Abstract
sep1+ encodes a Schizosaccharomyces pombe homolog of the HNF-3/forkhead family of the tissue-specific and developmental gene regulators identified in higher eukaryotes. Its mutant allele sep1-1 causes a defect in cytokinesis and confers a mycelial morphology. Here we report on genetic interactions of sep1-1 with the M-phase initiation mutations wee1−, cdc2-1w, and cdc25-22. The double mutants sep1-1 wee1− and sep1-1 cdc2-1w form dikaryon cells at high frequency, which is due to nuclear division in the absence of cell division. The dikaryosis is reversible and suppressible by cdc25-22. We propose that the genes wee1+, cdc2+, cdc25+, and sep1+ form a regulatory link between the initiation of mitosis and the initiation of cell division.
Most eukaryotic cells undergo a cytoplasmic division (cytokinesis) late in the M phase of the cell cycle. In animal cells, it is a contractile process brought about by a transient structure composed of circumferentially aligned microfilaments encircling the furrow where constriction and cleavage occur. A wide range of proteins (actin, myosin, septin, caldesmon, calmodulin, cofilin, profilin, coronin, etc.) are involved in the process (for recent reviews, see references 9 and 32). Although many structural components of the process are known, there is little information on the signals and mechanisms that coordinate it with nuclear division. Recent observations suggest that the maturation-promoting factor, a complex of a regulatory cyclin and the p34cdc2 kinase that catalyzes entry into mitosis, also initiates the cell division pathway (for a review, see reference 9). This complex phosphorylates a number of substrates in the prophase of mitosis, among them the regulatory light chain of myosin II, spectrin, and caldesmon, which are involved in cytokinesis (10, 14, 33).
The unicellular eukaryote Schizosaccharomyces pombe provides a technically convenient system for the genetic and molecular biological analyses of cytokinesis. It has a structure, the medial division septum, which is analogous to the cleavage furrow seen in the mammalian cells. Basically, its cell division is separable into two major stages: (i) elaboration of the septum and (ii) separation of the daughter cells by hydrolysis of the central layer of the septum (reviewed in reference 17). The first process is usually called septation, whereas cell separation is frequently referred to as cytokinesis. Septum formation requires two at least partially separable processes: formation of an actin ring in the middle of the cylindrical cells and deposition of the septal material at the actin ring. A number of genes are known to be involved in the assembly and correct positioning of the actin ring (cdc3, -4, -8, -12, and -15, dmf1/mid1, etc.). Other genes (cdc7, -11, -14, etc.) are thought to be required for the production and deposition of the septal material (for a review, see reference 34).
Recently, we isolated a novel category of mutants, sep1− mutants, which form septa but do not undergo cytokinesis and thus develop a mycelium (40). However, certain observations suggest that their cytokinesis defect is pleiotropic rather than a direct consequence of the inactivation of sep1+. For example, the cells of the mycelium separate when the culture enters stationary phase and the sep1-1 mutation genetically interacts with cdc4-8, a mutation of cdc4+ which encodes an EF-hand protein involved in formation of the actin ring (22). This interaction suggests that the function of sep1+ is not specific for cytokinesis; it may also be involved in an early event of cell division. sep1+ has been cloned and found to encode a homolog of the HNF-3/forkhead family of tissue-specific and developmental gene regulators identified in higher eukaryotes (27).
In this report, we show that sep1-1 uncouples nuclear division and cell division in a wee1− or cdc2-1w background. The double mutants frequently skip septation and produce binucleate cells (dikaryons). The product of cdc2+ (p34cdc2 or cdk1) is a protein kinase whose activation is required for the onset of mitosis and destruction coincides with the onset of septum formation (for a review, see reference 3). The product of wee1+ (p107wee1) is a negative regulator of p34cdc2 (31). If the cdc25+ phosphatase, an activator of p34cdc2 (30), is also inactivated, septation becomes regular. The genetic interactions indicate that these genes may be involved in the coordination of cell division with nuclear division.
MATERIALS AND METHODS
Strains and media.
The strains used in this study are described in Table 1. The media YEL, EMM, and MEA have been described by Gutz et al. (12) and Sipiczki and Ferenczy (38). Strains were constructed by standard genetic methods as described by Gutz et al. (12), but crosses were usually done by protoplast fusion (38).
TABLE 1.
Strains used
| Genotype | Origin |
|---|---|
| L972 h− (wild type) | U. Leupold |
| L975 h90 (wild type) | U. Leupold |
| cdc2-1w | P. Fantes |
| cdc2-3w h90 | P. Fantes |
| cdc2-3w leu1-32 h− | P. Fantes |
| cdc10-129 lys1-131 h− | P. Fantes |
| cdc25-22 lys3 h− | P. Fantes |
| cwg1-1 h− | A. Duran |
| cwg2-1 h− | A. Duran |
| cyr1::LEU2 leu1-32 ura4-D18 h+ | P. Nurse |
| orb1-1 h− | P. Nurse |
| sph1-1 h− | A. Duran |
| sep1-1 h90 | This study |
| sep1-1 leu1-32 h− | This study |
| sep1-1 cdc2-1w leu1-32 h− | This study |
| sep1-1 cdc2-3w lys1-32 h− | This study |
| sep1-1 cdc2-3w h90 | This study |
| sep1-1 cdc10-129 wee1-112 ura5 h− | This study |
| sep1-1 cdc25-22 wee1-50 ade1 h+ | This study |
| sep1-1 cwg1-1 h− | This study |
| sep1-1 cwg2-1 h− | This study |
| sep1-1 cyr1::LEU2 leu1-32 h− | This study |
| sep1-1 orb1-1 h− | This study |
| sep1-1 sph1-1 h− | This study |
| sep1-1 wee1-50 ade1 h+ | This study |
| sep1-1 wee1-50 h90 | This study |
| sep1-1 wee1-112 h− | This study |
| sep1-1 wee1-112 h90 | This study |
| sep1-1 wee1-112 ura5 h− | This study |
| wee1-50 h− | P. Fantes |
| wee1-50 h90 | P. Fantes |
| wee1-112 h90 | P. Fantes |
| wee1-50 cdc25-22 h− | This study |
| wee1-112 leu1-32 h− | P. Fantes |
Double and triple mutants containing sep1-1 and the nonlethal cell size mutations wee1-112, wee1-50, cdc2-1w, and cdc2-3w were selected from tetrads which showed nonparental ditype segregation of markers (12). In these tetrads, one pair of spores had wild-type morphology. The other pair was assumed to contain the required combination of markers. To verify the genotypes, each clone was also backcrossed to the wild type and tested for segregation of the markers.
