Abstract
During a helminthological survey of snakes in the Cerrado Biome in Maranhão State, Brazil, we found intestinal nematodes in Leptodeira annulata (Linnaeus), belonging to the genus Oxyascaris Travassos, 1920. We observed that the specimens found are distinct from their congeners by the combination of presented characters, mainly the cuticular expansion at the anterior region of the body, presence of a single papilla at the anterior cloacal lip, number, and arrangement of caudal papillae, presence of somatic papillae along body cuticle, as well as some morphometric characters. Thus, we describe the new species using light and scanning electron microscopy and, revise the morphological characters used to identify Oxyascaris spp. and propose a key to the species of the genus. Therefore, we describe the seventh species in the genus, the second reported to parasitize snakes, the sixth species recorded in Brazil, and the first described in the Cerrado Biome.
Keywords: Snake nematode, Cerrado biome, Oxyascaris
Graphical abstract
Highlights
-
•
A new species of Oxyascaris from Brazil.
-
•
The seventh species of Oxyascaris.
-
•
Key to species of Oxyascaris.
1. Introduction
Leptodeira annulata (Linnaeus), the cat-eyed snake, is widely distributed throughout Brazil and found in all Brazilian biomes (Costa and Bérnils, 2018). Studies regarding this species have been focusing on foraging, diet, and reproduction, with a small number of studies addressing its helminth fauna (Sprent, 1988; Mesquita et al., 2013; Silva-Neta et al., 2015; Carvalho et al., 2018; Ferreira-Silva et al., 2022).
Nematodes parasites of Leptodeira annulata are poorly known; however, some studies have already reported nematodes parasitizing this host, such as Hexametra boddaertii Baird 1860, Ophidascaris trichuriformis Vaz 1935, Raillietnema spectans Gomes 1964, Oswaldocruzia sp., Oxyascaris sp., Physaloptera sp., and Cosmocerca podicipinus Baker and Vaucher (1985); Sprent (1988); Carvalho et al. (2018); Ferreira-Silva et al. (2022).
Oxyascaris Travassos, 1920 is a genus of nematodes of the family Cosmocercidae Travassos, 1925 found parasitizing amphibians and reptiles (Baker and Vaucher, 1985; Vicente et al., 1993). Currently, there are six species described for this genus: Oxyascaris caatingae Felix-Nascimento, Vieira, Muniz-Pereira, Moura, Ribeiro, Oliveira, 2020), Oxyascaris caudacutus (Freitas, 1958; Baker and Vaucher 1985), Oxyascaris oxyascaris Travassos, 1920 , Oxyascaris similis (Travassos, 1920), Oxyascaris longum (Alcantara and Silva, 2021) = Aplectana longa Alcantara and Silva 2021 and Oxyascaris mcdiarmidi Bursey and Goldberg, 2007. All known species are distributed across the Neotropical region, in both South and Central America, reported from amphibians and snakes (Freitas, 1958; Bursey and Goldberg, 2007; Felix-Nascimento et al., 2020; Alcantara et al., 2021; Santos et al., 2023).
During a helminthological survey of snakes in the Cerrado Biome in Northeast Brazil, we found intestinal nematodes in Leptodeira annulata, which we assigned to the genus Oxyascaris and the species found herein are morphologically distinct from its congeners. Therefore, we describe these nematodes based on morphological analyses under light and scanning electron microscopy, and we propose a key to the species of Oxyascaris.
2. Materials and methods
Two specimens of L. annulata were manually captured through an active/visual search during a survey of the parasitic fauna of anurans and reptiles at Riachão municipality, Maranhão state, Brazil. After collection, the host specimens were anesthetized with an injection of 2% lidocaine hydrochloride, measured, weighed, and necropsied for endoparasites. The internal organs were removed, dissected, and analyzed under a Leica EZ4 stereomicroscope (Leica Microsystems, Wetzlar, Germany). The nematodes found in the small intestine were collected, rinsed in saline solution, killed in heated saline solution, and preserved in microtubes with 70% ethanol.
