:100; Abcam). Then, the antibodies were detected via streptavidin–biotin–horseradish peroxidase complex formation (Zhongshan‐jinqiao). The immunostaining was considered to be positive when brown cells appeared following a reaction with the reagent 3′,3′‐diaminobenzidine. Replacement of the primary antibodies with isotype‐matched immunoglobulin G was used as a negative control. The sections were examined via light microscopy by two blinded investigators who were unaware of the clinical data. The number of HNE‐positive cells was counted in 10 high‐power fields (HPFs, ×400) and averaged. Neutrophilic CRSwNP was identified when the average HNE‐positive cell number was above or equal to 4 (cutoff value) per HPFs in polyp tissues, as we previously described.
Cell culture and treatment
Human bronchial epithelial cell line 16 human bronchial epithelial (HBE) cells were grown in complete RPMI‐1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and streptomycin (Invitrogen). Rottlerin and curcumin (Sigma‐Aldrich) were dissolved in dimethyl sulfoxide according to the manufacturer's protocols for use. When a confluence of 80%–90% was reached, the cells were washed with PBS and were divided into three groups: rottlerin or curcumin‐treated or control groups, respectively. Next, rottlerin and curcumin (10 μM) were added into 16 HBE cells for 24 h, then cell pellets and supernatants were harvested and kept for further use.
Statistical analysis
Data were expressed as medians and interquartile ranges. These data were analyzed using the Kruskal–Wallis H test and the nonparametric Mann–Whitney U test or Student's t test. A p value of less than 0.05 was considered statistically significant. Predictive values of gene expression for the better or poor steroid response among CRSwNP patients were assessed with the receiver operating characteristic (ROC) curve analysis. The area under the curve (AUC) has a value from 0.5 to 1.0, AUC greater than 0.9 is considered excellent, ranges from 0.8 to 0.9 very good, 0.7 to 0.8 good, 0.6 to 0.7 average, and <0.6 poor.
RESULTS
The TOS and TAS levels in sinonasal tissues of CRSwNP, CRSsNP patients, and normal controls
To determine the role of oxidative stress in the pathogenesis of CRSwNP, we first examined the levels of TOS and TAS levels in the supernatants of homogenized sinonasal tissues of CRSwNP, CRSsNP patients, and normal controls. As shown in Figure 1, the levels of TOS in CRSwNP (5.811 ± 2.273) and CRSsNP (4.146 ± 0.971) patients were significantly higher than that in the normal (2.307 ± 1.261) controls (p < 0.05), whereas the levels of TAS in CRSwNP (0.386 ± 0.240) and CRSsNP (0.400 ± 0.244) patients were significantly lower than that in the normal (1.151 ± 0.374) controls (p < 0.05). When compared with CRSsNP patients, we found the level of TOS in CRSwNP patients was significantly higher than that in CRSsNP patients (p < 0.05), and the level of TAS in CRSwNP patients was significantly lower than that in CRSsNP patients (p < 0.05).
Figure 1.
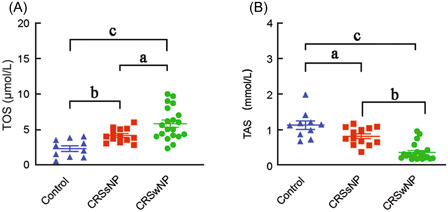
The TOS and TAS levels in sinonasal tissues of CRSwNP and CRSsNP patients and normal controls. The TOS levels (A) and TAS levels (B) in sinonasal tissues of CRSwNP and CRSsNP patients and normal controls. CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; TAS, total antioxidant status; TOS, total oxidant status; a p < 0.05, b p < 0.01, c p < 0.001.
