Abstract
Increasing FtsZ induces the formation of minicells at cell poles but does not increase the frequency or timing of central divisions. A coordinate increase in both FtsZ and FtsA, however, increases the frequency of both polar and central divisions.
The capacity of Escherichia coli cells to form new septa is normally limited to one per cell cycle (10, 12, 14). In the normal cell cycle, these septa form in the cell center, to give two equal-size daughter cells, but in minB mutants, there is an equal probability for the septum to form at either of the two cell poles or at the cell center (i.e., in normal-length dividing cells the probability of minicell formation is two-thirds [10, 14]). Ward and Lutkenhaus (17) and Bi and Lutkenhaus (4) showed that this limited division capacity was partly a function of the level of FtsZ protein, because cells overexpressing FtsZ formed minicells in addition to central septa. They also showed that a plasmid (pZAQ) overexpressing FtsZ, FtsA, and FtsQ was able to increase the frequency of division in minB cells to allow both central and polar septa to be formed in every cell cycle (5, 17).
Later work (6, 8) showed that normal cell division requires a particular ratio of FtsA to FtsZ protein. We have reexamined the requirements for increased division capacity in the light of this and show here that, while a six- to sevenfold increase in FtsZ alone does indeed induce minicell formation in wild-type cells, it does not increase the frequency of central divisions in either wild-type or minB cells, an observation which contradicts previous conclusions (4, 5). However, we confirm that a coordinate increase in FtsZ and FtsA together, which also induces minicell formation, also increases the frequency of central divisions in both wild-type and minB cells (4, 5). We conclude therefore that the capacity to make septa at new, central sites is determined by the levels of both FtsZ and FtsA but that increased FtsZ by itself can overcome the Min block to division at the cell poles (7) without altering the frequency of divisions at new sites.
MATERIALS AND METHODS
Growth conditions and medium.
Cells were grown with rotary shaking in Oxoid nutrient broth no. 2 at 37°C.
Strains.
E. coli K-12 strains JM101 [F′ traD36 lacIq Δ(lacZ)M15 proA+B+/supE thi Δ(lac-proAB)], C600 (F− leuB6 thr-1 lacY1 thi-1 supE44 rfbD1 fhuA21], P678 (thr leu thi), and P678-54 (P678 minB) (3) were used in various experiments, as were the derivatives described in the text.
Plasmid construction.
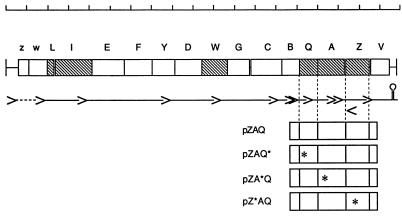
To inactivate ftsZ, ftsA, and ftsQ, the pZAQ plasmid (4) was cut with BstBI, BglII, or MluI endonuclease, filled in with Klenow enzyme, and religated, giving pZ*AQ, pZA*Q, and pZAQ*, respectively. This procedure gives 2- or 4-bp insertions, generating frameshifts in ftsZ, ftsA, and ftsQ, respectively. DNA in these plasmids was sequenced to confirm the changes.
Determination of FtsZ.
Amounts of FtsZ were determined with the ECL Western blot detection system (Amersham) according to the manufacturer’s instructions. Quantitative results were obtained by comparing sample band intensities on the scanned blot images with twofold dilutions of a standard (the sample with the highest concentration of FtsZ) by using the NIH image program (http://rsb.info.nih.gov/nih-image). Correction for loading errors was made by normalizing results to the amounts of GroEL protein detected with polyclonal anti-GroEL antibody (StressGen product no. SPA-804). F168-12 monoclonal anti-FtsZ antibody (16) was a gift from N. Nanninga.
RESULTS
Construction of plasmids expressing combinations of ftsQ, ftsA, and ftsZ.
