Abstract
A Fur titration assay was used to isolate DNA fragments bearing putative Fur binding sites (FBS) from a partial Bordetella bronchiseptica genomic DNA library. A recombinant plasmid bearing a 3.5-kb DNA insert was further studied. Successive deletions in the cloned fragment enabled us to map a putative FBS at about 2 kb from one end. Sequence analysis revealed the presence of an FBS upstream from a new gene encoding an AraC-type transcriptional regulator. The deduced protein displays similarity to PchR, an activator of pyochelin siderophore and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. Homologous genes in Bordetella pertussis and Bordetella parapertussis were PCR amplified, and sequence comparisons indicated a very high conservation in the three species. The B. pertussis and B. bronchiseptica chromosomal genes were inactivated by allelic exchange. Under low-iron growth conditions, the mutants did not secrete the alcaligin siderophore and lacked AlcC, an alcaligin biosynthetic enzyme. Alcaligin production was restored after transformation with a plasmid bearing the wild-type gene. On the basis of its role in regulation of alcaligin biosynthesis, the new gene was designated alcR. Additional sequence determination showed that alcR is located about 2 kb downstream from the alcABC operon and is transcribed in the same orientation. Two tightly linked open reading frames, alcD and alcE, were identified between alcC and alcR. AlcE is a putative iron-sulfur protein; AlcD shows no homology with the proteins in the database. The production of major virulence factors and colonization in the mouse respiratory infection model are AlcR independent.
To succeed in colonization of the host and subsequently cause disease, bacterial pathogens must first adhere to target tissues and concomitantly obtain nutrients which are essential for their growth. Iron is usually one such essential nutrient, and the ability of a pathogen to scavenge iron is an important virulence trait (55). In animals, the iron is not freely available to microorganisms, as it is bound to proteins such as transferrin (TF) and lactoferrin (LF) in the serum and other secretory fluids. Therefore, in order to survive, bacteria have evolved various iron uptake mechanisms. Some species, e.g., Escherichia coli and Pseudomonas spp., secrete low-molecular-weight siderophores which display a high affinity for ferric ions (36). These molecules can remove Fe(III) from TF or LF, and iron-loaded siderophores can bind to specific receptors on the bacterial surface to finally deliver the iron into the cell. Other bacteria, e.g., Neisseria spp. and Haemophilus influenzae, do not synthesize siderophores but produce receptors for the TF- and LF-iron complexes allowing iron uptake through direct contact between these host iron-binding proteins and the bacterial cell surface (9, 23, 46, 47). Bordetella pertussis, the etiologic agent of whooping cough in humans, and Bordetella bronchiseptica, the causative agent of swine atrophic rhinitis and kennel cough, may possess both iron uptake systems, since they synthesize alcaligin, a hydroxamate-type siderophore (1, 21, 34), as well as an outer membrane LF-binding protein (31, 43).
Iron uptake systems are usually expressed only under iron-limited growth conditions. In several species, this regulation mechanism involves the Fur (ferric ion uptake regulation) protein (24). In iron-rich growth conditions, the Fur repressor chelates Fe(II), binds to operator sequences in the promoter region of its target genes, and blocks transcription. These operators are called Fur-binding sites (FBS) or iron boxes. Under low-iron conditions, Fur is unable to bind to the FBS (14). Fur and iron may also modulate the expression of genes encoding virulence factors unrelated to iron metabolism, such as exotoxin A in Pseudomonas aeruginosa (40, 41), Shiga-like toxin in E. coli (13), or pH-regulated proteins in Salmonella typhimurium (17). Thus, the iron status of the environment appears to be used as a signal to trigger the expression of virulence genes in many pathogens.
Little is known about iron regulation in the bordetellae. The fur genes of B. pertussis, B. bronchiseptica, and Bordetella parapertussis have been cloned and sequenced recently (4, 12, 39). Several iron-repressed or iron-induced proteins have been detected (1, 3, 31), but only a few Fur target genes have been identified so far. Among them is the alcABC operon, coding for the first three enzymes of the alcaligin siderophore biosynthesis pathway (20, 28). Other cloned Fur-repressed genes encode outer membrane proteins BfeA, BfrB, and BfrC, receptors for ferric enterobactin and other hydroxamate siderophores in B. pertussis and B. bronchiseptica (5, 7), and BfrA, an unidentified exogenous siderophore receptor specific to B. bronchiseptica (6). The alcaligin receptor and its structural gene have not been characterized yet. To further elucidate the iron regulatory network in bordetellae and to study its involvement in virulence expression, we used the Fur titration assay (FURTA) of Stojiljkovic et al. to isolate Fur target genes (54). The same genetic approach has led to the recent identification of the B. pertussis sodA gene, encoding an Mn-containing superoxide dismutase (22). We present here the cloning and sequencing of a new Fur-repressed gene, alcR, encoding an AraC-type transcriptional regulator in B. bronchiseptica, B. pertussis, and B. parapertussis. This gene is located 2 kb downstream from the alcaligin biosynthesis operon. Sequence analysis of the alcABC-alcR intergenic region suggests that the alc operon may contain two additional open reading frames (ORFs). Construction and characterization of B. pertussis and B. bronchiseptica alcR mutants showed that AlcR is necessary for expression of the alcABC operon and thus required for alcaligin production but that it is not involved in the expression of the major B. pertussis virulence factors, filamentous hemagglutinin (FHA), pertussis toxin (PTX), pertactin (PRN), and adenylate cyclase hemolysin (AC-Hly). In vivo studies revealed that AlcR is not required for colonization in the mouse respiratory infection model.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this work are listed in Table 1. E. coli strains were grown at 37°C in Luria-Bertani (LB) medium (33) or on solid media obtained by addition of Bacto-Agar (1.5% [wt/vol]; Difco). Bordetella strains were grown at 37°C on Bordet-Gengou (BG) (10) agar base plates (Difco) supplemented with 1% glycerol and 15% sheep blood. Liquid cultures were grown in Stainer-Scholte (SS) medium (51) containing 10 μg of FeSO4 · 7H2O per ml (iron-rich SS medium) or in SS medium without addition of FeSO4 · 7H2O (iron-limited SS medium). Bordetella avium was grown in SS medium supplemented with 2 mg of 2-ketoglutarate per ml, 2 mg of pyruvate per ml, 10 μg of pantothenate per ml, 20 μg of l-phenylalanine per ml, and 0.5 mg of nicotinamide per ml. When necessary, antibiotics were included in the growth media at the following concentrations (in micrograms per milliliter): ampicillin, 150; chloramphenicol, 30; gentamicin, 10; kanamycin, 30; nalidixic acid, 30; streptomycin, 100; and tetracycline, 20.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant featuresa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| H1717 | aroB fhuF::λplacMu; Apr | 54 |
| XL1-Blue | High-efficiency transformation; Tcr | Stratagene |
| SM10 | Mobilizing strain; Kmr | 50 |
| S17.1 | Mobilizing strain; Smr | 50 |
| B. avium 103004 | Smr | Institut Pasteur, Paris, France |
| B. bronchiseptica | ||
| BB1015 | Smr derivative of NL1015 but not rpsL | This study |
| BBEP205 | Derivative of BB1015 but alcR::Kmr | This study |
| B. parapertussis PEP | 37 | |
| B. pertussis | ||
| BPSM | Tohama I rpsL; Smr Nalr | 32 |
| BPEP184 | Derivative of BPSM but alcR::Kmr | This study |
| BP953 | Tohama I rpsL fhaB::lacZ ptx::phoA; Smr Nalr | 53 |
| BPEP214 | Derivative of BP953 but alcR::Kmr | This study |
| Plasmids | ||
| pBBR1MCS | Broad-host-range vector; Cmr | 29 |
| pBCSK+ | High-copy-number cloning vector; Cmr | Stratagene |
| pEP279 | pBCSK+ containing a 3.5-kb PstI fragment carrying the B. bronchiseptica alcR gene; Cmr | This study |
| pEP300 | Derivative of pEP279 with a Kmr cassette inserted in the alcR gene; Cmr Kmr | This study |
| pEP301 | pBBR1MCS carrying a 1.6-kb KpnI-PstI fragment derived from pEP279; Cmr | This study |
| pEP308 | Derivative of pJQ200KS+ carrying a Kmr cassette flanked by alcR-derived sequences; Gnr Kmr | This study |
| pEP319 | Derivative of pSS1129 carrying a Kmr cassette flanked by alcR-derived sequences; Gnr Apr Kmr | This study |
| pJQ200KS+ | Bordetella suicide vector; Gnr | 42 |
| pSS1129 | Bordetella suicide vector; Gnr Apr | 52 |
| pUC4K | Source of Kmr cassette | Pharmacia |
Apr, Cmr, Gnr, Kmr, Nalr, Smr, and Tcr, resistance to ampicillin, chloramphenicol, gentamicin, kanamycin, nalidixic acid, streptomycin, and tetracycline, respectively.
