Highlights
-
•
In the adult, brain apolipoprotein E levels are determined by genotype.
-
•
Apolipoprotein E ε2, the protective allele, is most abundant in the brain.
-
•
Increased dietary fat alters brain apolipoprotein E levels.
-
•
The impact of dietary fat on brain protein is apolipoprotein E allele specific.
Keywords: Alzheimer’s disease, Apolipoprotein E, Western diet, Regular diet
Abstract
Human apolipoprotein E (APOE) is the greatest determinant of genetic risk for memory deficits and Alzheimer’s disease (AD). While APOE4 drives memory loss and high AD risk, APOE2 leads to healthy brain aging and reduced AD risk compared to the common APOE3 variant. We examined brain APOE protein levels in humanized mice homozygous for these alleles and found baseline levels to be age- and isoform-dependent: APOE2 levels were greater than APOE3, which were greater than APOE4. Despite the understanding that APOE lipoparticles do not traverse the blood–brain barrier, we show that brain APOE levels are responsive to dietary fat intake. Challenging mice for 6 months on a Western diet high in fat and cholesterol increased APOE protein levels in an allele-dependent fashion with a much greater increase within blood plasma than within the brain. In the brain, APOE2 levels responded most to the Western diet challenge, increasing by 20 % to 30 %. While increased lipoparticles are generally deleterious in the periphery, we propose that higher brain APOE2 levels may represent a readily available pool of beneficial lipid particles for neurons.
1. Introduction
The apolipoprotein E (APOE) protein plays an important role in the delivery of cholesterol and other lipids to various cells and tissues throughout the body, including within the brain [37]. Peripheral APOE exists within blood plasma lipoprotein particles and is mainly produced by liver hepatocytes, with small contributions from monocytes and macrophages [2], [6], [39]. The blood–brain barrier (BBB), which regulates the passage of materials between the peripheral bloodstream and the central nervous system (CNS), does not allow the passage of APOE lipoparticles into the brain [32], [34], rendering the brain dependent on in situ APOE production. Brain APOE is a product of local synthesis by astrocytes, but also by microglia, and to a lesser degree by neurons during stress or injury [7], [38], [65]. The primary function of astrocytic APOE is to transfer lipids to neurons for receptor-mediated uptake into the endolysosomal system to meet the brain’s substantial demands for cholesterol and cholesterol metabolites. Most cholesterol in the CNS exists in the myelin sheaths of oligodendrocytes [51], although the more dynamic cholesterol pool within neuronal membranes is critical for synaptic transmission, receptor localization, and endolysosomal function [5], [13], [31]. While there is a limited exchange of cholesterol between the brain and the periphery, diffusion-mediated efflux of the brain cholesterol metabolite 24S-hydroxycholesterol across the BBB helps regulate brain cholesterol levels by offering a pathway for its removal [4]. Conversely, the elevation of dietary cholesterol levels in the plasma is not thought to robustly alter intracerebral cholesterol levels [5], [13], [48], although flux of the peripheral cholesterol metabolite 27-hydroxycholesterol into the brain has been suggested to be a mechanism of CNS lipid changes that are relevant to AD pathobiology [4], [24].
The human APOE gene occurs in three alleles: ε2, ε3 and ε4 [37], and the respective protein isoforms APOE2, APOE3, and APOE4 differ structurally from each other due to amino acid substitutions at residues 112 and 158. These differences impact APOE receptor- and lipid-binding affinities, as well as their associated risk for neurodegenerative and cardiovascular disease during aging [7], [31], [33], [37], [38]. APOE2 is protective against Alzheimer’s disease (AD), the common APOE3 isoform is risk-neutral, and APOE4 increases the risk of developing sporadic AD while decreasing the age of disease onset in a gene-dose dependent manner [3], [11], [17], [31], [38]. APOE4 also perturbs memory in humans and knock-in mice independent of AD pathology when compared to the APOE3 and APOE2 alleles [8], [18], [38]. These include effects on olfactory perception and habituation prior to other forms of cognitive impairment, including those associated with AD-related amyloid β and tau pathology [1], [14], [20], [22], [29], [41], [45], [46].
Diet can modulate cognition in aged individuals, including those with AD [30], with many studies showing a correlation between a higher-fat Western diet and cognitive impairment (reviewed in [12], [64]). Modeling in rodents has consistently shown that a Western-diet compromises learning and memory in a range of tasks, including when the dietary challenge is for a period of weeks (reviewed in [16], [64]). In rodent models expressing familial-AD mutations, Western diets have been shown to increase cognitive deficits and AD-like pathologies [16], [27], [58], [62], a process that has been linked to increased neuroinflammatory markers [40], [61], [66] as well as synaptic changes [16], although some studies have not found these effects [21]. Given that APOE plays an integral role in lipid homeostasis and hyperlipidemia, that high-fat diets are risk factors for AD [12], and that APOE genotype has been shown to interact with dietary risk ([10], [44], reviewed in [54]), we asked whether a high-fat Western diet affects APOE levels in the brain. Additionally, we investigated whether this effect is isoform-specific, a reasonable assumption given that baseline APOE levels in targeted-replacement mice are isoform-dependent [50], [55], [59]. Extending these prior studies, we show in targeted-replacement APOE mice that brain APOE levels are isoform-dependent across a broad age range, with APOE2 > APOE3 > APOE4. Additionally, our findings demonstrate that a Western-diet impacts brain APOE levels, with the greatest increase seen in APOE2 mice and a smaller increase in APOE4. Thus, while APOE does not cross the BBB [34], a diet high in lipids increases APOE protein levels in the brain in an isoform-specific manner.
