Abstract
Background
Neck pain is common, disabling and costly. The effectiveness of electrotherapy as a physiotherapeutic option remains unclear. This is an update of a Cochrane review first published in 2005 and previously updated in 2009.
Objectives
This systematic review assessed the short, intermediate and long‐term effects of electrotherapy on pain, function, disability, patient satisfaction, global perceived effect, and quality of life in adults with neck pain with and without radiculopathy or cervicogenic headache.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, MANTIS, CINAHL, and ICL, without language restrictions, from their beginning to August 2012; handsearched relevant conference proceedings; and consulted content experts.
Selection criteria
Randomized controlled trials (RCTs), in any language, investigating the effects of electrotherapy used primarily as unimodal treatment for neck pain. Quasi‐RCTs and controlled clinical trials were excluded.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration. We were unable to statistically pool any of the results, but we assessed the quality of the evidence using an adapted GRADE approach.
Main results
Twenty small trials (1239 people with neck pain) containing 38 comparisons were included. Analysis was limited by trials of varied quality, heterogeneous treatment subtypes and conflicting results. The main findings for reduction of neck pain by treatment with electrotherapeutic modalities were as follows.
Very low quality evidence determined that pulsed electromagnetic field therapy (PEMF) and repetitive magnetic stimulation (rMS) were more effective than placebo, while transcutaneous electrical nerve stimulation (TENS) showed inconsistent results.
Very low quality evidence determined that PEMF, rMS and TENS were more effective than placebo.
Low quality evidence (1 trial, 52 participants) determined that permanent magnets (necklace) were no more effective than placebo (standardized mean difference (SMD) 0.27, 95% CI ‐0.27 to 0.82, random‐effects model).
Very low quality evidence showed that modulated galvanic current, iontophoresis and electric muscle stimulation (EMS) were not more effective than placebo.
There were four trials that reported on other outcomes such as function and global perceived effects, but none of the effects were of clinical importance. When TENS, iontophoresis and PEMF were compared to another treatment, very low quality evidence prevented us from suggesting any recommendations. No adverse side effects were reported in any of the included studies.
Authors' conclusions
We cannot make any definite statements on the efficacy and clinical usefulness of electrotherapy modalities for neck pain. Since the evidence is of low or very low quality, we are uncertain about the estimate of the effect. Further research is very likely to change both the estimate of effect and our confidence in the results. Current evidence for PEMF, rMS, and TENS shows that these modalities might be more effective than placebo. When compared to other interventions the quality of evidence was very low thus preventing further recommendations.
Funding bias should be considered, especially in PEMF studies. Galvanic current, iontophoresis, EMS, and a static magnetic field did not reduce pain or disability. Future trials on these interventions should have larger patient samples, include more precise standardization, and detail treatment characteristics.
Keywords: Humans, Magnets, Electric Stimulation Therapy, Electric Stimulation Therapy/methods, Iontophoresis, Iontophoresis/methods, Magnetic Field Therapy, Magnetic Field Therapy/methods, Musculoskeletal Pain, Musculoskeletal Pain/therapy, Neck Pain, Neck Pain/therapy, Randomized Controlled Trials as Topic, Whiplash Injuries, Whiplash Injuries/therapy
Plain language summary
Electrotherapy for neck pain
Background
Neck pain is common, disabling and costly. Electrotherapy is an umbrella term that covers a number of therapies using electric current that aim to reduce pain and improve muscle tension and function.
Study characteristics
This updated review included 20 small trials (N = 1239). We included adults (> 18 years old) with acute whiplash or non‐specific neck pain as well as chronic neck pain including degenerative changes, myofascial pain or headaches that stem from the neck. No index for severity of the disorders could be specified. The evidence was current to August 2012. The results of the trials could not be pooled because they examined different populations, types and doses of electrotherapy and comparison treatments, and measured slightly different outcomes.
Key results
We cannot make any definitive statements about the efficacy of electrotherapy for neck pain because of the low or very low quality of the evidence for each outcome, which in most cases was based on the results of only one trial.
For patients with acute neck pain, TENS possibly relieved pain better than electrical muscle stimulation, not as well as exercise and infrared light, and as well as manual therapy and ultrasound. There was no additional benefit when added to infrared light, hot packs and exercise, physiotherapy, or a combination of a neck collar, exercise and pain medication. For patients with acute whiplash, iontophoresis was no more effective than no treatment, interferential current, or a combination of traction, exercise and massage for relieving neck pain with headache.
For patients with chronic neck pain, TENS possibly relieved pain better than placebo and electrical muscle stimulation, not as well as exercise and infrared light, and possibly as well as manual therapy and ultrasound. Magnetic necklaces were no more effective than placebo for relieving pain; and there was no additional benefit when electrical muscle stimulation was added to either mobilisation or manipulation.
For patients with myofascial neck pain, TENS, FREMS (FREquency Modulated Neural Stimulation, a variation of TENS) and repetitive magnetic stimulation seemed to relieve pain better than placebo.
Quality of the evidence
About 70% of the trials were poorly conducted studies. The trials were very small, with a range of 16 to 336 participants. The data were sparse and imprecise, which suggests that results cannot be generalized to the broader population and contributes to the reduction in the quality of the evidence. Therefore, further research is very likely to change the results and our confidence in the results.
Summary of findings
Summary of findings for the main comparison. Summary of findings: EMS.
| EMS + another treatment compared with that same treatment for neck pain | |||
|
Patient or population: Patients with subacute/chronic neck pain with or without radicular symptoms and cervicogenic headache Settings: Community USA Intervention: Electrical Muscle Stimulation (EMS) + another treatment Comparison: that same treatment | |||
| Outcomes | Effect | No of Participants (studies) | Quality of the evidence (GRADE) |
| Pain Intensity ‐ intermediate‐term follow‐up (about 6 months) | One trial with factorial design (multiple treatment meta‐analysis, I2 = 0%) showed no difference in pain intensity (pooled SMD 0.09, 95% CI Random ‐0.15 to 0.33) |
269 (1 study with factorial design of 4 independent comparisons) | ⊕⊕⊝⊝
low Design: 0 Limitations: 0 Inconsistency: 0 Indirectness: 0 Imprecision: ‐1 Other: ‐1 |
|
Function intermediate‐term follow‐up |
One trial with factorial design (multiple treatment meta‐analysis, I2 = 0%) showed no difference in pain intensity (pooled SMD 0.09, 95% CI Random ‐0.15 to 0.33) |
269 (1 study with factorial design of 4 independent comparisons) | ⊕⊕⊝⊝
low Design: 0 Limitations: 0 Inconsistency: 0 Indirectness: 0 Imprecision: ‐1 Other: ‐1 |
| Global Perceived Effect | Not measured | ||
|
Satisfaction intermediate‐term follow‐up |
One trial with factorial design (multiple treatment meta‐analysis, I2 = 0%) showed no difference in pain intensity (pooled SMD 0.02, 95% CI Random ‐0.22 to 0.26) |
269 (1 study with factorial design of 4 independent comparisons) | ⊕⊕⊝⊝
low Design: 0 Limitations: 0 Inconsistency: 0 Indirectness: 0 Imprecision: ‐1 Other: ‐1 |
| Quality of life | Not measured | ||
| Adverse effects | No known study related adverse events | ||
|
Low quality: 1. Imprecision: Sparce EMS‐related data (‐1) 2. Other: 2x2x2 factorial design (8 groups; 3 of them with EMS plus another treatment; N= 336) No setting parameters for EMS; Treatment schedule unclear: " ...at least 1 treatment..." (manip / mob) No maximum, no average number of treatments reported (‐1) | |||
Summary of findings 2. Summary of findings: static magnetic field (necklace).
| Static magnetic field (necklace) compared with placebo for neck pain | |||
|
Patient or population: Patients with chronic non‐specific neck pain Settings: Community USA ‐ Rehabilitation Institute Intervention: Static magnetic field (necklace) Comparison: placebo | |||
| Outcomes | Effect | No of Participants (studies) | Quality of the evidence (GRADE) |
|
Pain Intensity immediate post‐treatment (3 weeks) |
One trial showed no difference in pain intensity (SMD 0.27, 95% CI Random ‐0.27 to 0.82) |
52 (1 study) | ⊕⊕⊝⊝
low Design: 0 Limitations: 0 Inconsistency: 0 Indirectness: ‐1 Imprecision: ‐1 Other: 0 |
| Function | Not measured | ||
|
Global Perceived Effect immediate post‐treatment (3 weeks) |
One trial showed no difference in global perceived effect (RR 0.85, 95% CI Random 0.48 to 1.50) |
52 (1 study) | ⊕⊕⊝⊝
low Design: 0 Limitations: 0 Inconsistency: 0 Indirectness: ‐1 Imprecision: ‐1 Other: 0 |
| Satisfaction | Not measured | ||
| Quality of life | Not measured | ||
| Adverse effects | Not reported | ||
|
Low quality: 1. Imprecision: Sparce data (‐1) 2. Directness: Single small trial (‐1) | |||
Background
For many years, electrotherapy has been commonly used as one of the physiotherapeutic options to treat neck pain. In contrast, little is known about its efficacy and efficiency. In our first review, published in 2005 (Kroeling 2005) and evaluating 11 publications, we found the evidence for all described modalities of electrotherapy either lacking, limited or conflicting. Our first update (Kroeling 2009) replaced the 2005 review and added seven recent publications, including studies on a new modality. Four studies (Ammer 1990; Chee 1986; Persson 2001; Provinciali 1996) that were included in the first review were excluded in the 2009 update, because studies of multimodal treatment were excluded; the unique contribution of the electrotherapy could not be identified. In our 2009 update (Kroeling 2009)18 small trials (1093 people with neck pain) were included. Analysis was limited by trials of varied quality, heterogeneous treatment subtypes and conflicting results.
Description of the condition
We studied neck pain that could be classified as either:
non‐specific mechanical neck pain, including whiplash associated disorders (WAD) category I and II (Spitzer 1987; Spitzer 1995), myofascial neck pain, and degenerative changes including osteoarthritis and cervical spondylosis (Schumacher 1993);
cervicogenic headache (Olesen 1988; Olesen 1997; Sjaastad 1990; or
neck disorders with radicular findings (Spitzer 1987; Spitzer 1995).
It can be classified as acute (less than 30 days), subacute (30 to 90 days) or chronic duration (longer than 90 days). Neck pain is typically provoked by neck movements and by physical examination provocation tests, and is located between the occiput to upper thoracic spine with the associated musculature.
Description of the intervention
Electrotherapy is a treatment category and may include: direct current, iontophoresis, electrical nerve stimulation, electrical muscle stimulation, pulsed electromagnetic fields, repetitive magnetic stimulation, and permanent magnets. Their underpinning mechanisms vary and are described in the following section.
How the intervention might work
1) Galvanic current for pain control
Treatment by direct current (DC), so‐called Galvanic current, reduces pain by inhibiting nociceptor activity (Cameron 1999). This effect is restricted to the area of current flow through the painful region. The main indication for Galvanic current is the treatment of acute radicular pain and inflammation of periarticular structures such as tendons and ligaments. Because DC enhances the transport of ionised substances through the skin, it can also be used to promote resorption of topical treatments, especially anti‐inflammatory drugs (iontophoresis).
2) Electrical nerve stimulation (ENS) or transcutaneous electrical nerve stimulation (TENS) for pain control
Alternating electrical current (AC) or modulated DC (so‐called Galvanic stimulation), mostly in the form of rectangular impulses, may inhibit pain‐related potentials at the spinal and supraspinal level, known as 'gate control'. This underpins all classical forms of stimulating electrotherapy (for example diadynamic current), as well as a modern form called TENS (including Ultra‐Reiz). While Galvanic current efficacy is restricted to the area of current flow, analgesic effects of ENS can be observed in the whole segmental region, both ipsilateral and contralateral (Cameron 1999; Kroeling 1998; Stucki 2000; Stucki 2007; Walsh 1997).
3) Electrical muscle stimulation
Most characteristics of EMS are comparable to TENS. The critical difference is in the intensity, which leads to additional muscle contractions. Primary pain relief via gate control can be obtained by EMS, TENS or other forms of ENS (Hsueh 1997). Rhythmic muscle stimulation by modulated DC, AC or interferential current probably increases joint range of motion (ROM), re‐educates muscles, retards muscle atrophy, and increases muscle strength. The circulation can be increased and muscle hypertension decreased, which may lead to secondary pain relief (Tan 1998).
4) Pulsed electromagnetic fields (PEMF) and permanent magnets
Electricity is always connected with both electrical and magnetic forces. Alternating or pulsed electromagnetic fields induce electric current within the tissue. Even though these currents are extremely small, we recognize PEMF and the application of permanent magnets as forms of electrotherapy. Their main therapeutic purpose is for enhancement of bone or tissue healing and pain reduction.
5) Repetitive magnetic stimulation
Repetitive magnetic stimulation (rMS), in contrast to PEMF therapy, is a rather new (mid‐1980s) neurophysiologic technique that allows the transcutaneous induction of nerve stimulating electric currents. This technique requires extremely strong and sharp magnetic impulses (for example 15,000 amperes peak current; 2.5 T field strength; < 1 msec) applied by specially designed coils (< 10 cm) over the target area. Modern devices allow the repetition of up to 60 impulses per second. Mainly developed to study and influence brain functions, rMS also stimulates spinal chord fibres and peripheral nerves. Initial studies used peripheral rMS for therapeutic reasons, such as in myofascial pain syndrome (Pujol 1998; Smania 2003; Smania 2005). Since the resulting small electric impulses are the nerve stimulating factor, rMS effects may be similar to TENS and EMS.
Why it is important to do this review
Neck disorders with episodic pain and functional limitation (Hogg‐Johnson 2008) are common in the general population (Carroll 2008a; US Census Bureau 2012), in workers (Côté 2008) and in whiplash associated disorders (WAD) (Carroll 2008b). In a Canadian study, about 5% of cases revealed a clinically important disability (Côté 1998). There is a great impact on the work force; and 3% to 11% of claimants are off work each year (Côté 2008). Direct and indirect costs are substantive (Hogg‐Johnson 2008). Chronic pain accounts for about USD 150 to USD 215 billion each year in economic loss (that is lost workdays, therapy, disability) (NRC 2001; US Census Bureau 1996). The annual expenditure on medical care for back and neck conditions adjusted for inflation per patient increased by 95%, from USD 487 in 1999 to USD 950 in 2008 (Davis 2012). Yet very little is known about the effectiveness of most of the numerous available treatments still. Two systematic reviews have been published subsequent to ours. Teasell 2010 investigated acute whiplash while Leaver 2010 reviewed non‐specific neck pain. Neither review revealed any new data and agreed with our former update. There continues to be very little information on this topic. Therefore ongoing updates of this review are necessary.
Objectives
This systematic review assessed the short, intermediate and long‐term effects of electrotherapy on pain, function, disability, patient satisfaction, global perceived effect, and quality of life in adults with neck pain with and without radiculopathy or cervicogenic headache.
Methods
Criteria for considering studies for this review
Types of studies
We included published randomized controlled trials (RCTs) in any language. Quasi‐RCTs and controlled clinical trials (CCTs) were excluded.
Types of participants
The participants were adults, 18 years or older, who suffered from acute (less than 6 weeks), subacute (6 to 12 weeks) or chronic (longer than 12 weeks) neck pain categorized as:
non‐specific mechanical neck pain, including WAD category I and II (Spitzer 1987; Spitzer 1995), myofascial neck pain, and degenerative changes including osteoarthritis and cervical spondylosis (Schumacher 1993);
cervicogenic headache (Olesen 1988; Olesen 1997; Sjaastad 1990; and
neck disorders with radicular findings (Spitzer 1987; Spitzer 1995).
Studies were excluded if they investigated neck pain with definite or possible long tract signs, neck pain caused by other pathological entities (Schumacher 1993), headache that was not of cervical origin but was associated with the neck, co‐existing headache when either the neck pain was not dominant or the headache was not provoked by neck movements or sustained neck postures, or 'mixed' headaches.
Types of interventions
All studies used at least one type of electrotherapy: direct current, iontophoresis, electrical nerve stimulation; electrical muscle stimulation; pulsed electromagnetic fields (PEMF), repetitive magnetic stimulation (rMS) and permanent magnets.
Interventions were contrasted against the following comparisons:
electrotherapy versus sham or placebo (e.g. TENS versus sham TENS or sham ultrasound);
electrotherapy plus another intervention versus that same intervention (e.g. TENS + exercise versus exercise);
electrotherapy versus another intervention (e.g. TENS versus exercise);
one type of electrotherapy versus another type (e.g. modulated versus continuous TENS).
Exclusion criteria
Other forms of high frequency electromagnetic fields, such as short wave diathermy, microwave, ultrasound and infrared light, were not considered in this review because their primary purpose is to cause therapeutic heat. Since electro‐acupuncture is a special form of acupuncture, it was also excluded. Multimodal treatment approaches that included electrotherapy were excluded if the unique contribution of electrotherapy could not be determined.
Types of outcome measures
The outcomes of interest were pain relief (for example a Numerical Rating Scale), disability (for example Neck Disability Index), function (for example activities of daily living) including work‐related outcomes (for example return to work, sick leave), patient satisfaction, global perceived effect and quality of life. Adverse events as well as costs of care were reported if available. The duration of follow‐up was defined as:
immediate post‐treatment (within one day);
short‐term follow‐up (closest to four weeks);
intermediate‐term follow‐up (closest to six months); and
long‐term follow‐up (closest to12 months).
Primary outcomes
The outcomes of interest were pain relief, disability, and function including work‐related outcomes.
Secondary outcomes
Patient satisfaction, global perceived effect and quality of life.
Search methods for identification of studies
References of retrieved articles were independently screened by two review authors. Note that our systematic review methodological design is consistent with the Cochrane Back Group methods.
Electronic searches
A research librarian searched computerized bibliographic databases without language restrictions for medical, chiropractic, and allied health literature. The search for this review was part of a comprehensive search on physical medicine modalities. These databases were searched for this update from December 2008 to August 2012.
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid), EMBASE (Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Manual Alternative and Natural Therapy (MANTIS). Subject headings (MeSH) and key words included anatomical terms (neck, neck muscles, cervical plexus, cervical vertebrae, atlanto‐axial joint, atlanto‐occipital joint, spinal nerve roots, brachial plexus); disorder and syndrome terms (arthritis, myofascial pain syndromes, fibromyalgia, spondylitis, spondylosis, spinal osteophytosis, spondylolisthesis, headache, whiplash injuries, cervical rib syndrome, torticollis, cervico‐brachial neuralgia, radiculitis, polyradiculitis, polyradiculoneuritis, thoracic outlet syndrome); treatment terms (multimodal treatment, electric stimulation therapy, transcutaneous electric nerve stimulation, rehabilitation, ultrasonic therapy, phototherapy, lasers, physical therapy, acupuncture, biofeedback, chiropractic, electric stimulation therapy); and methodological terms. See Appendix 1 for the full MEDLINE search strategy. We also searched trial registers such as ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
Searching other resources
We communicated with identified content experts, searched conference proceedings of the World Confederation for Physical Therapy 2011 and International Federation of Orthopaedic and Manipulative Therapists 2008. In addition, we searched our own personal files for grey literature.
Data collection and analysis
Selection of studies
Two review authors independently conducted citation identification and study selection. All forms were pre‐piloted. Each pair of review authors met for consensus and consulted a third author when there was persisting disagreement. Agreement (yes, unclear, no) was assessed for study selection using the quadratic weighted Kappa statistic, Cicchetti weights (Landis 1977).
Data extraction and management
Two review authors independently conducted data abstraction. Forms used were pre‐piloted. Data were extracted on the methods (RCT type, number analysed, number randomized, intention‐to‐treat analysis), participants (disorder subtype, duration of disorder), interventions (treatment characteristics for the treatment and comparison groups, dosage and treatment parameters, co‐intervention, treatment schedule), outcomes (baseline mean, reported results, point estimate with 95% confidence intervals (CI), power, side effects, costs of care) and notes (if authors were contacted or points of consideration related to the RCT). These factors are detailed in the 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
We conducted the 'Risk of bias' assessment for RCTs using the criteria recommended by The Cochrane Collaboration (Higgins 2011) and the Cochrane Back Review Group (Furlan 2009) (see Appendix 2). At least two review authors independently assessed the risk of bias. A consensus team met to reach a final assessment. The following characteristics were assessed for risk of bias: randomisation; concealment of treatment allocation; blinding of patient, provider, and outcome assessor; incomplete data: withdrawals, dropout rate and intention‐to‐treat analysis; selective outcome reporting; other including similar at baseline, similar co‐interventions, acceptable compliance, similar timing of assessment. A study with a low risk of bias was defined as having low risk of bias on six or more of these items and no fatal flaws.