DNA techniques.
Plasmid DNA was routinely isolated by the alkaline lysis method (45) or purified by using a Nucleobond AX kit (Macherey-Nagel, Hoerdt, France) for sequencing. Restriction endonucleases and T4 DNA ligase were obtained from Boehringer Mannheim and used according to standard procedures (45). The PstI DNA fragment inserted into pEP279 and appropriate subclones was sequenced by using a T7 polymerase kit from Pharmacia, α-35S-dCTP, and a combination of universal, reverse, and custom-synthesized primers (Pharmacia). The sequence was later confirmed by using an ABI PRISM Dye Terminator Cycle Sequencing kit and an ABI PRISM 377 sequencer (Perkin-Elmer). The B. pertussis and B. parapertussis alcR chromosomal locus was amplified by PCR with Vent DNA polymerase (New England Biolabs Inc., Beverly, Mass.) and oligonucleotides B10 (5′-GACGATGAAATCGGTGAGCGC-3′) and B3′ (5′-GCGCCGAAGGCTGGCAGGTAG-3′), which start at positions 1826 and 3306 (complementary strand) in the deposited B. bronchiseptica sequence, respectively. PCR products were cloned into the EcoRV site of pBCSK+. For each Bordetella species, sequence analysis was carried out on two independently isolated recombinant plasmids bearing inserts in the opposite orientation.
FURTA.
The FURTA was essentially performed as described by Stojiljkovic et al. (54). A partial B. bronchiseptica BB1015 genomic library had been constructed previously to isolate the fur gene in this organism (38). This strain is an Smr derivative of NL1015 (31). Chromosomal PstI DNA fragments with sizes ranging from 2 to 4 kb had been cloned into the high-copy-number plasmid pBCSK+ (Stratagene, San Diego, Calif.). E. coli H1717 carrying the chromosomal Fur-repressible fhuF::lacZ fusion was transformed with this partial library, and Cmr transformants were screened for the Lac+ phenotype on MacConkey lactose agar plates (Difco) containing 50 μM FeCl3. Four red colonies (Lac+) were isolated. Restriction mapping of the four recombinant plasmids (pEP276 to pEP279) showed that each one contained a distinct DNA insert. The recombinant plasmid conferring the strongest Lac+ phenotype in the assay, pEP279, was further studied.
Computer analysis of sequences.
The nucleotide and protein sequences were analyzed by using DNA Strider 1.2 software (Service de Biochimie et de Génétique Moléculaire du CEA, Saclay, France). Sequence homology was identified with the help of the BLASTN and BLASTP programs (2).
Construction of alcR mutants.
A HincII DNA restriction fragment of 1.3 kb containing the Kmr cassette from pUC4K (Pharmacia) was inserted into the unique NruI site of pEP279. The resulting plasmid, pEP300, was digested with BamHI and SalI, and the 4.8-kb DNA fragment bearing alcR::Kmr was subcloned into Gnr suicide vector pJQ200KS+ (42). The obtained plasmid, pEP308, was transformed into E. coli S17.1 (50) and then transferred to B. bronchiseptica BB1015 by conjugation as previously described (52). Exconjugants bearing the pEP308 insertion in the chromosome were selected on BG agar-streptomycin-gentamicin plates. The Bacillus subtilis sacB gene present on pJQ200KS+ had been shown to confer sucrose sensitivity to several gram-negative bacteria (42). We tried to select for double recombinants by plating exconjugants on plates of BG agar plus kanamycin or LB plus kanamycin supplemented with 5 or 10% sucrose, but all exconjugants were sucrose resistant. This suggests that, in contrast to the case for E. coli, the sacB gene was not expressed from its own promoter in B. bronchiseptica. To isolate alcR mutants without the help of counterselection, one exconjugant was grown to stationary phase in iron-rich SS medium plus streptomycin, and culture dilutions were plated onto BG agar plus streptomycin plus kanamycin. Of 333 clones tested, 15 spontaneous Kmr Smr Gns mutants were isolated. One of the alcR::Kmr mutants was designated BBEP205.
For the allelic exchange in B. pertussis, we constructed pEP319, a derivative of suicide vector pSS1129 (52). For this purpose, pEP300 was digested with EcoRI, and the resulting 3.4-kb EcoRI DNA fragment carrying alcR::Kmr was cloned into pSS1129 to give pEP319. E. coli SM10 (50) was transformed with pEP319 and used as a donor in conjugation with B. pertussis BPSM. Exconjugants were selected on BG agar-nalidixic acid-kanamycin plates. One such exconjugant was grown to stationary phase, and culture dilutions were plated onto BG agar plus kanamycin. Of 116 isolated colonies tested, only one, designated BPEP184, showed a Smr Kmr Gns double-recombination phenotype. Correct allelic exchange in BBEP205 and BPEP184 was confirmed by Southern blot hybridization with the KpnI-PstI fragment of pEP279 carrying alcR (data not shown). For complementation studies, replicative plasmids pBBR1MCS and pEP301 were conjugated into alcR mutants, with SM10 used as a donor.
