Abstract
Objectives
IBoerhaave’s syndrome (BS) is a rare, but potentially fatal condition, characterized by barogenic esophageal rupture and carries a high mortality. We aimed to study our institutional experience of managing patients with BS.
Material and Methods
A retrospective review of patients with BS presenting to a tertiary care centre from 2005 to 2018 was carried out in this study. Clinical presentation, diagnostic evaluations, treatments received, and treatment outcomes were studied. Perforations were classified as early (<24 hours) and delayed (>24 hours), based on the time elapsed. Surgical complications were graded using Clavien-Dindo grade. The Pittsburgh perforation severity score was correlated with short-term treatment outcomes.
Results
Of the 12 patients [male, 75%; mean (range) age, 53 (28-80) years] included, 10 patients had a delayed (>24 hours) presentation. Chest pain was the dominant symptom (58.3%); six patients presented either in shock (n= 1) or with organ failure (n= 3) or both (n= 2). All the perforations were sited in the lower thoracic esophagus, of which three were contained and nine were uncontained. The seal of the perforation was achieved by surgical repair in four patients (primary repair, 2; repair over a T-tube, 2) and endoscopic techniques in four patients (clipping, 1; stenting, 3). Sepsis drainage [surgical, 7 (open-5, minimally-invasive-2); non-surgical, 5] and feeding jejunostomy were performed in all patients. Five (41.7%) patients received a re-intervention. Median (range) hospital stay was 25.5 (12-101) days, 30-day operative morbidity was 50%, and there was one in-hospital death. The Pittsburgh perforation severity score was as follows: 2-5 in two patients and >5 in 10 patients; there were more delayed presentations, increased surgical interventions, post-procedure morbidity, and in-hospital mortality in the latter group, but the differences were statistically not significant. In 11 patients followed-up [median (range):1507 (17-5929) days], there was no disease recurrence, symptomatic reflux or dysphagia.
Conclusion
Favourable treatment outcomes, including reduced mortality and organ preservation can be achieved for Boerhaave’s perforations, through a multimodality approach. Minimally invasive, endoluminal or open surgical techniques may be safely utilized in its management. The Pittsburgh severity score can be a useful clinical tool that can be used to select the initial intervention and to predict treatment outcomes.
Keywords: Boerhaave’s syndrome, spontaneous esophageal perforation, surgery, therapeutic endoscopy, Pittsburgh perforation severity score
Introduction
Boerhaave’s syndrome (BS) is a rare, but potentially fatal condition, characterized by transmural esophageal rupture, secondary to the sudden rise in intraluminal pressure, as in forceful emesis (1). The perforation leads to contamination of the surrounding space with esophago-gastric contents, leading to local sepsis, organ failure, and a mortality rate of 24-50% in delayed presentations (2-4). BS diagnosis is often delayed because of its rarity, non-specific symptoms, and frequent initial diagnostic errors (5). Prompt diagnosis and timely intervention correlate with favorable treatment outcomes (3).
Due to its rarity, there is lack of standard guidelines for the optimal treatment of BS. Treatment options vary from conservative treatment to surgery as radical as esophagectomy. Surgical options include primary repair, tube esophagostomy, esophageal exclusion/diversion, and esophagectomy, combined with drainage and debridement of pleuro-mediastinal cavities (5-7). In a series of 88 patients with BS by Yan et al., the best operative outcomes including reduced postoperative esophageal leak, and shorter hospital and intensive care stays have been obtained in patients presenting early and receiving a primary repair, compared to buttressed repairs in the delayed group (8). In another series by Sutcliffe et al., immediate surgery was feasible in all eight patients presenting early but it was possible only in 6/10 patients presenting late (9). The rates of postoperative leak (78% vs 12.5%; p <0.05) and mortality (40% vs. 0%; p <0.05) were higher in the late referral group, and within the delayed referral subgroup, the worst mortality was seen in those managed conservatively. The authors have reiterated that the operative principles for BS are pleuro-mediastinal decontamination, debridement or resection of devitalized tissues, primary perforation repair (when feasible), gastric decompression, and enteral feeding access. Aggressive surgery including resection is suggested in delayed cases with extensive esophageal tissue loss (1).
With recent advances in minimally invasive surgery and therapeutic endoscopy, there is a paradigm shift towards a more conservative treatment approach for this condition. The safety and effectiveness of minimally invasive surgical approaches have been demonstrated by Haveman et al., Aref et al., Lee et al., Cho et al., and in a recent review by Aiolfi et al (10- 14). Minimally invasive surgery can potentially reduce surgical trauma, but the choice of operative access depends on the site of the perforation, the extent of pleuro-mediastinal contamination/necessity for pleural drainage. Similarly, several authors have reported the role of endoscopic therapy for the management of BS, particularly that of esophageal stents which are utilized both as a primary intervention and also as a salvage procedure for persistent leak following surgical repair (6,15,16).
Although a multitude of treatment options are available for BS and controversy exists concerning the best treatment modality, particularly for delayed presentations, optimal treatment outcomes are often achieved through a multimodality approach. Hence, patients should be managed at a centre, with appropriate facilities and the expertise to deal with this challenging condition.
In this study, it was aimed to review our institutional experience of managing patients with BS over a 13-year period, focusing on their clinical presentation, diagnostic evaluations, treatment approaches and their outcomes. We also attempted to retrospectively grade the severity of the perforation using the Pittsburgh perforation severity score (PPSS) which is a valid tool to grade the severity of esophageal perforations (17,18). PPSS has been shown to correlate with the time interval to presentation, choice of initial therapy and treatment outcomes, particularly in patient subgroups with BS (18-20).
Patients and Methods
A retrospective review of all adult patients treated for BS in the esophagogastric surgery unit of our centre from January 2005 to January 2018 was performed. The relevant data were retrieved from the hospital’s electronic medical records and included demographic details, clinical and laboratory characteristics, details of diagnostic evaluations and treatment, intensive care unit (ICU) stay details, LoHS, 90-day morbidity (including 30-day operative morbidity), 90-day and in-hospital mortality. The study was approved by the institutional review board [Min.No.13062 (retro) dated 24.06.2020].
