Abstract
The current research was conducted to determine and frequency of aflatoxins (B1, B2, G1, G2), in main feed ingredients (corn and soybean meal) and poultry finished feed (in mash and pellet forms). Eighty-five samples of corn, soybean meal, and poultry finished feed was randomly collected from feed mills in Iran. Regarding macro and microscopic morphological criteria, Aspergillus isolates were identified, and aflatoxins were determined by thin-layer chromatography and high-performance liquid chromatography (HPLC). All of poultry feed samples were contaminated with different levels of aflatoxins, ranging from ND (they were not detected in those samples) to 5.58 µg/kg. At all stages of processing, the poultry feed had lower levels of aflatoxins in comparison with the accepted/residue levels of poultry feed mills. Higher amounts of aflatoxins (B1, B2, G1, G2, and total) were detected in pelleted feed, compared to other poultry samples (P < 0.05). The total toxin level in mash feed samples reached a maximum of 3.31 ppb. The results indicate that finished feed samples in pellet form may pose a greater risk than their individual ingredients in poultry feed, particularly when suboptimal conditions exist for eliminating fungal populations. So, the prevention and reduction of (Aspergillus section Flavi) are highly important in maintaining quality control of poultry feed, as the production of aflatoxins can occur during the process of converting raw ingredients into finished feed.
Key words: natural aflatoxin, poultry feed, feed ingredient, finished feed
INTRODUCTION
Mycotoxins are secondary metabolites produced by various genera of fungi, and their presence in crops, processed food, and feed poses a significant global risk due to their highly toxic nature. Among the mycotoxins, aflatoxins are considered one of the most hazardous groups, capable of causing substantial contamination, as recognized by the World Health Organization (WHO, 2011; Omotayo et al., 2019). In addition, aflatoxins have been classified by the International Agency for Research on Cancer (IARC) as highly problematic carcinogens capable of causing liver damage in humans (IARC, 1987; Yilmaz et al., 2017). Specifically, aflatoxin B1 (AFB1) has been identified by the IARC as a type-A human liver carcinogen (IARC, 1987; WHO, 2011). Aflatoxins can be produced by various species of Aspergillus, including Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius. These fungi commonly contaminate cereals throughout their growth, harvest, storage, transport, and processing stages (Bryden, 2007). Among these species, Aspergillus flavus is a prevalent pathogen in animal feed and can exist in both toxigenic and nontoxigenic strains, potentially present in poultry feed. Studies have indicated that approximately 98% of the ingredients used in animal diets contain aflatoxins (including B1, B2, G1, and G2), with AFB1 being the most prevalent. Corn grain, in particular, is highly susceptible to fungal growth and mycotoxin production (Rodrigues et al., 2012; Ariyo et al., 2013). Aflatoxin contamination is a major concern to human health as AFB1 could be passed on to humans from poultry products. Research has indicated that AFB1 exists in a wide range of feed, depending on the region and climate (Labuda and Tanvinova, 2006).
Aflatoxin-producing fungi exhibit varying responses under different conditions, which can be influenced by factors such as storage, sampling techniques, geographical regions, seasonal climate changes, and temperature variations. The moisture content of feedstuffs has been identified as one of the most significant predisposing factors for contamination (Kana et al., 2013; Abdallah et al., 2015). In poultry, it is crucial to measure the concentrations of different types of aflatoxins (B1, B2, G1, and G2) as well as total aflatoxin levels in feedstuffs, as these serve as critical indicators of health (Wu, 2015).
Research conducted in recent decades has provided evidence of the detrimental effects of aflatoxins on poultry performance. Chronic aflatoxin poisoning in poultry has been observed to weaken the immune system, and these metabolic compounds possess toxic, carcinogenic, and mutagenic properties (Kumar et al., 2009; Sirajudeen et al., 2011). However, only a few attempts have been made to evaluate the presence of Aspergillus species and identify different types of aflatoxins in poultry feed. Furthermore, corn grain and soybean meal serve as the primary ingredients in Iranian-made poultry feed, with a significant portion being imported from other countries. There are variations in the initial safety of feed ingredients based on their country of origin, as well as differences in the potential for contamination, transportation conditions, and storage practices. Although these ingredients may be susceptible to fungal contamination, the extent of damage can also vary throughout the feed preparation process. The aim of this study was to assess the frequency, levels, and natural occurrence of aflatoxins (B1, B2, G1, and G2) as well as total aflatoxins, in corn grain, soybean meal, and poultry finished feed (in both mash and pellet forms).