Fluorescence microscopy.
Nuclei were stained with DAPI (4′,6′-diamino-2-phenylindole) after fixation with 70% ethanol (24). Calcofluor staining was carried out as described by Johnson et al. (16). Staining for actin was performed by indirect immunofluorescence as described by Alfa et al. (1).
Flow cytometry.
Cells for fluorescence-activated cell sorting (FACS) analysis were fixed, stained with propidium iodide, and analyzed on a Becton Dickinson FACScan (24). Since S. pombe is a unicellular organism, the DNA content of its cells can easily be determined in a flow cytometer. The sep1-1 cultures, however, form mycelia which must be first fragmented into separate cells. This can be achieved by short treatment with Novozym (2 mg/ml) at room temperature after fixation.
RESULTS
Inactivation of wee1+ causes frequent skip of septation in sep1-1.
To test the effect of the acceleration of M-phase initiation on septation in sep1-1 cells, double mutants were constructed by crossing sep1-1 h+ cells with wee1-50 h− and wee1-112 h− cells. Both the sep1-1 wee1-112 and the sep1-1 wee1-50 (at 35°C, restrictive for wee1-50) cultures showed the mycelial morphology typical of sep1-1, but their cells were somewhat shorter (Fig. 1). When stained with DAPI and calcofluor, many cells of the filaments contained two nuclei (Fig. 1). As shown in Table 2, the proportion of binucleate cells was three to seven times higher in the sep1− wee1− cultures than in the control sep1-1, wee1−, and wild-type cultures. Cells containing three or four nuclei also occurred, but never at a frequency exceeding 2%. Then the cultures were subjected to analysis by flow cytometry. Figure 2 shows that a considerable proportion of the sep1− wee1− cells had a 4C DNA content. The relative height of the 4C peak was proportional to the percentage of dikaryons, suggesting that most of the dikaryotic cells contained pairs of 2C nuclei. 4C cells were rare in the controls; they formed no discernible peak. These data indicate that the dikaryosis of the sep1− wee1− cells was caused by occasional skips of septation rather than by a late-M-phase arrest.
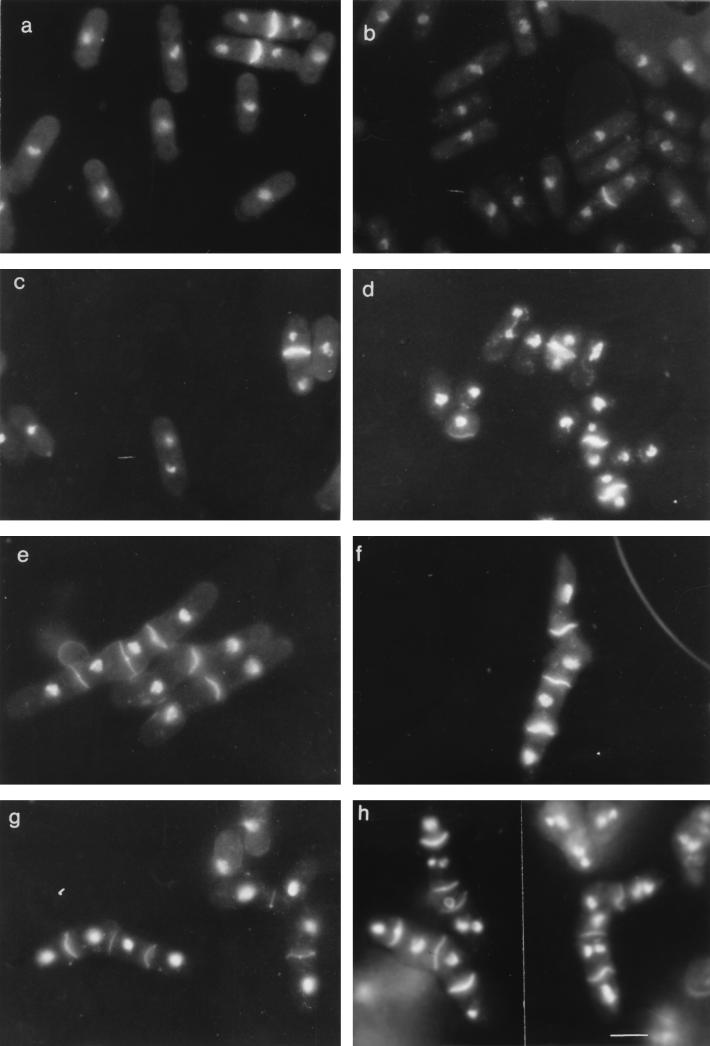
FIG. 1.
Formation of binucleate cells. Cells of exponentially growing cultures were stained with DAPI and calcofluor. (A) Wild type (L972) at 25°C; (B) wild type at 35°C; (C) wee1-50 h− strain at 25°C; (D) wee1-50 h− strain at 35°C; (E) sep1-1 leu1-32 h− strain at 25°C; (F) sep1-1 leu1-32 h− strain at 35°C; (G) sep1-1 wee1-50 ade1 h− strain at 25°C; (H) sep1-1 wee1-50 ade1 h− strain at 35°C. The bar represents 10 μm.
TABLE 2.