We cleared the nematodes with Amann's lactophenol for morphological and morphometric analyses mounted on temporary slides. The slides were observed with an Olympus BX41 microscope (Olympus, Tokyo, Japan) attached to a drawing tube and an Olympus BX53 (Olympus, Tokyo, Japan) equipped with an image capture system to obtain morphological measurements and images using the software cellSens (Olympus). All measurements through the text are given in micrometers and presented as the holotype/allotype, followed by the range and mean of the whole type series in parenthesis. The type material was deposited in the Collection of Other Invertebrates of the Museu Paraense Emílio Goeldi (MPEG), Belém, Pará state, Brazil.
For Scanning electron microscopy analyses, some specimens were post-fixed in 1% OsO4 (Osmium Tetroxide), dehydrated in an increasing ethanol series, and dried in a CO2 critical point dryer. Subsequently, the helminths were mounted on aluminum supports, coated with a thin layer of gold/palladium, and analyzed in a Tescan VEGA3 (Tescan, Brno, Czech Republic) scanning electron microscope at the Laboratory of Structural Biology, Biological Sciences Institute, Federal University of Pará (Universidade Federal do Pará — UFPA).
Cosmocercidae Travassos, 1925
Genus Oxyascaris Travassos, 1920
Oxyascaris annulatum n. sp. Santos, Melo & Fernandes, 2023
Type-host: Leptodeira annulata Linnaeus, 1758
Site in host: small intestine
Type-locality: Riachão, Maranhão (7°30′33.8″ S 46°23′02.9″W)
Type-material: The holotype (MPEG285) and paratypes (MPEG 288), the allotype (MPEG 286) and paratypes (MPEG 287) are deposited in the Museu Paraense Emílio Goeldi (MPEG) Belém, Pará, Brazil.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of
the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Oxyascaris annulatum is urn:lsid:zoobank.org:pub:3FC55064-3729-43D0-8D9A-A95B7D9665B7.
Etymology: The name of the species is given in reference to the type host Leptodeira annulata.
Fig. 1.
Line drawings of Oxyascaris annulatum n. sp. A – Male, whole view; B – Male, anterior end, lateral view; C – Male, ventral view of tail; D – Male, spicules; E − Female, anterior end, lateral view; F – Female, reproductive system; G – Female, tail, lateral view; H – Female, detail of uterine dilation; I – Larvated egg. Scale bars – A, F, H: 350 μm; B, E: 250 μm; C, G: 150 μm; D, I: 50 μm.
Fig. 2.
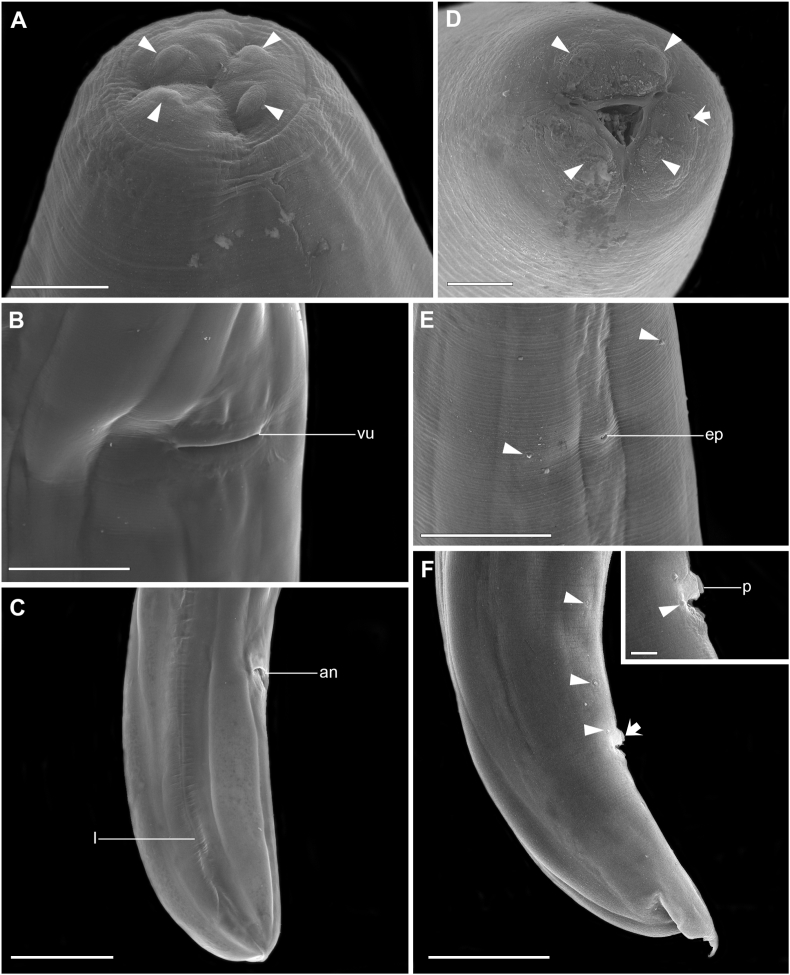