Plasmid pZAQ was modified by the introduction of 2- or 4-bp inserts to create frameshifts that inactivate ftsZ, ftsA, or ftsQ, giving plasmids pZ*AQ, pZA*Q, and pZAQ*, respectively (Fig. 1). Western blotting with antibody to FtsZ showed that the level of FtsZ was increased about six- to sevenfold in cells containing an intact plasmid-encoded FtsZ gene. For plasmids pBR322, pZAQ, pZ*AQ, pZA*Q, and pZAQ*, the relative amounts of FtsZ were 1.0, 6.9, 1.2, 7.4, and 6.4, respectively. Values were corrected for loading relative to GroEL, which was assayed with anti-GroEL antibodies, and normalized to the value for the strain carrying the vector plasmid pBR322. These figures are similar to those estimated for cells carrying pZAQ by Bi and Lutkenhaus (4) and show that there are no significant polar effects of the frameshift mutations in ftsQ or ftsA on expression of ftsZ. Complementation experiments with temperature-sensitive mutants ftsZ84, ftsA13, ftsA22, ftsA16, and ftsQ1 confirmed that each frameshift mutation inactivated only the gene in which it had been inserted.
FIG. 1.
The mra locus and the plasmids used in this work. The scale at the top is in kilobase pairs. Coding regions are shown as boxes, labelled as follows: z, mraZ; w, mraW; L, ftsL; I, ftsI (pbpB); E, murE; F, murF; Y, mraY; D, murD; W, ftsW; G, murG; C, murC; B, ddlB; Q, ftsQ; A, ftsA; Z, ftsZ; V, envA. The approximate positions of known promoters are shown as the points of the arrowheads, and the terminator is shown as a stem loop. The positions of frameshift insertions in plasmids are shown as asterisks.
Coordinate overproduction of both FtsA and FtsZ is necessary for enhanced cell division.
Ward and Lutkenhaus (17) and Bi and Lutkenhaus (4) previously reported that a plasmid, pZAQ, carrying the complete ftsQ, ftsA, and ftsZ genes together with the two promoters in the upstream ddlB gene (Fig. 1), induces minicell production and also causes host cells to divide at a reduced length. We confirmed this for strain JM101 (Table 1), showing that the pZAQ plasmid reduced average cell length by 29% and doubled the proportion of visibly constricted (dividing) cells, of which about 38% were constrictions near the cell poles (which would give rise to minicells).
TABLE 1.
Effect on average cell length and proportion of constricted cells
| Strain | Mean cell length (μm) | % Central Constriction | % Polar constriction |
|---|---|---|---|
| JM101 | 4.8 | 16 | 0 |
| JM101/pZAQ | 3.4 | 20 | 12 |
| JM101/pZ*AQ | 4.3 | 17 | 0 |
| JM101/pZA*Q | 5.1 | 13 | 7 |
| JM101/pZAQ* | 3.9 | 22 | 10 |
To show the contribution of each of the three cell division genes to the small-cell phenotype, we compared the effects of the four different pZAQ derivatives. Table 1 shows that pZAQ and pZAQ* cause a similar reduction in cell length and an increase in both polar and central constrictions, showing that overexpression of the ftsQ gene is not required to produce extra divisions. In contrast, pZA*Q does not cause any reduction in average cell length or any increase in central constrictions, although it does cause formation of polar septa (Table 1). Thus, overexpression of ftsA in addition to ftsZ is required for increased internal septa and reduced cell size but not for minicell production.
Overexpression of ftsA and ftsZ together increases the capacity of minB cells to form nonpolar divisions, but overexpression of ftsA or ftsZ alone does not.