Formation of binucleate cells in exponentially growing cultures
| Genotype | Binucleate cells (%)
|
||
|---|---|---|---|
| 25°C | 31°C | 36°C | |
| (Strain L972) h− | 2.6 | 2.9 | 4.2 |
| sep1-1 leu1-32 h− | 3.8 | 2.9 | 5.1 |
| wee1-50 h− | 2.3 | 3.5 | 3.8 |
| sep1-1 wee1-50 ade1 h− | 13.3 | 25.6 | 29.2 |
| wee1-112 leu1-32 h− | 3.5 | 2.6 | 3.8 |
| sep1-1 wee1-112 ura5 h− | 21.1 | 34.2 | 29.5 |
| cdc2-1w h− | NDa | 2.0 | 5.1 |
| sep1-1 cdc2-1w leu1-32 h− | ND | 28.1 | 29.0 |
| cdc2-3w leu1-32 h− | 3.5 | ND | 3.6 |
| sep1-1 cdc2-3w lys3 h− | 5.1 | ND | 13.0 |
ND, not determined.
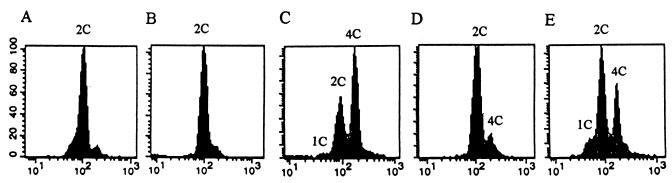
FIG. 2.
FACS analysis of strains. DNA contents of exponentially growing cells of the wild-type L972 (h−) (A) and the sep1-1 leu1-32 h− (B), sep1-1 wee1-112 h− (C), and sep1-1 wee1-50 ade1 h− (D) mutants at 25°C and after shift-up to 35°C (E) were analyzed with a Becton Dickinson FACScan.
To examine the nature of the septation defect, we examined the distribution of F-actin. In wild-type cells, actin is localized at the growing ends during interphase; then at the initiation of mitosis, it relocalizes from the ends of the cell to form an equatorial ring surrounding the dividing nucleus and anticipating the site of septum formation (21). Many dikaryons, however, showed neither actin-specific fluorescence between the nuclei (Fig. 3) nor deposition of septal material that could be stained with calcofluor (Fig. 1). Thus, the formation of binucleate cells in the sep1-1 wee1− cultures must be due to an early failure in the septation process.
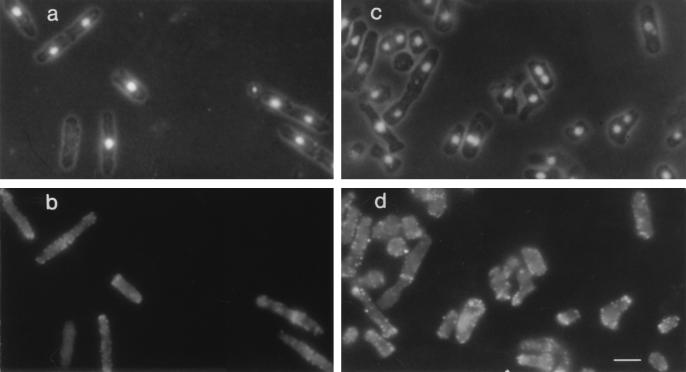
FIG. 3.
Localization of actin in wild-type and sep1-1 wee1-112 h− cells by immunofluorescence microscopy. (a and b) Exponentially growing wild-type cells stained with DAPI (a) and fluorescence-labeled antiactin antibody (b); (c and d) sep1-1 wee1-112 h− cells stained with DAPI (c) and fluorescence-labeled antiactin antibody (d). The hyphae characteristic of sep1− strains are broken because they were treated with a cell wall lytic enzyme to facilitate the uptake of antibodies. The bar represents 8 μm.
Reduction of cell size does not affect cell division in sep1-1.
A possible reason for the frequent failure of septation and cell division in sep1-1 wee1− cells is the reduction of cell size caused by the mutations in wee1+. It has been shown that the premature entry into M phase reduces the size of the wee1− cells by as much as 50% (25). wee1− cells have also been reported to diploidize at a higher than wild-type frequency (43), which was supposed to result from the smaller size and the “confusion” of the cytoskeleton (13). It is conceivable that the size reduction is more deleterious in a sep1− background.
To address this possibility, we constructed a sep1-1 cyr1::LEU2 double mutant. The cyr1+ gene encodes the catalytic subunit of adenylate cyclase which is involved in the adjusting of the rate of division to the nutritional conditions (20). The cyr1− cells behave as if they were starved even in rich medium and divide at a small size. If the failure of cell division in sep1-1 wee1− cells is due to reduced cell size, a similar phenomenon can be expected in the sep1-1 cyr1− cells. We found no significant difference between the sep1-1 cyr1+ and sep1-1 cyr1::LEU2 cultures in the frequency of binucleate cells, although the latter had almost as small cells as the sep1-1 wee1-50 culture (Table 3). Consistent with this, the cultivation of the sep1-1 cells in nitrogen-limited EMM (starvation-induced reduction of cell size [7]) did not increase the proportion of dikaryons either.
TABLE 3.