2. Materials and methods
2.1. Animals and brain homogenate preparation
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Nathan S. Kline Institute. Mice were group-housed under controlled temperature and lighting conditions with same-sex littermates and given free access to food and water. The APOE targeted-replacement mice used in this study are on a C57BL/6 background and express human APOE in place of the mouse Apoe gene under the control of the endogenous murine promoter [56], allowing for the expression of human APOE at physiologically regulated levels in the same temporal and spatial pattern as endogenous murine Apoe. These animals do not develop brain Aβ plaques or tau pathology [56], [57]. Mice homozygous for either the human APOE2, APOE3, or APOE4 genes are referred to in the text as APOE2, APOE3, and APOE4 mice, respectively. A total of 42 APOE2 (28 males, 14 females), 41 APOE3 (21 males, 20 females) and 42 APOE4 (27 male, 15 female) mice were used in this study. The experimental n for each study is given in the figure legend. Both males and females were examined as indicated in the figures; analysis of data by sex in this study did not reveal significant differences, and the results were pooled. The APOE genotypes of the mice were confirmed by restriction fragment length polymorphism analysis as previously described [25]. Hemibrains lacking the cerebellum and olfactory bulbs were dissected and snap-frozen on dry ice and later homogenized as previously described [42]. Briefly, 10 % (weight/volume) homogenates were prepared on ice using a dounce homogenizer in a buffer containing 8.5 % sucrose, 20 mM Tris (pH 7.4), 1 mM EDTA, 1 mM EGTA and a protease inhibitor cocktail (phenylmethylsulfonyl fluoride (PMSF), leupeptin, antipain, pepstatin) [53]. Homogenates were aliquoted and stored at −80 °C prior to use.
2.2. Diet
APOE mice were maintained on a normal chow diet (Purina LabDiet, Prolab RMH 3000, St. Louis, MO, US) containing 26 % of calories as protein, 60 % as carbohydrate, and 14 % as fat. These control-diet fed mice of both sexes were analyzed at 6, 12, and 18 months of age. For the dietary challenge, mice were placed on a Western diet (Purina Test Diet #18110842, Somerville, NJ, US) containing 39.1 % of calories from fat, 45.1 % from carbohydrate, and 15.8 % from protein beginning at 6 months of age until 12 months of age, when they were analyzed.
2.3. Western blot analysis
Freshly thawed homogentate containing equal protein amounts (Pierce BCA Protein Assay Kit, Thermo Scientific, Rockford, IL) were diluted into reducing Laemmli SDS sample buffer containing β-mercaptoethanol as we have previously described [42]. Proteins were separated by electrophoresis using Criterion 4–20 % Tris-HCl electrophoresis gels (Bio-Rad, Hercules, CA, US) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore Sigma, Burlington, MA, US). Anti-human APOE monoclonal antibodies (HJ15.3 and HJ15.7), used for Western blot analysis and ELISA, were the kind gift of Dr. David Holzman (Washington University, St. Louis, MO) [26], [63]. Membranes were incubated with primary antibodies overnight followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson Immuno Research, West Grove, PA, US) for one hour. Chemiluminescence (Pierce, Rockford, IL, US) was detected on Reflection Autoradiography films. Band intensity was analyzed with the open-source software ImageJ (National Institute of Health (NIH), Bethesda, MD, US) and normalized to β-Actin levels (Abcam #ab8227, Cambridge, UK).
2.4. APOE ELISA
Human APOE protein levels in diethylamine (DEA) extracted murine brain homogenate [43] and blood plasma were determined by sandwich ELISA using mouse monoclonal antibodies HJ15.3 as the capture antibody and HJ15.7 for detection, as has been previously described [26], [63]. The ELISA was performed using a protocol modified from the laboratory’s extensively characterized ELISAs to detect brain Aβ [52]. These antibodies recognize the three human APOE alleles, and do not react with murine apoE ([26], [63]; additional data not shown). Briefly, Nunc-Immuno Plates (MaxiSorp surface, Nunc A/S, Roskilde, Denmark) were coated with 100 µL/well of HJ15.3 (2.5 µg/mL) in bicarbonate buffer (30 mM NaHCO3, 70 mM Na2CO3, 0.05 % NaN3, pH 9.6) overnight at 4˚C with rocking. Recombinant human APOE (Abcam ab55210; APOE2 sequence) standards were serially diluted in phosphate buffer (20 mM sodium phosphate, 2 mM EDTA, 140 mM NaCl, 0.2 % BSA, 0.05 % CHAPS, 0.4 % Block Ace, 0.05 % NaN3, pH 7.0) from 0 ng to 400 ng. DEA extracted murine brain homogenate and blood plasma were diluted in phosphate buffer (1:10 and 1:100, respectively) prior to loading in triplicate. Dilutions were determined in preliminary experiments to insure that both standards and samples were within the linear-detection range of the ELISA. Wells were washed twice with PBS and non-specific binding was blocked by loading 200 µL/well of Block Ace Solution (1 % Block Ace; Sumitomo Dainippon Pharma Co., Osaka, Japan, 0.05 % NaN3, PBS, pH 7.4) and incubating for 4 h at room temperature with rocking. Immediately after removal of the blocking solution, 100 µL of standards, blanks, and samples in triplicate were added to the wells for overnight incubation at 4˚C with rocking. Wells were then washed twice with PBS-0.2 % Tween and once with PBS before the addition of 100 µL HRP-conjugated HJ15.7 detection antibody diluted 1:4000 in Buffer C (20 mM sodium phosphate, 2 mM EDTA, 140 mM NaCl, 1 % BSA, pH 7.0) and incubated for 4 h at room temperature with rocking. Wells were then washed twice in PBS-0.2 % Tween and once in PBS. Development of the ELISA was completed by adding 100 µL/well of TMB Substrate/H2O2 Solution (TMB Microwell Peroxidase Substrate System, Kirkegaard & Perry Laboratories, Maryland, USA) until sufficient color change was observed, and the reaction was stopped by the addition of 100 µL/well of 5.7 % O-Phosphoric Acid. Samples were read with a microplate reader at OD450. Brain ELISA results are reported as the mean ± SEM in µg APOE normalized to brain weight, based on standard curves using the recombinant human APOE.