The severity of the comorbidities was classified using Charlson’s comorbidity index (CCI) (21). Based on the time elapsed from the symptom onset to diagnosis, perforations were classified as early (<24 hours) and delayed (>24 hours). Shock was defined as systolic blood pressure <90 mmHg. Diagnosis was confirmed using contrast-esophagography and/or thoracic computed tomography (CT) and an occasional endoscopy. Perforation was classified as uncontained if there was a large amount of contrast extravasation into the pleural space or a large area of mediastinal air-fluid collections regardless of the pleural involvement; contained if there was no contrast extravasation or minimal contrast extravasation with the limited mediastinal air-fluid collection, not breaching the pleural space. Primary intervention was defined as the index procedure(s) aimed at sealing the perforation and/or drainage of sepsis and a re-intervention was defined as any subsequent procedure(s) performed to achieve similar goals. Post-procedure morbidity was recorded at the 90-day mark. Thirty-day postoperative complications were classified using Clavien-Dindo grade (CDG) and a major complication was defined as CDG≥ 3 (22).
The severity of the perforation at admission was retrospectively calculated using PPSS. PPSS was calculated by assigning points to each clinical variable to a total score of 18 and three patient risk categories were identified (PPSS: <2, low risk; 2-5 intermediate risk; >5, high risk) (17,18). PPSS category was correlated with time to diagnosis, choice of primary intervention (operative vs. non-operative), the requirement for ICU stay, LoHS, and in-hospital mortality. Follow-up data were obtained from medical records and were strengthened by telephonic conversation.
Categorical variables were expressed as frequencies with percentages, and continuous variables were expressed as mean with standard deviation or median with range. To find associations between two categorical variables, Fisher’s exact test or proportion test was used. The differences were considered significant if p <0.05.
Results
Baseline demography, clinical profile and details of radiological evaluations are summarized in Table 1.
Table 1. Demography, clinical profile and details of initial diagnostic evaluations.
| Variable | n= 12 |
| Year of presentation 2005-2010 2011-2015 2015-2018 |
3 (25.0%) 7 (58.3%) 2 (16.7%) |
| Age; years | 53.75 ± 14.96 (28-80) |
| Sex Male Female |
10 (83.3%) 2 (16.7%) |
| CCI CCI< 2 CCI> 2 |
7 (58.3%) 5 (31.7%) |
| Time intervala Early (<24 hours) Delayed (>24 hours) |
2 (16.7%) 10 (83.3%) |
| Dominant symptom Chest pain Abdomen pain Dyspnoea | 7 (58.4%) 4 (33.3%) 1 (8.3%) |
| Precipitating factor Alcohol + retching/vomiting No alcohol but retching/vomiting No precipitating factor reported |
6 (50.0%) 2 (16.7%) 4 (33.3%) |
| Initial admitting department Surgical unit Medical specialities0 | 7 (58.3%) 5 (41.7%) |
| Presence of shock/organ failure at admission Shock alone Organ failure0 alone Shock and organ failurec |
1 (8.3%) 3 (25.0%) 1 (16.7%) |
| Laboratory evaluations Hemoglobin (g/dL) Albumin (g/dL) Creatinine (mg/dL) Total leukocyte count (cells/cu mm). |
13.01 ± 3.17 3.1 ± 0.95 1.08 ± 0.47 12.400 (4.400-19.600) |
| Diagnostic modality Chest radiograph Normal Pleural effusion (Left, right, bilateral) Diagnostic endoscopy Contrast-esophagography Thoracic CT scan |
12 1 11 (7, 3, 1) 3 2 12 |
| Location of perforation Lower thoracic esophagus |
12 |
| Perforation contained?d Yes No |
3 (25.0%) 9 (75.0%) |
| CCI: Charlson comorbidity index, CT: Computed tomography. Values expressed in n, n (%), mean ± SD or median (range) as appropriate. a: Time interval, from the onset of symptoms to diagnosis/initiation of treatment, b: Admitted initially under medical specialities with an alternate diagnosis, c: Acute respiratory failure with hypoxia in four patients; acute renal failure in one patient, d: As determined by the radiological evaluations. | |
Demography and Clinical Presentation
Twelve patients [male:female, 10:2; mean (range) age, 53.75 ± 14.96 (28-80) years] were included. Ten (83.3%) patients had delayed diagnosis. Five patients were referred to our centre following an initial intervention elsewhere (Table 2). Five patients were erroneously diagnosed with pulmonary conditions and were initially admitted under medical specialities. The dominant presenting symptom was chest pain (58.4%), and Mackler’s triad (chest pain, vomiting, subcutaneous emphysema) was present in two (16.7%) patients. A total of six patients presented either in shock (n= 1) or with organ failure (n= 3) or both (n= 2).
Table 2. Individual patient profile and treatment details.