BPEP214 was obtained by conjugating BP953 (53) and SM10(pEP319) and selecting for the alcR::Kmr allelic exchange as described above.
CAS assay.
The chrome azurol S (CAS) assay (48) was used to assess alcaligin siderophore production by Bordetella cells as described previously (3). Briefly, cells were grown to stationary phase in iron-limited SS medium. A 0.5-ml volume of culture supernatant was added to 0.5 ml of CAS solution, and the A630 of the CAS dye was measured after incubation for 4 h at room temperature.
Cell fractionation and protein analysis.
Cells from 20-ml B. bronchiseptica or B. pertussis cultures were resuspended in 4 ml of HEPES (50 mM; pH 7.4) and disrupted with a French pressure cell (SLM-Aminco, Rochester, N.Y.). The pressates were centrifuged at 2,065 × g for 5 min at 4°C to sediment cellular debris and unbroken cells. Whole-cell lysates (WCLs) were saved or centrifuged at 111,000 × g for 1 h at 4°C to separate soluble and insoluble cell fractions.
Proteins (30 μg loaded per lane) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 5% stacking gel and a 12% separating gel (45). Following electrophoresis, proteins were visualized by Coomassie blue staining.
β-gal and AP assays.
β-Galactosidase (β-gal) and alkaline phosphatase (AP) specific activities generated by fhaB::lacZ and ptx::phoA fusions in strains BP953 and BPEP214 were measured on WCLs as previously described (11, 33), except that samples were incubated at 37 instead of 28°C to facilitate detection of the low-level AP activity in these strains.
Colonization assay in the murine model.
BPSM or BPEP184 cells were grown for 24 h on BG agar, and then 3- to 4-week-old mice were intranasally challenged with 5 × 106 cells from one of the strains. Infected mice were sacrificed by cervical dislocation 1 h after exposure and at 5, 8, 12, and 19 days thereafter (four mice per time point). The lungs were removed and homogenized in saline with tissue grinders. Enumeration of bacteria was performed on BG agar. To assess the stability of the alcR mutation, bacteria reisolated from the lungs of BPEP184-infected mice were tested for their resistance to kanamycin and absence of siderophore production. Both phenotypes had been retained.
Nucleotide sequence accession number.
The nucleotide sequence of the B. bronchiseptica 3,526-bp PstI DNA fragment in pEP279 has been assigned EMBL accession no. AJ000061.
RESULTS
Isolation of a Fur-repressed gene.
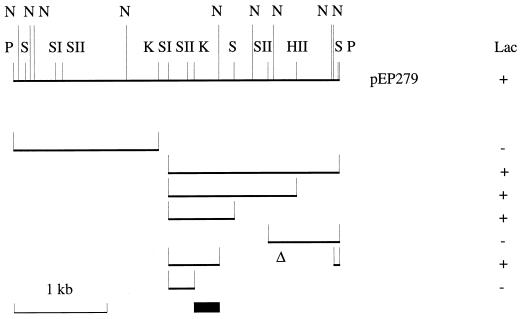
We used the FURTA system (54) to isolate potential Fur-binding fragments in a partial B. bronchiseptica genomic DNA library that we had previously constructed (38). E. coli H1717 bearing the Fur-repressible fhuF::lacZ fusion was transformed with the pool of recombinant plasmids, and four red colonies (Lac+ phenotype) were isolated on iron-rich MacConkey agar plates, suggesting that the cloned B. bronchiseptica sequences were interfering with Fur repression of the chromosomal lac fusion. One such plasmid, pEP279, was studied further since it gave the strongest Lac+ phenotype in the assay. Restriction mapping showed that pEP279 contained a 3.5-kb PstI DNA fragment. Successive deletions in the insert enabled the region conferring the Lac+ phenotype to be localized to a 0.6-kb SacI-NarI fragment (Fig. 1). The SacI-KpnI portion derived from this fragment did not confer a Lac+ phenotype to H1717; thus, the potential FBS was mapped to the other 0.3-kb half, between the KpnI and NarI sites, as shown in Fig. 1.
FIG. 1.
Mapping of the region of pEP279 conferring a Lac+ phenotype in the FURTA. Subclones of the 3.5-kb PstI insert were constructed by deletions in pEP279 using the following restriction enzymes: HII, HincII; K, KpnI; N, NarI; P, PstI; S, SmaI; SI, SacI; and SII, SacII. The recombinant plasmid-associated Lac phenotypes are indicated on the right. The deduced localization of the FBS is also shown (black box).
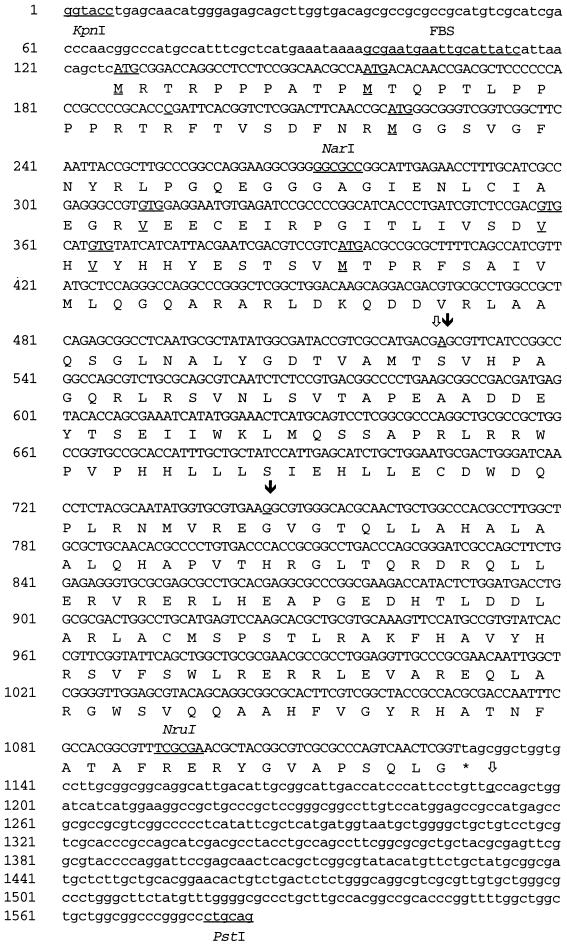
In order to characterize the putative Fur-repressed gene in pEP279, we determined the nucleotide sequence of the 1.5-kb KpnI-PstI fragment bearing the potential FBS (Fig. 2). A sequence homologous to the E. coli Fur-binding consensus GATAATGATAATCATTATC (13 of 19 matches) was identified between the KpnI and NarI restriction sites, in agreement with the FURTA system genetic data (Fig. 2). Sequence analysis revealed an ORF starting 120 bp downstream from the KpnI site and stretching approximately 1 kb towards the PstI site. In Fur-repressed promoters, FBS usually overlap the AT-rich −10 promoter region. A close examination of the proposed FBS sequence GCGAATGAATTGCATTATC revealed several putative −10 boxes; among them, the TATCAT sequence was the closest to the E. coli TATAAT −10 consensus (25). Twenty nucleotides upstream from this hexamer lies the sequence ATGAAA, sharing four nucleotides with the E. coli −35 consensus TTGACA. These determinants may constitute the promoter region. Six in-frame codons (three ATGs and three GTGs [Fig. 2]) could initiate translation, but four of them are not preceded by a sequence close to the canonical AAGGAGG E. coli ribosome binding site (RBS). Thus, translation most probably initiates either at the third ATG, located 10 bp downstream from a putative GGA RBS, or further down at the first GTG, situated 5 bp downstream from another GAGG potential RBS. However, the use of other triplets as start codons cannot be ruled out. The calculated molecular mass of the predicted translation product would then be 34 or 31 kDa, respectively.
