Abstract
Background:
The association of prenatal exposure to organophosphate esters (OPEs) and replacement brominated flame retardants (RBFRs) with respiratory outcomes has not been previously investigated in humans, despite reports that these chemicals can cross the placenta and alter lung development as well as immune functions.
Methods:
In a cohort of 342 pregnant women recruited between 2003 and 2006 in the greater Cincinnati, Ohio Metropolitan area, we measured indoor dust OPEs and RBFRs at 20 weeks of gestation and urinary OPEs at 16 and 26 weeks of gestation and at delivery. We performed generalized estimating equations and linear mixed models adjusting for covariates to determine the associations of prenatal OPEs and RBFRs exposures with adverse respiratory outcomes in childhood, reported every six months until age 5 years and with lung function at age 5 years. We used multiple informant modeling to examine time-specific associations between maternal urinary OPEs and the outcomes.
Results:
Dust concentrations of triphenyl phosphate (TPHP) (RR: 1.40, 95% CI: 1.18–1.66), 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (RR: 1.51, 95% CI: 1.23–1.85), and bis(2-ethylhexyl) tetrabromophthalate (RR: 1.57, 95% CI: 1.28–1.94) were associated with higher risk of wheezing during childhood. Dust TPHP concentrations were associated with higher risk of respiratory infections (RR: 1.43, 95% CI: 1.08–1.94), and dust tris-(2-chloroethyl) phosphate concentrations were associated with hay fever/allergies (RR: 1.11, 95% CI: 1.01–1.21). We also found that dust tris-(2-chloroethyl) phosphate loadings were associated with lower lung function. Urinary OPEs mainly at week 16 of gestation tended to be associated with adverse respiratory outcome, while bis(1-chloro-2-propyl) phosphate and diphenyl phosphate at delivery were associated with lower risk of hay fever/allergies.
Conclusions:
In-utero exposure to OPEs and RBFRs may be a risk factor for adverse respiratory outcomes in childhood, depending on the timing of exposure.
Keywords: Replacement Flame Retardants, Respiratory Health, Early Life Exposures, Prenatal Exposures
Introduction
Organophosphate esters (OPEs) are chemicals widely used as flame retardants and plasticizers that can be found in furniture, carpets, plastic, car seats, textiles, electric and electronic appliances, and several other consumer products (Ospina et al., 2018). They leach from household products and accumulate in house dust where they are universally found (Van den Eede et al., 2011). Until recently, polybrominated diphenyl esters (PBDEs) were used as flame retardants but were found to be associated with endocrine, metabolic, reproductive, neurodevelopmental, and carcinogenic effects in humans (Linares et al., 2015). Since the PBDEs ban and phase-out, the use of OPEs and replacement brominated flame retardants (RBFRs) as substitutes has significantly increased, causing widespread exposure to these chemicals, with OPEs levels sometimes orders of magnitude higher than those of PBDEs during their peak use (Blum et al., 2019; Zuiderveen et al., 2020).
Due to their ability to cross the placenta, exposure to OPEs and RBFRs can occur as early as during the in-utero period which is characterized by windows of vulnerability with unique health consequences (Bonds & Midoro-Horiuti, 2013; Zhao et al., 2017). These chemicals exhibit estrogenic (mostly OPEs) or antiestrogenic (RBFRs) effects and differential interactions with estrogen receptors (ER) α (Dong et al., 2021; Zhang et al., 2014). The role of estrogen in respiratory diseases is complex. On one hand, xenoestrogens may activate ER in immunomodulatory cells and promote T-helper 2 (Th2) cell responses to cause immunoglobulin E production and mast cell as well as basophil degranulation leading to allergies and asthma (Bonds & Midoro-Horiuti, 2013). On the other hand, ER antagonists may cause contraction in bronchial rings to induce bronchoconstriction (Bonds & Midoro-Horiuti, 2013). Moreover, in-utero exposure to xenoestrogens stimulates the production of lung surfactant and the development of alveolar crests, type II alveolar epithelial cells, and lamellar bodies (Trotter et al., 2009). Timing of exposure is also crucial; during gestation, lung formation begins with the occurrence of primary lung buds at approximately 4 weeks, airway smooth muscles begin developing between 8 and 10 weeks, and alveolar development begins at about 24 weeks (Sly & Flack, 2008). The amniotic sac contains amniotic fluid which contributes to lung growth and maturation. It forms around the 12th day of pregnancy, and fetal respiration begins in the second trimester (Sly & Flack, 2008). Therefore, prenatal exposures affecting the respiratory system may have different effects depending on the timing of exposure (Sly & Flack, 2008).
Yet, the association of prenatal exposure to OPEs and RBFRs with respiratory health has not been previously investigated. We tested the hypothesis that prenatal OPEs and RBFRs are associated with adverse respiratory health using prenatal dust and urinary assessments of the exposures at different windows during pregnancy as well as repeated measures of respiratory outcomes during childhood. This study has important public health relevance, as these chemicals are now ubiquitously found in our environment, and because respiratory conditions in childhood may lead to increased risk of chronic diseases in adulthood (Mendy & Mersha, 2022a).
Methods
HOME Study Cohort
The Health Outcomes and Measures of the Environment (HOME) Study is an ongoing prospective pregnancy and birth cohort that recruited pregnant women between March 2003 and January 2006 from the Greater Cincinnati, Ohio metropolitan area. It was designed to assess the health effects of early life environmental exposures on children (Braun et al., 2017). Among the 401 pregnant mother-child pairs initially included in the HOME Study, 342 had data on dust OPEs and RBFRs, as well as urinary OPEs measured prenatally, and on covariates and respiratory outcomes through follow-up until age 5 years. Details on the HOME Study cohort are published elsewhere (Braun et al., 2017).
Dust OPEs and RBFRs and Urinary OPEs
Using a High-Volume Surface Sampler, settled dust was sampled from a 1-meter squared floor area for a median sampling time of 275 seconds in the main activity room of participants’ homes, at around 20 weeks of gestation. Whole dust was stored at −20 °C until shipping to the Virginia Institute of Marine Sciences, William & Mary for analysis. The dust was sieved and extracted, extracts were purified and OPEs (tris-(2-chloroethyl) phosphate [TCEP], tris(1-chloro-2-propyl) phosphate [TCIPP], tris(1,3-dichloroisopropyl) phosphate [TDCIPP], and TPHP) as well as RBFRs (2-ethylhexyl-2,3,4,5-tetrabromobenzoate [EH-TBB] and bis(2-ethylhexyl) tetrabromophthalate [BEH-TEBP) concentrations were determined by high-performance liquid chromatography mass spectrometry (HPLC-MS), with a limit of detection (LOD) of 0.10 μg/g dust for OPEs and machine reading limit of 0.002 μg/g dust for RBFRs. In quality control performed on all batches, the recovery limits were 100% ± 2 standard deviations and the levels that had recovery rates outside these limits were excluded (4.2%). Exposure concentrations were divided by recovery rates for correction and the batches were adjusted by deducting the blank value from the uncorrected measurements if the blank value was >0.1 μg/g (Hornung & Reed, 1990). Dust OPEs and RBFRs dust loadings were estimated as (Concentration [ng/g dust]) × (sieved dust weight [g dust]) / dust sampling area (m2) (Mendy et al., 2023).
Spot urines from pregnant women were sampled at 16 and 26 weeks of gestation and within 48 hours of delivery and were frozen at −20 °C until analysis at the Centers for Disease Control and Prevention’s (CDC) National Center for Environmental Health. Urinary bis-2-chloroethyl phosphate (BCEP) (TCEP metabolite), bis(1-chloro-2-propyl) phosphate (BCIPP) (TCIPP metabolite), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) (TDCIPP metabolite), diphenyl phosphate (DPHP) (TPHP metabolite) were measured using HPLC-MS. The LOD was 0.10 μg/L and samples with concentrations <LOD were imputed with LOD/√2 (Hornung & Reed, 1990). Details of the analysis and quality control procedures for dust OPEs and RBFRs as well as urinary OPEs are described elsewhere (Jayatilaka et al., 2019; Percy et al., 2020).
Respiratory Outcomes
Standardized questions assessing respiratory symptoms (wheeze, respiratory infections, and hay fever/allergies) were based on the National Health and Nutrition Examination Survey, designed by the CDC to assess the health status of the non-institutionalized U.S. population (Mendy & Mersha, 2022). The questionnaires were administered every six months until the child reached age 5 years. At age 5 years, children completed forced expiratory volume in one second (FEV1) as well as peak expiratory flow (PEF) with a portable spirometer, and the three measures acceptable according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) Task Force criteria were recorded by trained research assistants (Miller et al., 2005). We used FEV1 % predicted calculated as the FEV1 of the participant divided by the reference FEV1 in children of similar age, sex, height, and race ethnicity. The values for reference FEV1 were estimated using the Global Lung Initiative equations (Quanjer et al., 2012).