Reduction of cell size and formation of binucleate cells
| Genotype | Temp (°C) | Cell length (μm) | Binucleate cells (%) |
|---|---|---|---|
| sep1-1 wee1-50 ade1 h+ | 28.5 | 5.3 | 16.1 |
| cyr1::LEU2 leu1-32 ura4-D18 h+ | 28.5 | 6.6 | 3.0 |
| sep1-1 cyr1::LEU2 leu1-32 h− | 28.5 | 6.4 | 3.0 |
| sep1-1 wee1-50 ade1 h+ | 29.5 | 5.4 | 23.9 |
| cyr1::LEU2 leu1-32 ura4-D18 h+ | 29.5 | 6.4 | 5.4 |
| sep1-1 cyr1::LEU2 leu1-32 h− | 29.5 | 5.8 | 3.3 |
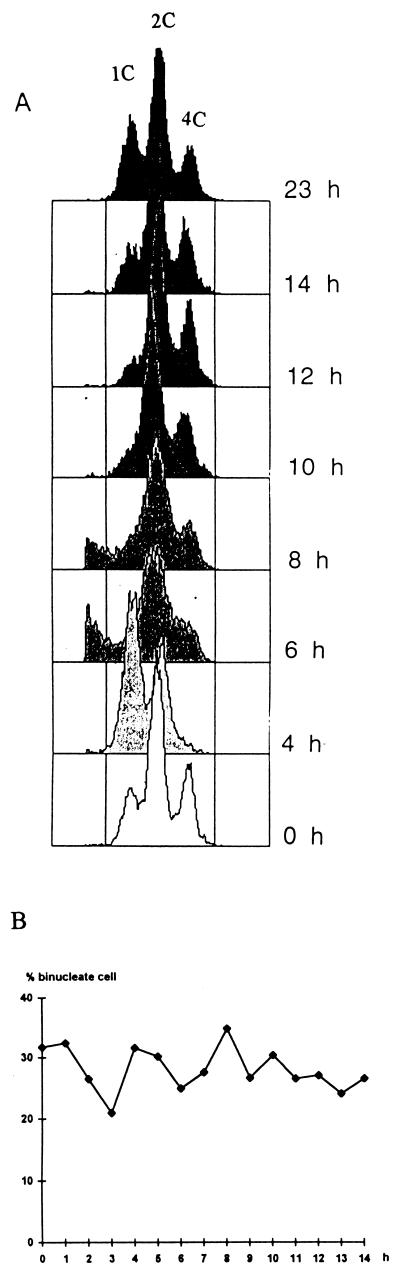
The alternative approach to address the effect of cell size was to increase it in the sep1-1 wee1− cells. For this purpose, we constructed the sep1-1 wee1-112 cdc10-129 ura5 h− strain. At 35°C, permissive for cdc10-129, the triple mutant contained 31.7% binucleate cells. When shifted to the restrictive temperature of 35°C, the cells showed the cdc10 phenotype: they arrested at the G1/S transition and elongated. FACS analysis showed (Fig. 4) that all cells arrested, and dikaryon cells with 2 × 1C, uninucleate cells with 1C DNA content, and the 4C peak (representing dikaryons being in G2) gradually disappeared. After 4 h, the culture was shifted down to the permissive temperature (25°C) and the DNA content and percentage of binucleates were monitored. The cells resumed propagation, and after 10 h their FACS pattern became similar to that seen at 0 h. Assuming that the shift-down resets the cell cycle time to that characteristic for 25°C, we supposed that they completed the third cell cycle by this time. Unfortunately, this could not be verified by direct measurement of cell number growth because the filamentous morphology conferred by sep1-1 did not allow precise determination of cell number. The G1 peak (1C) characteristic of wee1+ mutants appeared only at a time by which cells probably completed the first cell cycle following shift-down, since due to the elongation during the cdc10ts arrest, the daughter cells were larger than the necessary minimal cell size for entry into S. This interpretation of the FACS patterns was confirmed by direct measurement of cell size. The percentage of binucleate cells remained high after the culture was returned to the permissive temperature (Fig. 4), demonstrating that the extension of cell length cannot suppress the dikaryosis caused by the interaction of sep1-1 and wee1-112. Its fluctuation can be attributed to the synchronizing effect of the ts arrest.
FIG. 4.
DNA content and binucleate percentage in a sep1-1 wee1-112 cdc10-129 culture. The culture grown at 25°C was shifted to 35°C (0 h) for 4 h and then shifted back to 25°C. (A) FACS analysis; (B) percentage of binucleate cells.
Reduction of G2 does not affect cell division in sep1-1.
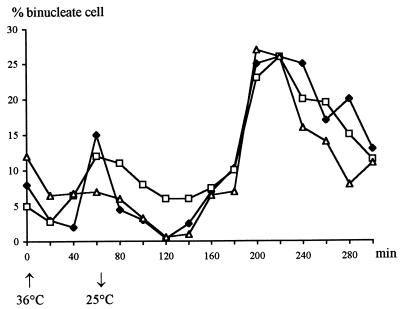
A major consequence of the accelerated entry into M provoked by wee1− is a drastic reduction of the length of G2 (2, 8). To examine if the frequent skip of septation in the sep1-1 wee1− cells could be attributed to the shortening of G2, we made use of the observation of Fantes and Nurse (8) that the proportion of G2 in a cell cycle can be reduced to a limited extent without consequences on the timing on cell division. They found that shift-ups of cdc10-129 cells to a restrictive temperature for short periods delayed S and reduced G2 without causing a delay in cell division. We shifted exponentially growing wild-type, sep1-1, and sep1-1 cdc10-129 cultures from 25 to 36°C for 60 min and then shifted them back to 25°C. In all cultures, a temporary inhibition of nuclear division was observed shortly after the shift-up (Fig. 5), most probably due to a resetting of the mitotic size control to a higher value typical of the higher temperature (43, 44). However, by the time of shift-down, the nuclear division (appearance of binucleate cells) resumed at rates comparable to that measured before shift-up. At 25°C, the percentage of binucleate cells first dropped drastically and began to increase again only after 120 min. A similar phenomenon was previously observed by Fantes and Nurse (8) in the wild type and in a cdc10-129 strain. The period that elapsed in sep1-1 cdc10-129 cells between the shift-down and the increase in nuclear division was 60 min shorter (120 min) than the length of G2 at this temperature in the wild type (180 min as calculated by Fantes and Nurse [8]). Since the peak in sep1-1 and sep1-1 cdc10-129 cells was not higher than in the wild type, we concluded that this sort of shortening of G2 does not affect septation. Consistent with this, after peaking the proportion of binucleate cells fell at the same rate in sep1-1 cdc10-129 cells and in the wild type.