FIG. 2.
Nucleotide sequence of alcR of B. bronchiseptica and deduced amino acid sequence of AlcR. Selected restriction enzyme sites, the putative FBS, and possible initiation codons are indicated (underlined). Differences detected in the B. pertussis and B. parapertussis sequences are also shown (underlined and labelled and , respectively).
Scanning of the Swiss Protein Data Base revealed that the C-terminal amino acid sequence deduced from the ORF is homologous to that of PchR, an AraC family activator of pyochelin siderophore and ferripyochelin receptor synthesis in P. aeruginosa (26, 27). An alignment of the conserved region is shown in Fig. 3. To a lesser extent, the C-terminal domain of the ORF is also homologous to that of YbtA, another AraC-type regulator controlling pesticin siderophore and yersiniabactin receptor synthesis (16). A second ORF, starting 126 bp downstream from the first one and running to the PstI site, was detected. The deduced 110-residue sequence shows 56% similarity to the N-terminal region of the 337-residue E. coli Bcr protein conferring bicyclomycin and sulfonamide resistance (8). Thus, this second gene could encode an inner membrane drug translocase of the Bcr family.
FIG. 3.
Alignment of the deduced C-terminal amino acid sequences of AlcR of B. bronchiseptica and PchR of P. aeruginosa. The final 180 residues of each protein are shown. The putative helix-turn-helix and AraC signature motifs (underlined), exact matches, and conserved changes (+) are indicated.
Presence of the Fur-repressed gene in other Bordetella genomes.
B. pertussis and B. parapertussis are human pathogens closely related to B. bronchiseptica, while B. avium, a poultry pathogen, is more distant, according to phylogenetic analysis (35). PCR experiments were performed on genomic DNA from B. pertussis BPSM, B. parapertussis PEP, and B. avium 103004, with oligonucleotides hybridizing to the flanking sequences of the B. bronchiseptica Fur-repressed gene. PCR amplification products of the expected size were obtained with chromosomal DNA from B. pertussis and B. parapertussis, but under the same conditions, no amplification product was generated with B. avium DNA (data not shown). Furthermore, B. avium genomic DNA did not hybridize to the KpnI-PstI fragment of pEP279 used as a probe in Southern blot experiments (data not shown). Thus, B. avium 103004 may lack this gene, or the sequence may be too divergent to be detected under the hybridization conditions tested.
Sequence comparison of the B. bronchiseptica DNA fragment with the cloned B. pertussis and B. parapertussis 1.48-kb PCR products revealed only two differences in each fragment (Fig. 2). In the B. pertussis sequence, the first distinction was an A-to-G alteration in the ORF (Ser-to-Gly change in the deduced protein), and the second was a G-to-A switch, 61 bp downstream from the stop codon. In the B. parapertussis sequence, both differences mapped in the ORF; the first one was identical to that in the B. pertussis gene, and the second change, a G-to-A switch, leads to a substitution of Ser for Gly in the deduced product. Ser-Gly substitutions are considered conservative changes; thus, the predicted proteins probably have the same function in B. bronchiseptica, B. pertussis, and B. parapertussis.
Characterization of B. pertussis and B. bronchiseptica alcR mutants.
Under low-iron growth conditions, B. pertussis and B. bronchiseptica have been shown to secrete the same alcaligin siderophore (Sid+ phenotype) (34). To our knowledge, B. parapertussis and B. avium siderophores have not been isolated. We found that B. parapertussis grown under iron-restricted conditions produced siderophore, but, interestingly, no siderophore was detected in the culture supernatant of B. avium 103004 (data not shown). In order to determine whether the cloned gene is involved in siderophore production, B. pertussis and B. bronchiseptica mutants were generated by exchange with an interrupted allele. In this construct, a kanamycin resistance cassette was inserted at the NruI site located in the 3′ region of the ORF (Fig. 2). This insertion disrupted the AraC signature motif of the putative regulatory protein (Fig. 3).
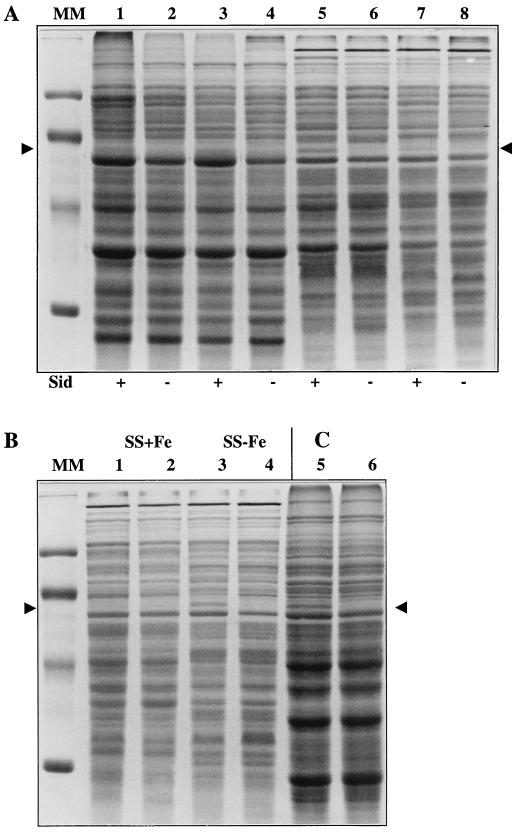
Isogenic B. pertussis and B. bronchiseptica wild-type and mutant strains were grown to stationary phase in low-iron SS medium. No difference in growth rate or in final yields between members of isogenic pairs could be detected, even after growth for several generations in such an iron-restricted medium (data not shown). Bacterial cells and culture supernatants were separated by centrifugation and saved. WCLs were subjected to SDS-PAGE analysis, and culture supernatants were tested for siderophore activity in the CAS assay (Fig. 4A, lanes 1, 2, 5, and 6). No siderophore activity was detected in the culture supernatant of either mutant (Sid− phenotype; Fig. 4A, lanes 2 and 6). The isogenic pairs presented similar protein profiles except for a 60-kDa polypeptide synthesized by wild-type B. pertussis and B. bronchiseptica strains (Fig. 4A, lanes 1 and 5) which was clearly absent in BPEP184 and BBEP205 (lanes 2 and 6).