Covariates
The HOME Study collected extensive data on variables such as child’s sex, child race/ethnicity, median household income at baseline, duration of breastfeeding, gestational age, and maternal and paternal allergy and asthma using questionnaires. Birth weight was extracted from the birth medical record, and prenatal exposure to active smoking and second-hand smoke was assessed using mothers’ serum cotinine measured at gestational weeks 16 and 26. A detailed description of these covariates’ measurements has been published elsewhere (Braun et al., 2017).
Statistical Analysis
We performed the descriptive statistics of the distributions of dust OPEs and RBFRs as well as urinary OPEs standardized by specific gravity to adjust for dilution, overall and by characteristics of study participants. The geometric means (GM) along with corresponding standard errors (SE) were reported and P-values for exposure level differences were estimated using the Wilcoxon Rank Test. Given their skewed distribution, dust OPEs and RBFRs and urinary OPEs exposure levels were log10-transformed to improve normality, and we explored the intercorrelation between the exposures using Pearson correlations. We also examined the intraclass correlation (ICC) of the for the single measures urinary OPEs across the three sampling periods points using mixed effect modeling with an ICC of ≥ 0.7 was interpreted as high.
To examine the association of the prenatal exposures with the repeated reports of wheezing, respiratory infections, and hay fever or allergy during childhood, we used generalized estimating equation (GEE) analysis. We selected a binomial distribution with a log link function for the models due to the repeated measures of the binary respiratory outcomes. Unstructured working matrix and robust variance estimators were specified to estimate the β coefficients exponentiated to obtain the relative risks (RR) and their corresponding 95% confidence intervals. To examine the association of the prenatal exposures with lung function as continuous variable, we performed linear regression modeling. Dust OPEs and RBFRs as well as urinary BCEP, BDCIPP, and DPHP, which had high detection frequencies (>80%), were used as continuous log10-transformed variables, while urinary BCIPP was used as a binary variable (detected versus non-detected) due to its low detection frequency. To investigate respiratory associations with high OPEs levels at multiple time points, we dichotomized the exposures into categories < or ≥ GM for each time point.
We determined time-specific associations between urinary OPEs and the respiratory outcomes using multiple informant models with GEEs for respiratory symptoms and with mixed linear regressions for lung function. We then estimated the exposure-outcomes associations for each OPE for high compared with low exposures at 16 and 26 weeks, at 16 weeks and delivery, at 26 weeks and delivery, and at 16 and 26 weeks and delivery at all time points. We adjusted all models for child’s birth weight, gestational age, family household income at baseline, duration of breastfeeding, prenatal exposure to cigarette smoking measured by maternal serum cotinine, and total dust weight, used as continuous variables, as well as for child’s sex, child race/ethnicity, and maternal and paternal allergy used as categorical variables. All these factors were previously reported to be associated with exposure and/or outcomes (Rosas-Salazar & Hartert, 2017; Mendy al., 2022b; Mendy et al., 2022c). We tested sex for effect modification on the association of the exposures with wheeze and hay fever/allergies and stratified analyses by sex were performed when we found significant effect modification. Effect modification testing on the association with respiratory infections was not examined because of the smaller number of outcome events limiting subgroup analyses. We performed the analyses in SAS (SAS Institute, Cary, NC), with p < 0.05 considered significant in all analyses.
Results
Descriptive Results
Among the 342 children included in the study, OPEs dust concentrations and loadings were highest for TCIPP, followed by TDCIPP, TPHP, and TCEP. For RBFRs, dust concentrations and loadings were higher for BEH-TEBP than for EH-TBB. Throughout pregnancy, urinary levels of DPHP were consistently the highest of all OPEs, followed by those of BDCIPP and BCEP. These OPEs were detected in 84.1% to 99.4% of the urinary samples; BCIPP was only detected in 12.5% to 32.7% of the samples (Table 1). Although TCIPP had the highest concentrations and loadings in dust, its metabolites in urine (BCIPP) was the lowest of all urinary OPEs. Urinary metabolites for TPHP (DPHP) were the highest of all urinary OPEs, while dust TPHP was lower than dust TCIPP and TDCIPP.
Table 1:
Distribution of dust OPEs and RBFRs and Urinary OPEs (N = 342)
| Exposure | Parent Compound | LOD or machine reading limita | % Detected | GM (SE) | 5th-95th percentile |
|---|---|---|---|---|---|
| Dust concentrations (μg/g dust) | |||||
| TCEP | TCEP | 0.10 | 78.8 | 0.67 (0.07) | 0.06 – 9.31 |
| TCIPP | TCIPP | 0.10 | 97.4 | 2.05 (0.17) | 0.23 – 22.56 |
| TDCIPP | TDCIPP | 0.10 | 97.4 | 1.87 (0.15) | 0.25 – 13.20 |
| TPHP | TPHP | 0.10 | 98.2 | 1.29 (0.10) | 0.17 – 11.19 |
| EH-TBB | EH-TBB | 0.002 | 100.0 | 0.06 (0.004) | 0.01 – 0.57 |
| BEH-TEBP | BEH-TEBP | 0.002 | 100.0 | 0.14 (0.01) | 0.02 – 0.73 |
| Dust loadings (mg/m2) | |||||
| TCEP | TCEP | – | – | 0.15 (0.02) | 0.01 – 3.34 |
| TCIPP | TCIPP | – | – | 0.45 (0.04) | 0.06 – 9.24 |
| TDCIPP | TDCIPP | – | – | 0.41 (0.04) | 0.04 – 5.73 |
| TPHP | TPHP | – | – | 0.28 (0.02) | 0.03 – 2.41 |
| EH-TBB | EH-TBB | – | – | 0.01 (0.001) | 0.001 – 0.16 |
| BEH-TEBP | BEH-TEBP | – | – | 0.03 (0.003) | 0.003 – 0.25 |
| Urinary OPEs (ug/L) | |||||
| At 16 weeks gestation | |||||
| BCEP | TCEP | 0.10 | 88.5 | 0.54 (0.04) | 0.07 – 5.19 |
| BCIPP | TCIPP | 0.10 | 32.7 | – | – |
| BDCIPP | TDCIPP | 0.10 | 95.7 | 0.69 (0.04) | 0.11 – 4.72 |
| DPHP | TPHP | 0.10 | 98.9 | 1.54 (0.10) | 0.23 – 9.70 |
| At 26 weeks gestation | |||||
| BCEP | TCEP | 0.10 | 84.1 | 0.50 (0.04) | 0.07 – 7.45 |
| BCIPP | TCIPP | 0.10 | 18.8 | – | – |
| BDCIPP | TDCIPP | 0.10 | 89.4 | 0.54 (0.04) | 0.07 – 4.85 |
| DPHP | TPHP | 0.10 | 97.6 | 1.10 (0.08) | 0.15 – 6.16 |
| At delivery | |||||
| BCEP | TCEP | 0.10 | 87.5 | 0.54 (0.04) | 0.07 – 7.09 |
| BCIPP | TCIPP | 0.10 | 12.5 | – | – |
| BDCIPP | TDCIPP | 0.10 | 92.5 | 0.62 (0.05) | 0.07 – 5.83 |
| DPHP | TPHP | 0.10 | 99.4 | 1.52 (0.10) | 0.23 – 11.26 |
| Average | |||||
| BCEP | TCEP | 0.10 | – | 0.72 (0.05) | 0.13 – 6.98 |
| BCIPP | TCIPP | 0.10 | – | – | – |
| BDCIPP | TDCIPP | 0.10 | – | 0.81 (0.05) | 0.16 – 4.55 |
| DPHP | TPHP | 0.10 | – | 1.79 (0.09) | 0.46 – 7.31 |
Abbreviations: GM: geometric mean, SE: standard error, TCEP: tris(2-chloroethyl) phosphate, TCIPP: tris(1-chloro-2-propyl) phosphate, TDCIPP: tris(1,3-dichloroisopropyl) phosphate, TPHP: triphenyl phosphate, EH-TBB: 2-ethylhexyl-2,3,4,5-tetrabromobenzoate, BEH-TBP: bis(2-ethylhexyl) tetrabromophthalate; BCEP: bis-2-chloroethyl phosphate, BCIPP: bis(1-chloro-2-propyl) phosphate, BDCIPP: bis(1,3-dichloro-2-propyl) phosphate, DPHP: diphenyl phosphate. Urinary OPEs adjusted for specific gravity.