MATERIALS AND METHODS
The study was performed in accordance with the guidelines of the Institute of Agricultural Education and Extension, Agricultural Research, Education and Extension Organization (AREEO) and of the Iranian Veterinary Organization (IVO) and Food and Agriculture Organization (FAO) of the United Nations (FAO, 2004). Iran's largest and most important feed mills are situated in the central region of the country, primarily in Tehran, Qom, and Alborz provinces. These factories have a crucial role in importing and handling a majority of feed ingredients, including corn and soybean meal, within Iran. Additionally, Temperature plays an important role in the growth of fungi and the increase in contamination of food materials with fungal toxins. Fungi generally thrive better in warmer and more humid conditions. Therefore, this study was conducted during the summer, considering a total of 10 important feed mills located in the central part of the country.
Sampling Preparation
Poultry feed samples were randomly selected from feed mill factories licensed by the Official Veterinary Authority. The sampling process included the random selection of samples from the main raw materials consisted of corn, soybean meal, and both mash and pelleted forms as completed feed. Selecting areas based on the volume of high production of poultry feed and these factories also tried to reduce the storage time and keep it up to date for this condition. The sampling method was conducted in line with the feed production process, specifically during manufacturing, at various points along the feed flowing line. Samples were collected in the path of material movement within the feed production line to ensure representative sampling. Three times sampling per month is conducted during the summer, and a total of 85 samples were collected, consisting of n = 22 samples (corn, soybean meal, mash) and n = 19 samples (pellet) each month. Presamples weighing 10 kg were collected, homogenized, and mixed so working samples weighing 500 g were obtained. The samples were prepared in sterile plastic bags and subsequently transported to the Faroogh Laboratory in Tehran, Iran.
Fungal Isolation
To isolate the fungi, sub-samples of each feed were cultured separately on agar media using the spread-plating method, following the procedure described by Samson et al. (2004). Briefly, samples were mixed by a high-speed mill and dilution in 9 mL of sterile distilled water into a test tube. Diluted samples were cultured on three cultures consists of Sabouraud Dextrose Agar plates with 0.05% chloramphenicol, Dichloran Rose-Bengal Chloramphenicol Agar, and Aspergillus flavus and Parasiticus Agar at 28ºC for 7 to 10 d. The final identification of Aspergillus section flavi was performed by considering a combination of macroscopic and microscopic characteristics based on species criteria, as outlined in the works of Samson et al. (2004), Atehnkeng et al. (2008), and Razzaghiabyaneh et al. (2006).
Toxine Isolation
The aflatoxin-producing ability of A. flavus samples was determined on yeast extract sucrose. All isolates were cultured on a yeast extract broth medium, containing 2% yeast extract and 18% sucrose, according to Razzaghi-Abyaneh (2006). Aflatoxin production was first screened by a thin layer chromatography on silica gel plates. Thin-layer chromatography plates were developed using chloroform-methanol (98:2, v/v) in the mobile phase and were checked regularly for blue spots of AFB1 or AFB2 under UV light. In infected samples, AFB and aflatoxin G (AFG) were quantified by high-performance liquid chromatography (HPLC).
For quantifying the level of aflatoxins by HPLC, samples were ground, sieved through 2 mm meshes, and analyzed according to the method was validated for accuracy and precision as described in the guidelines of waters 2695 and following a standard method of analysis (AOAC international method) (Trucksess et al., 1994; AOAC, 2000). All reagents were of HPLC standard grade and standards of aflatoxins were purchased from Sigma Chemical Company (Roedermark, Germany). The food analysis method involved three steps: extraction, purification, and determination of toxin content. To ensure the sensitivity and precision of the analysis, a validation test was conducted following the guidelines established by the International Union of Pure and Applied Chemistry (Durham, NC), as outlined by Yakubu et al. (2020). The limit of detection of aflatoxin mass fraction was 0.5 ug/kg and the aflatoxin content in the samples was calculated using specific equations:
-
1)
Concentration of aflatoxins in ppm = Standard peak height × Sample peak height/Standard peak height × Final volume of sample
-
2)
Total aflatoxins = The sum of B1, B2, G1, and G2
Statistical Analysis
The data regarding aflatoxin content in poultry feed ingredients and finished feed were subjected to descriptive statistics through SPSS software (version 16.0) (SPSS, Illinois, IL). Frequency distribution procedure in MS Excel 2016 were used for the processing and tabulation of obtained data.