The OSI levels in sinonasal tissues of CRSwNP, CRSsNP patients, and normal controls
We next used OSI (the ratio of TOS and TAS) as an indicator to further evaluate the importance of oxidase stress in the pathogenesis of CRSwNP. As shown in Figure 2A, we found the OSI levels in CRSwNP (18.847 ± 9.550) and CRSsNP (5.454 ± 2.518) patients were significantly higher than that in the normal (2.444 ± 1.770) controls (p < 0.05). When compared with CRSsNP patients, we found the level of OSI in CRSwNP patients was significantly higher than that in CRSsNP patients (p < 0.05). By setting the OSI median level as the cut‐off value, we subdivided CRSwNP patients into two subgroups (OSIhigh and OSIlow) and examined the association between OSI level and tissue neutrophilia. As shown in Figure 2B, WE found the neutrophil number was significantly elevated in the OSIhigh subgroup compared with the OSIlow subgroup of CRSwNP patients (p < 0.05), and significantly increased OSI levels were found in neutrophilic (23.899 ± 8.047) and poor‐steroid‐response (22.049 ± 10.197) CRSwNP patients when compared with the nonneutrophilic (11.268 ± 6.052) and better‐steroid‐response (15.645 ± 8.122) CRSwNP patients, respectively (p < 0.05), see Figure 2C,D.
Figure 2.
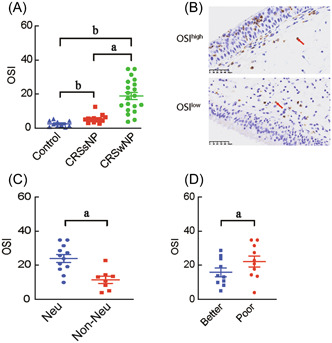
The OSI is associated with tissue neutrophilia and steroid response in CRSwNP patients. (A) The OSI levels in sinonasal tissues of CRSwNP and CRSsNP patients and normal controls. (B) Representative histological images of HNE‐positive cells (arrow indicating neutrophils) in polyp tissue of OSIhigh and OSIlow CRSwNP patients. (C) The OSI levels in sinonasal tissues of neutrophilic and non‐neutrophilic CRSwNP patients. (D) The OSI levels in sinonasal tissues of CRSwNP patients with better or poor steroid response. CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; Neu, neutrophilic; OSI, oxidative stress index; a p < 0.05, b p < 0.01.
The mRNA levels of ALDH1A1, MGST1, and SOD2 in sinonasal tissues of CRSwNP patients and normal controls
To further determine the importance of antioxidant system genes in CRSwNP patients, we examined the mRNA levels of ALDH1A1, MGST1, and SOD2 in sinonasal tissues of CRSwNP, CRSsNP patients, and normal controls. As shown in Figure 3, we found the mRNA levels of ALDH1A1 (1.003 ± 0.595), MGST1 (0.988 ± 0.501), and SOD2 (0.988 ± 0.552) in sinonasal tissues of CRSwNP were significantly lower than those in normal controls (3.885 ± 1.552, 2.277 ± 0.974, 1.719 ± 0.848, respectively, all p < 0.05). Next, we evaluated the ROC curves of ALDH1A1, MGST1, and SOD2 mRNA levels to determine the steroid response of CRSwNP patients. As shown in Figure 4, the AUCs of ALDH1A1, MGST1, and SOD2 in the CRSwNP group for poor steroid response were 0.8229, 0.6333, and 0.6250, respectively (p < 0.05).
Figure 3.
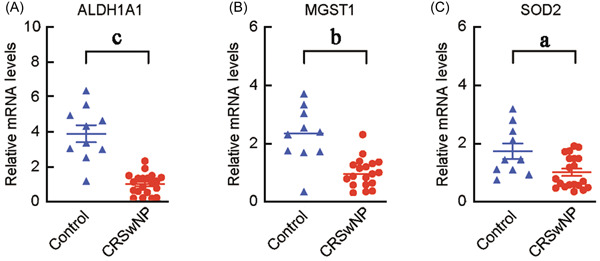
The mRNA levels of ALDH1A1 (A), MGST1 (B), and SOD2 (C) in sinonasal tissues of CRSwNP and normal controls. ALDH1A1, aldehyde dehydrogenase 1; CRSwNP, chronic rhinosinusitis with nasal polyps; MGST1, microsomal glutathione S‐transferase 1; mRNA, messenger RNA; SOD2, superoxide dismutase 2; a p < 0.05, b p < 0.01, c p < 0.001.
Figure 4.