FIG. 5.
Effect of delaying S phase on the formation of binucleate cells. Exponentially growing cultures were shifted from 25 to 36°C for 60 min and then returned to 25°C. The percentage of binucleate cells was determined at regular intervals in samples stained with DAPI. ⧫, wild-type L972 (h−); □, sep1-1 h− strain; ▿ sep1-1 cdc10 strain.
The change of cell shape to spherical does not affect cell division in sep1-1 cells.
The reduction of cell size conferred by wee1− entails a change of cell shape from cylindrical to almost spherical (43). The shape of the sep1-1 wee1− cells is similarly altered: the hyphae are more densely septated (shorter cells) than the hyphae of the sep1-1 cultures (Fig. 1). In principle, this change of shape might cause the septation defect. The inner cells of the hyphae can resume growth after completion of a cell cycle only at subapical positions because their ends are covered with unsplit septa (40). This requires some reorientation of the interphase cytoskeleton (39), which might be more difficult when larger parts of the apical regions are covered by intact septa left behind by the previous cell cycles. To examine whether the change of cell shape might affect septation in the sep1− cells, we constructed sep1-1 h− strains carrying one or the other of the mutations cwg1-1, cwg2-1, sph1-1, and orb1-1. These mutations change the cylindrical cell morphology to spherical or at least to oval (28, 29, 41). All double mutants formed hyphae composed of round cells, but none of them showed a 4C peak in flow cytometric analysis or a proportion of binucleate cells higher than in sep1-1 h− (data not shown). These results suggest that it is not the spherical shape that impairs the septation in the sep1-1 wee1− cells. A synthetic phenotype was observed only in sep1-1 orb1-1 cells which did not grow at 35°C, although neither sep1-1 nor orb1-1 is lethal at this temperature. However, these cells died with single nuclei (data not shown).
cdc2-1w and cdc2-3w act differently in sep1-1 cells.
The experiments described above establish that the formation of dikaryons is a primary consequence of the lack of wee1+ function rather than an effect of cell size, cell shape, or the reduction of G2. Certain mutant alleles of cdc2+ called cdc2-w are also known to accelerate division and reduce cell size (5, 43). To examine if these alleles can also affect cell division in sep1-1 cells, we constructed sep1-1 cdc2-1w and sep1-1 cdc2-3w double mutants. cdc2-1w caused an increase of dikaryon percentage comparable to that provoked by wee1− mutations, whereas cdc2-3w had only a modest effect (Table 2).
Inactivation of cdc25+ restores normal septation in sep1-1 wee1− cells.
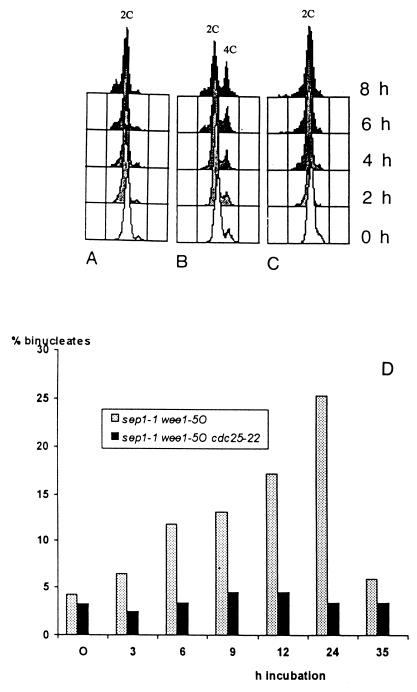
Both wee1+ and cdc2+ encode regulators of mitosis initiation (30). Another player in the initiation process is the tyrosine phosphatase p80cdc25, a positive effector of p34cdc2 (31). To test its effect on septation, an exponentially growing culture of the sep1-1 wee1-50 cdc25-22 h− triple mutant was shifted from 25 to 35°C. At 35°C, restrictive for both wee1-50 and cdc25-22, the cells propagated at a rate comparable to that of the wild-type L972 and the control sep1-1 wee1-50 h− and cdc25-22 wee1-50 h− strains, which is consistent with the earlier observation that the inactivation of wee1+ is epistatic over cdc25-22 (4, 15). The medium cell lengths were 21.44 μm for cdc25-22 h− cells but only 9.44 and 7.39 μm for sep1-1 wee1-50 cdc25-22 h− and sep1-1 wee1-50 h− cells, respectively. Surprisingly, wee1-50 did not provoke dikaryosis in the triple mutant, as revealed by FACS analysis (Fig. 6A) and nuclear staining (Fig. 6B).
FIG. 6.
Suppression of binucleate formation by cdc25-22. (A to C) FACS analysis of cdc25-22 wee1-50 h− (A), sep1-1 wee1-50 h− (B), and sep1-1 cdc25-22 wee1-50 h− (C) cultures after the shift from 25 to 35°C; (D) percentage of binucleate cells.
Conversion of dikaryons to monokaryons.
Upon a shift to the restrictive temperature, the proportion of binucleate cells increased to 25 to 40% in sep1-1 wee1-50 cultures but did not increase any further. Obviously, a process which counteracts the production of binucleates must exist. The dikaryons either die or convert to monokaryons. Since the sep1-1 wee1− cultures grew as fast as the sep1-1 cultures, conversion is more likely. In principle, the conversion might take place in one of two ways: either by nuclear fusion or by division in the subsequent cell cycle. If the former is true, the sep1-1 wee1− cultures must contain a high percentage of diploid cells. To test this possibility, we determined the percentage of diploid cells by counting the azygotic asci in homothallic cultures. In S. pombe, the diploid cells form so-called azygotic asci, which are clearly distinguishable from the zygotic asci which arise from conjugating haploids (18). We counted about nine times more azygotic asci in the homothallic sep1-1 wee1-112 cultures than in the homothallic sep1-1 control (Table 4). Although these asci might have resulted from diploid cells, we believe that fusion of nuclei cannot be the major way of restoring monokaryosis because it would lead to a gradual increase in ploidy. This in turn would result in giant asci containing di- or polyploid spores (23), which we have never observed. Thus, the azygotic asci resulted from dikaryons, in which the nuclei fused just before the onset of meiosis rather than from diploid cells. It has been shown that in multinucleate syncytia, the nuclei of S. pombe cells can fuse and undergo a subsequent meiosis-sporulation without sexual conjugation of cells (39).