2.5. Statistical analysis
NIH ImageJ (http://rsb.info.nih.gov) was used to quantify Western blots. Images were converted to grayscale (8-bit) and contrast was adjusted for background subtraction. A rectangular box was drawn across the row containing the bands of interest and the lanes were plotted. The base of each peak was enclosed using the straight-line tool to threshold the signal intensity over background. The resulting area under each peak was quantified as the signal intensity. Genotype and diet differences were analyzed with a two-tailed t-test as well as a two-way ANOVA using GraphPad Prism 8.4.2. Results are expressed as the mean ± standard error of the mean. Statistically significant findings are indicated as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. The value of n represents the number of animals and is given in the main text. Comparison of male and female results showed no significant differences between the sexes and the results were pooled.
3. Results and discussion
3.1. APOE genotype determines brain APOE levels independent of age
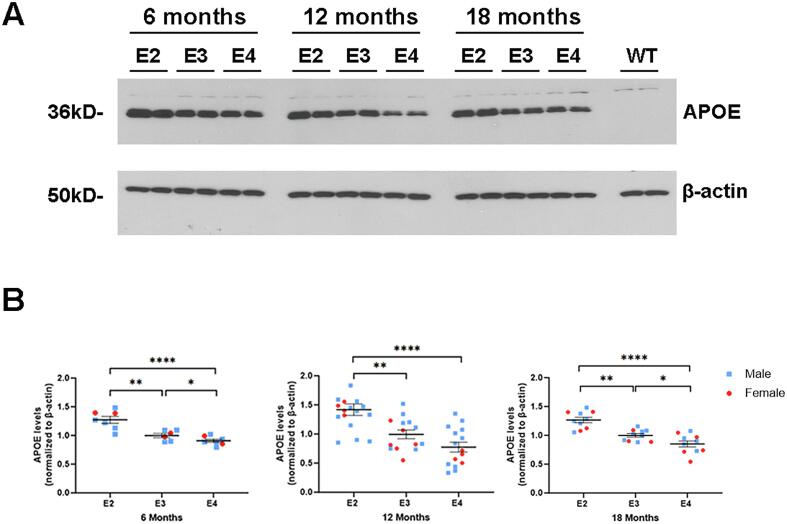
Previous mouse studies have suggested that brain APOE levels are determined by the specific APOE allele expressed [50]. We extended these findings to determine if genotype-dependent variability in brain APOE levels is modified by aging, given that APOE genotype modifies age-associated brain disease risk [3], [11], [17]. To this end, we examined brain APOE levels in targeted replacement APOE2, APOE3 and APOE4 mice at 6, 12 and 18 months of age by Western blot analysis (Fig. 1A, B shows quantitative data including additional Western blot analyses). Repeated measures 3 x 3 ANOVA (genotype x age) on Western blot analyses of APOE protein levels revealed a main effect of genotype (F(2,87) = 25.23, p < 0.0001), but not age independent of genotype. Post-hoc t-tests revealed higher APOE levels in APOE2 vs APOE3 mice beginning at 6 months of age (APOE2 = 129.6 ± 4.7 % of APOE3; t(20) = 5.138, p < 0.0001; Fig. 1B). Differences in APOE3 vs APOE4 levels at 6 months of age were smaller, with APOE4 levels significantly lower than APOE3 (APOE3 = 111.7 ± 3.1 % of APOE4; t(20) = 2.703, p = 0.0137; APOE2 compared to APOE4: APOE2 = 143.4 ± 5.2 % of APOE4; t(22) = 7.569, p < 0.0001; Fig. 1B). The same pattern of APOE2 > APOE3 > APOE4 brain protein level was observed at 12 and 18 months of age (see the legend for Fig. 1B for details regarding 12- and 18-month-old comparisons; see also ELISA data in Fig. 3 for control mice at 12 months of age). Thus, brain APOE2 levels were found to be significantly greater than APOE3 or APOE4 levels across all ages with consistently lower brain APOE levels in APOE4 compared with APOE3 mice. Thus, we show that APOE protein levels in the brain are allele-specific and constant across adult ages. While APOE genotype-effects in the brain are often linked with aging [33], [38], including by our group in these mouse models [47], differences in APOE protein levels occur early and remain consistent throughout adulthood in targeted-replacement APOE mice.
Fig. 1.