Measures of treatment effect
Standardized mean differences (SMD) with 95% confidence intervals (95% CI) were calculated for continuous data while relative risks (RR) were calculated for dichotomous outcomes. We selected SMD over weighted mean difference (WMD) because we were looking across different interventions and most interventions used different outcome measures with different scales. For outcomes reported as medians, effect sizes were calculated using the formula by Kendal 1963 (p 237). When neither continuous nor dichotomous data were available, we extracted the findings and the statistical significance as reported by the author(s) in the original study.
In the absence of clear guidelines on the size of a clinically important effect, a commonly applied system by Cohen 1988 was used: small (0.20), medium (0.50) and large (0.80). A minimal clinically important difference between treatments for the purpose of the review was 10 points on a 100‐point pain intensity scale (small: WMD < 10%; moderate: 10% ≤ WMD < 20%; large: 20% ≤ WMD of the visual analogue scale (VAS)). For the neck disability index, we used a minimum clinically important difference of 7/50 neck disability index units. It is noted that the minimal detectable change varies from 5/50 for non‐complicated neck pain to 10/50 for cervical radiculopathy (MacDermid 2009). To translate effect measures into clinically meaningful terms and give the clinician a sense of the magnitude of the treatment effect, we calculated the number needed to treat (NNT) when the effect size was statistically significant (NNT: the number of patients a clinician needs to treat in order to achieve a clinically important improvement in one) (Gross 2002).
Unit of analysis issues
We performed one multiple treatment meta‐analysis for the Hurwitz 2002 trial that used a factorial design. We used a random‐effects model to allow for heterogeneity within each subgroup. An I2 statistic was also computed for subgroup differences. The data in the subgroups were independent.
Dealing with missing data
Where data were not extractable primary authors were contacted. See the 'Characteristics of included studies' table, 'Notes' for details. Missing data from Hurwitz 2002 and Chiu 2005 were obtained in this manner. No other data were requested. Missing data that were greater than 10 years old were not requested.
Assessment of heterogeneity
Prior to calculation of a pooled effect measure, we assessed the reasonableness of pooling on clinical grounds. The possible sources of heterogeneity considered were: symptom duration (acute versus chronic); subtype of neck pain (for example WAD); intervention type (for example DC versus pulsed); characteristics of treatment (for example dosage, technique); and outcomes (pain relief, measures of function and disability, patient satisfaction, quality of life). We were unable to perform any of these calculations because the data were incompatible.
Assessment of reporting biases
Occurrences of reporting biases were noted in the text and 'Characteristics of included studies' tables, 'Notes' column. Our review search methods addressed language bias; no additional languages were selected for this review. Funding bias was possible in three trials (Sutbeyaz 2006; Thuile 2002; Trock 1994). One trial from Spain was judged to have serious flaws and high risks of bias which may represent reporting bias (Escortell‐Mayor 2011).
Data synthesis
We assessed the quality of the body of the evidence using the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted in the updated Cochrane Back Review Group (CBRG) method guidelines (Furlan 2009). Domains that may decrease the quality of the evidence are: 1) study design, 2) risk of bias, 3) inconsistency of results, 4) indirectness (not to generalize), 5) imprecision (insufficient data), and 6) other factors (for example reporting bias). The quality of the evidence was reduced by a level based on the performance of the studies against these five domains (see Appendix 3 for definitions of these domains). All plausible confounding factors were considered as were their potential effects on the demonstrated treatment responses and the treatment dose‐response gradient (Atkins 2004). Levels of quality of evidence were defined as the following.
High quality evidence: there are consistent findings among at least 75% of RCTs with low risk of bias; consistent, direct and precise data; and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate quality evidence: one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality evidence: three of the domains are not met. We are very uncertain about the results.
No evidence: no RCTs were identified that addressed this outcome.
We also considered a number of factors to place the results into a larger clinical context: temporality, plausibility, strength of association, dose response, adverse events, and cost. Clinical relevance was addressed for individual trials and reported either in the 'Characteristics of included studies' table or in the text.
Subgroup analysis and investigation of heterogeneity
We had also planned to assess the influence of risk of bias (concealment of allocation, blinding of outcome assessor), duration (acute, subacute, chronic), and subtypes of the disorder (non‐specific, WAD, degenerative change‐related, radicular findings, cervicogenic headache), but again data were too sparse. Since a meta‐analysis was not possible, sources of heterogeneity were not explored.
Sensitivity analysis
Sensitivity analysis or meta‐regression for (1) symptom duration, (2) methodological quality, and (3) subtype of neck disorder were planned but were not carried out because we did not have enough data in any one category.
Results
Description of studies
Twenty trials (1239 participants) were selected (Figure 1). The duration of the disorder, disorder subtypes and electrotherapy subtypes were as follows.
1.
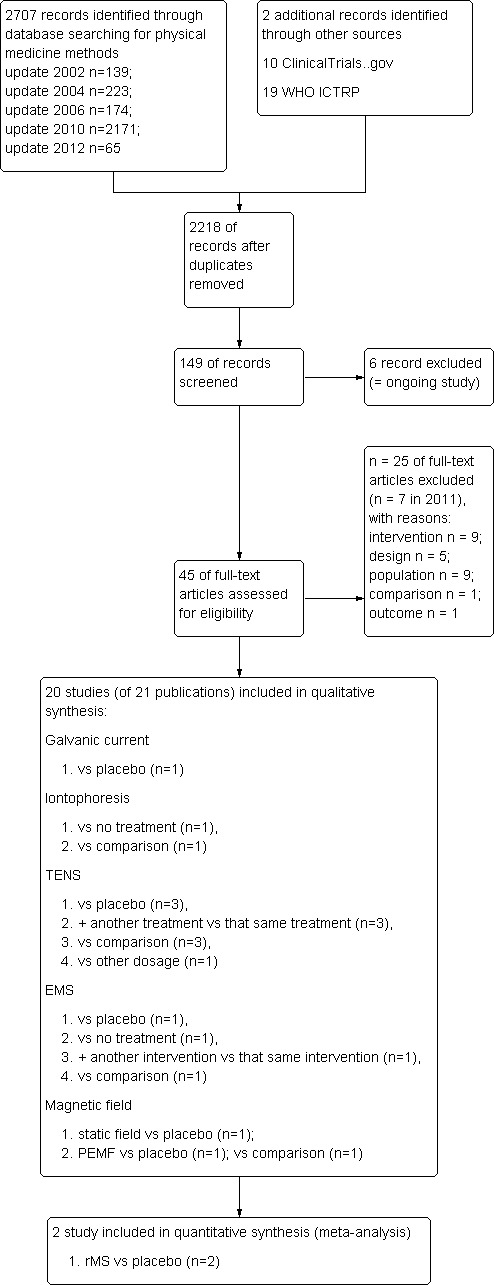
Study flow diagram.
Acute whiplash associated disorders (WAD) with or without cervicogenic headache (n = 4): Fialka 1989, electrical muscle stimulation (EMS) and iontophoresis; Hendriks 1996, transcutaneous electric nerve stimulation (TENS); Foley‐Nolan 1992, pulsed electromagnetic field (PEMF); Thuile 2002, PEMF.
Acute non‐specific neck pain (n = 1): Nordemar 1981, TENS.
Chronic myofascial neck pain (n = 5): Farina 2004, TENS; Hsueh 1997, TENS; Hou 2002, TENS; Smania 2003, repetitive magnetic stimulation (rMS); Smania 2005, rMS.
Chronic neck pain due to osteoarthritic cervical degenerative changes (n = 2): Trock 1994, PEMF; Sutbeyaz 2006, PEMF.
Chronic non‐specific neck pain (n = 5): Chiu 2005, TENS; Flynn 1987, TENS; Foley‐Nolan 1990, PEMF; Hong 1982, static magnetic field; Philipson 1983, modulated galvanic current.
Subacute or chronic neck pain with or without cervicogenic headache and radicular findings (n = 1): Hurwitz 2002, EMS.
Subacute or chronic non‐specific neck pain (n = 1): Escortell‐Mayor 2011.
One trial was translated from Danish (Philipson 1983). Three further non‐English trials (two French, one Italian) were subsequently excluded because they did not meet our criteria.
Six ongoing trials have been registered but not published (Triano 2009; Escortell 2011; Guayasamín 2013; Taniguchi 2010; Weintraub 2007).
Excluded studies
Twenty‐five studies were excluded (n = 7 in 2011). The reasons were: the intervention (n = 9) (Ammer 1990; Fernadez‐de‐las Penas2004; Forestier 2007a; Forestier 2007b; Klaber‐Moffett 2005; Persson 2001; Provinciali 1996; Vas 2006; Vikne 2007); population (n = 9) (Chen 2007; Coletta 1988; Gabis 2003; Hansson 1983; Jahanshahi 1991; Porzio 2000; Rigato 2002; Wang 2007; Wilson 1974); design (n = 5) (Chee 1986; Gonzales‐Iglesias 2009; Lee 1997; Vitiello 2007; Yip 2007); comparison (n = 1) (Dusunceli 2009); outcome (n = 1) (Garrido‐Elustondo 2010).
Risk of bias in included studies
Allocation
The allocation of concealment and reports on adequate randomisation were unclear in 60% of the trials (see also Figure 2).
2.
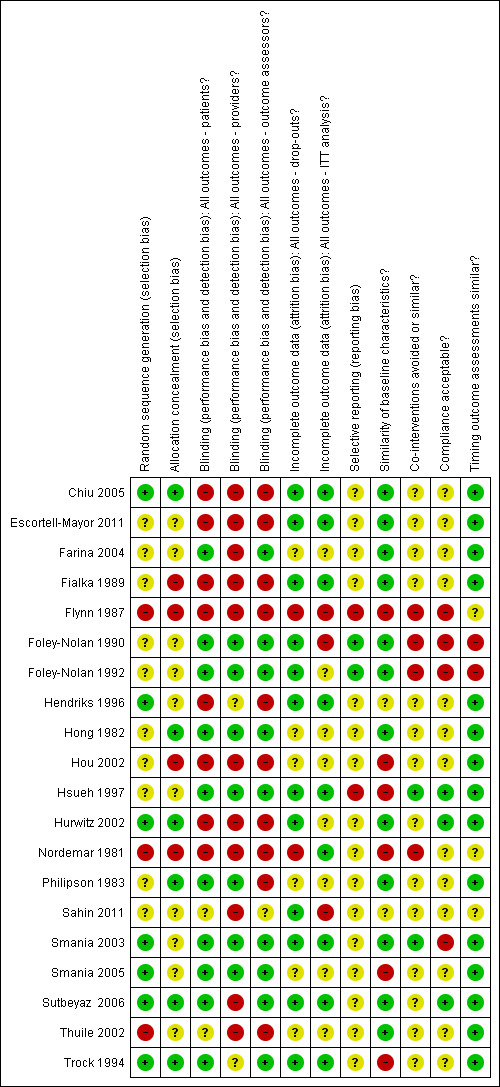
Risk of bias summary: review authors' judgements about each item for each included study.
Blinding
There was no clear reporting of blinding of patients (50%), providers (60%) or observers (60%).
Incomplete outcome data
In 50% of the trials there was attrition bias, when considering both dropouts (30%) and intention‐to‐treat (ITT) analysis (50%).
Selective reporting
Selective reporting was present or unclear in 80% of the trials.
Other potential sources of bias
In Trock 1994 their research support was listed as Bio‐Magnetic Systems, Inc. (co‐author Markoll was the principle shareholder of Bio‐Magnetic Sytems; Markoll and Trock were sentenced in 2001 for billing unapproved electromagnetic therapy (see FDA report: http://www.fda.gov/ora/about/enf_story/archive/2001/ch6/oci6.htm).
Effects of interventions
Galvanic current
1. Modulated Galvanic current versus placebo
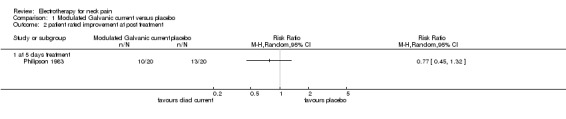
One study with a high risk of bias (Philipson 1983) assessed the effects of 'diadynamic' modulated Galvanic current (50 or 100 Hz) against placebo for patients with chronic pain in trigger points of the neck and shoulders.
Pain relief
No difference (RR 0.69, 95% CI 0.39 to 1.24, random‐effects model) between the groups was found after a one‐week treatment.
Global perceived effect
No difference between the groups was noted immediately post‐treatment.
Conclusion: there was very low quality evidence of no difference in pain or global perceived effect when diadynamic modulated Galvanic current was evaluated at immediate post‐treatment.
Iontophoresis
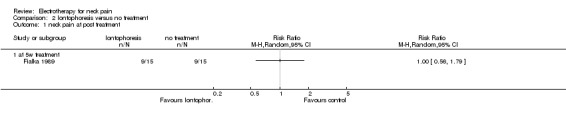
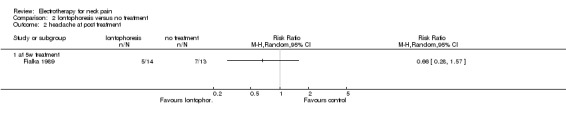
1. Iontophoresis versus no treatment
One study with a high risk of bias (Fialka 1989) assessed the effects of iontophoresis (DC combined with diclofenac gel) compared to no treatment for patients with acute WAD pain with or without cervicogenic headache.
Pain relief:
No difference between the groups was determined after a five‐week treatment.
Cervicogenic headache
No difference (RR 0.66, 95% CI 0.28 to 1.57, random‐effects model) between the groups was reported after a five‐week treatment.
Conclusion: very low quality evidence suggested that iontophoresis when compared to no treatment improved pain and headache for patients with acute WAD with or without cervicogenic headache.
2. Iontophoresis versus comparison
One study with a high risk of bias (Fialka 1989) assessed the effects of iontophoresis (DC combined with diclofenac gel, same as above) against two other treatments: a) interferential current, and b) multimodal treatment (traction + therapeutic exercise + massage) for patients with acute WAD.
Pain relief
No difference between the groups was determined after a five‐week treatment period.
Cervicogenic headache
No difference between the groups was reported after five weeks of treatment.
Conclusion: there was very low quality evidence that iontophoresis improved pain or headache when contrasted against either interferential or a multimodal approach for acute WAD or cervicogenic headache.
Transcutaneous electrical nerve stimulation (TENS)
1. TENS versus placebo (sham control)
Two studies with low risk of bias (Hsueh 1997; Smania 2005) and two with high risk of bias (Flynn 1987; Sahin 2011) compared TENS to sham controls for patients with chronic neck pain.
Pain relief
All four trials reported immediate post‐treatment pain relief favouring TENS. The results varied and they could not be combined since they assessed outcomes of very different treatment schedules. One trial also reported short‐term pain relief, but our calculations did not support that (SMD ‐0.52, 95% CI ‐1.24 to 0.20, random‐effects model) (Smania 2005).
Conclusion: there was very low quality evidence (four trials with sparse and non‐generalizable data; group sizes between 7 and 22 participants) showing varied results for TENS therapy, with different frequencies and treatment schedules, immediately post‐treatment for patients with chronic neck pain.
2. TENS plus another treatment versus that same treatment
Three studies with high risk of bias utilized TENS (80 to 100 Hz) for individuals with chronic neck pain (Chiu 2005), myofascial neck pain (Hou 2002), and acute neck pain (Nordemar 1981). Another trial assessed TENS (Ultra‐Reiz, 143 Hz) for patients with acute WAD (Hendriks 1996). In these trials, TENS was added to other interventions received by both comparison groups (Chiu 2005: Infrared; Hou 2002: hot pack, exercises; Nordemar 1981: neck collar, exercises, analgesic; Hendriks 1996: standard physiotherapy).
Pain relief
Three trials reported no benefit of TENS at post‐treatment (Hou 2002), short (Nordemar 1981) and intermediate‐term (Chiu 2005) follow‐up. One trial (Hendriks 1996) favoured Ultra‐Reiz for pain relief in the short term. Due to different dosage parameters, data were not pooled.
Conclusion: there was very low quality evidence (two trials with group sizes between 10 and 13, one with no blinding and different treatment regimens) that the addition of TENS had no additional significant effect on pain relief in patients with acute to chronic neck pain, and that Ultra‐Reiz reduced pain for patients with acute WAD (one trial, 2 X 8 participants).
3. TENS versus comparison
Three studies with high risk of bias compared TENS to EMS (Hsueh 1997), ultrasound (Flynn 1987) and manual therapy (Nordemar 1981) for treatment of acute and chronic neck pain. One study with high risk of bias (Escortell‐Mayor 2011) compared TENS to manual therapy for subacute and chronic neck pain.
Pain relief
TENS seemed superior to EMS (Hsueh 1997), but there was little or no difference between TENS and manual therapy (Nordemar 1981; Escortell‐Mayor 2011) or ultrasound (Flynn 1987).
Conclusion: there was very low quality evidence (trials with group sizes between 7 and 43 participants, sparse and non‐generalizable data) that TENS may relieve pain better than EMS, but there was little or no difference between the effects of TENS and manual therapy (low quality evidence) or ultrasound (very low quality evidence) for patients with acute or chronic neck pain. Due to different comparative treatments, the results of the trials could not be pooled.
4. TENS versus TENS (with different parameters)
One study with a low risk of bias (Farina 2004) examined the effects of TENS (100 Hz) against FREMS (a frequency and intensity varying TENS modification, 1 to 40 Hz) for chronic myofascial pain. Another study with high risk of bias (Sahin 2011) compared conventional TENS (100 Hz) with both acupuncture like (AL)‐TENS (4 Hz) and burst‐mode (Burst)‐TENS (100 Hz, 2 Hz) for chronic myofascial pain.
Pain relief
TENS and FREMS were both reported to be significantly effective for pain relief (VAS) after one week of treatment, and at one and three‐month follow‐up (Farina 2004). Conventional TENS showed no significant difference over AL‐TENS or Burst‐TENS after three weeks of treatment (Sahin 2011).
Conclusion: there was very low quality evidence (one trial, 19 + 21 participants; insufficient data reported) that FREMS and TENS were similarly effective for the treatment of chronic myofascial neck pain. There was very low quality evidence (one trial, two comparisons with 37 participants) that conventional TENS was similar to Burst‐TENS or AL‐TENS for chronic myofascial pain immediately post‐treatment.
Electrical Muscle Stimulation (EMS)
1. EMS versus placebo (sham control)
One trial with a low risk of bias (Hsueh 1997) studied the effects of a single EMS treatment (20 minutes,10 Hz) for chronic neck pain with cervical trigger points compared to sham control.
Pain relief
No difference for pain intensity and pressure threshold was found.
Conclusion: there was very low quality evidence (one trial, 22 + 18 participants) that a single treatment of EMS had no effect on trigger point tenderness compared to placebo treatment in patients with chronic neck pain.
2. EMS (interferential current) versus no treatment
One study with a high risk of bias described the effect of EMS (stereodynamic 50 Hz interferential current) (Fialka 1989) for acute WAD versus no treatment.
Pain relief
No difference between treated and untreated control patients was found for neck pain relief and headache.
Conclusion: there was very low quality evidence (one trial, 2 x 15 participants) that EMS neither reduced neck pain nor cervicogenic headache in patients with acute WAD, compared to no treatment.
3. EMS plus another treatment versus the same treatment
One 2 x 2 x 2 factorial design study with a low risk of bias (Hurwitz 2002) compared the effects of additional EMS on two independent groups with mobilisation and two independent groups with manipulation (each arm with or without moist heat) for patients with subacute to chronic neck pain with and without cervicogenic headache or radicular symptoms.
Pain relief
No differences between the groups were found at post‐treatment, short‐term and intermediate‐term follow‐up (Figure 3). A multiple treatment meta‐analysis from one factorial design of independent groups was pooled (SMD 0.09, 95% CI ‐0.15 to 0.33, random‐effects model) with an I2 of 0% at intermediate‐term follow‐up (Figure 4).
3.