Scanning Electron Microscopy of O. annulatum n. sp. A – Female, anterior end, detailing cephalic papillae (arrowhead), apical view; B – Female, vulva (vu), ventrolateral view; C – Female, posterior end, anus (an) and lateral line (l); D – Male, anterior end apical view, cephalic papillae (arrowhead) and amphid (arrow); E − Male, detail of excretory pore (ep) and somatic papillae (arrowhead); F – Male, posterior end, precloacal papillae (arrowhead), unpaired papilla (arrow), insert – adcloacal papillae (arrowhead), and detail of unpaired papilla (p). Scale bars – A: 20 μm; B, F: 100 μm; C: 200 μm; D: 10 μm; E: 50 μm; insert: 20 μm.
2.1. General
Evident sexual dimorphism, mature females four times larger than males in total length. Females presenting a vesicle at anterior region of body, with a wider portion beginning at cephalic region, extending to nerve ring, narrowing towards esophageal bulb (Fig. 1E). Oral opening at cephalic end, triangular in shape, with three lips; dorsal lip with a pair of papillae; lateroventral lips with one papilla and one amphid each (Fig. 1, Fig. 2A, D). Esophagus divided into pharynx, corpus, isthmus and bulb (Fig. 1B, E). Nerve ring at posterior third of esophagus in males and at middle of esophagus in females (Fig. 1B, E). Excretory pore post-bulbar or at the bulb level in males and pre-bulbar in females (Fig. 1, Fig. 2E). Numerous somatic papillae present at cuticular surface in both sexes, randomly distributed (Fig. 2E). Discrete lateral line in females (Fig. 2C), absent in males. Tail conical, robust, tapering abruptly to a short filament in both sexes (Fig. 1, Fig. 2C, F).
2.2. Males (based on the holotype and 6 paratypes)
Total body length 5.5 mm (3.8–5.7 mm; 5.08) mm, body width at level of esophagus-intestinal junction 179 (116–202; 168). Esophagus 434 (387–547; 463) long; pharynx 42 (37–55; 45) × 47 (37–47; 42), corpus 324 (314–418; 361) × 53 (53–79; 59); isthmus 47 (32–79; 50) × 42 (36–47; 40), bulb 66 (66–84; 75) × 68 (58–76; 72) respectively. Nerve ring at 253 (166–284; 242) and excretory pore 550 (390–518; 471) from anterior end, respectively. Caudal papillae slightly larger in size than somatic papillae, 17 in total (3 + 1:1:3): three pairs of ventral precloacal papillae; one large unpaired medial precloacal papilla at anterior border of cloacal aperture (Fig. 2G); one pair of ventral adcloacal papillae (Fig. 2G); three pairs of postcloacal papillae (the first postcloacal pair ventral, the second lateroventral, and the third pair ventral) (Fig. 1, Fig. 2F, G). Two small lateral phasmids located at tail tip. Spicules curved distally, right spicule 149 (84–174; 137) and left spicule 184 (120–180; 152) long (Fig. 1D). Gubernaculum absent. Tail 287 (234–334) long (Fig. 1, Fig. 2F).