TABLE 4.
Azygotic sporulation in homothallic double mutants
| Genotype | Temp (°C) | % of azygotic asci
|
||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||
| h90 (strain L975) | 30 | 0.2 | ||
| sep1-1 h90 | 30 | 1.4 | 2.5 | 7.4 |
| wee1-112 h90 | 30 | 4.7 | 3.2 | |
| sep1-1 wee1-112 h90 | 30 | 15.4 | 67.1 | |
| wee1-50 h90 | 32 | 2.9 | ||
| sep1-1 wee1-50 h90 | 32 | 28.3 | ||
| cdc2-3w h90 | 30 | 1.0 | ||
| sep1 cdc2-3w h90 | 30 | 26.9 | ||
DISCUSSION
In this work, we report on the genetic interactions of a mutant allele of the cell division gene sep1+ with mutations in the M-phase initiation genes cdc2+, cdc25+, and wee1+. We show that the wee1− and cdc2-1w mutations impair septation initiation in sep1-1 background, which suggests that these genes are involved in a regulatory link that couples nuclear division and cell division in early M phase.
The failure of septum formation is most probably due to the inability of the cells to initiate septum synthesis. Although many binucleate cells had no actin between their nuclei, it is not clear whether they failed to form actin rings or they formed the rings but then dissolved them to relocate the actin to the poles starting to grow in the new cell cycle. In wild-type cells, actin moves from the middle of the mother cell to the old ends of the daughter cells after completion of cell division (21).
The defect of septum formation does not prevent the cells from entering a new cell cycle. Both nuclei of the dikaryons undergo DNA replication and even proceed to another mitosis. Remarkably, these dikaryons seem to be more efficient in septation. The very low percentage of three- and four-nucleate cells and the lack of a detectable number of cells with DNA contents higher than 4C suggest that they do not frequently skip septation. We hypothesize that the dikaryons can divide and produce two septa, one at each dividing nucleus, resulting in two monokaryons and one dikaryon (Fig. 7). If they formed only one, the percentage of binucleate cells would continuously increase. If each monokaryon produces one dikaryon and each dikaryon forms two monokaryons and one dikaryon, the number of monokaryons and dikaryons in a population after n generations can be calculated with the following formulas: Mn = 2 × Dn − 1 and Dn = Mn − 1 + Dn − 1, where Mn is the number of monokaryons and Dn is the number of dikaryons. Using these formulas, one gets the 1:1 proportion of binucleates and mononucleates after a few generations regardless of their proportion in the zero generation. In our experiments, the actual proportion was usually lower than 1:1 (more cells were mononucleate than binucleate), which suggests that the block of septation was not absolute. A significant fraction of mononucleate cells could form septa and mononucleate daughter cells.
FIG. 7.
Hypothetical model of conversions between binucleate and uninucleate states in sep1-1 wee1− cultures. A monokaryon can produce a dikaryon (1A) or two monokaryons if septum is formed (1B). The two nuclei of a binucleate cell can divide and produce a four-nucleate cell (2). Two septa are formed following both mitoses, resulting in two monokaryons and one dikaryon (3).
In principle, a number of cellular changes caused by the mutations wee1− and cdc2-1w could account for the failure of septation in sep1-1 background. For example, both mutations change the cell shape from cylindrical to almost spherical, reduce cell size, and shorten G2 (5, 7, 43). We found that other mutations which also confer a roundish morphology did not affect septation. The size reduction can also be ruled out because an almost equal size reduction achieved by lowering the intracellular cyclic AMP level did not increase binucleate percentage and the increase of cell length by a ts mutation did not reduce it. The shortening of G2 by delaying S did not provoke dikaryosis either. Thus, these pleiotropic effects of wee1− and cdc2-1w are unlikely to be responsible for the defect of septation in sep1-1 background; more specific interactions must account for it.
We suggest that there is a mechanism in early M which coordinates the initiation of nuclear division and the initiation of septation. cdc2+, cdc25+, and sep1+ seem to be involved, directly or indirectly, in this mechanism. When the cdc2+ protein kinase or its positive regulator the cdc25+ phosphatase is inactive, neither mitosis nor septation can be initiated (30, 35). When wee1+, the negative regulator cdc2+, is inactive, both mitosis and septation begin prematurely (31). Besides, the wee1− mutants show a somewhat increased frequency of spontaneous endodiploidization (43). It is not known whether these endodiploids are due to skipped mitoses or rather to failures of septation. It has been found that certain regulatory mutations or the overexpression of certain cell cycle genes can provoke repeated replication in the absence of mitosis by releasing the block of DNA synthesis operating in G2 and S (42). Since this is a lethal phenotype but the septation defects are not lethal (34), the occasional skip of septation is more likely to be responsible for the endodiploidization. In this case, a dikaryon must be formed first, whose nuclei then fuse. It has been demonstrated by protoplast fusion experiments that vegetative nuclei can fuse in S. pombe (37). The formation of dikaryons in the sep1-1 wee1− cultures may have the same mechanism but intensified by the mutation in sep1+ which is supposed to delay the process of septation relative to the nuclear events of M phase (40).
sep1-1 also impairs septation in sep1-1 cdc2-1w cells but has only a slight effect in a cdc2-3w background. Since both cdc2 mutations reduce cell size and show wee1− morphology (15), the difference between their actions must be due to a difference between the activities of the mutant gene products. It has been shown that cdc2-1w makes the initiation of mitosis insensitive to inhibition by wee1+, whereas cdc2-3w renders it insensitive to activation by cdc25+ (31). In other words, the cdc2-1w cells behave like wee1− cells, whereas the cdc2-3w cells behave like cells in which cdc25+ is overexpressed (30). Since wee1− and cdc2-1w have the same effect on septation in sep1-1 background, it is tempting to conclude that wee1+ acts via cdc2+ in the initiation of septum formation.
