Abstract
Background
Multi-targeted tyrosine kinase inhibitors (TKIs) of the vascular endothelial growth factor receptor (VEGFR) pathway have activity in differentiated thyroid cancer (DTC). Lenalidomide demonstrated preliminary efficacy in DTC, but its safety and efficacy in combination with VEGFR targeted TKIs is unknown. We sought to determine the safety and efficacy of cediranib, a VEGFR targeted TKI, with or without lenalidomide, in the treatment of iodine 131-refractory differentiated thyroid cancer.
Patients and Methods
In this multicenter, open-label, randomized phase II clinical trial, 110 patients were enrolled and randomized to cediranib alone or cediranib with lenalidomide. The primary endpoint was progression-free survival (PFS). Secondary endpoints included response rate, duration of response, toxicity, and overall survival. Patients (≥18 years) with DTC who were refractory to further surgical or radioactive iodine (RAI) therapy as reviewed at a multispecialty tumor board conference, and evidence of disease progression within the previous 12 months and no more than 1 prior line of systemic therapy were eligible.
Results
Of the 110 patients, 108 started therapy and were evaluable for efficacy. The median PFS was 14.8 months (95% CI 8.5 to 23.8) in the cediranib arm and 11.3 months (95% CI 8.7 to 18.9) in the cediranib with lenalidomide arm (p=0.36). The 2-year overall survival was 64.8% (95% CI 43.3–86.4) and 75.3% (95% CI 59.4–91.0), respectively (p=0.80). The serious adverse event rate was 41% in the cediranib arm and 46% in the cediranib with lenalidomide arm.
Conclusions
Single-agent therapy with cediranib showed promising efficacy in RAI refractory DTC similar to other VEGFR targeted TKIs, while the addition of lenalidomide did not result in clinically meaningful improvements in outcomes.
Clinical trial registration
Clnicaltrials.gov identifier: NCT01208051
Introduction
Differentiated thyroid cancer (DTC) accounts for approximately 90% of thyroid cancer diagnoses. Despite a favorable prognosis for the majority of patients with surgery, thyroid hormone therapy, and radioactive iodine (RAI) therapy, a subset of patients develops progressive recurrent and/or metastatic disease that is refractory to RAI and have limited treatment options.1, 2 Historically, cytotoxic chemotherapy such as doxorubicin was associated with poor response rates, short-lived activity, and substantial treatment-related toxicity. Given the high vascularity of thyroid cancers, there has been interest in the use of tyrosine kinase inhibitors (TKIs) that target angiogenic signaling in the tumor microenvironment, particularly vascular endothelial growth factor receptor (VEGFR). At the time of study conception, no TKIs had been approved for the treatment of RAI refractory DTC. Since that time, randomized trials have demonstrated a benefit for TKIs such as lenvatinib and sorafenib associated with median PFS of 11–18 months and which are now Food and Drug Administration (FDA) approved in this setting.3–5 Despite these advances, improved therapeutic strategies are needed.
Cediranib is a potent inhibitor of all three VEGF receptors (VEGFR-1, −2, and −3). Given the activity of TKIs targeting VEGFR in RAI refractory DTC, it was hypothesized that cediranib may be active in this setting. Lenalidomide, a derivative of thalidomide, has immunomodulatory properties, inhibits angiogenesis, modulates stem cell differentiation, and has anti-cancer properties particularly in the treatment of multiple myeloma. At the time of study conception, phase II data supported single-agent activity for thalidomide and lenalidomide in the treatment of DTC.6, 7 The combination of cediranib and lenalidomide was thus seen as potentially promising and had not been evaluated in the treatment of DTC. To evaluate the safety and efficacy of cediranib and lenalidomide in RAI refractory DTC, we performed a phase I and subsequent randomized phase II study of cediranib, with or without lenalidomide.
Patients and methods
Patients
Eligible patients were ≥ 18 years of age with an Eastern Cooperative Oncology Group (ECOG) score of 0–1, a life expectancy of >12 weeks, and a histologically or cytologically confirmed differentiated thyroid cancer (papillary, follicular, papillary/follicular variant, and Hürthle cell carcinoma subtypes). Furthermore, the disease must have been radiographically measurable, with evidence of disease progression in the preceding 12 months by standard Response Evaluation Criteria of Solid Tumors (RECIST) criteria. Patients were required to have normal organ and bone marrow function, and ineligible for further curative-intent surgery and/or radioactive iodine (RAI) treatment as reviewed at a multispecialty tumor board conference.
Patients who received chemotherapy or radiotherapy within 4 weeks prior to entering study, or with prior use of thalidomide or lenalidomide were excluded, as were patients with known brain metastases, poorly controlled hypertension, proteinuria >=1.5 gram protein/24 hours, any condition resulting in malabsorption, serious non-healing wound or ulcer, history of abdominal fistula or gastrointestinal perforation, history of cerebrovascular accident or transient ischemic attack, history of myocardial infarction, cardiac arrhythmias, stable or unstable angina, symptomatic congestive heart failure, or coronary or peripheral artery bypass graft or stenting within the 12 months prior to study entry. Prior treatment with VEGF pathway inhibitors or BRAF inhibitors was permissible.
Study design and conduct
This trial was designed as a phase I dose escalation with primary endpoint of maximally tolerated dose (MTD) and recommended phase 2 dose (RP2D), followed by a randomized phase II trial with a primary endpoint of progression-free survival (PFS). Secondary endpoints included objective response rate (ORR), overall survival (OS), percent change in tumor size from baseline to the end of cycle 2, and safety and tolerability of cediranib, with or without lenalidomide. The study was conducted as a multicenter, open-label, phase I dose escalation and randomized phase II, comparative trial recruiting patients from twelve centers in the United States and Canada. Enrollment was performed at a participating site following approval by their respective institutional review boards, and all patients were provided written informed consent to participate.
Treatment
The phase I trial used a 3+3 design with the MTD defined as the highest dose level such that <2 of 6 patients experience dose-limiting toxicity (DLT) evaluating cediranib doses ranging from 20mg to 30mg daily, and lenalidomide doses ranging from 10mg on days 1–21 of a 28-day cycle to 20mg daily. In the phase I study of 15 evaluable patients, 4 were enrolled at the starting dose, 6 were enrolled in the +1 dose modification (cediranib 30mg daily and lenalidomide 15mg on days 1–21 of 28-day cycle), and 5 in the +2 dose modification (cediranib 30mg daily and lenalidomide 15mg daily). DLTs in the +2 dose modification were grade 3 fatigue and grade 3 mucositis. The DLT in the +1 modification was grade 3 fatigue. Since only 1 of 6 patients developed a DLT in the +1 group (cediranib 30mg daily and lenalidomide 15mg on days 1–21 of 28-day cycle) was determined as the dose schedule for the phase II portion of the trial.