| Year | Case No. | Age/ Sex | Type of intervention before referral | Time of presentation (Early vs. Delayed) | Location of perforation3 | Perforation size | Contained perforation (Yes/No) | Site of pleural collection | PPSS | Details of primary intervention(s) | Re-intervention |
| 2005 | 1 | 67/M | Non-referral | Early | Lower thoracic, 2 cm above GEJ |
3 cm | Yes | None1’ | 4 | Laparotomy, trans-hiatal primary repair, omentoplasty and FJ | Tube thoracostomy for left-sided pleural effusion1’ |
| 2008 | 2 | 80/F | Right-sided, tube thoracostomy | Delayed | Lower thoracic | N/l | No | Right | 10 | Right thoracotomy, drainage, decortication and FJ | None |
| 3 | 62/M | Left-sided, tube thoracostomy | Delayed | Lower thoracic | N/A | Yes | Left | 3 | Left-sided, tube thoracostomy, gastrostomy and FJ | None | |
| 2010 | 4 | 28/M | Non-referral | Delayed | Lower thoracic | N/A | Yes | Left | 9 | Left-sided, tube thoracostomy, FJ | None |
| 2011 | 5 | 47/M | Right thoracotomy, pleural drainage alone | Delayed | Lower thoracic | N/M | No | Right | 9 | Right thoracotomy, drainage, decortication, esophageal stenting (FCSEMS) and FJ | Stent migration ~^· endoscopic repositioning |
| 6 | 41/M | Left-sided, tube thoracostomy | Delayed | Lower thoracic, 2 cm above GEJ |
3 cm | No | Left | 10 | Laparotomy, trans-hiatal repair over aT-tube, left thoracotomy, drainage, decortication, gastrostomy and FJ | None | |
| 2012 | 7 | 61/M | Non-referral | Delayed | Lower thoracic, 37-39 cm |
2 cm | No | Left | 6 | Left-sided, tube thoracostomy, esophageal stenting (FCSEMS), FJ | None |
| 2013 | 8 | 50/M | Non-referral | Delayed | Lower thoracic | 3 cm | No | Left | 11 | Laparotomy, trans-hiatal repair over aT-tube, left thoracotomy, drainage, decortication, gastrostomy and FJ | None |
| 9 | 47/M | Non-referral | Delayed | Lower thoracic, 2 cm above the GEJ |
3 cm | No | Bilateral | 12 | Laparotomy, trans-hiatal primary repair, omentoplasty, bilateral thoracoscopic drainage, gastrostomy and FJ | None | |
| 2014 | 10 | 55/M | Right thoracotomy, pleural drainage | Delayed | Lower thoracic, 34-36 cm |
2 cm | No | Left | 6 | Left-side, tube thoracostomy, esophageal stenting (ECSEMS) and EJ | Left-sided residual pleural collection ~^· image-guided drainage |
| 2016 | 11 | 70/M | Non-referral | Delayed | Lower thoracic, just above the GEJ | 1 cm | No | Left | 12 | On-table endoscopy, left-thoracoscopic drainage, decortication and EJC | Post-op contrast study showed a leak —> stented (ECSEMS) stent migration -^· endoscopic repositioning |
| 2018 | 12 | 37/F | Non-referral | Early | Lower thoracic, 31-33 cm |
2 cm | No | Right | 8 | On-table endoscopy, clipping of the perforation, right-thoracoscopic drainage and EJ | Persistent leak -^· stented (FCSEMS) stent migration -»endoscopic repositioning |
| PPSS: Pittsburgh perforation severity score, M: Male, F: Female, GEJ: Gastro-esophageal junction, FJ: Feeding jejunostomy, FCSEMS: Fully covered self-expanding metal stent, N/l: Not identified (The perforation is not identified intra-op), N/A: Not applicable (No endoscopy or esophageal surgery attempted; hence size of the perforation cannot be commented on), N/M: Not mentioned (Although perforation identified either in endoscopy or intra-op, its size was not mentioned). a:The location of the perforation was identified using either radiological or endoscopic or intra-op assessment or their combination b: Pleural effusion developed following the primary intervention, but there was no evidence of a postoperative esophageal leak in the re-imaging c: On-table endoscopy showed an erosion in the lower thoracic esophagus, but the CT showed no obvious contrast leak and the perforation was not identified intra-op; hence stenting was not considered during the index operation. | |||||||||||
Laboratory and Radiological Evaluations
Median total leukocyte count was 12.400 cells/cu mm. The commonest finding in chest radiography was pleural effusion (11 patients; left, 7; right, 3; bilateral 1). Contrast-esophagography was performed in two patients, and contrast extravasation was seen in both. Thoracic CT was performed in all patients; all the perforations were localized to the lower thoracic esophagus and nine (75.0%) perforations were uncontained.
Treatment Details and Outcomes
Individual patient profile and treatments received are detailed in Table 2.
Sealing of the Perforation
Ten patients belonged to the delayed diagnosis group (perforation type: contained, 2; uncontained, 8) and two patients belonged to the early diagnosis group (perforation type: contained, 1; uncontained, 1). Two patients (SL No. 2 and 11) in the delayed but uncontained group, received surgical drainage, debridement and a feeding jejunostomy (FJ) alone. The initial sealing of the perforation was performed in the remaining six patients of the delayed but uncontained group, either using covered stents (n= 3) or surgery (n= 3). Two patients in the delayed but contained group received no esophageal intervention and were managed with a tube thoracostomy and FJ. In the early diagnosis group (n= 2), endo-clipping (n= 1) and surgery (n= 1) were utilized to seal the perforation.
All surgical repairs were performed through an open transhiatal approach. A reinforced primary repair was performed in a patient who presented early but with a contained perforation. Among the three patients in the delayed presentation group receiving esophageal surgery, a buttressed primary repair was performed in one patient and a T-tube repair was performed in two patients.
Drainage of Sepsis
In the uncontained perforation group (n= 9), thoracic drainage was achieved using either thoracoscopy/thoracotomy (n= 7) or a tube thoracostomy (n= 2). In the delayed but contained perforation group (n= 2), a tube thoracostomy alone was used to drain the reactive effusion. In a patient belonging to the early but contained perforation group receiving surgical repair of the perforation, there was no concomitant pleural drainage indicated initially. However, the patient developed a left-sided, serous effusion later, which was drained using a tube thoracostomy.
Feeding Procedure and Gastrostomy
An FJ was performed in all patients, and a surgical venting gastrostomy was created in four patients.
Re-Interventions
A total of five patients required re-intervention; one patient developed migration of the covered esophageal stent, managed by endoscopic stent repositioning, and two patients required stenting following the primary esophageal intervention (failure of clipping, 1; postoperatively evident leak, 1). The patient who received stenting following the failed clipping developed distal migration of the stent and required endoscopic repositioning. In the patient who was stented for an esophageal leak (revealed postoperatively), there were three instances of stent migration, necessitating endoscopic repositioning. Two patients developed residual pleural collection following the primary intervention and required additional drainage procedures (tube thoracostomy, 1; image-guided drainage, 1).
