Abstract
Objective:
We explored the associations of dual sensory impairment (DSI) with long-term depressive and anxiety symptoms as well as low perceived social support (LPSS) as a modifier of these associations.
Methods:
Multinomial logistic regression models were used to examine the associations of DSI and single sensory impairment (hearing [pure-tone average > 25 dB] and vision [impaired visual acuity and/or contrast sensitivity]) with long-term depressive symptom (≥8 on the 10-item Center for Epidemiologic Studies-Depression Scale) and anxiety symptom (present on the Hopkins Symptom Checklist) latent classes from group-based trajectory models (rare/never; mild/moderate increasing; chronically high) among 2102 Health, Aging and Body Composition Study participants (mean age:74.0 ± 2.8 years; 51.9 % female) over 10 years. Models were adjusted by demographic characteristics and cardiovascular risk factors, and LPSS. An additional model evaluated the two-way interaction between DSI and LPSS.
Results:
DSI was associated with increased risk of being chronically depressed (Risk Ratio, RR = 1.99, 95 % Confidence Interval, CI: 1.25, 3.17), not mild/moderate increasingly depressed (RR = 1.25, 95 % CI: 0.91, 1.71). DSI had increased risk of being mild/moderate increasingly anxious (RR = 1.60, 95 % CI: 1.16, 2.19) and chronically anxious (RR = 1.86, 95 % CI: 1.05, 3.27) groups, as compared to no impairments. Hearing impairment was associated with being mild/moderate increasingly anxious (RR = 1.34, 95 % CI: 1.01, 1.79). No other associations were found for single sensory impairments. LPSS did not modify associations.
Limitations:
Sensory measures were time-fixed, and LPSS, depression and anxiety measures were self-reported.
Conclusions:
Future research is warranted to determine if DSI therapies may lessen long-term chronically high depressive and anxiety symptoms.
Keywords: Dual sensory impairment, Depressive symptoms, Anxiety, Hearing, Vision, Perceived social support
1. Introduction
Prevalence of depressive and anxiety symptoms is common among older adults, and it increases with age (Yu et al., 2020). Approximately 26 % of males report depressive and anxiety symptoms, while 27 % and 29 % of females report depressive and anxiety symptoms respectively (Curran et al., 2020). Similar studies report 26 % of older adults report anxiety symptoms (Beekman et al., 1999; Boehlen et al., 2020; De Beurs et al., 2000; Forsell and Henderson, 1998). A potentially underappreciated risk factor for depressive (Brewster et al., 2018; Fiske et al., 2009; McDonnall, 2009a) and anxiety (Contrera et al., 2017; Contrera et al., 2016) symptoms is dual sensory impairment (DSI), which is typically defined as the co-occurrence of hearing and vision impairments, both of which are highly and increasingly prevalent with advancing age (Saunders and Echt, 2007). Prevalence of DSI is <1 % among Americans aged 70 years and younger, but DSI increases to 11.3 % among Americans aged 80 years and older (Swenor et al., 2013).
While several cross-sectional studies have evaluated the association of DSI with depression and anxiety (Capella-McDonnall, 2005; Heine and Browning, 2014; Lupsakko et al., 2002; McDonnall, 2009b), few have considered the longitudinal associations of DSI with depressive and anxiety symptoms (Cosh et al., 2018; Kiely et al., 2013; McDonnall, 2009a). These studies used linear mixed-effects models to evaluate the rate of change in depressive symptoms as a function of DSI, and only one study (Cosh et al., 2018) evaluated the rate of change in anxiety symptoms as a function of DSI as well. Linear mixed-effects models can evaluate the change in the number of depressive symptoms, but it does not consider whether a person would have long-term depressive or anxiety symptoms and it assumes that the rate of change in depressive symptoms is linear when it is not. Older adults may experience different patterns of depressive symptoms over time (Byers et al., 2012; Cui et al., 2008; Kuchibhatla et al., 2012). While the prevalence of major depressive disorder among older adults is rare, the presence of clinically relevant depressive symptoms is common among older adults. Depressive symptoms have been associated with adverse outcomes, such as mobility difficulty (Murphy et al., 2016) and dementia (Snowden et al., 2015). To date, there is a dearth of studies evaluating the association of DSI with long-term depressive and anxiety symptoms among White and Black older adults without functional limitations living in the United States and countries similar to the United States.
Hearing impairment (HI) and vision impairment (VI) separately have been linked to higher depressive and anxiety symptoms over time. Previous studies using data from the Health Aging and Body Composition (HABC) study found HI cross-sectionally is associated with higher depression and anxiety symptoms (Contrera et al., 2017; Contrera et al., 2016) and longitudinal depressive symptom trajectories (Brewster et al., 2018). HI also is associated with lower emotional vitality in an aged population, potentially linked with mental health and well-being (Contrera et al., 2016). Potential mediating pathways linking HI to depressive and anxiety symptoms could be cognitive decline (Lin et al., 2013), social isolation (Mick et al., 2014), and physical disability (Chen et al., 2015). VI is also associated with depressive and anxiety symptoms (Cosh et al., 2018; Simning et al., 2019), and those with VI are likely to report future depressive symptoms (Carrière et al., 2013; Frank et al., 2019). Potential mechanisms underlying this association are burden of care related to regular hospital visits to receive medical treatments, fear of intravitreal injections, fear of future blindness, uncertainty about disease prognosis, and poor self-esteem (Grant et al., 2021; Hernández-Moreno et al., 2021; Maaswinkel et al., 2020; Senra et al., 2017).
We examined whether DSI is associated with greater risk of long-term depressive and anxiety symptoms in a biracial sample of older adults. We hypothesize that those with DSI are at increased risk of higher long-term depressive and anxiety symptom patterns, as compared to those without hearing and vision impairments. Additionally, since DSI could limit opportunities for social interaction and support, we evaluated whether decreased perceived social support modified the associations of DSI with long-term depressive and anxiety symptom patterns as an exploratory analysis.
2. Methods
2.1. Characteristics of the study sample
Participants came from the HABC Study, a prospective cohort study designed to assess age-related changes in physical function and body composition in Black and White older adults without functional limitations. Details pertaining to HABC methodology and selection criteria have been published previously (Cesari et al., 2003; Katsiaras et al., 2005). Briefly, 3075 participants were initially recruited from field centers in Pittsburgh, PA and Memphis, TN between 1997 and 1998 (Year 1). Eligibility requirements for the study consisted of (i) no reported difficulty in activities of daily living (ADL) and (ii) no reported mobility limitation (difficulty walking ¼ mile or 10 stairs without rest). The study was approved at the study sites by the respective Institutional Review Boards, and participants provided written consent form. The final analytic sample included 2102 participants (mean age: 74.0 ± 2.8 years; 51.9 % female) with available measures on hearing, vision, depression, and anxiety (Supplementary Fig. 1).
2.2. Dual sensory impairment
Hearing Impairment.
In Year 5 (2001–2002), audiometric assessments were conducted in a sound-treated booth. Since hearing impairment progresses slowly in older age (Morrell et al., 1996), audiometric findings were used to define hearing status at study baseline (Year 1). Using headphones at frequencies from 0.25 to 8 k Hertz (kHz) (TDH 39; Telephonics Corporation) and an audiometer (MA40; Maico Diagnostics) calibrated to the standards of the American National Standards Institute (S3.6- 1996), pure-tone air conduction thresholds were collected in both left and right ears. A four-frequency pure tone average (PTA) was calculated from audiometric threshold at 0.5, 1, 2, and 4 kHz for the better hearing ear. HI is defined as PTA > 25 dB, since this cut-off has been used in previous Health ABC studies (Armstrong et al., 2020; Chen et al., 2015; Lin et al., 2013).