RESULTS
The results regarding the frequency of Aspergillus isolates from ingredients and finished poultry feed are presented in Table 1. Among the feed ingredients, corn grains exhibited a higher frequency of toxigenic isolates compared to soybean meal (64.2% vs. 12.5 %, respectively). The pellet form demonstrated a higher frequency of toxigenic isolates compared to mash feed (38.1% vs. 33.3%, respectively). Notably, corn samples had the highest proportion of aflatoxigenic isolates, accounting for the majority of the total isolates (64.2%), followed by pelleted feed (38.1%) and mash feed (33.3%), while soybean meal displayed the lowest level (12.5%).
Table 1.
Isolates obtained from ingredients and finished poultry feed.
| Samples | Ingredients |
Finished feed |
||
|---|---|---|---|---|
| Corn | Soybean | Mash | Pellet | |
| Total isolates | 64.2 (%) | 12.5 (%) | 33.3 (%) | 38.1 (%) |
The analysis of aflatoxins in corn samples (Table 2) revealed that approximately 9.09% of the corn samples (n = 22) were found to be contaminated. No contaminants of other aflatoxins (B2, G1, and G2) were detected in the corn samples. The concentration of aflatoxins in the studied corn samples did not exceed the standard limits set for corn aflatoxin in the European Union (EU, 1993; European Food Safety Authority (EFSA) 2014a, European Food Safety Authority (EFSA), 2014b). The analysis of aflatoxins in soybean meal samples (Table 3) revealed concentrations of 0.61 ppb for AFB1, 0.71 ppb for aflatoxin G1 (AFG1), and 1.05 ppb for aflatoxin G2 (AFG2). Aflatoxin B2 was not detected in the soybean samples. The frequency of contaminated samples for total aflatoxins was approximately 40.9%. Interestingly, the frequency of contaminated samples for AFG2 was higher than that of AFB1 and AFG1, with percentages of 36.36% compared to 13.63% for each. The concentration of aflatoxins in the soybean samples of this study did not exceed the standard limits set for corn aflatoxin allowance by the EU (2002; EFSA, 2004a,b). Different types of aflatoxins (B1, B2, G1, and G2) were detected in the finished feed samples in the form of mash (Table 4). The maximum level of total aflatoxin in the mash feed samples was found to be 3.31 ppb. Interestingly, the samples contaminated with AFG2 had higher levels compared to those contaminated with other aflatoxins in the mash feed samples. The frequency of contaminated samples with total aflatoxins in the mash feed was approximately 54.54%. However, the aflatoxin concentrations in the mash feed samples did not exceed the permitted limit set by the EU (EFSA, 2004a,b; EU, 2009).
Table 2.
Aflatoxins contamination in corn samples (n = 22).
| Aflatoxins | N1 (%)2 | Min3_max4 (ppb)5 | AF contaminated samples higher than standard level (%)6 |
|---|---|---|---|
| B1 | 2 (9.09) | ND7—0.45 | 0 |
| B2 | 0 (0) | ND—ND | 0 |
| G1 | 0 (0) | ND—ND | 0 |
| G2 | 0 (0) | ND—ND | 0 |
| Total8 | 2 (9.09) | ND—0.45 | 0 |
Number of contaminated samples.
Frequency of contaminated samples (aflatoxins infected samples × 100/total sample of corn).
Min: minimum.
Max: maximum.
ppb: part per billion.
Frequency of contaminated samples that containing AF level higher than the maximum tolerance of AF in the European Union.
Not-detectable.
Sum of all kinds of aflatoxins B1, B2, G1, and G2.
Table 3.
Aflatoxins contamination in soybean meal samples (n = 22).
| Aflatoxins | N1 (%)2 | Min3_– max4 (ppb)5 | AF contaminated samples higher than standard level (%)6 |
|---|---|---|---|
| B1 | 3 (13.63) | ND7—0.61 | 0 |
| B2 | 0 (0) | ND—ND | 0 |
| G1 | 3 (13.63) | ND—0.71 | 0 |
| G2 | 8 (36.36) | ND—1.05 | 0 |
| Total8 | 9 (40.91) | ND—2.37 | 0 |
Number of contaminated samples.
Frequency of contaminated samples (aflatoxins infected samples × 100/total sample of soybean).
Min: minimum.
Max: maximum.
ppb: part per billion.
Frequency of contaminated samples that containing AF level higher than the maximum tolerance of AF in the European Union.
Not-detectable.
Sum of all kinds of aflatoxins B1, B2, G1, and G2.