In the phase II portion, patients were randomized in a 1:2 ratio to cediranib, with or without lenalidomide, stratified by prior therapy with a VEGF-pathway tyrosine kinase inhibitor (yes vs. no), and ECOG performance status (0 and 1 vs. 2). Treatment was continued until disease progression as assessed by RECIST, unacceptable toxicity, or patient or physician-initiated discontinuation.
Following the interim analysis, patients assigned to arm B discontinued lenalidomide and continued on cediranib alone.
Dose modifications for toxicities
Toxicities were evaluated using criteria from common terminology for adverse events (CTCAE) version 4.0. Dose reductions were required for grade ≥3 toxicities, with protocol management of hypertension, neutropenia, thrombocytopenia, fatigue, hand-foot-skin reaction, liver function test abnormalities, proteinuria, and venous thromboembolic events. Two dose reductions were permitted for cediranib to 20 mg and 15 mg daily, and two dose reductions were permitted for lenalidomide to 10 mg and 5 mg on days 1–21 of 28-day cycle.
Response assessment
Initial imaging evaluation was required within 28 days of enrollment. After starting treatment, disease re-evaluation occurred every 8 weeks using RECIST guidelines, and every 16 weeks after 18 cycles. Patients without evidence of anti-thyroglobulin antibodies continued to have thyroglobulin levels measured at each response assessment. All patients received thyroxine suppression therapy and thyroid stimulation hormone (TSH) levels were monitored pre-therapy and every 8 weeks during therapy. All patients included in the study were assessed for response to treatment. All patients who met eligibility criteria and started therapy were included in the main analysis of response rate.
Statistical analysis
The primary endpoint of this study was PFS, which was estimated by the Kaplan-Meier method and compared between the two treatment arms using a stratified logrank test. PFS was calculated as the time from randomization to disease progression or death from any cause. A sample size of 110 patients (36 randomized to arm A and 74 to arm B) would have 85% power to detect a 75% improvement in median from 40 weeks (null hypothesis) to 70 weeks, corresponding to a hazard ration of 1.75, based on a one-sided test at alpha=0.10 significance level. An interim futility analysis was planned after half of the total number of projected events (40 of 80 projected events). The trial was planned to be stopped for futility if the conditional power at this time point was 15% or less, reducing the overall power of the study by 2–3%. Overall survival was analyzed in a manner similar to PFS. Objective response rates (CR or PR) were compared between the two treatment arms by chi-square test. Percent change in tumor size was compared using a nonparametric, Wilcoxon rank-sum test. Two patients who died prior to the cycle 2 were ranked at the extreme end of the distribution. Adverse event rates were tabulated by type, grade, and attribution to treatment.
Results
Patients and treatment
One hundred ten patients with RAI-refractory DTC were enrolled between 2010 and 2015 in phase II and randomized to treatment arm, 39 on arm A (cediranib alone), and 71 on arm B (cediranib with lenalidomide). Two patients withdrew consent prior to starting therapy and were considered non-evaluable, leaving 108 assessable for the primary endpoint, 39 on arm A and 69 on arm B. The futility analysis was performed after 40 events occurred, at which point a total of 39 patients had been randomized to arm A and 68 to arm B. One patient started treatment on arm B while the futility analysis was ongoing. 39 patients on arm A and 69 patients on arm B were analyzed for the final analysis (see Fig. 1).
Figure 1:
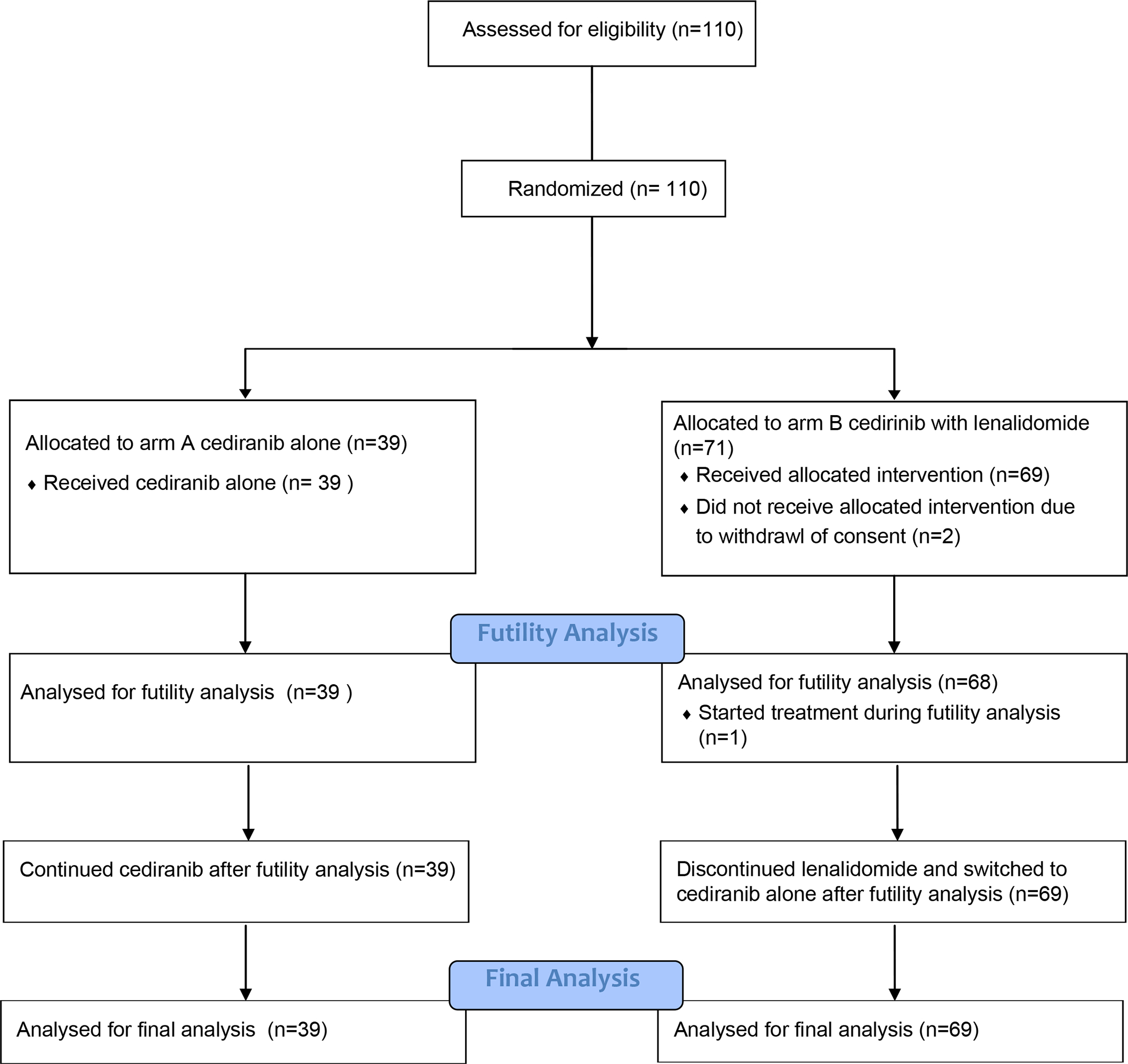
CONSORT diagram demonstrating patient flow through enrollment, randomization, assigned treatment, futility analysis, and final analysis.