LOD used for OPEs or machine reading limits used for RBFRs
Prenatal house dust TCEP was higher in non-Hispanic White or Hispanic/Other children and in those with a household income ≥ $75,000. Prenatal house dust TCIPP was higher in non-Hispanic White children, whereas prenatal house dust TPHP was higher in girls than in boys. Prenatal dust EH-TBB was higher in children with low; Prenatal dust BEH-TEBPs was higher in non-Hispanic White children, in breastfed children, and in those with serum cotinine <10 ng/ml. However, urinary OPEs were higher in non-Hispanic Black or Hispanic/Other race/ethnicity children, in those with low birth weight, and in those with an annual household income <$50,000 for BCEP. They were also higher in breastfed children for DPHP and in those with high prenatal exposure to smoking for BCEP and DPHP (Table 2). The distribution of dust chemicals’ loadings by the participants’ characteristics was similar to that of urinary OPEs (Supplemental Table 1).
Table 2:
Dust OPEs and RBFRs and urinary OPEs in pregnant mothers overall and by children’s characteristics – HOME Study (N = 342)
| Characteristics | % | Dust Flame-Retardant Concentrations (μg/g dust) | Urinary OPEs adjusted for specific gravity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPEs | RBFRs | ||||||||||
| TCEP | TCIPP | TDCIPP | TPHP | EH-TBB | BEH-TEBP | BCEP | BCIPP | BDCIPP | DPHP | ||
| Child’s sex | |||||||||||
| Female | 55.3 | 0.65 (0.08) | 2.03 (0.22) | 1.81 (0.19) | 1.38 (0.13) | 0.06 (0.01) | 0.13 (0.01) | 0.72 (0.07) | 0.10 (0.01) | 1.09 (0.09) | 1.79 (0.12) |
| Male | 44.7 | 0.67 (0.09) | 2.17 (0.28) | 1.65 (0.18) | 1.12 (0.12) | 0.06 (0.01) | 0.13 (0.01) | 0.72 (0.07) | 0.11 (0.01) | 0.99 (0.09) | 1.79 (0.14) |
| Race/ethnicity | |||||||||||
| NH-White | 64.3 | 0.80 (0.09) | 2.65 (0.28) | 1.94 (0.19) | 1.28 (0.12) | 0.07 (0.01) | 0.17 (0.01) | 0.60 (0.05) | 0.11 (0.01) | 0.99 (0.08) | 1.68 (0.11) |
| NH-Black | 28.9 | 0.41 (0.06) | 1.41 (0.20) | 1.38 (0.19) | 1.20 (0.16) | 0.05 (0.01) | 0.08 (0.01) | 1.03 (0.14) | 0.10 (0.004) | 1.17 (0.14) | 2.08 (0.17) |
| Hispanic/Othera | 6.7 | 0.91 (0.34) | 1.47 (0.38) | 1.71 (0.48) | 1.29 (0.43) | 0.05 (0.01) | 0.10 (0.03) | 1.13 (0.34) | 0.10 (0.01) | 1.05 (0.22) | 1.95 (0.37) |
| Term | |||||||||||
| Preterm | 9.4 | 0.46 (0.12) | 1.64 (0.34) | 2.03 (0.36) | 1.41 (0.30) | 0.07 (0.01) | 0.14 (0.02) | 0.50 (0.10) | 0.12 (0.02) | 1.13 (0.25) | 1.71 (0.27) |
| Normal term | 90.6 | 0.68 (0.07) | 2.15 (0.19) | 1.71 (0.14) | 1.24 (0.10) | 0.06 (0.01) | 0.13 (0.01) | 0.74 (0.06) | 0.10 (0.004) | 1.03 (0.07) | 1.80 (0.09) |
| Birth weight | |||||||||||
| Low | 5.6 | 0.52 (0.17) | 1.55 (0.42) | 2.46 (0.66) | 2.19 (0.69) | 0.11 (0.03) | 0.18 (0.04) | 0.77 (0.20) | 0.10 (0.01) | 1.24 (0.33) | 1.98 (0.47) |
| Normal | 79.5 | 0.69 (0.07) | 2.23 (0.21) | 1.73 (0.15) | 1.16 (0.09) | 0.05 (0.01) | 0.12 (0.01) | 0.66 (0.05) | 0.11 (0.01) | 1.01 (0.07) | 1.70 (0.09) |
| High | 14.9 | 0.52 (0.15) | 1.66 (0.36) | 1.51 (0.32) | 1.57 (0.34) | 0.07 (0.02) | 0.15 (0.03) | 1.10 (0.20) | 0.10 (0.01) | 1.14 (0.16) | 2.25 (0.26) |
| Household income | |||||||||||
| <$50,000 | 43.6 | 0.51 (0.07) | 1.73 (0.23) | 1.67 (0.22) | 1.19 (0.13) | 0.05 (0.01) | 0.11 (0.01) | 1.01 (0.10) | 0.10 (0.005) | 1.09 (0.10) | 2.10 (0.16) |
| $50,000 to $74,999 | 16.9 | 0.74 (0.15) | 2.41 (0.38) | 1.85 (0.29) | 1.43 (0.26) | 0.06 (0.01) | 0.14 (0.02) | 0.76 (0.13) | 0.11 (0.01) | 1.10 (0.13) | 1.59 (0.18) |
| $75,000+ | 39.5 | 0.83 (0.13) | 2.44 (0.31) | 1.76 (0.19) | 1.26 (0.14) | 0.06 (0.01) | 0.15 (0.02) | 0.51 (0.05) | 0.11 (0.01) | 0.97 (0.10) | 1.62 (0.13) |
| Breastfed | |||||||||||
| No | 17.0 | 0.64 (0.14) | 1.54 (0.29) | 1.56 (0.31) | 0.98 (0.19) | 0.05 (0.01) | 0.08 (0.01) | 0.73 (0.13) | 0.09 (0.005) | 1.30 (0.21) | 2.41 (0.30) |
| Yes | 83.0 | 0.66 (0.07) | 2.23 (0.20) | 1.78 (0.15) | 1.32 (0.10) | 0.06 (0.01) | 0.14 (0.01) | 0.72 (0.11) | 0.11 (0.01) | 0.99 (0.06) | 1.69 (0.09) |
| Serum cotinine (ng/mL) | |||||||||||
| < 0.10 | 68.1 | 0.70 (0.08) | 2.28 (0.21) | 1.78 (0.16) | 1.27 (0.11) | 0.07 (0.01) | 0.15 (0.01) | 0.63 (0.05) | 0.11 (0.01) | 1.02 (0.07) | 1.64 (0.10) |
| 0.10 to 10 | 23.4 | 0.62 (0.12) | 1.97 (0.39) | 1.71 (0.29) | 1.27 (0.19) | 0.05 (0.01) | 0.10 (0.01) | 0.94 (0.14) | 0.11 (0.01) | 1.04 (0.13) | 2.18 (0.21) |
| > 10 | 8.5 | 0.44 (0.13) | 1.22 (0.34) | 1.50 (0.42) | 1.09 (0.26) | 0.04 (0.01) | 0.09 (0.02) | 1.08 (0.25) | 0.09 (0.005) | 1.20 (0.26) | 2.29 (0.34) |
Abbreviation: NH: Non-Hispanic; TCEP: tris(2-chloroethyl) phosphate, TCIPP: tris(1-chloro-2-propyl) phosphate, TDCIPP: tris(1,3-dichloroisopropyl) phosphate, TPHP: triphenyl phosphate, EH-TBB: 2-ethylhexyl-2,3,4,5-tetrabromobenzoate, BEH-TBP: bis(2-ethylhexyl) tetrabromophthalate; BCEP: bis-2-chloroethyl phosphate, BCIPP: bis(1-chloro-2-propyl) phosphate, BDCIPP: bis(1,3-dichloro-2-propyl) phosphate, DPHP: diphenyl phosphate.
‘Other’ race/ethnicity included Native Hawaiian or Other Pacific Islander or Asian participants
Bold indicates significant difference in dust flame retardant concentrations or loadings across participants’ characteristics.
Intercorrelations between dust OPEs and RBFRs and Urinary OPEs and Intraclass Correlations
The intercorrelation between dust RBFRs concentrations (Figure 1A), between dust RBFRs loadings (Figure 1B), and the correlations of dust OPEs and RBFRs concentrations with their loadings (Figure 1C) were moderate to high (r ≥ 0.50). The remaining correlations between different urinary OPEs and between urinary OPEs and dust OPE concentrations or loadings were low (Figures 1D, 1E, & 1F). The ICC between urinary OPEs across the different sampling periods was low (0.38 for BCEP, 0.32 for BCIPP, 0.32 for BDCIPP, and 0.22 for DPHP), indicating poor reproducibility.