Post-Treatment Outcomes and Follow-Up
Post-treatment outcome and follow-up are elaborated in Table 3. There was one re-operation for intraperitoneal bleeding, three ventilator-associated pneumonia requiring tracheostomy, one central-line associated infection, and paroxysmal supraventricular tachycardia. Thirty-day postoperative complication was 50.0%, and all were CDG≥ 3 complications. All patients but two required ICU stay, and median (range) LoHS was 25.5 (12-101) days. Among patients who completed 90-day follow-up (n= 9), there was no 90-day mortality. One patient, who required multiple stent re-positioning, succumbed to multi-organ failure on the 101st postoperative day.
Table 3. Post-treatment outcomes and follow-up.
| Patient SL. No.a | Post-procedure additional morbidityb | CDG | PPSS | PPSS risk category (Intermediate; 2-5 vs. high; >5) | ICU (Yes/No) | LoHS (Days) (Days) | Mortality (90-day, in-hospital) | Follow-up (Days) | Recurrence (Yes/No) |
| 1 | Tracheostomy | 3a | 4 | Intermediate | Yes | 31 | No, No | 5929 | No |
| 2 | CLABSI, VAP, multi-organ failure | 4b | 10 | High | Yes | 68 | No, No | 1507 | No |
| 3 | None | N/A | 3 | Intermediate | Yes | 18 | No, No | 272 | No |
| 4 | None | N/A | 9 | High | No | 17 | No, No | 49 | No |
| 5 | None | N/A | 9 | High | Yes | 20 | No, No | 3467 | No |
| 6 | VAP, tracheostomy | 3b | 10 | High | Yes | 38 | No, No | 17 | No |
| 7 | None | N/A | 6 | High | Yes | 19 | No, No | 65 | No |
| 8 | Bleeding from a hiatal vessel needing laparoscopic ligation | 3b | 11 | High | Yes | 40 | No, No | 3007 | No |
| 9 | PSVT treated medically | 3a | 12 | High | Yes | 12 | No, No | 2956 | No |
| 10 | None | N/A | 6 | High | No | 32 | No, No | 2630 | No |
| 11 | VAP, tracheostomy | 5 | 12 | High | Yes | 101 | No, Yes | N/A | N/A |
| 12 | None | N/A | 8 | High | Yes | 17 | No, No | 1142 | No |
| CDG: Clavien-Dindo grading, ICU: Intensive care unit, LoHS: Length of hospital stay, CLABSI: Central-line associated bloodstream infection, VAP: Ventilator-associated pneumonia, PSVT: Paroxysmal supraventricular tachycardia, N/A: Not applicable. a: Patient serial number in the same order as in Table 2. b: Additional post-procedure morbidity (excluding any form of esophago-pleural re-interventions). | |||||||||
All esophageal stents were retrieved in the outpatient clinic, and no patient had a residual leak in the follow-up contrastesophagography. Among the survivors (n= 11), the median (range) follow-up was 1507 (17-5929) days; one patient died of community-acquired pneumonia at nine months following discharge, and the remaining patients were alive with no recurrence, dysphagia or symptomatic reflux.
The Clinical Significance of PPSS
PPSS was 2-5 in two patients and >5 in 10 patients. When PPSS >5 and 2-5 patient groups were compared, there were more delayed presentation (90.0% vs. 50.0%; p= 0.165), more surgical interventions (70.0% vs. 0.0%), increased rate of overall postprocedure morbidity (70.0% vs. 50.0%; p= 0.583) and in-hospital mortality (10.0% vs. 0.0%) in the former group (Table 4). Eight (80%) patients with PPSS> 5 and all patients with PPSS 2-5 required ICU stay (p= 0.488). There was no clinically relevant difference in re-intervention rate (50.0% vs. 50%) or LoHS (24.5 days vs. 26 days) between PPSS patient groups.
Table 4. PPSS and its correlation with treatment selection and post-treatment outcomes.
| PPSS (n= 12) | p | ||
| Variable | Intermediate risk (2, 5) (2, 16.7%) | High risk (>5) (10, 83.3%) | |
| Time of presentation <24 hours >24 hours |
1 (50%) 1 (50%) |
1 (10%) 9 (90%) |
0.165 |
| Primary intervention(s) Surgery ± other interventions Endoscopic alone Radiological alone Endoscopic & Radiological |
0 (0%) 0 (0%) 2 (100%) 0 (0%) |
7 (70%) 0 (0%) 0 (0%) 3 (30%) |
- |
| Re-intervention requirement3 Yes No |
1 (50%) 1 (50%) |
4 (40%) 6 (60%) |
- |
| Post-procedure morbidityb Yes No |
1 (50%) 1 (50%) |
7 (70%) 3 (30%) |
0.583 |
| Need for ICU stay Yes No |
2 (100%) 0 (0%) |
8 (80%) 2 (20%) |
0.488 |
| Median LoHS, days | 24.5 | 26 | - |
| Mortality 90-day In-hospital |
0% (0%) 0% (0%) |
0 (0%) 1 (10%) |
- |
| PPSS: Pittsburgh severity score, ICU: Intensive care unit, LoHS: Length of hospital stay. a: Includes the patient in whom a re-intervention was warranted (stenting for persistent esophago-pleural fistula) but refused. b: Post-procedure morbidity includes re-interventions also. PPSS was calculated by assigning points to each clinical variable to a total score of 18 and three patient risk categories were identified (low risk <2, intermediate risk 2-5, high risk >5): 1 = age >75 years, heart rate >100 beats per minute, white cell count >10 x 109/mL, pleural effusion; 2= fever (>38.5 °C), uncontained leak (radiological studies), respiratory compromise (respiratory rate >30 per minute, need for increasing oxygen or mechanical ventilation), time of diagnosis >24 h; 3= oesophageal cancer, hypotension (17,18). | |||
Discussion
Early detection and timely management of BS can reduce its morbidity and mortality, but the optimal therapeutic approach remains controversial (18). Traditionally, aggressive surgical approaches including resection were favoured. However, with recent developments in endoluminal therapy and minimally invasive surgery, there is a paradigm shift in the management approach to this condition. This case series reports the treatment outcomes of BS, from an upper gastrointestinal surgical unit, over a period of 13 years. All available treatment options have also evolved over the period of the study.