Median age was 63 years, with a range from 24 to 86 years. Male patients were 41%, and females were 59%. 82% of patients were Caucasian. 96 patients enrolled in the United States and 12 enrolled in Canada. 23% of patients had received prior VEGF targeted therapy in both arms, and ECOG performance status was 0 or 1 in 95% and 96% in arm A and arm B, respectively.
Complete demographic data is listed in Table 1.
Table 1:
Patient demographics
| Arm A (cediranib alone) | Arm B (cediranib with lenalidomide) | Total | |
|---|---|---|---|
| No. (overall %, n=39) | No. (overall %, n=69) | No. (overall %, n=108) | |
| Sex | |||
| Male | 24 (62%) | 40 (58%) | 64 (59%) |
| Female | 16 (38%) | 29 (42%) | 44 (41%) |
| Age, years | Median (range) | Median (range) | Median (range) |
| 62 (27 to 86) | 64 (24–83) | 63 (24–86) | |
| ECOG PS | |||
| 0 or 1 | 37 (95%) | 66 (96%) | 103 (95%) |
| 2 | 2 (5%) | 3 (4%) | 5 (5%) |
| Race | |||
| White | 30 (77%) | 59 (86%) | 89 (82%) |
| Asian | 3 (8%) | 4 (6%) | 7 (6%) |
| Black or African American | 5 (13%) | 2 (3%) | 7 (6%) |
| American Indian or Alaskan native | 1 (3%) | 0 (0%) | 1 (1%) |
| More than one race | 0 (0%) | 1 (1%) | 1 (1%) |
| Unknown or not reported | 0 (0%) | 3 (4%) | 3 (3%) |
| Region of enrollment | |||
| United States | 34 (87%) | 62 (90%) | 96 (89%) |
| Canada | 5 (13%) | 7 (10%) | 12 (11%) |
| Prior VEGF targeted therapy | |||
| Yes | 9 (23%) | 16 (23%) | 25 (23%) |
| No | 30 (77%) | 53 (77%) | 83 (77% |
| Thyroid cancer subtype | |||
| Papillary | 19 (73%) | 26 (44%) | 45 (64%) |
| Follicular | 1 ( 4%) | 7 (16%) | 8 (11%) |
| Squamous cell | 2 ( 8%) | 1 ( 2%) | 3 ( 4%) |
| Adenocarcinoma | 0 ( 0%) | 2 ( 5%) | 2 ( 3%) |
| Not otherwise specified | 4 (15%) | 8 (18%) | 12 (17%) |
| Missing | 13 | 25 |
Response and survival
At time of futility analysis, the median PFS in arm A and arm B were 20.9 months (95% CI 10.9 to not reached) and 10.6 months (95% CI 7.3 to 14.6), respectively, with a stratified logrank test p-value of 0.26. The conditional power was 7.8%, reaching the futility boundary of <15%. Subsequently, patients on the combination arm discontinued lenalidomide and continued cediranib alone.
The final follow-up survival analyses in the intention-to-treat population occurred after a median follow-up of 11 months, at which time there were 81 PFS events. The median PFS in arm A was 14.8 months (95% CI 8.5 to 23.8), and in arm B was 11.3 months (95% CI 8.7 to 18.9), logrank p=0.36. The Kaplan-Meier curves for PFS can be seen in figure 2.
Figure 2:
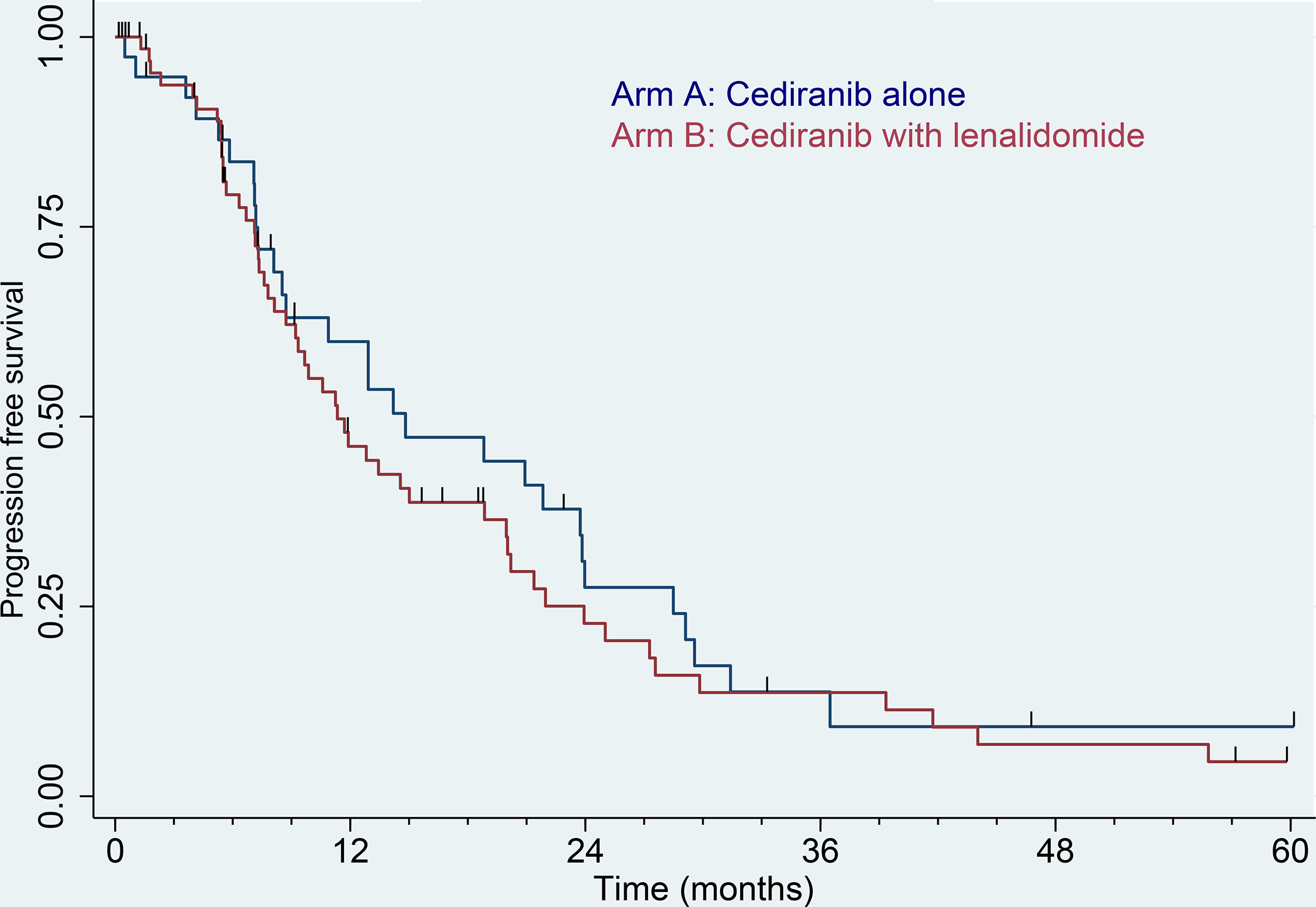
Kaplan–Meier curve for progression-free survival by RECIST. Blue: Arm A (cediranib alone), N = 39. Red: Arm B (cediranib with lenalidomide), N = 69. ECIST = Response Evaluation Criteria in Solid Tumors. Tic marks denote censored observations.