2.3. Female (based on allotype and 4 paratypes)
Total body length 22 mm (18–24; 21), body width at level of esophagus-intestinal junction 295 (213–350; 272). Esophagus 668 (739–929; 802) long; pharynx 68 (53–74; 62) × 74 (58–74; 64); corpus 666 (571–724; 637) × 129 (100–132; 124); isthmus 58 (26–108; 64) × 73 (63–73; 70); bulb 124 (92–105; 99) × 118 (108–139; 120). Nerve ring at 347 (276–418; 342) and excretory pore at 634 (516–671; 579) from anterior end, respectively. Vulva pre-equatorial, lips not protruded (Fig. 2B), located at 8 mm (6.4–8.2, corresponding 34–35% of the total body length; 7.3) from anterior end. Didelphic and amphidelphic genital apparatus. Vagina muscular, directed anteriorly, flexed to posterior region throughout most of length, giving rise to one uterine branche directed anteriorly and one branch directed posteriorly. Uterine branches associated with an ovary each. Anterior and posterior region of uterus branch with a dilated portion, flexed posteriorly, usually filled with spermatozoa (Fig. 1F, H). Body width at the level of vulva 547 (500–639; 576). Eggs oval, often larvated 82 (82–103) long and 66 (65–68) wide (Fig. 1I). Tail 1 mm (0.974–1.1 mm; 480) long (Fig. 1F; 2C).
3. Remarks
We assigned the nematodes presented in this study to the Cosmocercidae by the evident sexual dimorphism compared to other cosmocercid genera, primarily the total length of females (mature females more than twice as large as males). Additionally, the nematodes have three distinct lips, females didelphic amphidelphic, uteri with one branch directed anteriorly and one posteriorly filled with numerous small eggs; tail conical robust, males without pre-anal pseudosucker. According to Travassos (1920), Freitas (1958), and Bursey and Goldberg (2007), those morphological traits can be attributed to species of the genus Oxyascaris.
The species of this genus are distinguished based on a combination of the following morphological characters: presence or absence of unpaired medial papillae located at the anterior cloacal lip, the number and distribution of caudal papillae, the presence or absence of lateral body alae in males and females, presence or absence of gubernaculum, length of spicules in males, and position of the vulva relative to the anterior end of the body in females (Freitas 1958; Baker and Vaucher 1985; Bursey and Goldberg 2007).
Oxyascaris comprises six known species: Oxyascaris caatingae, O. caudacutus, O. oxyascaris, O. similis, O. longum and O. mcdiarmidi). The new species is distinguished from O. mcdiarmidi by the absence of the gubernaculum (present in O. mcdiarmidi) and the presence of an unpaired papilla on the upper lip of the cloaca (absent in O. mcdiarmidi). The new species is similar to O. oxyascaris by lacking gubernaculum, but differs by the presence of the unpaired papilla on the upper lip of the cloaca (Freitas, 1958; Bursey and Goldberg, 2007; Felix-Nascimento et al., 2020; Alcantara et al., 2021; Santos et al., 2023).
The new species shares the presence of an unpaired papilla at the anterior cloacal lip with O. caatingae, O. caudacutus, and O. similis. However, Oxyascaris annulatum n. sp. differs from O. caatingae by the total number of caudal papillae pairs (7 pairs + 1 unpaired vs 15 pairs + 1, respectively), as well as in the distribution of papillae (3:1:3 vs 5:1:9 in O. caatingae, papillae distribution formula pre-: ad-: post-cloacal). Additionally, the males of the new species lack gubernaculum, while it is present in males of O. caatingae (Felix-Nascimento et al., 2020).
The males of Oxyascaris annulatum n. sp. and O. caudacutus do not have gubernaculum (based on Freitas, 1958). However, they differ in the total number of papillae pairs (7 pairs + 1 vs 13 pairs + 1 in O. caudacutus), and the distribution of papillae (3:1:3 vs 6:0:7 in O. caudacutus) and also by females with a discreet lateral line in the new species (wide lateral alae in O. caudacutus). Additionally, the new species is larger than O. caudacutus (males: 3.8–5.7 mm and females: 18–24 mm vs males: 2.78–3.62 mm and females: 5.63–16.21 mm, respectively). In both species, the vulva is located in the pre-equatorial region of the body. Still, they differ in the distance of the vulva from the anterior region (6.4–8.2 mm, corresponding 34–35% of the total body in the new species vs 2.01–5.33, corresponding 25–32% of the whole body in O. caudacutus) (Freitas, 1958).