Table 4.
Aflatoxins contamination in mash feed samples (n = 22).
| Aflatoxins | N1 (%)2 | Min3_– max4 (ppb)5 | AF contaminated samples higher than standard level (%)6 |
|---|---|---|---|
| B1 | 2 (9.09) | ND7—0.42 | 0 |
| B2 | 1 (4.54) | ND—0.33 | 0 |
| G1 | 3 (13.36) | ND—0.69 | 0 |
| G2 | 9 (40.91) | ND—1.87 | 0 |
| Total8 | 12 (54.54) | ND—3.31 | 0 |
Number of contaminated samples.
Frequency of contaminated samples (aflatoxins infected samples × 100/total sample of mash feed).
Min: minimum.
Max: maximum.
ppb: part per billion.
Frequency of contaminated samples that containing AF level higher than the maximum tolerance of AF in the European Union.
Not-detectable.
Sum of all kinds of aflatoxins B1, B2, G1, and G2.
Different types of aflatoxins (B1, B2, G1, and G2) were detected in the finished feed samples in pellet form (Table 5). The concentration (3.01 ppb) and frequency (47.36%) of samples contaminated with AFG2 were found to be higher than those contaminated with other aflatoxins in the pelleted feed samples. The frequency of samples contaminated with total aflatoxins was 57.89%. The maximum level of total aflatoxin in the pellet feed samples was 5.58 ppb. Importantly, the concentration of aflatoxins in the pellet feed samples did not exceed the standard levels set by the EU (1993, 2002, 2009; EFSA, 2004a,b).
Table 5.
Aflatoxins contamination in pellet feed samples (n = 19).
| Aflatoxins | N1 (%)2 | Min3_– max4 (ppb)5 | AF contaminated samples higher than standard level (%)6 |
|---|---|---|---|
| B1 | 5 (26.32) | ND7—0.97 | 0 |
| B2 | 2 (10.53) | ND—0.62 | 0 |
| G1 | 3 (15.79) | ND—0.98 | 0 |
| G2 | 9 (47.36) | ND—3.01 | 0 |
| Total8 | 11 (57.89) | ND—5.58 | 0 |
Number of contaminated samples.
Frequency of contaminated samples (aflatoxins infected samples × 100/total sample of pellet feed).
Min: minimum.
Max: maximum.
ppb: part per billion.
Frequency of contaminated samples that containing AF levels higher than the maximum tolerance of AF in the European Union.
Not-detectable.
Sum of all kinds of aflatoxins B1, B2, G1, and G2.
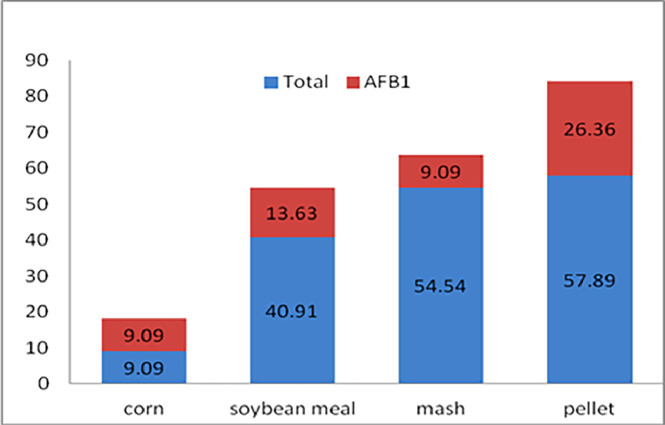
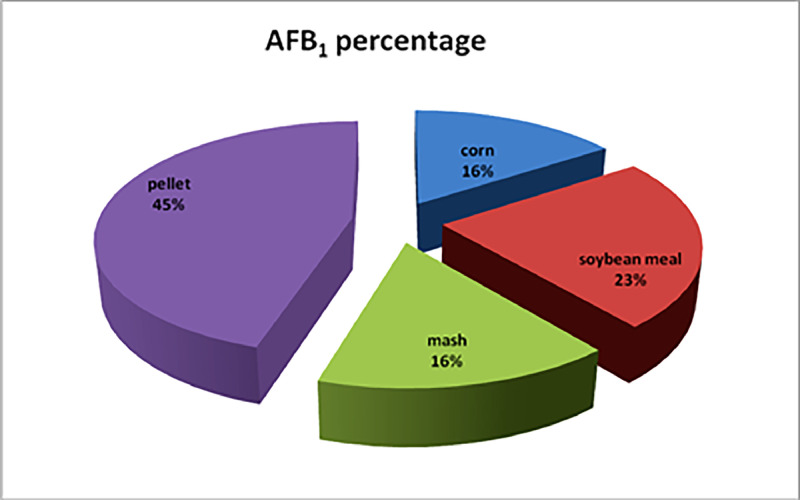
The frequency of sample contamination with any toxin increased in the finished feed (mash and pellet) compared to corn and soybean meal (Figure 1). As shown in Figure 1, the percentages of total aflatoxin contamination in corn, soybean meal, mash feed, and pellet feed samples were 9.09%, 40.91%, 54.54%, and 57.89%, respectively. Additionally, the contamination of corn, soybean meal, mash feed, and pellet feed samples with AFB1 was found to be 9.09%, 13.63%, 9.09%, and 26.32%, respectively. When aggregating the data to obtain overall statistics for all aflatoxins, the results indicate that corn had the lowest presence of aflatoxins (B1, B2, G1, G2, and total) compared to other feed samples. The frequency of contamination with AFB1 in pellet feed was higher than in corn, soybean meal, and the mash form (Figure 2).