Figure 1:
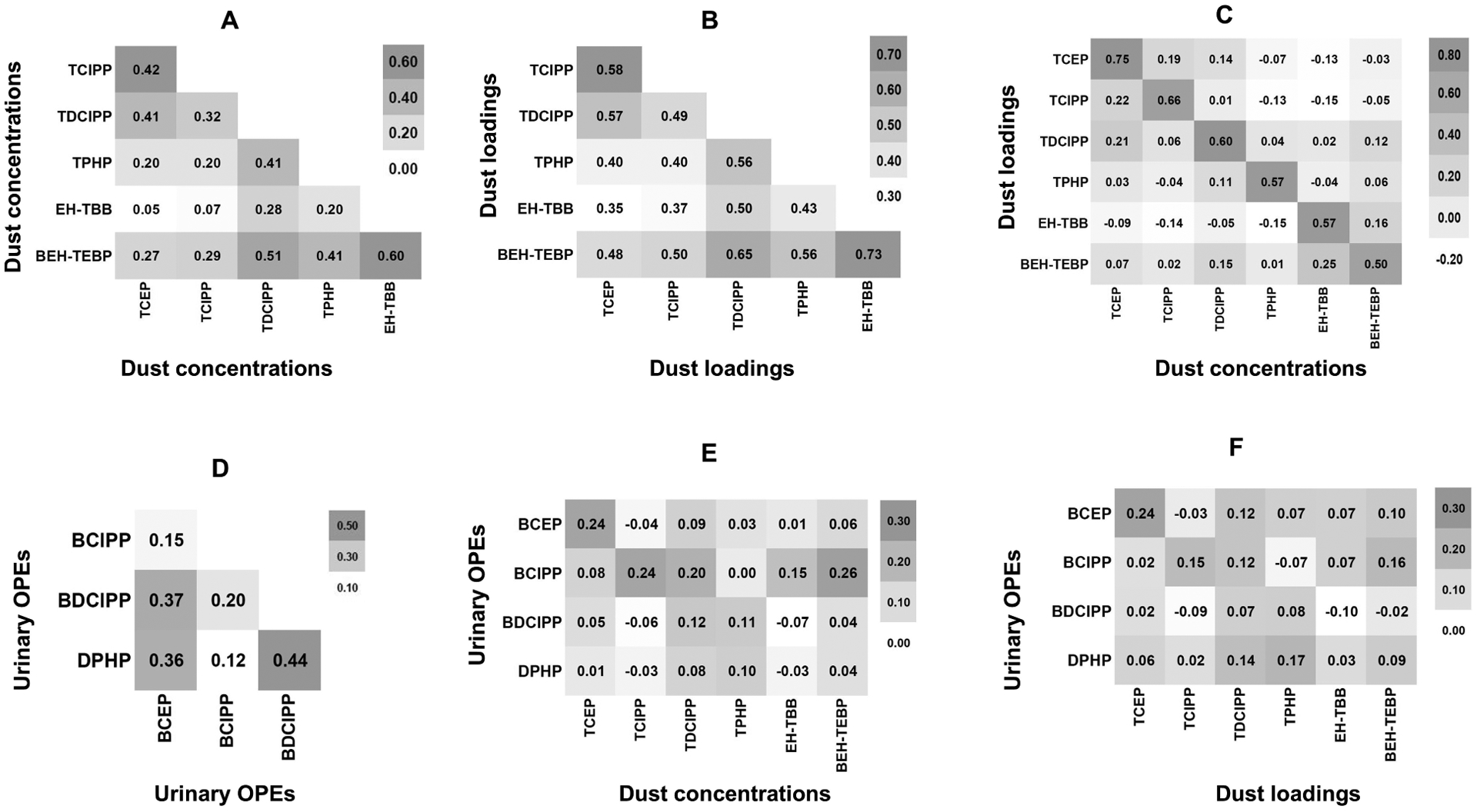
Heatmap for intercorrelation between dust OPEs and RBFRs and urinary OPEs
Dust OPEs and RBFRs, Urinary OPEs and Respiratory Outcomes
The risk of wheezing during childhood increased with prenatal dust concentrations of TPHP (RR: 1.40, 95% CI: 1.18–1.66), EH-TBB (RR: 1.51, 95% CI: 1.23–1.85), and BEH-TEBP (RR: 1.57, 95% CI: 1.28–1.94), while the risk of respiratory infections increased with higher prenatal dust TPHP concentrations (RR: 1.43, 95% CI: 1.08–1.94). Prenatal dust TCEP concentrations were associated with higher risk of hay fever/allergies (RR: 1.11, 95% CI: 1.01–1.21). Similar associations were found for dust OPEs and RBFRs loadings. However, TPHP and/or RBFRs dust loadings were associated with attenuated risks of wheeze (23% to 28%) or respiratory infections (31%). Additionally, the association of TPHP dust loadings with hay fever or allergies reached significance (RR: 1.13, 95% CI: 1.00–1.25) (Figure 2).
Figure 2:
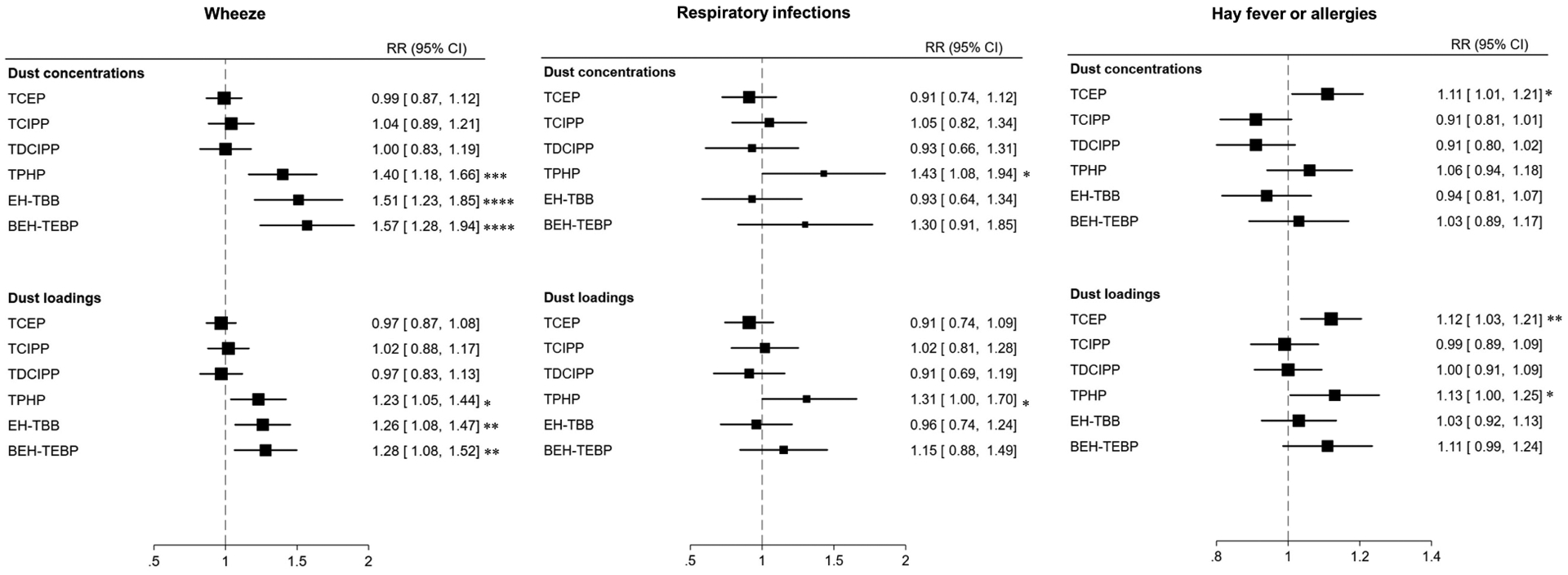
Forest plots for the association of dust OPEs and RBFRs with wheeze, respiratory infections, and hay fever or allergies. Models adjusted for child’s sex, child’s race/ethnicity, birth weight, gestational term, family income, and child receiving breast milk. *P < 0.05 ** P < 0.01 ***P < 0.001 ****P < 0.0001
The association of urinary OPEs with reported respiratory outcomes was dependent on the timing of sampling during pregnancy. Urinary BCIPP detection at 16 weeks of gestation (RR: 1.21, 95% CI: 1.03–1.43), 26 weeks of gestation (RR: 1.37, 95% CI: 1.14–1.65), or at delivery (RR: 1.43, 95% CI: 1.15–1.77) was associated with higher risk of wheeze. Urinary BDCIPP levels at 16 weeks of gestation were associated with higher risk of wheeze (RR: 1.29, 95% CI: 1.07–1.56), but associations were null at other time points. Urinary BCEP and BDCIPP levels at 16 weeks of gestation were associated with respiratory infections (RR: 1.43, 95% CI: 1.08–1.90 and 1.59, 95% CI: 1.08, 2.33, respectively), while no associations were observed at other time points. Lower risk of hay fever/allergies were observed with urinary BCIPP detection (RR: 0.67, 95% CI: 0.54–0.84) and DPHP levels (RR: 0.85, 95% CI:0.77–0.95) at delivery, but there were no associations for urinary BCIPP and DPHP sampled at other time points (Figure 3).
