Abstract
Background
Migraine is the world’s second most common disabling disorder, affecting 15% of UK adults and costing the UK over £1.5 billion per year. Several costly new drugs have been approved by National Institute for Health and Care Excellence.
Aim
To assess the cost-effectiveness of drugs used to treat adults with chronic migraine.
Methods
We did a systematic review of placebo-controlled trials of preventive drugs for chronic migraine. We then assessed the cost-effectiveness of the currently prescribable drugs included in the review: Onabotulinum toxin A (BTA), Eptinezumab (100mg or 300mg), Fremanezumab (monthly or quarterly dose), Galcanezumab or Topiramate, each compared to placebo, and we evaluated them jointly. We developed a Markov (state-transition) model with a three-month cycle length to estimate the costs and quality-adjusted life years (QALYs) for the different medications from a UK NHS and Personal Social Services perspective. We used a two-year time horizon with a starting age of 30 years for the patient cohort. We estimated transition probabilities based on monthly headache days using a network meta-analysis (NMA) developed by us, and from published literature. We obtained costs from published sources and applied discount rates of 3.5% to both costs and outcomes.
Results
Deterministic results suggest Topiramate was the least costly option and generated slightly more QALYs than the placebo, whereas Eptinezumab 300mg was the more costly option and generated the most QALYs. After excluding dominated options, the incremental cost-effectiveness ratio (ICER) between BTA and Topiramate was £68,000 per QALY gained and the ICER between Eptinezumab 300mg and BTA was not within plausible cost-effectiveness thresholds. The cost-effectiveness acceptability frontier showed that Topiramate is the most cost-effective medication for any amount the decision maker is willing-to-pay per QALY.
Conclusions
Among the various prophylactic medications for managing chronic migraine, only Topiramate was within typical cost-effectiveness threshold ranges. Further research is needed, ideally an economic evaluation alongside a randomised trial, to compare these newer, expensive CGRP MAbs with the cheaper oral medications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-023-01686-y.
Keywords: Chronic migraine, Prophylactic medications, Cost-effectiveness
Introduction
Migraine is one of the most common and debilitating neurological disorders globally and is the second leading cause of years lived with disability worldwide [1]. Average global migraine prevalence is reported as 12% (range: 2.6% and 21.7%), with variation between countries and between studies conducted in the same country [2]. Migraine is more common among women [3, 4] and also more prevalent among the 35–42 years age group [5]. The frequency of migraine episodes determines its classification: up to 14 migraine days per month is classified as ‘episodic’, while a headache occurring on 15 or more days per month, with at least 8 days meeting migraine criteria, is classified as ‘chronic’[6].
Chronic migraine has a disabling impact on people’s health and quality of life [7]. The global prevalence of chronic migraine is between 1.4% to 2.2% [8]. From a societal view, the more prevalent chronic migraine is, the greater the consumption of health care resources and more productivity losses. There are substantial differences in the health impact of migraine on people experiencing ≥ 15 monthly headaches days compared with people with 1–3 monthly headaches days in terms of quality of life, presenteeism of work, and total work productivity losses [5]. In the United Kingdom (UK), approximately one in six adults are affected by migraines (both episodic and chronic), predominantly young adults with personal (i.e. family) and professional responsibilities. This results in an economic burden of over £1.5 billion annually in the UK, [9] this includes both direct costs such as hospitalisation and medications, and indirect costs resulting from work presenteeism and absenteeism [10–12].
Pharmacological drugs for chronic migraine aim to reduce the frequency and severity of migraine attacks and alleviate associated symptoms such as headaches, nausea and sensitivity to light and sound. However, the current state of the evidence for migraine prevention is poor, making it difficult for those affected and clinicians to make decisions about which medications to consider. Several drugs are recommended by Health Technology Assessment (HTA) agencies within the UK: the National Institute for Health and Care Excellence (NICE) in England and the Scottish Intercollegiate Guidelines Network (SIGN) in Scotland. These include various oral medications used to treat chronic migraine such as Topiramate, Propranolol, Tricyclic antidepressants [9, 13]. The treatment pathway for people with chronic migraine is typically that they have tried at least three of the older, cheap, oral medications before they are able to access Onabotulinum toxin A injections (BTA). Since 2020, calcitonin gene-related peptide (CGRP) monoclonal antibodies (MAbs), such as Erenumab, Fremanezumab, and Galcanezumab have become available and they are usually given as monthly injections [14–17]. These treatment options are more expensive than the earlier generation of oral prophylactic medications. In people with chronic migraine, they are currently reserved for people who have not benefitted from BTA treatment. The availability of these diverse medicines means that there are more choices for healthcare professionals, policymakers and of course, the patients for managing and preventing chronic migraine. Chronic migraine was introduced as a concept in 2007, so many of the oral drugs in earlier studies, have not been trialled under the definition of ‘chronic migraine’. Hence, the current evidence base for the use of oral medications in chronic migraine comes almost exclusively from data extrapolated from trials on episodic migraine.
Evidence regarding the cost-effectiveness of these different pharmacological drugs is also lacking. There are several economic evaluations comparing single prophylactic drugs against another drug or a placebo [13, 18–22]; however, given the range of available treatments, there is an absence of comparing more than one drug. Thus, in this study, based on available evidence we present a more comprehensive economic analysis comparing various prophylactic drugs for chronic migraine in the adult population.
Methods
The study is reported as per Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Statement [23]. We have included those drugs included in our network meta-analysis of randomised controlled trials of prophylactic drugs for chronic migraine (manuscript submitted for publication) [24].