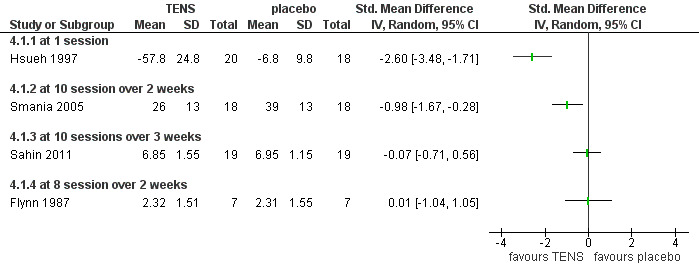
Forest plot of comparison: 4 TENS versus placebo or sham, outcome: 4.1 pain intensity at post‐treatment.
4.
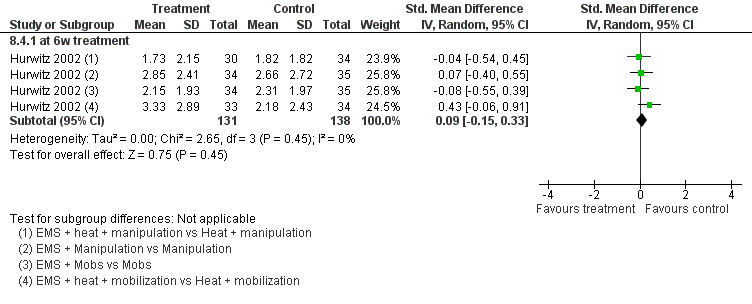
Forest plot of comparison: 8 EMS + another intervention versus that same intervention, outcome: 8.4 pain intensity at IT (6‐month) follow‐up.
Function
No differences between the groups were found (pooled SMD 0.09, 95% CI ‐0.15 to 0.33, random‐effects model; I2 = 0%) at post‐treatment, short‐term and intermediate‐term follow‐up (Figure 5).
5.
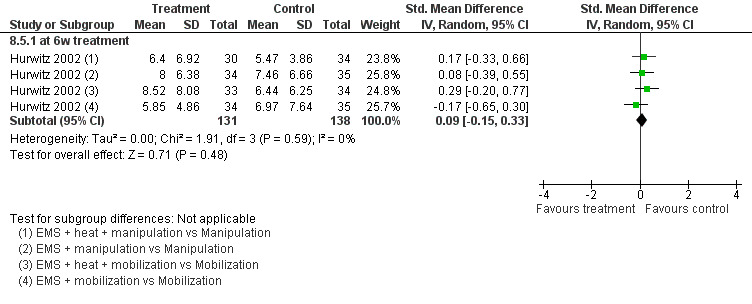
Forest plot of comparison: 8 EMS + another intervention versus that same intervention, outcome: 8.5 function at IT (6‐month) follow‐up.
Patient satisfaction
No differences between the groups were found (pooled SMD 0.02, 95% CI ‐0.22 to 0.26, random‐effects model; I2 = 0%) at post‐treatment, short‐term and intermediate‐term follow‐up (Figure 6).
6.
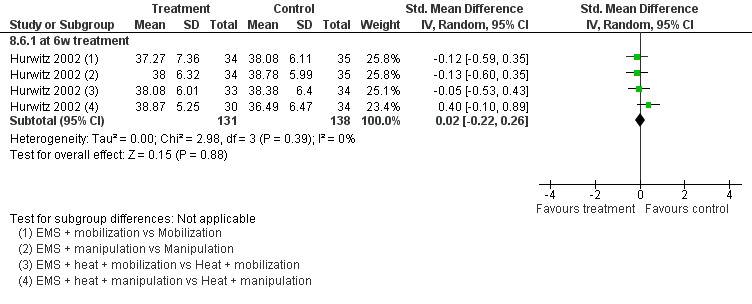
Forest plot of comparison: 8 EMS + another intervention versus that same intervention, outcome: 8.6 patient satisfaction at post‐treatment.
Conclusion: there was low quality evidence (1 factorial designed trial, N = 336; three EMS groups, N = ˜ 40; no EMS settings or treatment schedules reported) that EMS had no significant impact on pain relief, disability and patient satisfaction when used as an adjunct to cervical mobilisation and manipulation, at post‐treatment, short‐term and intermediate‐term follow‐up.
4. EMS versus comparison
One study with a low risk of bias compared the effect of EMS to TENS for chronic myofascial pain (Hsueh 1997), and one study with a high risk of bias to treatment with iontophoresis for patients with acute WAD (Fialka 1989; see above).
Pain relief
EMS was found to be inferior to TENS for pain relief immediately following treatment. No difference was found between EMS and Iontophoresis at post‐treatment and short‐term follow‐up.
Conclusion: there was very low quality evidence (one trial, 20 + 18 participants; one treatment only; poor clinical relevance) that EMS was inferior to TENS for pain relief of chronic neck pain. There was very low quality evidence (one trial, 2 x 15 participants) that there was no significant difference between EMS and iontophoresis for pain relief in acute WAD.
Pulsed electromagnetic field (PEMF)
1. PEMF versus placebo or sham control
Two studies with a low risk of bias examined the efficacy of non‐thermal, high frequency PEMF (miniaturized high frequency (HF) generator, 27 MHz, 1.5 mW/cm²) treatment on patients with chronic neck pain (Foley‐Nolan 1990) and acute WAD (Foley‐Nolan 1992). Two other trials with a low risk of bias studied the efficacy of low frequency PEMF therapy (< 100 Hz) for participants with chronic cervical osteoarthritis pain (Sutbeyaz 2006; Trock 1994). All four studies were sham‐controlled by inactive devices.
Pain relief
In their first trial, the authors (Foley‐Nolan 1990) found that pain intensity (VAS) was reduced post‐treatment. In their second trial (Foley‐Nolan 1992) no relevant effects were found. Trock 1994 reported significant pain relief after four to six weeks of treatment, but not at the one‐month follow‐up. Sutbeyaz 2006 reported significant pain relief, favouring the active PEMF group, after three weeks of treatment.
Function
Trock 1994 reported no differences in improvement in function.
Global perceived effects
Trock 1994 and Sutbeyaz 2006 reported no differences in effects.
Conclusion: there was very low quality evidence that non‐thermal high frequency PEMF (27 MHz) reduced acute or chronic neck pain, and that low frequency PEMF (< 100 Hz) may have reduced pain from cervical spine osteoarthritis after some weeks of treatment. Even though these trials were rated as having a low risk of bias by our validity assessment team, they were imprecise, inconsistent and may have been influenced by other biases. The evidence rating was therefore reduced from moderate quality to very low for the following reasons: funding bias may have been present in Trock 1994 and Sutbeyaz 2006; the PEMF application (in a cervical collar worn 24 hours per day) in Foley‐Nolan 1990 and Foley‐Nolan 1992 was a very uncommon PEMF method using diathermy‐like HF‐pulses but with intensities far below the thermal threshold. The biological rationale for the chosen treatment was based on literature from 1940 and remains unclear.
2. PEMF versus comparison
One study with a high risk of bias (Thuile 2002) compared low frequency PEMF (< 100 Hz) treatment versus a standard therapy for WAD patients.
Pain relief
Reported results favoured PEMF therapy for neck pain relief and headache reduction in patients with WAD.
Conclusion: there was very low quality evidence (one trial, 44 + 48 participants; no placebo control; funding bias unclear) that PEMF may have reduced WAD‐related neck pain and headache compared to a standard therapy.
Repetitive magnetic stimulation (rMS)
1. rMS versus placebo
Two similar studies with a low risk of bias (Smania 2003; Smania 2005) evaluated rMS therapy (400 mT, 4000 pulses per session) for patients with myofascial neck pain against placebo ultrasound.
Pain relief and function
Pain and disability (VAS, Neck Pain Disability (NPD)) reduction by rMS was more effective than placebo for the treatment of myofascial neck pain at two weeks, one month (Figure 7) (pooled SMD ‐1.35, 95% CI ‐1.96 to ‐0.74, random‐effects model), and three months follow‐up.
7.
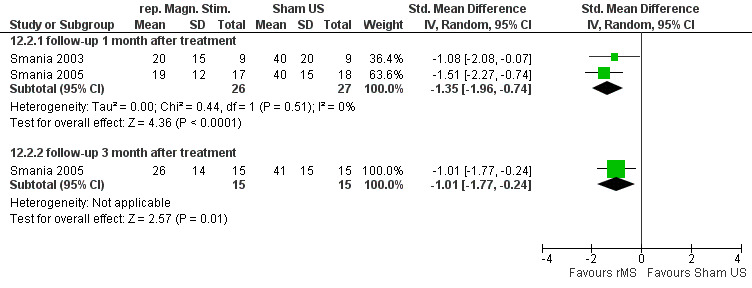
Forest plot of comparison: 12 Repetitive magnetic stimulation (rMS) versus placebo ultrasound, outcome: 12.2 pain and function at ST follow‐up.
Conclusion: we found very low quality evidence (two trials from the same research group, with sparse and non‐generalizable data, 9 to 16 participants in either group) that rMS was effective for a short‐term reduction of chronic neck pain and disability compared to placebo. However, although the NNT = 3 and treatment advantage was 46% to 56%, because of the low quality of the evidence one should treat the results with caution. Publication bias may be considered. Funding was not reported.
Static magnetic field
1. Static magnetic field (permanent magnets, necklace) versus sham control
One study with a low risk of bias (Hong 1982) investigated the efficacy of a magnetic necklace (120 mT) on patients with chronic neck and shoulder pain compared to a sham control group with identical but non‐magnetic necklaces.
Pain relief
No differences (SMD 0.27, 95% CI ‐0.27 to 0.82, random‐effects model) were found between the groups (Figure 8).
8.

Forest plot of comparison: 13 Static magnetic field (necklace) versus placebo, outcome: 13.1 pain intensity at post‐treatment.
Conclusion: there was low quality evidence (one trial, 27 + 25 participants) that permanent magnets were not effective for chronic neck and shoulder pain relief.
Side effects
No adverse side effects were reported in any of the included studies evaluated above. However, studies were too small for a valid evaluation of adverse effects.
Costs
No costs were reported in any of the included studies evaluated above.
Discussion
Electrotherapy has been developed during the last two centuries. The systematic use of electric currents for therapeutic reasons began shortly after Luigi Galvani's observations (1780) that electric currents cause muscle contractions if stimulating efferent nerves. Since then, a growing variety of methods, including electromagnetic and magnetic agents, have been developed for a manifold of therapeutic reasons. Only a small selection of these methods have been investigated by the trials described above, direct or modulated Galvanic currents, iontophoresis, transcutaneous electrical nerve stimulation (TENS), electrical muscle stimulation (EMS), low or high frequency pulsed electromagnetic fields (PEMF), repetitive magnetic stimulation (rMS) and permanent magnets. A great deal of research in these fields has been published in the past 25 years (Cameron 1999), however only 20 trials examining the treatment of neck pain met our review criteria. Therefore, evidence for any of the modalities was found to be of low or very low quality, due to the size of the trials and the heterogeneity of the populations, interventions and outcomes. This precluded meta‐analysis and resulted in sparse data. The average sample size over all treatment groups was about 20 participants.
Summary of main results
For this review, there were 20 trials with 38 comparisons that met our inclusion criteria. No outcomes had high or moderate strength of evidence. The evidence for all electrotherapy interventions for neck pain is of low or very low quality, which means that we are very uncertain about the estimate of effect. Further research is very likely to have an important impact on this and our confidence in the results. Therefore, no conclusions can be drawn regarding the effectiveness of electrotherapy for neck pain based on the available small trials. Large randomized controlled trials are needed to get a valid and precise estimate of the effect of electrotherapy for neck pain.
Overall completeness and applicability of evidence
In general, convincing, high or moderate quality evidence for any of the described modalities was lacking. Thirty‐eight comparisons in 20 studies examined seven different forms, and their modifications, of electrotherapy. Of the few studies that examined the same modalities, conclusions were limited by the heterogeneity of the treatment parameters or population. For example, the frequency for TENS ranged from 60 Hz to 143 Hz, with disorders from acute WAD to chronic myofascial pain. This heterogeneity made it impossible to pool the data and difficult to interpret the applicability of the results. More research needs to be done in order to confirm the positive findings, and to determine which treatment parameters are the most applicable and for which disorders.
Quality of the evidence
Performance and detection bias are the two dominant biases influencing our systematic review findings. Specifically, blinding of the patients and providers are essential considerations for future trials. Co‐interventions need to be avoided to establish clearer results.
Potential biases in the review process
Language bias was avoided by including all languages during study selection, however non‐English databases were not searched (that is Chinese databases).
Agreements and disagreements with other studies or reviews
The evidence presented in this review needs to be compared to the evidence described in other reviews. The limited number of reviews on the subject makes it difficult to carry out that comparison. There was conflicting evidence in the results on PEMF (Sutbeyaz 2006; Thuile 2002; Trock 1994), such that the positive findings for PEMF were strongly doubted in other reviews (Hulme 2002; Schmidt‐Rohlfing 2000). We also have these concerns and caution the reader that funding bias may be present. In particular, research support was declared as being provided by Bio‐Magnetic Systems, Inc. Co‐author Markoll was principle shareholder of Bio‐Magnetic Sytems; Markoll and Trock were sentenced in 2001 for billing unapproved electromagnetic therapy (see FDA report:http://www.fda.gov/ora/about/enf_story/archive/2001/ch6/oci6.htm).
Authors' conclusions
Implications for practice.
We cannot make any definitive statements on the efficacy and clinical usefulness of electrotherapy modalities for neck pain. Since the quality of the evidence is low or very low, we are uncertain about the estimates of the effect. Further research is very likely to change both the estimate of effect and our confidence in the results. Current evidence for rMS, TENS and PEMF shows that these modalities might be more effective than placebo but not other interventions, and funding bias has to be considered, especially in PEMF studies. Galvanic current, iontophoresis, electric muscle stimulation (EMS) and a static magnetic field did not reduce pain or disability.
Implications for research.
Due to a lack of consensus on parameters, and the restricted quality of most of the publications, additional studies need to be done to confirm the results described in this review. Possible new trials examining these specific interventions should include more participants and correct the internal validity and reporting shortcomings found in earlier randomized controlled trials. They should include more precise standardization and description of treatment characteristics.
What's new
| Date | Event | Description |
|---|---|---|
| 4 July 2013 | New citation required but conclusions have not changed | Updated literature search from 2009 to August 2012, 2 new publications were included, 7 publications were excluded. |
| 4 July 2013 | New search has been performed | 20 studies (21 publications) included in qualitative synthesis: galvanic current versus placebo (n = 1); iontophoresis versus no treatment (n = 1), versus comparison (n = 1); TENS versus placebo (n = 3), + another treatment versus that same treatment (n = 3), versus comparison (n = 3), versus other dosage (n = 1); EMS versus placebo (n = 1), versus no treatment (n = 1), + another intervention versus that same intervention (n = 1), versus comparison (n = 1); Static magnetic field versus placebo (n = 1); PEMF versus placebo (n = 1), versus comparison (n = 1); |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 4 August 2009 | New citation required but conclusions have not changed | Inclusion criteria modified and contracted to clearly isolate the unique effect of electrotherapy, resulting in four publications excluded from the 2005 version of the review. An additional kind of electrotherapy was also identified (repetitive magnetic stimulation, rMS). However, there were no essential changes in conclusions ‐ the evidence neither supports nor refutes the efficacy of electrotherapy for the management of neck pain. Further research is very likely to change both the estimate of effect and our confidence in the results. |
| 15 June 2008 | Amended | Converted to new review format. |
| 14 June 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank our volunteer translators and the Cochrane Back Review Group editors.
Appendices
Appendix 1. MEDLINE search strategy
Physical Medicine‐COG_NeckPain_
July 11 2010
1. Neck Pain/
2. exp Brachial Plexus Neuropathies/
3. exp neck injuries/ or exp whiplash injuries/
4. cervical pain.mp.
5. neckache.mp.
6. whiplash.mp.
7. cervicodynia.mp.
8. cervicalgia.mp.
9. brachialgia.mp.
10. brachial neuritis.mp.
11. brachial neuralgia.mp.
12. neck pain.mp.
13. neck injur*.mp.
14. brachial plexus neuropath*.mp.
15. brachial plexus neuritis.mp.
16. thoracic outlet syndrome/ or cervical rib syndrome/
17. Torticollis/
18. exp brachial plexus neuropathies/ or exp brachial plexus neuritis/
19. cervico brachial neuralgia.ti,ab.
20. cervicobrachial neuralgia.ti,ab.
21. (monoradicul* or monoradicl*).tw.
22. or/1‐21
23. exp headache/ and cervic*.tw.
24. exp genital diseases, female/
25. genital disease*.mp.
26. or/24‐25
27. 23 not 26
28. 22 or 27
29. neck/
30. neck muscles/
31. exp cervical plexus/
32. exp cervical vertebrae/
33. atlanto‐axial joint/
34. atlanto‐occipital joint/
35. Cervical Atlas/
36. spinal nerve roots/
37. exp brachial plexus/
38. (odontoid* or cervical or occip* or atlant*).tw.
39. axis/ or odontoid process/
40. Thoracic Vertebrae/
41. cervical vertebrae.mp.
42. cervical plexus.mp.
43. cervical spine.mp.
44. (neck adj3 muscles).mp.
45. (brachial adj3 plexus).mp.
46. (thoracic adj3 vertebrae).mp.
47. neck.mp.
48. (thoracic adj3 spine).mp.
49. (thoracic adj3 outlet).mp.
50. trapezius.mp.
51. cervical.mp.
52. cervico*.mp.
53. 51 or 52
54. exp genital diseases, female/
55. genital disease*.mp.
56. exp *Uterus/
57. 54 or 55 or 56
58. 53 not 57
59. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 58
60. exp pain/
61. exp injuries/
62. pain.mp.
63. ache.mp.
64. sore.mp.
65. stiff.mp.
66. discomfort.mp.
67. injur*.mp.
68. neuropath*.mp.
69. or/60‐68
70. 59 and 69
71. Radiculopathy/
72. exp temporomandibular joint disorders/ or exp temporomandibular joint dysfunction syndrome/
73. myofascial pain syndromes/
74. exp "Sprains and Strains"/
75. exp Spinal Osteophytosis/
76. exp Neuritis/
77. Polyradiculopathy/
78. exp Arthritis/
79. Fibromyalgia/
80. spondylitis/ or discitis/
81. spondylosis/ or spondylolysis/ or spondylolisthesis/
82. radiculopathy.mp.
83. radiculitis.mp.
84. temporomandibular.mp.
85. myofascial pain syndrome*.mp.
86. thoracic outlet syndrome*.mp.
87. spinal osteophytosis.mp.
88. neuritis.mp.
89. spondylosis.mp.
90. spondylitis.mp.
91. spondylolisthesis.mp.
92. or/71‐91
93. 59 and 92
94. exp neck/
95. exp cervical vertebrae/
96. Thoracic Vertebrae/
97. neck.mp.
98. (thoracic adj3 vertebrae).mp.
99. cervical.mp.
100. cervico*.mp.
101. 99 or 100
102. exp genital diseases, female/
103. genital disease*.mp.
104. exp *Uterus/
105. or/102‐104
106. 101 not 105
107. (thoracic adj3 spine).mp.
108. cervical spine.mp.
109. 94 or 95 or 96 or 97 or 98 or 106 or 107 or 108
110. Intervertebral Disk/
111. (disc or discs).mp.
112. (disk or disks).mp.
113. 110 or 111 or 112
114. 109 and 113
115. herniat*.mp.
116. slipped.mp.
117. prolapse*.mp.
118. displace*.mp.
119. degenerat*.mp.
120. (bulge or bulged or bulging).mp.
121. 115 or 116 or 117 or 118 or 119 or 120
122. 114 and 121
123. intervertebral disk degeneration/ or intervertebral disk displacement/
124. intervertebral disk displacement.mp.
125. intervertebral disc displacement.mp.
126. intervertebral disk degeneration.mp.
127. intervertebral disc degeneration.mp.