Figure 3:
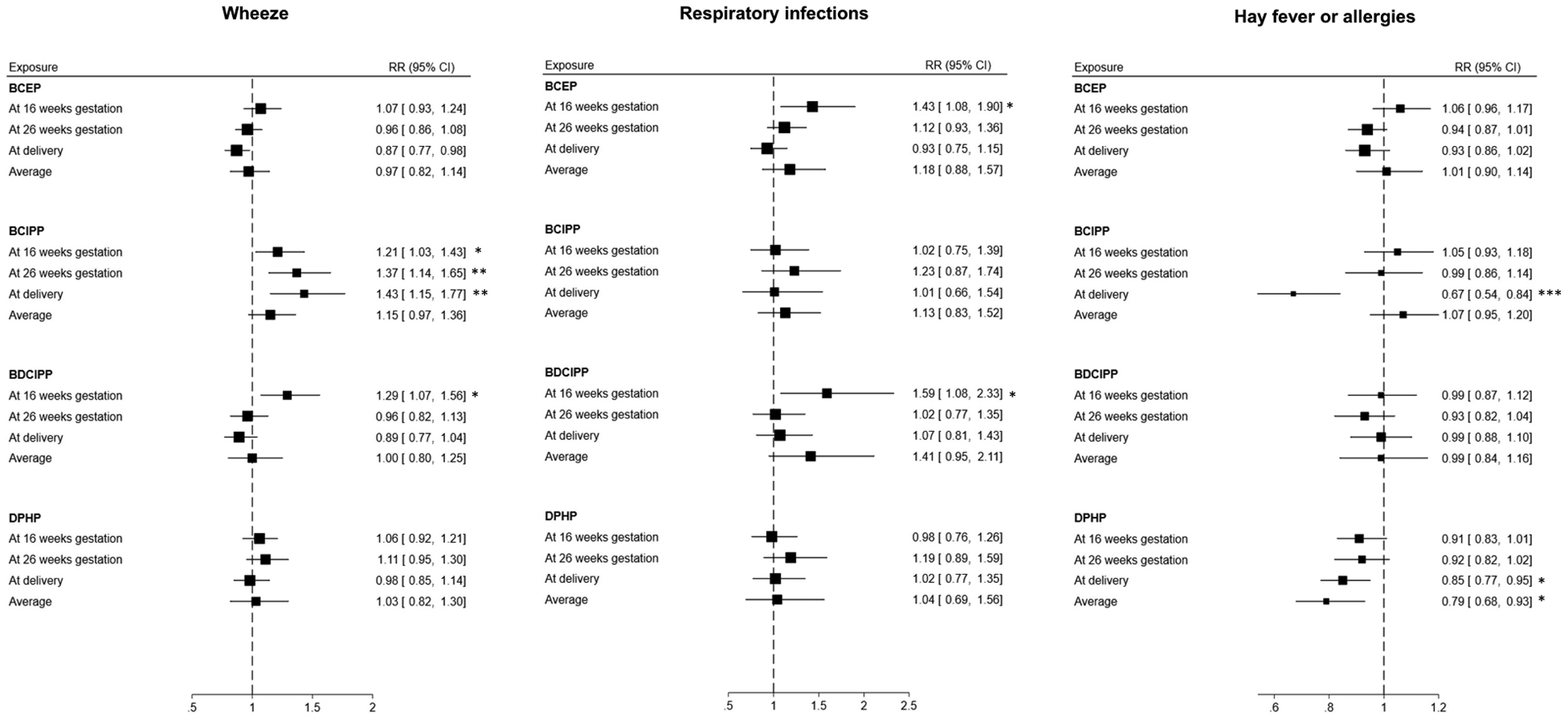
Forest plots for the association of urinary OPEs by times of sampling with wheeze, respiratory infections, and hay fever or allergies. Models adjusted for child’s sex, child’s race/ethnicity, birth weight, gestational term, family income, and child receiving breast milk. *P < 0.05 **P < 0.01 ***P < 0.001
Prenatal dust TCEP loadings (β: −6.17, 95% CI: −11.09, −1.25) were associated with lower FEV1 % at age 5 years [indicative of a detrimental association]. However, urinary BCEP at 26 weeks of gestation (β: 4.88, 95% CI: 0.04–9.73) and DPHP at delivery (β: 8.29, 95% CI: 1.33, 15.26) were associated with higher FEV1 % [indicative of beneficial associations] (Figure 4).
Figure 4:
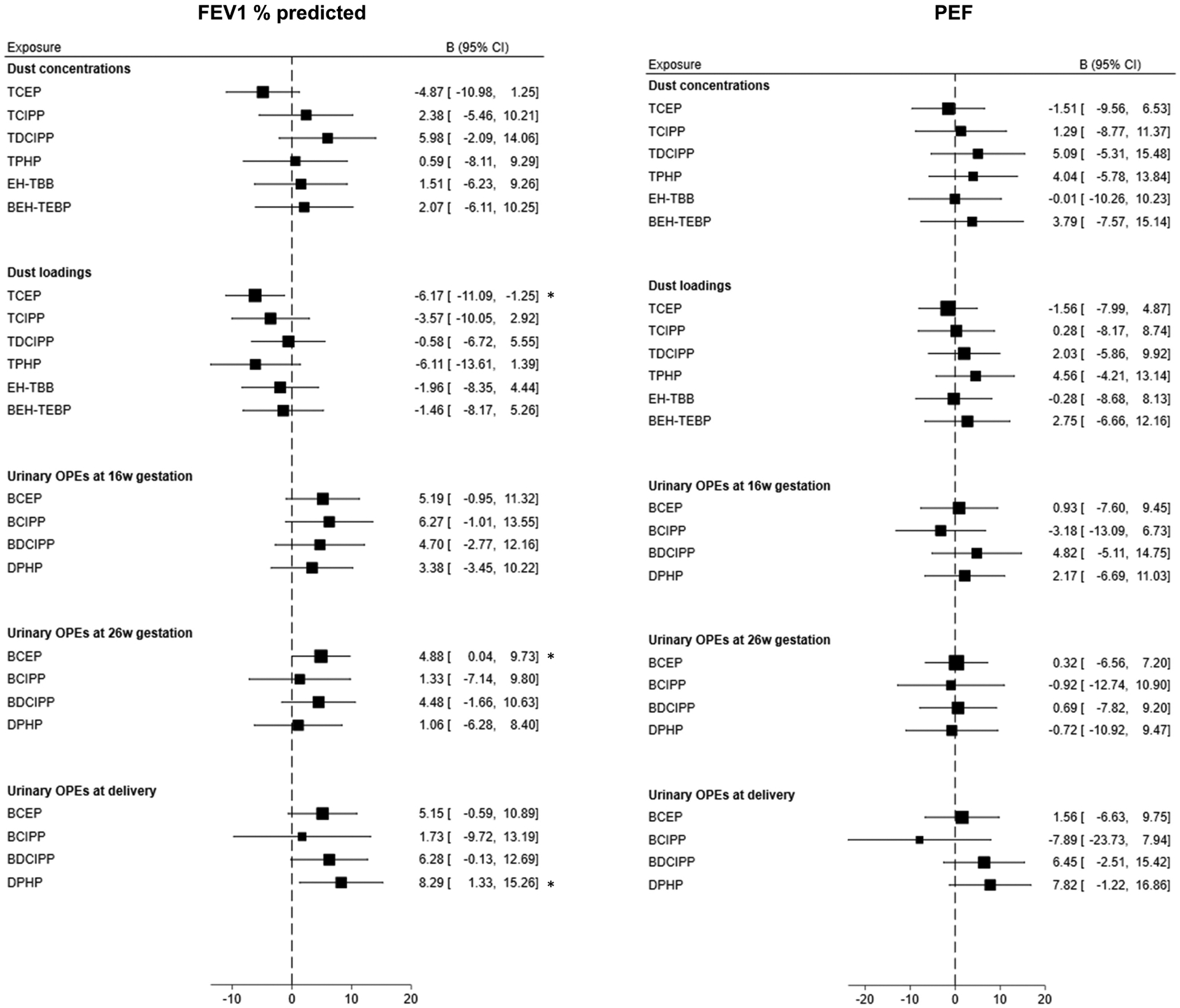
Forest plots for the association of dust OPEs and RBFRs and urinary OPEs with FEV1 % predicted and PEF. Models adjusted for child’s sex, child’s race/ethnicity, birth weight, gestational term, family income, and child receiving breast milk. *P < 0.05
High Urinary OPEs at Multiple Time Points and Respiratory Outcomes
High urinary BCEP concentrations at gestational weeks 16 and 26 were associated with higher risk of wheeze (RR: 1.63, 95% CI: 1.12–2.36). High urinary BCEP and BDCIPP concentrations at gestational weeks 16 and 26 were associated with a higher risk of respiratory infections (RR: 1.78, 95% CI: 1.04–3.07, and 3.38, 95% CI: 1.27–9.00, respectively). High BCEP at week 16 of gestation and at delivery was associated with higher risk of wheeze (RR: 1.88, 95% CI: 1.18–2.99) and hay fever/allergies (RR: 1.65, 95% CI: 1.08–2.51). High urinary concentrations of some OPEs across all time points were associated higher risk of wheeze (RR: 1.51, 95% CI: 1.05–2.18 for DPHP), respiratory infections (RR: 3.53, 95% CI: 1.36–9.14 for BDCIPP) and hay fever/allergies (RR: 1.49, 95% CI: 1.04–2.14 for BDCIPP) (Figure 3).