Model structure and assumptions
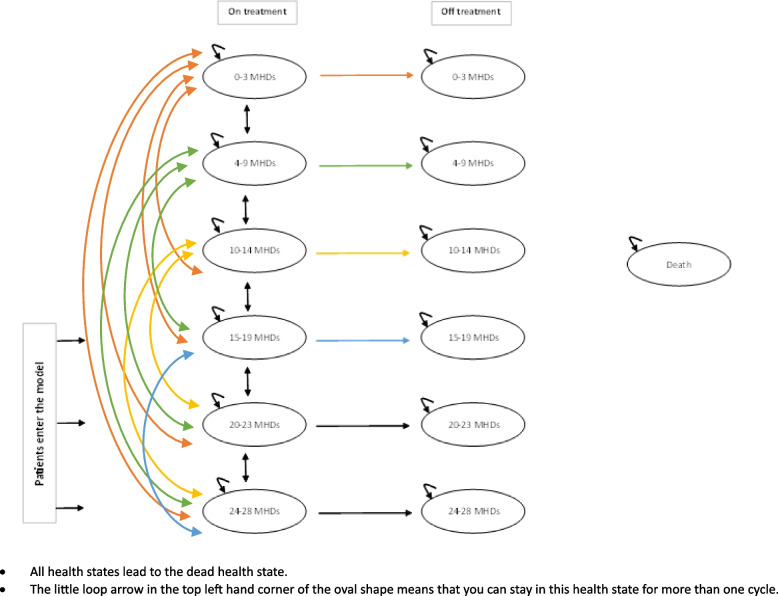
We built a Markov state-transition model to illustrate the progression of chronic migraine as measured by the number of monthly headache days (MHDs). The model was developed based on a systematic review of economic evaluations of pharmacological drugs for adults with chronic migraine, literature and input from our project team [9, 18, 25]. We created two parallel models for on-treatment and off-treatment scenarios, with MHDs as health states and an additional health state for all-cause mortality. The cycle length for the model was 12 weeks (Fig. 1).
Fig. 1.
Economic model structure
The model starts by assigning a hypothetical cohort of 1,000 people with chronic migraine into one of three chronic migraine health states based on the PREEMPT trial as it is one of the largest chronic migraine trials: 15–19 MHDs – 530 patients; 20–23 MHDs – 280 patients; and 24–28 MHDs—190 patients [26, 27]. We compared the following prophylactic medications in the base-case analysis: i) Onabotulinum toxin A (BTA), ii) Eptinezumab 100mg, iii) Eptinezumab 300mg, iv) Fremanezumab (monthly dose), v) Fremanezumab (quarterly dose), vi) Galcanezumab (120mg), vii) Topiramate (100mg); and viii) placebo. We also compared Erenumab (70mg and 140mg) with data from monthly migraine days in a sensitivity analysis.
Model inputs
Transition probabilities
To estimate transition probabilities: firstly, we digitised the transition probabilities from a paper by Batty et al. [18] which showed a visual representation of the PREEMPT trial [26, 27] transition probabilities for the placebo group. Secondly, for all other pharmacological medications, we derived post-treatment distributions of MHDs for each health state, based on differences in the mean of number of headache days from the network meta-analysis (NMA) conducted as part of our overall chronic migraine project [28]. In other words, for each of the six health states in the ‘on treatment’ arm (0–3, 4–9, 10–14, 15–19, 20–23, 24–28 MHDs) we converted these health states into more granular data. Instead of a cluster of days, a single-day band was used, e.g., 1 headache day per month, 2 headache days per month, and so on. Using this information, we then calculated the transitions (improvement in health, impairment in health and remaining in the same health) required from each headache day per month to either a better or worse health state. For example, a patient having 16 headache days per month (belonging to 15–19 MHDs health state), they would need to reduce their headaches by 2.5 days in a month in order to move to a better health state (10–14 MHDs) or their headaches would need to increase by 3.5 days in order to move into worse health state (20–23 MHDs). Using this information, for each prophylactic medication we worked out the probabilities for a person to move to a better, or to a worse, or to remain in each health state, by calculating weighted probabilities from the distribution. Thirdly, we then applied a discontinuation rate of 10% for BTA and 20% for all other medications based on input from project clinicians which reflected real-life clinical practice. Finally, we multiplied the transition probabilities for the placebo group with the transition probabilities of each pharmacological treatment to obtain transition probabilities for each individual prophylactic drug (see online Supplementary file).
Health-related quality of life (HRQoL)
The utility values for each of the MHD health states were based on the EQ-5D-5L questionnaire responses from a randomised trial for educational and supportive self-management intervention for people with chronic headaches (CHESS) [29]. The EQ-5D-5L questionnaire includes five questions addressing mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with each dimension assessed at five levels: from no to extreme problems [30]. The EQ-5D-5L responses were converted into health state utilities based on values mapped onto the EQ-5D-3L descriptive system using the the Hernandez-Alava crosswalk algorithm [31]. We assumed that HRQoL was the same for all drugs but varied by MHD health states that the participant was in (see Table 1 for details).
Table 1.
Utility values used in the base-case analysis
| Health states | Mean | SE |
|---|---|---|
| 0–3 MHD on-treatment | 0.7573 | 0.1662 |
| 4–9 MHD on-treatment | 0.6449 | 0.2817 |
| 10–14 MHD on-treatment | 0.6764 | 0.2458 |
| 15–19 MHD on-treatment | 0.6420 | 0.2543 |
| 20–23 MHD on-treatment | 0.5916 | 0.2549 |
| 24–28 MHD on-treatment | 0.5040 | 0.2835 |
| 0–3 MHD off-treatment | 0.7573 | 0.1662 |
| 4–9 MHD off-treatment | 0.6449 | 0.2817 |
| 10–14 MHD off-treatment | 0.6764 | 0.2458 |
| 15–19 MHD off-treatment | 0.6420 | 0.2543 |
| 20–23 MHD off-treatment | 0.5916 | 0.2549 |
| 24–28 MHD off-treatment | 0.5040 | 0.2835 |
Resource Utilisation and Costs
We obtained drug costs from the British National Formulary [32] and computed them for three-month cycles. Topiramate was the only orally administered drug. All the other medications (except BTA and Eptinezumab), we assumed that the first injection/infusion would be administered by a nurse (30-min) and who would also train the patient on self-administration. We assumed that 10% of patients would not be able to self-administer and accounted for this in each subsequent cycle [33, 34]. For BTA and Eptinezumab, these drugs are administered only in hospitals/clinics (we assumed these would be 15-min appointments with a nurse). The hourly cost of the nurse's time was obtained from the Unit Costs of Health and Social Care 2021 [35]. Costs were adjusted to the 2021/22 price year and any costs outside this period were inflated using the NHS cost inflation index [35] (see Table 2).
Table 2.