The response rates were similar for cediranib alone versus cediranib with lenalidomide. In arm A with cediranib alone, 17/39 patients had a partial response corresponding to a 44% objective response rate. In arm B with cediranib plus lenalidomide, 30/69 patients had a complete (1 patient) or partial response corresponding to a 43% objective response rate (p=0.99) (see waterfall plots in figure 3.
Figure 3:
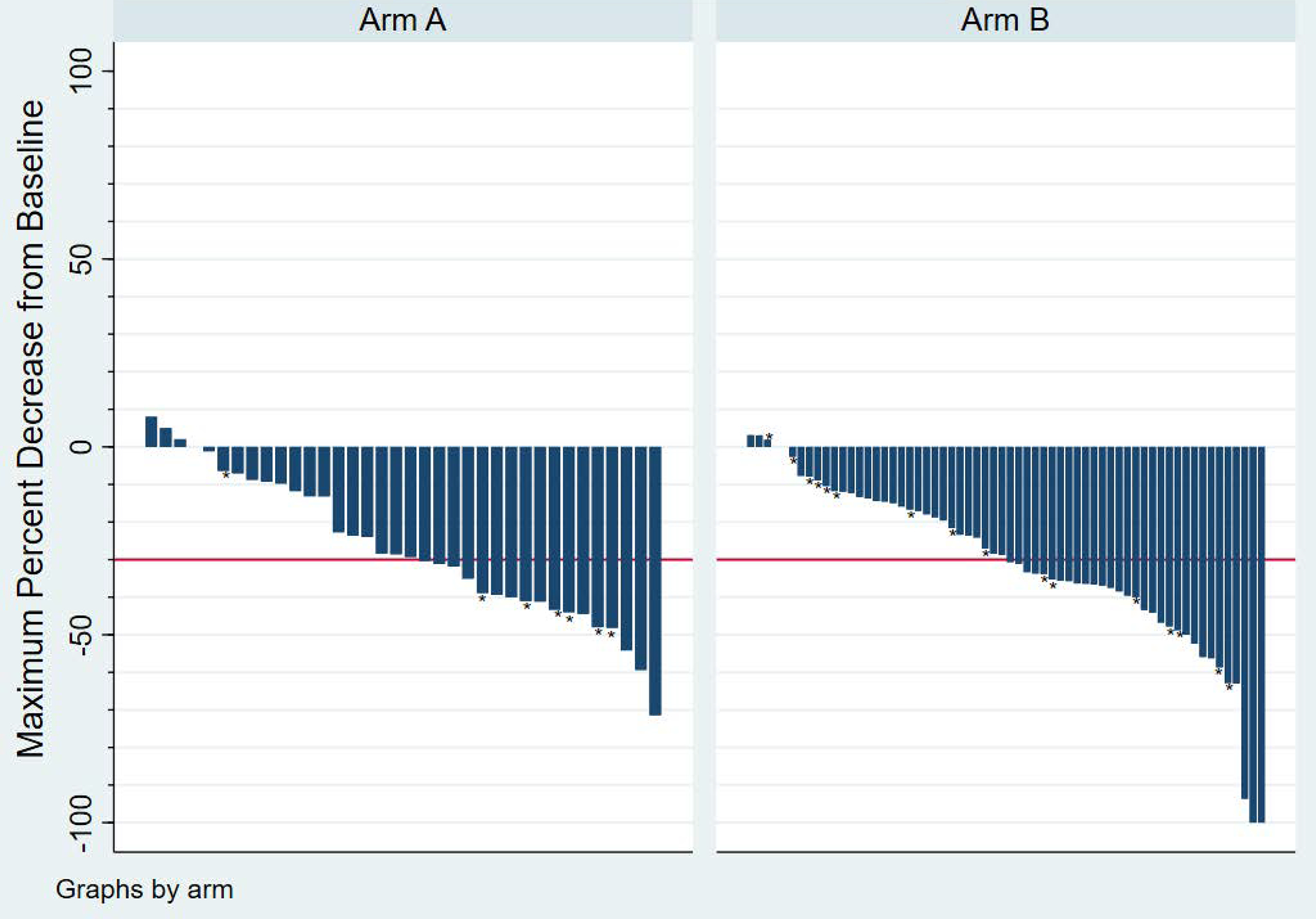
Waterfall plots demonstrating best overall response by RECIST in Arm A and Arm B respectively. Two additional patients who died prior to two months are excluded (both Arm A). * denotes patients who had a new lesion.
Twenty-five patients had received prior VEGF therapy while 83 patients were first line. PFS in the two treatment arms by prior VEGF treatment is shown in Figures 4A and 4B. First-line patients fared better than second line, but there were no significant differences between the two treatment arms (p=0.11 and p=0.17, respectively). Response rates in first-line patients were 16/30 (53%) in arm A vs. 23/53 (43%) in arm B, p=0.49. For second-line patients, response rates were 1/9 (11%) and 7/16 (44%), respectively, p=0.18.