Effect modification by sex
In effect modification testing, the association of prenatal dust EH-TBB concentrations and loadings (Pinteraction<0.001 for both) and dust TPHP loadings (Pinteraction=0.04), as well as the association of hay fever/allergies with dust TCEP concentrations and loadings (Pinteraction<0.001 for both) and dust TCIPP loadings (Pinteraction=0.01) differed by sex. Dust EH-TBB concentrations (RR: 2.36, 95% CI: 1.88–2.97) and loadings (RR: 1.86, 95% CI: 1.36–2.55) and dust TPHP concentrations (RR: 2.13, 95% CI: 1.66–2.72) were associated with higher risk in males but not in females. However, dust TCEP concentrations (RR: 1.29, 95% CI: 1.16–1.44) and loadings (RR:1.33, 95% CI: 1.21–1.47) were associated with higher risk of hay fever/allergies if females but not in males. Dust TCIPP loading were not associated with hay fever/allergies in males or females (Supplemental Table 2). The associations of dust OPEs and RBFRs and urinary OPEs with FEV1% predicted or PEF did not differ by sex (Supplemental Table 3).
Discussion
This is the first study to examine the associations of in-utero exposure to OPEs and RBFRs with respiratory outcomes in childhood. We found that dust TPHP, TCEP and RBFRs were associated with higher risk of wheeze, respiratory infections, and hay fever/allergies. Additionally, dust TCEP was associated with higher risk of hay fever/allergies in females, but not in males. Urinary BCIPP at all time points as well as urinary BCEP and BDCIPP at 16 weeks of gestation were associated with higher risk of wheeze and/or respiratory infections. However, we found inverse association of BCEP at delivery with wheeze as well as of BCIPP and DPHP at delivery with hay fever/allergies.
Prenatal Dust OPEs Exposure and Adverse Respiratory Outcomes
Evidence of potential adverse health effects of prenatal exposure to OPEs in humans is scarce and limited to reports of associations with neurodevelopmental outcomes and with low birth weight, especially when exposure occurred during the second semester of pregnancy (Luo et al., 2020; Luo et al., 2021). However, animal and in-vitro studies suggest that prenatal OPEs such as TPHP may cause adverse pregnancy outcomes after placental accumulation through several processes that involve PPARγ activation, lipid accumulation, placental function impairment, hormone secretion, cell apoptosis, and endoplasmic reticulum stress (Hong et al., 2022). Consistent with our finding of a strong association between in utero TPHP exposure and childhood wheeze, PPARγ activation could interfere with alveolar type II cell function necessary for alveolar repair; epigenetic changes in the PPARγ gene may also lead to the differentiation of alveolar mesenchymal fibroblasts into myofibroblasts to cause chronic lower respiratory diseases (Guo et al., 2022; Hong et al., 2022). Likewise, endoplasmic reticulum stress can cause inflammation, hypersecretion of mucus in airways as well as airway hyperresponsiveness and remodeling, and epithelial cell apoptosis, all of which play a role in the pathogenesis of chronic lower respiratory diseases such as asthma (Miao et al., 2020). Moreover, TPHP, TCEP, TDCIPP reportedly act as ER-α agonists and may cause increased estradiol synthesis through various enzymatic pathways (Ji et al., 2022; Sutha et al., 2022). Other chemicals with similar endocrine-disrupting activity have been shown to have immune effects such as Th1/Th2 cells modifications, reduction in T regulatory cells, TH17 changes, impaired innate immunity, higher B-cells, and higher expression of genes related to immunity (Casas & Gascon, 2020).
Prenatal Dust RBFRs Exposure and wheezing
This study is the first to report adverse respiratory effects of prenatal dust RBFRs exposure in humans, despite demonstrated placental transfer of the chemicals (Phillips et al., 2016). In vitro, EH-TBB has been shown to exhibit antiestrogenic effects in the yeast estrogen screen assay, while BEH-TEBP produced antiandrogenic effects in the yeast androgen screen assay and both EH-TBB and BEH-TEBP increased estradiol levels (Saunders et al., 2013). In animal models, prenatal exposure to brominated flame-retardant chemicals such as PBDEs induced epigenetic changes in asthma related genes such as Bdnf and Tnfa (Vuong et al., 2017). Bdnf is expressed in the lungs and in neurons from the parasympathetic nervous system which plays a role in breathing, mucus secretion, airway smooth muscle contraction, and coughing reflex (Aven & Ai, 2013). During embryogenesis, Bdnf acts as a target-derived neurotrophic signal for airway smooth muscle extrinsic innervation (Aven & Ai, 2013). The Tnfa gene codes for tumor necrosis factor (TNF)-α, a modulator of immune and inflammatory responses (Noguchi et al., 2002). It is found in the human major histocompatibility complex of the chromosome 6p21.3, purportedly associated with allergic asthma. A polymorphism in the promoter region of the Tnfa gene (308G/A) has also previously been linked to asthma and airway hyperreactivity (Noguchi et al., 2002). Furthermore, both EH-TBB and BEH-TEBP can induce oxidative and endoplasmic reticulum stress, leading to increased free radicals’ levels and reduced antioxidant capacity that can cause lipid peroxidation and ultimately histopathological changes in lung tissues as well as cell and tissue damage (Salo et al., 2022).
Respiratory Outcomes associated with Urinary OPEs Depending on Exposure Timing
We observed differential associations between urinary OPEs and respiratory outcomes by timing of sampling during pregnancy. Few studies have examined the differences in association of respiratory conditions with environmental exposures occurring at different periods of pregnancy. For instance, a Dutch birth cohort reported increased odds of persistent wheeze and of asthma in 6-year-old children if their mothers smoked cigarettes throughout the pregnancy, but not when they smoked only in the third trimester (Rosas-Salazar & Hartert, 2017). In a Chinese cohort, ambient air pollution was associated with higher odds of eczema when exposure happened during the first trimester, with higher odds of asthma when exposure occurred in the second trimester, and with allergic rhinitis when the exposure was documented in the third trimester (Deng et al., 2016). In a U.S. cohort, exposure to particulate matter ≤ 2.5 μm at 16 to 25 weeks of gestation was associated with increased risk of asthma by the age of 6 years (Leon Hsu et al., 2015). In Spain, NO2 exposure in the second or third trimesters of pregnancy was associated with increased eczema risk of lower respiratory tract infections in infants aged 12–18 months (Aguilera et al., 2013). The reason for respiratory outcomes being associated with some chemicals when exposure occurred early in pregnancy but with others when the exposure occurred later in pregnancy is unknown. Exposure to some chemicals before 18 weeks of gestation may retard growth of tracheobronchial tree and large blood vessels, whereas chemical exposures occurring later in the pregnancy retard growth of pulmonary capillaries and lung tissue (Sly & Flack, 2008). Some studies suggest that the Th1/Th2 alterations causing allergic diseases that may result from environmental chemicals are more likely to happen when exposure occurs early in pregnancy versus later (Prescott et al., 2003). Our results of an inverse association between OPEs and allergy outcomes are consistent with the findings of Donohue et al. who reported lower risk of asthma with bisphenol A (Donohue et al., 2013). They hypothesized that maternal urinary chemical concentrations late in pregnancy may underestimate fetal exposure or that the late pregnancy might not be a relevant window of vulnerability for this outcome (Donohue et al., 2013).
