Abstract
Background and Objectives:
High-risk infant follow-up programs (HRIF) are a recommended standard of care for all extremely low birth weight (ELBW) infants to help mitigate known risks to long-term health and development. However, participation is variable with known racial and ethnic inequities, though hospital-level drivers of inequity remain unknown. We conducted a study using a large multicenter cohort of ELBW infants to explore within- and between-hospital inequities in HRIF participation.
Methods:
Vermont Oxford Network collected data on 19503 ELBW infants born between 2006–2017 at 58 United States hospitals participating in the ELBW Follow-up Project. Primary outcome was evaluation in HRIF at 18–24 months corrected age. The primary predictor was infant race and ethnicity, defined as maternal race (non-Hispanic white, non-Hispanic Black, Hispanic, Asian, Native American, Other). We utilized generalized linear mixed models to test within- and between-hospital variation and inequities in HRIF participation.
Results:
Among the 19503 infants, 44.7% (IQR 31.1, 63.3) were seen in HRIF. Twenty six percent of the total variation in HRIF participation rates was due to between-hospital variation. In adjusted models, Black infants had a significantly lower odds of HRIF participation compared to white infants (aOR 0.73, 95% CI 0.64, 0.83). The within-hospital effect of race varied significantly between hospitals.
Conclusions:
There are significant racial inequities in HRIF participation with notable variation within and between hospitals. Further study is needed to identify potential hospital-level targets for interventions to reduce this inequity.
Article Summary
We identified significant racial and ethnic inequities, within- and between-hospitals, in high-risk infant follow-up program participation in a nationally representative sample.
Introduction
Extremely low birth weight (ELBW) infants and those born extremely preterm have an increased risk of developing neurodevelopmental delays, chronic medical problems, and functional impairments later in life.1–3 Because these impairments are often not evident at Neonatal Intensive Care Unit (NICU) discharge, the American Academy of Pediatrics recommends that all high-risk infants participate in high-risk infant follow-up (HRIF) programs after NICU discharge.4,5 While their composition varies, most HRIF programs are multidisciplinary clinics that provide enhanced neurodevelopmental and functional screening, assessment, and diagnosis as complement to standard care provided by primary pediatricians.6,7 One role of HRIF is early referral to appropriate developmental services, such as Early Intervention (EI), which is associated with improved outcomes.4,8–10 While limited by small sample sizes and non-random loss to follow up, some studies suggest that early and consistent participation in HRIF is associated with improved outcomes for high-risk infants.11,12 A new conceptualization of HRIF prioritizing a “follow-through” paradigm examines both medical and social factors to further optimize pediatric health.13
Despite the importance of HRIF, only approximately 50% of eligible infants participate.6,14,15 Medical factors such as younger gestational age, lower birth weight, and medical sequelae of prematurity such as bronchopulmonary dysplasia (BPD) and intraventricular hemorrhage (IVH) have been associated with increased HRIF participation, as have social factors such as maternal age, socioeconomic status, and post-NICU discharge neighborhood opportunity.6,14–18 Furthermore, racial and ethnic inequities exist in HRIF which may contribute to long-term health inequity across the pediatric life-course, though the drivers of these inequities are unknown.6,15–17,19
Race is a social construct and inequities in health and healthcare utilization are the result of institutional, structural, interpersonal, and internalized racism.20–22 Inequities can originate and be propagated at multiple levels, and hospital variation in healthcare quality has been shown to be an important driver of racial and ethnic health inequities.23–27 Using a large multicenter cohort of ELBW infants, we tested the hypothesis that the effect of race and ethnicity on HRIF participation varies significantly both within- and between-hospitals and that hospital-level factors contribute to racial and ethnic inequities in HRIF participation.
Methods
Data Source
VON is a voluntary worldwide community of practice dedicated to improving the quality, safety, and value of newborn care through a coordinated program of data-driven quality improvement, education, and research. Of the 819 centers in the US participating in VON from 2006 to 2017, 58 centers participated in the VON ELBW Follow-up Project and contributed data for this study (Supplementary Table 1) (N=29421).
Population
Participating centers reported demographic information and neurodevelopmental outcomes at 18–24 months corrected age among infants who were 401–1000 grams or 22–27 completed weeks at birth and seen for follow-up from 2007–2019.28
We limited our study to infants born at US VON participating centers. We excluded infants who died prior to NICU discharge (N=6960) or HRIF program visit (N=211), or who had unknown hospital disposition or outcome data (N=38). We also excluded infants who had incomplete race and ethnicity data (N=59). The University of Vermont and Beth Israel Deaconess Medical Center Institutional Review Boards (IRB) determined that use of data from the VON Research Repository for this analysis is human subjects research that meets the criteria for exempt status.
Race and Ethnicity
Race is a social construct reflecting hierarchies of power. Health differences between racial and ethnic populations are not reflective of biology, genetics, or individual behavior. Rather, these differences represent the effects of racism and we therefore include race as a proxy for racism in our analysis.29,30 Participating centers submitted data on race (Black or African American; white; Asian; American Indian or Alaskan native; native Hawaiian or other Pacific Islander; other) and ethnicity (Hispanic; not Hispanic) based on the 2010 US Census definitions.31 Abstractors were instructed to obtain the information by personal interview with the mother, review of the birth certificate, or medical record, in that order.32 We combined race and ethnicity to yield non-Hispanic white, non-Hispanic Black, non-Hispanic Asian or Pacific Islander, non-Hispanic American Indian or Alaskan native, Hispanic, or other which we refer to as white, Black, Asian, Native American, Hispanic, and other respectively.
Variables
The primary outcome for the study was participation in HRIF at 18–24 months corrected for gestational age which is the standard timepoint for evaluation in HRIF per VON ELBW Follow-up Project protocol. Participating institutions coordinated follow-up, and informed consent for inclusion in the ELBW Follow-up Project was obtained according to each institution’s IRB specifications. Centers attempted to schedule follow-up with the families or caregivers of all infants who were alive at hospital discharge.