Figure 1.
The ratio of the percentage of the aflatoxins B1/total aflatoxins in poultry feed.
Figure 2.
The ratio of percentage AFB1 contamination in poultry feed.
DISCUSSION
Aflatoxins, among the mycotoxins, are widely recognized for their association with various health and disease risks in poultry and livestock studies. The presence of aflatoxins in poultry feed and feed ingredients is a global concern due to their negative impact on poultry performance and the potential transfer of aflatoxin residues into the human food chain.
The Codex Alimentarius Commission, which plays a central role in the Joint FAO/WHO Food Standards Program, has established guidelines regarding the maximum permissible levels of aflatoxins (B1, B2, G1, G2, and M1) in feedstuffs and complementary feed. According to the Codex Alimentarius Commission's guidelines, the maximum acceptable limit for aflatoxins in these products is set at 20 parts per billion (ppb) (Kotinagu et al., 2015). Many countries have implemented regulations to establish maximum permissible levels of AFBs in food and feed products as a means to mitigate this potential hazard. The specific regulations governing aflatoxin levels vary among countries, often influenced by factors such as economic considerations (Adeniran et al., 2013; Medina et al., 2014; Yakubu et al., 2020).
For instance, the Institute of Standards and Industrial Research of Iran has set a Maximum Residue Level of 20 µg/kg for aflatoxins in poultry feed. The Food and Drug Administration has established a regulatory limit of 20 ppb for total aflatoxins in both food and feed. This limit serves as the minimum acceptable level for animal feed according to the Food and Drug Administration (FDA, 2000) guidelines.
In this study, it was found that the contamination of corn with Aspergillus flavus was the highest among the sampled feed ingredients. However, interestingly, the levels of aflatoxins (specifically AFB1, AFB2, AFG1, AFG2, and total aflatoxins) in this study were the lowest among the tested feed samples. Furthermore, the study observed that the concentration and frequency of samples contaminated with AFB1 were higher compared to samples contaminated with AFB2, regardless of the type of feed sample (such as corn, soybean meal, mash, or pellet).
The levels of toxins in poultry feed samples can be influenced by various natural conditions. Several factors contribute to these variations, including the quantity and composition of proteins and fats in the ingredients, as well as their proportions in the final feed formulation. Probst et al. (2011) conducted a study that revealed that nontoxigenic isolates of A. flavus, along with the presence of other fungi, have the potential to influence the natural production of aflatoxins in food and feed. The study further demonstrated that the application of different chemicals resulted in the reduction of fungal toxins or the elimination of aflatoxins, particularly through the use of mycotoxin binders or preservatives in incomplete feed.
The findings of the study demonstrated that the ability of fungi to produce AFB1 (aflatoxin B1) does not necessarily correlate with the level of toxin present. Therefore, the presence of A. flavus fungi in poultry feed does not always indicate the presence of aflatoxin in the substrate. The production of aflatoxin depends on specific conditions required for fungal growth and toxin production. It is essential to carefully screen high-risk ingredients, such as corn and other nonprocessed ingredients, due to the diverse range of fungal contaminations observed in different types and volumes of poultry feed worldwide. The study also observed that finished poultry feed in pelleted form was contaminated, likely due to the widespread distribution of A. flavus spores in the environment. As previously mentioned, temperature plays a crucial role in fungal growth and the contamination of feed ingredients with fungal toxins. Fungi tend to thrive in warmer and more humid conditions. After the production and storage of final feeds, fungal growth can occur due to increased nutrient availability. It is important to note that while heating feed to boiling point for at least 30 min can eliminate living Aspergillus organisms, it does not eliminate spores that can germinate later. During the transfer of pellets from the Pelletizer machine to the cooling machine, exposure to saprophytic Aspergillus in the environment can lead to growth during storage. Malfunctions in the equipment can also impact the feed preparation process, potentially affecting the overall quality (Ghaemmaghami et al., 2020).
