Abstract
Objectives:
Osteoarthritis (OA) has been the common cause to lead to chronic pain. Transcranial direct current stimulation (tDCS) is effective in the treatment of chronic pain, but its analgesic mechanism is still unclear. This study observed the analgesic effects of tDCS in rats to explore the top-down analgesic modulation mechanism of tDCS.
Methods:
Monosodium iodoacetate (MIA) was used to establish OA chronic pain model. After 21 days, the rats received tDCS for 14 consecutive days (20 min/day). We assessed the pain-related behaviors of rats at different time points. Western blot and Immunohistochemistry were performed to observe the expression level of NMDAR2B in the spinal cord after tDCS treatment.
Results:
After MIA injection, rats developed apparent mechanical hyperalgesia and thermal hyperalgesia. However, the pain-related behaviors of rats were significantly improved after tDCS treatment. In addition, the expression of NMDAR2B and the proportion of positive stained cells of NMDAR2B were reversed by tDCS treatment.
Conclusions:
The results demonstrated that tDCS can attenuate OA-induced chronic pain in rats via reducing NMDAR2B expressions in the spinal cord. We believe that this may be the result of tDCS participating in the top-down modulation of pain pathway in the endogenous analgesic system.
Keywords: Chronic Pain, NMDAR2B Receptors, Osteoarthritis, tDCS
Introduction
Osteoarthritis (OA) is the most common chronic degenerative joint disease[1]. Pain is the main symptom of OA, as well as the most relevant cause of disability and poor quality of life in the affected patients[2]. Currently, medical treatment often provided a limited improvement with processive pain and may also lead to lots of adverse effects over time[3,4]. The joint replacement operation is considered to be an effective surgical intervention to solve OA pain, but studies have found that 20% of patients still have pain after joint replacement[5,6].
The mechanism of OA pain is complex, involving pain sensitization and the change in endogenous analgesic system[7,8]. Studies showed there are several pathways from prefrontal areas and the ACC to the periaqueductal gray area (PAG), which may serve as a framework for tuning somatosensory information at the spinal cord level[9,10]. The periaqueductal gray (PAG)-rostral ventromedial medulla (RVM)-spinal dorsal horn (DH) pathway was considered to be the key in endogenous analgesic system[11]. The PAG receives the nociceptive information from the spinal cord DH, and it projects antinociceptive transmission to the RVM and lower brainstem, then the RVM projects the information to the spinal cord DH[12,13]. L4-L6 spinal cord is the intumescentia lumbalis of the spinal cord in rats, which contains a large number of nerve cells and fibers, and is also a key part of the endogenous analgesic system. Previous research has found N-methyl-D-aspartate (NMDA) receptors may contribute to the development of pain behavior[14]. Moreover, in four NMDA receptors, the NR2B-containing NMDA receptor played an essential role in pain regulation, and it was considered one of the best potential targets of pain[15-17].
Transcranial direct current stimulation (tDCS) is a non-invasive neuromodulatory technique that has been used to treat chronic disorders[18]. TDCS regulates neuronal excitability by applying direct current to the scalp using two electrodes. Anodal stimulation enhances the excitability and cathodal stimulation decreases the excitability[19]. Some studies have found that tDCS may modulate pain pathway through endogenous analgesic system[20,21]. Studies have indicated that electrical stimulation in the cerebral cortex is able to modulate remote areas of the neuroaxis, such as the brainstem and the spinal cord[22]. This exogenous stimulus may use similar pathways involved in the top-down modulation found across sensory systems[23]. In our previous study, we demonstrated that tDCS can alleviate OA-induced chronic pain in rats by modulating the expression of NMDA receptors in PAG to play an analgesic role[24]. However, whether the analgesic effect of tDCS can work in the spinal cord is still unclear.
In this study, we aim to explore the mechanism of tDCS by the test of the pain-related behaviors and the expression level of NMDAR2B in the spinal cord, which will provide a better understanding and clinical application of tDCS.
Materials and Methods
Animals
Sprague–Dawley rats (n=40, weight 200 ± 20 g) were provided by the Experimental Animal Center. All the rats were housed in animal facilities with sufficiently controlled temperature (24 ± 1°C) and humidity (50–60%) under a 12/12-h light/ dark cycle and had access to chow and water. Excluding rats that died unexpectedly and failed to establish models, only a portion of the rats were included in the experiment.