Figure 4:
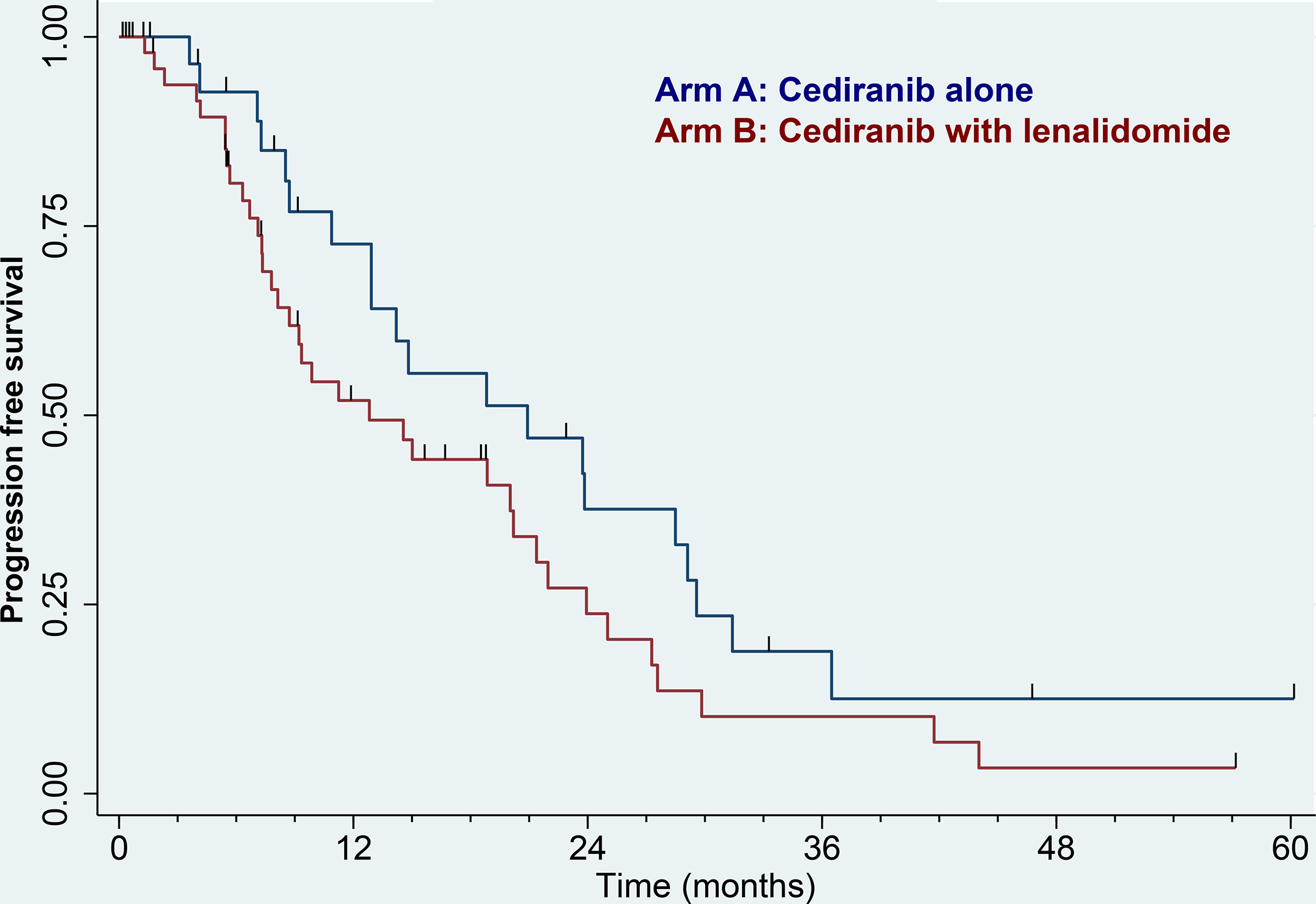
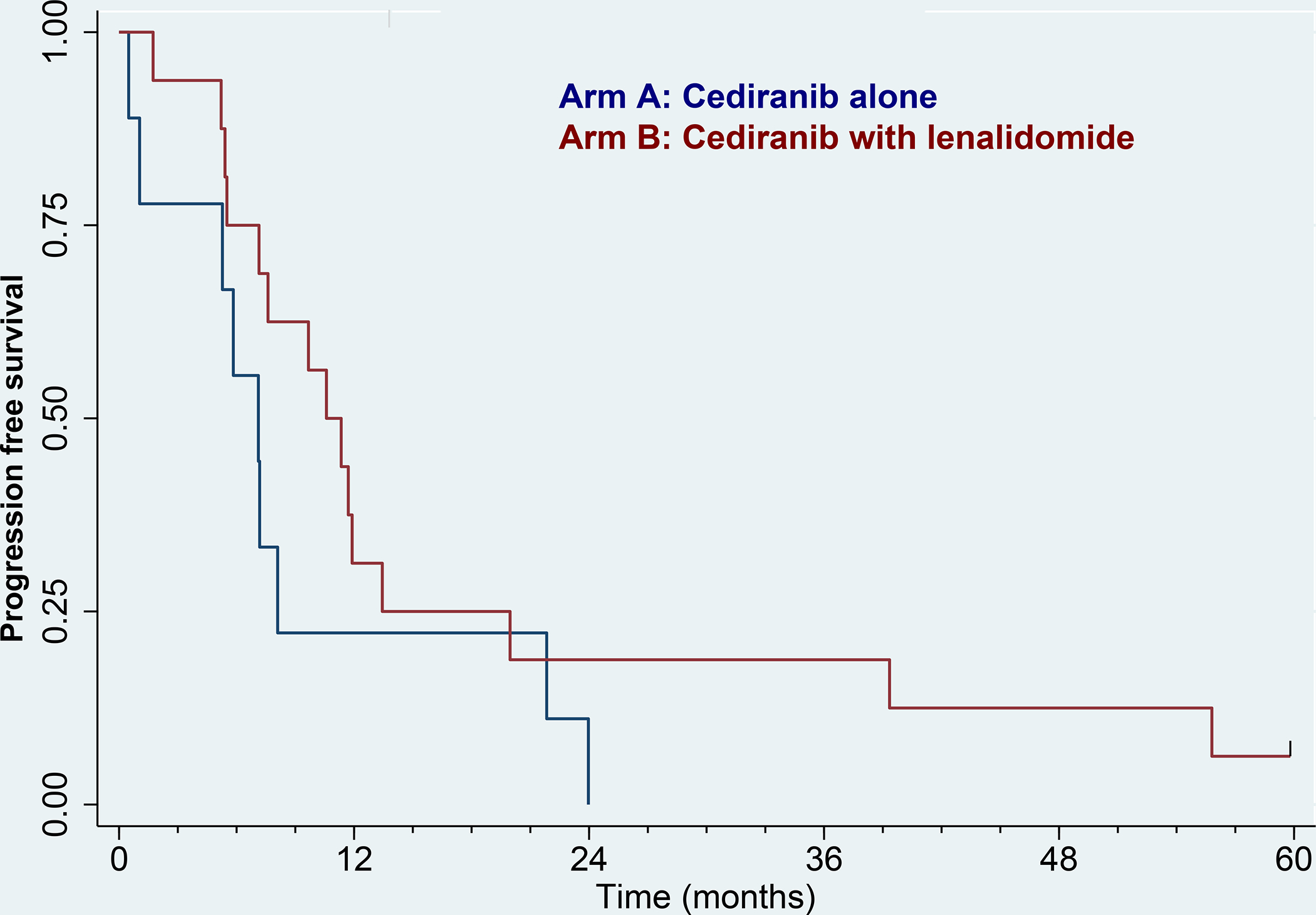
Kaplan–Meier curve for progression-free survival by RECIST. 4A) No prior VEGF therapy. Blue: Arm A (cediranib alone), N = 30. Red: Arm B (cediranib with lenalidomide), N = 53. 4B) Prior VEGF therapy. Blue: Arm A (cediranib alone), N = 9. Red: Arm B (cediranib with lenalidomide), N = 16. Tic marks denote censored observations.