In our series, majority of the patients were middle-aged males, the diagnosis was often delayed, chest pain was the dominant symptom, and all perforations were sited in the distal thoracic esophagus; a trend concurrent with the reported literature (1,5,20,23). Abnormal chest radiography findings in BS include pleural effusion (Figure 1A), pneumomediastinum, subcutaneous emphysema, hydropneumothorax, and rarely pneumoperitoneum. In this series, chest radiography showed pleural effusion in all patients. Although contrast-esophagography (Figure 1B) is a useful investigation to confirm diagnosis, falsenegative rates can reach 15-25% and its application is often limited by the patient’s ability to swallow the contrast; it was possible in two of our patients, confirming the diagnosis in both (5,24). Thoracic CT (Figure 1C) gives valuable information regarding the site of perforation, its contained vs. uncontained nature, and the presence of additional esophageal pathologies and can guide effective sepsis drainage (1,24). In our experience, thoracic CT had excellent sensitivity in detecting perforation, and majority of the perforations (75.0%) were uncontained type. Endoscopy has a sensitivity of 100% and specificity of 80-93% in diagnosis, but can potentially worsen the esophageal tear (1,3,5). Routine diagnostic endoscopy is not performed in our centre but was utilized for intra-operative localization of the perforation in three patients (Figure 1D), where an immediate endoluminal intervention was followed.
Figure 1. A. Chest radiograph of a patient with Boerhaave’s perforation, showing a left-sided pleural effusion. B. Contrast esophagography showing contrast extravasation (black arrow) from the distal thoracic esophagus, into the left pleural space. C. Contrast-enhanced, thoracic computed tomography showing contrast extravasation (black arrow) from the distal thoracic esophagus and a left-sided pleural collection (white star). D. Intraoperative endoscopy showing the distal esophageal perforation (E: Esophageal lumen, P: Perforation).
Initial management of BS consists of fluid resuscitation, antibiotics and antifungals, acid suppression, analgesia, and cardio-respiratory support. The specific treatment approach depends on the patient’s general condition and comorbidities, the location and extent of the perforation, esophageal viability, the extent of pleuro-mediastinal soiling, and the availability of expertise (24). The treatment of BS is primarily aimed at three important steps: 1. sealing the perforation and maintaining the luminal continuity, 2. drainage of sepsis, and 3. nutritional support.
In our series, either surgery or endoscopic interventions were performed to achieve the sealing of the perforation. Although transthoracic approach is considered to be the standard operative approach for BS, trans-hiatal approach was found to be feasible and safe in our experience, as also reported by others (2,4,9,25). In addition to providing direct access to the perforation, this operative approach allows for the drainage of the mediastinum, placement of an omental patch, performance of a concomitant FJ, and an occasional gastrostomy. During the early part of the series, surgery was often utilized to achieve sealing of the perforation and the technique of repair was either a primary buttressed repair or a T-tube esophagostomy. Primary repair in patients presenting early is reported to have low postoperative leakage, shorter LoHS and ICU stay, and the reinforcement of the repair using vascularized tissues can reduce the postoperative leakage (4,8). A reinforced primary repair was possible in two patients, one each in the early and delayed diagnosis groups, and adequate sealing was achieved in both. Key steps of primary repair include debridement of non-viable tissues, esophageal myotomy on either end of the perforation to expose healthy mucosal edges and a meticulous closure, preferably in double layers (1,24). Trans-thoracic repair can be reinforced with a pleural, pericardial or intercostal muscle flap or a gastric fundal wrap, whereas an omental or a gastric fundal wrap may be utilized to buttress a trans-hiatal repair (1,12). Although delayed diagnosis does not preclude a primary repair, a high risk of a postoperative leak, re-interventions and mortality is reported, particularly when the delay is >48 hours (1,5,8,9,25). In such a scenario, repair over a T-tube is preferred, which creates a controlled esophago-cutaneous fistula; a technique that had successful outcomes in two of our patients presenting late (2,4,7,9). In our opinion, this technique is an attractive alternative in patients with delayed diagnosis, where the feasibility of a primary repair is limited, due to edematous and friable tissues, thereby avoiding or delaying other morbid operative procedures (diversion, exclusion or resection).
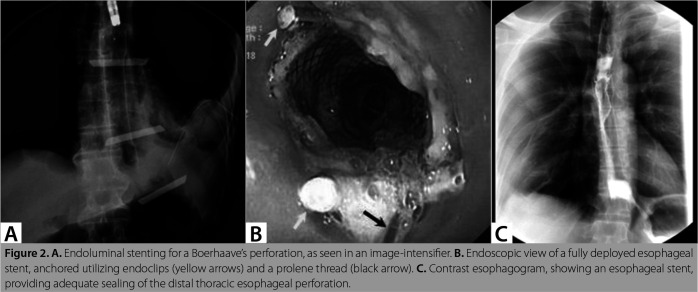
During the later phase of the study, endoscopic stenting with covered self-expanding metal stents (Figure 2) was more commonly used. Stenting is an effective, minimally invasive, primary modality for sealing the perforation in BS. Initial successful stenting can avoid radical surgeries, facilitates early oral alimentation, and can shorten the LoHS, ICU stay, and ventilator days (15,16,26). It can also be a salvage option in patients with persistent postoperative leak (6,7). In three of our patients with delayed diagnosis, the initial sealing of the perforation could be achieved by stenting. Additionally, one patient received stenting for a perforation that was not localized during the index operation but revealed later (Patient SL No. 11). Endoscopic clips, particularly over the scope clips are recommended for early perforations, measuring up to 30 mm (1,3,24). Although we utilized this technique in one patient who presented early, a persistent leak warranted stent implantation later. This patient had an uncontained perforation which perhaps increased the likelihood of a re-leak, despite initial thoracic drainage.