Infant-level demographic and NICU-comorbidity information were linked in the VON Very Low Birth Weight (VLBW) database. Covariables of interest included gestational age, sex, multiple gestation, inborn status, grade 3 or 4 IVH, presence of periventricular leukomalacia (PVL), BPD, necrotizing enterocolitis (NEC), NEC requiring surgical intervention, focal intestinal perforation, severe retinopathy of prematurity (sROP), and patent ductus arteriosus (PDA) requiring surgical ligation. Additionally, we included the need for durable medical equipment (DME) following NICU discharge in the analysis. Need for DME included 1) gastrostomy or jejunostomy tube, 2) home oxygen, 3) tracheostomy or tracheotomy, or 4) discharge with apnea/cardiorespiratory monitoring. Because of the de-identified nature of the data, no measures of residential racism or opportunity could be included. Data were collected by participating center staff using uniform definitions until neonates were discharged from the hospital, died, or reached one year of age in the hospital.32 Measures were reported using standardized data collection tools and each data item was defined in the ELBW Infant Follow-Up Manual of Operations.28
Hospital-level covariables of interest obtained from the VON Annual Member Survey included hospital type, (Government-Owned, Non-Profit, Private, Other) and academic hospital (presence of residents or fellows). Average number of ELBW admissions annually and proportion of white NICU ELBW admissions were obtained from infant data.
Analysis
We performed bivariate analyses using Student’s t test for continuous variables and Chi-square test for categorical variables between all infant-level variables and the primary outcome. We used generalized linear models to determine the association between hospital-level variables and the primary outcome accounting for hospital-level clustering. We derived the list of candidate variables for our multivariable model from bivariate analyses with threshold for inclusion of p<0.10.
The data were structured in two levels, with individual infants at level one and hospitals at level two. The clustering of infants within hospitals informed the analytic approach, affording the opportunity to quantify the relative contributions of covariables at each level.
We used generalized linear mixed effect models to test within- and between-hospital variation in HRIF participation. We first created a null model with random hospital-level intercepts to calculate the interclass correlation coefficient (ICC). The ICC is a ratio of the between-hospital variance to the total variance, to determine the proportion of the total variance attributable to hospital only. We then added race/ethnicity to the model to estimate the effect of racism on HRIF participation. Because racism may vary between different hospitals, we included a random term for race/ethnicity to allow the effect of race (i.e. racism) to vary between hospitals. Because we were interested in inequity of at-risk infants compared to white infants, we coded white as the reference category. We then included all infant-level variables from the candidate list. To identify hospital-level factors associated with HRIF participation, we added hospital-level variables one at a time. Infants with missing data were excluded from the model.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Our study sample included 19503 infants. Seven thousand two hundred eighteen infants were excluded because of unknown disposition or death, and 2,711 were excluded because they were born in a non-U.S. center (N=2,654), had unknown race (N=59), or had unknown follow up status (N=1) (See Supplemental Figure 1). Of the included 19503 infant, the mean gestational age was 26 weeks (Standard Deviation (SD) 1.87) and mean birth weight was 829.3g (SD 191g). There were 9477 (48.6%) white, 6346 (32.5%) Black, 2675 (13.7%) Hispanic, 639 (3.3%) Asian, and 147 (0.8%) Native American infants. Two hundred-nineteen (1.1%) were identified as other. The complete characteristics of the study population are shown in Table 1.
Table 1.
Patient demographics and baseline characteristics
| Number or mean | Percent or Standard Deviation | |
|---|---|---|
| Maternal race | ||
| White | 9477 | 48.6% |
| Black | 6346 | 32.5% |
| Hispanic | 2675 | 13.7% |
| Asian | 639 | 3.3% |
| Native American | 147 | 0.8% |
| Other | 219 | 1.1% |
| Infant factors | ||
| Gestational age (weeks) | 26.1 | (1.9) |
| Birthweight (grams) | 829.3 | (191.1) |
| SGA (<10%ile) | 3007 | 15.5% |
| Male | 9960 | 51.1% |
| Multiple gestation | 4853 | 24.9% |
| Inborn | 15801 | 81.0% |
| Presence of NICU co-morbidity | ||
| IVH grade 3–4 | 1832 | 9.5% |
| Periventricular leukomalacia | 755 | 3.9% |
| Bronchopulmonary Dysplasia | 8864 | 46.4% |
| Necrotizing enterocolitis | 1273 | 6.5% |
| Necrotizing enterocolitis requiring surgery | 998 | 5.1% |
| Focal intestinal perforation | 796 | 4.1% |
| Severe ROP | 2647 | 14.0% |
| PDA requiring surgical ligation | 3137 | 16.1% |
| Need for durable medical equipment at discharge | ||
| Gastrostomy or Jejunostomy tube | 1274 | 6.5% |
| Home oxygen | 5105 | 28.6% |
| Tracheostomy/Tracheotomy | 336 | 1.7% |
| Apnea/Cardiorespiratory monitoring | 7327 | 41.1% |
| Hospital factors | ||
| Hospital type | ||
| Government-Owned | 5 | 8.6% |
| Non-Proft | 47 | 81.0% |
| Private | 4 | 6.9% |
| Other | 2 | 3.5% |
| Academic Hospital | 42 | 72.4% |
| Avg number of ELBW admissions annually | 62.5 | 31.2 |
| % white ELBW admissions | 48.2% | 21.6 |
| Participation in HRIF | 8721 | 44.7% |
Of the 58 hospitals included in the analysis, 18 (31.0%) contributed between 1 to 3 years of data, 11 hospitals (19.0%) contributed 4 to 7 years of data, 9 hospitals (15.5%) contributed 8 to 10 years of data, and 20 hospitals (34.5%) contributed 11 or more years of data. Eighteen hospitals (31.0%) contributed to all 12 years of data. The overall follow up rate did not vary by year over the study period. (See Supplementary Table 2) The majority of birth hospitals for eligible infants were classified as not-for-profit (47, 81.0%) and academic (42, 72.4%). The average ELBW admissions annually was 63 (SD 32) and the percent of white ELBW admissions was 48.2% (SD 21.6%).