Ghaemmaghami et al. (2016) conducted a study to determine the levels of fungal contamination in poultry feed. The research identified a total of 384 fungal isolates across different feed ingredients, including corn (124 isolates), soybean meal (92 isolates), mash feed (72 isolates), and pellet feed (96 isolates). These isolates belonged to seven different genera. The findings of our study revealed that corn, soybean meal, and finished poultry feed exhibited varying degrees of contamination with natural aflatoxins. These results align with similar findings reported by researchers from other countries, such as Charoenpornsook (2006) and Fraga et al. (2007), who also observed comparable levels of aflatoxin in certain feed samples. Jelinek et al. (1989) conducted a study where they observed variations in the levels of aflatoxin in corn across different locations and years. The average amount of aflatoxin detected in corn samples ranged from 1.0 to 80 µg/kg. Furthermore, their findings indicated that the majority of feed samples contained aflatoxin levels ranging from 5 to 20 µg/kg.
In the study conducted by Jindal et al. (1993) on Indian samples, a total of 240 poultry feed samples were analyzed. The results indicated that all of the samples were contaminated with aflatoxins, with levels ranging from 7 to 11,600 µg/kg. It was observed that 76% of the samples had aflatoxin amounts exceeding 30 µg/kg. According to the findings reported by Shetty et al. (1987), 19% of the 31 samples collected in Nigeria were found to contain aflatoxin levels ranging from 30 to 1,610 µg/kg. Similarly, Purwoko et al. (1991) reported that 19% of the 31 feed samples analyzed in Indonesia had aflatoxin levels ranging from 22 to 6,171 µg/kg. According to Hegazy et al. (1991), 7.30% of the 1,175 samples collected from chicken farms in Egypt were found to be contaminated with aflatoxin.
In the present study, the contamination caused by aflatoxins did not exceed 5.58 µg/kg in any of the samples analyzed. In a study conducted by López Grío et al. (2010), levels of aflatoxin B1, B2, G1, and G2 were measured in animal feed samples. Among the 19 samples tested, it was found that two of them had aflatoxin G2 levels exceeding the standard limit. However, the levels of other aflatoxins were present in negligible amounts in the tested samples.
CONCLUSIONS
This study indicates that corn, as an unprocessed ingredient in poultry feed, warrants additional evaluations, particularly when conditions are not optimal for preserving the feed. The assessment showed that corn had the highest percentage of Aspergillus spp. mold contamination, while pellet feed had the highest aflatoxin B1 concentration levels. These findings highlight the importance of considering both fungal contamination and aflatoxin concentration levels when evaluating the risk associated with different feed ingredients, enabling effective management and mitigation of aflatoxin risks in poultry feed production. The prevention and reduction of A. flavus and other aflatoxingenic fungi such as A. parasiticus as well as other Aspergillus section flavi are highly important in ensuring the quality control of poultry feed. Therefore, the findings suggest that finished feed, especially in pellet form, carries a higher risk of aflatoxin contamination compared to the individual ingredients in poultry feed. This increased risk is particularly evident when suboptimal conditions are present for controlling fungal populations during the manufacturing and storage processes. Therefore, special attention should be given to the quality control measures applied to finished feed to minimize the potential for aflatoxin production and ensure the safety of poultry feed.
ACKNOWLEDGMENTS
The authors wish to acknowledge the financial support received from the Institute of Agricultural Education and Extension, Agricultural Research, Education and Extension Organization (AREEO).
DISCLOSURES
The authors declare no potential conflict of interest.