Sex-Specific Associations of Dust TCEP, TPHP and EH-TBB with Wheeze and Allergy
We observed that associations of dust EH-TBB and TPHP with wheeze as well as the associations of dust TCEP and TCIPP with hay fever/allergies were sex-specific. TPHP dust loadings as well as EH-TBB dust concentrations and loadings were association with wheeze in males but not in females, whereas TCEP dust concentrations and loadings were associated with hay fever/allergies in females but not in males. Consistent with our findings on sex-specific association with wheeze, other xenoestrogens such as bisphenol S have been reported to be associated with asthma in males but not in females (Mendy et al., 2020). Likewise, environmental phenols and phthalates were also found to be associated with asthma in males, but not in females (Buckley et al., 2018). Also similar to the sex-specific associations we found with hay fever/allergies, bisphenol A has been observed to be associated with atopic disorders predominantly in females (Wang et al., 2016; Zhou et al., 2017). The underlying mechanisms for these sex-specific associations are not fully understood. A possible hypothesis is the mediation by endogenous hormones on the effects of xenoestrogens on the immune system. Xenoestrogens could interfere with and inhibit androgen production and function, which could explain the associations with wheeze being predominant in males (Mendy et al., 2020). On the other hand, xenoestrogens purportedly induce a higher production of B-cell-activating factor in females than in males, which may result in higher B-cell survival and maturation and higher antibody production in females than in males (Mendy et al., 2020).
Dust versus Urinary OPEs and Respiratory Outcomes
Among OPEs, we found that dust TPHP was associated with higher risk of wheeze, respiratory infections, as well as hay fever or allergy and dust TCEP was associated with higher risk of hay fever or allergy and lower FEV1 % predicted. Dust TCIPP and TDCIPP were not associated with any respiratory outcome. Consistent with the associations observed for dust, the urinary metabolite TCEP (BCEP) at 16 weeks was associated with risk of respiratory infections. However, in contrast to the results observed for dust TCIPP, urinary metabolites of this chemical (BCIPP) were associated with higher risk of wheeze, whether they were sampled at 16 or 26 weeks of gestation or at delivery. Likewise, dust TDCIPP was not associated with not associated with respiratory outcomes, while the urinary metabolite for TDCIPP (BDCIPP) at 16 weeks of gestation was associated with higher risk of wheeze and respiratory infections. Despite the association of dust TPHP with wheeze, respiratory infections, and hay fever or allergy, urinary TPHP metabolite (DPHP) at delivery was associated with lower risk of haver or allergies. The reason for these discrepancies is unknown. We found low correlation between dust OPEs and their urinary levels and there was low reproducibility in urinary OPEs across the different sampling timing points. Although dust OPEs may reflect long-term exposure to these chemicals, they only capture exposure to indoor sources and may not account for outdoor and dietary sources (Mitro et al., 2016). Urinary OPEs on the other hand, capture personal OPE exposure regardless of sources but may only reflect short term exposure due to the short half-lives (Kuiper et al., 2020). In addition to the different sources, other factor that could explain the low correlation between dust and urinary OPEs include interindividual variations in toxicokinetics (absorption, metabolism, and activity pattern) or differences in environmental matrices containing the OPEs (Hou et al., 2021). In a same individual, urinary OPE metabolites have also been reported to vary by sampling time (Wang et al., 2019). Albeit urinary OPE’s short half-lives, they were useful for determining the variation in the risk of outcome across different sampling windows during pregnancy.
Limitations and strengths
Our study had some limitations. Dust OPEs and RBFRs were sampled only once at the baseline of the study and not repeatedly at different time points of the pregnancy. However, there are indications that chemicals’ concentrations measured in settled dust are representative of long-term exposures (Whitehead et al., 2012). RBFRs levels were only measured in house dust and not in any biological sample due to the lack of reliable exposure biomarkers. Therefore, house dust RBFRs only reflected domestic indoor exposure and did not capture exposure from outdoor or other indoor sources. We had limited power to examine the association of high urinary OPEs at multiple time points of urinary sampling with lung function at child age 5 years. Also, depending on when urinary samples were collected around delivery (before, during or after), they may be less representative of in utero exposure. As for any observational study, we cannot rule-out completely residual confounding and dust flame retardants and urinary OPEs could be correlated with other co-exposures also associated with respiratory outcomes. Our statistical analysis included multiple testing which might have increased the probability of incidental findings at α level of significance < 0.05. However, some of our results such as the association of dust TPHP and RBFRs with wheeze were highly significant and not likely to be explained by chance. Nonetheless, the prospective design and multiple exposure assessment as well as repeated outcome measures are major strengths of this study. It also combines dust assessment of exposure to OPEs and RBFRs as well as urinary OPE measurements at different timepoints during pregnancy that allowed us to identify potential windows of susceptibility for the studied exposure-outcome relationships. We investigated prenatal exposure to both dust concentrations and loading of OPEs and RBFRs since there are reports that dust loadings may be better exposure predictors that account for the dust chemicals’ concentrations as well as the amount of dust amount per sampled area, and we found consistent results (De Voogt, 2015). We additionally observed that dust TCEP dust loadings but not concentrations were associated with lower FEV1 %. We adjusted for a wide variety of covariates such as socioeconomic and early childhood factors previously reported to be associated with the exposures and/or the outcomes, which minimized residual confounding. In addition to respiratory symptoms reported every six months until child age 5 years, our study included objective measures such as lung function.
Conclusions
In-utero OPEs and RBFRs exposure is associated with adverse respiratory outcomes and the relationship of prenatal OPEs with adverse respiratory outcomes is contingent to the timing of exposure during pregnancy. If the associations observed in the present study are causal, reducing prenatal exposure to the chemicals could prevent adverse respiratory outcomes in children and subsequent chronic conditions in adulthood.
Supplementary Material
Highlights.
In-utero exposure to replacement brominated flame retardants and organophosphate esters tends to be associated with adverse childhood respiratory outcomes.
Urinary bis(1-chloro-2-propyl) phosphate (BCIPP) detection was associated with higher risk of wheeze at any of the prenatal sampling time points.
Urinary bis(1,3-dichloro-2-propyl) phosphate (BDCIPP) at 16 weeks of gestation was associated with higher risk of wheeze.
Urinary bis-2-chloroethyl phosphate and BDCIPP at 16 weeks of gestation were associated with higher risk of respiratory infections
BDCIPP and diphenyl phosphate around delivery were associated with lower risk of hay fever or allergies
Funding:
This work was supported by the National Institute of Environmental Health Sciences (Grant Numbers R01ES034049, P01ES011261, R01ES014575, R01ES028277). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CRediT author statement
Angelico Mendy: Conceptualization, Formal analysis, Resources, Writing Original draft, Writing – Review & editing, visualization, funding acquisition. Zana Percy: Writing – Review & editing. Joseph M. Braun: Methodology, Investigation, Resources, Writing – Review & editing. Bruce Lanphear: Investigation, Resources, Writing – Review & editing. Mark J. La Guardia: Methodology, Writing – Review & editing. Robert Hale: Methodology, Writing – Review & editing. Kimberly Yolton: Methodology, Investigation, Resources, Writing – Review & editing. Aimin Chen: Methodology, Investigation, Resources, Writing – Review & editing.