Experimental design
There is a habituation period of one week for rats. After the adaptation period, the rats were randomly divided into four groups: Sham group, MIA group, MIA+tDCS group and MIA+StDCS group. Except for the Sham group, the rats in other three groups were injected with MIA into the articular cavity. Then the tDCS and StDCS sessions were applied for 14 days (20 min/day) after 21 days of MIA injection (Figure 1).
Figure 1.
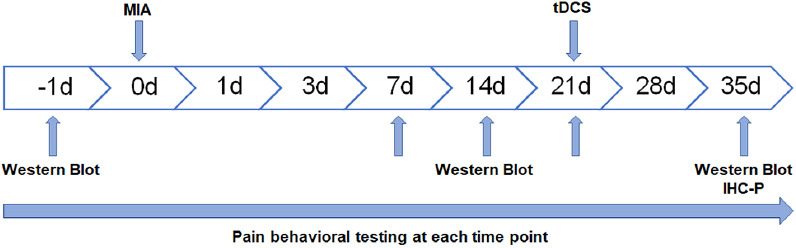
Experimental design (MIA: Monosodium Iodoacetate tDCS: transcranial direct current stimulation IHC-P: Immunohistochemistry-Paraffin sections).
Animal model
OA chronic pain model was induced after the rats were light anesthetized with 5% isoflurane in O2, then rats were injected with 60 µl 80 mg/ml monosodium iodoacetate (MIA, Sigma, USA) into the left ankle joint cavity. As normal control group, the rats in Sham group were injected with saline into the identical parts.
Transcranial direct current stimulation (tDCS)
After 21 days of MIA injection, the rats in MIA+tDCS and MIA+StDCS groups were subjected to a constant direct current of 0.5 mA (20 minutes/day) for 14 consecutive days[25]. We used bandages to bind rats’ limbs to prevent them from moving after the rats were transiently anesthetized by 5% isoflurane in O2. In order to fit the rats’ heads, we reduced the size of the electrodes to 1.5 cm2 and maintained the stimulation parameters at 0.33 mA/cm2 with no lesions for the brain. In addition, the rats’ heads were shaved for better adherence before application. The anodal electrode was placed between the ears, on the neck of the rat (parietal cortex) and the cathodal electrode was positioned at the midpoint between the lateral angles of both eyes (supraorbital area). After being positioned, the electrodes were fixed onto the head using adhesive tape. Sham-stimulated (StDCS) rats underwent similar procedures, but the stimulator was turned off throughout the experiments.
Behavioral testing
Mechanical allodynia
In order to assess mechanical allodynia, the Von Frey hair (North Coast, USA) based on the up-down method has been adopted[26]. Behavioral test was performed in a blinded manner, the observer was not clear about the grouping in advance. Before testing, rats were placed individually on a suspended wire cage with a mesh-bottom and allowed to acclimate for 10 min. A series of Von Frey filaments, with calibrated bending force ranging from 0.16 to 26 g, were then applied perpendicularly to the plantar surface of the left hind paw. The tests invariably began with 2 g, and each hair was applied 5 times with an interval of 5 minutes between the two stimuli. For the mechanical stimulation, retreat or paw licking after stimulation is considered a positive reaction. If the paw is not retracted, the next stronger stimulus is applied. Instead, a weaker stimulus was chosen. If we observed positive responses from a particular hair 3 out of the five consecutive applications, the value of a particular hair in that gram was considered to be the paw withdrawal threshold (PWT). According to the up-down method, the 50% response threshold was interpolated using the formula: 50% g threshold = (10[Xf+K&])/10,000. Ultimately, the measurements were averaged in each group.
Thermal hyperalgesia
All rats were exposed to a hot plate (HP) for 5 min to adapt to the hot plate 24 h before testing. On the test day, the temperature of the hot plate was maintained at 55 ± 1 °C. The rats were placed in glass funnels on the heated surface, and the time in seconds for quickly pulling, licking, or contracting its extremities was recorded as the paw withdrawal latency (PWL).