Resource use and unit costs
| Resource use item | Unit cost | Source |
|---|---|---|
| Prophylactic drugs (3 monthly cycle) – 2022 prices | ||
| BTAc | £276.40 | https://bnf.nice.org.uk/ [32] |
| Eptinezumabc 100mg | £1,350.00 | |
| Eptinezumabc 300mg | £4,050.00 | |
| Fremanezumab—monthly | £1,350.00 | |
| Fremanezumab—quarterly | £1,350.00 | |
| Galcanezumab | £1,350.00a | |
| Topiramate | £5.10 | |
| Staff time in 2021/2022 prices | ||
| Nurse (hourly cost) | £42.00 | Unit Costs of Health and Social Care, 2021 [35] |
| Specialist consultant – neurologist (hourly cost) | £122.00b | Latest tariff did not include costs for neurology outpatient therefore assumed to be a Follow Up Attendance—Single Professional (WF01A) for a Neurology outpatient visits (code 400) [36] |
| Other resource items in 2021/2022 prices | ||
| GP visit | £39.23 | Unit Costs of Health and Social Care, 2021 [35] |
| A&E visit | £165.00 | A&E worksheet. 'VB08Z', Emergency Medicine, Category 2 Investigation with Category 1 Treatment [37] |
| Hospital admission | £618.00 | Non-elective tariff for code AA31E (Headache, Migraine or Cerebrospinal Fluid Leak, with CC Score 0–6) in worksheet “1 APC & OPROC” HRG code: AA31E [37] |
| Triptan usage | £3.99 | The cost of triptans per attack was based on the weighted average of triptan costs in the UK, taken from NHS Prescriptions Cost Analysis [18, 25] |
aThe cost of maintenance dose in each subsequent cycle
bUprated to 2021/2022 prices
cDrugs administered in hospital
Additionally, we allocated a cost of care to each health state for each 12-week cycle, regardless of the prophylactic medication. This cost included visits to GPs, Accident and Emergency (A&E), hospital admissions, and triptan use. The usage frequency of these resources was obtained from the International Burden of Migraine study (IBMS) for UK patients and in line with published NICE guidance [25, 38–40].
We also consulted the NICE guidance [25, 39, 40] for the different prophylactic medications and included any additional visits from neurology consultants and nurses (see Table 2).
All-cause mortality
The model used age-specific mortality rates obtained from the Office for National Statistics (ONS) in the UK [41]. The rates were based on general population lifetime tables and averaged for males and females. Mortality rates increase as the cohort ages over the model's time horizon.
Base-case and sensitivity analysis
The Markov model adopted a UK NHS and Personal Social Service (PSS) perspective to analyse the costs and quality-adjusted life years (QALYs) of various prophylactic drugs for chronic migraine. The analysis used a two-year time horizon and a starting age of 30 years for the patient cohort. The costs were measured in 2021/2022 prices and health outcomes in QALYs. The cost-effectiveness analysis was measured in terms of an incremental cost per QALY gained (ICER), with a discount rate of 3.5% applied to both costs and outcomes.
To account for uncertainty in model parameters and sampling variability, we did a probabilistic sensitivity analysis (PSA) using Monte Carlo simulations with 1,000 iterations for all model inputs, except for drug costs which were fixed values. A gamma distribution was applied for costs, and a beta distribution was used for utility values. A cost-effectiveness acceptability frontier (CEAF) was used to summarise the uncertainty for the different medications jointly, by indicating which medication is preferred at different threshold values for cost-effectiveness. The cost per QALY threshold by NICE for England and Wales is between £20-30k.
Scenario and sensitivity analyses
We did scenario and sensitivity analyses by altering base-case inputs into the model:
Changing time horizon – from a 2-year time horizon to a 5-year and a life-time horizon.
Utility inputs – using van-Hout crosswalk algorithm [42] instead of the Hernandez-Alava crosswalk algorithm [31].
Monthly Migraine Days (MMDs) – using MMDs as the outcome measure instead of MHDs, allowed us to include Erenumab—70mg and 140mg in the analysis. Additionally, we utilised utility values based on MMDs from the Lipton et al. study [43].
Reducing drug costs for CGRP MAbs– confidential discounts are agreed via the Patient Access Scheme between the NHS and manufacturers, but their actual value is not available. We reduced the costs of the following drugs by 50%: Eptinezumab 100mg and 300mg, Fremanezumab monthly and quarterly, and Galcanezumab.
Results
Base-case analysis – comparing each medication separately to placebo
The deterministic discounted results showed that Topiramate dominated placebo as it was cheaper (£104 less expensive) and more effective (0.0464 more QALYs). The other medications were more expensive than placebo, however, they generated additional QALYs when compared to placebo. BTA was more cost-effective than placebo at the £30k threshold with an ICER of £25,238 per QALY gained. The other five medications (Fremanzumab monthly, Fremanzumab quarterly, Eptinezumab 100mg, Eptinezumab 300mg and Galcanuzmab) when compared with placebo had ICERs which would not be considered cost-effective if using a £20-30k ($50k or $100) per QALY threshold used by NICE in the UK (threshold values used in the USA [44]). Probabilistic results where similar to deterministic results (see Table 3).
Table 3.