FIG. 4.
Effect of alcR inactivation on protein and siderophore production in B. pertussis and B. bronchiseptica as determined by SDS-PAGE analysis. (A) WCLs of cultures grown in iron-limited SS medium. Lanes: 1, BPSM (wild type); 2, BPEP184 alcR::Kmr; 3, BPEP184(pEP301); 4, BPEP184(pBBR1MCS); 5, BB1015 (wild type); 6, BBEP205 alcR::Kmr; 7, BBEP205(pEP301); 8, BBEP205(pBBR1MCS). Siderophore (Sid) production in matching culture supernatants tested by the CAS assay is indicated below the gel (+, high level of activity; −, no siderophore activity detected). (B) WCLs of BB1015 (lanes 1 and 3) and BBEP205 alcR::Kmr (lanes 2 and 4) grown in iron-rich SS medium (SS+Fe) (lanes 1 and 2) or iron-limited SS medium (SS-Fe) (lanes 3 and 4). (C) Soluble proteins prepared from BPSM (lane 5) and BPEP184 alcR::Kmr (lane 6) grown in iron-limited SS medium. For both gels, the molecular masses of markers in lane MM are 97.4, 66.2, 45, and 31 kDa, from top to bottom. The 60-kDa iron-repressed protein is indicated (arrowheads).
Each mutant was then transformed with pBBR1MCS or with pEP301, a pBBR1MCS derivative bearing an intact copy of the B. bronchiseptica Fur-repressed gene. The transformants were grown in iron-limited SS medium, and WCLs were analyzed by SDS-PAGE, while culture supernatants were tested in the CAS assay (Fig. 4A, lanes 3, 4, 7, and 8). BPEP184(pEP301) and BBEP205(pEP301) produced siderophores and synthesized the 60-kDa polypeptide (Fig. 4A, lanes 3 and 7), whereas no siderophore and no 60-kDa polypeptide were synthesized by BPEP184 and BBEP205 bearing pBBR1MCS (lanes 4 and 8). This complementation experiment indicated that neither the Sid− phenotype nor the absence of the 60-kDa protein resulted from a polar effect of the disruption on downstream genes. Both phenotypes were directly linked to the absence of a functional Fur-repressed gene. This new gene was designated alcR owing to its involvement in alcaligin siderophore production.
The AlcR-dependent 60-kDa polypeptide is a soluble iron-repressed protein.
To investigate whether the production of the 60-kDa protein was iron regulated, B. bronchiseptica BB1015 and BBEP205 were grown in iron-rich and iron-limited SS media. SDS-PAGE analysis of WCLs revealed that the AlcR-dependent 60-kDa protein was synthesized by BB1015 only under low-iron growth conditions (Fig. 4B, compare lanes 1 and 3). Thus, this iron-repressed protein was designated IRP60. In agreement with our previous observations, BBEP205 AlcR− grown in iron-limited or iron-rich SS medium did not produce IRP60 (Fig. 4B, lanes 2 and 4). To determine the IRP60 cell location, soluble and membrane protein fractions were prepared from lysates of BPSM and BPEP184 cells grown in iron-limited SS medium. The protein samples were analyzed by SDS-PAGE. The 60-kDa protein was identified in the soluble protein fraction from BPSM, while it was absent in the BPEP184 extract (Fig. 4C, compare lanes 5 and 6). IRP60 was not detected in membrane preparations (data not shown). These observations suggest that IRP60 is a cytoplasmic or periplasmic protein.
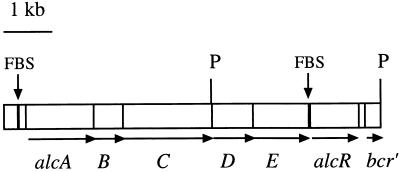
The alcR gene is located downstream from the alcABC operon.
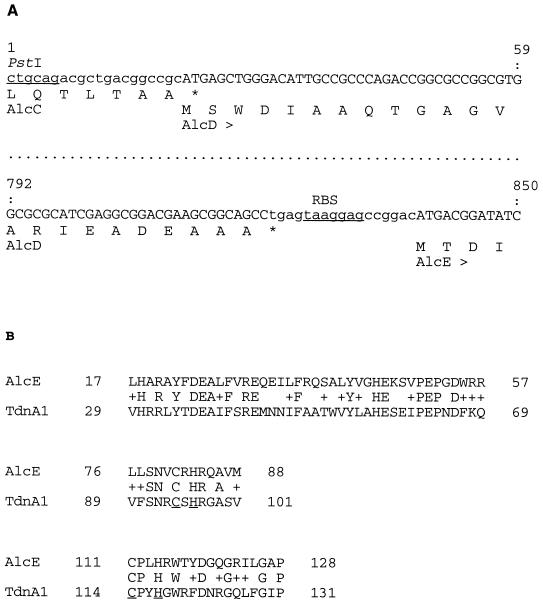
Structural genes of regulatory proteins sometimes lie in the vicinity of their target genes. To identify putative AlcR-regulated genes, the nucleotide sequence upstream from alcR in pEP279 was determined up to the first PstI cloning site shown in Fig. 1. A BLASTN search (2) in the nonredundant GenBank/EMBL/DDBJ/PDB library revealed a 300-bp sequence overlap with the 3′ end of the alcABC operon encoding alcaligin biosynthesis enzymes (20, 28). Thus, alcR was mapped about 2 kb downstream from the alcC gene on the chromosome and in the same orientation as the alcABC operon (Fig. 5). We noticed a few discrepancies between the B. bronchiseptica alcC downstream sequence deposited in the databank by Giardina et al. (20) and our sequence determination. The differences corresponded to three base substitutions, two base insertions, and one base inversion. Sequence analysis of the alcR upstream region revealed two tightly linked ORFs oriented in the same direction as alcC (Fig. 5). The first ORF contains an ATG codon overlapping the alcC stop codon (Fig. 6A) and could encode a 29-kDa polypeptide. The second ORF, 14 bp downstream from the stop codon of the preceding ORF and 6 bp downstream from a putative TAAGGAG RBS (Fig. 6A), could specify a 45-kDa protein. Such a tight organization suggests that these genes are part of the alcABC operon. Thus, they were designated alcD and alcE. Amino acid sequences deduced from alcD (AlcD) and alcE (AlcE) were subjected to a BLASTP search (2) in the library cited above. Homology between AlcE and TdnA1, the large subunit of Pseudomonas putida terminal dioxygenase, was detected. TdnA is an iron-sulfur protein involved in aniline degradation (18). A high degree of similarity was observed around the putative iron-sulfur center of TdnA1, as shown in Fig. 6B, suggesting that AlcE might also be an iron-sulfur protein. A scan with AlcD failed to reveal any homology with sequences in the bank.