Western blot analysis
Rats (n=3/group) were deeply anesthetized and sacrificed. The L4-L6 spinal cord tissues were quickly removed and stored at -80°C. These tissues were homogenized in a mixture of RIPA lysis buffer containing proteinase inhibitor, and centrifuged at 4°C at 12,000 rpm for 15 min in order to collect the supernatants. Protein content was quantified using a BCA protein assay kit (Solarbio, China). After that, each sample, containing 20 µg protein, was loaded into 10% SDS-polyacrylamide gels for electrophoresis, then transferred to a polyvinylidene difluoride (PVDF) membrane (Sigma, USA). The membranes were blocked with 5% non-fat dry milk in TBST at room temperature for 2 hours, and incubated with the following primary antibodies at 4°C overnight: NMDAR2B (diluted 1:5000, Abcam) or GAPDH (diluted 1:5000, Abways). Next, the membranes were incubated with HRP-labeled goat anti-rabbit IgG secondary antibody (diluted 1:5000, Bioss) for 2 hours after washing with TBST. Each membrane was washed three times with TBST and visualized using an enhanced chemiluminescence ECL reagent (Millipore, USA). Images were analyzed by Image J software.
Immunohistochemistry analysis
The rats in four groups (n=4/group) were deeply anesthetized and then perfused transcardially with cold saline followed by 4% cold paraformaldehyde (pH 7.4). Subsequently, the L4-L6 spinal cord tissues were quickly removed and placed in the perfusion fixative (4°C) for 24 h. Paraffin-embedded sections of the spinal cord were cut into 5-µm-thick sections and treated with 0.3% Triton X-100 and 3% H2O2 in PBS for 1 h, and processed for 2 h in 5% normal goat serum, then incubated overnight with primary antibodies at 4°C at the following dilutions: anti-NR2B (diluted 1:1000, Abcam). This was followed by incubation with secondary antibodies (HRP-conjugated goat anti-rabbit IgG, diluted 1:1000, Abcam) for 1 h after washing with PBS and subsequently reacted with DAB for color development. Next, these sections were redyed with hematoxylin after flushing with running water for 30 minutes, then dehydrated through a series of ethanol solutions, cleared in xylene. Finally, images were obtained on a confocal Olympus Fluoview IX73 microscope.
Statistical analysis
The sample size of rats was determined using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). A power of 80% and significance of 0.05 was considered acceptable for the power calculation. All results were presented as Mean ± SEM and analyzed by GraphPad Prism 8.0 software (GraphPad Software, CA, USA). One-way ANOVA were used for analyzing the differences between the groups for the Western blot and Immunohistochemistry staining. Two-way repeated measures of ANOVA (two-way RMANOVA) was used to test the differences in pain thresholds. For all comparisons P<0.05 was considered significant.
Results
PWT and PWL were significantly decreased after MIA injection in rats
Before MIA injection, there was no significant difference in PWT and PWL between the four groups. The tests of pain-related behaviors after MIA injection showed that compared with the Sham group, the PWT (F7,80=131.9, P<0.0001) and PWL (F7,80=17.59, P<0.0001) of MIA-induced OA rats were significantly decreased in the whole process (Figure 2A and 2B). These results indicated that MIA may induce mechanical allodynia and thermal hyperalgesia in rats.
Figure 2.
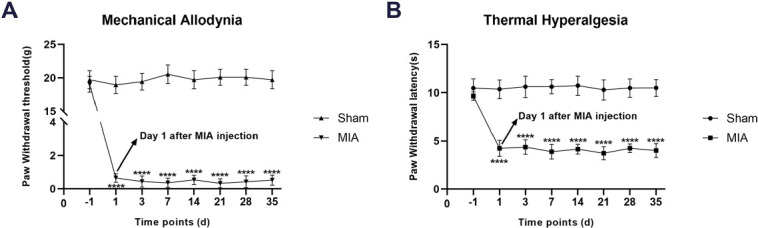
A-B: Effects of tDCS on mechanical allodynia (presented by PWT) and thermal hyperalgesia (presented by PWL) in MIA-induced chronic pain rats were shown in the figure. Compared with the Sham group, there were significant decreases on PWT and PWL after MIA injection. Data were presented as the mean ± SEM, (n=6/group). ****P<0.0001 represented comparison of MIA with Sham group.
The tDCS treatment improved the pain-related behaviors
21 days after MIA injection, the rats received tDCS or StDCS treatment. The analysis of behavioral testing showed that compared with the MIA group, the PWT (F14,120=1.862, P<0.05) in MIA+tDCS group dramatically decreased on 14 days after tDCS treatment. Similarly, compared with the MIA group, the PWL (F14,120=2.553, P<0.01) in MIA+tDCS group was obviously reversed by tDCS treatment. Moreover, there was no obvious difference for PWT and PWL between the MIA+StDCS and the MIA groups (Figure 3A and 3B). Our findings suggested that the tDCS treatment may have a significant analgesic effect on chronic pain induced by MIA in rats.