128. 123 or 124 or 125 or 126 or 127
129. 109 and 128
130. 28 or 70 or 93 or 122 or 129
131. animals/ not (animals/ and humans/)
132. 130 not 131
133. exp *neoplasms/
134. exp *wounds, penetrating/
135. 133 or 134
136. 132 not 135
137. Neck Pain/rh [Rehabilitation]
138. exp Brachial Plexus Neuropathies/rh
139. exp neck injuries/rh or exp whiplash injuries/rh
140. thoracic outlet syndrome/rh or cervical rib syndrome/rh
141. Torticollis/rh
142. exp brachial plexus neuropathies/rh or exp brachial plexus neuritis/rh
143. 137 or 138 or 139 or 140 or 141 or 142
144. Radiculopathy/rh
145. exp temporomandibular joint disorders/rh or exp temporomandibular joint dysfunction syndrome/rh
146. myofascial pain syndromes/rh
147. exp "Sprains and Strains"/rh
148. exp Spinal Osteophytosis/rh
149. exp Neuritis/rh
150. Polyradiculopathy/rh
151. exp Arthritis/rh
152. Fibromyalgia/rh
153. spondylitis/rh or discitis/rh
154. spondylosis/rh or spondylolysis/rh or spondylolisthesis/rh
155. or/144‐154
156. 59 and 155
157. exp Combined Modality Therapy/
158. Exercise/
159. Physical Exertion/
160. exp Exercise Therapy/
161. exp Rehabilitation/
162. exp Physical Therapy Modalities/
163. Hydrotherapy/
164. postur* correction.mp.
165. Feldenkrais.mp.
166. (alexander adj (technique or method)).tw.
167. Relaxation Therapy/
168. Biofeedback, Psychology/
169. or/157‐168
170. 136 and 169
171. 143 or 156 or 170
172. animals/ not (animals/ and humans/)
173. 171 not 172
174. exp randomized controlled trials as topic/
175. randomized controlled trial.pt.
176. controlled clinical trial.pt.
177. (random* or sham or placebo*).tw.
178. placebos/
179. random allocation/
180. single blind method/
181. double blind method/
182. ((singl* or doubl* or trebl* or tripl*) adj25 (blind* or dumm* or mask*)).ti,ab.
183. (rct or rcts).tw.
184. (control* adj2 (study or studies or trial*)).tw.
185. or/174‐ 184
186. 173 and 185
187. limit 186 to yr="2006 ‐Current"
188. limit 186 to yr="1902 ‐ 2005"
189. guidelines as topic/
200. practice guidelines as topic/
201. guideline.pt.
202. practice guideline.pt.
203. (guideline? or guidance or recommendations).ti.
204. consensus.ti.
205. or/189‐204
206. 173 and 205
207. 136 and 205
208. 206 or 207
209. limit 208 to yr="2006 ‐Current"
210. limit 208 to yr="1902 ‐ 2005"
211. meta‐analysis/
212. exp meta‐analysis as topic/
213. (meta analy* or metaanaly* or met analy* or metanaly*).tw.
214. review literature as topic/
215. (collaborative research or collaborative review* or collaborative overview*).tw.
216. (integrative research or integrative review* or intergrative overview*).tw.
217. (quantitative adj3 (research or review* or overview*)).tw.
218. (research integration or research overview*).tw.
219. (systematic* adj3 (review* or overview*)).tw.
220. (methodologic* adj3 (review* or overview*)).tw.
221. exp technology assessment biomedical/
222. (hta or thas or technology assessment*).tw.
223. ((hand adj2 search*) or (manual* adj search*)).tw.
224. ((electronic adj database*) or (bibliographic* adj database*)).tw.
225. ((data adj2 abstract*) or (data adj2 extract*)).tw.
226. (analys* adj3 (pool or pooled or pooling)).tw.
227. mantel haenszel.tw.
228. (cohrane or pubmed or pub med or medline or embase or psycinfo or psyclit or psychinfo or psychlit or cinahl or science citation indes).ab.
229. or/211‐228
230. 173 and 229
231. limit 230 to yr="2006 ‐Current"
Appendix 2. Criteria for assessing risk of bias for internal validity (Higgins 2011)
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/ care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel or care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
for patient‐reported outcomes in which the patient was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if drop‐outs are very large, imputation using even "acceptable" methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and drop‐outs should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective Reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of patients with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat‐analysis
There is low risk of bias if all randomized patients were reported/analysed in the group to which they were allocated by randomisation.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Appendix 3. Grading the quality of evidence ‐ definition of domains
Factors that might reduce the quality of the evidence
Study Design refers to type of study (i.e. randomized, observational study)
Limitations within Study Design (Quality) refers to the 12 risk of bias criteria noted in Appendix 2.
Consistency (heterogeneity) refers to the similarity of results across studies. When all studies are included in the meta‐analysis, ‘consistency’ is defined as absence of statistical heterogeneity. In the case that not all studies are combined in a meta‐analysis, ‘consistency’ is defined when all studies for the specific outcome lead to the same decision or recommendation, and ‘inconsistency’ is present if the results of two or more studies lead to clinically different decisions or recommendations. Authors use their judgment to decide if there is inconsistency when only one study leads to clinically different decision or recommendation.
Directness (generalizability) refers to the extent to which the people, interventions and outcome measures are similar to those of interest.
Precision of the evidence relates to the number of studies, patients and events for each outcome. Imprecise data is defined as:
Only one study for an outcome, regardless of the sample size or the confidence interval.
Multiple studies combined in a meta‐analysis: the confidence interval is sufficiently wide that the estimate is consistent with conflicting recommendations. For rare events one should consider the confidence interval around the risk difference rather than the confidence interval around the relative risk.
Multiple studies not combined in a meta‐analysis: the total sample size is underpowered to detect a clinically significant difference between those who received the index intervention compared to those who received the control intervention. In this case, a post‐hoc sample size calculation should be performed to determine the adequate sample size for each outcome.
Reporting (publication) bias should only be considered present if there is actual evidence of reporting bias rather than only speculation about reporting bias. The Cochrane Reporting Bias Methods Group describes the following types of Reporting Bias and Definitions:
Publication Bias: the publication or non publication of research findings, depending on the nature and direction of the results.
Time Lag Bias: the rapid or delayed publication of research findings, depending on the nature and direction of the results.
Language Bias: the publication of research findings in a particular language, depending on the nature and direction of the results.
Funding Bias: the reporting of research findings, depending on how the results accord with the aspirations of the funding body.
Outcome Variable Selection Bias: the selective reporting of some outcomes but not others, depending on the nature and direction of the research findings.
Developed Country Biases: the non publication or non indication of findings, depending on whether the authors were based in developed or in developing countries.
Data and analyses
Comparison 1. Modulated Galvanic current versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at post treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 at 5 days treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 patient rated improvement at post treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 at 5 days treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
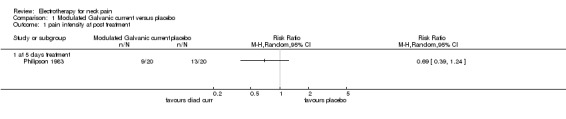
Comparison 1 Modulated Galvanic current versus placebo, Outcome 1 pain intensity at post treatment.
1.2. Analysis.
Comparison 1 Modulated Galvanic current versus placebo, Outcome 2 patient rated improvement at post treatment.
Comparison 2. Iontophoresis versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 neck pain at post treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 at 5w treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 headache at post treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 at 5w treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 Iontophoresis versus no treatment, Outcome 1 neck pain at post treatment.
2.2. Analysis.
Comparison 2 Iontophoresis versus no treatment, Outcome 2 headache at post treatment.
Comparison 3. Iontophoresis versus comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 neck pain at post treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 vs Interferential current ‐ at 5w treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 vs traction + therapeutic exercise + massage ‐ at 5w treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
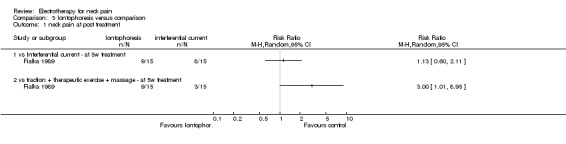
Comparison 3 Iontophoresis versus comparison, Outcome 1 neck pain at post treatment.
Comparison 4. TENS versus placebo or sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at post treatment | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 1 session | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 at 10 session over 2 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 at 10 sessions over 3 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 at 8 session over 2 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 pain intensity at ST follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 at 3 month follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 pressure pain threshold at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 at 1 session | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
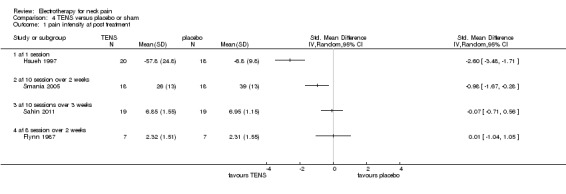
Comparison 4 TENS versus placebo or sham, Outcome 1 pain intensity at post treatment.
4.2. Analysis.

Comparison 4 TENS versus placebo or sham, Outcome 2 pain intensity at ST follow‐up.
4.3. Analysis.

Comparison 4 TENS versus placebo or sham, Outcome 3 pressure pain threshold at post treatment.
Comparison 5. TENS + another intervention versus that same intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at post treatment | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 1 session post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 at 1w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 at 6w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 pain intensity at IT (6 month) follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 at 6w treatment + 6 month follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
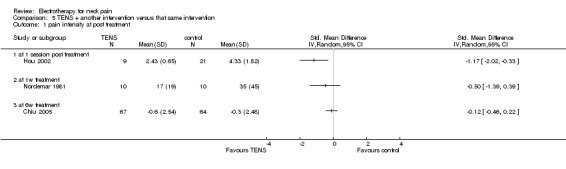
Comparison 5 TENS + another intervention versus that same intervention, Outcome 1 pain intensity at post treatment.
5.2. Analysis.

Comparison 5 TENS + another intervention versus that same intervention, Outcome 2 pain intensity at IT (6 month) follow‐up.
Comparison 6. TENS versus comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at post treatment | 5 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 vs EMS ‐ at 1 session | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 vs Mobilization ‐ at 1w treatment vs Mobilisation | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 vs US ‐ at 2w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 vs Manual Therapy ‐ at 4w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 vs AL‐TENS at 3W treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 vs Burst TENS at 3W treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 pain at IT (5 month) follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 vs Manual Therapy ‐ at 4w treatment + 5 month follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 function at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 vs Manual Therapy ‐ at 4w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 function at IT (5 month) follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 vs Manual Therapy ‐ at 4w treatment + 6 month follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 QoL at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 vs Manual Therapy ‐ at 4w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 QoL at IT (5 month) follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 vs Manual Therapy ‐ at 4w treatment + 6 month follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
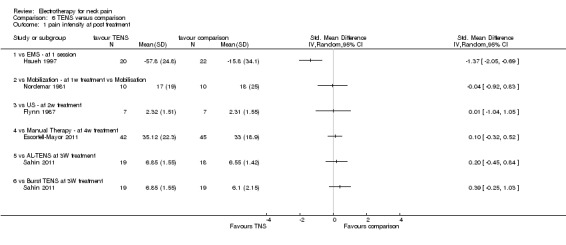
Comparison 6 TENS versus comparison, Outcome 1 pain intensity at post treatment.
6.2. Analysis.
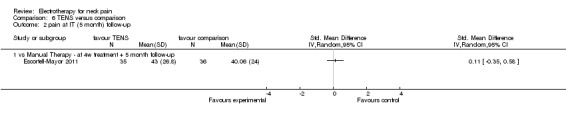
Comparison 6 TENS versus comparison, Outcome 2 pain at IT (5 month) follow‐up.
6.3. Analysis.
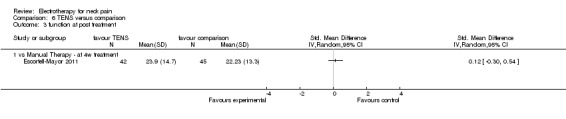
Comparison 6 TENS versus comparison, Outcome 3 function at post treatment.
6.4. Analysis.
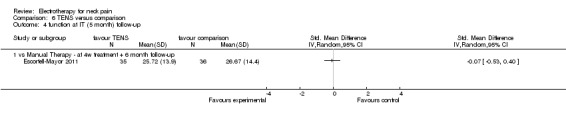
Comparison 6 TENS versus comparison, Outcome 4 function at IT (5 month) follow‐up.
6.5. Analysis.
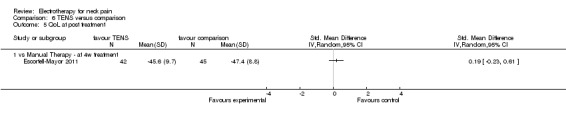
Comparison 6 TENS versus comparison, Outcome 5 QoL at post treatment.
6.6. Analysis.
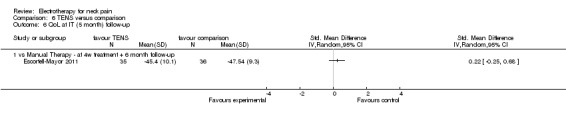
Comparison 6 TENS versus comparison, Outcome 6 QoL at IT (5 month) follow‐up.
Comparison 7. EMS versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 1 session | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 pressure pain threshold at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 at 1 session | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
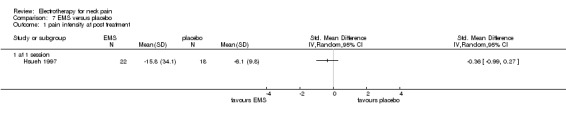
Comparison 7 EMS versus placebo, Outcome 1 pain intensity at post treatment.
7.2. Analysis.
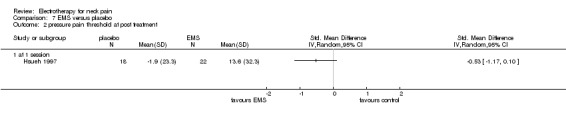
Comparison 7 EMS versus placebo, Outcome 2 pressure pain threshold at post treatment.
Comparison 8. EMS + another intervention versus that same intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at IT (6month) follow‐up | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.15, 0.33] |
| 1.1 EMS + Manip vs Manip: at ?6w treatment | 1 | 69 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.40, 0.55] |
| 1.2 EMS + Mobs vs Mobs: at ?6w treatment | 1 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.55, 0.39] |
| 1.3 EMS + heat + manip vs Heat + manip: at ?6w treatment | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.54, 0.45] |
| 1.4 EMS + heat + mobs vs Heat + mobs: at ?6w treatment | 1 | 67 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.06, 0.91] |
| 2 function at IT (6 months) follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 EMS + manip vs Manip: at ?6w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 EMS + mobs vs Mobs: at ?6w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 EMS + heat + manip vs Heat + manip: at ?6w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 EMS + heat + mobs vs Heat + mobs: ?6w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 patient satisfaction at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 EMS + manip vs Manip: at 4w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 EMS + mobs vs Mobs: at 4w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 EMS + heat + manip vs Heat + manip: at 4w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 EMS + heat + mobs vs Heat + mobs: at 4w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 pain intensity at IT (6month) follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 at 6w treatment | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.15, 0.33] |
| 5 function at IT (6 months) follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 at 6w treatment | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.15, 0.33] |
| 6 patient satisfaction at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 at 6w treatment | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.22, 0.26] |
8.1. Analysis.
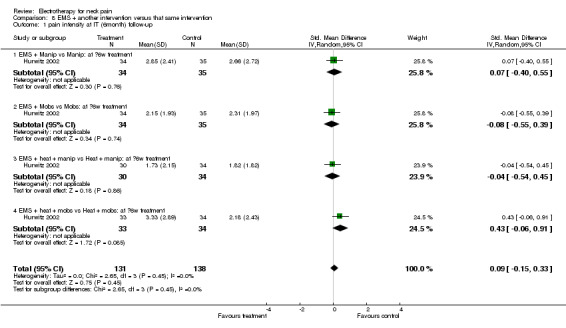
Comparison 8 EMS + another intervention versus that same intervention, Outcome 1 pain intensity at IT (6month) follow‐up.
8.2. Analysis.
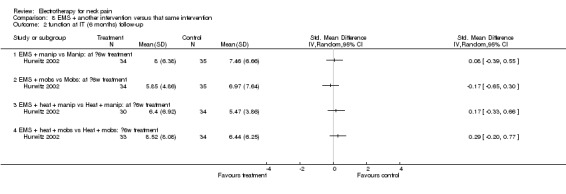
Comparison 8 EMS + another intervention versus that same intervention, Outcome 2 function at IT (6 months) follow‐up.
8.3. Analysis.
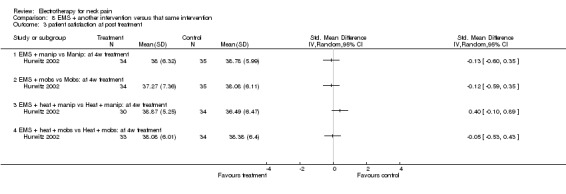
Comparison 8 EMS + another intervention versus that same intervention, Outcome 3 patient satisfaction at post treatment.
8.4. Analysis.
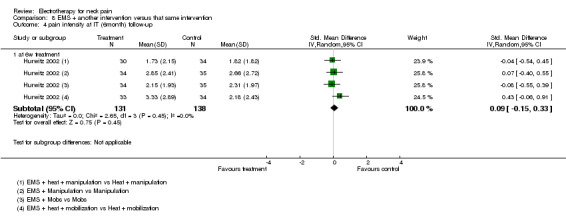
Comparison 8 EMS + another intervention versus that same intervention, Outcome 4 pain intensity at IT (6month) follow‐up.
8.5. Analysis.
Comparison 8 EMS + another intervention versus that same intervention, Outcome 5 function at IT (6 months) follow‐up.
8.6. Analysis.
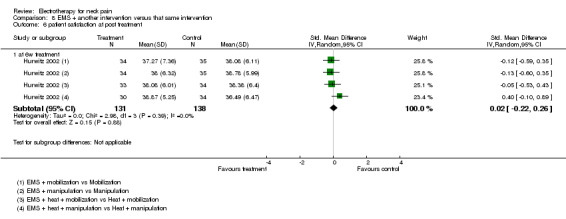
Comparison 8 EMS + another intervention versus that same intervention, Outcome 6 patient satisfaction at post treatment.
Comparison 9. EMS (inferential current) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 neck pain at post treatment | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 at 5w treatment | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 headache at post treatment | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 at 5w treatment | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
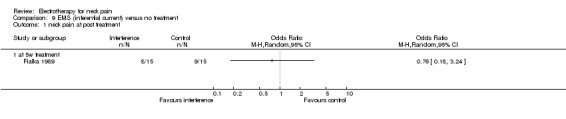
Comparison 9 EMS (inferential current) versus no treatment, Outcome 1 neck pain at post treatment.
9.2. Analysis.
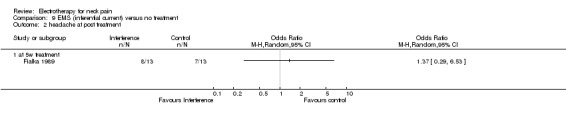
Comparison 9 EMS (inferential current) versus no treatment, Outcome 2 headache at post treatment.
Comparison 10. PEMF (low frequency) versus sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at post treatment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 30 sessions over 3 weeks treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 18 sessions over 4 to 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 pain intensity at ST follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 18 sessions over 4 to 6 weeks treatment + 4 week follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 function at post treatment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 30 sessions over 3 weeks treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 18 sessions over 4 to 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 function at ST follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 18 sessions over 4 to 6 weeks treatment + 4 week follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 global percieved effect at post treatment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 30 sessions over 3 weeks treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 18 sessions over 4 to 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 global percieved effect at ST follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 18 sessions over 4 to 6 weeks treatment + 4 week follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
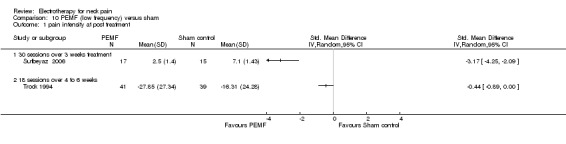
Comparison 10 PEMF (low frequency) versus sham, Outcome 1 pain intensity at post treatment.
10.2. Analysis.
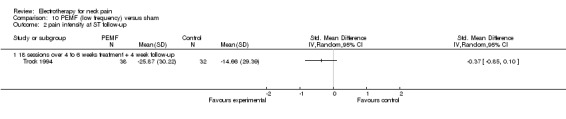
Comparison 10 PEMF (low frequency) versus sham, Outcome 2 pain intensity at ST follow‐up.
10.3. Analysis.
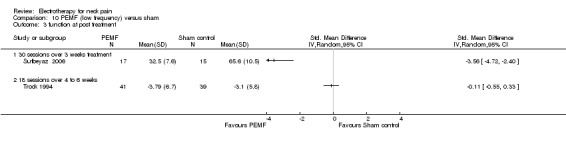
Comparison 10 PEMF (low frequency) versus sham, Outcome 3 function at post treatment.