Bivariate Analyses
Infants who had a HRIF visit at 18–24 months corrected age were more likely to be of white race (p<0.001), younger gestational age (26.0 weeks [SD 1.8] vs. 26.3 weeks [SD 1.9], p<0.001), a product of multiple gestation (27.0% v. 23.2%, p<0.001), and born in the hospital they were cared for (inborn) (84.5% v. 78.2%, p<0.001). They were more likely to have BPD (47.2% v. 44.0%, p=0.001), sROP (15.1% v. 13.1%, p<0.001), and a PDA requiring surgical ligation (17.5% v. 15.0%, p<0.001). Following discharge, infants that participated in HRIF were more likely to have been discharged with an apnea/cardiorespiratory monitor (43.6% v. 39.0%, p<0.001). Infants in hospitals with a higher proportion of white ELBW admissions were also more likely to participate in HRIF. (Table 2)
Table 2.
Bivariate analysis of infant and hospital factors with HRIF participation
| Not seen in HRIF (N=10790) | Seen in HRIF (N=8721) | ||||
|---|---|---|---|---|---|
| Number or mean | Percent or Standard Deviation | Number or mean | Percent or Standard Deviation | p-value | |
| Maternal Race | |||||
| White | 4877 | 45.2% | 4600 | 52.8% | <0.001 |
| Black | 3759 | 34.8% | 2587 | 29.7% | |
| Hispanic | 1557 | 14.4% | 1118 | 12.8% | |
| Asian | 339 | 3.1% | 300 | 3.4% | |
| Native American | 95 | 0.9% | 52 | 0.6% | |
| Other | 155 | 1.4% | 64 | 0.7% | |
| Infant factors | |||||
| Gestational age (weeks) | 26.3 | (1.9) | 26.0 | (1.8) | <0.001 |
| SGA (<10%ile) | 1691 | 15.7% | 1316 | 15.1% | 0.27 |
| Male | 5543 | 51.4% | 4417 | 50.7% | 0.29 |
| Multiple gestation | 2499 | 23.2% | 2354 | 27.0% | <0.001 |
| Inborn | 8439 | 78.2% | 7362 | 84.5% | <0.001 |
| Presence of NICU co-morbidity | |||||
| IVH grade 3–4 | 1035 | 9.6% | 797 | 9.1% | 0.24 |
| Periventricular leukomalacia | 417 | 3.9% | 338 | 3.9% | 0.98 |
| Bronchopulmonary Dysplasia | 4748 | 44.0% | 4116 | 47.2% | 0.001 |
| Necrotizing enterocolitis | 737 | 6.8% | 536 | 6.2% | 0.052 |
| Necrotizing enterocolitis requiring surgery | 579 | 5.4% | 419 | 4.8% | 0.074 |
| Focal intestinal perforation | 443 | 4.1% | 353 | 4.1% | 0.83 |
| Severe ROP | 1357 | 13.1% | 1290 | 15.1% | <0.001 |
| PDA requiring surgical ligation | 1615 | 15.0% | 1522 | 17.5% | <0.001 |
| Need for durable medical equipment at discharge | |||||
| Gastrostomy or Jejunostomy tube | 723 | 3.7% | 551 | 6.3% | 0.27 |
| Home oxygen | 2754 | 28.7% | 2351 | 28.6% | 0.96 |
| Tracheostomy/Tracheotomy | 199 | 1.9% | 137 | 1.6% | 0.14 |
| Apnea/Cardiorespiratory monitoring | 3747 | 39.0% | 3580 | 43.6% | <0.001 |
| Hospital factors (N=58) | |||||
| Hospital type | |||||
| Government-Owned | 864 | 8.0% | 945 | 10.8% | 0.33 |
| Non-Profit | 7955 | 73.7% | 6938 | 79.6% | |
| Private | 1303 | 12.1% | 490 | 5.6% | |
| Other | 663 | 6.1% | 339 | 3.9% | |
| Academic Hospital | 7296 | 67.6% | 6215 | 71.3% | 0.63 |
| Number of ELBW admissions annually | |||||
| Lowest Quintile (Q1) | 529 | 4.9% | 545 | 6.3% | 0.76 |
| Middle Quintiles (Q2-Q4) | 6996 | 64.8% | 5176 | 59.4% | |
| Highest Quintile (Q5) | 3266 | 30.3% | 2991 | 34.3% | |
| % White ELBW admissions | |||||
| Lowest Quintile (Q1) | 1973 | 18.3% | 1064 | 12.2% | 0.092 |
| Middle Quintiles (Q2-Q4) | 2693 | 25.0% | 5915 | 67.9% | |
| Highest Quintile (Q5) | 1419 | 13.2% | 1733 | 19.9% | |
p-value for maternal race, infant factors, presence of NICU co-morbidity, and need for durable equipment at discharge computed using Chi square test or Student’s t test for categorical or continuous variables respectively. P-value for hospital factors computed using generalized linear models accounting for hospital-level clustering
Generalized Linear Mixed Models
In our null model with random hospital-level intercepts, the ICC was 0.26 indicating that 26% of the variability in the outcome was due to hospital alone. (Figure 1) After including race/ethnicity with a random term to the model, we found that compared to white infants, Black infants had an adjusted odds ratio (aOR) of participating in HRIF of 0.72 (95% Confidence Interval (CI) [0.63,0.81], p<0.0001) and Hispanic infants had an aOR of 0.83 (95% CI [0.72, 0.96], p=0.01). The covariance parameter for the random race/ethnicity term was statistically significant, indicating that the effect of race/ethnicity significantly varied between hospitals. (Figure 2)
Figure 1.