The cdc25+ protein phosphatase also seems to be involved because the mutation cdc25-22 restores regular septation in the sep1-1 wee1− cells. In this respect, cdc25-22 is epistatic over wee1−. At the initiation of M phase, the opposite happens: the wee1− mutations release the cell cycle block conferred by cdc25-22 (4, 15). In fact, the sep1-1 wee1− cdc25-22 cells simultaneously show both types of interactions: wee1− suppresses the lethal effect of cdc25-22, and cdc25-22 suppresses the septation deficiency caused by wee1−. This duality might be due to the hypothesized dual effect (cell cycle defect and allosuppression of protein synthesis) of the cdc25-22 mutation (26) or to the delay of M phase and the concomitant increase of cell size caused by cdc25-22 at the restrictive temperature (15). A mechanism similar to the latter might also account for the improvement of septation in dikaryons. As suggested above, these divide at the second M phase, after the completion of two sets of G phases during which they could grow. Their size is thus closer to that of the wild type at the entry into M phase. This size increase affects the cell cycle in ways presumably unrelated to wee1+ functions but makes the wee1− cells resemble wild-type cells in the timing of certain cell cycle events such as mitosis and septation. It has been reported that the wee1− phenotype can be suppressed indirectly by conditions that protract S phase and thus allow the cells to attain the wild-type cell size (36).
A number of different models could be proposed to explain the observed genetic interactions. One possibility is that the M-phase initiation genes wee1+, cdc2+, and cdc25+ regulate septum initiation by controlling the activity of sep1+. We previously reported genetic interaction between sep1-1 and cdc4-8 (40). The cdc4+ protein shows a significant homology to the myosin regulatory light chain (22), a substrate of cdc2+ in vertebrate cells (33). Therefore, it is tempting to speculate that the cdc2+ kinase also regulates sep1+. The product of sep1+ is a forkhead (HNF-3) transcription factor homolog (27), which might be a link between the M-phase initiation machinery and the genes controlling septation initiation. An alternative model assumes a regulatory pathway coupling a very early event required for septum formation to nuclear division. This pathway mediates information about septum initiation to nuclear division and can delay nuclear division when septation is delayed to ensure the proper synchrony of the two processes. The premature entry into nuclear division (provoked by wee1−) in cells with delayed septation initiation (caused by sep1-1) makes the coordination highly inefficient. A recent report on the existence of a novel class of cell cycle checkpoint control in Saccharomyces cerevisiae (19) describes a similar mechanism. It was found that ts mutants that cannot form a bud at the restrictive temperature cause a dramatic delay in nuclear division. The delay arises through the regulation of Cdc28 and can be eliminated by overexpression of the MIH1 phosphatase gene (the Saccharomyces cerevisiae homolog of cdc25+) or by the mutation CDC28Y19F that renders Cdc28 resistant to inhibition by phosphorylation (functionally similar to cdc-1w). The abolishment of the delay was accompanied by the generation of binucleate cells. We hope to be able to resolve which of these interpretations has merit by testing other sep− mutants (11), overexpressing sep1+ in different genetic backgrounds and by identifying the substrates of the sep1+ protein.
ACKNOWLEDGMENTS
We thank Angel Duran, Peter Fantes, Urs Leupold, and Paul Nurse for strains and Paul Nurse for admitting A.G. to his laboratory to learn FACS analysis. We also thank Ilona Lakatos for excellent technical assistance.
This work was financed by grants from National Fund for Scientific Research and the Hungarian Academy of Sciences.
REFERENCES
- 1.Alfa C, Fantes P, Hyams J, Mcleod M, Warbrick E. Experiments with fission yeast. A laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- 2.Creanor J, Mitchison J M. The kinetics of H1 histone kinase activation during the cell cycle of wild-type and wee mutants of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1994;107:1197–1204. doi: 10.1242/jcs.107.5.1197. [DOI] [PubMed] [Google Scholar]
- 3.Fankhauser C, Simanis V. Cold fission: splitting the pombe cell at room temperature. Trends Cell Biol. 1994;4:96–101. doi: 10.1016/0962-8924(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 4.Fantes P. Epistatic gene interactions in the control of division in fission yeast. Nature. 1979;279:428–430. doi: 10.1038/279428a0. [DOI] [PubMed] [Google Scholar]
- 5.Fantes P. Isolation of cell size mutants of a fission yeast by a new selective method: characterization of mutants and implications for division control mechanisms. J Bacteriol. 1981;146:746–754. doi: 10.1128/jb.146.2.746-754.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fantes P, Nurse P. Controls over the timing of DNA replication during the cell cycle of fission yeast. Exp Cell Res. 1977;107:365–375. doi: 10.1016/0014-4827(77)90358-5. [DOI] [PubMed] [Google Scholar]
- 7.Fantes P, Nurse P. Control of cell size at division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1977;107:377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- 8.Fantes P, Nurse P. Control of the timing of cell division in fission yeast. Cell size mutants reveal a second control pathway. Exp Cell Res. 1978;115:317–329. doi: 10.1016/0014-4827(78)90286-0. [DOI] [PubMed] [Google Scholar]