Figure 3.
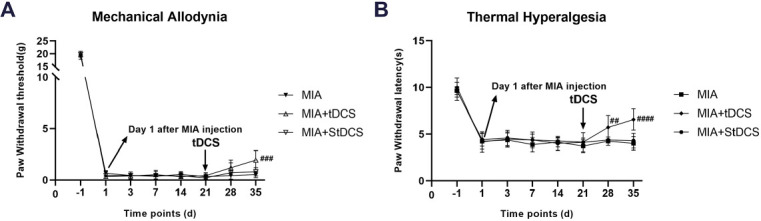
A-B: After tDCS treatment, PWT and PWL dramatically increased compared with the MIA group, but no difference was observed between the MIA and MIA+StDCS groups. Data were presented as the mean ± SEM, (n=6/group). ##P<0.01, ###P<0.001, ####P<0.0001 represented comparison of MIA+tDCS with MIA group.
The tDCS treatment down-regulated NMDAR2B expression in the spinal cord of rats
The Western blot results indicated that compared with the day before MIA injection, there was a remarkable increase in NMDAR2B level on days 7, 14, and 21 after MIA injection (Figure 4A). In addition, the Figure 4B showed that tDCS treatment substantially down-regulated the expression of NMDAR2B in the spinal cord compared with the MIA group. Furthermore, there was no significant difference between the MIA+StDCS and the MIA groups. The result analysis of Immunohistochemistry also suggested that the proportion of positive stained cells significantly decreased after tDCS treatment and there was no significant difference in the proportion of positive stained cells between the MIA and MIA+StDCS group (Figure 5). The results of Western blot and Immunohistochemistry showed that the expression of NMDAR2B in the spinal cord increased after MIA injection. TDCS treatment could down-regulate the expression of NMDAR2B.
Figure 4.
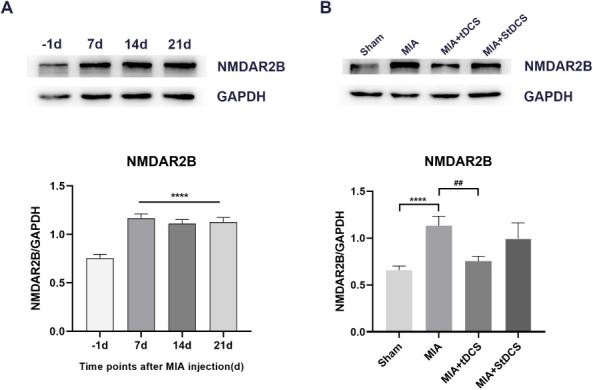
A: Effect of MIA on NMDAR2B protein in the spinal cord by western blot analysis. Data were presented as the mean ± SEM (n=3/group). ****P<0.0001, represented comparison of 7 days, 14 days and 21 days after MIA injection with 1 day before MIA injection. B: The expression of NMDAR2B protein in the spinal cord was measured at 14 days after tDCS treatment. Data were presented as the mean ± SEM (n=3/group). ****P<0.0001, MIA group vs. Sham group; ##P<0.01, MIA+tDCS group vs. MIA group.
Figure 5.
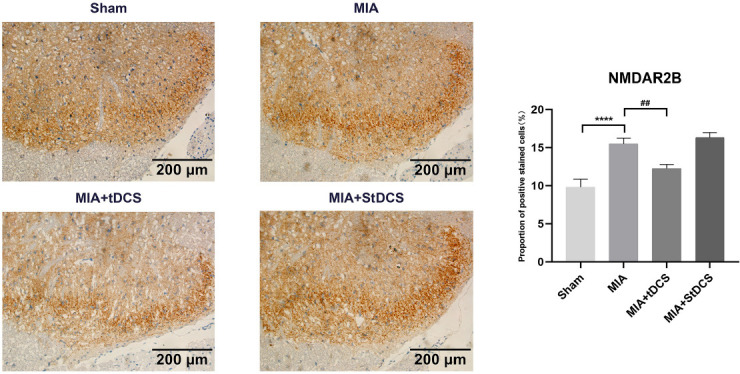
Immunohistochemical staining for NMDAR2B in all groups. Scale bars: 200 µm. The proportion of positive stained cells were presented as the mean ± SEM (n=4/group). ****P<0.0001, MIA group vs. Sham group; ##P<0.01, MIA+tDCS group vs. MIA group.