10.4. Analysis.
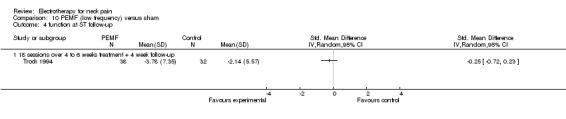
Comparison 10 PEMF (low frequency) versus sham, Outcome 4 function at ST follow‐up.
10.5. Analysis.
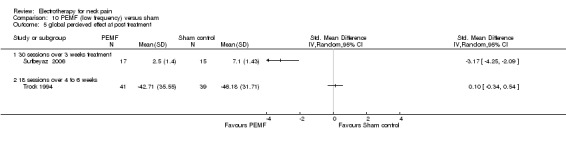
Comparison 10 PEMF (low frequency) versus sham, Outcome 5 global percieved effect at post treatment.
10.6. Analysis.
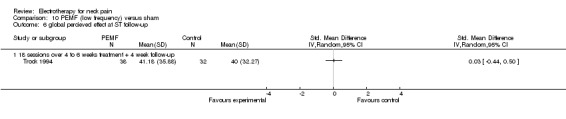
Comparison 10 PEMF (low frequency) versus sham, Outcome 6 global percieved effect at ST follow‐up.
Comparison 11. PEMF (low frequency) versus comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 neck pain at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 2w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.
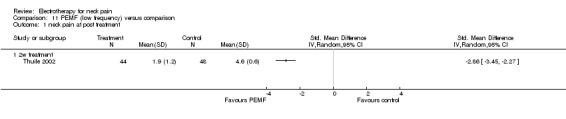
Comparison 11 PEMF (low frequency) versus comparison, Outcome 1 neck pain at post treatment.
Comparison 12. Repetitive magnetic stimulation (rMS) versus placebo ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain/function at post treatment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 post 2w treatment | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.37, ‐0.24] |
| 2 pain/function at ST follow‐up | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 follow‐up 1 month after treatment | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.96, ‐0.74] |
| 2.2 follow‐up 3 month after treatment | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.77, ‐0.24] |
| 3 headache at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 2w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
12.1. Analysis.
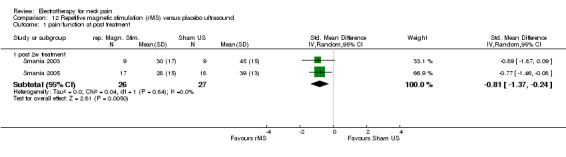
Comparison 12 Repetitive magnetic stimulation (rMS) versus placebo ultrasound, Outcome 1 pain/function at post treatment.
12.2. Analysis.
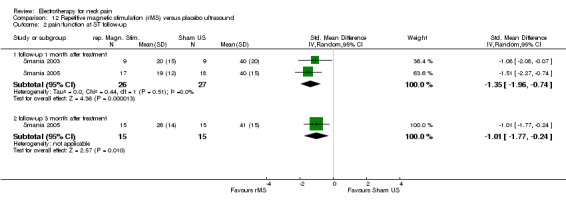
Comparison 12 Repetitive magnetic stimulation (rMS) versus placebo ultrasound, Outcome 2 pain/function at ST follow‐up.
12.3. Analysis.
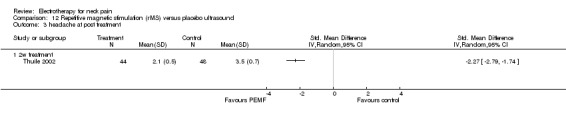
Comparison 12 Repetitive magnetic stimulation (rMS) versus placebo ultrasound, Outcome 3 headache at post treatment.
Comparison 13. Static magnetic field (necklace) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pain intensity at post treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 3w treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 global perceived effect at post treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 at 3w treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
13.1. Analysis.
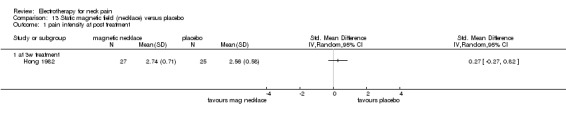
Comparison 13 Static magnetic field (necklace) versus placebo, Outcome 1 pain intensity at post treatment.
13.2. Analysis.

Comparison 13 Static magnetic field (necklace) versus placebo, Outcome 2 global perceived effect at post treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chiu 2005.
| Methods | RCT Number Analysed/Randomized: 109/145 Intension‐to‐treat Analysis: calculated Power Analysis: 90% power | |
| Participants | Chronic neck pain (not specified)
Duration of Complaint for Cases at baseline: Subacute (>3 months)
Duration of Complaint for Control at baseline: Subacute (>3 months) G1: N = 73 G2: N = 67 G3: N = 78 |
|
| Interventions | G1: TENS (TENS)
a. 30 minutes of dual channel portable TENS unit (ITO model 1302). Continuous trains of 150ms square pulse at 80 Hz. 4 Electrodes (4x4cm); b) infrared irradiation, 20 min; c) education on neck care G2: Exercise Program (Ex) + IR a. deep neck flexor‐using pressure sensor @20mmHg x10 min (10 sec on/15 sec off) b.Strengthening using a Multi Cervical Rehabilitation Unit (MCRU). 15 reps of flexion, extension at 20% of Peak Isometric Strength (PIS) as warm up Then Dynamic flexion and extension with variable resistance x 0‐12 reps c. Infrared irradiation d. 35 minutes of exercise per session G3: a) Infrared Irradiation, 20 min; b) education on neck care Duration of Treatment: 6 weeks, 2 sessions/week Duration of Follow‐up: 6 months CO‐INTERVENTION: Infrared Irradiation |
|
| Outcomes | PAIN (VAS, 0 to 10)
Baseline Median: G1 4.69, G2 4.61, G3 4.26
Reported Results: NS (between the three groups)
SMD(Ex+IR versus TENS): ‐0.13 (95% CI:‐0.51 to 0.26) FUNCTION (Chinese version of Northwick Park Questionnaire, 0 to 4) Baseline Median: G1 1.39, G2 1.55, G3 1.36 Reported Results: Ex + IR was favoured over TENS (P=0.02) SMD(Ex+IR versus TENS): ‐1.10(95% CI:‐1.51 to ‐0.69) REASON FOR DROPOUTS: Reported SIDE EFFECT: No complications occurred COST OF CARE: NR |
|
| Notes | Different treatment times for TENS+IR and IR control group. Pathology of patients completely unknown (only selection criteria: neck pain > 3 months) MISSING DATA: A request was made to clarify data that differed slightly in two reports. Dr Chui responded to a request for clarification. "Please be informed that the subjects were the same groups (exercise and control) of patients as reported in spine but the TENS group was introduced in the Clinical Rehab article and different methods of calculation/ analysis of the neck muscle strength were used in the Clinical Rehab. article." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | reported in text |
| Allocation concealment (selection bias) | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | reported in text |
| Selective reporting (reporting bias) | Unclear risk | no steady protocol available |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | unclear, not described |
| Compliance acceptable? | Unclear risk | not reported for exercise |
| Timing outcome assessments similar? | Low risk | reported in text |
Escortell‐Mayor 2011.
| Methods | RCT Number Analysed/Randomized: 71/90 Intension‐to‐treat Analysis: calculated Power Analysis: 47.5% power | |
| Participants | subacute or chronic neck pain (grade I and II)
Duration of Complaint for Cases at baseline: chronic (mean 20 weeks)
Duration of Complaint for Control at baseline: chronic (mean 22 weeks) G1 TENS: N = 43 |
|
| Interventions | G1: TENS (+ exercise)
a. TENS electrode placement in the painful area in the metamere or in the nerve's pathway (Adel and Luykey 1996) portable digital TENS unit (Manufacturer: Enraf‐Nonius; model TENSMED911). 150 microsecond pulse duration, 80Hz, adjustable amplitude, 30 minutes duration, 10 sessions on alternate days for about 1 month b. Exercise: isometric exercise, neck exercise and postural skills in the form of a handout and explained individually over two sessions to perform at home. G2: Manual Therapy (MT) + exercise a. Neuromuscular technique, post isometric stretching , spray and stretch, Jones technique (Chaitow 1991, Girardin 2004), 30 minute duration, 10 sessions on alternate days for about 1 month b. Exercise: isometric exercise, neck exercise and postural skills in the form of a handout and explained individually over two sessions to perform at home Duration of Treatment: 3 to 4 weeks, 10 sessions Duration of Follow‐up: 6 months CO‐INTERVENTION: medication consumption of anti‐inflammatory, analgesics, and muscle relaxants; no significant difference between groups |
|
| Outcomes | PAIN Intensity (VAS, 0 to 100 mm, high score indicates worse) Baseline mean: TENS (+exercise) 56.4; MT (+exercise) 54.9 Reported results: Comparison between TENS and MT group: P = 0.9 (NS) SMD TENS versus MT : 0.11 [‐0.35, 0.58] FUNCTION (NDI, 0 to 50, high score indicates worse) Baseline Mean: TENS (+exercise) 34.4; MT (+exercise) 31.63 Reported results: Comparison between TENS and MT group: P = 0.67 (NS) SMD TENS versus MT: ‐0.07 [‐0.53, 0.40] PCS (SF‐12 Physical component SF 12 summary, 0 to 50, high score indicates better) Baseline Mean: TENS (+exercise) 42.7; MT (+exercise) 43.3 Reported results: Comparison between TENS and MT group: P = 0.45 (NS) SMD TENS versus MT: 0.19 [‐0.23, 0.61] REASON FOR DROPOUTS: Reported SIDE EFFECTS: no important side effects in either group COST OF CARE: NR |
|
| Notes | Location of Study: Madrid Region, Spain; the paper was judged to have serious flaws and high risks of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of block randomisation is not clearly stated; it is not clear that complete blocks were done at each centre |
| Allocation concealment (selection bias) | Unclear risk | envelopes were not numbered |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | not possible due to design |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | not possible due to design |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | not possible due to design |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | see Figure 1 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | page 69 paragraph 2 |
| Selective reporting (reporting bias) | Unclear risk | no protocol provided |
| Similarity of baseline characteristics? | Low risk | see Table 1 |
| Co‐interventions avoided or similar? | Unclear risk | not reported |
| Compliance acceptable? | Unclear risk | exercise compliance not reported |
| Timing outcome assessments similar? | Low risk | baseline one month and six months |
Farina 2004.
| Methods | RCT Analysed/randomized: 40/40 blinding: patients evaluation examiner regarding treatment therapist regarding clinical status | |
| Participants | Myofascial pain syndrome (upper m. trapezius) G1: N = 21 G2: N = 19 |
|
| Interventions | G1: TENS (Phyacton 787, Uniphy, Netherlands) 100 Hz, 0.25 ms pulse width; placement: negative electrode on most painful trigger point; intensity: below muscular contraction (< 39mA), at patient's comfort G2: FREMS; a variation of TENS: FREquency Modulated Neural Stimulation (ETS 501‐Physioflog, Lorenz Biotech, Italy); high voltage (>300V) low intensity (< 0.01 mA) and short duration impulses (0.01 msec); programmed frequency variations 1‐40 Hz; placement: positive electrode at most painful trigger point Co‐interventions: all patients were instructed to avoid PT for 2 months and analgesic medication for 2 weeks Treatment schedule: 10 treatment sessions, 5 days a week, for 2 consecutive weeks; duration 20 minutes each |
|
| Outcomes | NECK PAIN AND DISABILITY (NPDVAS; 0 to 10)
(means only; SD not reported!) Baseline mean: G1 5.29; G2 4.81 At 1week treatment: G1 2.81; G2 2.46 Follow up 1 month: G1 3.24; G2 1.29 Follow up 3 months: G1 4.09; G2 2.73 Reported statistical analysis results: baseline versus 1 week/ 1 month/ 3 months: all P < 0,001 (except P < 0.05 for TENS 3 at months) Further outcome parameters: Algometry; Cervical ROM; Triggerpoint characteristics; similar results |
|
| Notes | Conclusion of authors: Both TENS and FREMS have positive short‐term effects on MPS, but medium‐term effects were achieved only with FREMS. However, no statistical significant differences between TENS and FREMS have been observed in most outcome parameters. Means and statistical results are reported, but SD values are missing (though announced). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | describes a simple random scheme |
| Allocation concealment (selection bias) | Unclear risk | p 295 described as "patients were informed that they would be submitted to 1 of 2 possible treatments" |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | 2 treatment groups involved 2 different machines |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | not described in results |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | no ITT analysis described |
| Selective reporting (reporting bias) | Unclear risk | not described |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | not described |
| Compliance acceptable? | Unclear risk | not described |
| Timing outcome assessments similar? | Low risk | reported in text |
Fialka 1989.
| Methods | RCT Blinding: not patient, not observer Analysed/randomized: 60/60 patients |
|
| Participants | acute whiplash (>5 <10 days), cervicogenic headache G1: N = 15 G2: N = 15 G3: N = 15 G4: N = 15 |
|
| Interventions | G1: Stereodynamic 50 Hz interferential current (Stereodynator, Siemens), treatment duration 15 minutes, 2 triple electrodes on neck and dorsal spine; intensity not reported G2: Iontophoresis: DC, duration 20 minutes, diclofenac‐gel on a filter paper, placed under the electrodes on the neck, intensity 0.1 mA/cm2 G3: Multimodal treatment : Traction, therapeutic exercise, massage (THGM) G4: Control group; no therapy Treatment schedule: start of treatment after first investigation (5‐10 days after car accident); number of treatments and end of treatment not reported. Second investigation after 35 days |
|
| Outcomes | PAIN (neck; headache; patient's report) Baseline Mean: not reported Reported Results: improvement, significance not specified RR (G1 versus G4 for neck pain): 0.76 (95% CI Random: 0.18, 3.24) RR (G1 versus G4 for headache): 1.37 (95% CI Random: 0.29, 6.53) RR (G2 versus G4 for neck pain): 1.00 (95% CI Random: 0.42, 2.40) RR (G2 versus G4 for headache): 0.66 (95% CI Random: 0.28, 1.57) SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | No number of treatments reported No adequate statistical evaluation No use of VAS for neck pain or headache | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | details not reported |
| Allocation concealment (selection bias) | High risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | reported in text |
| Selective reporting (reporting bias) | Unclear risk | unclear |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | not reported |
| Compliance acceptable? | Unclear risk | not reported |
| Timing outcome assessments similar? | Low risk | reported in text |
Flynn 1987.
| Methods | RCT (pilot study) Blinding: not reported Analysed/randomized: 21/21 patients |
|
| Participants | whiplash associated pain (duration not reported) G1: N = 7 G2: N = 7 G3: N = 7 |
|
| Interventions | G1: Ultra‐Reiz 143 Hz (Endomed 404) intensity as tolerated, < 35mA; electrodes with viscose sponge at painful area; duration: 14 minutes G2: ultrasound (Multiphon unit) 3 MHZ, pulsed 1:1, intensity 0.5 W/ cm2; duration 6 minutes G3: same treatment as in G2, but intensity 0.0 W/cm2 (placebo) Co‐interventions for all groups: posture advices and neck care including collar. Home exercises twice a day; continuing any medication as before, but not starting with new medication Treatment schedule: G1:8 times in 2 weeks G2 and G3: 8 times in 3 weeks |
|
| Outcomes | NECK PAIN (VAS 0 to 10cm) reported pre / post results (SD): G1: 7.42 (1.30) / 2.32 (1,51) (P < 0.05) G2: 5.1 (2.72) / 4.07 ( 2.73) (n.s.) G3: 4.43 (1.49) / 2.31 (2.31) (n.s.) reported baseline group differences: G1 versus G2: P < 0,05 G1 versus G3: P < 0,002 SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | Different treatment times for G1 (2 weeks) and G2/G3 (3 weeks) The author characterized the investigation as a "pilot study" (small and uneven groups). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | method not reported |
| Allocation concealment (selection bias) | High risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | not reported |
| Selective reporting (reporting bias) | High risk | not reported, no protocol |
| Similarity of baseline characteristics? | High risk | significant baseline group differences, especially G1 versus G3 |
| Co‐interventions avoided or similar? | High risk | intention to avoid medication changes, but no details reported |
| Compliance acceptable? | High risk | not reported |
| Timing outcome assessments similar? | Unclear risk | different treatment duration (2 weeks and 3 weeks) |
Foley‐Nolan 1990.
| Methods | RCT Blinding: patients, observer Analysed/randomized: 20/20 patients |
|
| Participants | Chronic non‐specific neck pain G1: N = 10 G2: N = 10 |
|
| Interventions | G1: HF‐PEMF therapy by a collar, fitted with a miniaturized short wave (HF‐) generator; frequency: 27 MHz; pulse width: 0.06 ms; repetition frequency: 450/ second; mean power: 1.5 mW/cm2 G2: placebo HF‐PEMF Co‐interventions G1and G2: anti‐inflammatory analgesics, depending on pain intensity Treatment schedule: G1: 3 times in 3 weeks active G2: 3 weeks placebo and 3 weeks active; 8 hours daily |
|
| Outcomes | PAIN INTENSITY (VAS 10 cm):
Baseline Mean: not reported
Reported Results: significant at 3 weeks of treatment
P < 0.05 SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | This therapy is an uncommon PEMF method, using diathermy‐like HF‐pulses, but with intensities far below the thermal threshold. The reason for the chosen treatment is only based on a literature remark in 1940 and remains unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomisation, not specified |
| Allocation concealment (selection bias) | Unclear risk | the method is unclear |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | not reported |
| Selective reporting (reporting bias) | Low risk | reported in text |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | High risk | 2‐8 paracetamol tablets were allowed according to actual pain |
| Compliance acceptable? | High risk | not reported |
| Timing outcome assessments similar? | High risk | not reported |
Foley‐Nolan 1992.
| Methods | RCT Blinding: patients, observer Analysed/randomized: 40/40 patients |
|
| Participants | Acute whiplash injury (<3 days) G1: N = 20 G2: N = 20 |
|
| Interventions | G1: HF‐PEMF therapy (see: Foley‐Nolan 1990) G2: placebo HF‐PEMF Co‐interventions G1+G2: optional anti‐inflammatory analgesics; optional physiotherapy treatment after 4 weeks, if progress not satisfying Treatment schedule: 12 weeks; 8 hours daily |
|
| Outcomes | PAIN INTENSITY (VAS 10 cm):
Baseline Mean: not reported
Reported Results: not significant SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | This therapy is an uncommon PEMF method, using diathermy‐like HF pulses, but with intensities far below the thermal threshold. The reason for the chosen treatment is only based on a literature remark in 1940 and remains unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomisation, not specified |
| Allocation concealment (selection bias) | Unclear risk | this is unclear and poorly described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | not reported |
| Selective reporting (reporting bias) | Low risk | reported in text |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | High risk | analgesics consumption (mefenamid acid) depending on pain |
| Compliance acceptable? | High risk | not reported |
| Timing outcome assessments similar? | High risk | not reported |
Hendriks 1996.
| Methods | RCT Blinding: none Analysed/randomized: 16/16 |
|
| Participants | Acute whiplash (< 3 days) G1: n = 8 G2: n = 8 |
|
| Interventions | G1: group 2 treatment, plus Ultra‐Reiz current 143 Hz, intensity individually graduated, 2 6x8 cm electrodes with viscose sponge placed paravertebral (C4 to T3), duration 15 minutes G2: standard physiotherapy: ice 15 minutes in clinic and 1 time per day at home; ROM exercises at home; advice on neck care, posture, use of collar Co‐interventions: prescribed drugs were continued as instructed by medical staff Treatment schedule: 5 sessions within 1 week measurements: immediately after 5th session Follow‐up: 6 weeks after final treatment |
|
| Outcomes | PAIN INTENSITY (VAS 100mm)
Baseline Mean: not reported
Reported Results: significant difference (P < 0.05) favouring Group 1 immediately post‐treatment (N = 16) and at 6 weeks (N=14) follow‐up (P < 0.005) SIDE EFFECT: not reported COST OF CARE: not reported |
|
| Notes | Only unrelated t‐test values for A/B comparison, but no specific VAS data reported. Single group sizes not clearly specified No numbers of patients were given in tables for each group; authors failed to present information for a large number of criteria |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | reported in text |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | due to difference in treatment method, not possible to blind the patient |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | only the treatment method was described but no report of who gave the treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | pain score rated by patient |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Tables 1‐3 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | not described |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available or referenced |
| Similarity of baseline characteristics? | Unclear risk | only post treatment measurements were reported |
| Co‐interventions avoided or similar? | Unclear risk | no information given |
| Compliance acceptable? | Unclear risk | no information given |
| Timing outcome assessments similar? | Low risk | P13Lp2 |
Hong 1982.