Figure 2. A. Endoluminal stenting for a Boerhaave’s perforation, as seen in an image-intensifier. B. Endoscopic view of a fully deployed esophageal stent, anchored utilizing endoclips (yellow arrows) and a prolene thread (black arrow). C. Contrast esophagogram, showing an esophageal stent, providing adequate sealing of the distal thoracic esophageal perforation.
The selection of primary esophageal intervention should be individualized based on patient and perforation characteristics. In a recent meta-analysis, surgery has been found to be the most favoured therapeutic approach, being utilized in 76% of the patients with BS, particularly when the diagnosis is made early; endoluminal techniques and non-operative management (NOM) are utilized predominantly in case of a delayed diagnosis (27). We prefer surgical repair over stenting for patients presenting early. However, in the early phase of this study, surgery was also performed in patients with a delayed diagnosis if the perforations were localized near the gastro-esophageal junction (GEJ) and patients could tolerate esophageal surgery, a practice similarly reported by others (8,9,25). Stenting, as the primary treatment modality for BS, has been demonstrated to have favourable clinical success, irrespective of the time to diagnosis (6,15,16,26). However, when the diagnosis is delayed, stenting may be associated with increased re-interventions, morbidity and mortality (16,26). In our recent experience, stenting was often utilized in delayed presentations, particularly if the perforations were localized away from the GEJ or the local esophageal condition and if the patient’s poor general condition did not permit an immediate esophageal surgery. However, we ensured adequate pleuro-mediastinal drainage ± debridement in all patients receiving stenting, which perhaps contributed to the absence of persistent leakage in this subgroup. Distal migration can frequently be seen in patients with BS receiving stenting (20-33%) and a high risk of persistent dysphagia is expected following a failed endoluminal stenting (3,6,15,16,26). Although stent migration developed in three of our patients, these were successfully re-positioned endoscopically, and immediate additional interventions were not indicated in any patient, except one. There were no instances of esophageal stenosis and symptomatic reflux in the stented group. In our opinion, appropriate patient and stent type selection, the availability of expertise, and adequate sepsis drainage are paramount to minimise stent failure and its complications.
Adequate pleuro-mediastinal sepsis control is the key component in the treatment of BS and is a mandatory step to improve the success of any esophageal interventions. Tube thoracostomy or image-guided drainage is an accepted initial modality for drainage of localized contaminations, as utilized in five of our patients. However, since perforation in BS is barogenic, thoracic cavity is frequently contaminated with alimentary contents, warranting surgical debridement and drainage at some time point. In our series, majority of the patients with an uncontained perforation received surgical drainage and debridement, particularly when the diagnosis was delayed or when the pleural collections were loculated.
Nutritional access is a key treatment component for BS. We performed tube jejunostomy in all patients to facilitate early enteral nutrition. A nasojejunal tube is also an accepted alternative for feeding. Venting gastrostomy can reduce the incidence of postoperative reflux, particularly in perforations near the GEJ, and was performed in four of our patients. We generally avoid routine feeding or venting gastrostomy for perforations related to B, since it allows gastric preservation, for esophageal reconstruction, if an esophagectomy is indicated.
Surgical repair of esophageal perforation and sepsis drainage could also be achieved with minimally invasive surgery (10,12,13,19,23). Haveman et al. have demonstrated comparable effectiveness and safety of pleural sepsis drainage utilizing video-assisted thoracoscopic surgery (VATS) and open thoracotomy techniques (10). In a recent review, minimally invasive surgery has been found to be feasible and safe for esophageal repair and thoracic debridement/drainage, especially in patients presenting early with stable vitals (14). Recently, VATS is our preferred operative approach for addressing thoracic sepsis, as in three of our patients. Although we preferred laparotomy for the repair of the perforations, the feasibility and safety of laparoscopic, trans-hiatal, and videoassisted trans-thoracic repairs have been shown by other authors, including in patients with delayed diagnosis (12,13,19,23).
In carefully selected patients with BS, successful treatment outcomes are achievable by NOM, provided the following criteria are satisfied: a contained perforation within the mediastinum and drainage flowing back to the esophageal lumen, minimal symptoms, no overt signs of systemic sepsis, and availability of appropriate radiological studies and thoracic surgery expertise (28). A true NOM consist of nil by mouth, intravenous fluids, anti-acid therapy, broad-spectrum antibiotics and enteral tube feeding, but is rarely possible in BS, due to its frequent delayed presentation. No patient received a true NOM in our series, but two patients with delayed but contained type perforation with no overt signs of sepsis, received conservative treatment approach including tube thoracostomy and nutritional access, without esophageal interventions, and their recovery was uneventful. An initial esophageal intervention is preferably avoided in this sub-group of patients and favourable treatment outcomes could result from drainage and nutritional support alone (1,2).
PPSS is a valuable clinical tool to stratify risk among patients with esophageal perforation, particularly in the context of BS (17-20). Patients who present early with a contained leak and do not have overt signs of sepsis often have a low PPSS and are ideal candidates for initial NOM in specialized esophageal centres (1). An operation in this subset of patients may have a worse treatment outcome (17,23). In our series, none of the patients had PPSS of ≤2 and no patient received a true NOM. The severity of complications, LoHS and mortality is shown to correlate with PPSS (17). In this study, there were more delayed presentations, increased surgical interventions, increased postprocedure morbidity, and in-hospital mortality in those patients with a PPSS≥ 5, compared to those with PPSS 2-5 but these differences were not statistically significant. Further analysis to evaluate the effect of PPSS on treatment selection and outcomes was not feasible in this study, considering its small study population.