Vision Impairment.
In Year 3 (1999–2000), visual acuity, contrast sensitivity, and stereo acuity measures were administered to participants (Swenor et al., 2015). The Bailer-Lovie distance visual acuity test, converted to Snellen equivalents was used, with an acuity equivalent of 20/50 or worse being defined as impaired (Bailey and Lovie, 1976; Lovie-Kitchin, 1988). Additionally, Pelli-Robson contrast sensitivity was used, with 1.3 log units or less being defined as impaired (log Contrast units, higher values = better sensitivity) (Elliott et al., 1990; Pelli et al., 1988). Participants were asked about any previous impairments to their vision such as cataracts, glaucoma, and/or retinopathy. VI was defined as having impaired visual acuity or contrast sensitivity. As with HI, we assumed that this would be similar to values in Year 1.
Dual Sensory Impairment.
Using these defined cut-offs for HI and VI, we created a categorical variable to examine DSI: both hearing and vision, hearing only, vision only, and neither (reference). Both hearing and vision are time-invariant, as they were measured once in HABC.
2.3. Depressive symptoms
One of our outcomes of interest was classes related to the longitudinal trajectories of depressive symptoms. Depressive symptoms were measured using the Center for Epidemiologic Studies-Depression scale (CES-D) (Andresen et al., 1994; Radloff, 1977). The standard 20-item CES-D was administered in Years 1, 4, 6, 8, and 10 with scores ranging from 0 to 60. The 10-item CES-D (mCES-D) was administered in Years 3, 5, and 11 with scores ranging from 0 to 30 and reports measures of depressive symptoms experienced during the previous week through self-assessment. On a scale of 0 (rarely or none of the time) to 3 (most or all of the time), questions on the report range from mood (five items), irritability (one item), calories (energy, two items), concentration (one item), and sleep (one item). The maximum number of points allotted is 30, with higher scores indicating greater depressive symptoms. We used the items from the 20-item CES-D that matched the mCES-D to obtain the long-term depressive trajectories classes. That way, we had data on the mCES-D at Years 1, 3, 4, 5, 6, 8, 10, 11 (Supplementary Fig. 2). We used mCES-D cut-off score ≥ 8 for the presence of clinically relevant symptoms for the latent trajectory classes, as this has been established and used in a previous HABC study (Andresen et al., 1994; Armstrong et al., 2020).
There has been previous research using data from the full HABC study analyzing depression trajectories over 10 years by longitudinal latent class analysis (Brewster et al., 2018; Kaup et al., 2016). Brewster et al. (2018) found that depression trajectories consisted of one of three categories: (i) increasing probability of depressive symptoms, (ii) consistently high probability of depressive symptoms, or (iii) consistently low probability of depressive symptoms.
2.4. Anxiety symptoms
The second outcome of interest was classes related to the longitudinal trajectories of anxiety symptoms. Anxiety symptoms were measured using three items from the Hopkins Symptom Checklist that queried about presence of anxiety symptoms in the previous week (Derogatis et al., 1974). Participants were asked “during the past week: (i) have you felt fearful?; (ii) have you felt nervous/shaky inside?; (iii) have you felt tense/keyed up?”. Answers to these questions were on a scale of 1 to 4: no (1), a little (2), quite a bit (3), or extremely (4). We distinguished an anxiety threshold if participants rated any symptom occurring at least “quite a bit” or any two symptoms occurring at least “a little” (Guralnik et al., 1995; Mehta et al., 2003; Simonsick et al., 2015). Prior analysis has established the effectiveness of using the Hopkins Symptom Checklist as a screening tool for anxiety levels (Brenes et al., 2005; Mehta et al., 2007; Tambs, 2004). Anxiety symptoms were measured in Years 1, 3, 5, 6, 7 and 9 (Supplementary Fig. 2).
2.5. Measures of perceived social support
Perceived social support was defined by responses to questions related to satisfaction with social contact, adequacy of social contact, and emotional support. Satisfaction with social contact was defined by responses to the following questions: (1) “How satisfied are you with how often you see or talk to your family and friends?” (2) “How satisfied are you with the help you get from your friends and family?”. These were rated on a scale from 0 being dissatisfied and 10 being very satisfied. Adequacy of social contact was defined by responses to two questions about how often in a week do you see friends and relatives respectively. Responses included at least once a day, 4–6 times a day, 2–3 times a day, one time per week, and less than once per week. Definitions for adequacy of social contact were used previously in Simonsick et al. (1998). Emotional support was defined by responses to the following question: “In the past year, could you have used more emotional support than you received?” The responses were: (1) you needed a lot more; (2) some more; (3) a little more; (4) none. These questions were administered in odd years, but for this study, we used data from Years 1 (1997–1998) and 5 (2001–2002). Low perceived social support was defined by the following categorizations: dissatisfaction defined as the lower 10th percentile of the sample distribution on both questions (score < 6), low social contact defined as one time per week/less than once per week for friends and/or relatives, and low emotional support defined as needing more emotional support. Since few people had combinations of these components (Supplementary Table 1), low perceived social support was defined by the presence of one, two, and all three components versus absence of any component.
2.6. Covariates
Covariates included baseline demographic characteristics (age, sex [female vs. male], self-reported race [Black vs. White], marital status [married vs. not], postsecondary education [attained college/graduate degree vs. not]), current smoking status, history of Type II diabetes and hypertension, and body mass index (BMI). History of Type II diabetes was determined through self-reporting of diagnosis, use of Type II diabetes drugs, or fasting glucose ≥126 mg/dL at baseline. History of hypertension was determined by having systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure > 90 mm Hg, or by self-report of diagnosis of hypertension with or without antihypertensive medication use. About 2.4 % (n = 50) reported antidepressant use, so antidepressant use was not included as a covariate.
2.7. Trajectory group membership for depressive and anxiety symptoms
To identify distinct trajectory patterns of depressive and anxiety symptoms, we used a latent group-based univariate trajectory modeling approach under the assumption that the sample is drawn from a discrete set of subpopulations, each one having a distinct trajectory pattern, to approximate an underlying continuous distribution with a complex structure (Murphy et al., 2016; Nagin and Odgers, 2010; Nagin and Tremblay, 2001). We modeled depressive and anxiety symptoms data separately to find the best-fit group-based univariate trajectory models. The optimal number of trajectory classes and functional form (linear or polynomial) were determined. Criteria for the determination of best fit were average posterior probability of trajectory group membership >0.7 for all trajectory groups, Bayesian information criteria closest to zero, presence of at least 5 % of participants in each trajectory group, and the odds of correct classification >5.0 (Nagin and Odgers, 2010; Nagin and Tremblay, 2001). For depressive and anxiety symptoms, a logit model was used to describe the probability of clinically relevant symptoms at each visit up to 10 years.
2.8. Statistical analysis
Using two-sample t-tests for continuous variables and Chi-squared tests for categorical variables, baseline characteristics for each DSI category were evaluated. After assigning group membership for long-term depressive and anxiety symptoms, multivariable multinomial logistic regression models were used to determine if DSI was associated with long-term depressive and anxiety symptoms groups. Models were adjusted by baseline covariates. We calculated the relative excess risk due to interaction (RERI) and the synergy index (SI) to assess the biological interaction between hearing and vision impairment on an additive scale (Rothman et al., 2008). RERI >0 and SI > 1 mean that there is a positive interaction or more than additivity; RERI < 0 and SI < 1 mean negative interaction or less than additivity; RERI = 0 and SI = 1 mean no interaction or exactly additivity. We used multivariable multinomial logistic models to examine the association between DSI and low perceived social support cross-sectionally at Years 1 and 5. Then, we evaluated whether low perceived social support modified the association of DSI with long-term depressive and anxiety symptoms, by adding a two-way interaction term between low perceived social support and DSI as an exploratory analysis.