Base-case cost-effectiveness results comparing each medication separately
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER: cost per QALY gained (£) | |
|---|---|---|---|---|---|
| Deterministic results—discounted | |||||
| Placebo | £1,729 | 1.3531 | - | - | - |
| Topiramate | £1,624 | 1.3995 | -£104 | 0.0464 | Dominated |
| Placebo | £1,729 | 1.3531 | - | - | - |
| BTA | £3,654 | 1.4294 | £1,925 | 0.0763 | £25,238 |
| Placebo | £1,729 | 1.3531 | - | - | - |
| Fremanezumab (monthly) | £10,155 | 1.4307 | £8,427 | 0.0776 | £108,604 |
| Placebo | £1,729 | 1.3531 | - | - | - |
| Fremanezumab (quarterly) | £10,193 | 1.4224 | £8,465 | 0.0693 | £122,126 |
| Placebo | £1,729 | 1.3531 | - | - | - |
| Eptinezumab 100 | £10,216 | 1.4239 | £8,487 | 0.0708 | £119,796 |
| Placebo | £1,729 | 1.3531 | - | - | - |
| Galcanezumab | £10,640 | 1.4229 | £8,912 | 0.0698 | £127,649 |
| Placebo | £1,729 | 1.3531 | - | - | - |
| Eptinezumab 300 | £27,401 | 1.4403 | £25,672 | 0.0873 | £294,151 |
| Probabilistic results—discounted | |||||
| Placebo | £1,728 | 1.3460 | - | - | - |
| Topiramate | £1,624 | 1.4045 | -£104 | 0.0584 | Dominated |
| Placebo | £1,728 | 1.3460 | - | - | - |
| BTA | £3,654 | 1.4270 | £1,926 | 0.0810 | £23,775 |
| Placebo | £1,728 | 1.3460 | - | - | - |
| Fremanezumab (monthly) | £10,161 | 1.4350 | £8,433 | 0.0890 | £94,748 |
| Placebo | £1,728 | 1.3460 | - | - | - |
| Fremanezumab (quarterly) | £10,196 | 1.4273 | £8,467 | 0.0812 | £104,251 |
| Placebo | £1,728 | 1.3460 | - | - | - |
| Eptinezumab 100 | £10,221 | 1.4199 | £8,492 | 0.0739 | £114,894 |
| Placebo | £1,728 | 1.3460 | - | - | - |
| Galcanezumab | £10,646 | 1.4161 | £8,917 | 0.0701 | £127,279 |
| Placebo | £1,728 | 1.3460 | - | - | - |
| Eptinezumab 300 | £27,411 | 1.4365 | £25,683 | 0.0904 | £284,030 |
Base-case analysis – comparing all medications together
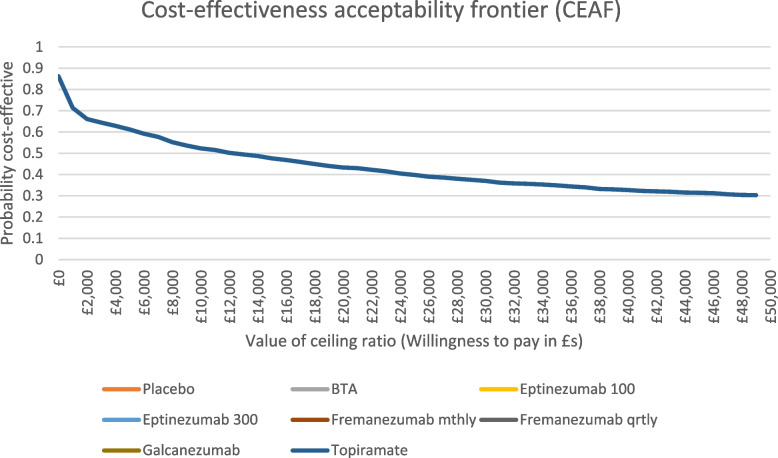
Table 4 shows the discounted deterministic results when comparing all medications ranked by the least costly option. Topiramate was the least costly option and had slightly more QALYs than the placebo, whereas Eptinezumab 300mg was the more costly option and generated the most QALYs. Options placebo (dominated by Topiramate), Fremanezumab quarterly, Eptinezumab 100mg and Galcanezumab (all dominated by Fremanezumab monthly) were all eliminated as they were dominated by other medications. We then compared Topiramate, BTA, Fremanezumab monthly and Eptinezumab 300mg. Fremanezumab monthly was extendedly dominated (where any interventions that have an ICER which is greater than that of a more effective intervention is ruled out) by a linear combination of BTA and Eptinezumab 300mg and was therefore eliminated. The ICER between BTA and Topiramate was £68,000 per QALY gained and the ICER between Eptinezumab 300mg and BTA was not within plausible cost-effectiveness thresholds. The probabilistic results were similar to the deterministic results. The CEAF shows that Topiramate is the most cost-effective medication for any amount the decision maker is willing-to-pay per QALY (see Fig. 2).
Table 4.
Base-case cost-effectiveness results comparing all medications
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER: cost per QALY gained (£) | Comparison | |
|---|---|---|---|---|---|---|
| Deterministic results—discounted | ||||||
| Topiramate | £1,625 | 1.3995 | - | - | - | |
| Placebo | £1,729 | 1.3531 | £104 | -0.0464 | Dominated | Placebo vs. Topiramate |
| BTA | £3,654 | 1.4294 | £2,029 | 0.0298 | £68,002 | BTA vs. Topiramate |
| Fremanezumab (monthly) | £10,155 | 1.4403 | £6,501 | 0.0013 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | £10,193 | 1.4224 | £38 | -0.0083 | Dominated | Fremanezumab (quarterly vs. monthly) |
| Eptinezumab 100 | £10,216 | 1.4239 | £22 | -0.0067 | Dominated | Eptinezumab 100 vs Fremanezumab (monthly) |
| Galcanezumab | £10,640 | 1.4229 | £485 | -0.0078 | Dominated | Galcanezumab vs. Fremanezumab (monthly) |
| Eptinezumab 300 | £27,401 | 1.4403 | £17,246 | 0.0097 | £2,160,037 | Eptinezumab 300 vs BTA |
| Probabilistic results—discounted | ||||||
| Topiramate | £1,624 | 1.4045 | - | - | - | |
| Placebo | £1,728 | 1.3460 | £104 | -0.0584 | Dominated | Placebo vs. Topiramate |
| BTA | £3,654 | 1.4270 | £2,030 | 0.0226 | £89,939 | BTA vs. Topiramate |
| Fremanezumab (monthly) | £10,161 | 1.4350 | £6,507 | 0.0080 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | £10,196 | 1.4273 | £34 | -0.0078 | Dominated | Fremanezumab (quarterly vs. monthly) |
| Eptinezumab 100 | £10,221 | 1.4199 | £59 | -0.0151 | Dominated | Eptinezumab 100 vs Fremanezumab (monthly) |
| Galcanezumab | £10,646 | 1.4161 | £485 | -0.0189 | Dominated | Galcanezumab vs. Fremanezumab (monthly) |
| Eptinezumab 300 | £27,411 | 1.4365 | £17,250 | 0.0014 | £2,524,429 | Eptinezumab 300 vs BTA |
Extendedly dominated is where any interventions that have an ICER which is greater than that of a more effective intervention is ruled out
Fig. 2.