Despite advances in therapeutics for BS, treatment-related morbidity can reach 70.0%, major operative morbidity can reach 36.0%, re-intervention rate can be 40%, mortality can be 8-40%, and prolonged ICU care and LoHS is not uncommon (10,13,19,20,23,27). In this study, overall post-treatment complication was 50% (CDG ≥3, 100%), and majority of the patients had a prolonged LoHS. However, despite these adverse outcomes, there was only one death (8.3%) and the esophagus could be salvaged in all patients. No patients required esophageal re-interventions following discharge; all stents were successfully retrieved and there were no stent-related complications, except the migrations. Further, there were no instances of dysphagia, symptomatic reflux or recurrent perforation.
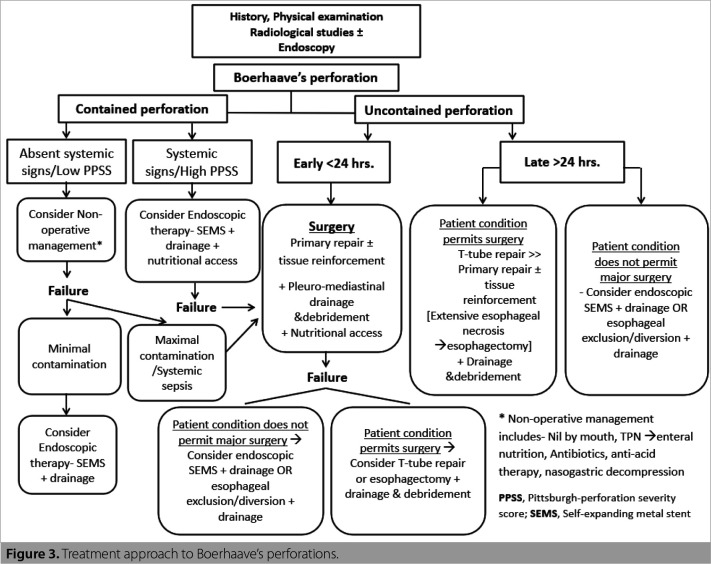
Owing to the rarity and life-threatening nature of this condition, prospective studies to evaluate the effects of different treatment approaches are difficult to execute, and hence, the current evidence concerning the efficacy and safety of various treatment options are retrospective studies. A treatment algorithm based on our experience and the data available in the literature is formed (Figure 3).
Figure 3. Treatment approach to Boerhaave’s perforations.
This study had a few limitations. Firstly, it was a single institution, retrospective study with a relatively small number of patients, and hence, has its inherent biases. Secondly, a few patients were referred to us following some form of primary intervention at the index hospital. Hence, PPSS at the presentation in our centre is not a true reflection of their actual PPSS. Lastly, various patient and treatment-related factors which can help choose a particular treatment strategy and predict the treatment success could not be established, due to the small study population. Keeping aside the limitations, the current study focused solely on BS-related perforations, from a low-middle-income country, where timely access to a specialized esophageal centre is often limited. Also, the results from this study do support the view that favourable treatment outcomes could be achieved, by utilizing hybrid therapeutic techniques. We feel that future studies should focus on a multidimensional approach to BS, rather than comparing various therapeutic approaches.
Conclusion
Boerhaave’s syndrome is a rare esophageal emergency and remains a diagnostic and therapeutic challenge. Despite an increased disease and treatment-related morbidity and prolonged hospital stay, successful treatment outcomes including reduced mortality, organ preservation, and better functional outcomes could be achieved through timely, individualized, multimodality management. Recent advances in minimally invasive, endoluminal and surgical techniques can further improve treatment outcomes. Pittsburgh severity score is a useful tool to select the initial treatment strategy and can possibly predict treatmentrelated outcomes.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Peer Review: Externally peer-reviewed.
Ethics Committee Approval: This study was approved by Christian Medical College Ethics Committee (Decision no: 13062, Date: 24.06.2020).
Author Contributions: Concept - SS, CV, MY, IS; Design - SS, CV, MY, IS; Supervision - IS; Materials - SS, CV, MY, NP, SC, AJ, EGS, IS; Data Collection and/ or Processing - SS, CV, MY, NP, SC, AJ, EGS, IS; Analysis and/or Interpretation - SS, CV, MY, NP, SC, AJ, EGS, IS; Literature Search - SS, CV, MY, NP, SC, AJ, EGS, IS; Writing Manuscript - SS, CV; Critical Reviews - SS, MY, NP, SC, AJ, EGS, IS.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Chirica M, Kelly MD, Siboni S, Aiolfi A, Riva CG, Asti E, et al. Esophageal emergencies: WSES guidelines. World J Emerg Surg. 2019;14(1):26–26. doi: 10.1186/s13017-019-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connelly C, Lamb P, Paterson-Brown S. Outcomes following Boerhaave’ syndrome. Ann R Coll Surg Engl. 2013;95(8):557–560. doi: 10.1308/rcsann.2013.95.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloreidi K, Patel B, Ridgway T, Yeager T, Atiq M. Non-surgical management of Boerhaave’s syndrome: A case series study and review of the literature. E92-7Endosc Int Open. 2018;6(1) doi: 10.1055/s-0043-124075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulpice L, Dileon S, Rayar M, Badic B, Boudjema K, Bail JP, et al. Conservative surgical management of Boerhaave’s syndrome: Experience of two tertiary referral centers. Int J Surg. 2013;11(1):64–67. doi: 10.1016/j.ijsu.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Han D, Huang Z, Xiang J, Li H, Hang J. The role of operation in the treatment of Boerhaave’s syndrome. BioMed Res Int. 2018;2018:8483401–8483401. doi: 10.1155/2018/8483401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauge T, Kleven OC, Johnson E, Hofstad B, Johannessen HO. Outcome after stenting and débridement for spontaneous esophageal rupture. Scand J Gastroenterol. 2018;53(4):398–402. doi: 10.1080/00365521.2018.1448886. [DOI] [PubMed] [Google Scholar]