As a sensitivity analysis, we examined the associations between DSI with depressive and anxiety symptom trajectory classes four years from baseline to determine if inferences change over time. This was done to account for the single hearing assessment occurring four years after baseline.
All analyses were performed in Stata 16.0 (StataCorp, 2019). A p value <0.05 guided statistical interpretation. Access to the dataset can be requested by submitting a request to: https://healthabc.nia.nih.gov/eform/submit/analysis-plan.
3. Results
3.1. Study participants
The sample characteristics can be seen in Table 1. Those with DSI were older and more likely to be male and white compared to the other groups. Also, those with DSI were more likely to have a lower level of postsecondary education and were at a higher risk of having Type II diabetes. Additionally, DSI-affected individuals were more likely to have a history of cataracts, macular degeneration, and glaucoma (Table 1).
Table 1.
Sample characteristics of Health Aging and Body Composition participants (N = 2102).
| Overall | No Hearing or Vision Impairment | Hearing Impairment Only | Vision Impairment Only | Dual Sensory Impairment | p-value for the difference | |
|---|---|---|---|---|---|---|
| Sample characteristics | N = 2102 | n = 538 | n = 743 | n = 330 | n = 491 | |
| Age, in years, mean (SD) | 74.0 (2.8) | 72.9 (2.5) | 74.0 (2.7) | 73.9 (2.8) | 75.1 (2.9) | <0.001 |
| Female, n (%) | 1090 (51.9) | 346 (64.3) | 320 (43.07) | 190 (57.6) | 234 (47.7) | <0.001 |
| African American, n (%) | 766 (36.4) | 229 (42.6) | 190 (25.6) | 166 (50.3) | 181 (36.9) | <0.001 |
| Postsecondary education, n (%) | 959 (45.6) | 248 (46.1) | 357 (48.1) | 155 (47.0) | 199 (40.5) | 0.080 |
| Married, n (%) | 1110 (52.8) | 262 (48.7) | 438 (59.0) | 157 (47.6) | 253 (51.5) | <0.001 |
| Current smoking status, n (%) | 167 (7.9) | 36 (6.7) | 49 (6.6) | 35 (10.6) | 47 (9.6) | 0.046 |
| Body mass index, in kg/m2, mean (SD) | 27.4 (4.7) | 27.5 (4.9) | 27.2 (4.5) | 27.4 (4.7) | 27.5 (4.5) | 0.697 |
| Type II Diabetes Mellitus, n (%) | 359 (17.1) | 72 (13.4) | 123 (16.6) | 62 (18.8) | 102 (20.8) | 0.050 |
| Hypertension, n (%) | 1314 (62.5) | 330 (61.3) | 448 (60.3) | 222 (67.3) | 314 (64.0) | 0.086 |
| Hearing Aid Use, n (%) | 190 (9.0) | 4 (0.7) | 118 (15.9) | 2 (0.6) | 66 (13.4) | <0.001 |
| Pure-tone average, in decibels of hearing level, mean (SD) | 30.3 (13.4) | 18.0 (5.0) | 38.7 (11.0) | 18.6 (5.0) | 39.1 (10.0) | <0.001 |
| Impaired visual acuity, n (%) | 87 (4.1) | 0 (0.0) | 0 (0.0) | 32 (9.7) | 55 (11.2) | <0.001 |
| Impaired contrast sensitivity, n (%) | 813 (38.7) | 0 (0.0) | 0 (0.0) | 325 (98.5) | 488 (99.4) | <0.001 |
| History of cataracts, n (%) | 1142 (54.3) | 263 (48.9) | 367 (49.4) | 198 (60.0) | 314 (64.0) | <0.001 |
| History of macular degeneration, n (%) | 125 (6.0) | 22 (4.1) | 26 (3.5) | 26 (7.9) | 51 (10.4) | <0.001 |
| History of glaucoma, n (%) | 266 (12.7) | 41 (7.6) | 87 (11.7) | 53 (16.1) | 85 (17.3) | <0.001 |
| Antidepressant use, n (%) | 50 (2.4) | 10 (1.9) | 23 (3.1) | 3 (0.9) | 14 (2.9) | 0.194 |
| Presence of depressive symptoms at Year 1, n (%) | 185 (8.8) | 42 (7.8) | 57 (7.7) | 28 (8.5) | 58 (11.8) | 0.032 |
| Presence of depressive symptoms at Year 5, n (%) | 484 (23.0) | 116 (21.6) | 156 (21.0) | 69 (20.9) | 143 (29.1) | 0.005 |
| Presence of anxiety symptoms at Year 1, n (%) | 370 (17.7) | 89 (16.7) | 132 (17.8) | 50 (15.2) | 99 (20.2) | 0.270 |
| Presence of anxiety symptoms at Year 5, n (%) | 140 (6.7) | 46 (8.6) | 39 (5.3) | 20 (6.1) | 35 (7.1) | 0.001 |
| Low perceived social support in Year 1, n (%) | 0.195 | |||||
| At least one component present | 721 (34.3) | 169 (31.4) | 243 (32.7) | 131 (39.7) | 178 (36.3) | |
| Two components present | 145 (6.9) | 42 (7.8) | 46 (6.2) | 21 (6.4) | 36 (7.3) | |
| Three components present | 23 (1.1) | 9 (1.7) | 6 (0.8) | 2 (0.6) | 6 (1.2) | |
| Low perceived social support in Year 5, n (%) | 0.028 | |||||
| At least one component present | 694 (33.0) | 170 (31.6) | 237 (31.9) | 106 (32.1) | 181 (36.9) | |
| Two components present | 144 (6.9) | 42 (7.8) | 39 (5.3) | 27 (8.2) | 36 (7.3) | |
| Three components present | 18 (0.9) | 5 (0.9) | 5 (0.7) | 7 (2.1) | 1 (0.2) | |
| Years on study, mean (SD) | 9.3 (1.9) | 9.6 (1.6) | 9.4 (1.8) | 9.2 (2.0) | 8.8 (2.2) | <0.001 |
| Number of visits, n (%) | 0.513 | |||||
| 1 | 2102 (100.0) | 538 (100.0) | 743 (100.0) | 330 (100.0) | 491 (100.0) | |
| 2 | 2102 (100.0) | 538 (100.0) | 743 (100.0) | 330 (100.0) | 491 (100.0) | |
| 3 | 2102 (100.0) | 538 (100.0) | 743 (100.0) | 330 (100.0) | 491 (100.0) | |
| 4 | 2102 (100.0) | 538 (100.0) | 743 (100.0) | 330 (100.0) | 491 (100.0) | |
| 5 | 2033 (96.7) | 528 (98.1) | 721 (97.0) | 319 (96.7) | 465 (94.7) | |
| 6 | 1862 (88.6) | 496 (92.2) | 671 (90.3) | 289 (87.6) | 406 (82.7) | |
| 7 | 1675 (79.7) | 468 (87.0) | 607 (81.7) | 255 (77.3) | 345 (70.3) | |
| 8 | 1493 (71.0) | 422 (78.4) | 556 (74.8) | 226 (68.5) | 289 (58.9) |
There was missingness on post-secondary education (n = 5), marital status (n = 140), Type II diabetes (n = 1), Center for Epidemiologic Studies – Depression Scale at Year 1 (n = 9), Center for Epidemiologic Studies – Depression Scale at Year 5 (n = 5), social support at Year 1 (n = 5), social support at Year 6 (n = 236), anxiety measures at Year 5 (n = 1337), visual acuity (n = 3), cataracts (n = 16), glaucoma (n = 9), macular degeneration (n = 21), antidepressant use (n = 4). p-values for differences across groups were derived from ANOVA (continuous variables) and chi-squared tests (categorical variables). Low perceived social support is defined by the following: (i) dissatisfaction defined as the lower 10th percentile of the sample distribution on both questions (score < 6), (ii) low social contact defined as one time per week/less than once per week for friends and/or relatives, and (iii) low emotional support defined as needing more emotional support.