| Methods | RCT Blinding: patients, observer Analysed/randomized: 52/52 patients (2 of 4 groups evaluated) |
|
| Participants | Chronic non‐specific neck and shoulder pain G1: N = 27 G2: N = 25 |
|
| Interventions | G1: necklace with magnetic samarium cobalt elements; field strength: 1200 Gauss (120 mT) flux density at surface G2: placebo necklace Treatment schedule: 3 weeks; 24 hours daily |
|
| Outcomes | PAIN INTENSITY (4‐point rating scale):
Baseline Mean: magnetic 2.84, non‐magnetic 3.10
End of Study Mean: magnetic 2.56, non‐magnetic 2.74
Absolute Benefit: magnetic 0.10, non‐magnetic 0.37
Reported Results: not significant, SMD: 0.27 (95% CI Random: ‐0.27, 0.82)
Power: 82% PATIENT PERCEIVED IMPROVEMENT: Baseline Mean: NR, Reported Results: magnetic 52% improved, non‐magnetic 44% improved RR: 0.86 (95% CI Random: 0.51, 1.45) SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | Two ignored groups had no pain (controls with active and placebo necklace) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomisation, method not described |
| Allocation concealment (selection bias) | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | not described |
| Selective reporting (reporting bias) | Unclear risk | not described |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | not described |
| Compliance acceptable? | Unclear risk | not described |
| Timing outcome assessments similar? | Low risk | reported in text |
Hou 2002.
| Methods | RCT Blinding: none Analysed/randomized: 71/71 |
|
| Participants | Myofascial neck pain; duration of disorder not specified G1: N = 9 G2: N = 9 G3: N = 9 G4: N = 21 G5: N = 13 G6: N = 10 |
|
| Interventions | G1: TENS 100 Hz/ 0.25 ms, ischemic compression, hot pack 20 minutes, Active ROM exercise G2: TENS 100 Hz/ 0.25 ms, spray and stretch, hot pack for 20 minutes, Active ROM exercises G3: interferential current (100 Hz interfering wave for 20 minutes), myofascial release technique, hot pack for 20 minutes, Active ROM exercises G4: hot pack 20 minutes, Active ROM exercise G5: ischemic compression, hot pack 20 minutes, Active ROM exercise G6: spray and stretch by Simon et al, hot pack for 20 minutes, Active ROM exercise Treatment schedule: 1 session |
|
| Outcomes | PAIN INTENSITY (VAS) reported results: all groupsG1‐G6 have significantly improved concerning pre/post treatment (P < 0.05) G4 versus G3: RR: ‐1,20 ( 95% CI Random: ‐2.50, ‐0.36) = hot pack versus interference (P < 0.05) G4 versus G2: RR: ‐1,17 ( 95% CI Random: ‐2.02, ‐ 0.33 = hot pack versus TENS (P < 0.05) Reported Results: G1 versus G2 not significant,G2 versus G6 not significant, G3 versus G4 significant favouring G3, G1 versus G5 not significant SMD (G1 versus G5): 0.56 (95% CI Random: ‐1.43, 0.31) SMD (G2 versus G6): ‐0.72 (95% CI Random: ‐1.65, 0.22) SMD (G3 versus G4): ‐1.20 (95% CI Random: ‐2.05, ‐0.36) SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | 20 minutes TENS treatment time appears to be extremely short designed, compared to usual recommendations (at least 30 minutes for TENS) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomisation not described |
| Allocation concealment (selection bias) | High risk | not concealed |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | VAS assessed by patients |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | unclear, not described in results |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | not described |
| Selective reporting (reporting bias) | Unclear risk | no protocol |
| Similarity of baseline characteristics? | High risk | table 1: seven groups are different in age |
| Co‐interventions avoided or similar? | Unclear risk | not described |
| Compliance acceptable? | Unclear risk | not reported |
| Timing outcome assessments similar? | Low risk | reported in text |
Hsueh 1997.
| Methods | RCT Blinding: patients, observer Analysed/randomized: 60/60 patients |
|
| Participants | Chronic myofascial neck pain with trigger points at trapezius muscle G1: N = 22 G2: N = 20 G3: N = 18 |
|
| Interventions | G1: Group A or TENS (60 Hz) at trapezius muscle; feel strong stimulation without muscle contraction G2: Group B or EMS (electrical muscle stimulation); 10 Hz; visible trapezius muscle stimulation G3: Group C or sham electrotherapy at trapezius muscle Treatment schedule: 1 session, 20 minutes |
|
| Outcomes | PAIN INTENSITY (VAS):
Baseline Mean: not reported
Reported Results: significant improvement favouring group B versus C; not significant group A versus C
SMD (A versus C): ‐0.36 (95% CI Random: ‐0.99, ‐0.27)
SMD (B versus C): ‐2.60 (95% CI Random: ‐3.48, ‐1.71)
Power: 6% SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | 20 minutes TENS treatment time appears to be extremely short, compared to usual recommendations (at least 30 minutes for TENS) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | type of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | unclear as described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | reported in text |
| Selective reporting (reporting bias) | High risk | most data given as percentage change only |
| Similarity of baseline characteristics? | High risk | most data given as percentage change only |
| Co‐interventions avoided or similar? | Low risk | reported in text |
| Compliance acceptable? | Low risk | reported in text |
| Timing outcome assessments similar? | Low risk | reported in text |
Hurwitz 2002.
| Methods | RCT (2x2x2 factorial design) Analysed/Randomized: 269/336 |
|
| Participants | Subacute and chronic neck pain with or without radicular symptoms and cervicogenic headache Manipulaton groups G1/G2/G5 /G6 total N = 171 Mobilisation groups G3/ G4/ G7/ G8 total N = 165 Single groups: N = NR |
|
| Interventions | G1: Manipulation with electrical muscle stimulation (Manip/EMS): 10‐minute application of EMS before manipulation; EMS parameters not reported G2: Manipulation with electrical muscle stimulation (Manip/EMS) and heat: 10‐minute moist heat application and EMS simultaneously before mobilisation G3: Mobilisation with EMS (Mob/EMS): 10‐minute application of this modality before mobilisation; parameters NR G4: Mobilisation with heat and electrical muscle stimulation (Manip/EMS) G5: Manipulation (Manip): at least 1 controlled, dynamic thrust applied with high velocity low amplitude force, directed at 1 or more restricted upper thoracic or cervical spine joint segments G6: Manipulation with heat (Manip/Heat): 10‐minute moist heat application before manipulation G7: Mobilisation (Mob): 1 or more low velocity, variable amplitude movements directed to 1 or more restricted upper thoracic or cervical spine joint segments G8: Mobilisation with heat (Mob/Heat): 10‐minute moist heat application before mobilisation Co‐intervention: All participants received information on posture and body mechanics and one or more of the following: stretching, flexibility, or strengthening exercises and advice about ergonomic and workplace modifications Treatment schedule: unclear: "...at least 1 treatment..." (manip / mob) No maximum, no average number of treatments reported Measurements / follow up: 2 weeks; 6 weeks; 3 months; 6 months |
|
| Outcomes | PAIN INTENSITY (most severe pain, NRS 0 to10)
Baseline Mean: Not reported for each subgroup
Reported Results: no significant differences e.g.: at 6 months SMD (EMS + manip versus manip): 0.07 (95% CI ‐0.40 to 0.55) DISABILITY (NDI 0 to 50) Reported Results: no significant difference at 6 months SMD (EMS + manip versus manip): 0.08 (95% CI: ‐0.39 to 0.55) PATIENT SATISFACTION Reported Results: no significant difference at 4 weeks SMD (EMS + manip versus manip): ‐0.13 (95% CI: ‐0.60 to 0.35) SIDE EFFECTS: interviewed at 4 weeks of care, no known study‐related adverse events |
|
| Notes | Factorial design. No relevant differences between EMS (G3, G4, G7, G8) v no EMS (G1, G2, G5, G6) "At least 1 treatment", but no maximum, no average number of treatments by mob/ manip or modalities reported 10 minutes modalities treatment time appears extremely short design, compared to usual recommendations (at least 30 minutes). No setting parameters for EMS were reported Missing Data: a request to clarify the specific treatment parameters was sent but no response received. However, a request for data (end of study mean and SD for each outcome) was sent and response received from Hurwitz 2002. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | reported in text |
| Allocation concealment (selection bias) | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | not possible; differences in treatment methods |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | subjective rating of pain |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | not described |
| Selective reporting (reporting bias) | Unclear risk | not described |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | not described |
| Compliance acceptable? | Low risk | reported in text |
| Timing outcome assessments similar? | Low risk | reported in text |
Nordemar 1981.
| Methods | RCT Blinding: none Analysed/randomized: 30/30 patients |
|
| Participants | Acute non‐specific neck pain (< 3 days; without radiation) G1: N = 10 G2: N = 10 G3: N = 10 |
|
| Interventions | G1: TENS: 80 Hz; intensity just below pain threshold; neck collar, rest, exercise, analgesic G2: Manual Therapy (MT): soft tissue treatment, manual traction, neuromuscular mobilization, collar, rest, exercise, analgesic G3: Neck collar, rest, exercise, analgesic Treatment schedule: G1: 3 times per week; 15 minutes G2: 3 times per week; 30 minutes G3: intermittent collar use over 2 weeks Follow‐up: after 6 weeks |
|
| Outcomes | PAIN INTENSITY (VAS 100 mm):
Baseline Mean: TENS 83, MT 97, collar 90
End of Study Mean: TENS 0, MT 0, collar 0
Absolute Benefit: TENS 83, MT 97, collar 90
Reported Results: no significant difference
SMD (TENS versus collar): ‐0.50 (‐1.39, 0.39)
SMD (TENS versus MT): ‐0.04 (‐0.92, 0.83)
Power: 8% SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | Most patients had no need of treatment after first week in all groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | consecutive distribution to three groups |
| Allocation concealment (selection bias) | High risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | quick recovery of most cases within one week, while therapy was planned for 3 weeks (many dropouts) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | reported in text |
| Selective reporting (reporting bias) | Unclear risk | unclear |
| Similarity of baseline characteristics? | High risk | strong deviations because of small group size |
| Co‐interventions avoided or similar? | High risk | self medication allowed |
| Compliance acceptable? | Unclear risk | not reported |
| Timing outcome assessments similar? | Unclear risk | not reported |
Philipson 1983.
| Methods | RCT Blinding: patients Analysed/Randomized: 40/40 patients |
|
| Participants | Chronic non‐specific neck and shoulder pain G1: N = 20 G2: N = 20 |
|
| Interventions | G1: Diadynamic Current (LP) G2: Placebo group: current turned up until patient felt sensation in neck, then turned off Treatment schedule: 4 minutes each at 3 trigger points; 5 consecutive days |
|
| Outcomes | PAIN INTENSITY (VAS):
Baseline Mean: not reported
Reported Results: not significant difference
RR: 0.69 (95% CI Random: 0.39, 1.24)
Power: 13% PATIENT RATED IMPROVEMENT (5‐point scale): Baseline Mean: not reported Reported Results: no significant difference; RR: 0.07 (95% CI Random: 0.33, 1.47) SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | not reported |
| Compliance acceptable? | Unclear risk | not reported |
| Timing outcome assessments similar? | Low risk | reported in text |
Sahin 2011.
| Methods | RCT Blinding: none |
|
| Participants | Forty patients with cervical myofascial pain syndrome [MPS] > 3 months Groups, randomized / analysed G1 n = 20 / 19 G2 n = 20 / 18 G3 n = 20 / 19 G4 n = 20 / 19 |
|
| Interventions | G1: Conventional TENS with a frequency of 100 Hz, 40 μs duration, low amplitude G2: Acupuncture‐like TENS (AL‐TENS) with a frequency of 4 Hz, 250 μs duration, high amplitude G3: Burst TENS with high [100 Hz] and low [2 Hz] frequency, 40 μs, high amplitude G4: Placebo TENS: electrical stimulation until patients sensation, then turned down to zero Treatment schedule: 30 minutes, 3 times a week, until 10 sessions completed Follow up: Not reported |
|
| Outcomes | PAIN INTENSITY (VAS 0 to 10) BASELINE Mean G1 conventional TENS (n =19) 7.12 G2 AL‐TENS (n = 18) 6.15 G3 Burst TENS (n = 19) 6.85 G4 Placebo TENS (n = 19) 7.56 Reported Results: no significant difference SMD (G1 versus G4) ‐0.07 [95% CI Random: ‐0.71 to 0.56] SMD (G1 versus G2) 0.20 [95% CI Random: ‐0.45 to 0.84] SMD (G1 versus G3) 0.39 [95% CI Random: ‐0.25 to 1.03] SIDE EFFECTS: not found COST OF CARE: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | provider would likely know what settings are used on the TENS unit |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | not reported |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Similarity of baseline characteristics? | Unclear risk | unclear |
| Co‐interventions avoided or similar? | Unclear risk | not reported |
| Compliance acceptable? | Unclear risk | not reported |
| Timing outcome assessments similar? | Unclear risk | not reported |
Smania 2003.
| Methods | RCT Blinding: patients examiner regarding treatment therapist regarding clinical status Analysed/Randomized: 18/18 |
|
| Participants | Myofascial neck pain (trigger points at upper trapezius; duration not specified) G1: N = 9 G2: N = 9 |
|
| Interventions | G1: Repetitive Magnetic Stimulation (rMS), Magstim Super Rapid Stimulator by Magstim company, intensity up to 400 mT (4000 G), 4000 pulses, administered in 5 sec trains at 20 Hz, separated by 25 sec pauses G2: detuned ultrasound (Supersonic 1010, Italy) Co‐interventions: avoid any PT for 2 months, refrain from taking any analgesic drug for 15 days, no other treatment during study Treatment schedule: 2 weeks, 10 sessions; duration: 20 minutes each Follow up: 1 week and 1 month after treatment |
|
| Outcomes | NECK PAIN AND DISABILITY (NPDVAS 0‐100)
Baseline Mean and other values: graphed post‐treatment; SMD: ‐0.89 (95% CI Random:‐ 1.87, 0.09) follow‐up 1 week after treatment; SMD: ‐1.39 (95% CI Random: ‐2.44, ‐0.33) follow‐up 1 month after treatment: SMD ‐1.08 (95% CI Random: ‐2.08, ‐0.07); NNT 3; treatment advantage 56% SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | Amelioration from "after treatment" to 1‐month follow‐up reported. Pilot study with small groups; see also Smania 2005, similar trial with 53 patients. Funding not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | reported in text |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | reported in text |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Low risk | no medication during trial |
| Compliance acceptable? | High risk | not reported |
| Timing outcome assessments similar? | Low risk | timing of final outcome is unclear |
Smania 2005.
| Methods | RCT Blinding: patients examiner regarding treatment therapist regarding clinical status Analysed/Randomized: 53/53 |
|
| Participants | Myofascial neck pain syndrome (trigger points at upper trapezius; duration not specified) G1: N = 17 G2: N = 18 G3: N = 18 at 3 month follow‐up: G1: N = 15 G2: N = 16 G3: N = 15 32 patients excluded (from 85) before randomization (53) |
|
| Interventions | G1: Repetitive Magnetic Stimulation (rMS), Magstim Super Rapid Stimulator by Magstim company, intensity up to 400 mT (4000 G), 4000 pulses, administered in 5 sec trains at 20 Hz, separated by 25 sec pauses; 20 minutes duration G2: TENS (Phyacton 787; Uniphy, Netherlands) 100 Hz; 0,25 ms pulse width; asymmetrical rectangular biphasic wave form; intensity at comfort below muscular contraction; placement: negative electrode over most painful trigger point G3: detuned ultrasound (Supersonic 1010, Italy) Co‐interventions: no PT for 2 months, no analgesic drug for 15 days, no other treatment during study Treatment schedule: 2 weeks, 10 sessions; duration: 20 minutes each Follow up: 1 week, 1 month and 3 months after treatment |
|
| Outcomes | NECK PAIN AND DISABILITY (NPDVAS 0‐100)
Baseline Mean and other values: graphed rMS versus Placebo US: G1 versus G3: post‐treatment; SMD: ‐0.77 (95% CI Random:‐ 1.46, ‐0.08) G1 versus G3: follow‐up 1 month after treatment; SMD‐1.51 (95% CI Random: ‐2.27, ‐0.74); NNT 3; treatment advantage: 45.6% G1 versus G3: follow‐up 3 month after treatment: SMD ‐1.01 (95% CI Random: ‐1.77, ‐0.24) TENS versus Placebo US: G2 versus G3: post‐treatment; SMD: ‐0.89 (95%CI Random: ‐1.76, ‐0.28) G2 versus G3: follow‐up 1 month after treatment; SMD ‐0.65 (95% CI Random: ‐1.32, ‐0.02) G2 versus G3: follow‐up 3 month after treatment: SMD ‐0.52 (95% CI Random: ‐1.24, ‐0.20) SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | See also Smania 2003, similar trial with 18 patients in total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | reported in text |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | not described, but reported in fig. 1 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | not described |
| Selective reporting (reporting bias) | Unclear risk | not described |
| Similarity of baseline characteristics? | High risk | table 1 age: much different in the placebo group |
| Co‐interventions avoided or similar? | Unclear risk | no medication during trial |
| Compliance acceptable? | Unclear risk | not described |
| Timing outcome assessments similar? | Low risk | reported in text |
Sutbeyaz 2006.
| Methods | RCT Blinding: patients, observer Analysed/randomized: 32/34 (27 patients excluded before randomizations) |
|
| Participants | Cervical osteoarthritis G1: N = 17 G2: N = 15 |
|
| Interventions | G1: PEMF System: Wave Ranger Professional (MRS 2000+ Home, FL‐9492 Eschen); intensity 0,04 mT; frequency range 0.1‐ 64Hz, applied frequency not reported; application by whole body mat 1.8x0.6 m size G2: same conditions as in G1, PEMF inactivated (sham control) Co‐interventions: NSAIDs if necessary, need recorded at end of study Treatment schedule: 3 weeks, 2 times a day; duration 30 minutes |
|
| Outcomes | PAIN INTENSITY (VAS; 0 to 10 points)
after 3w treatment; SMD: ‐3.17(95% CI Random:‐ 4.25 to ‐2.09) NECK PAIN AND DISABILITY SCORE (NPDS; 0 to 100 points) after 3w treatment; SMD: ‐3.56 (95% CI Random:‐ 4.72 to ‐2.40) Reported statistical analysis: G1 pre/post: P<0.001 for all items G2 pre/post: not significant for all items Baseline values differences: not significant for all items GLOBAL PERCEIVED EFFECT (0 to 3, more is better) after 3w treatment; SMD: ‐3.17(95% CI Random:‐4.25 to ‐2.09) SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | The credibility of the results, strongly favouring PEMF and contrasting with no sham control effects, seems very low. Support e.g. by MRS 2000 company is neither reported, nor excluded, so funding bias has to be taken in account. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | reported in text |
| Allocation concealment (selection bias) | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | primary outcome VAS assessed by patients |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | reported in text |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | not reported |
| Compliance acceptable? | Low risk | reported in text |
| Timing outcome assessments similar? | Low risk | reported in text |
Thuile 2002.
| Methods | RCT Blinding: none Analysed/Randomized: 92/92 |
|
| Participants | Whiplash I and II (WAD) pain in neck and/or "in the back of the head" (duration not specified) G1: N = 44 G2: N = 48 |
|
| Interventions | G1: PEMF System, MRS 2000 plus MED (Vitalife Inc, Austria); intensity 0,01 to 0,03 mT, basic frequency 64Hz; duration: 16 minutes local magnetic cushion application, followed by 8 minutes whole body mat treatment ; medication: diclofenac, tizanidine G2: Standard Therapy, diclofenac, tizanidine (no sham control) Treatment schedule: 2 weeks, 2 times per day (G1) |
|
| Outcomes | PAIN INTENSITY (VAS, 0‐10) Baseline Mean: G1 6.3, G2 5.3 End of Study Mean: G1 1.9, G2 4.6 Absolute Benefit: G1 4.4, G2 0.7 Reported Results: significant differences, P<0.03 each SMD (neck pain): ‐2.86 (95% CI Random: ‐2.79, ‐1.74) SMD (headache): ‐2.27 (95% CI Random: ‐2.81, ‐1.75) SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | Control group with standard medication only, no placebo magnetic field therapy; The credibility of the results appears to be very low. Support, e.g. by Vitalife Inc, Austria, is neither reported, nor excluded, so funding bias is possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | patients were assigned on a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | blinding of observer not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | pain score rated by patient |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | not described in results, but in methods on page 64 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | not described |
| Selective reporting (reporting bias) | Unclear risk | not described |
| Similarity of baseline characteristics? | Low risk | reported in text |
| Co‐interventions avoided or similar? | Unclear risk | not described |
| Compliance acceptable? | Unclear risk | not described |
| Timing outcome assessments similar? | Low risk | reported in text |
Trock 1994.