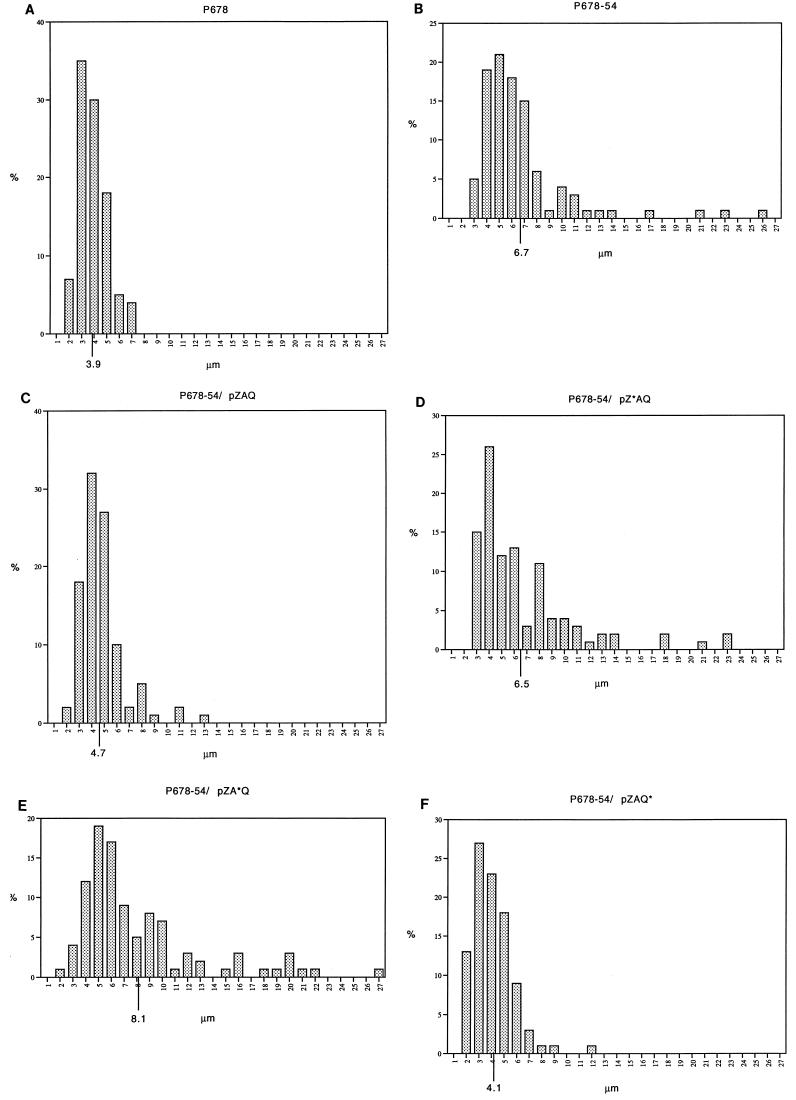
minB mutants have the same capacity for cell division as normal cells but waste about half of this capacity in the formation of minicells at the cell poles (10, 14) because these old sites are no longer blocked for reuse. Bi and Lutkenhaus (5) showed that pZAQ could increase the total division potential of minB cells so that extra divisions take place at nonpolar sites, causing the average cell length to be reduced to normal. Table 2 shows this effect in the original minB strain, P678-54 (3), and shows that pZAQ* is also able to increase the division capacity of the minB mutant, so that its average cell length is reduced to close to that of its parent strain (P678, minB+). In contrast, neither the plasmid that lacks ftsZ (pZ*AQ) nor that which lacks functional ftsA (pZA*Q) is able to cause any reduction in average cell length. (The presence of pZA*Q actually increases average cell length.) Thus, contrary to earlier conclusions but in agreement with the previously published data (4, 5, 17) we see that an increase in division capacity requires an increase in both FtsZ and FtsA.
TABLE 2.
Effect on cell length
| Strain | Mean cell length (μm) (excluding minicells) |
|---|---|
| P678 | 3.9 |
| P678-54 (minB) | 6.7 |
| P678-54/pZAQ | 4.7 |
| P678-54/pZ*AQ | 6.5 |
| P678-54/pZA*Q | 8.1 |
| P678-54/pZAQ* | 4.1 |
Figure 2 shows the cell length distributions for these strains. Plasmids that express both ftsA and ftsZ increase the division capacity of minB cells, so that their length distributions are close to that of the minB+ strain; in contrast, plasmids that encode either ftsZ or ftsA alone do not increase the division capacity of minB cells. (The presence or absence of extra copies of the ftsQ+ allele does not affect the number of polar or central divisions.)
FIG. 2.
Increased division in minB mutants overexpressing ftsA and ftsZ. Cell length distributions of exponential populations in nutrient broth at 37°C. (A) P678 (parental strain); (B) P678-54 (minB); (C) P678-54/pZAQ; (D) P678-54/pZ*AQ; (E) P678-54/pZA*Q; (F) P678-54/pZAQ*.