Base-case cost-effectiveness acceptability frontier
Sensitivity analysis
Table 5 shows the results for the discounted probabilistic sensitivity analysis when comparing all medications together (the discounted deterministic results were similar and have not been presented here). For all the different scenarios, and in line with the base-case results, Topiramate was the least costly option and had slightly more QALYs than placebo; whereas Eptinezumab 300mg was the more costly option. For all scenarios, when removing the dominated options, BTA was more cost-effective than Topiramate; however, the cost per QALY gained was not within plausible thresholds unless a lifetime horizon was used. After removing the dominated options, when BTA was compared with either Fremanezumab monthly or Eptinezumab 300mg, the ICERs were not within plausible cost-effectiveness threshold ranges.
Table 5.
Sensitivity analysis results comparing all medications
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER: cost per QALY gained (£) | Comparison | |
|---|---|---|---|---|---|---|
| a) 5-year time horizon | ||||||
| Probabilistic results – discounted | ||||||
| Topiramate | £3,159 | 3.1717 | - | - | - | - |
| Placebo | £3,491 | 3.0348 | £333 | -0.1369 | Dominated | Placebo vs. Topiramate |
| BTA | £6,383 | 3.2497 | £3,224 | 0.0779 | £41,366 | BTA vs. Topiramate |
| Fremanezumab (monthly) | £16,039 | 3.2483 | £9,656 | -0.0014 | Dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | £16,120 | 3.2283 | £9,737 | -0.0214 | Dominated | Fremanezumab (quarterly) vs BTA |
| Eptinezumab 100 | £16,145 | 3.2163 | £9,762 | -0.0334 | Dominated | Eptinezumab 100 vs. BTA |
| Galcanezumab | £16,577 | 3.2071 | £10,194 | -0.0425 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | £42,184 | 3.2573 | £35,801 | 0.0076 | £4,707,286 | Eptinezumab 300 vs. BTA |
| b) Lifetime horizon | ||||||
| Probabilistic results—discounted | ||||||
| Topiramate | £13,351 | 15.7628 | - | - | - | - |
| Placebo | £15,138 | 15.1467 | £1,787 | -0.6161 | Dominated | Placebo vs. Topiramate |
| BTA | £16,381 | 16.2613 | £3,030 | 0.4985 | £6,077 | BTA vs. Topiramate |
| Fremanezumab (monthly) | £27,469 | 16.1774 | £11,088 | -0.0840 | Dominated | Fremanezumab (monthly) vs. BTA |
| Eptinezumab 100 | £27,846 | 16.1319 | £11,465 | -0.1294 | Dominated | Fremanezumab (quarterly) vs BTA |
| Fremanezumab (quarterly) | £27,840 | 16.0931 | £11,459 | -0.1682 | Dominated | Eptinezumab 100 vs. BTA |
| Galcanezumab | £28,194 | 16.1418 | £11,813 | -0.1195 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | £57,609 | 16.3428 | £41,228 | 0.0815 | £505,711 | Eptinezumab 300 vs. BTA |
| c) Utility inputs—van-Hout crosswalk algorithm | ||||||
| Probabilistic results—discounted | ||||||
| Topiramate | £1,627 | 1.4063 | - | - | - | - |
| Placebo | £1,723 | 1.3807 | 96 | -0.0256 | Dominated | Placebo vs. Topiramate |
| BTA | £3,656 | 1.4475 | £2,029 | 0.0412 | £49,265 | BTA vs. Topiramate |
| Fremanezumab (monthly) | £10,161 | 1.4608 | £6,505 | 0.0133 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | £10,193 | 1.4532 | £32 | -0.0076 | Dominated | Fremanezumab (quarterly) vs Fremanezumab (monthly) |
| Eptinezumab 100 | £10,221 | 1.4346 | £60 | -0.0262 | Dominated | Eptinezumab 100 vs. Fremanezumab (monthly) |
| Galcanezumab | £10,650 | 1.4436 | £489 | -0.0172 | Dominated | Galcanezumab vs. Fremanezumab (monthly) |
| Eptinezumab 300 | £27,411 | 1.4512 | £17,250 | -0.0096 | £6,353,726 | Eptinezumab 300 vs. BTA |
| d) Using MMDs instead of MHDs | ||||||
| Probabilistic results—discounted | ||||||
| Topiramate | £1,585 | 1.3220 | - | - | - | - |
| Placebo | £1,731 | 1.2245 | £146 | -0.0975 | Dominated | Placebo vs. Topiramate |
| BTA | £3,645 | 1.3566 | £2,060 | 0.0346 | £59,596 | BTA vs. Topiramate |
| Erenumab 70 | £8,944 | 1.3754 | £5,299 | 0.0188 | Extendedly dominated | Erenumab 70 vs BTA |
| Erenumab 140 | £8,949 | 1.3749 | £5 | -0.0005 | Dominated | Erenumab 140 vs Erenumab 70 |
| Fremanezumab (monthly) | £10,072 | 1.3916 | £1,128 | 0.0162 | £183,732 | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | £10,140 | 1.3644 | £68 | -0.0272 | Dominated | Fremanezumab (quarterly) vs Fremanezumab (monthly) |
| Eptinezumab 100 | £10,188 | 1.3584 | £116 | -0.0332 | Dominated | Eptinezumab 100 vs. Fremanezumab (monthly) |
| Galcanezumab | £10,610 | 1.3584 | £538 | -0.0332 | Dominated | Galcanezumab vs. Fremanezumab (monthly) |
| Eptinezumab 300 | £27,377 | 1.3850 | £17,305 | -0.0065 | Dominated | Eptinezumab 300 vs. Fremanezumab (monthly) |
| e) Reducing costs of MAbs by 50% | ||||||
| Probabilistic results—discounted | ||||||
| Topiramate | £1,625 | 1.4078 | - | - | - | - |
| Placebo | £1,729 | 1.3415 | £105 | -0.0663 | Dominated | Placebo vs. Topiramate |
| BTA | £3,653 | 1.4218 | £2,028 | 0.0140 | £144,881 | BTA vs. Topiramate |
| Fremanezumab (monthly) | £5,835 | 1.4395 | £2,182 | 0.0177 | £123,111 | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | £5,869 | 1.4321 | £34 | -0.0074 | Dominated | Fremanezumab (quarterly) vs Fremanezumab (monthly) |
| Eptinezumab 100 | £5,896 | 1.4210 | £61 | -0.0185 | Dominated | Eptinezumab 100 vs. Fremanezumab (monthly) |
| Galcanezumab | £6,097 | 1.4272 | £261 | -0.0123 | Dominated | Galcanezumab vs. Fremanezumab (monthly) |
| Eptinezumab 300 | £14,455 | 1.4358 | £8,620 | -0.0037 | Dominated | Eptinezumab 300 vs. Fremanezumab (monthly) |
Extendedly dominated is where any interventions that have an ICER which is greater than that of a more effective intervention is ruled out
Discussion
In this economic evaluation we aimed to determine the cost-effectiveness of different pharmacological drugs for managing chronic migraine. With numerous drugs available for chronic migraine management in the UK, it can be challenging to determine the most cost-effective option, while ensuring that the limited resources and finite budget meets the needs of chronic migraine patients. Our 2022 review of existing economic analyses for chronic migraine prophylactic medications revealed a lack of comprehensive evaluations that compared more than three medications against each other [9]. In the absence of such evidence, this study provides more comprehensive insights into managing a common neurological disorder. It also has important implications for policymakers in helping them making informed decisions and allocating scarce resources for chronic migraine management. It can help optimise patient access to effective treatments while ensuring efficient utilisation of healthcare resources. This approach has the potential to enhance the overall quality of care provided to individuals suffering from chronic migraine, leading to better access to treatments, improved quality of life and better allocation of limited healthcare resources.