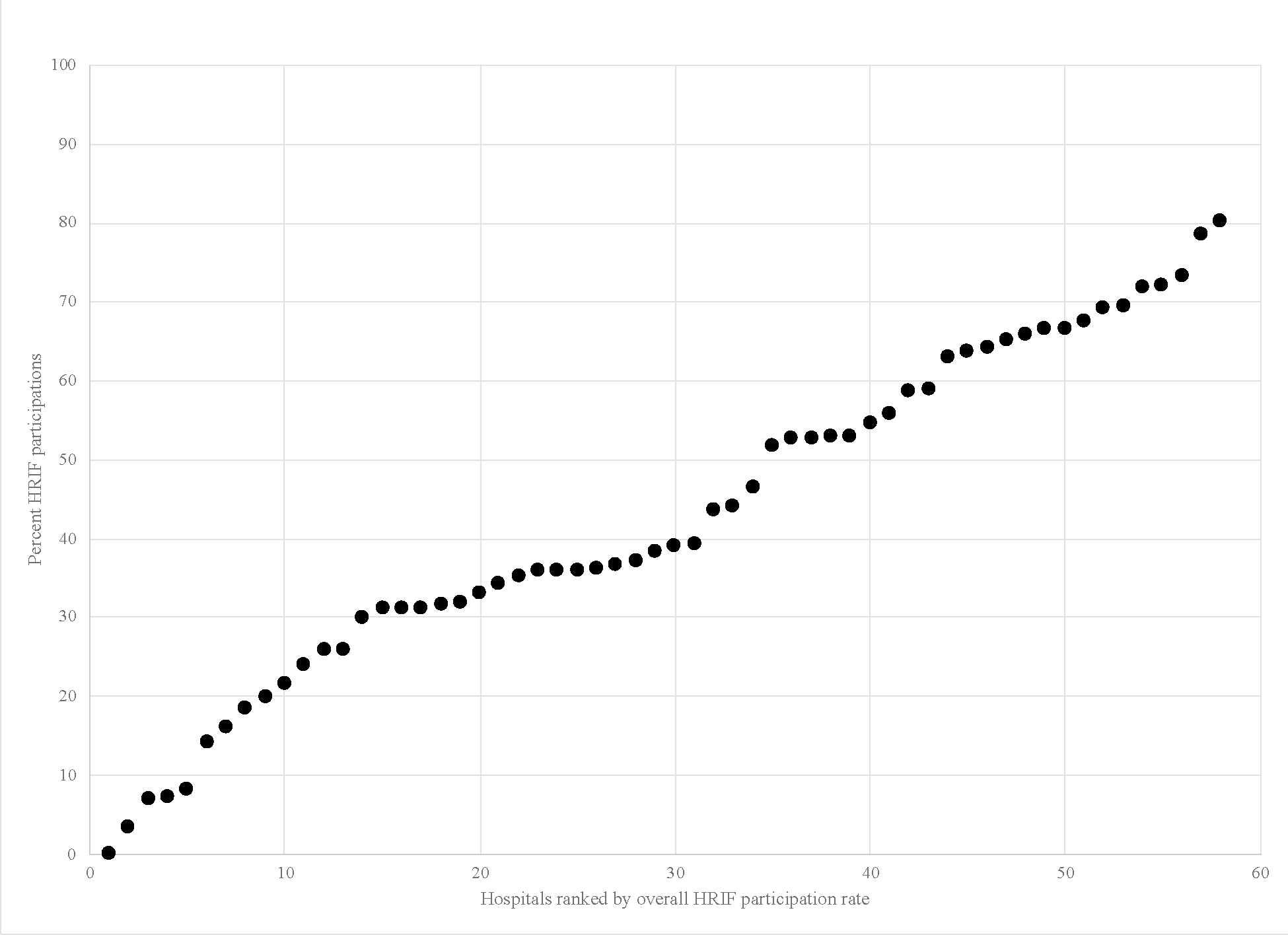
Between Hospital Variation in HRIF Participation
Figure 2.
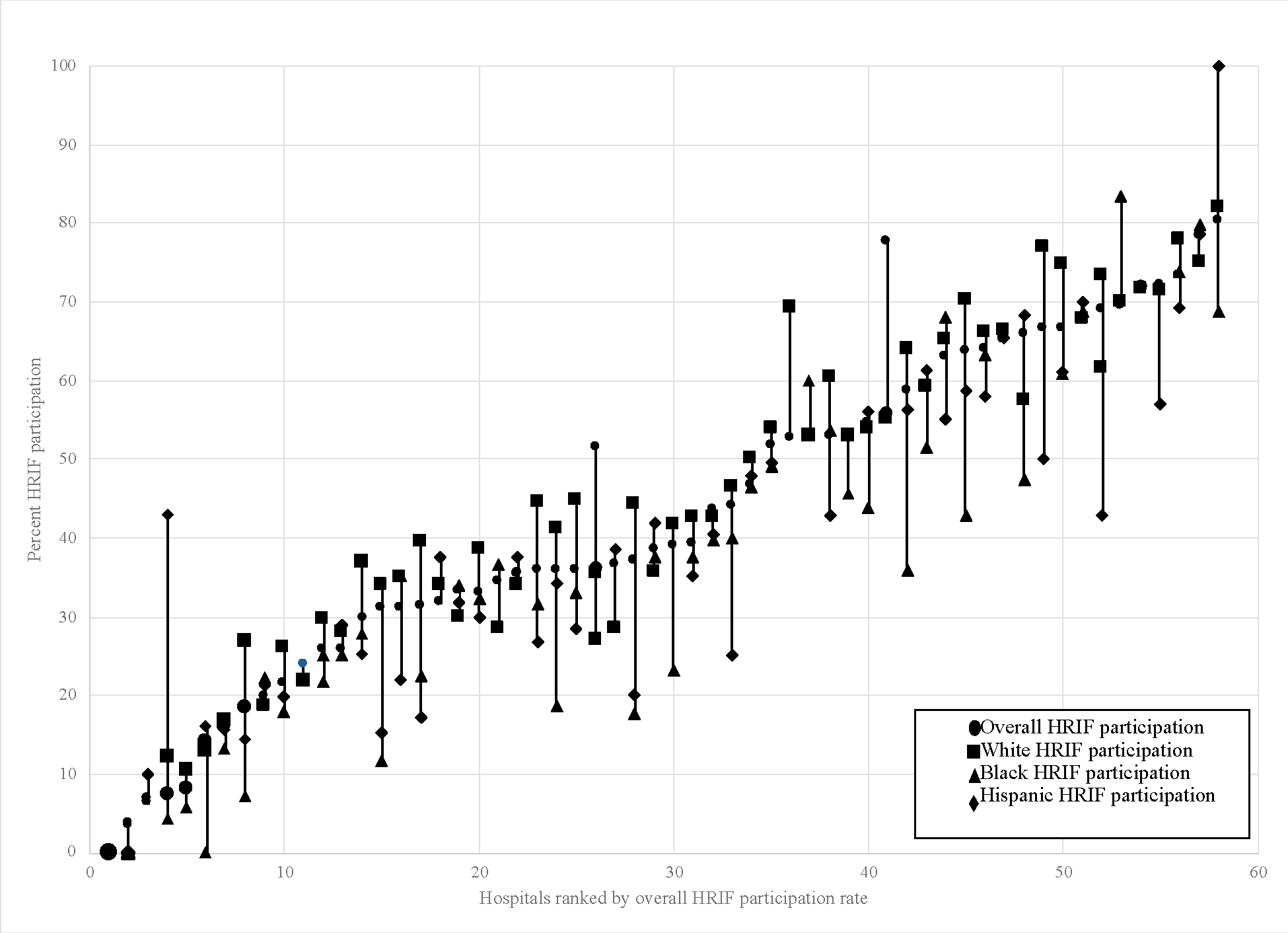
Within and Between Hospital Inequity in HRIF Participation
Upon adding the infant-level covariables, race/ethnicity continued to be a significant predictor of HRIF participation (Table 3). In addition, infants of younger gestational ages and those who were a product of a multiple gestation, were inborn compared to outborn, and had a PDA requiring surgical ligation were more likely to participate in HRIF. Infants discharged from the NICU with apnea/cardiorespiratory monitoring were also more likely participate in HRIF.
Table 3.
Results of the multivariable generalized mixed effects model of HRIF with hospital-level intercepts and random term for race
| aOR | 95% CI | p-value | Wald Type 3 p-value | |
|---|---|---|---|---|
| Maternal race | ||||
| White | ref | <0.001 | ||
| Black | 0.73 | (0.64, 0.83) | <0.001 | |
| Hispanic | 0.87 | (0.75, 1.01) | 0.07 | |
| Asian | 0.98 | (0.79, 1.22) | 0.88 | |
| Native American | 0.74 | (0.48, 1.14) | 0.02 | |
| Other | 0.69 | (0.48, 0.99) | 0.04 | |
| Infant factors | ||||
| Gestational age (weeks) | 0.93 | (0.92, 0.95) | <0.001 | |
| Multiple gestation | 1.14 | (1.06, 1.23) | <0.001 | |
| Inborn | 1.42 | (1.28, 1.58) | <0.001 | |
| Presence of NICU co-morbidity | ||||
| Bronchopulmonary Dysplasia | 1.04 | (0.96, 1.13) | 0.30 | |
| Necrotizing enterocolitis | 0.93 | (0.79, 1.09) | 0.36 | |
| Necrotizing enterocolitis requiring surgery | 0.91 | (0.76, 1.08) | 0.27 | |
| Severe ROP | 1.04 | (0.94, 1.16) | 0.42 | |
| PDA requiring surgical ligation | 1.12 | (1.01, 1.23) | 0.03 | |
| Need for durable medical equipment at discharge | ||||
| Apnea/Cardiorespiratory monitoring | 1.21 | (1.11, 1.31) | <0.001 | |
| Hospital factors | ||||
| Proportion white ELBW admissions | ||||
| Lowest Quintile (Q1) | 0.59 | (0.29, 1.19) | 0.14 | 0.11 |
| Middle Quintiles (Q2-Q4) | ref | |||
| Highest Quintile (Q5) | 1.51 | (0.74, 3.06) | 0.26 | |