3.2. Trajectory group membership for depressive and anxiety symptoms
Fig. 1 shows the crude best-fit group-based univariate curves of trajectory classes for long-term depressive and anxiety symptoms. The optimal univariate trajectory model fit for depression and anxiety was achieved when three trajectory groups for both were specified (Supplementary Tables 2 and 3). The three patterns for the overall sample were rare/never (61.1 %), mild/moderately increasing (28.6 %), and chronically high (10.3 %). The three patterns for the overall sample for anxiety symptoms were rare/never (61.6 %), mild/moderately increasing (27.5 %), and chronically high (10.9 %).
Fig. 1.
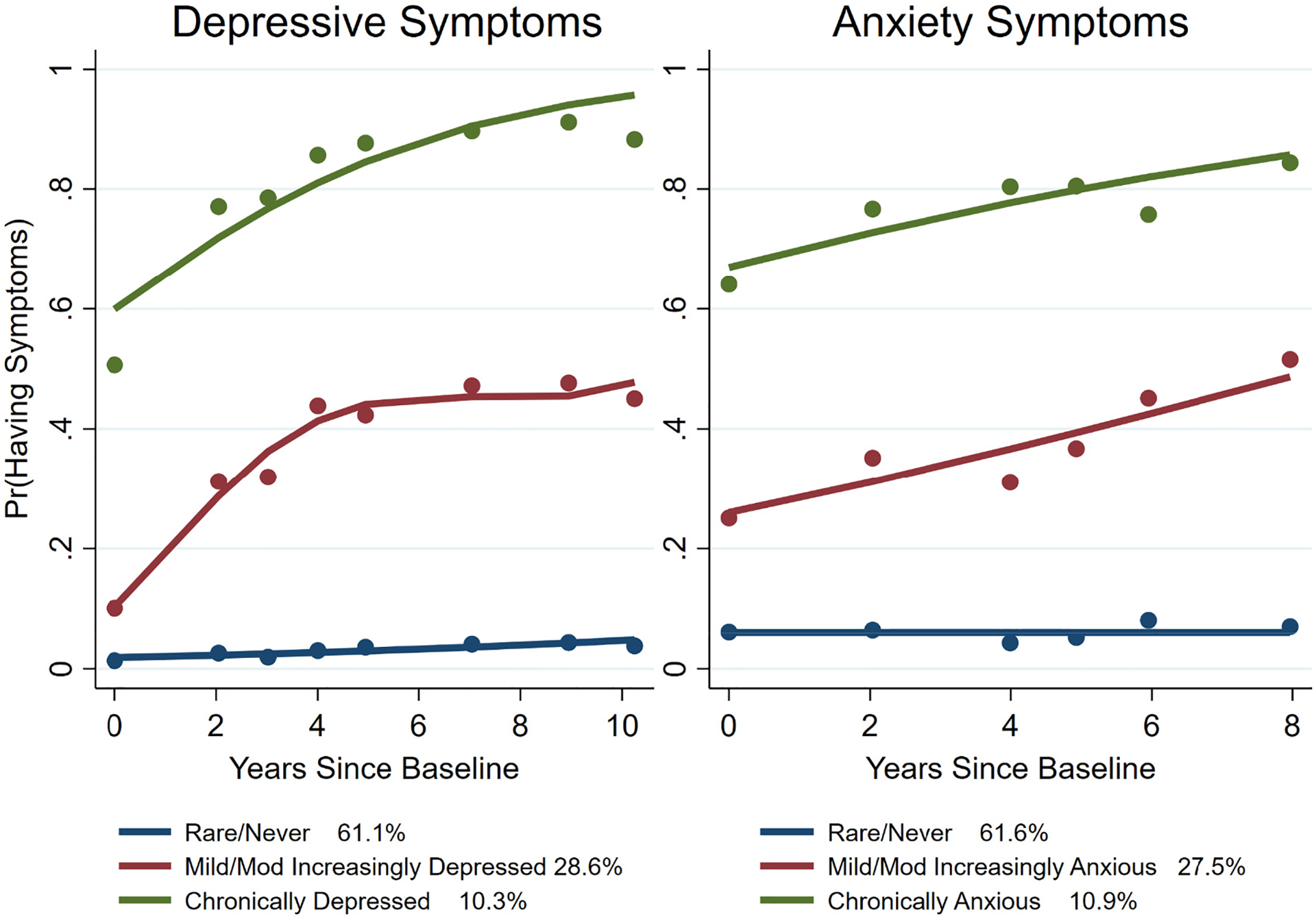
Trajectory classes for long-term depressive and anxiety symptoms.
Note: Pr(Having Symptoms) means prevalence for having either depressive or anxious symptoms at the timepoints on the x-axis. Mod means moderately. Anxiety symptoms were defined as the presence of anxiety symptoms in the previous week through the Hopkins Symptom Checklist. The anxiety threshold was defined as present if participants rated any symptom occurring at least “quite a bit” or any two symptoms occurring at least “a little”. Presence of clinically relevant depressive symptoms was defined as the cut-off score ≥ 8 on the 10-item Center for Epidemiologic Studies – Depression Scale.
3.3. Relationship of dual sensory impairment with depressive and anxiety symptom trajectory classes
Table 2 shows the associations of DSI categories with depressive and anxiety symptom trajectory classes. While there were no associations between DSI categories and the mild/moderately increasing depressive symptoms in the unadjusted and adjusted analyses, DSI was associated with being chronically depressed after covariate adjustment (Relative Risk, RR = 1.99, 95 % Confidence Interval, CI: 1.25, 3.17). The RERI between DSI and chronically depressed was 0.58 (95 % CI: −0.23, 1.38), and the SI was 3.24 (95 % CI: −7.16, 13.64). The linear combination of the effects for HI and VI is RR = 1.40 (95 % CI: 0.60, 3.27), which is less than the estimate for DSI (RR = 1.99), suggesting the presence of DSI contributes more risk than the two sensory impairments individually.
Table 2.