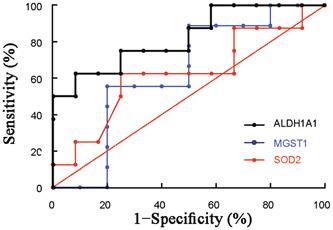
The ROC curves of ALDH1A1, MGST1, and SOD2 mRNA levels in CRSwNP patients. The AUCs of ALDH1A1, MGST1 and SOD2 in the CRSwNP group for poor steroid response were 0.8229, 0.7500, and 0.9085, respectively. ALDH1A1, aldehyde dehydrogenase 1; AUC, area under the curve; CRSwNP, chronic rhinosinusitis with nasal polyps; MGST1, microsomal glutathione S‐transferase; mRNA, messenger RNA; ROC, receiver operating characteristic; SOD2: superoxide dismutase 2.
The effects of rottlerin and curcumin on oxidase stress in 16 HBE cells in vitro
To evaluate the potential therapeutic effects of rottlerin and curcumin on oxidase stress in CRSwNP patients, we then examined the levels of TOS and TAS levels in 16 HBE cells after treatment with rottlerin or curcumin for 24 h. As shown in Figure 5, the TOS level in 16 HBE cells after treatment with rottlerin (0.511 ± 0.328) or curcumin (7.651 ± 1.118) was significantly decreased (p < 0.05), whereas the TAS level in 16 HBE cells after treatment with rottlerin (0.734 ± 0.248) or curcumin (5.656 ± 1.579) was significantly increased when compared to the control (p < 0.05). Consistently, the mRNA levels of ALDH1A1 (2.048 ± 0.274 for rottlerin and 2.583 ± 0.462 for curcumin) and MGST1 (1.540 ± 0.189 for rottlerin 1.695 ± 0.101 for curcumin), but not SOD2 (1.128 ± 0.193 for rottlerin 1.129 ± 0.315 for curcumin) in 16 HBE cells after treatment with rottlerin or curcumin was significantly increased when compared to the control (p < 0.05), see Figure 6.
Figure 5.
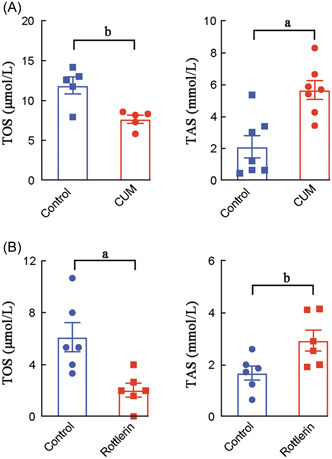
The TOS and TAS levels in 16 HBE cells after treatment with rottlerin or curcumin. (A) The TOS and TAS levels in 16 HBE cells after treatment with rottlerin; (B) the TOS and TAS levels in 16 HBE cells after treatment with curcumin. CUM, curcumin; HBE, human bronchial epithelial; TAS, total antioxidant status; TOS, total oxidant status; a p < 0.05, b p < 0.01.
Figure 6.
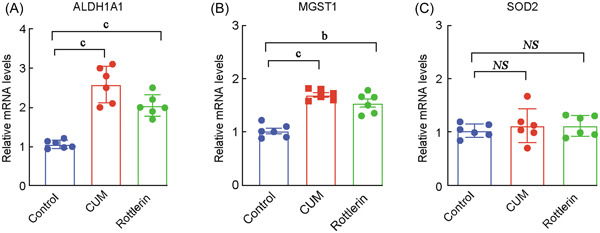
The mRNA expression levels of ALDH1A1, MGST1 and SOD2 after treatment with rottlerin or curcumin. The mRNA levels of ALDH1A1 (A) and MGST1 (B), and SOD2 (C) in 16 HBE cells after treatment with curcumin or rottlerin. ALDH1A1, aldehyde dehydrogenase 1; CUM, curcumin; MGST1, microsomal glutathione S‐transferase; NS, no significant difference; SOD2, superoxide dismutase 2; a p < 0.05, b p < 0.01, c p < 0.001
DISCUSSION
In this study, we provided evidence that the levels of TOS and OSI were significantly increased in CRSwNP patients, and OSI in polyps tissues was positively associated with tissue neutrophilia and poor steroid response. These findings might improve our understanding of how oxidase stress is involved in the pathogenesis of neutrophilic mucous inflammation and poor steroid response in Eastern CRSwNP patients.