FIG. 5.
Physical map of the alc locus of B. bronchiseptica. Blocks representing ORFs are drawn to scale from the 4.3-kb alcABC sequence determined by Giardina et al. (20) and from the sequence data for the 3.5-kb PstI DNA fragment presented in this study. PstI restriction sites (P), FBS upstream from alcA and alcR (black bars), and bcr′, the 5′ extremity of a putative bicyclomycin resistance gene downstream from alcR, are indicated.
FIG. 6.
(A) Flanking sequences of the alcD gene and deduced amino acid sequences. The tight genetic organization of the alcC, alcD, and alcE genes is shown. The PstI restriction site (underlined), the 3′-terminal sequence of alcC (lowercase), and the deduced C-terminal sequence of AlcC and the alcD and alcE putative coding sequences and predicted translation products (uppercase) are shown. A potential RBS upstream from alcE is also indicated (underlined). (B) Partial amino acid sequence alignments of the putative AlcE protein with P. putida TdnA1. C and H residues forming the predicted iron-sulfur reaction center in TdnA1 (underlined) and conservative changes (+) are indicated.
AlcR is not involved in FHA, AC-Hly, PRN, or PTX production or in colonization.
Since in other pathogens the production of important virulence factors may be iron regulated, we investigated whether AlcR is involved in the production of FHA, AC-Hly, PRN, or PTX in B. pertussis. The WCLs and culture supernatants of BPSM and BPEP184 were compared by SDS-PAGE and immunoblot analyses. The two strains produced similar amounts of FHA, AC-Hly, PRN, and PTX, indicating that AlcR is not required for the synthesis of these four virulence factors (data not shown). The results for FHA and PTX were confirmed by using transcriptional reporter gene fusions to the fhaB and ptx chromosomal genes. For this purpose, the alcR::Kmr mutation was introduced into BP953 (fhaB::lacZ ptx::phoA) (53) by allelic exchange to generate BPEP214. The β-gal and AP activities of the isogenic AlcR+-AlcR− strains were then compared (data not shown). The alcR disruption had no significant effect on β-gal and AP activities, indicating that AlcR is not involved in fhaB or ptx expression.
FHA, PRN, PTX, and AC-Hly are the major adhesins and toxins in B. pertussis and as such play an important role in the initiation, amplification, and persistence of the bacterial infection in the mouse respiratory infection model. As none of these virulence factors proved to be AlcR dependent, we tested whether AlcR, as an activator of siderophore synthesis, was required for efficient colonization of B. pertussis in this animal model. Mice were infected with either BPSM or BPEP184. The two strains presented similar colonization profiles, with a 3-log increase in bacterial counts during the first week after exposure followed by a slow decline over the subsequent 2 weeks (data not shown). Similar results were obtained with the B. bronchiseptica AlcR+-AlcR− isogenic pair (data not shown). Therefore, at least in the mouse model, AlcR plays no important role in colonization.
DISCUSSION
As a first step to elucidate the iron regulatory network in Bordetella spp., we chose to identify target genes of the global iron regulator Fur. We have isolated a clone bearing an FBS from a B. bronchiseptica partial library by using the E. coli FURTA (54). The FBS in the plasmid was mapped about 2 kb downstream from one end of the cloned 3.5-kb fragment. Sequence analysis of the 1.5-kb region downstream of the FBS revealed an ORF, alcR, which could encode an AraC-like regulatory protein homologous to PchR, a regulator of pyochelin and ferripyochelin receptor synthesis in P. aeruginosa (26, 27, 49), and to YbtA, a regulator of pesticin and yersiniabactin receptor synthesis in Yersinia pestis (16). No significant ORF running in the opposite direction in the 2-kb flanking region was detected, strongly suggesting that the target of Fur repression is the downstream alcR gene. In the same orientation, and about 120 bp downstream from alcR, an ORF (bcr) encoding a putative inner membrane drug resistance translocase was detected. The large spacing between alcR and bcr suggests that bcr is transcribed from its own promoter, but since no obvious terminator was found in the alcR-bcr intergenic sequence, the possibility that alcR and bcr are cotranscribed and form an operon cannot be ruled out. The alcR upstream sequence in the cloned 3.5-kb fragment was shown to contain the very end (7 C-terminal amino acid residues) of the previously identified alcC gene (20, 28), followed by two tightly linked ORFs running in the same direction. Such a spatial arrangement suggests that these ORFs are part of the alcaligin biosynthesis alcABC operon (28). We designated them alcD and alcE. A homology search indicated that alcE could encode an iron-sulfur protein. No homology was detected for the putative AlcD protein. In their paper reporting the identification of the alcABC operon, Kang et al. suggested that in addition to AlcA, AlcB, and AlcC, at least two other enzymes are required to complete the siderophore biosynthesis pathway from succinyl-hydroxy-putrescine to alcaligin (28). The alcD and alcE genes could code for these enzymes. Experiments are in progress to determine whether the alcD and alcE ORFs are involved in alcaligin biosynthesis.
Sequence comparison of the B. bronchiseptica, B. pertussis, and B. parapertussis alcR genes showed that the predicted product is highly conserved in these species. Both B. bronchiseptica and B. pertussis produce alcaligin (34), and in this paper we show that B. parapertussis also synthesizes siderophores. It is highly probable that this siderophore is alcaligin. In contrast, no siderophore production was observed in the culture supernatant of B. avium 103004 grown in iron-restricted conditions. Concomitantly, no sequence hybridizing to an alcR probe could be detected in Southern blot experiments with B. avium genomic DNA. Another B. avium strain in our collection presented also a Sid− phenotype (38). In neither of these B. avium strains could the production of siderophore be induced by a plasmid-borne copy of the B. bronchiseptica alcR gene (38), suggesting that they were not simply alcR mutants. To our knowledge, B. avium iron uptake systems have not been studied yet, and we do not know whether the absence of siderophore production is a physiological trait of the B. avium species. An LF-binding protein has been identified in the B. avium outer membrane (30), but the role of this protein in iron uptake remains to be elucidated.