The overall survival was assessed at time of final analysis and was similar between cediranib alone versus cediranib plus lenalidomide and was associated with 2-year survival rates of 64.8% (95% CI 43.3–86.4) and 75.3% (95% CI 59.4–91.0), respectively (logrank p=0.80). Kaplan-Meier curves for overall survival for arms A and B can be observed in figure 5.
Figure 5.
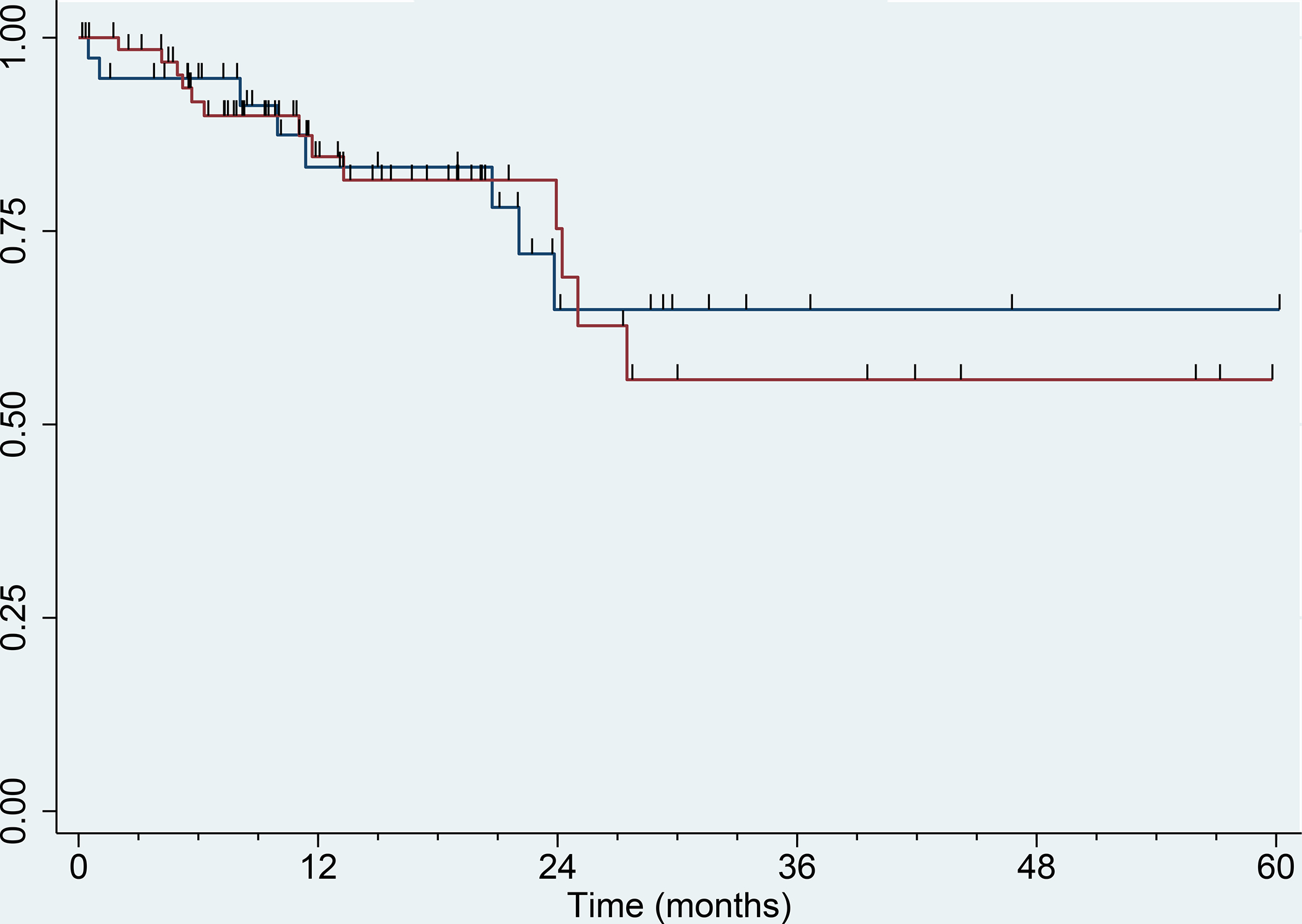
Kaplan–Meier curve for overall survival. Blue: Arm A (cediranib alone), N = 39. Red: Arm B (cediranib with lenalidomide), N = 69. Tic marks denote censored observations.
The secondary endpoint of percent change in tumor size was assessed comparing baseline to the end of cycle 2, or two months after starting therapy. 38 patients in arm A and 63 patients in arm B had assessable imaging at the 2-month time-point for this endpoint. The median percent change in tumor size in arm A with cediranib alone was −12.5% (Interquartile range [IQR] −26.0% to 0.0%), and in arm B with cediranib plus lenalidomide was −4.2% (IQR −25.0% to 0.0%), Wilcoxon p=0.83).
Toxicity
Rates and types of treatment-related adverse events (AEs) during phase II were comparable to those seen in other studies of cediranib and lenalidomide 8–10. Table 2 lists AEs prior to the discontinuation of lenalidomide that were grade 3 or higher and at least possibly related to the study drugs. The most common grade 3 or higher treatment-related AEs included fatigue (26% in both arms), hypertension (26% in arm A and 28% in arm B), and diarrhea (15% in arm A and 12% in arm B). Notable treatment-related grade 3 or higher AEs that were more common with cediranib plus lenalidomide compared with cediranib alone included proteinuria (5.1% in arm A compared with 8.7% in arm B), generalized muscle weakness (0% in arm A compared with 5.8% in arm B), and cytopenias including neutropenia (2.6% in arm A compared with 16% in arm B), thrombocytopenia (0% in arm A compared with 4.3% in arm B), and lymphopenia (0% in arm A compared with 4.3% in arm B). In arm B, after discontinuation of lenalidomide, there was one additional grade 3 hypercalcemia, one grade 3 hyponatremia, and one grade 3 leukocytosis reported.