CRSwNP is to be well known to be characterized by type 2 inflammation and tissue eosinophilia, 1 whereas the pathogenesis of neutrophilic CRSwNP has not been fully understood. Intranasal or oral steroid is currently the mainstay of treatment for CRSwNP patients recommended by the international consensus, but the steroid sensitivity is variable, and the mechanisms underlying steroid insensitivity remains elusive. 14 , 15 In our previous study, we demonstrated that enhanced neutrophilia in polyp tissues might cause poor steroid response, 5 and provided an alternative to restore steroid sensitivity by adding clarithromycin. 5 Consistently, Kim et al. 7 observed that HNE‐positive neutrophils were associated with poor treatment outcomes in Asians with CRSwNP, and Wang et al. 16 also showed that the IL‐36γ/IL‐36R pathway may contribute to the development of neutrophilic inflammation and corticosteroid resistance in CRSwNP patients. Collectively, these findings indicate polyp neutrophils may play a heretofore unrecognized meaningful role in the pathogenesis of CRSwNP. 17
Free oxygen radicals have important roles in the regulation of cell functions, such as intracellular signaling, transcription activation, cell proliferation, inflammation, and apoptosis. Oxidative stress is present in airway diseases such as severe asthma or chronic obstructive pulmonary disease and contributes to the low response to glucocorticoids through the downregulation of histone deacetylase activity. 18 Up to now, only limited studies reported the importance of oxidative stress in the pathogenesis of CRSwNP. Oxidative stress previously has been demonstrated to exacerbate mucous inflammation by inducing expression of unfolding protein response and driving endoplasmic reticulum stress in nasal polyps tissues. 11 In another two studies, Bozkus et al. 12 reported higher TOS, OSI levels, and lower TAS in both serum and polyps tissues in CRSwNP patients, and Topal et al. 13 demonstrated the severity of oxidative stress, in the forms of TAS and NO, is significantly correlated with the severity of the nasal obstruction and congestion, respectively. However, whether oxidative stress exerts a promotive effect on tissue neutrophilia and poor steroid response in CRSwNP patients remains unclear. In this study, our findings further show that OSI was significantly increased in CRSwNP patients and OSI in polyps tissues was positively associated with tissue neutrophilia and poor steroid response, providing a therapeutic target for improving the clinical efficacy of CRSwNP.
It is known that ALDH1A1 plays a role in preventing oxidative damage by free‐radical species. One study showed increased expression of the ALDH1A1 gene in ALCs patients, which may be ascribed to the involvement of ALDH1A1 in the metabolism of vitamin A and participation in the second and third lines of antioxidant defense. 19 MGST1 gene is a member of the membrane‐associated proteins in the eicosanoid and glutathione metabolism family of a transmembrane protein, it is ubiquitous in human tissue and cell lines, and is localized to the endoplasmic reticulum and outer mitochondrial membrane to protect these membranes from oxidative stress. 20 In our study, in addition to increased TOS and OSI, we observed significantly decreased expression of ALDH1A1, MGST1, and SOD2 mRNA in CRSwNP patients as well. Moreover, by applying analysis of ROC curves of these two genes, it is interesting for us to observe the ALDH1A1, MGST1, and SOD2 mRNA levels showed comparable accuracy as predictors of poor steroid response indicated by AUC. Therefore, evaluating the mRNA levels of ALDH1A1, MGST1, and SOD2 might be helpful for us to determine the steroid response earlier and design a precise therapeutic strategy for the difficult‐to‐treat CRSwNP.