Associations of dual sensory impairment with depressive and anxiety symptom trajectory classes.
| Depressive symptom trajectory classes | Anxiety symptom trajectory classes | |||||||
|---|---|---|---|---|---|---|---|---|
| Mild/moderate increasingly depressed | Chronically depressed | Mild/moderate increasingly anxious | Chronically anxious | |||||
| Type of Sensory Impairment | Relative risk | 95 % Confidence Interval | Relative risk | 95 % Confidence Interval | Relative risk | 95 % Confidence Interval | Relative risk | 95 % Confidence Interval |
| Unadjusted | ||||||||
| Hearing Impairment Only | 0.84 | (0.64, 1.08) | 0.99 | (0.66, 1.47) | 1.12 | (0.86, 1.45) | 1.22 | (0.76, 1.96) |
| Vision Impairment Only | 1.09 | (0.80, 1.49) | 1.03 | (0.63, 1.69) | 1.14 | (0.83, 1.57) | 1.01 | (0.55, 1.85) |
| Dual Sensory Impairment | 1.33 | (1.01, 1.75) | 1.78 | (1.18, 2.67) | 1.45 | (1.09, 1.92) | 1.62 | (0.98, 2.67) |
| Adjusted | ||||||||
| Hearing Impairment Only | 0.96 | (0.72, 1.28) | 1.30 | (0.84, 2.03) | 1.34 | (1.01, 1.79) | 1.66 | (0.99, 2.81) |
| Vision Impairment Only | 1.08 | (0.77, 1.51) | 1.08 | (0.63, 1.84) | 1.19 | (0.84, 1.68) | 1.13 | (0.60, 2.16) |
| Dual Sensory Impairment | 1.25 | (0.91, 1.71) | 1.99 | (1.25, 3.17) | 1.60 | (1.16, 2.19) | 1.86 | (1.05, 3.27) |
Note: Depressive and anxiety symptom trajectory classes were assessed from baseline to Year 11 on study. Bolded estimates and their respective 95 % confidence intervals indicate that the 95 % confidence intervals did not overlap with 1. Multivariable multinomial logistic models were adjusted by baseline demographic characteristics (age, sex [female vs male], race [black vs. white], marital status [married vs. not], postsecondary education [attained vs. not]), current smoking status, history of Type II diabetes and hypertension, body mass index (BMI), and social support. Low perceived social support is defined by the following: (i) dissatisfaction defined as the lower 10th percentile of the sample distribution on both questions (score < 6), (ii) low social contact defined as one time per week/less than once per week for friends and/or relatives, and (iii) low emotional support defined as needing more emotional support.
Those with DSI were at an increased risk of being mild/moderate increasingly anxious (RR = 1.60, 95 % CI: 1.16, 2.19) and chronically anxious (RR = 1.86, 95 % CI: 1.05, 3.27) groups, as compared to the group without HI and VI. HI was associated with an increased risk of being mild/moderate increasingly anxious (RR = 1.34, 95 % CI: 1.09, 1.92), but not the chronically anxious (RR = 1.62, 95 % CI: 0.98, 2.67). VI was not associated with increased risk of having either mild/moderate increasingly or chronically high anxiety symptoms (Table 2). The RERI between DSI and mild/moderate increasingly anxious was −0.01 (95 % CI: −1.14, 1.11), and the SI was 0.98 (95 % CI: −0.31, 2.28). The estimate of both HI and VI (RR = 1.60, 95 % CI: 0.92, 2.76) was the same estimate for DSI (RR = 1.60), suggesting the presence of DSI did not contribute more risk than the two sensory impairments individually. The RERI between DSI and chronically anxious group was 0.21 (95 % CI: −0.25, 0.68), and the SI was 4.44 (95 % CI: −32.46, 41.33). The estimate of both HI and VI (RR = 1.89, 95 % CI: 0.69, 5.19) was less than the estimate for DSI (RR = 1.86), suggesting the presence of DSI did not contribute more risk than the two sensory impairments individually.
We evaluated whether low perceived social support modified the association of DSI with long-term depressive and anxiety symptom trajectory groups as an exploratory analysis. None of the two-way interaction terms between DSI and low perceived social support were significant (all p’s > 0.05) with the exception of the two-way interaction between VI and at least one low perceived social support component (RR = 5.16, 95 % CI: 1.45, 18.29). No one had VI only and 3 low perceived social support components.
As a sensitivity analysis, we derived the trajectory classes for depressive and anxiety symptoms at Year 5. The number of trajectory classes for depressive symptoms decreased from 3 to 2 groups, while the number of trajectory classes for anxiety remained the same. While there were no associations between DSI categories and the depressive symptoms group, there were associations of HI with increased risk of having chronically high anxiety symptoms (RR = 1.51, 95 % CI: 1.07, 2.11), starting in Year 5 (Supplementary Table 4).
3.4. Relationship of dual sensory impairment with social support
Supplementary Table 1 shows the frequencies of the combinations of the measures of low perceived social support for the overall sample and by DSI. Most of the sample (57.7 %; n = 1212) did not have any components of low social support (dissatisfaction, low emotional support, and low social contact). About 18.1 % of the sample reported low emotional support only, and 15.4 % reported low social contact only. All other combinations and dissatisfaction only were all below 5 %. In Table 1, approximately 1.1 % (n = 23) had all three components of low social support; 6.9 % (n = 145) had two components of low social support; 34.4 % (n = 721) had one component of low social support. The frequencies did not differ between Years 1 and 5. However, low social support measures differed across DSI categories (Table 1).
We examined the relationship between DSI and low perceived social support at Years 1 and 5 cross-sectionally (Table 3). There were no associations between the DSI categories and number of components of low perceived social support cross-sectionally in Years 1 and 5, except for VI and one component of low perceived social support in Year 1 (OR = 1.41, 95 % CI: 1.03, 1.92).
Table 3.
Cross-sectional association between dual sensory impairment and low perceived social support at Year 1 and Year 5 on study.
| Low perceived social support | Hearing Impairment Only | Vision Impairment Only | Dual Sensory Impairment | |||
|---|---|---|---|---|---|---|
| Relative Risk | 95 % Confidence Interval | Relative Risk | 95 % Confidence Interval | Relative Risk | 95 % Confidence Interval | |
| Year 1 | ||||||
| None of the components | REF | REF | REF | REF | REF | REF |
| At least one component is present | 1.10 | (0.85, 1.44) | 1.41 | (1.03, 1.92) | 1.25 | (0.93, 1.67) |
| Two components are present | 0.76 | (0.47, 1.24) | 0.86 | (0.49, 1.53) | 0.87 | (0.52, 1.47) |
| All three components are present | 0.38 | (0.13, 1.14) | 0.14 | (0.02, 1.14) | 0.52 | (0.17, 1.63) |
| Year 5 | ||||||
| None of the components | REF | REF | REF | REF | REF | REF |
| At least one component is present | 1.07 | (0.82, 1.39) | 1.01 | (0.74, 1.39) | 1.24 | (0.93, 1.66) |
| Two components are present | 0.65 | (0.40, 1.06) | 0.97 | (0.56, 1.67) | 1.02 | (0.61, 1.70) |
| All three components are present | 0.67 | (0.17, 2.62) | 2.48 | (0.70, 8.82) | 0.19 | (0.02, 1.78) |
Note: Multivariable multinomial logistic models were adjusted by sex, race, postsecondary education, age, body mass index, history of Type II diabetes and hypertension, and marital status (married vs. not). All continuous variables were mean-centered. Low perceived social support is defined by the following: (i) dissatisfaction defined as the lower 10th percentile of the sample distribution on both questions (score < 6), (ii) low social contact defined as one time per week/less than once per week for friends and/or relatives, and (iii) low emotional support defined as needing more emotional support. Bolded estimates and their respective 95 % confidence intervals indicate that the 95 % confidence intervals did not overlap with 1.
4. Discussion
DSI was associated with greater risk of having chronically high depressive symptoms and mild/moderate increasingly and chronically highly anxious symptoms. DSI was associated with an ~99 % increased risk of having chronically depressive symptoms group as well as with a ~60 % increased risk of being mild/moderate increasingly anxious and ~86 % increased risk of being chronically anxious. Interestingly, DSI contributed greater risk of having chronically high depressive symptoms and mild/moderate increasingly anxious symptoms than the individual sensory impairments. Low perceived social support does not appear to modify the association of DSI with the long-term depressive and anxiety symptom trajectory classes.