DISCUSSION
It has been known for some time that increased amounts of FtsZ protein have dramatic effects on cell division (4, 17). Increasing amounts of FtsZ (up to seven times the normal level) cause increasing numbers of additional septa to form near the cell poles, producing minicells. A plasmid, pZAQ, carrying the complete ftsQ, ftsA, and ftsZ genes (Fig. 1) increased FtsZ levels by about sevenfold and increased the frequency of both polar and centrally located septa (4). The increase in both types of septa was originally attributed to increased FtsZ (4), but our new results show that an increase in both FtsZ and FtsA is required to cause early central division.
(Interestingly, in the experiments reported by Ward and Lutkenhaus [17] an increase in central divisions was not seen in cells containing a plasmid, pJW5, which has a deletion in the beginning of the ftsQ gene but carries intact ftsA and ftsZ genes. This plasmid overproduced FtsZ to a level comparable to that by pZAQ but failed to increase the frequency of central divisions. This result therefore seemed to contradict both the conclusion of Ward and Lutkenhaus [17], that increased FtsZ alone can reduce the length of cells at division, and that of the present paper, that increased FtsA plus FtsZ is sufficient to do this. We think that the explanation for this anomaly is that the ftsA gene is not expressed, or at least not expressed at a high level, from pJW5, because we have earlier shown that deletion of the upstream part of ftsQ, at the same BamHI site used to construct pJW5, abolishes the expression of the downstream ftsA gene [9]. Overexpression of ftsZ would still take place in pJW5 because of transcription from promoters that lie within ftsA [13, 18]. In the corresponding plasmid used in this work, pZAQ*, both ftsA and ftsZ are expressed because we used a frameshift mutation, rather than a deletion, to inactivate ftsQ. Table 1 shows that this plasmid is indeed capable of inducing early central division in wild-type cells.)
We have previously suggested (10, 14) that cell poles represent used division sites. Such sites can be reactivated by newly synthesized FtsZ protein (17), although this is normally prevented by the action of the Min proteins (7). Overproduction of FtsZ by itself can overcome the inhibitory action of the Min proteins and allow septa to form at the cell poles (4, 17), but overproduction of FtsA cannot. In contrast, as we show here, coordinate overproduction of FtsA and FtsZ together not only allows the use of cell poles for septation but also enables new, nonpolar septation sites to be activated earlier in the cell cycle (Table 1). In minB mutants, polar sites and newly formed sites compete equally for a limited amount of division potential (10, 14), so that minicells form at the expense of normal divisions. Bi and Lutkenhaus (5) showed that division potential can be increased in minB mutants by increasing the production of FtsZ and FtsA but not by increasing FtsA alone. We have shown here that an increase in FtsZ alone also does not increase the capacity of cells to make new septa at internal (nonpolar) sites and that, to do this, coordinate overexpression of both FtsA and FtsZ is required. Therefore, division potential, or the capacity of a cell to make a particular number of septa at new division sites, is limited by both FtsA and FtsZ proteins.
Rings of FtsZ can form at division sites in the absence of functional FtsA, but these rings are unable to contract (1). FtsA also forms rings at division sites but only in the presence of an FtsZ ring (2). The experiments that we report here may be understood if a critical ratio of FtsA to FtsZ is required for the function of the FtsA-FtsZ ring, as previously suggested (6, 8).
Further studies are required to find out how FtsA and FtsZ interact and how it is that excess FtsZ can apparently reactivate old division sites but requires an additional increase in FtsA in order to activate new sites. One possibility is that FtsA is sequestered in old division sites, as has been suggested by the finding that FtsA protein must be remade in every cycle (11), and that some temperature-sensitive mutant forms of FtsA can cause long-lasting inactivation of division sites formed at the restrictive temperature (15). Alternatively, perhaps the entire FtsA-FtsZ complex is sequestered at the cell poles but blocked by the interaction between MinC and FtsZ; production of excess FtsZ might then titrate out the MinC and release the activity of the sequestered division proteins.