The new species resembles O. similis by the absence of the Gubernaculum, but they can be easily distinguished by a discrete lateral line in females of O. annulatum n. sp., while in O. similis the lateral alae is present in both sexes, and they differ by the number and distribution of caudal papillae pairs (7 + 1/3:1:3 in the new species vs 13 + 1/6:0:7 in O. similis). The position of the vulva differs in both species (6.4–8.2 mm, corresponding 34–35% of the total body in the new species vs 3.52–14.07 mm, corresponding 43–51% of the whole body in O. similis), as well as the position of the nerve ring in males and females (males: 166–284 and females: 276–418 in Oxyascaris n. sp. vs males: 270–460 and females: 310–700 in O. similis) (Freitas, 1958).
Oxyascaris longum was originally described as Aplectana longa, and in a recent study conducted by Santos et al. (2023) (in press), the species was reallocated into the genus Oxyascaris. Therefore, the new species differs from O. longum by the absence of a gubernaculum (present in O. longum), the presence of discrete lateral alae (lateral line) in O. annulatum n. sp. (absent in O. longum), and the pattern of caudal papillae, which in the new species is 3:1:3 + 1, whereas, in O. longum, it is 9:0:6 + 1. Additionally, O. annulatum n. sp. has a pre-equatorial vulva; in O. longum, the vulva is equatorial (Alcantara et al., 2021).
4. Discussion
The morphological identification and classification of species of Oxyascaris is challenging and has been a subject of discussion since the proposition of the genus to allocate Oxyascaris oxyascaris (Travassos, 1920) The genus was initially assigned within Oxyascarididae, then reclassified as Kathlanidae Lane, 1914, and finally placed within Cosmocercidae as proposed by Baker and Vaucher (1985). Freitas (1958) and Baker and Vaucher (1985) also debated the validity of the genus because the species morphology resembles the species of Aplectana Railliet and Henry, 1916.
Freitas (1958) proposed that species of Oxyascaris have a reduced esophageal bulb compared to other Cosmocercinae species. However, according to Baker and Vaucher (1985), the bulb of O. caudacutus is proportionally larger than in other species of Cosmocercinae. We recently observed that species of Aplectana can also be misidentified as Oxyascaris (see the recent reallocation of Aplectana longa to Oxyascaris longum) and they have a more dilated esophageal bulb than Oxyascaris (see Santos et al., 2023). Therefore, based on the recently described species (Oxyascaris caatingae, O. mcdiarmidi) and the original descriptions by Freitas (1958), we propose that the esophageal bulb is reduced in Oxyascaris spp. We also observed that females of Oxyascaris have a conical and robust tail, usually tapering abruptly to a short filament, while Aplectana has a long caudal filament. Thus, we add to the generic diagnosis of Oxyascaris the morphological traits of a reduced esophageal bulb and the morphology of the tail.
Additionally, we highlight that the female specimens of Oxyascaris annulatum n. sp. have a dilation by the end of each uterine branch, similar to a spermatheca. We cannot state that this is a generic character, as it was first observed in our specimens and was not represented in line drawings or reported in descriptions of previously known species. However, the authors should be cautious and try to observe this morphological character in future species descriptions and identifications. Thus, we also propose a key for species identification.
The species of the genus Oxyascaris are distributed in Argentina, Brazil, Costa Rica, Central America, and Paraguay (Baker and Vaucher 1985; Bursey and Goldberg 2007; Goldberg and Bursey 2008). Among the six previously described species, five were recorded in Brazil (O. caatinga, O. caudacutus, O. longum, O. oxyascaris, and O. similis), five were found parasitizing amphibians (O. caatingae, O. caudacutus, O. longum, O. mcdiarmidi, and O. similis) and only one was reported in snakes (O. oxyascaris) (Felix-Nascimento et al., 2020). Thus, O. annulatum n. sp. represents the seventh species in the genus, the second reported parasitizing snake host, the sixth species recorded in Brazil, and the first described in the Cerrado Biome.
Therefore, considering the great diversity of potential host species and the comparatively small number of Oxyascaris species described so far, the diversity within this genus might still be underestimated, and new species are awaiting to be discovered.