References
- Abdallah M.F., Girgin G., Baydar T. Occurrence, prevention, and limitation of mycotoxins in feeds. Anim. Nut. Feed. Tech. 2015;15:471–490. [Google Scholar]
- Adeniran L.A., Makun H.A., Muhammad H.L. Survey of mycotoxigenic fungi in concentrated poultry feed. Niger. J. Food. Res. 2013;2:128–138. [Google Scholar]
- AOAC (Association of Official Analytical Chemists) 17th ed. Vol. 27. Association of Official Analytical Chemists; Baltimore, MD: 2000. pp. 827–994. (Official Methods of Analysis of AOAC International). [Google Scholar]
- Ariyo L.A., Anthony M.H., Lami M.L. Survey of mycotoxigenic fungi in concentrated poultry feed in Niger State. Niger. J. Food. Res. 2013;2:128–138. [Google Scholar]
- Atehnkeng J., Ojiambo P.S., Donner M., Ikotun T. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food. Microbio. 2008;122:74–84. doi: 10.1016/j.ijfoodmicro.2007.11.062. [DOI] [PubMed] [Google Scholar]
- Bryden W.L. Mycotoxins in the food chain: human health implications. Asia. Paci. J. Clin. Nutr. 2007;16:95–101. [PubMed] [Google Scholar]
- Charoenpornsook K., Kavisarasai P. Mycotoxins in animal feedstuffs of Thailand. Curr. Appl. Sci. Tech. 2006;6:25–28. [Google Scholar]
- European Food Safety Authority (EFSA) Opinion of the Scientific Panel on contaminants in the food chain (CONTAM Panel) related to aflatoxin B1 as undesirable substance in animal feed. EFSA. J. 2004;2:35–39. [Google Scholar]
- European Food Safety Authority (EFSA) Opinion of the Scientific Panel on contaminants in the food chain (CONTAM Panel) related to deoxynivalenol (DON) as undesirable substance in animal feed. EFSA. J. 2004;2:36–73. [Google Scholar]
- European Food Safety Authority (EFSA) Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA. J. 2014;12:3690–3699. [Google Scholar]
- European Food Safety Authority (EFSA) Guidance on data exchange version 2.0. EFSA J. 2014;12:3939–3945. [Google Scholar]
- EU (European Union) Council Regulation (EEC) No 315/93 of February 1993 laying down community procedures for contaminants in food. Off. J. Euro. Uni. L. 1993;37:1–3. [Google Scholar]
- EU (European Union) Directive 2002/32/EC of the European Parliament and the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Euro. Uni. L. 2002;140:10–21. [Google Scholar]
- EU (European Union) Commission Regulation (EC) No 386/2009 of 12 May 2009 amending Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the establishment of a new functional group of feed additives. Off. J. Euro. Uni. L. 2009;118:50–66. [Google Scholar]
- FAO (Food and Agriculture Organization) Worldwide regulations for mycotoxins in food and feed in 2003. Food. Nut. 2004;81:3–5. [Google Scholar]
- FDA (Food and Drug Administration) Guidance for industry: action levels for poisonous or deleterious substances in human food and animal feed. FDA. J. 2000;4:1950–1956. [Google Scholar]
- Fraga M.E., Curvello F., Gatti M.J., Cavaglieri L.R. Potential aflatoxin and ochratoxin a production by Aspergillus species in poultry feed processing. Vet. Res. Commun. 2007;31:343–348. doi: 10.1007/s11259-006-3434-x. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S.S., Modirsaneii M., Khosravi A., Razzaghiabyaneh M. Study on mycoflora of poultry feed ingredients and finished feed in Iran. Iran. J. Microbio. 2016;8:47–54. [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S.S., Pashootan N., Razzaghi-Abyaneh M. Toxigenicity and phylogeny of Aspergillus section Flavi in poultry feed in Iran. Curr. Med. Mycol. 2020;6:22–29. doi: 10.18502/cmm.6.1.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy S.M., Azzam A., Gabal M.A. Interaction of naturally occurring aflatoxins in poultry feed and immunization against fowl cholera. Poult. Sci. 1991;70:2425–2428. doi: 10.3382/ps.0702425. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Aflatoxins. In overall evaluations of carcinogenicity. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans, suppl. Int. Agen. Res. Cance. 1987;7:83–87. [Google Scholar]
- Jelinek C.F., Pohland A.E., Wood G.E. Worldwide occurrence of mycotoxins in foods and feeds-an update. J. Assoc. Off. Anal. Chem. 1989;72:223–230. [PubMed] [Google Scholar]