Except for clarithromycin, rottlerin and curcumin are known to have antioxidant functions as natural plant polyphenols with a long tradition in folk medicine. Especially, rottlerin is able to prevent deoxycholate‐induced reactive oxygen species generation in human colon epithelial cells and prevent the free radical generation, but its importance in the management of CRSwNP remains unknown. In our previous study, we have shown IL‐17‐ and IL‐6‐induced hypoxia‐inducible factor (HIF)‐1α was significantly inhibited by steroid and clarithromycin and curcumin in cultured nasal epithelial cells, and observed an additive effect of combined steroid with clarithromycin and curcumin on IL‐17A‐induced HIF‐1α protein levels in vitro. 5 Be consistent with the previous findings, we here provided the first evidence that the TOS level was significantly decreased after treatment with rottlerin or curcumin in cultured 16 HBE cells, and the TAS level and mRNA levels of ALDH1A1 and MGST1 (but not SOD2) were significantly increased after treatment with rottlerin or curcumin. These results collectively demonstrated rottlerin and curcumin might be used as an add‐on therapy of steroids to improve the clinical efficacy of poor‐steroid‐response (or neutrophilic) CRSwNP patients.
We here acknowledged this study might include some limitations. First, we collected the nasal polyps from CRSwNP patients, and use 16 HBE cells for the in vitro validation, although 16 HBE is widely used as a model for respiratory epithelial diseases and barrier function, 6 we are still considering setting up the mouse model of neutrophilic nasal polyps, and treat the mouse with rottlerin and curcumin to check whether we could achieve the same phenotype we got in vitro, and this experiment will allow us to know better about the mechanism why rottlerin and curcumin could treat neutrophilic nasal polyps. Second, we set the exclusion criteria that patients with a history of FESS surgery should be excluded, actually, there is a population of recurrent CRSwNP patients who could barely benefit from steroid treatment, we think it is important to recruit this group of patients, and test the TOS and OSI, as well as the mRNA expression level of ALDH1A1, MGST1, SOD2.
CONCLUSION
In the present study, we found that the levels of TOS and OSI were significantly increased in CRSwNP patients, and OSI was positively associated with tissue neutrophilia and poor steroid response. Moreover, the mRNA levels of SOD2, ALDH1A1, and MGST1 were significantly lower in CRSwNP patients and showed comparable accuracy as predictors of poor steroid response. These findings raise the possibility that antioxidative stress agents could be used as an add‐on therapy of steroids to improve the clinical efficacy of poor‐steroid‐response CRSwNP patients.
AUTHOR CONTRIBUTIONS
Xian‐Ting Hu, Bai‐Wen Chen, Hua‐Bin Li, and De‐Hui Wang conceived the project. Xian‐Ting Hu, Bai‐Wen Chen, and Yu‐Jie Cao carried out the investigations. Hua‐Bin Li and De‐Hui Wang provided the resources. Xian‐Ting Hu and Chun Zhou carried out the formal analysis. Bai‐Wen Chen and Chun Zhou curated the data. Xian‐Ting Hu and Bai‐Wen Chen wrote the original draft of the paper. Hua‐Bin Li and De‐Hui Wang supervised, wrote, edited, and reviewed the paper.
CONFLICT OF INTEREST
Professor Hua‐Bin Li is a member of World Journal of Otorhinolaryngology – Head & Neck Surgery (WJOHNS) editorial board and is not involved in the peer review process of this article. The other authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the ethical committee of Eye and ENT Hospital, Fudan University, and written informed consent was obtained from all the subjects.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Grants of China (81725004, 81870703, 82271138) and Shanghai Science and Technology Committee Grants (19XD4010000, 20MC1920200).
Hu X‐T, Chen B‐W, Cao Y‐J, Zhou C, Li H‐B, Wang D‐H. Enhanced oxidative stress is associated with tissue neutrophilia and poor steroid response in chronic rhinosinusitis with nasal polyps. World J Otorhinolaryngol Head Neck Surg. 2023;9:320‐327. 10.1002/wjo2.91
Xian‐Ting Hu and Bai‐Wen Chen contributed equally to this study.
[Correction added on 24 August 2023, after first online publication: CONFLICT OF INTEREST section is updated.]
Contributor Information
Hua‐Bin Li, Email: allergyli@163.com.
De‐Hui Wang, Email: wangdehuient@sina.com.