Declaration of interests
Joseph M. Braun has been compensated as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera I, Pedersen M, Garcia-Esteban R, Ballester F, Basterrechea M et al. (2013). Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environmental Health Perspectives, 121(3), 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aven L, & Ai X (2013). Mechanisms of respiratory innervation during embryonic development. Organogenesis, 9(3), 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Behl M, Birnbaum LS, Diamond ML, Phillips A et al. (2019). Organophosphate ester flame retardants: Are they a regrettable substitution for polybrominated diphenyl ethers? Environmental Science & Technology Letters, 6(11), 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds RS, & Midoro-Horiuti T (2013). Estrogen effects in allergy and asthma. Current Opinion in Allergy and Clinical Immunology, 13(1), 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S et al. (2017). Cohort profile: The health outcomes and measures of the environment (HOME) study. International Journal of Epidemiology, 46(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Quirós-Alcalá L, Teitelbaum SL, Calafat AM, Wolff MS et al. (2018). Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environment international, 115, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, & Gascon M (2020). Prenatal exposure to endocrine-disrupting chemicals and asthma and allergic diseases. Journal of Investigational Allergology & Clinical Immunology, 30(4), 215–228. [DOI] [PubMed] [Google Scholar]
- De Voogt P (2015). Reviews of environmental contamination and toxicology volume 236 Springer. [Google Scholar]
- Deng Q, Lu C, Li Y, Sundell J, & Norbäck D (2016). Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environmental Research, 150, 119–127. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang S, Qu J, You H, & Liu D (2021). New understanding of novel brominated flame retardants (NBFRs): Neuro (endocrine) toxicity. Ecotoxicology and Environmental Safety, 208, 111570. [DOI] [PubMed] [Google Scholar]
- Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA et al. (2013). Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. Journal of Allergy and Clinical Immunology, 131(3), 736–742. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wu J, He Q, Zhang M, Li H, & Liu Y (2022). The potential role of PPARs in the fetal origins of adult disease. Cells, 11(21), 3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Jiang M, Guo L, Lin J, Wang Y, Tang H, & Liu X (2022). Prenatal exposure to triphenyl phosphate activated PPARγ in placental trophoblasts and impaired pregnancy outcomes. Environmental Pollution, 301, 119039. [DOI] [PubMed] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene, 5(1), 46–51. [Google Scholar]
- Hou M, Fang J, Shi Y, Tang S, Dong H et al. (2021). Exposure to organophosphate esters in elderly people: Relationships of OPE body burdens with indoor air and dust concentrations and food consumption. Environment International, 157, 106803. [DOI] [PubMed] [Google Scholar]
- Jayatilaka NK, Restrepo P, Davis Z, Vidal M, Calafat AM, & Ospina M (2019). Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere, 235, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Li N, Ma M, Li X, Zhu K et al. (2022). Comparison of the mechanisms of estrogen disrupting effects between triphenyl phosphate (TPhP) and tris (1, 3-dichloro-2-propyl) phosphate (TDCIPP). Ecotoxicology and Environmental Safety, 229, 113069. [DOI] [PubMed] [Google Scholar]
- Kuiper JR, Stapleton HM, Wills-Karp M, Wang X, Burd I et al. (2020). Predictors and reproducibility of urinary organophosphate ester metabolite concentrations during pregnancy and associations with birth outcomes in an urban population. Environmental health, 19, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon Hsu H, Mathilda Chiu Y, Coull BA, Kloog I, Schwartz J et al. (2015). Prenatal particulate air pollution and asthma onset in urban children. identifying sensitive windows and sex differences. American Journal of Respiratory and Critical Care Medicine, 192(9), 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares V, Bellés M, & Domingo JL (2015). Human exposure to PBDE and critical evaluation of health hazards. Archives of Toxicology, 89(3), 335–356. [DOI] [PubMed] [Google Scholar]
- Luo D, Liu W, Tao Y, Wang L, Yu M et al. (2020). Prenatal exposure to organophosphate flame retardants and the risk of low birth weight: A nested case-control study in china. Environmental Science & Technology, 54(6), 3375–3385. [DOI] [PubMed] [Google Scholar]
- Luo D, Liu W, Wu W, Tao Y, Hu L et al. (2021). Trimester-specific effects of maternal exposure to organophosphate flame retardants on offspring size at birth: A prospective cohort study in china. Journal of Hazardous Materials, 406, 124754. [DOI] [PubMed] [Google Scholar]
- Mendy A, Salo PM, Wilkerson J, Feinstein L, Ferguson KK et al. (2020). Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes. Environmental research, 183, 108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A, & Mersha TB (2022a). Comorbidities in childhood-onset and adult-onset asthma. Annals of Allergy, Asthma & Immunology, 129(3), 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A, Merianos AL, Mersha TB, & Mahabee-Gittens EM (2022b). Blood volatile organic compounds associated with non-reversible and reversible airflow obstruction in US adults. European Respiratory Journal, 60(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A, Burcham S, Merianos AL, Mersha TB, Mahabee-Gittens EM, Chen A, & Yolton K (2022c). Urinary volatile organic compound metabolites and reduced lung function in US adults. Respiratory Medicine, 205, 107053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A, Percy Z, Braun JM, Lanphear B, La Guardia MJ et al. (2023). Exposure to dust organophosphate and replacement brominated flame retardants during infancy and risk of subsequent adverse respiratory outcomes. Environmental Research, 235, 116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao K, Zhang L, Pan T, & Wang Y (2020). Update on the role of endoplasmic reticulum stress in asthma. American Journal of Translational Research, 12(4), 1168–1183. [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F et al. (2005). Standardization of spirometry. European Respiratory Journal, 26(2), 319–338. [DOI] [PubMed] [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF et al. (2016). Consumer product chemicals in indoor dust: a quantitative meta-analysis of US studies. Environmental science & technology, 50(19), 10661–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi E, Yokouchi Y, Shibasaki M, Inudou M, Nakahara, et al. (2002). Association between TNFA polymorphism and the development of asthma in the Japanese population. American Journal of Respiratory and Critical Care Medicine, 166(1), 43–46. [DOI] [PubMed] [Google Scholar]
- Ospina M, Jayatilaka NK, Wong L, Restrepo P, & Calafat AM (2018). Exposure to organophosphate flame-retardant chemicals in the US general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environment International, 110, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy Z, La Guardia MJ, Xu Y, Hale RC, Dietrich KN et al. (2020). Concentrations and loadings of organophosphate and replacement brominated flame retardants in house dust from the home study during the PBDE phase-out. Chemosphere, 239, 124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, & Stapleton HM (2016). Editor’s highlight: Transplacental and lactational transfer of firemaster® 550 components in dosed wistar rats. Toxicological Sciences, 153(2), 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, King B, Strong T, & Holt P (2003). The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy, 58(11), 1187–1194. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL et al. ERS Global Lung Function Initiative. (2012). Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. The European Respiratory Journal, 40(6), 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Salazar C, & Hartert TV (2017). Prenatal exposures and the development of childhood wheezing illnesses. Current Opinion in Allergy and Clinical Immunology, 17(2), 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo PM, Mendy A, Wilkerson J, Molsberry SA, Feinstein L et al. (2022). Serum antioxidant vitamins and respiratory morbidity and mortality: A pooled analysis. Respiratory Research, 23(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DM, Higley EB, Hecker M, Mankidy R, & Giesy JP (2013). In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicology Letters, 223(2), 252–259. [DOI] [PubMed] [Google Scholar]
- Sly PD, & Flack F (2008). Susceptibility of children to environmental pollutants. Annals of the New York Academy of Sciences, 1140(1), 163–183. [DOI] [PubMed] [Google Scholar]
- Sutha J, Anila PA, Gayathri M, & Ramesh M (2022). Long term exposure to tris (2-chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (danio rerio): In vivo and computational analysis. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 254, 109263. [DOI] [PubMed] [Google Scholar]
- Trotter A, Hilgendorff A, Kipp M, Beyer C, Kueppers E et al. (2009). Gender-related effects of prenatal administration of estrogen and progesterone receptor antagonists on VEGF and surfactant-proteins and on alveolarisation in the developing piglet lung. Early Human Development, 85(6), 353–359. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Dirtu AC, Neels H, & Covaci A (2011). Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environment International, 37(2), 454–461. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Braun JM, Yolton K, Xie C, Webster GM et al. (2017). Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environmental Research, 153, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IJ, Chen CY, & Bornehag CG (2016). Bisphenol A exposure may increase the risk of development of atopic disorders in children. International journal of hygiene and environmental health, 219(3), 311–316. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li W, Martínez-Moral MP, Sun H, & Kannan K (2019). Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. Environment international, 122, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Nuckols JR, Ward MH, & Rappaport SM (2012). Carpet-dust chemicals as measures of exposure: Implications of variability. Emerging Themes in Epidemiology, 9(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lu M, Dong X, Wang C, Zhang C, Liu W, & Zhao M (2014). Potential estrogenic effects of phosphorus-containing flame retardants. Environmental Science & Technology, 48(12), 6995–7001. [DOI] [PubMed] [Google Scholar]
- Zhao F, Chen M, Gao F, Shen H, & Hu J (2017). Organophosphorus flame retardants in pregnant women and their transfer to chorionic villi. Environmental Science & Technology, 51(11), 6489–6497. [DOI] [PubMed] [Google Scholar]
- Zhou A, Chang H, Huo W, Zhang B, Hu J et al. (2017). Prenatal exposure to bisphenol A and risk of allergic diseases in early life. Pediatric research, 81(6), 851–856. [DOI] [PubMed] [Google Scholar]
- Zuiderveen EA, Slootweg JC, & de Boer J (2020). Novel brominated flame retardants-A review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere, 255, 126816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