APOE genotype determines brain APOE protein levels during aging. (A) A representative Western blot analysis of homogenates prepared from APOE2, APOE3 and APOE4 mice hemibrains probed with anti-human APOE monoclonal antibody (HJ15.3). β-actin is shown as a loading control. (B) Quantification of APOE band intensity at 6, 12 and 18 months of age normalized to β-actin. Data are expressed as the mean ± SEM normalized to APOE3 at each age. n(6 months) = 12 APOE2, 10 APOE3, 12 APOE4; n(12 months) = 17 APOE2, 14 APOE3, 15 APOE4; n(18 months) = 10 each genotype; throughout data from individual male mice are graphed with a blue circle and from female with a red square. Post-hoc t-tests at 12 months of age: APOE2 = 142.6 ± 13.0 % of APOE3; t(29) = 3.279, p = 0.0027; APOE3 vs. APOE4 not significantly different (p = 0.066); and APOE2 = 183.0 ± 17.1 % of APOE4 t(30) = 4.835, p < 0.0001. At 18 months of age, APOE2 = 126.5 ± 5.7 % of APOE3; t(18) = 4.662, p = 0.0002); APOE3 = 117.1 ± 7.1 % of APOE4; t(18) = 2.405, p = 0.027; and APOE2 = 148.6 ± 8.4 % of APOE4 t(18) = 5.803, p = 0.0001. Throughout: *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
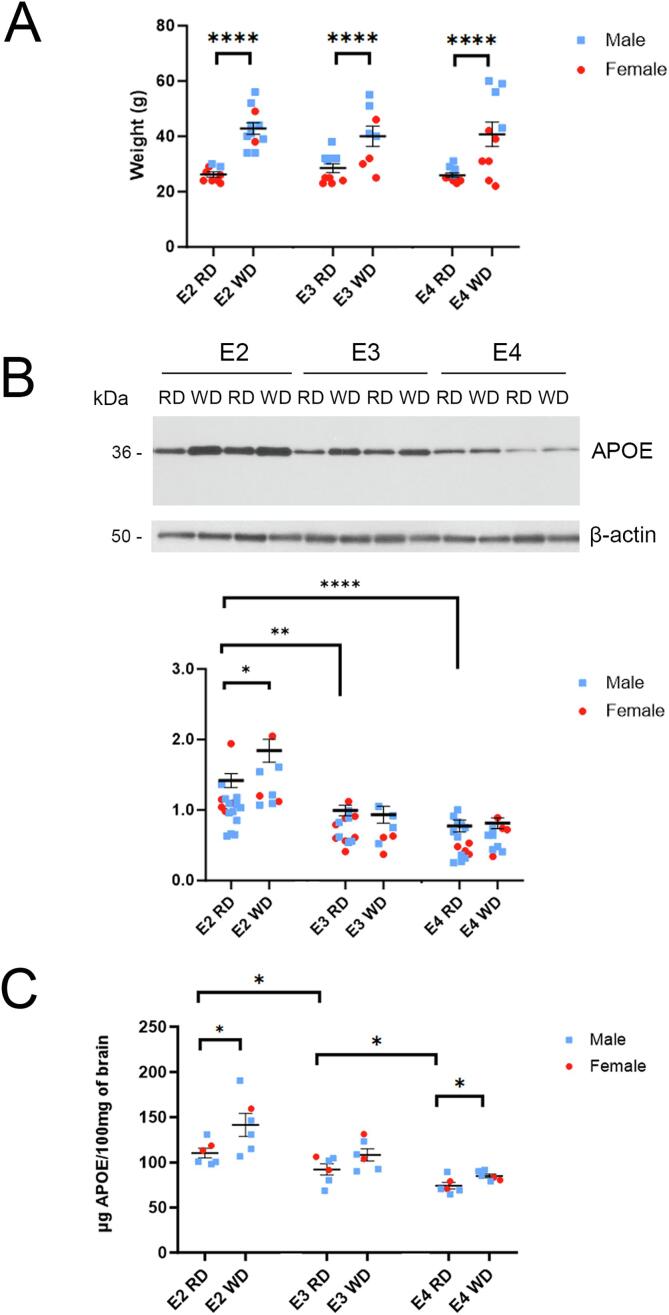
Fig. 3.
Brain APOE protein levels are altered in a genotype-dependent manner following a Western diet. (A) Body weight of APOE2, APOE3, and APOE4 mice at 12 months of age challenged with a regular diet (RD) or a Western high-fat diet (WD) for 6 months. No statistical difference in body weight by genotype was seen when comparing mice within each of the diet groups. (B) A representative Western blot analysis is shown of hemibrain homogenates from 12-month-old APOE2, APOE3 and APOE4 mice following RD or WD for 6 months. β-actin is shown as a loading control. Western blotting quantification of APOE band intensity normalized to β-actin is shown in the graph. n(APOE2) = 17RD, 8WD; n(APOE3) = 14 RD, 7WD; n(APOE4) = 15RD, 10WD (C) Quantification of human-APOE levels detected by sandwich ELISA from DEA-extracted hemibrain homogenates of APOE mice is shown. Data are expressed as the mean ± SEM normalized to APOE3 (RD); n = 6 for each genotype, each diet.
3.2. Western diet effects on plasma APOE levels are determined by APOE genotype
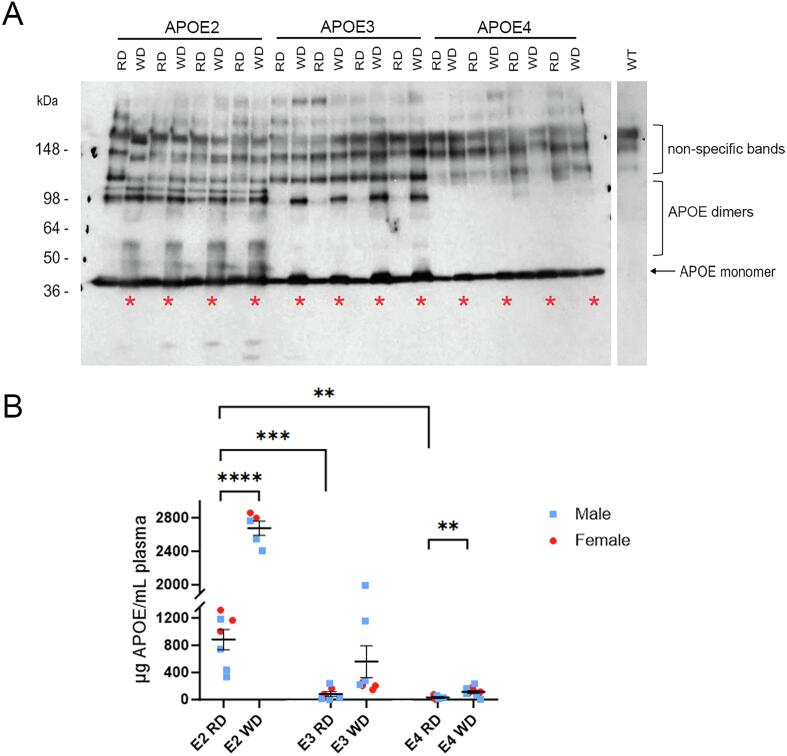
An individual’s risk for AD can be impacted by lipid dysmetabolism and a high fat diet [12], as well as by low plasma levels of APOE [19], [49]. We expected that APOE protein levels in this model would change in the periphery in response to dietary fat, as dietary fat is known to increase lipoparticle levels in the blood [23]. To determine if APOE genotype affects the response of APOE levels to dietary lipid intake, we placed APOE2, APOE3, and APOE4 mice on a Western diet high in fat and cholesterol from 6 months to 12 months of age in parallel with age-matched mice fed a standard rodent chow. Plasma levels of APOE protein by Western blot analysis appeared higher after a Western diet across all genotypes when compared with mice given a control diet, with the greatest differences in APOE2 mice (Fig. 2A). The Western diet, however, led to multiple higher molecular weight bands resolution by SDS-PAGE under reducing conditions that were genotype-dependent, appearing most prominently in APOE2 mice (Fig. 2A). APOE forms homo- and heterodimers [36], and, while unexpected, the preservation of such interactions following resolution by SDS-PAGE likely explains the genotype and diet-specific banding patterns seen. Interestingly, brain APOE subjected to the same detergents and resolved under the same conditions did not show any complex banding pattern indicative of dimers (see Fig. 1A and 3B), although APOE2 and APOE3 dimers in the brain have been reported [15]; reviewed in [18]. Given the complexity of quantifying within a linear range the multiple APOE bands of differing intensity detected by Western blot analysis, we used a human APOE sandwich ELISA that would allow for better quantification of blood plasma APOE levels. One-way ANOVA of ELISA on 12-month-old mice that were fed a standard diet (control mice) revealed a gradient in blood plasma levels that was determined by genotype, with APOE2 highest as in brain (APOE2 > APOE3 = APOE4; Fig. 2B). However, the magnitude of these differences was much greater in the blood plasma than in the brain. In particular, plasma APOE2 levels were an order of magnitude greater than in APOE3 (APOE2 = 1071.3 ± 181.7 % of APOE3 mice; t(12) = 5.345, p = 0.0002; APOE3 compared to APOE4 was not significantly different, p = 0.200; Fig. 2B). When mice fed a Western diet were added into the analysis, repeated measures 2 x 3 ANOVA (diet x genotype) revealed a main effect of both genotype (F(2,38) = 97.16, p < 0.0001) and diet (F (1, 38) = 56.91, p < 0.0001), as well as interaction between diet and genotype (F (2, 38) = 23.10, p < 0.0001). Post-hoc t-tests revealed a substantial increase in plasma APOE2 levels in response to a Western diet (WD = 303.0 ± 21.4 % of RD; t(10) = 9.483, p < 0.0001; Fig. 2B). APOE4 mice similarly showed an increase that was significant (WD = 360.9 ± 84.6 % of RD; t(15) = 3.084, p = 0.0076), while increased APOE levels in APOE3 mice with the Western diet did not reach significance (p = 0.086; Fig. 2B).