Table 2:
Adverse events that were grade 3 or higher and at least possibly related to study drug(s).
| Arm A (cediranib alone), n (%) | Arm B (cediranib with lenalidomide) (%) | |
|---|---|---|
| Fatigue | 10 (26) | 18 (26) |
| Hypertension | 10 (26) | 19 (28) |
| Diarrhea | 6 (15) | 8 (12) |
| Hand foot syndrome | 6 (15) | 5(7.2) |
| Oral mucositis | 3 (7.7) | 3 (4.3) |
| Proteinuria | 2 (5.1) | 6 (8.7) |
| Generalized muscle weakness | 0 (0) | 4 (5.8) |
| Anorexia | 3 (7.7) | 2 (2.9) |
| Syncope | 2 (5.1) | 1 (1.4) |
| Vomiting | 0 0) | 1 (1.4) |
| Hypophosphatemia | 1 (2.6) | 4 (5.8) |
| Hypokalemia | 1 (2.6) | 3 (4.3) |
| Thromboembolic event | 1 (2.6) | 2 (2.9) |
| Neutropenia | 1 (2.6) | 11 (16) |
| Thrombocytopenia | 0 (0) | 3 (4.3) |
| White blood cell count decreased | 0 (0) | 3 (4.3) |
| Stroke | 0 (0) | 1 (1.4) |
| Sinus bradycardia | 0 (0) | 1 (1.4) |
| Rash maculo-papular | 0 (0) | 2 (2.9) |
| Pneumothorax | 0 (0) | 1 (1.4) |
| Pharyngolaryngeal pain | 0 (0) | 1 (1.4) |
| Pancreatitis | 0 (0) | 1 (1.4) |
| Oral dysesthesia | 0 (0) | 1 (1.4) |
| Nausea | 0 (0) | 1 (1.4) |
| Muscle weakness upper limb | 0 (0) | 1 (1.4) |
| Lung infection | 0 (0) | 1 (1.4) |
| Leukocytosis | 0 (0) | 1 (1.4) |
| Hypophosphatemia | 0 (0) | 4 (5.8) |
| Hypokalemia | 0 (0) | 3 (4.3) |
| Hypocalcemia | 0 (0) | 3 (4.3) |
| Hypercalcemia | 0 (0) | 1 (1.4) |
| Hepatobiliary disorder-other | 0 (0) | 1 (1.4) |
| Fall | 0 (0) | 1 (1.4) |
| Ejection fraction increased | 0 (0) | 1 (1.4) |
| Ear pain | 0 (0) | 1 (1.4) |
| Dyspnea | 0 (0) | 2 (2.9) |
| Dizziness | 0 (0) | 1 (1.4) |
| Delirium | 0 (0) | 1 (1.4) |
| Dehydration | 0 (0) | 2 (2.9) |
| Creatinine increased | 0 (0) | 1 (1.4) |
| Colitis | 0 (0) | 1 (1.4) |
| Blood bilirubin increased | 0 (0) | 2 (2.9) |
| Aspartate aminotransferase increased | 0 (0) | 1 (1.4) |
| Anemia | 0 (0) | 1 (1.4) |
| Weight loss | 1 (2.6) | 3 (4.3) |
| Lymphocyte count decreased | 2 (5.1) | 3 (4.3) |
| Hyponatremia | 1 (2.6) | 1 (1.4) |
| Lymphopenia | 0 (0) | 3 (4.3) |
| Alanine aminotransferase increased | 0 | 3 (4.3) |
| Peripheral ischemia | 1 (2.6) | 0 (0) |
| Abdominal pain | 1 (2.6) | 1 (1.4) |
Discussion
Here we report the results of a large, randomized phase II trial of cediranib, with or without lenalidomide, in RAI-refractory recurrent and/or metastatic DTC. Despite results of early phase, single-arm trials, suggesting efficacy of lenalidomide and thalidomide in RAI refractory DTC6, 7, our randomized results did not demonstrate an improvement in PFS with the addition of lenalidomide to cediranib alone in this patient population, highlighting the importance of randomized trials in this setting.
Interestingly, our study demonstrated promising activity and tolerable safety profile for cediranib alone, which was associated with a median PFS of 14.8 months and an objective response rate of 44%. Since the completion of this study, randomized trials have demonstrated activity of multiple VEGF targeted inhibitors including lenvatinib3, 4, sorafenib5, and vandetanib11. The previously reported median PFS for lenvatinib ranges from 13 to 18 months and reported objective response rates ranging from 50% to 65%3, 4. Sorafenib in this patient population is associated with a median PFS of 10.8 months5, and vandetanib is associated with a median PFS of 11.1 months11. Other VEGF targeted TKIs such as axitinib, pazopanib, and sunitinib have been evaluated in non-randomized studies demonstrate median PFS ranging from 12.8 months to 18.1 months, and objective response rates ranging from 22% to 49%12–15. Our results for cediranib demonstrating a median PFS of 14.8 months and objective response rate of 44% seems to be in similar range compared with other multitargeted TKIs including angiogenesis and, specifically, VEGFR targeted pathways.
Recently, there has been enthusiasm towards mutation specific inhibitors in a subset of RAI-refractory recurrent and/or metastatic DTC. Rearrangements of one of the neurotrophic tropomyosin receptor kinase (NTRK) 1–3 fusions can occur in approximately 1–2% of DTC16. Although rare, when present highly selective inhibitors of TRK kinases have demonstrated impressive objectives response rates of around 71%17. Other targetable changes include rearrangements involving the RET gene, particularly in papillary thyroid cancer, in approximately 6–10%, in which selective RET kinase inhibitors have demonstrated objective response rates ranging from 79% to 89%18–20. Interestingly, inhibitors of BRAF, with or without a MEK inhibitor in RAI-refractory DTC with BRAFV600E mutations, are associated with lower objective response rates of approximately 30% to 54%21, 22. Similar to other multitargeted antiangiogenic TKIs, our objective response rate of 44% with cediranib is similar to BRAF targeting and remains lower than selective inhibitors targeting NTRK and RET and therefore these remain preferred treatments in the rare subset of patients with RAI refractory DTC with these specific targets.
Yet, despite recent advances in selective targeted treatments in a rare subset of RAI-refractory DTC, improved therapeutic strategies are urgently needed. Despite meaningful responses and improvements in PFS that have been demonstrated with multitargeted antiangiogenic TKIs, complete responses continue to be rare, and are associated with toxicities which have a substantial impact on quality of life, particularly with long-term use in the setting of RAI-refractory DTC23. This highlights the need for investigating novel therapeutic strategies in RAI-refractory DTC progressed on or ineligible for VEGF targeted TKIs.