- Jindal N., Mahipal S.K., Mahajan N.K. Occurrence of aflatoxin in compound poultry feeds in Haryana and effect of different storage conditions on its production. Indi. J. Anim. Sci. 1993;63:71–73. [Google Scholar]
- Kana J.R., Gnonlonfin G.J., Harvey J., Wainaina J., Wanjuki I., Skilton R.A. Assessment of aflatoxin contamination of maize, peanut meal and poultry feed mixtures from different agroecological zones in Cameroon. Toxi. J. 2013;5:884–894. doi: 10.3390/toxins5050884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontinagu K., Mohanamba T., Kumari L.R. Assessment of aflatoxin B1 in livestock feed and feed ingredients by high-performance thin layer chromatography. Vet. W. J. 2015;8:1396–1399. doi: 10.14202/vetworld.2015.1396-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Balachandran C. Histopathological changes in broiler chickens fed aflatoxin and cyclopiazonic acid. Vet. Arh. J. 2009;79:31–40. [Google Scholar]
- Labuda R., Tancinova D. Fungi recovered from Slovakian poultry feed mixtures and their toxinogenity. Ann. Agric. Environ. Med. 2006;13:193–200. [PubMed] [Google Scholar]
- Lopez Grio S.J., Garridofrenich A., Martinezvidal J.L., Romerogonzález R. Determination of aflatoxins B1, B2, G1, G2 and ochratoxin A in animal feed by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2010;33:502–508. doi: 10.1002/jssc.200900663. [DOI] [PubMed] [Google Scholar]
- Medina A., Rodriguez A., Magan N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbio. J. 2014;5:338–348. doi: 10.3389/fmicb.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omotayo O.P., Omotayo A.O., Mwanza M., Babalola O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019;35:1–7. doi: 10.5487/TR.2019.35.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C., Bandyopadhyay R., Price L.E., Cotty P.J. Identification of atoxigenic Aspergillus flavus isolates to reduce aflatoxin contamination of maize in Kenya. Plan. Disea. J. 2011;95:212–218. doi: 10.1094/PDIS-06-10-0438. [DOI] [PubMed] [Google Scholar]
- Purwoko H.M., Hald B., Wolstrup J. Aflatoxin content and number of fungi in poultry feedstuffs from Indonesia. Lett. Appl. Microbio. 1991;12:212–215. [Google Scholar]
- Razzaghiabyaneh M., Shamsghahfarokhi M., Allameh A., Kazeroonshiri A. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopath. J. 2006;161:183–192. doi: 10.1007/s11046-005-0242-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues I., Naehrer K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxi. J. 2012;4:663–675. doi: 10.3390/toxins4090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R.A., Hoekstra E.S., Frisvad J.C. Identification of the common food-and airborne fungi. Intr. Food. Airb. Fung. 2004;7:1–282. [Google Scholar]
- Shetty S.N., Asuzu I.U., Anika S.M. Aflatoxin contamination of animal feedstuffs in Anambra State. Trop. Vet. J. 1987;5:21–25. [Google Scholar]
- Sirajudeen M., Gopi K., Tyagi J.S., Moudgal R.P., Mohan J. Protective effects of melatonin in reduction of oxidative damage and immunosuppression induced by aflatoxin B1-contaminated diets in young chicks. Environ. Toxicol. J. 2011;26:153–160. doi: 10.1002/tox.20539. [DOI] [PubMed] [Google Scholar]
- Trucksess M.W., Stack M.E., Nesheim S., Albert R.H., Romer T.R. Multifunctional column coupled with liquid chromatography for determination of aflatoxins B1, B2, G1, and G2 in corn, almonds, Brazil nuts, peanuts, and pistachio nuts: collaborative study. J. AOAC. Int. 1994;77:1512–1521. [PubMed] [Google Scholar]
- WHO (World Health Organization) FAO/WHO guide for application of risk analysis principles and procedures during food safety emergencies. Food. Agric. Organ. Uni. Nat. 2011;54:24–74. [Google Scholar]
- Wu F. Global impacts of aflatoxin in maize: trade and human health. World Mycotoxin J. 2015;8:137–142. [Google Scholar]
- Yakubu A., Vyas A. In: Biotechnology: Basic Research and Applications. Singh J., Vyas A., Wang S., Prasad R., editors. Springer; Singapore: 2020. Aflatoxin: occurrence, regulation, and detection in food and feed; pp. 337–353. [Google Scholar]
- Yilmaz S., Kaya E., Kisacam M.A. Aflatoxin-Control, Analysis, Detection and Health Risks. InTech; 2017. The effect on oxidative stress of aflatoxin and protective effect of lycopene on aflatoxin damage [Internet] pp. 67–90. [Google Scholar]