In somewhat contrast to our findings, investigators from one study reported DSI was associated with longitudinal increases in depressive symptoms over a 16-year follow-up period, which were explained by difficulties with activities of daily living and social engagement (Kiely et al., 2013). Meanwhile, Chou (2008) did not find any associations between DSI and depressive symptoms over a two-year period. It could be that the timing was too short to detect associations. While Cosh et al. (2018) reported no associations between DSI and anxiety symptoms over time, we found associations of DSI with mild/moderate increasingly anxious and chronically anxious symptom classes. These findings are consistent with a prior longitudinal study using data from the Health and Retirement Study found that those with DSI had more depressive symptoms than those without DSI at baseline and the number of depressive symptoms increased at a faster rate among those with DSI over a 13-year follow-up period, as compared to those without HI and VI (McDonnall, 2009a). Similarly, a longitudinal population-based study reported that DSI was associated with increased depressive, but not anxiety, symptoms over a 6-year follow-up period (Cosh et al., 2018).
DSI was not associated with low perceived social support at Years 1 and 5. The prevalence of low social support was 42.3 % in Year 1 and 40.7 % in Year 5. Additionally, two-way interaction between DSI and low perceived social support in the main and sensitivity analyses was insignificant, suggesting that low perceived social support did not modify our associations. It could be that low objective social support may modify these associations, but the study was limited to using scales based on perceived social support. Our results are in contrast to those of another study where social activity engagement explained the association of DSI with increases in depressive symptoms (Kiely et al., 2013). Social activity engagement was reported via self-report questionnaire called the Adelaide activity profile, a validated measure of lifestyle activities for older adults (Clark and Bond, 1995). Participants reported their level of participation in social and mentally stimulating activities over a 3-month period. Our measure of low social support did not gauge the level of participation in various activities. Rather, it was more of a subjectively measured variable based on the participants’ perception of support, which could have biased findings.
There were strengths and limitations of this study. Our study defined long-term depressive and anxiety symptoms over a 10-year follow-up period to better classify these long-term symptoms, as opposed to examining a snapshot of depressive and anxiety symptoms in a short period of time. Longer follow-up time is needed, as evidenced by lack of associations in sensitivity analyses that utilized a shorter follow-up period among older adults without functional limitations. Other strengths included objective measures for hearing and vision. A limitation from our study is that our participants are from a healthier subset of the HABC cohort, thus limiting the generalizability of these findings. Other limitations include the sensory impairments being measured at two different time points, the use of time-fixed sensory impairments, and loss to follow-up. This could have led to some misclassification of sensory impairment, and we could not evaluate change from normal to sensory impairment. Loss to follow-up is associated with depressive and anxiety symptoms, so this could have affected the estimates in a conservative direction.
Although there is a high prevalence of DSI among older adults, it is poorly understood (Davila et al., 2009), under-recognized, and under-diagnosed (Heine and Browning, 2002). There is a lack of consensus as to what clinical cut points can be used to define HI and VI, and structural barriers may exist for those with HI and VI. Those with DSI may experience diminished quality of life (Brennan et al., 2006; Dalton et al., 2003). This feeling of diminished quality of life could be associated with the onset of depression and anxiety. By recognizing DSI as being part of the factors that influences depression and anxiety, this can lead to better rehabilitation options and increase communication-based situations (Heine and Browning, 2002). Rehabilitation can be employed for compensatory strategies, modifications in environment, and use of assistive tools on an as-needed basis. In turn, these can improve self-esteem and self-confidence as well as postpone cognitive decline, leading to independent living for longer periods of time (Guthrie et al., 2018; Guthrie et al., 2016) and better quality of life (Tseng et al., 2018). Screening older adults for DSI, depression, and anxiety may identify treatment opportunities earlier to optimize health and well-being. Further investigation is needed to see if screening affects these associations. Additionally, potential mediators, i.e., social support, loneliness, and falls/fractures, of the association of DSI with long-term depressive and anxiety symptoms could be explored in other prospective cohort studies.
Supplementary Material
Acknowledgments
We would like to thank the staff and participants of the Health Aging and Body Composition Study.
Funding
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; R01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and National Institute of Nursing Research NINR grant R01-NR012459 from the National Institutes of Health (NIH) in the USA. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. JAD was supported by NIH/NIA grant K01AG054693. WDB was supported by NIH/NIA grant K01AG062722.
Sponsor’s role
The authors’ sponsors had no role in the design, methods, data collection, analysis or preparation of this paper.
Conflict of Interest
Frank R. Lin is a consultant to Frequency Therapeutics, received speaker honoraria from Caption Call, and director of the Cochlear Center for Hearing and Public Health—a public health research center funded in part by a philanthropic gift from Cochlear Ltd. to the Johns Hopkins Bloomberg School of Public Health. All other authors have nothing additional to disclose.
Abbreviations:
- DSI
dual sensory impairment
- LPSS
low perceived social support
- HI
hearing impairment
- VI
visual impairment
- HABC
Health Aging and Body Composition Study
- RR
relative risk
- CI
confidence interval
- RERI
relative excess risk due to interaction
- SI
synergy index
Footnotes
CRediT authorship contribution statement
Study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision: Nicole Armstrong. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Camila Vieira Ligo Teixeira. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Colby Gendron. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Willa Brenowitz. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Frank Lin. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Bonnelin Swenor. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Jennifer Deal. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Danielle Powell. Study concept and design; analysis and interpretation of data; acquisition of data; critical revision of the manuscript for important intellectual content: Eleanor Simonsick. Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Rich Jones.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jad.2022.07.067.