Discussion
In the past research, we found tDCS can alleviate OA-induced chronic pain in rats by modulating the expression of NMDA receptors in PAG. The study illustrates the mechanism of tDCS at the top of the central nervous system (CNS), but how tDCS works at the bottom of the CNS is still unclear. Therefore, the current study aimed to explore the mechanism of top-down modulation of tDCS in the spinal cord. Our results showed that tDCS reverted mechanical allodynia and thermal hyperalgesia, it also decreased the NMDAR2B levels in spinal cord. We concluded that tDCS can modulate the expression of NMDA receptors in spinal cord and alleviate chronic pain. This research may be an important reference for clarifying the top-down modulation of tDCS.
In the current study, a 20-min session of tDCS treatment for 14 consecutive days was applied to the rats on day 21 after MIA injection. We observed that compared with the MIA group, the PWT of the MIA+tDCS group gradually increased and there was significant difference on the 14th day after the tDCS treatment. In addition, the results of pain-related behaviors showed that the significant improvement of PWL in MIA+tDCS group occurred on the 7th day after the treatment. There is a difference in the analgesic response of tDCS, which may be related to the fibers activated by mechanical and thermal stimulation. Thermal nociception is mediated by C- and Aδ-fibers and mechanical response is mediated by Aβ fibers[27,28]. Our results showed that tDCS relieved OA-induced mechanical allodynia and thermal hyperalgesia, but there may be differences in treatment time for alleviating pain.
Neurotransmission of pain from the periphery to the cortex relies upon integration and signal processing within the spinal cord, brain stem and via the thalamus to specific areas of the cortex. The anterior cingulate cortex and insular cortex are integral to nociception[28]. The brain regions activated by tDCS included several motor areas, such as the M1, the caudal portion of the anterior cingulate cortex, right parieto-occipital junction, superior temporal sulcus and cerebellum. This may in part be due to a modulation of the functional interaction between M1 and these areas via cortico–cortical and cortico–subcortical connections. Recent work has shown that glutamate is the major fast excitatory transmitter within these structures, which is also considered to be involved in the development of pain behavior[29,30]. A previous study found that tDCS has two effects, the short-term effects are mediated by ionic channel modulation, the long-term effects are mediated by NMDA receptors. N-methyl-D-aspartate (NMDA) receptors play a pivotal role in synaptic transmission and neural plasticity[31]. NMDA receptors containing the NR2B subunit constitute a major population in the adult mammalian forebrain and spinal cord[32]. Research found that the expression of NMDAR subunit 2B (NMDAR2B) in the in spinal dorsal horn (DH) is higher in mice models of diabetic neuropathy. In addition, Fifteen Aprila Fajrin et al. suggested that it can significantly ameliorated hyperalgesia and allodynia in mice model of PDN by reducing the expression of TRPV1 and NMDAR2B in the spinal cord[33]. In our research, The expression of NMDAR2B increased significantly during the establishment of OA pain model, accompanied by the remarkable increase of PWT and PWL. After tDCS treatment, the expression of NMDAR2B decreased in the spinal cord of rats, and the pain-related behaviors also improved significantly.
We believed that this result may be related to the changes of NMDA receptors in the top central nervous system. Studies have confirmed that selectively over-expressing the NR2B subunit protein in the mouse anterior cingulate cortex/insular cortex enhanced responsiveness to subsequent peripheral injection of inflammatory stimuli (chronic pain model), whereas no effect on acute pain models was reported[32]. There is also a growing body of evidence to suggest opioid systems in the midbrain are activated during tDCS and that patients receiving tDCS may require less opioid-analgesia[34]. A study found that the tDCS-mediated reduction in inflammatory cytokine levels supports the potential use of tDCS as a countermeasure against inflammation and offers additional support for the hypothesis that cytokines contribute to the modulation of synaptic plasticity[35]. In addition, another study has reported that tDCS effectively reduced the concentration of pro-inflammatory cytokines in the cortex, thalamus, midbrain, and medulla via promoting the phenotypic transformation of microglia[36]. TDCS has been found to modify microglia activation in cerebral cortex of mice and rats, and realize immunomodulatory effects by downregulating constitutive expression of Iba1 on microglia in the cortex of the mouse[37]. The analgesic effects of tDCS have also been enhanced when used alongside conditioned pain modulation (CPM) paradigms in healthy subjects suggesting bottom-up changes in supraspinal sites may be involved[38]. It is therefore possible that tDCS applied over the primary motor cortex may be involved in the top-down modulation of pain processing at the spinal level.