Fig. 2.
Plasma APOE protein levels are increased in APOE mice following 6 months on a Western diet. (A) A representative Western blot is shown of blood plasma from 12-month-old APOE2, APOE3 and APOE4 mice on a regular diet (RD) or a Western high-fat diet (WD; also marked by an asterisk) probed with the anti-human APOE monoclonal antibody HJ15.3. Western blot analysis of blood plasma from a wild-type mouse (right panel) is used as a negative control to show non-specific reactivity. The molecular weight of monomeric APOE protein is indicated, as are apparent higher molecular weight allele-dependent APOE homo- and/or heterdimers. (B) Quantification by sandwich ELISA showing APOE protein levels in blood plasma samples from APOE mice treated with regular diet (RD) or Western diet (WD). Data are expressed as the mean ± SEM. n(APOE2) = 7 RD, 5 WD; n(APOE3) = 7 RD, 8 WD; n(APOE4) = 5 RD, 11 WD.
3.3. Brain APOE protein levels are altered in a genotype-dependent manner following a Western diet
Given that APOE is the primary lipid carrier in the brain parenchyma, we hypothesized that diet-driven changes to brain lipid metabolism would affect brain APOE protein levels in a genotype-specific fashion, as in the periphery, if increased dietary fat and cholesterol impacted brain lipids. Mice fed the Western diet for 6 months gained on average 14 g relative to mice maintained on a standard diet (Fig. 3A). APOE genotype had no effect on weight at baseline or following the dietary challenge. As in plasma, we examined brain APOE protein levels using both Western blot analysis and ELISA in mice that were fed 6 months of a Western diet compared with a standard chow (Fig. 3B and C). In contrast to the Western blot analysis of APOE in blood plasma (Fig. 2), APOE protein in the brain was detected as a single band by Western blot analysis regardless of diet, consistent with reduced interactions with other apolipoproteins in the brain [18], interactions that form heterodimers with APOE in the periphery [60]. This finding is consistent with previous determinations that APOE in the blood and in the brain are distinct pools separated by the BBB [32], [34]. Consistent with the findings in Fig. 1, repeated measures 2 x 3 ANOVA (diet x genotype) on Western blot analyses revealed a main effect of genotype (F (2, 65) = 35.61, p < 0.0001) on brain APOE levels.
When challenged with a Western diet, posthoc t-test showed brain APOE levels that were significantly higher only in APOE2 mice (WD = 129.74 ± 12.96 % of RD; t(23) = 2.294, p = 0.031) (Fig. 3A). ELISA results yielded a repeated measures 2 × 3 ANOVA (diet x genotype) that revealed a main effect of both genotype (F (2, 30) = 22.50, p < 0.0001) and diet (F (1, 30) = 11.78, p = 0.0018). As by Western blot analysis, baseline APOE levels were higher in APOE2 compared with APOE3 mice fed a standard diet (APOE2 = 119.74 ± 8.23 % of APOE3; t(10) = 2.239, p = 0.049; Fig. 3B). ELISA in this cohort also showed significantly lower baseline APOE levels in APOE4 compared with APOE3 mice (APOE3 = 124.6 ± 10.0 % of APOE4; t(10) = 2.543, p = 0.029; with E2 = 149.3 ± 8.7 % of APOE4; t(10) = 5.680, p = 0.0002; Fig. 3B). Furthermore, ELISA revealed that a Western diet challenge significantly increases APOE levels in both APOE2 (WD = 128.2 ± 12.4 % of RD; t(10) = 2.268, p = 0.047) and APOE4 mice (WD = 114.9 ± 5.7 % of RD; t(10) = 2.637, p = 0.025; Fig. 3B), while APOE3 mice did not reach significance (p = 0.11). Previous work has shown that neither peripheral APOE nor dietary cholesterol crosses into the CNS through the BBB [5], [13], [32], [34], [48]. Nevertheless, our findings demonstrate that brain APOE levels respond in the same fashion as peripheral APOE levels to the Western diet, albeit to a lesser magnitude. Thus, while it appears that the BBB can dampen the effects of dietary lipids on the CNS, the brain is not fully protected from responding to peripheral dietary challenges.