B. bronchiseptica and B. pertussis alcR mutants, obtained by in vitro mutagenesis and gene replacement, were deficient in alcaligin production. They were also deficient in the synthesis of a soluble 60-kDa iron-repressed protein (IRP60). Both phenotypes were complemented by an intact plasmid-borne copy of alcR, showing that neither of them resulted from a polar effect of the alcR disruption. Thus, AlcR is required for alcaligin and IRP60 synthesis. Kang et al. demonstrated that AlcC is a soluble iron-repressed protein and that, although predicted to have a size of 70 kDa, AlcC migrates with an apparent molecular mass of 59 kDa during SDS-PAGE (28). It is therefore possible that IRP60 is in fact AlcC, suggesting that AlcR is an activator of the alcC gene. Complementation studies have shown that alcC is transcribed from the alcA promoter and that the alcABC locus constitutes an operon (28). Kang et al. also compared the protein profiles for two B. bronchiseptica mutants bearing polar mutations in the alcA gene with that for a wild-type strain. They reported that the only observed difference was the absence of the AlcC polypeptide in the alcA polar mutants (28). Since the B. pertussis and B. bronchiseptica alcR mutants described in this paper present the same protein profile phenotype as alcA mutants, it is very likely that AlcR is an activator of the whole alc operon in both species, including the putative new alcD and alcE genes located downstream from alcC. Regulatory genes are often autoregulated; however, a comparison of the alcA and alcR promoter regions did not reveal any potential AlcR binding sites. Overexpression and purification of AlcR will enable us to determine the N-terminal sequence of the protein as well as characterize its DNA binding sequence. Alternatively, AlcR may be an intermediate regulator and may activate the promoter of another, as-yetunidentified gene, the latter being the final activator of the alc operon. The identification of AlcR target genes will help establish its position in the iron regulatory network.
A putative FBS overlaps the −10 box of the alcABC promoter, and transcription of the alcABC genes is iron repressed (28). In addition, alcABC transcription appears to be AlcR dependent, suggesting the following model for very tight Fur repression of the alc operon. Under iron-rich growth conditions, the Fur-Fe(II) complex binds to the alcR promoter, generating an AlcR depletion which in turn shuts off alcABC operon transcription. Concomitantly, the Fur-Fe(II) complex binds directly to the alcABC promoter, ensuring a tight double-level repression. Under iron-restricted growth conditions, both promoters are derepressed and AlcR activates alcABC transcription, either directly or via another regulatory protein.
The iron regulatory network strongly influences the production of virulence factors in a number of gram-positive and gram-negative pathogenic bacteria, such as Corynebacterium diphtheria, E. coli, P. aeruginosa, and many others (for a review, see reference 15). The production of the major virulence factors FHA, PTX, PRN, and AC-Hly in B. pertussis was not affected by the inactivation of the alcR gene. The behaviors of the AlcR-deficient mutant and the parental strain in the murine respiratory model were similar probably because the expression of adhesins and toxins was neither enhanced nor repressed in the absence of AlcR. Siderophore production has been shown to contribute significantly to virulence in a number of bacterial pathogens (55), and, like most bacteria, Bordetella spp. also require iron for growth (31). The alcR mutation did not affect colonization in the mouse model, although the mutant strain was unable to secrete siderophore in vitro under iron-limited growth conditions. Our observations therefore suggest that, in addition to alcaligin production, B. pertussis has evolved a second iron uptake mechanism, unless low-level siderophore synthesis, below the detection limit of the CAS assay, occurs in the absence of the regulator. Beall and coworkers recently identified three B. pertussis iron-regulated genes encoding BfeA, BfrB, and BfrC, three outer membrane proteins homologous to receptors of enterobactin, ferrichrome, and another hydroxamate siderophore, respectively (5, 7). They also showed that, in addition to these proteins, B. bronchiseptica synthesizes BfrA, an unidentified exogenous siderophore receptor (6). Thus, via these specific receptors, Bordetella spp. may perhaps scavenge various heterologous ferrisiderophores secreted by the commensal flora of the airways. If the synthesis of these receptors is AlcR independent, the multiplicity of heterologous siderophore-mediated iron uptake systems could compensate for the absence of alcaligin in alcR mutants. Alternatively, Redhead and Hill have suggested that B. pertussis has recourse to a TF-LF receptor to scavenge iron from the host (43). Menozzi et al. have isolated LF-binding outer membrane proteins from both B. pertussis and B. bronchiseptica (31). It is therefore possible that Bordetella spp. use both siderophore-mediated and siderophore-independent iron uptake systems. The testing of mutants bearing deletions of the alcaligin biosynthesis genes in the mouse colonization model could help determine if the alcaligin system is essential.
Interestingly, in certain B. bronchiseptica strains, siderophore production is repressed by BvgA, a global activator of most Bordetella virulence factors (19). This implies that in these strains siderophores are produced only in the avirulent phase, suggesting that siderophores may interfere with colonization in certain circumstances. Consistent with this assumption, Register et al. observed that a B. bronchiseptica mutant deficient in siderophore synthesis in fact expressed enhanced virulence in neonatal pigs (44). The relationship between siderophore production and virulence thus appears to be quite complex in Bordetella spp. and deserves further study.
ACKNOWLEDGMENTS
We thank Klaus Hantke for providing the FURTA system, Scott Stibitz for the gift of strain BP953, and Michael Kovach for the gift of pBBR1MCS. We are grateful to Eve Willery and Nathalie Reveneau for technical assistance with automatic sequencing, to Sabine Thiberge for technical assistance with mouse experiments, and to Carine Capiau for the gift of antipertactin serum. We thank Emmanuelle Fort for photographic work and Franco Menozzi for critically reading the manuscript.
This work was supported by the INSERM, the Institute Pasteur de Lille, the Institut Pasteur de Paris, the Région Nord-Pas-de-Calais, and the Ministère de l’Enseignement Supérieur et de la Recherche.