For the base-case analysis, the deterministic results showed when comparing each of the medications seperately against placebo, Topiramate dominated placebo. The other drugs when compared separately, were more expensive than placebo, however, they generated more QALYs. In terms of the cost per QALY gained, BTA was more cost-effective than placebo at the £30k threshold with an ICER of £25,328 per QALY gained. The deterministic results when comparing all medications together, Topiramate was the cheapest, but generated the fewest QALYs (with the exception of placebo). On the other hand, Eptinezumab 300mg was the most expensive option and produced the most QALYs. The ICER for BTA vs Topiramate was estimated to be £68,000 per QALY gained, while the ICER for Eptinezumab 300mg vs BTA was not within plausible cost-effectiveness thresholds. The CEAF revealed that when comparing all medications, Topiramate was most likely to be the cost-effective medication for any amount the decision-maker is willing-to-pay per QALY. NICE typically uses a threshold range of £20-£30k per QALY gained as a reference range, but this range can be higher or lower depending on the circumstances. Base-case probabilistic results were consistent with the base-case deterministic results. Sensitivity and scenario analyses were conducted, primarily using MHDs as an outcome measure, and the results were mostly consistent with the base-case findings. The only important exception was that when using MMDs as an outcome measure, Fremanezumab monthly generated more QALYs than Eptinezumab 300mg.
Our results are in line with previous studies. Batty et al. (2013) concluded the use of BTA for chronic migraine resulted in an increase in costs of £1,367 and an improvement in QALYs of 0.1 compared to placebo, resulting in an ICER of £15,028. Specifically, treatment with BTA was associated with a reduction in headache days by approximately 38 days per year, at a cost of £18 per headache day avoided [18]. A 2018 study found that with an annual drug price of US$6,900 (£5,604 in 2017 prices) for Erenumab in 2017 prices, treatment with Erenumab compared to no preventive treatment is dominant from a societal perspective, meaning it is both cheaper and more effective for chronic migraine patients. When indirect costs were excluded, the ICERs were considered to be cost-effective for chronic migraine participants: comparing Erenumab to no preventive treatment the ICER was (US$23,079; £18,746 in 2017 prices) and when comparing Erenumab with BTA, although the ICER (US$65,720; £53,380 in 2017 prices) was considered cost-effective, it is not within current UK cost-effectiveness thresholds [22].
The 2022 systematic review on this topic by our team concluded that BTA is cost-effective when compared to a placebo, with an ICER ranging between £15,028 and £16,598 [9]. For individuals who did not respond to previous preventive treatments, Erenumab was shown to be a cost-effective alternative to placebo. However, when comparing Erenumab to BTA, the ICERs ranged from £59,712 to £182,128, exceeding the most commonly accepted willingness-to-pay (WTP) thresholds [9]. Under widely accepted WTP thresholds, all CGRP MAbs, including Erenumab, Galcanezumab, and Fremanezumab, were deemed cost-effective for the chronic migraine population who have failed BTA [9].
Strengths of the study
To our knowledge, this is the first study that encompasses five drugs (seven different dosing regimens) plus placebo for managing chronic migraine providing valuable insights into cost-effectiveness. The study addressed a gap in the literature by comparing multiple medications against each other, offering a more comprehensive analysis of available options. The study features sensitivity analyses, which enable a wide range of changes in the parameters of interest to be examined and their potential impact on the base-case results to be investigated. Sensitivity and scenario analyses confirmed the robustness of the findings as the probabilistic results were consistent with the base-case deterministic results.
Study limitations
Due to the lack of readily available evidence in the literature we had to employ some additional assumptions, some of which may not be true in current practice. Firstly, one assumption we used was when someone enters the ‘off-treatment’ health state, they cannot return to an ‘on-treatment’ health state. For example, we know that a participant can come off a prophylactic medication if their migraines are better, or if they cannot tolerate a medication; however, their migraine may return sometime later, and they may be prescribed another medication for their migraine.
Secondly, we assumed that the treatment effects were based on mean health differences from our NMA, where we assumed these effects would be uniformly distributed across all health states, regardless of the severity of the condition at the start. However, it is likely that there will be heterogeneity in the distribution of effects. Furthermore, in our NMA we have not included evidence on other oral medications (such as Amitriptyline, Candesartan Propranolol). We only included trials with at least 100 participants per arm meaning it was possible we excluded some smaller studies of other oral medications. However, on re-checking the excluded studies list there were no trials excluded from the NMA on the basis of size alone [28].