Key to the species of Oxyascaris (Cosmocercoidea)
| 1 - Gubernaculum present | 2 |
| Gubernaculum absent | 4 |
| 2 - Males with an unpaired papillae at anterior cloacal lip | 3 |
| Unpaired papillae at anterior cloacal lip absent, male caudal papilla pattern distribution 5:0:6; spicules 612–689 μm in length, vulva post equatorial, cephalic vesicle present | O. mcdiarmidi |
| 3 - Cephalic vesicle absent, vulva pre equatorial, male caudal papilla pattern distribution 5:1:9 + 1; spicules 95–109 μm in length | O. caatingae |
| Vulva equatorial; male caudal papilla pattern distribution 9:0:6 + 1; spicules 232–258 μm in length | O. longum |
| 4 - Males with an unpaired papillae at the anterior cloacal lip | 5 |
| Unpaired papillae at anterior cloacal lip absent, cephalic vesicle present; lateral alae absent, male caudal papilla pattern distribution 4:0:3; spicules 150–206 μm in total length | O. oxyascaris |
| 5 - Males with 13 pairs of caudal papillae | 6 |
| Males with 7 pairs of caudal papillae, caudal papillae pattern distribution 3:1:3 + 1, discreet lateral line only in females; spicules subequal, measuring right spicule 84–174 μm and left spicule 120–180 μm long | O. annulatum n. sp. |
| 6 - Lateral alae well developed, excretory pore close to isthmus-bulb region; male caudal papillae pattern distribution 6:0:7 + 1; spicules 122–134 μm in length; esophagus total length < 630 μm in females | O. caudacutus |
| Caudal papillae pattern distribution 6:0:7 + 1; spicules 160–193 μm in length; excretory pore in the middle portion of esophagus; esophagus total length ≥660 μm in females | O. similis |
Authors contributions
A. N. Santos wrote the main draft and prepared images. Y. Willkens helped with specimen observations and SEM analysis, reviewing and writing the manuscript. T. F. Fernandes, L. F. N. Silva writing the manuscript. F.T.V Melo wrote the manuscript, revised and prepared the line drawings. All authors reviewed the manuscript.
Funding
This study was supported by Coordination for the Improvement of High Higher Education Personnel (CAPES)/Postgraduate Program in the Biology of Infectious and Parasitic Agents (PPGBAIP)/UFPA, Research productivity scholarship of CNPq to F.T.V. Melo (Process number 314116/2021-4). Amazon Foundation for Research and Studies Support (FAPESPA), Programa de Apoio a Nucleos Emergentes (FAPESPA/CNPq PRONEM 01/2021, process number 51/2021. This study is part of the Ph.D. thesis of Ana Nunes dos Santos in PPGBAIP (Institute of Biological Sciences-UFPa).
Code availability
Not applicable.
Ethics approval
All procedures contributing to this work comply with all applicable institutional, national, and international guidelines for animal care and use Animal Research Ethics Committee, Universidade Estadual da região Tocantina do Maranhão (Uemasul), under license N 001/2021. The present study was approved by Chico Mendes Institute for Biodiversity Conservation (ICMBio), Brazil, and host specimens were collected under license number SISBIO: No 48102-2.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
We are grateful to Dra. Edilene Oliveira da Silva for his technical support with SEM analyses from the Laboratory of Cellular Structural Biology at the Federal University of Pará, Belém, for her technical support with the SEM analyses. We grateful to Dr. Marcelo Silva and Jeannie N. dos Santos for their contribution to identifying the specimens.