Classically, tDCS effects have been attributed to interactions between prosencephalon regions, such as the primary motor cortex, dorsal lateral prefrontal cortex, and cingulate cortex[39,40]. However, tDCS effects may involve projections to remote area, such as the periaqueductal gray area, which is part of the descending system to the spinal cord. More and more studies believed that tDCS could be effective in the direct contact area of the electrode and play a role in the distance[41,42]. In our previous study, we chose M1 as the stimulation site of tDCS and observed the changes of NMDA receptor in periaqueductal gray (PAG)[24]. It is believed that the stimulation of tDCS in M1 region can activate contiguous regions such as PAG. Other studies have also found tDCS delivered to the cerebral cortex is able to reduce pain sensitivity and modulate neuronal changes in the spinal cord and brainstem, probably by top-down systems[43]. Combined with our previous studies, we believe that there is a top-down modulation of tDCS. The fact that the changes of NMDA receptors in PAG and spinal cord further confirm that tDCS can exert effects from top to bottom.
Neuroimaging studies have shown that tDCS applied over the primary motor cortex can indirectly activate areas of the brain involved in the modulation of pain perception[44]. Previous studies conceptualized the effect of tDCS, believing that the stimulation of M1 is able to modify activities of cortical (ACC) and sub-cortical (thalamus) regions, and these three regions have direct connections to the spinal cord and are able to alter ascending information at that level. In the spinal cord, this top-down regulation may use different mechanisms, such as local circuits involving presynaptic (primary afferents) and postsynaptic sites (second order neurons), intrinsic inter-neurons, or interconnections between different ascending and descending pathways[23,45,46]. Combined with previous studies, we believed that the top-down regulatory mechanism of tDCS may be that changes in the top of the CNS and neurotransmitters indirectly affect the corresponding changes in the bottom of the CNS, which promotes the endogenous analgesic system to exert analgesic effects.
Our results further confirmed the possible mechanism of tDCS top-down regulation and provided new evidence for the existence of tDCS top-down regulation. We suggest that tDCS may play a key role in the top-down modulation of endogenous analgesic system. There are limitations inherent to the current study design and that several questions remain open. The use of NMDAR2B agonist treatment can further confirm our hypothesis from another perspective, and we will implement the study in the future. More research will be done in the future to explore how tDCS causes NMDA receptor changes at the different level.
This research demonstrated that tDCS can attenuate OA-induced chronic pain in rats via reducing NMDAR2B expressions in the spinal cord. We believe that this may be the result of tDCS participating in the top-down modulation of the endogenous analgesic system. More research is needed to confirm our conclusions in the future.
Ethics approval
All experimental methods and procedures were approved by the Animal Experimental Ethics Inspection of Qingdao University (The approval number: NO. 20200901SD4520210601004), in compliance with the Ethical Guidelines for the Use of Experimental Animals by Ministry of Science and Technology of the People’s Republic of China (2019).