Limitations of his study include that mutational status for patients was not known and there was no alternative antiangiogenic TKI as the backbone. Nevertheless, our study contributes to the expanding body of literature regarding the efficacy of VEGFR inhibitors in RAI-refractory DTC. The lack of improved efficacy with the addition of immunomodulatory agent lenalidomide, despite promising data regarding potential single-agent activity, highlights the need for investigating novel therapeutic strategies to improve patient outcomes in this setting in RAI-refractory recurrent and/or metastatic DTC.
Conclusion
Antiangiogenic TKIs are reaffirmed as the standard of care treatment approach in RAI-refractory DTC while the addition of immunomodulatory agents does not improve progression-free survival, demonstrating alternative combinatorial approaches warrant evaluation in large multicenter studies.
Highlights.
This randomized phase II trial demonstrated promising activity of cediranib with a median PFS of 14.8 months and an objective response rate of 44%.
The addition of lenalidomide to cediranib alone did not improve progression-free or overall survival.
This highlights the activity of antiangiogenic TKIs in RAI-refractory differentiated thyroid carcinoma, and the need for novel combinatorial therapeutic strategies in this disease.
Funding
The trial was sponsored by the University of Chicago with multi-institutional coordination services provided through the UCCCC (University of Chicago Comprehensive Cancer Center) in collaboration with AstraZeneca and Celgene, which provided funding and the drugs for the study. The funder collaborated with the co-authors in study design only. The funder had no role in data collection, data analysis, data interpretation or manuscript approval. All authors had full access to all the data reported in the study and the corresponding author had final responsibility for the decision to submit for publication.
Footnotes
Disclosure
AJR reports receiving consultation fees from EMD-Serono, Nanobiotix, Astellas, Novartis, Privo, and Galectin. WMS reports Consultant (DSMB): Astra-Zeneca, Merck, Pfizer, Treadwell Therapeutics; Consultant (other): Astra-Zeneca, Calico Life Sciences, Caremark/CVS, EMA Wellness, Fortress Biotech; Speakers Bureau: CME providers (sponsorship unknown): Applied Clinical Education, Dava Oncology, Global Academy for Medical Education, OncLive, PeerView, Research to Practice, Vindico; Grant/Research Support (to institution): Abbvie, Amgen, Astra-Zeneca, Astellas (Medivation), Bayer, Bristol-Myers-Squibb, Bellicum, Boehringer-Ingelheim, Calithera, Clovis, Corvus, Eisai, Exilixis, Genentech (Roche), Johnson & Johnson (Janssen), Merck, Novartis, Pfizer, Seattle Genetics, X4Pharmaceuticals; Stockholder: Fortress Biotech; Expert Witness: Apotex, DRL, Mylan, Sandoz; Miscellaneous/Editorial: Cancer (ACS), Up-To-Date, Kidney Cancer Journal. All remaining authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Comprehensive Cancer Network. Thyroid Carcinoma (Version 3.2022).
- 2.Fagin JA, Wells SA. Biologic and Clinical Perspectives on Thyroid Cancer. New England Journal of Medicine 2016; 375 (11): 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabanillas ME, Schlumberger M, Jarzab B et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer 2015; 121 (16): 2749–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlumberger M, Tahara M, Wirth LJ et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015; 372 (7): 621–630. [DOI] [PubMed] [Google Scholar]
- 5.Brose MS, Nutting CM, Jarzab B et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014; 384 (9940): 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ain KB, Lee C, Williams KD. Phase II trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid 2007; 17 (7): 663–670. [DOI] [PubMed] [Google Scholar]
- 7.Ain KB, Lee C, Holbrook KM et al. Phase II study of lenalidomide in distantly metastatic, rapidly progressive, and radioiodine-unresponsive thyroid carcinomas: preliminary results. Journal of Clinical Oncology 2008; 26 (15_suppl): 6027–6027. [Google Scholar]
- 8.Judson I, Morden JP, Kilburn L et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol 2019; 20 (7): 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy PL, Owzar K, Hofmeister CC et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366 (19): 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attal M, Lauwers-Cances V, Marit G et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366 (19): 1782–1791. [DOI] [PubMed] [Google Scholar]
- 11.Leboulleux S, Bastholt L, Krause T et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol 2012; 13 (9): 897–905. [DOI] [PubMed] [Google Scholar]
- 12.Bible KC, Suman VJ, Molina JR et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 2010; 11 (10): 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr LL, Mankoff DA, Goulart BH et al. Phase II Study of Daily Sunitinib in FDG-PET–Positive, Iodine-Refractory Differentiated Thyroid Cancer and Metastatic Medullary Carcinoma of the Thyroid with Functional Imaging Correlation. Clinical Cancer Research 2010; 16 (21): 5260–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravaud A, de la Fouchardière C, Caron P et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: mature data from the THYSU study. Eur J Cancer 2017; 76: 110–117. [DOI] [PubMed] [Google Scholar]
- 15.Cohen EE, Rosen LS, Vokes EE et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 2008; 26 (29): 4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greco A, Miranda C, Pierotti MA. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Molecular and Cellular Endocrinology 2010; 321 (1): 44–49. [DOI] [PubMed] [Google Scholar]
- 17.Waguespack SG, Drilon A, Lin JJ et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. European Journal of Endocrinology 2022; 186 (6): 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirth LJ, Sherman E, Robinson B et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. New England Journal of Medicine 2020; 383 (9): 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbiah V, Hu MI, Wirth LJ et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. The Lancet Diabetes & Endocrinology 2021; 9 (8): 491.. [DOI] [PubMed] [Google Scholar]
- 20.Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159 (3): 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brose MS, Cabanillas ME, Cohen EEW et al. Vemurafenib in patients with BRAFV600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. The Lancet Oncology 2016; 17 (9): 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busaidy NL, Konda B, Wei L et al. Dabrafenib Versus Dabrafenib + Trametinib in <i>BRAF</i>-Mutated Radioactive Iodine Refractory Differentiated Thyroid Cancer: Results of a Randomized, Phase 2, Open-Label Multicenter Trial. Thyroid 2022; 32 (10): 1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlumberger M, Jarzab B, Elisei R et al. PHASE III RANDOMIZED, DOUBLE-BLINDED, PLACEBO-CONTROLLED TRIAL OF SORAFENIB IN LOCALLY ADVANCED OR METASTATIC PATIENTS WITH RADIOACTIVE IODINE (RAI)-REFRACTORY DIFFERENTIATED THYROID CANCER (DTC) - EXPLORATORY ANALYSES OF PATIENT-REPORTED OUTCOMES, Presented at 83rd Annual Meeting of the American Thyroid Association in Puerto Rico, October 16–20, 2013. [Google Scholar]