References
- Andresen E, Malmgren J, Carter W, Patrick D, 1994. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am. J. Prev. Med 10 (2), 77–84. [PubMed] [Google Scholar]
- Armstrong NM, Deal JA, Betz J, Kritchevsky S, Pratt S, Harris T, Barry LC, Simonsick EM, Lin FR, 2020. Associations of hearing loss and depressive symptoms with incident disability in older adults: health, aging, and body composition study. J. Gerontol. A Biol. Sci. Med. Sci 75 (3), 531–536. 10.1093/gerona/gly251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey IL, Lovie JE, 1976. New design principles for visual acuity letter charts. Am. J. Optom. Physiol. Optic 53 (11), 740–745. 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- Beekman ATF, Bremmer MA, Deeg D, Van Balkom A, Smit JH, Beurs ED, Dyck RV, Tilburg WV, 1999. Anxiety disorders in later life: a report from the longitudinal aging study Amsterdam. Int. J. Geriatr. Psychiatry 13 (10), 717–726. . [DOI] [PubMed] [Google Scholar]
- Boehlen FH, Herzog W, Schellberg D, Maatouk I, Schoettker B, Brenner H, Wild B, 2020. Gender-specific predictors of generalized anxiety disorder symptoms in older adults: results of a large population-based study. J. Affect. Disord 262, 174–181. 10.1016/j.jad.2019.10.025. [DOI] [PubMed] [Google Scholar]
- Brenes GA, Guralnik JM, Williamson J, Fried LP, Penninx BWJH, 2005. Correlates of anxiety symptoms in physically disabled older women. Am. J. Geriatr. Psychiatry 13 (1), 15–22. 10.1097/00019442-200501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M, Su YP, Horowitz A, 2006. Longitudinal associations between dual sensory impairment and everyday competence among older adults. J. Rehabil. Res. Dev 43 (6), 777–792. 10.1682/jrrd.2005.06.0109. [DOI] [PubMed] [Google Scholar]
- Brewster KK, Ciarleglio A, Brown PJ, Chen C, Kim H-O, Roose SP, Golub JS, Rutherford BR, 2018. Age-related hearing loss and its association with depression in later life. Am. J. Geriatr. Psychiatry 26 (7), 788–796. 10.1016/j.jagp.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Vittinghoff E, Lui L-Y, Hoang T, Blazer DG, Covinsky KE, Ensrud KE, Cauley JA, Hillier TA, Fredman L, 2012. Twenty-year depressive trajectories among older women. Arch. Gen. Psychiatry 69 (10), 1073–1079. 10.1001/archgenpsychiatry.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-McDonnall ME, 2005. The effects of single and dual sensory loss on symptoms of depression in the elderly. Int. J. Geriatr. Psychiatry 20 (9), 855–861. 10.1002/gps.1368. [DOI] [PubMed] [Google Scholar]
- Carrière I, Delcourt C, Daien V, Pérès K, Féart C, Berr C, Laure Ancelin M, Ritchie K, 2013. A prospective study of the bi-directional association between vision loss and depression in the elderly. J. Affect. Disord 151 (1), 164–170. 10.1016/j.jad.2013.05.071. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx B, Newman AB, Kritchevsky SB, Nicklas B, Sutton-Tyrrell K, Rubin S, Ding J, Simonsick EM, Harris TB, Pahor M, 2003. Inflammatory markers and onset of cardiovascular events: results from the health ABC study. Circulation 108 (19). 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Chen DS, Betz J, Yaffe K, Ayonayon HN, Kritchevsky S, Martin KR, Harris TB, Purchase-Helzner E, Satterfield S, Xue QL, Pratt S, Simonsick EM, Lin FR, 2015. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J. Gerontol. A Biol. Sci. Med. Sci 70 (5), 654–661. 10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, 2008. Combined effect of vision and hearing impairment on depression in older adults: evidence from the english longitudinal study of ageing. J. Affect. Disord 106 (1–2), 191–196. 10.1016/j.jad.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Clark MS, Bond M, 1995. The Adelaide activities profile: a measure of the lifestyle activities of elderly people. Aging 7 (4), 174–184. 10.1007/BF03324332. [DOI] [PubMed] [Google Scholar]
- Contrera KJ, Betz J, Deal JA, Choi JS, Ayonayon HN, Harris T, Helzner E, Martin KR, Mehta K, Pratt S, Rubin SM, Satterfield S, Yaffe K, Garcia M, Simonsick EM, Lin FR, Health ABCS, 2016. Association of hearing impairment and emotional vitality in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci 71 (3), 400–404. 10.1093/geronb/gbw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrera KJ, Betz J, Deal J, Choi JS, Ayonayon HN, Harris T, Helzner E, Martin KR, Mehta K, Pratt S, Rubin SM, Satterfield S, Yaffe K, Simonsick EM, Lin FR, 2017. Association of hearing impairment and anxiety in older adults. Aging Health 29 (1), 172–184. 10.1177/0898264316634571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosh S, von Hanno T, Helmer C, Bertelsen G, Delcourt C, Schirmer H, 2018. The association amongst visual, hearing, and dual sensory loss with depression and anxiety over 6 years: the Tromsø study. Int. J. Geriatr. Psychiatry 33 (4), 598–605. 10.1002/gps.4827. [DOI] [PubMed] [Google Scholar]
- Cui X, Lyness JM, Tang W, Tu X, Conwell Y, 2008. Outcomes and predictors of late-life depression trajectories in older primary care patients. Am. J. Geriatr. Psychiatry 16 (5), 406–415. 10.1097/JGP.0b013e3181693264. [DOI] [PubMed] [Google Scholar]
- Curran E, Rosato M, Ferry F, Leavey G, 2020. Prevalence and factors associated with anxiety and depression in older adults: gender differences in psychosocial indicators. J. Affect. Disord 267, 114–122. 10.1016/j.jad.2020.02.018. [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, Nondahl DM, 2003. The impact of hearing loss on quality of life in older adults. Gerontologist 43 (5), 661–668. 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- Davila E, Caban-Martinez A, Muennig P, Lee D, Fleming L, Ferraro K, LeBlanc W, Lam B, Arheart K, McCollister K, Zheng D, Christ S, 2009. Sensory impairment among older US workers. Am. J. Public Health 99 (8), 1378–1385. 10.2105/AJPH.2008.141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beurs E, Beekman ATF, Deeg DJH, Van Dyck R, Van Tilburg W, 2000. Predictors of change in anxiety symptoms of older persons: results from the longitudinal aging study Amsterdam. Psychol. Med 30 (3), 515–527. 10.1017/S0033291799001956. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L, 1974. The Hopkins symptom checklist (HSCL): a self-report symptom inventory. Behav. Sci 19, 1–15. [DOI] [PubMed] [Google Scholar]
- Elliott D, Sanderson K, Conkey A, 1990. The reliability of the pelli-Robson contrast sensitivity chart. Ophthalmic Physiol. Opt 10 (1), 21–24. [PubMed] [Google Scholar]
- Fiske A, Wetherell JL, Gatz M, 2009. Depression in older adults. Annu. Rev. Clin. Psychol 5, 363–389. 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsell Y, Henderson AS, 1998. Epidemiology of paranoid symptoms in an elderly population. Br. J. Psychiatry 172 (5), 429–432. 10.1192/bjp.172.5.429. [DOI] [PubMed] [Google Scholar]
- Frank CR, Xiang X, Stagg BC, Ehrlich JR, 2019. Longitudinal associations of self-reported vision impairment with symptoms of anxiety and depression among older adults in the United States. JAMA Ophthalmol. 137 (7), 793–800. 10.1001/jamaophthalmol.2019.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Aubin MJ, Buhrmann R, Kergoat MJ, Freeman EE, 2021. Visual impairment, eye disease, and the 3-year incidence of depressive symptoms: the Canadian longitudinal study on aging. Ophthalmic Epidemiol. 28 (1), 77–85. 10.1080/09286586.2020.1823425. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Simonsick EM, Lafferty ME, Kasper JD, 1995. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. DIANE Publishing. [Google Scholar]
- Guthrie DM, Declercq A, Finne-Soveri H, Fries BE, Hirdes JP, 2016. The health and well-being of older adults with dual sensory impairment (DSI) in four countries. PLoS One 11 (5), e0155073. 10.1371/journal.pone.0155073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie DM, Davidson JGS, Williams N, Campos J, Hunter K, Mick P, Orange JB, Pichora-Fuller MK, Phillips NA, Savundranayagam MY, Wittich W, 2018. Combined impairments in vision, hearing and cognition are associated with greater levels of functional and communication difficulties than cognitive impairment alone: analysis of interRAI data for home care and long-term care recipients in Ontario. PLoS One 13 (2), e0192971. 10.1371/journal.pone.0192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine C, Browning CJ, 2002. Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disabil. Rehabil 24 (15), 763–773. 10.1080/09638280210129162. [DOI] [PubMed] [Google Scholar]