Our findings are consistent with APOE lipoparticles not crossing the BBB: the magnitude of changes in the periphery are vastly different than in the brain, and the APOE banding patterns by Western blot analysis in the periphery compared with the brain are also distinct. Studies have shown that the BBB is compromised specifically in APOE4 individuals [9]. As such, we would anticipate APOE levels in the brain to be most affected in APOE4 mice where APOE lipoparticles may “leak” across the BBB. In contrast, our findings showed that the greatest responses to a dietary challenge were found in APOE2 mice. While free cholesterol cannot cross the BBB, oxidative metabolism of cholesterol forms oxysterols (27-OHC in the periphery and 24-OHC in the brain) that can traverse biological membranes by diffusion [4]. Considering that a high-fat diet and hypercholesterolemia are often associated with elevated levels of 27-OHC, and that numerous studies have associated high levels of 27-OHC in the brain with memory deficits, AD, and other neurodegenerative processes [12], [35], we suggest that oxysterols may be the bridge between peripheral cholesterol and brain APOE levels, one that warrants further investigation.
Consistent with our study, prior work has shown that APOE2 is more abundant in humanized mouse brain than APOE3 [50], [55], and that APOE3 is more abundant than APOE4 [59]. Riddell and colleagues [50] showed in these mouse models that, independent of genotype, APOE levels were greater in the hippocampus than frontal cortex, and future studies examining whether “high-risk” brain regions such as the hippocampus respond differently to a Western diet in terms of APOE protein levels may be informative. That APOE2 is the AD-protective allele and our findings that APOE2 levels increased the most in the brain in response to a high-fat dietary challenge raises the possibility that higher levels of APOE lipoparticles in the brain parenchyma are not damaging. Rather, APOE lipoparticles in the brain represent a lipid pool available to neurons when needed for biosynthetic processes. While genotype-mediated differences in receptor binding and neuronal uptake may impact the ability of neurons to utilize APOE lipoparticles [28], the abundance of APOE lipoparticles within the brain is also likely to determine their availability for neuronal biosynthetic processes. Indeed, in contrast to the periphery where abundant circulating lipoparticles can lead to vascular disease, an increased pool of available lipoparticles in the brain may be beneficial by offering neurons lipids as needed. While studies have shown that humans expressing an APOE4 allele are likely to show greater neurocognitive vulnerability to a Western, high-fat diet than APOE3 individuals [16], data on APOE2 carriers is limited by the relatively rarity of this allele in the population. Having shown that APOE2 in the brain is more responsive to a Western diet than the other alleles emphasizes the importance of studying in greater detail the unique brain properties of the protective APOE2 in the context of life-style and metabolic challenges that are thought to increase AD risk for individuals expressing APOE3 and, particularly, APOE4.
Credit authorship contribution statement
Braison Liemisa: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Samantha F. Newbury: Data curation, Formal analysis, Investigation. Mariah J. Novy: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. Jonathan A. Pasato: Data curation, Formal analysis, Investigation, Validation. Jose Morales-Corraliza: Data curation, Investigation, Supervision. Katherine Y. Peng: Formal analysis, Methodology, Writing – review & editing, Supervision. Paul M. Mathews: Conceptualization, Formal analysis, Funding acquisition, Project administration, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by National Institutes of Health grants P01 AG017617 and RF1 AG057517 to PM and a National Institutes of Health postdoctoral research training grant T32 AG052909 (KP). The funding sources were not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors declare no competing financial interests.