| Methods | RCT Blinding: patients, observer Analysed/randomized: 70/81 Intention‐to‐treat: not reported |
|
| Participants | Chronic non‐specific neck pain with radiologic findings of degenerative changes G1: N = 42 G2: N = 39 |
|
| Interventions | G1: PEMF therapy (5/10/12 Hz, rectangular; 10 minutes for each frequency)
G2: sham PEMF Co‐interventions: medication, physiotherapy Treatment schedule: 18 sessions lasting 30 minutes each, over 4 to 6 weeks Follow‐up: 1 month after treatment |
|
| Outcomes | PAIN INTENSITY (VAS 100 mm):
Baseline Median: PEMF 72.02, placebo 62.30
End of Study Median: PEMF 46.16, placebo 47.64
Absolute Benefit: PEMF 25.88, placebo 14.66
at ST follow‐up; SMD:‐0.37 [95% CI Random:‐0.85 to 0.10] Reported Results: short term benefits, significant , P < 0.04 at end of treatment; not significant, P = 0.1 at 1 month follow‐up Power: 41% FUNCTION (Acivity of Daily Living; 0 to 24): Baseline Mean: PEMF 11.94, placebo 11.5 End of Study: PEMF 8.16, placebo 9.36 Absolute Benefit: PEMF 3.78, placebo 2.14 at ST follow‐up; SMD: ‐0.25 [95% CI Random:‐0.72 to 0.23] Reported Results: not significant GLOBAL RATING OF IMPROVEMENT (VAS 0 to 10 cm, more is better): at ST follow‐up; SMD 0.03 (95% CI Random: 0.03 (‐0.44 to 0.50) Reported Results: not significant SIDE EFFECTS: not reported COST OF CARE: not reported |
|
| Notes | Funding bias may be present. Research support declared as Bio‐Magnetic Systems, Inc. (Co‐author Markoll was principle shareholder of Bio‐Magnetic Sytems; Markoll and Trock were sentenced in 2001 for billing unapproved electro‐magnetic therapy (see FDA report: http://www.fda.gov/ora/about/enf_story/archive/2001/ch6/oci6.htm). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | reported in text |
| Allocation concealment (selection bias) | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | reported in text |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | reported in text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | reported in text |
| Selective reporting (reporting bias) | Unclear risk | not reported |
| Similarity of baseline characteristics? | High risk | table 1 showed differences in age table 2 showed differences in pain |
| Co‐interventions avoided or similar? | Unclear risk | instruction: not to change medication during trial |
| Compliance acceptable? | Unclear risk | unclear at least for medication (not controlled) |
| Timing outcome assessments similar? | Low risk | reported in text |
N = number of participants DC = direct current PT = physiotherapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ammer 1990 | Intervention: multimodal treatment; the unique contribution of electrotherapy could not be determined |
| Chee 1986 | Design: quasi‐RCT (drew cards and divided in two groups); extremely small sample size (7 versus 9 patients) Outcome: palpatory evaluation of the presence of trigger point was no a pain or surrogate pain intensity measure |
| Chen 2007 | Population: Headache only, unable to split cervicogenic headache data |
| Coletta 1988 | Population: Unable to split data |
| Dusunceli 2009 | Comparison: Both comparison studies received the same TENS treatment |
| Fernadez‐de‐las Penas2004 | Intervention: Multimodal treatment for control group; no description of specific parameters for electrotherapy; unable to split data; no further data from authors available on request |
| Forestier 2007a | Intervention: Thermal agent used (pulsed short wave, 200W) |
| Forestier 2007b | Intervention: Thermal agent used (pulsed short wave, 200W) |
| Gabis 2003 | Population: 20 patients, only three of them with cervicogenic headache |
| Gabis 2009 | Intervention: trancranial electrical stimulation is not classical TENS Population: chronic pain only 23 had cervical pain Outcome: insufficient data on neck |
| Garrido‐Elustondo 2010 | Outcome: Key parameter is only satisfaction of patients with all kinds of physiotherapy; TENS is only mentioned |
| Gemmell 2011 | No neck pain; just stimulated trigger points |
| Gonzales‐Iglesias 2009 | Intervention: Both comparison groups received TENS |
| Hansson 1983 | Population: Not neck pain (oro‐facial pain) |
| Jahanshahi 1991 | Population: Not neck pain |
| Klaber‐Moffett 2005 | Intervention: Multimodal treatment; unable to split data; less than 10% of patients received 6 different kinds of electrotherapy |
| Lee 1997 | Intervention: Small group size (4groups with a total of 26 patients); multimodal treatment (combination of medium frequency AC+DC electrotherapy plus ultrasound) |
| Persson 2001 | Intervention: Multimodal treatment; the unique contribution of electrotherapy could not be determined |
| Porzio 2000 | Population: Fewer than 80% of included patients had neck pain |
| Provinciali 1996 | Intervention: Multimodal treatment; the unique contribution of electrotherapy could not be determined |
| Rigato 2002 | Population: Fewer than 80% of included patients had neck pain |
| Vas 2006 | Intervention: Placebo TENS, no active intervention of electrotherapy |
| Vikne 2007 | Intervention: Electrotherapy mentioned, but no modality type or parameters mentioned |
| Vitiello 2007 | Design: Data were severely flawed in many points (recalculated and evaluated by a statistician). The communication with authors did not improve the credibility, neither of the data, nor of the results |
| Wang 2007 | Population: 4 x 30 patients with pain of neck, shoulder, loin and legs, treated with four different kinds of electro‐acupuncture (excluded in this review). Unable to split data |
| Wilson 1974 | Population: Not neck pain (soft tissue injury as a result of inversion injury of the ankle) |
| Yip 2007 | Design: Quasi‐RCT |
Characteristics of ongoing studies [ordered by study ID]
Guayasamín 2013.
| Trial name or title | Study of clinical documentation, controlled, double‐blind, randomized, multicenter, designed to evaluate the effectiveness and tolerance of fixed combination of thiocolchicoside plus diclofenac potassium in reduction of acute painful muscle contracture |
| Methods | Dr. Ivan Guayasamín, Medical Surgeon |
| Participants | Acute painful striated muscle contracture,(cervical pain, backache, low back pain without sciatica, etc.); age 18 to 58; male/female |
| Interventions | Group I (experimental): 1 single tablet that contains in combination thiocolchicoside 4mg plus potassium diclofenac 50 mg every 12 hours by mouth to complete 10 doses, 5 days of treatment (TIO+DICLOK). Group II (Control): placebo, 1 tablet inactive every 12 hours orally to complete 10 doses, 5 days of treatment. Group I and Group II: Acetaminophen 500 mg as rescue medication PRN; Sample size n=90 |
| Outcomes | Primary outcome(s): 1. Evaluation of efficacy 1.1 Muscular contracture degree by a visual inspection (Contracture visible muscle mass with fixed‐antalgic attitude, Contracture visible muscle mass without fixed‐antalgic attitude, No visual signs of muscle contracture). Measuring time: at baseline and after finished the treatment (Day 5). 1.2 Muscular contracture degree by palpation (Contracture severe with evoked pain during palpation, Contracture moderate with evoked pain during palpation, Contracture mild without evoked pain during palpation, Absence contracture). Measuring time: at baseline and after finished the treatment (Day 5). 1.3 Degree of overall pain intensity (Visual Analogue Scale (VAS) of 10 cm, ranging from no pain to worst pain imaginable very severe). Measuring time: at baseline and after finished the treatment (Day 5). 2. Evaluation of tolerability 2.1 Possible Adverse Reactions (AR). Measuring time: after finished the treatment (Day 5): ‐ Occurrence of some AR in the subject (yes / no) ‐ Nature of the AR (adverse event name) ‐ Intensity of AR (Mild, moderate, severe) ‐ Duration of AR (difference between the start date and the completion of the event) ‐ Causation (causal categories described by the Uppsala Monitoring Centre (WHO): Definite, probable, possible, unlikely, conditional, not evaluable) ‐ Treatment (medication withdrawal, other) ‐ Severity of AR (Severe / serious; Not severe / not serious) Key secondary outcomes: 1. Efficacy 1.1 Efficacy of treatment by the patient (Very effective, Effective, Moderately Effective, Not effective (Ineffective). Measuring time: at the end of the treatment (Day 5) 1.2 Rescue medication (yes / no). Measuring time: at the end of the treatment (Day 5) 1.3 Total Tablets of rescue medication (Number of tablets). Measuring time: at the end of the treatment (Day 5) 1.4 Daily Tablets of rescue medication (Number of tablets). Measuring time: daily during the treatment 2. Tolerability 2.1 Tolerability of treatment by the patient (Very good, Good, Fair, Poor). Measuring time: at the end of the treatment (Day 5) 2.2 Degree of alertness ‐ sleepiness (Visual Analogue Scale (VAS) of 10 cm, ranging from awake (alert) to severe sleepiness (sleeping)). Measuring time: at baseline and after finished the treatment (Day 5) 2.3 Psychomotor activity level (Tapping test (hitting the keyboard of a personal computer as soon as possible) for 30 seconds, record the number of hits). Measuring time: at baseline and after finished the treatment (Day 5) 2.4 Psychomotor activity level (Pauli Test for 3 minutes, recorded the number of successes achieved). Measuring time: at baseline and after finished the treatment (Day 5) 3. Efficacy and Tolerability 3.1 Overall rating of treatment by the investigator (Clinical Global Impression scale). Measuring time: at the end of the treatment (Day 5) 3.2 Overall treatment satisfaction by the investigator (Very Satisfied, Satisfied, Moderately satisfied, not satisfied (dissatisfied)). Measuring time: at the end of the treatment (Day 5) 3.3 Overall treatment satisfaction by the patient (Very Satisfied, Satisfied, Moderately satisfied, not satisfied (dissatisfied)). Measuring time: at the end of the treatment (Day 5) |
| Starting date | Recruitment status: Complete Date of first enrollment: 2012/04/15 |
| Contact information | First Name: Ana Middle Name: María Last Name: Fallas Quezada Affiliation: Gutis Ltda. Postal Address: Zona industrial de Pavas, 300 metros al oeste de las oficinas centrales de Pizza Hut City: San Jose País: Costa Rica Zip Code: Apdo. 5391‐1000 Telephone: +(506) 2549 8300 Dirección de correo electrónico: a.fallas@gutis.com |
| Notes | Ecuador |
Taniguchi 2010.
| Trial name or title | Effect of neck‐type magnetotherapeutic device (magneloop) for neck and shoulder pain |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Abstract of a congress presentation Unpublished data only [14th Congress of Asia Pacific League of Associations for Rheumatology, APLAR 2010 Hong Kong Hong Kong. Conference Start: 20100711 Conference End: 20100715. Conference Publication: 230.; Taniguchi N, Kanai S. In: International Journal of Rheumatic Diseases. 2010 |
Triano 2009.
| Trial name or title | InterX 5000 ‐ A new treatment technique for people with chronic neck and shoulder pain |
| Methods | a nerve stimulation device called the InterX 5000 |
| Participants | chronic neck and shoulder pain, > 3 months |
| Interventions | InterX 5000 neurostimulator; 3 consecutive sessions, 3 times per week, 6 weeks, 20 minute sessions |
| Outcomes | Electromyography scan or an EMG, the Neck Walk Index (NWl), the Upper Limb Coordination During an Overhead Reach (ULCS) test, and the Task Limitation (Tl)/Functional lmpairment Test‐Head and Neck, Shoulder, Arm (FlT‐HaNSA) |
| Starting date | 2007 to June 2011 |
| Contact information | Dr. John J. Triano, Canadian Memorial Chiropractic College Dr Linda Woodhouse at the School of Rehabilitation Science at McMaster University, 905‐525‐9140 Ext.2259 |
| Notes | Industry Funder: Neuro Resource Group INC Woodhouse L & Triano J. Proposal to evaluate the efficacy of the InterX5000 in the treatment of chronic neck and shoulder pain. Neuro Group Inc, $100,000, 2007‐06/2009‐05. |
Weintraub 2007.
| Trial name or title | Study on Magnetic Field Therapy to Improve Quality of Sleep and Reduction of Chronic Spine Pain (SLEEP/MAG) |
| Methods | Allocation: Randomized Endpoint Classification: Efficacy Study Intervention Model: Parallel Assignment Masking: Double‐Blind Primary Purpose: Treatment |
| Participants | 18 Years to 85 Years; male/female Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Treated subjects will receive a permanent/static magnetic sleeping pad with a nominal strength of less than 1000 Gauss. Control subjects will receive physically identical sleeping pad with a nominal surface field strength of 0 Gauss (placebo). The magnets will be contained in a standard mattress pad and subjects will sleep on the pad. |
| Outcomes | Primary Outcome Measures: VAS Pain scores/Pittsburgh Sleep scores/Insomnia sleep scores/SF 15 pain descriptor scores/PGIC/ Secondary Outcome Measures: Autonomic Nerve Functions |
| Starting date | September 26, 2007 |
| Contact information | Weintraub, Michael I., MD, FACP, FAAN; miwneuro@pol.net |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently. |
Differences between protocol and review
Assessment of risk of bias, GRADE method application, inclusion criteria
Contributions of authors
This review is one of a series of reviews being conducted by the Cervical Overview Group (COG): Burnarski D, Burnie S, Eddy A, Ezzo J, Goldsmith C, Graham N, Gross A, Haines T, Haraldsson B, Hoving J, Kay T, Kroeling P, Linge L, Peloso P, Miller J, Morien A, Perry L, Radylovick Z, Santaguida P, Szeto G,Trinh K, Wang E, White R.
The primary review authors for this review and their contributions are listed below.
Kroeling P: manuscript preparation, data extraction, synthesis, conclusion, recommendations, public presentation, publication, public responsibility
Gross A: grant submission, citation identification and selection, data entry, synthesis, conclusions, manuscript preparation and review, public presentation, project coordination
Graham N: electronic study searches, author communication, validity assessment, organisation
Burnie S: validity assessment, synthesis, manuscript review
Szeto G: risk of bias assessment; synthesis, final publication
Goldsmith CH: statistical analysis, grant submission, validity assessment, manuscript review
Haines T: identification and selection, synthesis, conclusions, manuscript review
Forget M: validity assessment, synthesis, conclusions, manuscript review
Sources of support
Internal sources
McMaster University, Canada.
Ludwig Maximilians University of Munich, Germany.
University of Western Ontario, Canada.
St. Josephs's Healthcare, Canada.
External sources
Consortial Centre for Chiropractic Research ‐ Nat. Institute of Health, Bethseda, MD, USA.
Hamilton Hospital Association, Ontario, Canada.
Declarations of interest
No conflicts of interest known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Chiu 2005 {published data only}
- Chiu TW, Hui‐Chan C, Cheing G. A randomized clinical trial of TENS and exercise for patients with chronic neck pain. Clinical Rehabilitation 2005;19:850‐60. [DOI] [PubMed] [Google Scholar]
Escortell‐Mayor 2011 {published data only}
- Escortell E. Manual therapy effectiveness in comparison with electric nerve stimulation (TENS) in patients with neck pain. ClinicalTrials.gov identifier: NCT01153737 Feb 2011.