Authors’ contributions
LW designed the study. ZL executed the study and acquired the data, XC interpreted the data, PC analyzed the data. All authors contributed to the article and approved the submitted version.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi: 10.1016/j.coph.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Previtali D, Capone G, Marchettini P, Candrian C, Zaffagnini S, Filardo G. High Prevalence of Pain Sensitization in Knee Osteoarthritis:A Meta-Analysis with Meta-Regression. Cartilage. 2022;13(1):19476035221087698. doi: 10.1177/19476035221087698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Y, Wu C, Ou Q, et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-beta1. Bioact Mater. 2023;19:444–457. doi: 10.1016/j.bioactmat.2022.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavares DRB, Okazaki JEF, Rocha AP, et al. Effects of Transcranial Direct Current Stimulation on Knee Osteoarthritis Pain in Elderly Subjects With Defective Endogenous Pain-Inhibitory Systems:Protocol for a Randomized Controlled Trial. JMIR Res Protoc. 2018;7(10):e11660. doi: 10.2196/11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawker GA. Who, when, and why total joint replacement surgery?The patient's perspective. Curr Opin Rheumatol. 2006;18(5):526–30. doi: 10.1097/01.bor.0000240367.62583.51. [DOI] [PubMed] [Google Scholar]
- 6.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty:who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haywood AR, Hathway GJ, Chapman V. Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci Rep. 2018;8(1):7122. doi: 10.1038/s41598-018-25581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnham LJ, Dickenson AH. The antinociceptive effect of milnacipran in the monosodium iodoacetate model of osteoarthritis pain and its relation to changes in descending inhibition. J Pharmacol Exp Ther. 2013;344(3):696–707. doi: 10.1124/jpet.112.199489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy SG, Leichnetz GR. Frontal cortical projections to the periaqueductal gray in the rat:a retrograde and orthograde horseradish peroxidase study. Neurosci Lett. 1981;23(1):13–7. doi: 10.1016/0304-3940(81)90183-x. [DOI] [PubMed] [Google Scholar]
- 10.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422(4):556–78. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Palazzo E, Luongo L, Novellis V, Rossi F, Maione S. The Role of Cannabinoid Receptors in the Descending Modulation of Pain. Pharmaceuticals (Basel) 2010;3(8):2661–2673. doi: 10.3390/ph3082661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74(4):1742–59. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 13.Beitz AJ. The sites of origin brain stem neurotensin and serotonin projections to the rodent nucleus raphe magnus. J Neurosci. 1982;2(7):829–42. doi: 10.1523/JNEUROSCI.02-07-00829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Hocevar M, Bie B, Foss JF, Naguib M. Cannabinoid Type 2 Receptor System Modulates Paclitaxel-Induced Microglial Dysregulation and Central Sensitization in Rats. J Pain. 2019;20(5):501–514. doi: 10.1016/j.jpain.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhuo M. Glutamate receptors and persistent pain:targeting forebrain NR2B subunits. Drug Discov Today. 2002;7(4):259–67. doi: 10.1016/s1359-6446(01)02138-9. [DOI] [PubMed] [Google Scholar]
- 16.Nakazato E, Kato A, Watanabe S. Brain but not spinal NR2B receptor is responsible for the anti-allodynic effect of an NR2B subunit-selective antagonist CP-101,606 in a rat chronic constriction injury model. Pharmacology. 2005;73(1):8–14. doi: 10.1159/000081069. [DOI] [PubMed] [Google Scholar]
- 17.Zhuo M. Plasticity of NMDA receptor NR2B subunit in memory and chronic pain. Mol Brain. 2009;2:4. doi: 10.1186/1756-6606-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fregni F, Gimenes R, Valle AC, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54(12):3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 19.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DosSantos MF, Oliveira AT, Ferreira NR, Carvalho ACP, Rosado de Castro PH. The Contribution of Endogenous Modulatory Systems to TMS- and tDCS-Induced Analgesia:Evidence from PET Studies. Pain Res Manag. 2018;2018:2368386. doi: 10.1155/2018/2368386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan R, Wang Y, Feng B, et al. Effect of High-definition Transcranial Direct Current Stimulation on Conditioned Pain Modulation in Healthy Adults:A Crossover Randomized Controlled Trial. Neuroscience. 2021;479:60–69. doi: 10.1016/j.neuroscience.2021.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Ahveninen J, Jaaskelainen IP, Raij T, et al. Task-modulated “what”and “where”pathways in human auditory cortex. Proc Natl Acad Sci U S A. 2006;103(39):14608–13. doi: 10.1073/pnas.0510480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spezia Adachi LN, Quevedo AS, de Souza A, et al. Exogenously induced brain activation regulates neuronal activity by top-down modulation:conceptualized model for electrical brain stimulation. Exp Brain Res. 2015;233(5):1377–89. doi: 10.1007/s00221-015-4212-1. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Zhou W, Wang L, Ye Y, Li T. Transcranial Direct Current Stimulation Alleviates the Chronic Pain of Osteoarthritis by Modulating NMDA Receptors in Midbrain Periaqueductal Gray in Rats. J Pain Res. 2022;15:203–214. doi: 10.2147/JPR.S333454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laste G, Caumo W, Adachi LN, et al. After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Exp Brain Res. 2012;221(1):75–83. doi: 10.1007/s00221-012-3149-x. [DOI] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 27.Millan MJ. The induction of pain:an integrative review. Prog Neurobiol. 1999;57(1):1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 28.Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci. 2018;19(8) doi: 10.3390/ijms19082164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna) 2014;121(8):799–817. doi: 10.1007/s00702-014-1180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira V, Goudet C. Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors. Front Mol Neurosci. 2018;11:464. doi: 10.3389/fnmol.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4(6) doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chazot PL. The NMDA receptor NR2B subunit:a valid therapeutic target for multiple CNS pathologies. Curr Med Chem. 2004;11(3):389–96. doi: 10.2174/0929867043456061. [DOI] [PubMed] [Google Scholar]
- 33.Fajrin FA, Nugroho AE, Nurrochmad A, Susilowati R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J Ethnopharmacol. 2020;249:112396. doi: 10.1016/j.jep.2019.112396. [DOI] [PubMed] [Google Scholar]
- 34.Stamenkovic DM, Mladenovic K, Rancic N, et al. Effect of Transcranial Direct Current Stimulation Combined With Patient-Controlled Intravenous Morphine Analgesia on Analgesic Use and Post-Thoracotomy Pain. A Prospective, Randomized, Double-Blind, Sham-Controlled, Proof-of-Concept Clinical Trial. Front Pharmacol. 2020;11:125. doi: 10.3389/fphar.2020.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ethridge VT, Gargas NM, Sonner MJ, et al. Effects of transcranial direct current stimulation on brain cytokine levels in rats. Front Neurosci. 2022;16:1069484. doi: 10.3389/fnins.2022.1069484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan M, Feng Z, Chen H, et al. Transcranial direct current stimulation regulates phenotypic transformation of microglia to relieve neuropathic pain induced by spinal cord injury. Front Behav Neurosci. 2023;17:1147693. doi: 10.3389/fnbeh.2023.1147693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pikhovych A, Stolberg NP, Jessica Flitsch L, et al. Transcranial Direct Current Stimulation Modulates Neurogenesis and Microglia Activation in the Mouse Brain. Stem Cells Int. 2016;2016:2715196. doi: 10.1155/2016/2715196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes SW, Ali M, Sharma P, Insan N, Strutton PH. Frequency-dependent top-down modulation of temporal summation by anodal transcranial direct-current stimulation of the primary motor cortex in healthy adults. Eur J Pain. 2018 doi: 10.1002/ejp.1238. [DOI] [PubMed] [Google Scholar]
- 39.Lang N, Siebner HR, Ward NS, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22(2):495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto S, Ishii D, Ishibashi K, Kohno Y. Transcranial Direct Current Stimulation of the Dorsolateral Prefrontal Cortex Modulates Cognitive Function Related to Motor Execution During Sequential Task:A Randomized Control Study. Front Hum Neurosci. 2022;16:890963. doi: 10.3389/fnhum.2022.890963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen JP, Nizard J, Keravel Y, Lefaucheur JP. Invasive brain stimulation for the treatment of neuropathic pain. Nat Rev Neurol. 2011;7(12):699–709. doi: 10.1038/nrneurol.2011.138. [DOI] [PubMed] [Google Scholar]
- 42.Alappat JJ. Motor cortex stimulation for chronic pain:systematic review and meta-analysis of the literature. Neurology. 2009;72(6):577. doi: 10.1212/01.wnl.0000344169.51931.e5. author reply 577. [DOI] [PubMed] [Google Scholar]
- 43.Bocci T, Barloscio D, Parenti L, Sartucci F, Carli G, Santarcangelo EL. High Hypnotizability Impairs the Cerebellar Control of Pain. Cerebellum. 2017;16(1):55–61. doi: 10.1007/s12311-016-0764-2. [DOI] [PubMed] [Google Scholar]
- 44.DaSilva AF, Datta A, Swami J, Kim DJ, Patil PG, Bikson M. The Concept, Development, and Application of a Home-Based High-Definition tDCS for Bilateral Motor Cortex Modulation in Migraine and Pain. Front Pain Res (Lausanne) 2022;3:798056. doi: 10.3389/fpain.2022.798056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stachowski NJ, Dougherty KJ. Spinal Inhibitory Interneurons:Gatekeepers of Sensorimotor Pathways. Int J Mol Sci. 2021;22(5) doi: 10.3390/ijms22052667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fung C, Vanden Berghe P. Functional circuits and signal processing in the enteric nervous system. Cell Mol Life Sci. 2020;77(22):4505–4522. doi: 10.1007/s00018-020-03543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