References
- Alcantara E.P., Ferreira-Silva C., Forti L.R., Morais D.H., Silva R.J. A new species of Aplectana (Nematoda: Cosmocercidae) in the marsupial frog Gastrotheca microdiscos (Amphibia: Hemiphractidae) from Brazil. Zootaxa. 2021;4908(3):426–434. doi: 10.11646/zootaxa.4908.3.7. [DOI] [PubMed] [Google Scholar]
- Baker M.R., Vaucher C. Parasitic helminths from Paraguay VII: systematic position of oxyascaris Travassos, 1920 (Nematoda: Cosmocercoidea) Rev. Suisse Zool. 1985;92:303–310. doi: 10.5962/bhl.part.81619. [DOI] [Google Scholar]
- Bursey C.R., Goldberg S.R. A new species of Oxyascaris (Nematoda, Cosmocercidae) in the Costa Rica brook frog, Duellmanohyla uranochroa (Anura, Hylidae) Acta Parasitol. 2007;52:58–61. doi: 10.2478/s11686-007-0007-2. [DOI] [Google Scholar]
- Carvalho E.F.F., Silva-Neta A.F.D., Silva C.S., Oliveira C.R., Nunes J.D.C.X., Souza T.G., Ávila R.W. Helminths infecting the cat-eyed snake Leptodeira annulata Linnaeus 1758 (Squamata: Dipsadidae) in a semiarid region of Brazil. Helminthologia. 2018;55(4):281–285. doi: 10.2478/helm-2018-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa H.C., Bérnils R.S. Répteis do Brasil e suas Unidades Federativas: Lista de espécies. Herpetol. Brasileira. 2018;7(1):11–57. [Google Scholar]
- Felix-Nascimento G., Vieira F., Muniz-Pereira L., Moura G., Ribeiro L., Oliveira J. Two new species of Cosmocercidae (Nematoda: Cosmocercoidea) of Leptodactylus macrosternum miranda-ribeiro (Anura: Leptodactylidae) from caatinga biome, Brazil. Zootaxa. 2020;4877(2):274–290. doi: 10.11646/zootaxa.4877.2.3. [DOI] [PubMed] [Google Scholar]
- Ferreira-Silva C., Alcantara E.P., Ávila R.W., Silva R.J. First record of Cosmocerca podicipinus (Nematoda: Cosmocercidae) parasitizing Leptodeira annulata (serpentes: Dipsadidae) in northeastern Brazil. Braz. J. Biol. 2022;82:1–4. [Google Scholar]
- Freitas J.F.T. Estudos sôbre “Oxyascarididae” (Travassos, 1920) (Nematoda, Subuluroidea) Mem. Inst. Oswaldo Cruz. 1958;56:489–515. doi: 10.1590/s0074-02761958000200008. [DOI] [PubMed] [Google Scholar]
- Goldberg S.R., Bursey C.R. Helminths from 10 species of Brachycephalid frogs (Anura, Brachycephalidae) from Costa Rica. Comp. Parasitol. 2008;75:255–262. doi: 10.1654/4327.1. [DOI] [Google Scholar]
- ICZN International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa. 2012;3450:1–7. doi: 10.3897/zookeys.219.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita P.C.M.D., Passos D.C., Borges-Nojosa D., Cechin Z. Ecologia e história natural das serpentes de uma área de Caatinga no nordeste brasileiro [Ecology and natural history of snakes from an area of caatinga in northestean Brazil] Papéis Avulsos Zool. (São Paulo) 2013;53(8):99–113. doi: 10.1590/S0031-10492013000800001. (In Portuguese) [DOI] [Google Scholar]
- Santos A.N., Borges E.S., Wilkens Y., Santos J.N., Costa-Campos C.E., Melo F.T.V. A New Species of Aplectana Railliet & Henry, 1916 (Nematoda: Cosmocercidae) Parasite of the Boana boans (Anura: Hylidae) (Linnaeus, 1758) from Brazilian Amazon. Braz J Vet Parasitol. 2023;32(4) doi: 10.1590/S1984-29612023074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Neta A.F., Claudio M.S., Ávila R.W. Leptodeira annulata (banded cat- eyed) Herpetol. Rev. 2015;46(3) [Google Scholar]
- Sprent J.F.A. Ascaridoid nematodes of amphibians and reptiles: Ophidascaris Baylis, 1920. Syst. Parasitol. 1988;11:165–213. [Google Scholar]
- Travassos L. Contribuiçôes para o conhecimento da fauna helmintologica brasileira. Archos Escola superior. Agronomia Medicina veteterinária Nictheroy. 1920;4:17–20. [Google Scholar]
- Vicente J.J., Rodrigues H.O., Gomes D.C., Pinto R.M. Nematóides do Brasil. Parte III: nematóides de répteis. Rev. Bras. Zool. 1993;10(1):19–168. doi: 10.1590/S0101-81751993000100003. [DOI] [Google Scholar]