No hospital-level covariables were significantly associated with the outcome in our multivariable model. However, upon adding a term for the proportion white ELBW admissions to the model, the hospital level variance decreased and the ICC decreased, indicating that a proportion of hospital-level variability was due to the percent of white infants in the NICU. (Results of the complete model-building process can be found in Supplementary Table 3)
Subgroup Analyses
We pursued exploratory subgroup analyses limited to infants identified as white and Black because this was the largest inequity in the population. There were no significant changes to the infant-level factors predicting HIRF participation. Upon adding the proportion of white ELBW infants into the model in quintiles, we identified a potential dose-response relationship, and thus included the variable in the model. Upon inclusion as a continuous variable, we found that increasing proportion of white ELBW was associated with a higher aOR of 3.91 (95% CI 1.06–14.47) of HRIF participation. This suggests an effect of the NICU racial composition on follow-up rates. We further explored whether there was a differential role of proportion white infants in the NICU on white vs. Black infants by including an interaction term (proportion white ELBW admissions × Black). This interaction term was not significant, meaning there was no difference in the effect of proportion white ELBWs on follow-up for Black compared to white infants. (Supplementary Table 4 and Table 5)
Discussion
We identified significant variation in HRIF participation among infants cared for in 58 NICUs in the United States from 2006–2017. The proportion of eligible infants with a HRIF visit ranged from zero percent to 80% by hospital. Hospital alone accounted for 26% of the variability in outcome. In multivariable models we did not identify specific hospitals characteristics associated with HRIF participation. In addition to low HRIF participation overall that has been previously reported in the literature, we identified significant racial and ethnic inequity in HRIF both between- and within-hospitals. Importantly, the effect of race significantly varied between hospitals.