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- 1. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1‐12. [DOI] [PubMed] [Google Scholar]
- 2. Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728‐732. [DOI] [PubMed] [Google Scholar]
- 3. Bachert C, Marple B, Hosemann W, Cavaliere C, Wen W, Zhang N. Endotypes of chronic rhinosinusitis with nasal polyps: pathology and possible therapeutic implications. J Allergy Clin Immunol Pract. 2020;8:1514‐1519. [DOI] [PubMed] [Google Scholar]
- 4. Wen W, Liu W, Zhang L, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129:1522‐1528. [DOI] [PubMed] [Google Scholar]
- 5. Yu Z, Wang Y, Hu X, et al. Overexpression of hypoxia‐inducible factor 1α is associated with neutrophilic inflammation in chronic rhinosinusitis with nasal polyps. Auris Nasus Larynx. 2020;47:401‐409. [DOI] [PubMed] [Google Scholar]
- 6. Ruan JW, Zhao JF, Li XL, et al. Characterizing the neutrophilic inflammation in chronic rhinosinusitis with nasal polyps. Front Cell Dev Biol. 2021;9:793073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim DK, Lim HS, Eun KM, et al. Subepithelial neutrophil infiltration as a predictor of the surgical outcome of chronic rhinosinusitis with nasal polyps. Rhinology. 2020;59:173‐180. [DOI] [PubMed] [Google Scholar]
- 8. Wu JQ, Kosten TR, Zhang XY. Free radicals, antioxidant defense systems, and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:200‐206. [DOI] [PubMed] [Google Scholar]
- 9. Strzalka‐Mrozik B, Prudlo L, Kimsa MW, et al. Quantitative analysis of SOD2, ALDH1A1 and MGST1 messenger ribonucleic acid in anterior lens epithelium of patients with pseudoexfoliation syndrome. Mol Vis. 2013;19:1341‐1349. [PMC free article] [PubMed] [Google Scholar]
- 10. Spector A. Review: oxidative stress and disease. J Ocul Pharmacol Ther. 2000;16:193‐201. [DOI] [PubMed] [Google Scholar]
- 11. Jeanson L, Kelly M, Coste A, et al. Oxidative stress induces unfolding protein response and inflammation in nasal polyposis. Allergy. 2012;67:403‐412. [DOI] [PubMed] [Google Scholar]
- 12. Bozkus F, San I, Ulas T, et al. Evaluation of total oxidative stress parameters in patients with nasal polyps. Acta Otorhinolaryngol Ital. 2013;33:248‐253. [PMC free article] [PubMed] [Google Scholar]
- 13. Topal O, Kulaksızoglu S, Erbek SS. Oxidative stress and nasal polyposis: does it affect the severity of the disease. Am J Rhinol Allergy. 2014;28:e1‐e4. [DOI] [PubMed] [Google Scholar]
- 14. Karatzanis A, Chatzidakis A, Milioni A, et al. Contemporary use of corticosteroids in rhinology. Curr Allergy Asthma Rep. 2017;17:11. [DOI] [PubMed] [Google Scholar]
- 15. Aversa S, Ondolo C, Abbadessa G, et al. Steroid resistance in nasal polyposis: role of glucocorticoid receptor and TGF‐beta1. Rhinology. 2012;50:427‐435. [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Li ZY, Jiang WX, et al. The activation and function of IL‐36γ in neutrophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141:1646‐1658. [DOI] [PubMed] [Google Scholar]
- 17. Lan F, Zhang L. Understanding the role of neutrophils in refractoriness of chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res. 2020;12:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milara J, Navarro A, Almudéver P, Lluch J, Morcillo EJ, Cortijo J. Oxidative stress‐induced glucocorticoid resistance is prevented by dual PDE3/PDE4 inhibition in human alveolar macrophages. Clin Exp Allergy. 2011;41:535‐546. [DOI] [PubMed] [Google Scholar]
- 19. Rasmussen CA, Kaufman PL. The trabecular meshwork in normal eyes and in exfoliation glaucoma. J Glaucoma. 2014;23:S15‐S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pawłowska‐Góral K, Kimsa‐Dudek M, Synowiec‐Wojtarowicz A, Orchel J, Glinka M, Gawron S. Effect of static magnetic fields and phloretin on antioxidant defense system of human fibroblasts. Environ Sci Pollut Res. 2016;23:14989‐14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