Thirdly, the small differences in QALYs between some of the medications namely Fremanezumab and BTA meant that they produced very large ICERs. Even quite small changes in the QALY estimate might substantially change the apparent cost-effectiveness. Fourthly, we used utility data based on MHDs based on the CHESS trial. There was limited data in the literature on utility values for MMDs; the majority of utility values for MMDs were based on data for episodic migraine [19, 22, 43] Also, there were no studies that mapped EQ-5D or SF-6D data to generate utility values for the specific headache day health states we have used in our model.
Fifthly, we only considered a NHS and PSS perspective. If we were to take a broader societal perspective, incorporating indirect costs such as productivity losses, the resulting ICERs may have been different. Finally, we excluded adverse events from the model, based on evidence from our systematic review on adverse events, we found that serious adverse events were not related to the medication itself and therefore were assumed to not influence health care resource usage [22, 28].
Conclusion
Among the different prophylactic medications for managing chronic migraine included in this study, it seems that Topiramate was the cheapest, however, it is not the most effective in terms of gained QALYs in comparison with other medications. On the other hand, Eptinezumab 300mg was more costly, however, it accrued the most QALYs. When comparing all medications, only Topiramate was within typical cost-effectiveness threshold ranges. Further research is needed, ideally an economic evaluation alongside a randomised trial, to compare these newer, expensive CGRP MAbs with the cheaper oral medications.
Supplementary Information
Additional file 1: Table A. Deterministic transition probabilities used in the base-case analysis.
Acknowledgements
We would like to thank Saval Khanal who helped to obtain inputs for the model. We are also grateful to James Mason and the Health Economics Study Group (HESG) Summer 2023 conference attendees who provided comments an on earlier draft version of this manuscript.
Authors’ contributions
Study concept and design: HM, MU, CD, JM, MM; Funding acquisition: HM, MU, CD, JM, MM; Methodology and analysis: HM, SN, MU, JM; Writing first draft: HM, SN; Edits and revisions of manuscript: HM, SN, MU, CD, JM, MM; All authors agreed to submit the current version of the manuscript to the journal.
Funding
This study/project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme – project reference NIHR132803.
Availability of data and materials
The datasets used and/or used during the current study are available from the data sharing committee on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
HM, SN, JM have no competing interests to declare.MU is chief investigator or co-investigator on multiple previous and current research grants from the UK National Institute for Health and Care Research, and is a co-investigator on grants funded by the Australian NHMRC and Norwegian MRC. He is a director and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd, funded by the European Social Fund, related to return to work initiatives. He is a co-investigator on two current and one completed NIHR funded studies that have, or have had, additional support from Stryker Ltd. CD is chair of Scottish Intercollegiate Guideline Network (SIGN) 155 and has provided advise on the use of BTA, CGRP MAbs and CGRP antagonists to the Scottish Medicines Consortium and on Eptinezumab to NICE. He is the Secretary for the British Association for the Study of Headache 2015-2022 and he is a Board member of Anglo Dutch Migraine Association. MM is the President of the medical advisory board of the CSF Leak Association. He has received consulting fees from AbbVie, TEVA, Lundbeck, Eli Lilly, Salvia, Pfizer. He has received payment for the development of educational presentations from AbbVie, Pfizer and Eli Lilly and support for attending a meeting from Pfizer. He has is on the advisory board for AbbVie, TEVA, Lunbeck, Eli Lilly, Salvia and Pfizer. He has the following patent issued WO2018051103A1: System and method for diagnosing and treating headaches. He has stock options with Tesla, Adobe, Nvidia, META and Microsoft. He has received grants from Abbott, Medtronic and Ehlers Danlos society.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steiner T, Stovner L, Jensen R, Uluduz D, Katsarava Z. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):1–4. doi: 10.1186/s10194-020-01208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. 2018;8(6):e00950. doi: 10.1002/brb3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, et al. Sex Differences in the Prevalence, Symptoms, and Associated Features of Migraine, Probable Migraine and Other Severe H eadache: Results of the A merican Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1278–99. [DOI] [PubMed]
- 4.Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631–649. doi: 10.1016/j.ncl.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Doane MJ, Gupta S, Fang J, Laflamme AK, Vo P. The humanistic and economic burden of migraine in Europe: a cross-sectional survey in five countries. Neurol Ther. 2020;9:535–549. doi: 10.1007/s40120-020-00196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lattanzi S, Trinka E, Altamura C, Del Giovane C, Silvestrini M, Brigo F, et al. Atogepant for the Prevention of Episodic Migraine in Adults: A Systematic Review and Meta-Analysis of Efficacy and Safety. Neurol Ther. 2022;11(3):1235–1252. doi: 10.1007/s40120-022-00370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil M, Moreno-Ajona D, Villar-Martínez MD, Greenwood F, Hoffmann J, Goadsby PJ. Erenumab in chronic migraine: Experience from a UK tertiary centre and comparison with other real-world evidence. Eur J Neurol. 2022;29(8):2473–2480. doi: 10.1111/ene.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natoli J, Manack A, Dean B, Butler Q, Turkel C, Stovner L, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30(5):599–609. doi: 10.1111/j.1468-2982.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 9.Khanal S, Underwood M, Naghdi S, Brown A, Duncan C, Matharu M, et al. A systematic review of economic evaluations of pharmacological treatments for adults with chronic migraine. J Headache Pain. 2022;23(1):122. doi: 10.1186/s10194-022-01492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg J. Economic Evidence in Migraine and Other Headaches: A Review. Eur J Health Econ. 2004;5:S43–54. doi: 10.1007/s10198-005-0288-z. [DOI] [PubMed] [Google Scholar]