- Escortell‐Major E, Lebrijo‐Perez G, Perez‐Martin Y, Asunsolo‐del Barco, Riesgo‐Fuertes R, Saa‐Requejo C, por el Gruppo TEMA‐TENS. Randomized clinical trial for primary care patients with neck pain: manual therapy versus electrical stimulation [Ensayo clinico aleatorizado en pacientes con cervicalgia mecanica en atencion primaria: terapia manual frente a electroestimulacion nerviosa transcutanea]. Atención Primaria 2008;40(7):337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escortell‐Mayor E, Riesgo‐Fuertes R, Garrido‐Elustondo S, Asunsolo‐del Barco A, Diaz‐Pulido B, Blanco‐Diaz M, Bejerano‐Alvarez E and TEMA‐TENS Group. Primary care randomized clinical trial: Manual therapy effectiveness in comparison with TENS in patients with neck pain. Manual Therapy 2011;16:66‐73. [DOI] [PubMed] [Google Scholar]
Farina 2004 {published data only}
- Farina S, Casarotto M, Benelle M, Tinassi M, Fiaschi A, Goldoni M, et al. A randomized controlled study on the effect of two different treatments in myofascial pain syndrome. Europa Medicophysica 2004;40:293‐301. [PubMed] [Google Scholar]
Fialka 1989 {published data only}
- Fialka V, Preisinger E, Bohler A. Zur physikalischen diagnostik und physikalischen therapie der distorsio columnae vertebralis cervicalis. Zeitschrift fur physikalische Medizin, Balneologie, medizinische Klimatologie 1989;18:390‐7. [Google Scholar]
Flynn 1987 {published data only}
- Flynn T. A comparative study between UltraReiz and Ultrasound in the treatment for relief of pain in whiplash injuries. Physiotherapy Ireland 1987;8(1):11‐4. [Google Scholar]
Foley‐Nolan 1990 {published data only}
- Foley‐Nolan D, Barry C, Coughlan RJ, O'Connor P, Roden D. Pulsed high frequency (27MHz) electromagnetic therapy for persistent neck pain: A double blind, placebo‐controlled study of 20 patients. Orthopaedics 1990;13:445‐51. [DOI] [PubMed] [Google Scholar]
Foley‐Nolan 1992 {published data only}
- Foley‐Nolan D, Moore K, Codd M, Barry C, O'Connaor P, Coughlan RJ. Low energy high frequency pulsed electromagnetic therapy for acute whiplash injuries. A double blind randomized controlled study. Scandinavian Journal of Rehabilitation Medicine 1992;24(1):51‐9. [PubMed] [Google Scholar]
Hendriks 1996 {published data only}
- Hendriks O, Horgan A. Ultra‐reiz current as an adjunct to standard physiotherapy treatment of the acute whiplash patient. Physiotherapy Ireland 1996;17(1):13‐7. [Google Scholar]
Hong 1982 {published data only}
- Hong CZ, Lin JC, Bender LF, Schaeffer JN, Meltzer RJ, Causin P. Magnetic necklace: Its therapeutic effectiveness on neck and shoulder pain. Archives of Physical Medicine and Rehabilitation 1982;63:462‐6. [PubMed] [Google Scholar]
Hou 2002 {published data only}
- Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger point sensitivity. Archives of Physical Medicine and Rehabilitation 2002;83:1406‐14. [DOI] [PubMed] [Google Scholar]
Hsueh 1997 {published data only}
- Hsueh TC, Cheng PT, Kuan TS, Hong CZ. The immediate effectiveness of electrical nerve stimulation and electrical muscle stimulation on myofascial trigger points. American Journal of Physical Medicine and Rehabilitation 1997;76:471‐6. [DOI] [PubMed] [Google Scholar]
Hurwitz 2002 {published data only}
- Hurwitz EL, Morgenstern H, Harber P, Kominski GF, Yu F, Adams AH. A randomized trial of chiropractic manipulation and mobilization for patients with neck pain: clinical outcomes from the UCLA Neck Pain Study. American Journal of Public Health 2002;92(10):1634‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nordemar 1981 {published data only}
- Nordemar R, Thorner C. Treatment of acute cervical pain ‐ A comparative group study. Pain 1981;10:93‐101. [DOI] [PubMed] [Google Scholar]
Philipson 1983 {published data only}
- Philipson T, Haagensen N, Laumann V, Nies M, Thorup K, Hansen TI. The effect of diadynamic current on chronic soft‐tissue pain in the neck and shoulder girdle [Effekten af diadynamisk stroem pa kroniske bloeddelsmerter i nakke‐skulderaget]. Ugeskrift for Laeger 1983;145:479‐81. [(Danish, translated)] [PubMed] [Google Scholar]
Sahin 2011 {published data only}
- Lidia L, Shklar B, Kesner Baruch Y, Raz R, Gabis E, Geva D. Effect of different transcutaneous electrical stimulation modalities on cervical myofascial pain syndrome. Journal of Rehabilitation Medicine 2011;41:256–61. [Google Scholar]
Smania 2003 {published data only}
- Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti S, Tinazzi M. Therapeutic effects of peripheral repetitive magnetic stimulation on myofascial pain syndrome. Clinical Neurophysiology 2003;114:350‐8. [DOI] [PubMed] [Google Scholar]
Smania 2005 {published data only}
- Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti SM, Tinazzi M. Repetetive magnetic stimulation: a novel therapeutic approach for myofascial pain syndrome. Journal of Neurology 2005;252:307‐14. [DOI] [PubMed] [Google Scholar]
Sutbeyaz 2006 {published data only}
- Sutbeyaz ST, Sezer N, Kuseoglu BF. The effect of pulsed electromagnetic fields in the treatment of cervical osteoarthritis: a randomized, double‐blind, sham controlled study. Rheumatology International 2006;26:320‐4. [DOI] [PubMed] [Google Scholar]
Thuile 2002 {published data only}
- Thuile CH, Walzi M. Evaluation of electromagnetic fields in the treatment of pain in patients with lumbar radiculopathy or the whiplash syndrome. NeuroRehabilitation 2002;17:63‐7. [PubMed] [Google Scholar]
Trock 1994 {published data only}
- Trock DH, Bollet AJ, Markoll R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and the cervical spine. Report of randomized, double blind, placebo controlled trials. Journal of Rheumatology 1994;21(10):1903‐11. [PubMed] [Google Scholar]
References to studies excluded from this review
Ammer 1990 {published data only}
- Ammer K, Rathkolb O. Physical therapy in occipital headaches [Physikalische Therapie bei Hinterhauptschmerzen]. Manual Medizin 1990;28:65‐8. [Google Scholar]
Chee 1986 {published data only}
- Chee E, Walter H. Treatment of trigger point with microamperage transcutaneous electrical nerve stimulation (TENS) ‐ (The electro‐acuscope 80). Journal Manipulative and Physiological Therapeutics 1986;9(2):131‐4. [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen L, Zhang X, Ding H, Tao Y, Zhan H. Comparative study on effects of manipulation treatment and TENS on patients with cervicogenic headache [Chinese language]. Journal of Chinese Integrative Medicine 2007;5(4):403‐6. [DOI] [PubMed] [Google Scholar]
Coletta 1988 {published data only}
- Coletta R, Maggiolo F, Tizio S. Etofenamate and transcutaneous electrical nerve stimulation treatment of painful spinal syndromes. International Journal of Clinical Pharmacology Research 1988;8(4):295‐8. [PubMed] [Google Scholar]
Dusunceli 2009 {published data only}
- Dusunceli Y, Ozturk C, Atamaz F, Hepguler S, Durmaz B. Efficacy of neck stabilisation exercises for neck pain: a randomized controlled study. Journal of Rehabilitation Medicine 2009;41:626‐31. [DOI] [PubMed] [Google Scholar]
Fernadez‐de‐las Penas2004 {published data only}
- Fernandez‐de‐las‐Penas C, Fernandez‐Carnero J, Palomque del Cerro L, Miangolarra‐Page JC. Manipulative treatment vs. conventional physiotherapy treatment in whiplash injury: a randomized controlled trial. Journal of Whiplash & Related Disorders 2004;3(2):73‐90. [Google Scholar]
Forestier 2007a {published and unpublished data}
- Forestier R, Francon A, Saint‐Arromand F, Bertolino C, Guillemot A, Graber‐Duvernay B, et al. Are SPA therapy and pulsed electromagnetic field therapy effective for chronic neck pain? Randomized clinical trial. First part: clinical evaluation. Annales de Readaptation et de Medecine Physique 2007;50:140‐7. [DOI] [PubMed] [Google Scholar]
Forestier 2007b {published and unpublished data}
- Forestier R, Francon A, Saint AF, Bertolino C, Graber‐Duvernay B, Guillemot A, et al. Are spa therapy and pulsed electromagnetic field therapy effective for chronic neck pain? Randomized clinical trial. Second part: medicoeconomic approach. Annales de Readaptation et de Medecine Physique 2007;50:148‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gabis 2003 {published data only}
- Gabis L, Shklar B, Geva D. Immediate influence of transcranial electrostimulation on pain and beta‐endorphin blood levels. An active placebo controlled study. American Journal of Physical Medicine and Rehabilitation 2003;82:81‐5. [DOI] [PubMed] [Google Scholar]
Gabis 2009 {published data only}
- Gabis L, Shklar B, Kesner Baruch Y, Raz R, Gabis E, Geva D. Pain reduction using transcranial electrostimulation: a double blind, active placebo controlled trial. Journal of Rehabilitation Medicine 2009;41:256–61. [DOI] [PubMed] [Google Scholar]
Garrido‐Elustondo 2010 {published data only}
- Garrido‐Elustondo S, Riesgo‐Fuertes R, Escortell‐Mayor E, Asunsolo del Barco A, Perez‐Martin Y, Martin‐Castro B. Satisfaction of patients with mechanical neck disorders attended to by primary care physical therapists. Journal of Evaluation in Clinical Practice 2010;16:445‐50. [DOI] [PubMed] [Google Scholar]
Gemmell 2011 {published data only}
- Gemmell H, Hilland A. Immediate effect of electric point stimulation (TENS) in treating latent upper trapezius trigger points: a double blind randomized placebo‐controlled trial. Journal of Bodywork and Movement Therapies 2011;15:348‐54. [DOI] [PubMed] [Google Scholar]
Gonzales‐Iglesias 2009 {published data only}
- Gonzales‐Iglesias J, Fernandez de‐Las ‐Penas C, Cleland J, Albuquerque‐Sendin F, Palomeque‐del‐Cerro L, Mendez‐Sanchez R. Inclusion of thoracic spine thrust manipulation into an electrotherapy/thermal program for the management of patients with acute mechanical neck pain: A randomized clinical trial. Manual Therapy 2009;14:306‐13. [DOI] [PubMed] [Google Scholar]
Hansson 1983 {published data only}
- Hansson P, Ekblom A. TENS as compared to placebo TENS for relief of acute oro‐facial pain. Pain 1983;15:157‐65. [DOI] [PubMed] [Google Scholar]
Jahanshahi 1991 {published data only}
- Jahanshahi M, Sartory G, Marsden CD. EMG biofeedback treatment of torticollis: a controlled outcome study. Biofeedback and Self‐Regulation 1991;16(4):413‐48. [CO97:359] [DOI] [PubMed] [Google Scholar]
Klaber‐Moffett 2005 {published data only}
- Klaber‐Moffett JA, Jackson DA, Richmond S, Hahn S, Coulton S, Farrin A, et al. Randomized trial of a brief physiotherapy intervention compared with usual physiotherapy for neck pain patients: outcomes and patients preference. BMJ 2005;75‐:1‐6. [doi: 10.1136/bmj.38268.82 (published 7 December 2004)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee JC, Lin DT, Hong CZ. The effectiveness of simultaneous thermotherapy with ultrasound and electrotherapy with combined AC and DC current on the immediate pain relief of myofascial trigger points. Journal of Musculoskeletal Pain 1997;5(1):81‐90. [Google Scholar]
Persson 2001 {published data only}
- Persson LCG, Carlsson CA, Carlsson JY. Long‐lasting cervical radicular pain managed with surgery, physiotherapy or a cervical collar. Spine 1997;22(7):751‐8. [DOI] [PubMed] [Google Scholar]
- Persson LCG, Lilja A. Pain, coping, emotional state and physical function in patients with chronic radicular neck pain. A comparison between patients treated with surgery, physiotherapy or neck collar. A blinded, prospective randomized study. Disability and Rehabilitation 2001;23(8):325‐35. [DOI] [PubMed] [Google Scholar]
- Persson LCG, Moritz U, Brandt L, Carlsson CA. Cervical radiculopathy: pain, muscle weakness and sensory loss in patients with cervical radiculopathy treated with surgery physiotherapy or cervical collar. European Spine Journal 1997;6(4):265‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porzio 2000 {published data only}
- Porzio F. Use of thermomagnetic bandages and belts in cervical and lumbar pain syndromes. Experimental study with double blind method versus placebo [Impiege di fasce e cinture termomagnetice nelle sindromi dolorose cervicali e lombari. Studio sperimentale con il metodo della doppia cecita vs. placebo]. La Clinica Terapeutica 2000;151:140‐53. [PubMed] [Google Scholar]
Provinciali 1996 {published data only}
- Provinciali L, Baroni M, Illuminati L, Ceravolo MG. Multimodal treatment to prevent the late whiplash syndrome. Scandinavian Journal of Rehabilitation Medicine 1996;28:105‐11. [PubMed] [Google Scholar]
Rigato 2002 {published data only}
- Rigato M, Battisti E, Fortunato M, Giordano N. Comparison between the analgesic and therapeutic effects of a musically modulated electromagnetic field (TAMMEF) and those of a 100 Hz electromagnetic field: blind experiment on patients suffering from cervical spondylosis or shoulder periarthritis. Journal of Medical Engineering and Technology 2002;26(6):253‐8. [DOI] [PubMed] [Google Scholar]
Vas 2006 {published data only}
- Vas J, Perea‐Milla E, Mendez C, et al. Efficacy and safety of acupuncture for chronic uncomplicated neck pain: A randomized controlled study. Pain 2006;126:245‐55. [DOI] [PubMed] [Google Scholar]
Vikne 2007 {published data only}
- Vikne J, Oedegaard A, Laerum E, Ihlebaek C, Kirkesola G. A randomized study of new sling exercise treatment vs traditional physiotherapy for patients with chronic whiplash‐associated disorders with unsettled compensation claims. Journal Rehabilitation Medicine 2007;39:252‐9. [DOI] [PubMed] [Google Scholar]
Vitiello 2007 {published and unpublished data}
- Vitiello AL, Bonello RP, Pollard HP. The effectiveness of ENAR(R) for the treatment of chronic neck pain in Australian adults: A preliminary single blind, randomized controlled trial. Chiropractic & Osteopathy 2007;15(9):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang Z, Chen L, Zhu W. Observation on the transient analgesic effect of abdominal acupuncture TENS on neck pain, shoulder, loin and legs. Chinese Acupuncture and Moxibustion 2007;27(9):657‐9. [PubMed] [Google Scholar]
Wilson 1974 {published data only}
- Wilson DH. Comparison of short wave diathermy and pulsed electromagnetic energy in treatment of soft tissue injuries. Physiotherapy 1974;60(10):309‐11. [PubMed] [Google Scholar]
Yip 2007 {published data only}
- Yip YB, Tse HS, Wu KK. An experimental study comparing the effects of combined transcutaneous acupoint electrical stimulation and electromagnetic millimeter waves for spinal pain in Hong Kong. Complementary Therapies in Clinical Practice 2007;13:4‐14. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Guayasamín 2013 {unpublished data only}
- Guayasamín I. Study of clinical documentation, controlled, double‐blind, randomized, multicenter, designed to evaluate the effectiveness and tolerance of fixed combination of thiocolchicoside plus diclofenac potassium in reduction of acute painful muscle contracture. http://rpcec.sld.cu/trials/RPCEC00000162‐En (accessed 21 August 2013).
Taniguchi 2010 {unpublished data only}
- Taniguchi N, Kanai S. Effect of neck‐type magnetotherapeutic device (magneloop) for neck and shoulder pain. International Journal of Rheumatic Diseases. Proceedings of the 14th Congress of Asia Pacific League of Associations for Rheumatology; 2010 July 11‐15, Hong Kong. 2010; Vol. 13:230.
Triano 2009 {unpublished data only}
- Triano JJ. Efficacy of the InterX 5000 in the Treatment of Chronic Neck Pain. http://ClinicalTrials.gov/show/NCT01382537 (accessed 21 August 2013).
- Woodhouse L, Triano J. Proposal to evaluate the efficacy of the InterX5000 in the treatment of chronic neck and shoulder pain [Industry Partner Grant: $100,000]. Neuro Group Inc 2007‐06/2009‐05; Vol. McMaster University, Canadian Memorial Chiropractic College, issue Hamilton, Ontario, Canada.
Weintraub 2007 {unpublished data only}
- Weintraub MI. Study on Magnetic Field Therapy to Improve Quality of Sleep and Reduction of Chronic Spine Pain (SLEEP/MAG). http://ClinicalTrials.gov/show/NCT00445133. (accessed 21 August 2013).
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, et al. GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58:1233‐40. [DOI] [PubMed] [Google Scholar]
Cameron 1999
- Cameron MH (editor). Physical agents in rehabilitation. From research to practice. 1st Edition. Philadelphia, New York, London: WB Saunders Company, 1999. [Google Scholar]
Carroll 2008a
- Carroll LJ, Hogg‐Johnson S, Velde G, Haldeman S, Holm LW, Carragee EJ, et al. Course and prognostic factors for neck pain in the general population: results of the Bone and Joint Decade 2000‐2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008;33(4S):S75‐S82. [DOI] [PubMed] [Google Scholar]
Carroll 2008b
- Carroll LJ, Holm LW, Hogg‐Johnson S, Côté P, Cassidy JD, Haldeman S, et al. Course and prognostic factors for neck pain in whiplash‐associated disorders (WAD); results of the Bone and Joint Decade 2000‐2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008; Vol. 33, issue 4S:S83‐92. [DOI] [PubMed]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioural sciences. 2nd Edition. Hilldale, NY: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Côté 1998
- Côté P, Cassidy D, Corroll L. The Saskatchewan health and back pain survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine 1998;23(15):1689‐98. [DOI] [PubMed] [Google Scholar]
Côté 2008
- Côté P, Kristman V, Vidmar M, Eerd D, Hogg‐Johnson S, Beaton D, et al. The prevalence and incidence of work absenteeism involving neck pain. A cohort of Ontario lost‐time claimants. Spine 2008;33(4S):S192‐8. [DOI] [PubMed] [Google Scholar]
Davis 2012
- Davis MA, Onega T, Weeks WB, Lurie JD. Where the United States spends Its spine dollars expenditures on different ambulatory services for the management of back and neck conditions. Spine 2012;37(19):1693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furlan 2009
Gross 2002
- Gross A, Kay T, Kennedy C, Gasner D, Hurley L, Yardley K, Hendry L. Clinical practice guideline on the use of manipulation or mobilization in the treatment of adults with mechanical neck disorder. Manual Therapy 2002;7(4):193‐205. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 www.cochrane‐handbook.org. The Cochrane Collaboration, March 2011 (updated). [Google Scholar]
Hogg‐Johnson 2008
- Hogg‐Johnson S, Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000‐2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008;33(4S):S39‐S51. [DOI] [PubMed] [Google Scholar]
Hulme 2002
- Hulme J, Robinson V, DeBie R, Wells G, Judd M, Tugwell P. Electromagnetic fields for treatment of osteoarthritis. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003523] [DOI] [PubMed] [Google Scholar]
Kendal 1963
- Kendal MG, Stuart A. The advanced theory of statistics: Distribution theory. 2nd Edition. Vol. 1, New York: Hofner Publishing Co., 1963:237. [Google Scholar]
Kroeling 1998
- Kroeling P, Gottschild S, Kosarev A, Pfeiffer R. TENS increases the pressure pain threshold in a tibia model. Kepplinger B (editor): Pain: clinical aspects and therapeutical issues. Linz: Edition Selva, 1998. [Google Scholar]
Landis 1977
- Landis RJ, Koch GG. The measure of observer agreement for categorical data. Biometrics 1977;33:159‐74. [PubMed] [Google Scholar]
Leaver 2010
- Leaver AM, Refshauge KM, Maher CG, McAuley JH. Conservative interventions provide short‐term relief for non‐specific neck pain: a systematic review. Journal of Physiotherapy 2010;56(2):73‐85. [DOI] [PubMed] [Google Scholar]
MacDermid 2009
- MacDermid JC, Walton DM, Avery S, Blanchard A, Etruw E, McAlpine C. Measurement properties of the neck disability index: a systematic review. Journal of Orthopaedic & Sports Physical Therapy 2009;39(5):400‐17. [DOI] [PubMed] [Google Scholar]
NRC 2001
- National Research Council. National Academy Press. Washington DC, 2001.
Olesen 1988
- Olesen J. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalgia 1988;8(7):61‐2. [PubMed] [Google Scholar]
Olesen 1997
- Olesen J, Gobel H. ICD‐10 Guide for Headaches. Guide to the classification, diagnosis and assessment of headaches in accordance with the tenth revision of the international classification of diseases and related health problems and its application to neurology. Cephalgia 1997;17 Suppl 19:29‐30. [Google Scholar]
Pujol 1998
- Pujol J, Pascual‐Leone A, Dolz C, Delgado E, Dolz JL, Aldomà J. The effect of repetitive magnetic stimulation on localized musculoskeletal pain. Neuroreport 1998;9(8):1745‐8. [DOI] [PubMed] [Google Scholar]
Schmidt‐Rohlfing 2000
- Schmidt‐Rohlfing B, Silny J, Niethard FU. Pulsating electromagnetic fields in the treatment of orthopaedic diseases: a review and meta‐analysis [Pulsierende elektromagnetische Felder in der Behandlung von Verletzungen und Erkrankungen der Bewegungsorgane ‐ eine Übersicht und Metaanalyse]. Zeitschrift fur Orthopadie und ihre Grenzgebiete 2000;138:379‐89. [DOI] [PubMed] [Google Scholar]
Schumacher 1993
- Schumacher HR, Klippel JH, Koopman WJ (eds). Primer on the Rheumatic Diseases. 10th Edition. Atlanta: Arthritis Foundation, 1993. [Google Scholar]
Sjaastad 1990
- Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic Headache: Diagnostic criteria. Headache 1990;30:725‐6. [DOI] [PubMed] [Google Scholar]
Spitzer 1987
- Spitzer WO, Leblanc FE, Dupuis M. Scientific approach to the assessment and management of activity related spinal disorders. Spine 1987;Suppl 7:S1‐S59. [PubMed] [Google Scholar]
Spitzer 1995
- Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, et al. Scientific monograph of the Quebeck Task Force on Whiplash‐Associated Disorders: Redefining "whiplash" and its management. Spine 1995;Suppl:1S‐73S. [PubMed] [Google Scholar]
Stucki 2000
- Stucki G, Kroeling P. Physical therapy and rehabilitation in the management of rheumatic disorders. Baillieres Best Practice and Research. Clinical Rheumatology 2000;14(4):751‐71. [DOI] [PubMed] [Google Scholar]
Stucki 2007
- Stucki G, Kroeling P. In: Hochberg M, Silman A, Smolen J, Weinblatt ME, Weisman MH editor(s). Rheumatology. 4th Edition. Vol. 1, Edinburgh: Mosby, 2007:557‐568. [Google Scholar]
Tan 1998
- Tan JC. Practical manual of physical medicine and rehabilitation. St. Louis, Missouri: Mosby, 1998. [Google Scholar]
Teasell 2010
- Teasell RW, McClure JA, Walton D, Pretty J, Salter K, Meyer M, et al. A research synthesis of therapeutic interventions for whiplash‐associated disorder: Part 2 ‐ interventions for acute WAD. Pain Research and Management (Canadian Pain Society) 2010;15(5):295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
US Census Bureau 1996
- United States Census Bureau. Statistical Abstract of the United States 1996. US Bureau of Census. 116. Washington DC: US Bureau of Census, 1996. [Google Scholar]
US Census Bureau 2012
- US Census Bureau. The 2012 Statistical Abstract of U.S. The National Data Book <www.census.gov/prod/www/abs/statab.html>;Section 3: Health and Nutrician:132 (retrieved 2013‐01‐16). [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Walsh 1997
- Walsh DM. TENS: Clinical applications and related theory. New York: Churchill Livingston, 1997. [Google Scholar]
References to other published versions of this review
Kroeling 2005
- Kroeling P, Gross AR, Goldsmith CH and the Cervical Overview Group. A Cochrane review of electrotherapy for mechanical neck disorders. Spine 2005;30(21):E641‐8. [DOI] [PubMed] [Google Scholar]
Kroeling 2009
- Kroeling P, Gross A, Goldsmith CH, Burnie SJ, Haines T, Graham N, Brant A . Electrotherapy for neck pain. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004251.pub4] [DOI] [PubMed] [Google Scholar]