- 7.Rokicki M, Rokicki W, Rydel M. Boerhaave’s syndrome-over 290 yrs of surgical experiences. Surgical, endoscopic and conservative treatment. Pol J Surg. 2016;88(6):365–372. doi: 10.1515/pjs-2016-0078. [DOI] [PubMed] [Google Scholar]
- 8.Yan XL, Jing L, Guo LJ, Huo YK, Zhang YC, Yan XW, et al. Surgical management of Boerhaaves syndrome with early and delayed diagnosis in adults: A retrospective study of 88 patients. Rev Esp Enfermedades Dig. 2020;112(9):669–674. doi: 10.17235/reed.2020.6746/2019. [DOI] [PubMed] [Google Scholar]
- 9.Sutcliffe RP, Forshaw MJ, Datta G, Rohatgi A, Strauss DC, Mason RC, et al. Surgical management of Boerhaave’s syndrome in a tertiary oesophagogastric centre. Ann R Coll Surg Engl. 2009;91(5):374–380. doi: 10.1308/003588409X428298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haveman J, Nieuwenhuijs V, Kobold J, Dam G, Plukker J, Hofker S. Adequate debridement and drainage of the mediastinum using open thoracotomy or video-assisted thoracoscopic surgery for Boerhaave’s syndrome. Surg Endosc. 2011;25:2492–2497. doi: 10.1007/s00464-011-1571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aref H, Yunus T, Alhallaq O. Laparoscopic management of Boerhaave’s syndrome: A case report with an intraoperative video. BMC Surg. 2019;19(1):109–109. doi: 10.1186/s12893-019-0576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AHH, Kweh BTS, Gillespie C, Johnson MA. Trans-hiatal repair for oesophageal and Junctional perforation: A case series. BMC Surg. 2020;20(1):41–41. doi: 10.1186/s12893-020-00702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JS, Kim YD, Kim JW, I HS, Kim MS. Thoracoscopic primary esophageal repair in patients with Boerhaave’s syndrome. Ann Thorac Surg. 2011;91(5):1552–1555. doi: 10.1016/j.athoracsur.2011.01.082. [DOI] [PubMed] [Google Scholar]
- 14.Aiolfi A, Micheletto G, Guerrazzi G, Bonitta G, Campanelli G, Bona D. Minimally invasive surgical management of Boerhaave’s syndrome: A narrative literature review. J Thorac Dis. 2020;12(8):4411–4417. doi: 10.21037/jtd-20-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. Esophageal stent placement for the treatment of spontaneous esophageal perforations. Ann Thorac Surg. 2009;88(1):194–198. doi: 10.1016/j.athoracsur.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Koivukangas V, Biancari F, Meriläinen S, Ala-Kokko T, Saarnio J. Esophageal stenting for spontaneous esophageal perforation. J Trauma Acute Care Surg. 2012;73(4):1011–1013. doi: 10.1097/TA.0b013e318265d176. [DOI] [PubMed] [Google Scholar]
- 17.Abbas G, Schuchert MJ, Pettiford BL, Pennathur A, Landreneau J, Landreneau J, et al. Contemporaneous management of esophageal perforation. Surgery. 2009;146(4):749–756. doi: 10.1016/j.surg.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 18.Schweigert M, Sousa HS, Solymosi N, Yankulov A, Fernández MJ, Beattie R, et al. Spotlight on esophageal perforation: A multinational study using the Pittsburgh esophageal perforation severity scoring system. J Thorac Cardiovasc Surg. 2016;151(4):1002–1009. doi: 10.1016/j.jtcvs.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 19.Elliott JA, Buckley L, Albagir M, Athanasiou A, Murphy TJ. Minimally invasive surgical management of spontaneous esophageal perforation (Boerhaave’s syndrome) Surg Endosc. 2019;33(10):3494–3502. doi: 10.1007/s00464-019-06863-2. [DOI] [PubMed] [Google Scholar]
- 20.Wigley C, Athanasiou A, Bhatti A, Sheikh A, Hodson J, Bedford M, et al. Does the Pittsburgh severity score predict outcome in esophageal perforation? Dis Esophagus Off. J Int Soc Dis Esophagus. 2019;32(2) doi: 10.1093/dote/doy109. [DOI] [PubMed] [Google Scholar]
- 21.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 22.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 23.Aref H, Yunus T, Alhallaq O. Laparoscopic management of Boerhaave’s syndrome: A case report with an intraoperative video. BMC Surg. 2019;19(1):109–109. doi: 10.1186/s12893-019-0576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalal UR, Dalal AK, Kaur R, An L, Dua A. Oesophageal perforation management in a tertiary care hospital. J Surg Anesth. 2020;4(1):1–10. [Google Scholar]
- 25.Wang Y, Zhang R, Zhou Y, Li X, Cheng Q, Wang Y, et al. Our experience on management of Boerhaave’s syndrome with late presentation. Dis Esophagus. 2009;22(1):62–67. doi: 10.1111/j.1442-2050.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 26.Glatz T, Marjanovic G, Kulemann B, Hipp J, Theodor Hopt U, Fischer A, et al. Management and outcome of esophageal stenting for spontaneous esophageal perforations: Esophageal stenting for spontaneous esophageal perforation. Dis Esophagus. 2017;30(3):1–6. doi: 10.1111/dote.12461. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen BD, van der Leeden B, Ali JT, Gudbjartsson T, Hermansson M, Low DE, et al. Early diagnosis is associated with improved clinical outcomes in benign esophageal perforation: An individual patient data meta-analysis. Surg Endosc. 2021;35(7):3492–3505. doi: 10.1007/s00464-020-07806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altorjay A, Kiss J, Vörös A, Bohák A. Nonoperative management of esophageal perforations. Is it justified. Ann Surg. 1997;225(4):415–421. doi: 10.1097/00000658-199704000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]