Previous studies have identified individual health-related factors associated with HRIF participation with varied results. While Mercier et. al. identified that inborn infants, multiple gestations, and younger gestational ages were associated with an increased likelihood of participation in HRIF, Nehra et. al. did not find similar patterns for infants of multiple gestations.14,33 Furthermore, while Hintz and others have identified that NICU co-morbidities such as IVH or BPD are associated with an increased likelihood of HRIF participation, Nehra et. al. did not.15,16,33 When accounting for the clustering effect of hospital, we identified few NICU co-morbidities that predict individual-level HRIF participation. Those co-morbidities that continue to be associated with HRIF, younger gestational age, a PDA requiring surgical ligation, and infants discharged from the NICU with an apnea/cardiorespiratory monitor may represent a subset of patients that are more ill, or perceived by caregivers to be more ill, at discharge and thus caregivers may place a higher priority on attending HRIF for developmental support than infants without those co-morbidities. Further research is needed to understand observed relationships, particularly caregiver perceptions of the role and value of HRIF, and to develop strategies to further increase HRIF attendance for infants without co-morbidities.
Our findings corroborate previous findings of inequities in HRIF participation.6,15–17 Though race has been conflated with poverty and other indicators of material deprivation, it is now being acknowledged that the social disadvantage associated with race is not truly synonymous with, nor a proxy for, race. Rather, those associations are the result of inequitable programs and policies favoring one group over another, that is, long-standing structural racism. Our unique methodologic approach employing multilevel mixed effects modeling enabled us to partition the many types of racism at multiple ecologic levels that drive inequities in HRIF participation in the US.
The fixed effect of race, consistent across individuals in our sample, may be conceptually understood to represent the impact of structural racism on healthcare access utilization. Structural racism refers to the ways in racial discrimination is perpetuated through mutually reinforcing systems such as healthcare, housing, and income inequality.22 Here, we imagine that such structural forces lead to an overall decreased odds of HRIF participation for Black ELBW infants in the US.
In subgroup analyses we saw that hospitals with more white infants had higher overall HRIF participation rates. This is consistent with previous data that has highlighted that hospital segregation is associated with care quality.23,25,35 In our study, the association between hospital demographics and HRIF participation may be considered evidence of institutional racism, the differential and racialized access to resources, where hospitals with more white patients deliver higher quality care.21 Exploring hospital-demographics as a driver of racial and ethnic inequities may prove useful in exploring the underpinnings of inequities in other health-outcomes and health service utilization.
Our results indicate that the effect of race significantly differed not just between hospitals but within hospitals, as well. Within-hospital inequity may represent local structural and institutional racism that may lead to differential HRIF participation due to in-hospital lack of social supports, diminished trust between providers and patients and families, and post-discharge decreased clinic accessibility, lack of transportation, or limited time off from work. Additionally, it may be that the within-hospital inequities are the effect of interpersonal racism. Interpersonal racism refers to personally-mediated prejudice, assumptions, and differential behaviors and actions based on race.21 Quantitative and qualitative research has found that within NICUs there is differential quality, treatment, and experiences of Black compared to white families.23,27,34 Interpersonal racism, experienced as either explicit or implicit racism, may explain how infants within a single NICU have different likelihoods of HRIF participation based on race.
Inequities in HRIF participation are important not only because they may lead to unmet needs and poorer quality care in the short-term, but also because of potential effects on long-term pediatric health. There are racial and ethnic inequities in NICU co-morbidities like IVH that are associated with poorer long-term outcomes.35–38 HRIF participation is designed for early detection of prematurity-associated neurodevelopmental and functional impairments and to facilitate referral to needed services. Inequities in HRIF participation may therefore serve to further widen the health equity gap that already exists between white and non-white infants at NICU discharge and potentially magnify the effects of inequitable perinatal health throughout the life-course.19
This work has three methodologic limitations. First, infant race and ethnicity was based on the classification of maternal race and ethnicity. Not only may there be misclassification by data abstractors, but identifying infant race as maternal race erases the social construction of race as well as the important role of non-birthing parents in a child’s identity formation. Furthermore, categorical, mutually exclusive definitions of race and ethnicity do not acknowledge individuals with multiple intersectional identities and obscures the important granularity of within-race subpopulation analysis. Second, our study lacked important social information, such as indicators of socioeconomic status, that may represent important mediators that lie downstream from structural, residential, and institutional racism.39 In line with investment and focus on equitable follow-through after NICU discharge, efforts are currently underway at VON to improve the collection of social and demographic data. Finally, we have measured follow-up visit at 18–24 months as our outcome and we are unable to know if non-participation resulted from lack of referral from the NICU, missed HRIF appointments after a referral was made, or follow up in a clinic that did not participate in the VON ELBW Follow-up Project.
Despite these limitations, our study has several strengths. We used a multilevel analytic approach to capitalize on the data structure and answer questions about the roles of individuals and hospitals in follow-up. Our work is both hypothesis-driven and grounded in social science theory. We utilized the social ecological model40 and the public health critical race praxis41 (PHCR) to develop our hypothesis, inform methodologic approaches, and aid in the interpretation of results. The social ecological model views ecological levels, in this case, the individual and the hospital, as each having distinct influences on health.40 PHCR is a “semi-structured process for conducting research that remains attentive to issues of both racial equity and methodologic rigor.”41 PHCR is based on the understanding that race is a social construct and that racism is ordinary and embedded in society. It orients research to exploring the underlying drivers of inequity and encourages researchers to consider research as a pathway to health equity and justice. Using these frameworks, we identified multiple levels at which racial and ethnic inequity is upheld and potential targets, at multiple ecological levels, for interventions to improve health equity. Improving HRIF participation may be critical in preventing the worsening of health equity between white and non-white high-risk preterm infants across the life-course.