References
- 1.Bacon A.W., Bondi M.W., Salmon D.P., Murphy C. Very early changes in olfactory functioning due to Alzheimer's disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–731. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- 2.Basu S.K., Ho Y.K., Brown M.S., Bilheimer D.W., Anderson R.G., Goldstein J.L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982;257(16):9788–9795. [PubMed] [Google Scholar]
- 3.Bertram L., McQueen M.B., Mullin K., Blacker D., Tanzi R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkhem I., Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 6.Braesch-Andersen S., Paulie S., Smedman C., Mia S., Kumagai-Braesch M. ApoE production in human monocytes and its regulation by inflammatory cytokines. PLoS One. 2013;8(11):e79908. doi: 10.1371/journal.pone.0079908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caselli R.J. Age-related memory decline and apolipoprotein E e4. Discov Med. 2009;8(41):47–50. [PubMed] [Google Scholar]
- 9.Chernick D., Ortiz-Valle S., Jeong A., Qu W., Li L. Peripheral versus central nervous system APOE in Alzheimer's disease: Interplay across the blood-brain barrier. Neurosci Lett. 2019;708 doi: 10.1016/j.neulet.2019.134306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen A., Pike C.J. APOE genotype affects metabolic and Alzheimer-related outcomes induced by Western diet in female EFAD mice. FASEB J. 2019;33(3):4054–4066. doi: 10.1096/fj.201801756R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 12.Dai L., Zou L., Meng L., Qiang G., Yan M., Zhang Z. Cholesterol Metabolism in Neurodegenerative Diseases: Molecular Mechanisms and Therapeutic Targets. Mol Neurobiol. 2021;58(5):2183–2201. doi: 10.1007/s12035-020-02232-6. [DOI] [PubMed] [Google Scholar]
- 13.Dietschy J.M., Turley S.D. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45(8):1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.East B.S., Fleming G., Peng K., Olofsson J.K., Levy E., Mathews P.M., et al. Human Apolipoprotein E Genotype Differentially Affects Olfactory Behavior and Sensory Physiology in Mice. Neuroscience. 2018;380:103–110. doi: 10.1016/j.neuroscience.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott D.A., Halliday G.M., Garner B. Apolipoprotein-E forms dimers in human frontal cortex and hippocampus. BMC Neurosci. 2010;11:23. doi: 10.1186/1471-2202-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fado R., Molins A., Rojas R., Casals N. Feeding the Brain: Effect of Nutrients on Cognition, Synaptic Function, and AMPA Receptors. Nutrients. 2022;14(19) doi: 10.3390/nu14194137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R., et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 18.Flowers S.A., Rebeck G.W. APOE in the normal brain. Neurobiol Dis. 2020;136 doi: 10.1016/j.nbd.2019.104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannisis A., Al-Grety A., Carlsson H., Patra K., Twohig D., Sando S.B., et al. Plasma apolipoprotein E levels in longitudinally followed patients with mild cognitive impairment and Alzheimer's disease. Alzheimers Res Ther. 2022;14(1):115. doi: 10.1186/s13195-022-01058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert P.E., Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer's disease, probable Alzheimer's disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26(6):779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- 21.Gratuze M., Julien J., Morin F., Calon F., Hebert S.S., Marette A., et al. High-fat, high-sugar, and high-cholesterol consumption does not impact tau pathogenesis in a mouse model of Alzheimer's disease-like tau pathology. Neurobiol Aging. 2016;47:71–73. doi: 10.1016/j.neurobiolaging.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Graves A.B., Bowen J.D., Rajaram L., McCormick W.C., McCurry S.M., Schellenberg G.D., et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53(7):1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- 23.Hayek T., Ito Y., Azrolan N., Verdery R.B., Aalto-Setala K., Walsh A., et al. Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a new animal model and mechanistic studies in human Apo A-I transgenic and control mice. J Clin Invest. 1993;91(4):1665–1671. doi: 10.1172/JCI116375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heverin M., Maioli S., Pham T., Mateos L., Camporesi E., Ali Z., et al. 27-hydroxycholesterol mediates negative effects of dietary cholesterol on cognition in mice. Behav Brain Res. 2015;278:356–359. doi: 10.1016/j.bbr.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Hixson J.E., Vernier D.T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 26.Huynh T.V., Wang C., Tran A.C., Tabor G.T., Mahan T.E., Francis C.M., et al. Lack of hepatic apoE does not influence early Abeta deposition: observations from a new APOE knock-in model. Mol Neurodegener. 2019;14(1):37. doi: 10.1186/s13024-019-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanova N., Liu Q., Agca C., Agca Y., Noble E.G., Whitehead S.N., et al. White matter inflammation and cognitive function in a co-morbid metabolic syndrome and prodromal Alzheimer's disease rat model. J Neuroinflamm. 2020;17(1):29. doi: 10.1186/s12974-020-1698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson L.A., Olsen R.H., Merkens L.S., DeBarber A., Steiner R.D., Sullivan P.M., et al. Apolipoprotein E-low density lipoprotein receptor interaction affects spatial memory retention and brain ApoE levels in an isoform-dependent manner. Neurobiol Dis. 2014;64:150–162. doi: 10.1016/j.nbd.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josefsson M., Larsson M., Nordin S., Adolfsson R., Olofsson J. APOE-varepsilon4 effects on longitudinal decline in olfactory and non-olfactory cognitive abilities in middle-aged and old adults. Sci Rep. 2017;7(1):1286. doi: 10.1038/s41598-017-01508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanoski S.E., Davidson T.L. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutsodendris N., Nelson M.R., Rao A., Huang Y. Apolipoprotein E and Alzheimer's Disease: Findings, Hypotheses, and Potential Mechanisms. Annu Rev Pathol. 2022;17:73–99. doi: 10.1146/annurev-pathmechdis-030421-112756. [DOI] [PubMed] [Google Scholar]
- 32.Linton M.F., Gish R., Hubl S.T., Butler E., Esquivel C., Bry W.I., et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88(1):270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M., Kuhel D.G., Shen L., Hui D.Y., Woods S.C. Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am J Physiol Regul Integr Comp Physiol. 2012;303(9):R903–R908. doi: 10.1152/ajpregu.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loera-Valencia R., Goikolea J., Parrado-Fernandez C., Merino-Serrais P., Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer's disease: Potential novel targets for treatment. J Steroid Biochem Mol Biol. 2019;190:104–114. doi: 10.1016/j.jsbmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Mahley R.W., Innerarity T.L., Rall S.C., Jr., Weisgraber K.H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984;25(12):1277–1294. [PubMed] [Google Scholar]