- 11.Lanteri-Minet M. Economic burden and costs of chronic migraine. Curr Pain Headache Rep. 2014;18(1):1–6. doi: 10.1007/s11916-013-0385-0. [DOI] [PubMed] [Google Scholar]
- 12.Lantéri-Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31(7):837–850. doi: 10.1177/0333102411398400. [DOI] [PubMed] [Google Scholar]
- 13.Hollier-Hann G, Curry A, Onishchenko K, Akehurst R, Ahmed F, Davies B, et al. Updated cost-effectiveness analysis of onabotulinumtoxinA for the prevention of headache in adults with chronic migraine who have previously received three or more preventive treatments in the UK. J Med Econ. 2020;23(1):113–123. doi: 10.1080/13696998.2019.1675417. [DOI] [PubMed] [Google Scholar]
- 14.Diener H-C, Charles A, Goadsby PJ, Holle D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14(10):1010–1022. doi: 10.1016/S1474-4422(15)00198-2. [DOI] [PubMed] [Google Scholar]
- 15.Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999–2008. doi: 10.1001/jama.2018.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–2132. doi: 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- 17.Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–2122. doi: 10.1056/NEJMoa1709038. [DOI] [PubMed] [Google Scholar]
- 18.Batty AJ, Hansen RN, Bloudek LM, Varon SF, Hayward EJ, Pennington BW, et al. The cost-effectiveness of onabotulinumtoxinA for the prophylaxis of headache in adults with chronic migraine in the UK. J Med Econ. 2013;16(7):877–887. doi: 10.3111/13696998.2013.802694. [DOI] [PubMed] [Google Scholar]
- 19.Giannouchos TV, Mitsikostas DD, Ohsfeldt RL, Vozikis A, Koufopoulou P. Cost-Effectiveness Analysis of Erenumab Versus OnabotulinumtoxinA for Patients with Chronic Migraine Attacks in Greece. Clin Drug Investig. 2019;39(10):979–990. doi: 10.1007/s40261-019-00827-z. [DOI] [PubMed] [Google Scholar]
- 20.Hansson-Hedblom A, Axelsson I, Jacobson L, Tedroff J, Borgstrom F. Economic consequences of migraine in Sweden and implications for the cost-effectiveness of onabotulinumtoxinA (Botox) for chronic migraine in Sweden and Norway. J Headache Pain. 2020;21(1):99. doi: 10.1186/s10194-020-01162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahon R, Lang A, Vo P, Huels J, Cooney P, Danyliv A, et al. Cost-Effectiveness of Erenumab for the Preventive Treatment of Migraine in Patients with Prior Treatment Failures in Sweden. Pharmacoeconomics. 2021;39(3):357–372. doi: 10.1007/s40273-020-00996-2. [DOI] [PubMed] [Google Scholar]
- 22.Sussman M, Benner J, Neumann P, Menzin J. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: Results from the US societal and payer perspectives. Cephalalgia. 2018;38(10):1644–1657. doi: 10.1177/0333102418796842. [DOI] [PubMed] [Google Scholar]
- 23.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value in health. 2022;25(1):10–31. doi: 10.1016/j.jval.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Naghdi S, Underwood M, Madan J, Brown A, Duncan C, Matharu M, et al. Clinical effectiveness of pharmacological interventions for managing chronic migraine in adults: a systematic review and network meta-analysis. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- 25.Royle P, Cummins E, Walker C, Chong S, Kandala N-B, Waugh N. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine - Single Technology Apprasial. Warwick Evidence; 2011.
- 26.Diener H, Dodick D, Aurora S, Turkel C, DeGryse R, Lipton R, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 27.Aurora S, Dodick D, Turkel C, DeGryse R, Silberstein S, Lipton R, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803. doi: 10.1177/0333102410364676. [DOI] [PubMed] [Google Scholar]
- 28.Mistry H, Naghdi S, Brown A, Rees S, Madan J, Grove A, et al. What is the comparative clinical and cost-effectiveness of pharmacological treatments for adults with chronic migraine? Health Technology Assessment. Manuscript submitted for publication.
- 29.Underwood M, Achana F, Carnes D, Eldridge S, Ellard DR, Griffiths F, et al. Supportive self-management program for people with chronic headaches and migraine: a randomized controlled trial and economic evaluation. Neurology. 2023;100(13):e1339–e1352. doi: 10.1212/WNL.0000000000201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández-Alava M, Pudney S. EQ5Dmap: a command for mapping between EQ-5D-3L and EQ-5D-5L. Stand Genomic Sci. 2018;18(2):395–415. [Google Scholar]
- 32.National Institute for Health and Care Excellence. British National Formulary (BNF) 2022 [updated updated 14th December 2022. Available from: https://bnf.nice.org.uk/.
- 33.National Institute for Health and Care Excellence. Single Technology Appraisal: Fremanezumab for preventing migraine [ID1368] - Committee Papers. London. 2019.
- 34.National Institute for Health and Care Excellence. Single Technology Appraisal: Galcanezumab for preventing migraine [ID1372] - Committee Papers. London. 2020.
- 35.Jones KC, Burns A. Unit costs of health and social care. 2021.
- 36.NHS England. NHS Tariff 2018/2019 2020 [Available from: https://www.england.nhs.uk/publication/past-national-tariffs-documents-and-policies/.
- 37.NHS England. NHS Tariff 2021/2022 2022 [Available from: https://www.england.nhs.uk/publication/past-national-tariffs-documents-and-policies/.
- 38.Blumenfeld A, Varon S, Wilcox T, Buse D, Kawata A, Manack A, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31(3):301–315. doi: 10.1177/0333102410381145. [DOI] [PubMed] [Google Scholar]
- 39.National Institute for Health and Care Excellence. Galcanezumab for preventing migraine. Technology appraisal guidance. 2020 18 November.
- 40.National Institute for Health and Care Excellence. Fremanezumab for preventing migraine. Technology appraisal guidance [TA764] 2022 [updated 02 February 2022. Available from: https://www.nice.org.uk/guidance/ta764.
- 41.Office for National Statistics. UK Interim Life Tables, 1980–1982 to 2018–2020. 2021.
- 42.Van Hout B, Janssen M, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value in health. 2012;15(5):708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Lipton RB, Brennan A, Palmer S, Hatswell AJ, Porter JK, Sapra S, et al. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: a US societal perspective. J Med Econ. 2018;21(7):666–675. doi: 10.1080/13696998.2018.1457533. [DOI] [PubMed] [Google Scholar]
- 44.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table A. Deterministic transition probabilities used in the base-case analysis.
Data Availability Statement
The datasets used and/or used during the current study are available from the data sharing committee on reasonable request.