Conclusions
We identified significant within- and between-hospital variation in the effect of race on HRIF participation among a national, multi-institutional cohort of ELBW infants. There are multiple mechanisms by which racism —interpersonal, institutional, and structural—and its downstream effects, may impact HRIF participation among high-risk infants. Further study is needed to identify actionable targets for interventions to address these inequities in care to close the equity gap.
Supplementary Material
What’s Known on This Subject
High-risk infant follow-up is a recommended component of post-discharge care of the preterm infant and may reduce the long-term impact of prematurity. However, only 50% of eligible infants participate and studies to identify factors associated with participation are limited.
What This Study Adds
Using a national sample and employing generalized linear mixed effects modeling, we identified significant between- and within-hospital variation in the effect of race/ethnicity on high-risk infant follow-up program participation. This study highlights how racism affects neonatal health service utilization.
Acknowledgments
We thank our colleagues who submit data to the VON on behalf of infants and their families. Participating centers are listed in Supplemental Table 1. We thank Lucy Greenberg for her statistical support.
Funding/Support:
Dr. Fraiman was supported by AHRQ grant number T32HS000063 as part of the Harvard-wide Pediatric Health Services Research Fellowship Program and by the American Academy of Pediatrics, Section on Neonatal Perinatal Medicine Marshall Klaus Perinatal Research Award. The authors received no additional funding.
Abbreviations:
- ELBW
Extremely Low Birth Weight
- NICU
Neonatal Intensive Care Unit
- HRIF
High-Risk Infant Follow-Up Program
- EI
early intervention
- BPD
bronchopulmonary dysplasia
- NEC
necrotizing enterocolitis
- IVH
intraventricular hemorrhage
- IRB
Institutional Review Board
- PVL
periventricular leukomalacia
- sROP
severe retinopathy of prematurity
- SD
standard deviation
- CI
confidence interval
Footnotes
Conflict of Interest Disclosures: Dr. Edwards receives salary support from the Vermont Oxford Network. Dr. Horbar is Chief Executive Officer, President, and Chief Scientific Officer of Vermont Oxford Network and an unpaid member of the Vermont Oxford Network Board of Directors. Dr. Soll is Vice President, Director of Clinical Trials and Follow-up, and an unpaid member of the Vermont Oxford Network Board of Directors. The other authors have no relevant financial conflicts of interest to disclose.
References
- 1.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 2.Spittle AJ, Cameron K, Doyle LW, Cheong JL. Motor Impairment Trends in Extremely Preterm Children: 1991–2005. Pediatrics. 2018;141(4):e20173410. doi: 10.1542/peds.2017-3410 [DOI] [PubMed] [Google Scholar]
- 3.Adams-Chapman I, Heyne RJ, DeMauro SB, et al. Neurodevelopmental Impairment Among Extremely Preterm Infants in the Neonatal Research Network. Pediatrics. 2018;141(5):e20173091. doi: 10.1542/peds.2017-3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Committee on Fetus and Newborn. Hospital Discharge of the High-Risk Neonate. Pediatrics. 2008;122(5):1119–1126. doi: 10.1542/peds.2008-2174 [DOI] [PubMed] [Google Scholar]
- 5.Follow-up care of high-risk infants. In: Pediatrics. Vol 114. American Academy of Pediatrics; 2004:1377–1397. doi: 10.1542/peds.2004-0866 [DOI] [Google Scholar]
- 6.Litt JS, Edwards EM, Lainwala S, et al. Optimizing High-risk Infant Follow-up in Nonresearch-based Paradigms. Pediatr Qual Saf. 2020;5(3):e287. doi: 10.1097/pq9.0000000000000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuppala VS, Tabangin M, Haberman B, Steichen J, Yolton K. Current state of high-risk infant follow-up care in the United States: Results of a national survey of academic follow-up programs. J Perinatol. 2012. doi: 10.1038/jp.2011.97 [DOI] [PubMed] [Google Scholar]
- 8.Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015. doi: 10.1002/14651858.CD005495.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick MC, Brooks-Gunn J, Buka SL, et al. Early intervention in low birth weight premature infants: Results at 18 years of age for the infant health and development program. Pediatrics. 2006;117(3):771–780. doi: 10.1542/peds.2005-1316 [DOI] [PubMed] [Google Scholar]
- 10.Litt JS, Glymour MM, Hauser-Cram P, Hehir T, McCormick MC. Early Intervention Services Improve School-age Functional Outcome Among Neonatal Intensive Care Unit Graduates. Acad Pediatr. 2018;18(4):468–474. doi: 10.1016/j.acap.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 11.Callanan C, Doyle L, Rickards A, Kelly E, Ford G, Davis N. Children followed with difficulty: How do they differ? J Paediatr Child Health. 2001;37(2):152–156. doi: 10.1046/j.1440-1754.2001.00621.x [DOI] [PubMed] [Google Scholar]
- 12.Tin W, Fritz S, Wariyar U, Hey E. Outcome of very preterm birth: children reviewed with ease at 2 years diVer from those followed up with diYculty. Arch Dis Child Fetal Neonatal Ed. 1998;79:83–87. doi: 10.1136/fn.79.2.F83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horbar JD, Edwards EM, Ogbolu Y. Our Responsibility to Follow Through for NICU Infants and Their Families. Pediatrics. 2020;146(6). doi: 10.1542/peds.2020-0360 [DOI] [PubMed] [Google Scholar]