REFERENCES
- 1.Agiato L A, Dyer D W. Siderophore production and membrane alterations by Bordetella pertussis in response to iron starvation. Infect Immun. 1992;60:117–123. doi: 10.1128/iai.60.1.117-123.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong S K, Clements M O. Isolation and characterization of Bordetella bronchiseptica mutants deficient in siderophore activity. J Bacteriol. 1993;175:1144–1152. doi: 10.1128/jb.175.4.1144-1152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall B, Sanden G N. Cloning and initial characterization of the Bordetella pertussis fur gene. Curr Microbiol. 1995;30:1–4. doi: 10.1007/BF00293637. [DOI] [PubMed] [Google Scholar]
- 5.Beall B, Sanden G N. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology. 1995;141:3193–3205. doi: 10.1099/13500872-141-12-3193. [DOI] [PubMed] [Google Scholar]
- 6.Beall B, Hoenes T. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology. 1997;143:135–145. doi: 10.1099/00221287-143-1-135. [DOI] [PubMed] [Google Scholar]
- 7.Beall B, Hoenes T. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Three unlinked genes in Bordetella encoding putative ferric siderophore receptors, abstr. B-239; p. 69. [Google Scholar]
- 8.Bentley J, Hyatt L S, Ainley K, Parish J H, Herbert R B, White G R. Cloning and sequence analysis of an Escherichia coli gene conferring bicyclomycin resistance. Gene. 1993;127:117–120. doi: 10.1016/0378-1119(93)90625-d. [DOI] [PubMed] [Google Scholar]
- 9.Bonnah R A, Yu R-H, Schryvers A B. Biochemical analysis of lactoferrin receptors in the Neisseriaceae: identification of a second bacterial lactoferrin receptor protein. Microb Pathog. 1995;19:285–297. doi: 10.1016/s0882-4010(96)80002-7. [DOI] [PubMed] [Google Scholar]
- 10.Bordet J, Gengou O. Le microbe de la coqueluche. Ann Inst Pasteur (Paris) 1906;20:731–741. [Google Scholar]
- 11.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 12.Brickman T J, Armstrong S K. Bordetella pertussis fur gene restores iron repressibility of siderophore and protein expression to deregulated Bordetella bronchiseptica mutants. J Bacteriol. 1995;177:268–270. doi: 10.1128/jb.177.1.268-270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderwood S B, Mekalanos J J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987;169:4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coy M, Neilands J B. Structural dynamics and functional domains of the Fur protein. Biochemistry. 1991;30:8201–8210. doi: 10.1021/bi00247a016. [DOI] [PubMed] [Google Scholar]
- 15.Crosa J H. Bacterial iron metabolism, plasmids and other virulence factors. In: Bullen J J, Griffiths E, editors. Iron and infection. Chichester, United Kingdom: John Wiley & Sons; 1987. pp. 139–170. [Google Scholar]
- 16.Fetherston J D, Bearden S W, Perry R D. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol Microbiol. 1996;22:315–325. doi: 10.1046/j.1365-2958.1996.00118.x. [DOI] [PubMed] [Google Scholar]
- 17.Foster J W, Hall H K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J Bacteriol. 1992;174:4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumori F, Saint C P. Nucleotide sequences and regulational analysis of genes involved in conversion of aniline to catechol in Pseudomonas putida UCC22(pTDN1) J Bacteriol. 1997;179:399–408. doi: 10.1128/jb.179.2.399-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giardina P C, Foster L-A, Musser J M, Akerley B J, Miller J F, Dyer D W. bvg repression of alcaligin synthesis in Bordetella bronchiseptica is associated with phylogenetic lineage. J Bacteriol. 1995;177:6058–6063. doi: 10.1128/jb.177.21.6058-6063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giardina P C, Foster L-A, Toth S I, Roe B A, Dyer D W. Identification of alcA, a Bordetella bronchiseptica gene necessary for alcaligin production. Gene. 1995;167:133–136. doi: 10.1016/0378-1119(95)00659-1. [DOI] [PubMed] [Google Scholar]
- 21.Gorringe A R, Woods G, Robinson A. Growth and siderophore production by Bordetella pertussis under iron-restricted conditions. FEMS Microbiol Lett. 1990;66:101–106. doi: 10.1016/0378-1097(90)90265-r. [DOI] [PubMed] [Google Scholar]
- 22.Graeff-Wohlleben H, Killat S, Banemann A, Guiso N, Gross R. Cloning and characterization of an Mn-containing superoxide dismutase (SodA) of Bordetella pertussis. J Bacteriol. 1997;179:2194–2201. doi: 10.1128/jb.179.7.2194-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 25.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinrichs D E, Poole K. Cloning and sequence analysis of a gene (pchR) encoding an AraC family activator of pyochelin and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. J Bacteriol. 1993;175:5882–5889. doi: 10.1128/jb.175.18.5882-5889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinrichs D E, Poole K. PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and as a repressor. J Bacteriol. 1996;178:2586–2592. doi: 10.1128/jb.178.9.2586-2592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang H Y, Brickman T J, Beaumont F C, Armstrong S K. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J Bacteriol. 1996;178:4877–4884. doi: 10.1128/jb.178.16.4877-4884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovach M E, Phillips R W, Elzer P H, Roop II R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 30.Menozzi, F. Unpublished data.
- 31.Menozzi F D, Gantiez C, Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991;59:3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 34.Moore C H, Foster L-A, Gerbig D G, Dyer D W, Gibson B W. Identification of alcaligin as the siderophore produced by Bordetella pertussis and B. bronchiseptica. J Bacteriol. 1995;177:1116–1118. doi: 10.1128/jb.177.4.1116-1118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller M, Hildebrandt A. Nucleotide sequence of the 23S rRNA genes from Bordetella pertussis, B. parapertussis, B. bronchiseptica, and B. avium, and their implications for phylogenic analysis. Nucleic Acids Res. 1993;21:3320. doi: 10.1093/nar/21.14.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neilands J B, Nakamura K. Detection, determination, isolation, characterization and regulation of microbial iron chelates. In: Winkelmann G, editor. Handbook of microbial iron chelates. Boca Raton, Fla: CRC Press; 1991. pp. 1–15. [Google Scholar]
- 37.Nordmann P, François B, Menozzi F D, Commare M C, Barois A. Whooping cough associated with Bordetella parapertussis in an HIV-infected child. Pediatr Infect Dis J. 1992;11:248. [PubMed] [Google Scholar]
- 38.Pradel, E. Unpublished observation.
- 39.Pradel E, Locht C. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Cloning and sequencing of the fur gene from Bordetella pertussis, abstr. B-357; p. 227. [Google Scholar]
- 40.Prince R W, Cox C D, Vasil M L. Regulation of toxA and regA by Escherichia coli fur gene and identification of a Fur homolog in Pseudomonas aeruginosa PA103 and PAO1. Mol Microbiol. 1991;5:2823–2831. doi: 10.1111/j.1365-2958.1991.tb01991.x. [DOI] [PubMed] [Google Scholar]
- 41.Prince R W, Storey D G, Vasil M L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993;175:2589–2593. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 43.Redhead K, Hill T. Acquisition of iron from transferrin by Bordetella pertussis. FEMS Microbiol Lett. 1991;77:303–308. doi: 10.1016/0378-1097(91)90570-z. [DOI] [PubMed] [Google Scholar]
- 44.Register K B, Ackermann M R, Dyer D W. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. Loss of siderophore production in Bordetella bronchiseptica affects virulence in neonatal pigs, abstr. B-173; p. 59. [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 46.Schryvers A, Morris L J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988;2:281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 47.Schryvers A B. Identification of the transferrin- and lactoferrin-binding proteins in Haemophilus influenzae. J Med Microbiol. 1989;29:121–130. doi: 10.1099/00222615-29-2-121. [DOI] [PubMed] [Google Scholar]
- 48.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 49.Serino L, Reimmann C, Visca P, Beyeler M, Della Chiesa V, Haas D. Biosynthesis of pyochelin and dihydroaeruginoic acid requires the iron-regulated pchDCBA operon in Pseudomonas aeruginosa. J Bacteriol. 1997;179:248–257. doi: 10.1128/jb.179.1.248-257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. BioTechnology. 1983;1:784–790. [Google Scholar]
- 51.Stainer D W, Scholte M J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 52.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 53.Stibitz S, Carbonetti N H. Hfr mapping of mutations in Bordetella pertussis that define a genetic locus involved in virulence gene regulation. J Bacteriol. 1994;176:7260–7266. doi: 10.1128/jb.176.23.7260-7266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stojiljkovic I, Baumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new Escherichia coli iron-regulated genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg E D. Acquisition of iron and other nutrients in vivo. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C: American Society for Microbiology; 1995. pp. 81–95. [Google Scholar]