- Heine C, Browning CJ, 2014. Mental health and dual sensory loss in older adults: a systematic review. Front. Aging Neurosci 6 10.3389/fnagi.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Moreno L, Senra H, Moreno N, Macedo AF, 2021. Is perceived social support more important than visual acuity for clinical depression and anxiety in patients with age-related macular degeneration and diabetic retinopathy? Clin. Rehabil 35 (9), 1341–1347. 10.1177/0269215521997991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsiaras A, Newman AB, Kriska A, Brach J, Krishnaswami S, Feingold E, Kritchevsky S, Li R, Harris TB, Schwartz A, Goodpaster BH, 2005. Skeletal muscle fatigue, strength, and quality in the elderly: the health ABC study. J. Appl. Physiol 99 10.1152/japplphysiol.01276.2004. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Byers AL, Falvey C, Simonsick EM, Satterfield S, Ayonayon HN, Smagula SF, Rubin SM, Yaffe K, 2016. Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry 73 (5), 525–531. 10.1001/jamapsychiatry.2016.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely K, Anstey K, Luszcz M, 2013. Dual sensory loss and depressive symptoms: the importance of hearing, daily functioning, and activity engagement. Front. Hum. Neurosci 7 (837) 10.3389/fnhum.2013.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhatla MN, Fillenbaum GG, Hybels CF, Blazer DG, 2012. Trajectory classes of depressive symptoms in a community sample of older adults. Acta Psychiatr. Scand 125 (6), 492–501. 10.1111/j.1600-0447.2011.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, Xue Q-L, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM, for the Health ABC Study Group, 2013. Hearing loss and cognitive decline in older adults. JAMA Intern. Med 173 (4), 293–299. 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovie-Kitchin J, 1988. Validity and reliability of visual acuity measurements. Ophthalmic Physiol. Opt 8 (4), 363–370. [DOI] [PubMed] [Google Scholar]
- Lupsakko T, Mäntyjäarvi M, Kautiainen H, Sulkava R, 2002. Combined hearing and visual impairment and depression in a population aged 75 years and older. Int. J. Geriatr. Psychiatry 17 (9), 808–813. 10.1002/gps.689. [DOI] [PubMed] [Google Scholar]
- Maaswinkel IM, van der Aa HPA, van Rens G, Beekman ATF, Twisk JWR, van Nispen RMA, 2020. Mastery and self-esteem mediate the association between visual acuity and mental health: a population-based longitudinal cohort study. BMC Psychiatry 20 (1), 461. 10.1186/s12888-020-02853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnall MC, 2009a. The effects of developing a dual sensory loss on depression in older adults: a longitudinal study. Aging Health 21 (8), 1179–1199. 10.1177/0898264309350077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnall MC, 2009b. Risk factors for depression among older adults with dual sensory loss. Aging Ment. Health 13 (4), 569–576. 10.1080/13607860902774410. [DOI] [PubMed] [Google Scholar]
- Mehta K, Simonsick E, Penninx B, Schulz R, Rubin S, Satterfield S, Yaffe K, 2003. Prevalence and correlates of anxiety symptoms in well-functioning older adults: findings from the health aging and body composition study. J. Am. Geriatr. Soc 51 (4), 499–504. 10.1046/j.1532-5415.2003.51158.x. [DOI] [PubMed] [Google Scholar]
- Mehta K, Yaffe K, Brenes G, Newman N, Shorr R, Simonsick E, Ayonayon H, Rubin S, Covinsky K, 2007. Anxiety symptoms and decline in physical function over 5 years in the health, aging and body composition study. J. Am. Geriatr. Soc 55 (2), 265–270. 10.1111/j.1532-5415.2007.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick P, Kawachi I, Lin FR, 2014. The association between hearing loss and social isolation in older adults. Otolaryngol. Head Neck Surg 150 (3), 378–384. [DOI] [PubMed] [Google Scholar]
- Morrell CH, Gordon-Salant S, Pearson JD, Brant LJ, Fozard JL, 1996. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. J. Acoust. Soc. Am 100 (4), 1949–1967. 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Hagaman AK, Reinders I, Steeves JA, Newman AB, Rubin SM, Satterfield S, Kritchevsky SB, Yaffe K, Ayonayon HN, Nagin DS, Simonsick EM, Penninx BWJH, Harris TB, Health ABC Study, 2016. Depressive trajectories and risk of disability and mortality in older adults: longitudinal findings from the Health, Aging, and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci 71 (2), 228–235. 10.1093/gerona/glv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin DS, Odgers CL, 2010. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol 6 (1), 109–138. 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE, 2001. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol. Methods 6 (1), 18–34. [DOI] [PubMed] [Google Scholar]
- Pelli D, Robson J, Wilkins A, 1988. The design of a new letter chart for measuring contrast sensitivity. Clin. Vis. Sci 2, 187–199. [Google Scholar]
- Radloff LS, 1977. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas 1 (3), 385–401. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL, 2008. Modern Epidemiology, Vol. 3. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Saunders GH, Echt KV, 2007. An overview of dual sensory impairment in older adults: perspectives for rehabilitation. Trends Amplif. 11 (4), 243–258. 10.1177/1084713807308365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senra H, Balaskas K, Mahmoodi N, Aslam T, 2017. Experience of anti-VEGF treatment and clinical levels of depression and anxiety in patients with wet age-related macular degeneration. Am J. Ophthalmol 177, 213–224. 10.1016/j.ajo.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Simning A, Fox ML, Barnett SL, Sorensen S, Conwell Y, 2019. Depressive and anxiety symptoms in older adults with auditory, vision, and dual sensory impairment. Aging Health 31 (8), 1353–1375. 10.1177/0898264318781123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Kasper JD, Phillips CL, 1998. Physical disability and social interaction: factors associated with low social contact and home confinement in disabled older women (The Women’s health and aging Study). J. Gerontol. B Psychol. Sci. Soc. Sci 53 (4), S209–S217. 10.1093/geronb/53b.4.s209. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Guralnik JM, Fried LP, 2015. Who walks? Factors associated with walking behavior in disabled older women with and without self-reported walking difficulty. J. Am. Geriatr. Soc 47 (6), 672–680. 10.1111/j.1532-5415.1999.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Snowden MB, Atkins DC, Steinman LE, Bell JF, Bryant LL, Copeland C, Fitzpatrick AL, 2015. Longitudinal association of dementia and depression. Am. J. Geriatr. Psychiatry 23 (9), 897–905. 10.1016/j.jagp.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp, 2019. Stata Statistical Software: Release 16. StataCorp LLC, College Station, TX. [Google Scholar]
- Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR, 2013. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern. Med 173 (4), 312–313. 10.1001/jamainternmed.2013.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenor BK, Simonsick EM, Ferrucci L, Newman AB, Rubin S, Wilson V, Health, Aging, Body Composition Study, 2015. Visual impairment and incident mobility limitations: the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc 63 (1), 46–54. 10.1111/jgs.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambs K, 2004. Moderate effects of hearing loss on mental health and subjective well-being: results from the Nord-Trøndelag hearing loss study. Psychosom. Med 66 (5), 776–782. 10.1097/01.psy.0000133328.03596.fb. [DOI] [PubMed] [Google Scholar]
- Tseng Y-C, Liu SH-Y, Lou M-F, Huang G-S, 2018. Quality of life in older adults with sensory impairments: a systematic review. Qual. Life Res 27 (8), 1957–1971. 10.1007/s11136-018-1799-2. [DOI] [PubMed] [Google Scholar]
- Yu B, Zhang X, Wang C, Sun M, Jin L, Liu X, 2020. Trends in depression among adults in the United States, NHANES 2005–2016. J. Affect. Disord 263, 609–620. 10.1016/j.jad.2019.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