- 37.Mahley R.W., Rall S.C., Jr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genom Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 38.Martens Y.A., Zhao N., Liu C.C., Kanekiyo T., Yang A.J., Goate A.M., et al. ApoE Cascade Hypothesis in the pathogenesis of Alzheimer's disease and related dementias. Neuron. 2022;110(8):1304–1317. doi: 10.1016/j.neuron.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Martinez A.B., Torres-Perez E., Devanney N., Del Moral R., Johnson L.A., Arbones-Mainar J.M. Beyond the CNS: The many peripheral roles of APOE. Neurobiol Dis. 2020;138 doi: 10.1016/j.nbd.2020.104809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattar J.M., Majchrzak M., Iannucci J., Bartman S., Robinson J.K., Grammas P. Sex Differences in Metabolic Indices and Chronic Neuroinflammation in Response to Prolonged High-Fat Diet in ApoE4 Knock-In Mice. Int J Mol Sci. 2022;23(7) doi: 10.3390/ijms23073921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesholam R.I., Moberg P.J., Mahr R.N., Doty R.L. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55(1):84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- 42.Morales-Corraliza J., Mazzella M.J., Berger J.D., Diaz N.S., Choi J.H., Levy E., et al. In vivo turnover of tau and APP metabolites in the brains of wild-type and Tg2576 mice: greater stability of sAPP in the beta-amyloid depositing mice. PLoS One. 2009;4(9):e7134. doi: 10.1371/journal.pone.0007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Corraliza J., Wong H., Mazzella M.J., Che S., Lee S.H., Petkova E., et al. Brain-Wide Insulin Resistance, Tau Phosphorylation Changes, and Hippocampal Neprilysin and Amyloid-beta Alterations in a Monkey Model of Type 1 Diabetes. J Neurosci. 2016;36(15):4248–4258. doi: 10.1523/JNEUROSCI.4640-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moser, V.A., Pike, C.J., 2017. Obesity Accelerates Alzheimer-Related Pathology in APOE4 but not APOE3 Mice. eNeuro 4(3). [DOI] [PMC free article] [PubMed]
- 45.Murphy C., Bacon A.W., Bondi M.W., Salmon D.P. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 46.Peng K.Y., Mathews P.M., Levy E., Wilson D.A. Apolipoprotein E4 causes early olfactory network abnormalities and short-term olfactory memory impairments. Neuroscience. 2017;343:364–371. doi: 10.1016/j.neuroscience.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng K.Y., Perez-Gonzalez R., Alldred M.J., Goulbourne C.N., Morales-Corraliza J., Saito M., et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain. 2019;142(1):163–175. doi: 10.1093/brain/awy289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfrieger F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60(6):1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen K.L., Tybjaerg-Hansen A., Nordestgaard B.G., Frikke-Schmidt R. Plasma apolipoprotein E levels and risk of dementia: A Mendelian randomization study of 106,562 individuals. Alzheimers Dement. 2018;14(1):71–80. doi: 10.1016/j.jalz.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Riddell D.R., Zhou H., Atchison K., Warwick H.K., Atkinson P.J., Jefferson J., et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28(45):11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saher G., Quintes S., Nave K.A. Cholesterol: a novel regulatory role in myelin formation. Neuroscientist. 2011;17(1):79–93. doi: 10.1177/1073858410373835. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt S.D., Mazzella M.J., Nixon R.A., Mathews P.M. Abeta measurement by enzyme-linked immunosorbent assay. Methods Mol Biol. 2012;849:507–527. doi: 10.1007/978-1-61779-551-0_34. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt S.D., Nixon R.A., Mathews P.M. Tissue processing prior to analysis of Alzheimer's disease associated proteins and metabolites, including Abeta. Methods Mol Biol. 2012;849:493–506. doi: 10.1007/978-1-61779-551-0_33. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan P.M. Influence of Western diet and APOE genotype on Alzheimer's disease risk. Neurobiol Dis. 2020;138 doi: 10.1016/j.nbd.2020.104790. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan P.M., Han B., Liu F., Mace B.E., Ervin J.F., Wu S., et al. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging. 2011;32(5):791–801. doi: 10.1016/j.neurobiolaging.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan P.M., Mezdour H., Aratani Y., Knouff C., Najib J., Reddick R.L., et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272(29):17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 57.Tai L.M., Youmans K.L., Jungbauer L., Yu C., Ladu M.J. Introducing Human APOE into Abeta Transgenic Mouse Models. Int J Alzheimers Dis. 2011;2011 doi: 10.4061/2011/810981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theriault P., ElAli A., Rivest S. High fat diet exacerbates Alzheimer's disease-related pathology in APPswe/PS1 mice. Oncotarget. 2016;7(42):67808–67827. doi: 10.18632/oncotarget.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vitek M.P., Brown C.M., Colton C.A. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009;30(9):1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weisgraber K.H., Shinto L.H. Identification of the disulfide-linked homodimer of apolipoprotein E3 in plasma. Impact on receptor binding activity. J Biol Chem. 1991;266(18):12029–12034. [PubMed] [Google Scholar]
- 61.Wieckowska-Gacek A., Mietelska-Porowska A., Wydrych M., Wojda U. Western diet as a trigger of Alzheimer's disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. 2021;70 doi: 10.1016/j.arr.2021.101397. [DOI] [PubMed] [Google Scholar]
- 62.Xiong J., Deng I., Kelliny S., Lin L., Bobrovskaya L., Zhou X.F. Long term high fat diet induces metabolic disorders and aggravates behavioral disorders and cognitive deficits in MAPT P301L transgenic mice. Metab Brain Dis. 2022;37(6):1941–1957. doi: 10.1007/s11011-022-01029-x. [DOI] [PubMed] [Google Scholar]
- 63.Xiong M., Wang C., Gratuze M., Saadi F., Bao X., Bosch M.E., et al. Astrocytic APOE4 removal confers cerebrovascular protection despite increased cerebral amyloid angiopathy. Mol Neurodegener. 2023;18(1):17. doi: 10.1186/s13024-023-00610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Lou I., Ali K., Chen Q. Effect of nutrition in Alzheimer's disease: A systematic review. Front Neurosci. 2023;17:1147177. doi: 10.3389/fnins.2023.1147177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Q., Bernardo A., Walker D., Kanegawa T., Mahley R.W., Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26(19):4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanguas-Casas N., Torres C., Crespo-Castrillo A., Diaz-Pacheco S., Healy K., Stanton C., et al. High-fat diet alters stress behavior, inflammatory parameters and gut microbiota in Tg APP mice in a sex-specific manner. Neurobiol Dis. 2021;159 doi: 10.1016/j.nbd.2021.105495. [DOI] [PubMed] [Google Scholar]