- 14.Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF, Vermont Oxford Network ELBW Infant Follow-Up Study Group. Neurodevelopmental Outcome of Extremely Low Birth Weight Infants from the Vermont Oxford Network: 1998–2003. Neonatology. 2010;97(4):329–338. doi: 10.1159/000260136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraiman YS, Stewart JE, Litt JS. Race, language, and neighborhood predict high-risk preterm Infant Follow Up Program participation. J Perinatol. 2021. doi: 10.1038/s41372-021-01188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hintz SR, Gould JB, Bennett MV., et al. Factors Associated with Successful First High-Risk Infant Clinic Visit for Very Low Birth Weight Infants in California. J Pediatr. 2019;210:91–98.e1. doi: 10.1016/j.jpeds.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 17.Swearingen C, Simpson P, Cabacungan E, Cohen S. Social disparities negatively impact neonatal follow-up clinic attendance of premature infants discharged from the neonatal intensive care unit. J Perinatol. 2020;40(5):790–797. doi: 10.1038/s41372-020-0659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmon SL, Conaway M, Sinkin RA, Blackman JA. Factors Associated With Neonatal Intensive Care Follow-up Appointment Compliance. Clin Pediatr (Phila). 2013;52(5):389–396. doi: 10.1177/0009922813477237 [DOI] [PubMed] [Google Scholar]
- 19.Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. July 2019. doi: 10.1038/s41390-019-0513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger N A glossary for social epidemiology. J Epidemiol Community Health. 2001;55(10):693–700. doi: 10.1136/jech.55.10.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CP. Levels of racism: A theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212–1215. doi: 10.2105/AJPH.90.8.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Profit J, Gould JB, Bennett M, et al. Racial/Ethnic Disparity in NICU Quality of Care Delivery. Pediatrics. 2017;140(3):e20170918. doi: 10.1542/peds.2017-0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horbar JD, Edwards EM, Greenberg LT, et al. Racial Segregation and Inequality in the Neonatal Intensive Care Unit for Very Low-Birth-Weight and Very Preterm Infants. JAMA Pediatr. 2019;173(5):455. doi: 10.1001/jamapediatrics.2019.0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glazer KB, Zeitlin J, Egorova NN, et al. Hospital Quality of Care and Racial and Ethnic Disparities in Unexpected Newborn Complications. Pediatrics. 2021;148(3). doi: 10.1542/peds.2020-024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in Morbidity and Mortality Rates in Black, White, and Hispanic Very Preterm Infants Among New York City Hospitals. JAMA Pediatr. 2018;172(3):269. doi: 10.1001/jamapediatrics.2017.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards EM, Greenberg LT, Profit J, Draper D, Helkey D, Horbar JD. Quality of care in US NICUs by race and ethnicity. Pediatrics. 2021;148(2). doi: 10.1542/peds.2020-037622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermont Oxford Network. Manual of Operations: The Infant Follow-up Project: Extremely-Low-Birth-Weight Infant Birth Year 2017 Cohort, Version 20. Burlington, VT: Vermont Oxford Network; 2018. [Google Scholar]
- 29.Boyd RW, Lindo EG, Weeks LD, McLemore MR. On Racism: A new standard for publishing on racial health inequities. Health Affairs Forefront. doi: 10.1377/hblog20200630.939347 [DOI] [Google Scholar]
- 30.Lett E, Asabor E, Beltrán S, Michelle Cannon A, Arah OA. Conceptualizing, Contextualizing, and Operationalizing Race in Quantitative Health Sciences Research. Ann Fam Med. January 2022:2792. doi: 10.1370/afm.2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humes KR, Jones NA, Ramirez RR. Overview of Race and Hispanic Origin: 2010. Washington, D.C.; 2011. [Google Scholar]
- 32.Vermont Oxford Network. Manual of Operations Part 2: Data Definitions and Infant Data Forms. Vol 23. Burlington, VT: Vermont Oxford Network; 2018. [Google Scholar]
- 33.Nehra V, Pici M, Visintainer P, Kase JS. Indicators of compliance for developmental follow-up of infants discharged from a regional NICU. J Perinat Med. 2009;37(6). doi: 10.1515/JPM.2009.135 [DOI] [PubMed] [Google Scholar]
- 34.Sigurdson K, Morton C, Mitchell B, Profit J. Disparities in NICU quality of care: a qualitative study of family and clinician accounts. J Perinatol. 2018;38(5):600–607. doi: 10.1038/s41372-018-0057-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in morbidity and mortality rates in black, white, and hispanic very preterm infants among New York City Hospitals. JAMA Pediatr. 2018;172(3):269–277. doi: 10.1001/jamapediatrics.2017.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janevic T, Zeitlin J, Auger N, et al. Association of Race/Ethnicity With Very Preterm Neonatal Morbidities. JAMA Pediatr. 2018;172(11):1061. doi: 10.1001/jamapediatrics.2018.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murosko D, Passerella M, Lorch S. Racial Segregation and Intraventricular Hemorrhage in Preterm Infants. Pediatrics. 2020;145(6):e20191508. doi: 10.1542/peds.2019-1508 [DOI] [PubMed] [Google Scholar]
- 38.Sigurdson K, Mitchell B, Liu J, et al. Racial/Ethnic Disparities in Neonatal Intensive Care: A Systematic Review. Pediatrics. July 2019:e20183114. doi: 10.1542/PEDS.2018-3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yearby R Structural Racism and Health Disparities: Reconfiguring the Social Determinants of Health Framework to Include the Root Cause. J Law, Med Ethics. 2020;48(3):518–526. doi: 10.1177/1073110520958876 [DOI] [PubMed] [Google Scholar]
- 40.Bronfenbrenner U Understanding Children in Context: The Ecological Model of Human Development; 1979.
- 41.Ford CL, Airhihenbuwa CO. The public health critical race methodology: Praxis for antiracism research. Soc Sci Med. 2010;71(8):1390–1398. doi: 10.1016/j.socscimed.2010.07.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