ACKNOWLEDGMENTS
This work was supported by a grant from the Medical Research Council and by studentships from the Darwin Trust (Y.N.) and the Nuffield Foundation (N.C.).
We thank Nanne Nanninga for the gift of anti-FtsZ antibodies.
REFERENCES
- 1.Addinall S G, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addinall S G, Lutkenhaus J. FtsA is localized to the septum in an FtsZ-dependent manner. J Bacteriol. 1996;178:7167–7172. doi: 10.1128/jb.178.24.7167-7172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler H I, Fisher W D, Cohen A, Hardigree A A. Miniature Escherichia coli cells deficient in DNA. Proc Natl Acad Sci USA. 1967;57:321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi E, Lutkenhaus J. FtsZ regulates the frequency of cell division in Escherichia coli. J Bacteriol. 1990;172:2765–2768. doi: 10.1128/jb.172.5.2765-2768.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi E, Lutkenhaus J. Interaction between the min locus and FtsZ. J Bacteriol. 1990;172:5610–5616. doi: 10.1128/jb.172.10.5610-5616.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai K, Lutkenhaus J. The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J Bacteriol. 1992;174:6145–6151. doi: 10.1128/jb.174.19.6145-6151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer P A J, Crossley R E, Rothfield L I. Central role of the Escherichia coli minC gene product in two different division-inhibition systems. Proc Natl Acad Sci USA. 1990;87:1129–1133. doi: 10.1073/pnas.87.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewar S J, Begg K J, Donachie W D. Inhibition of cell division by an imbalance in the ratio of FtsA to FtsZ. J Bacteriol. 1992;174:6314–6316. doi: 10.1128/jb.174.19.6314-6316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewar S J, Donachie W D. Regulation of expression of the ftsA cell division gene by sequences in upstream genes. J Bacteriol. 1990;172:6611–6614. doi: 10.1128/jb.172.11.6611-6614.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donachie W D, Begg K J. “Division potential” in Escherichia coli. J Bacteriol. 1996;178:5971–5976. doi: 10.1128/jb.178.20.5971-5976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donachie W D, Begg K J, Lutkenhaus J F, Salmond G P C, Martinez-Salas E, Vicente M. Role of the ftsA gene product in control of Escherichia coli cell division. J Bacteriol. 1979;140:388–394. doi: 10.1128/jb.140.2.388-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain K, Begg K J, Salmond G P C, Donachie W D. ParD: a new gene coding for a protein required for chromosome partitioning and septum localization in Escherichia coli. Mol Microbiol. 1987;1:73–81. doi: 10.1111/j.1365-2958.1987.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 13.Robinson A C, Kenan D J, Hatfull G F, Sullivan N F, Spiegelberg R, Donachie W D. DNA sequence and transcriptional organization of essential cell division genes ftsQ and ftsA of Escherichia coli: evidence for overlapping transcriptional units. J Bacteriol. 1984;160:546–555. doi: 10.1128/jb.160.2.546-555.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teather R M, Collins J F, Donachie W D. Quantal behavior of a diffusible factor which initiates septum formation at potential division sites in Escherichia coli. J Bacteriol. 1974;118:407–413. doi: 10.1128/jb.118.2.407-413.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tormo A, Vicente M. The ftsA gene product participates in formation of the Escherichia coli septum structure. J Bacteriol. 1984;157:779–784. doi: 10.1128/jb.157.3.779-784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voskuil J L A, Westerbeek C A M, Wu C, Kolk A H J, Nanninga N. Epitope mapping of Escherichia coli cell division protein FtsZ with monoclonal antibodies. J Bacteriol. 1994;176:1886–1893. doi: 10.1128/jb.176.7.1886-1893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward J E, Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in Escherichia coli. Cell. 1985;42:941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- 18.Yi Q-M, Rockenbach S, Ward J E, Jr, Lutkenhaus J. Structure and expression of the cell division genes ftsQ, ftsA, and ftsZ. J Mol Biol. 1985;184:399–412. doi: 10.1016/0022-2836(85)90290-6. [DOI] [PubMed] [Google Scholar]