- 9.Fishkind D J, Wang Y. New horizons for cytokinesis. Curr Opin Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- 10.Fowler V M, Adams E J H. Spectrin redistributes to the cytosol and is phosphorylated during mitosis in cultured cells. J Cell Biol. 1992;119:1559–1572. doi: 10.1083/jcb.119.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grallert A, Miklos I, Sipiczki M. Division-site selection, cell separation, and formation of anucleate minicells in Schizosaccharomyces pombe mutants resistant to cell-wall lytic enzymes. Protoplasma. 1977;198:218–229. [Google Scholar]
- 12.Gutz H, Heslot H, Leupold U, Loprieno N. Schizosaccharomyces pombe. In: King R C, editor. Handbook of genetics. New York, N.Y: Plenum Press; 1974. pp. 395–446. [Google Scholar]
- 13.Hagan I M, Hyams J S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya N, Hosoya H, Yamashiro S, Mohri H, Matsumura F. Localization of caldesmon and its dephosphorylation during cell division. J Cell Biol. 1993;121:1075–1082. doi: 10.1083/jcb.121.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson J D, Feilotter H, Young P G. stf1: non-wee mutations epistatic to cdc25 in the fission yeast Schizosaccharomyces pombe. Genetics. 1990;126:309–315. doi: 10.1093/genetics/126.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson B F, Calleja G B, Boisclair I, Yoo B Y. Cell division in yeasts. III. The biased, asymmetric location of the septum in the fission yeast cell, Schizosaccharomyces pombe. Exp Cell Res. 1979;123:253–259. doi: 10.1016/0014-4827(79)90466-x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson B F, Miyata M, Miyata H. Morphogenesis of fission yeasts. In: Nasim A, Young P, Johnson B F, editors. Molecular biology of the fission yeast. San Diego, Calif: Academic Press; 1989. pp. 332–366. [Google Scholar]
- 18.Leupold U. Methodisches zur Genetik von Schizosaccharomyces pombe. Schweiz Z Allg Pathol Bakteriol. 1955;18:1141–1146. [PubMed] [Google Scholar]
- 19.Lew D J, Reed S I. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda T, Mochizuki N, Yamamoto M. Adenylyl cyclase is dispensable for vegetative growth in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1990;87:7814–7818. doi: 10.1073/pnas.87.20.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks J, Hyams J S. Localization of F-actin through the cell division cycle of S. pombe. Eur J Cell Biol. 1985;39:27–32. [Google Scholar]
- 22.McCollum D, Balasubramanian M K, Pelcher L E, Hemmingsen S M, Gould K L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein for cytokinesis. J Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molnar M, Sipiczki M. Polyploidy in the haplontic yeast Schizosaccharomyces pombe: construction and analysis of strains. Curr Genet. 1993;24:45–52. doi: 10.1007/BF00324664. [DOI] [PubMed] [Google Scholar]
- 24.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 25.Nurse P, Thuriaux P. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics. 1980;96:627–637. doi: 10.1093/genetics/96.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nurse P, Thuriaux P. Temperature sensitive allosuppressor mutants of the fission yeast Schizosaccharomyces pombe influence cell cycle control over mitosis. Mol Gen Genet. 1984;196:332–338. [Google Scholar]
- 27.Ribar B, Banrevi A, Sipiczki M. sep1+ encodes a transcription-factor homologue of the HNF-3/forkhead DNA-binding-domain family in Schizosaccharomyces pombe. Gene. 1997;202:1–5. doi: 10.1016/s0378-1119(97)00390-9. [DOI] [PubMed] [Google Scholar]
- 28.Ribas J C, Diaz M, Duran A, Perez P. Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1-3)β-d-glucan. J Bacteriol. 1991;173:3456–3462. doi: 10.1128/jb.173.11.3456-3462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribas J C, Roncero C, Rico H, Duran A. Characterization of a Schizosaccharomyces pombe morphological mutant altered in the galactomannan content. FEMS Microbiol Lett. 1991;79:263–268. doi: 10.1016/0378-1097(91)90096-s. [DOI] [PubMed] [Google Scholar]
- 30.Russel P R, Nurse P. cdc25+ functions as an inducer of mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- 31.Russel P R, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- 32.Sanders S L, Field C M. Septins in common? Curr Biol. 1994;4:907–910. doi: 10.1016/s0960-9822(00)00201-3. [DOI] [PubMed] [Google Scholar]
- 33.Satterwhite L L, Lohka M J, Wilson K J, Scherson T Y, Cisek L J, Corden J L, Pollard T D. Phosphorylation of myosin-II regulatory light chain by cyclin p34cdc2: a mechanism for the timing of cytokinesis. J Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simanis V. The control of septum formation and cytokinesis in fission yeast. Semin Cell Biol. 1995;6:79–87. doi: 10.1016/1043-4682(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 35.Simanis V, Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986;45:261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- 36.Singer R A, Johnston G C. Indirect suppression of the wee1 mutant phenotype in Schizosaccharomyces pombe. Exp Cell Res. 1985;158:533–543. doi: 10.1016/0014-4827(85)90477-x. [DOI] [PubMed] [Google Scholar]
- 37.Sipiczki M, Creanor J, Fantes P. Fusion of cdc mutants: a possible effect of cell cycle on the fusion of yeast protoplasts. In: Potrykus I, Harms C T, Hinnen A, Huetter R, King P J, Shillito P D, editors. Protoplasts 83. Basel, Switzerland: Birkhaeuser Verlag; 1983. pp. 338–339. [Google Scholar]
- 38.Sipiczki M, Ferenczy L. Protoplast fusion of Schizosaccharomyces pombe auxotrophic mutants of identical mating type. Mol Gen Genet. 1977;151:77–81. doi: 10.1007/BF00446915. [DOI] [PubMed] [Google Scholar]
- 39.Sipiczki, M., and A. Grallert. Polarity, spatial organisation of cytoskeleton, and nuclear division in morphologically altered cells of Schizosaccharomyces pombe. Can. J. Microbiol., in press. [DOI] [PubMed]
- 40.Sipiczki M, Grallert B, Miklos I. Mycelial and syncytial growth in Schizosaccharomyces pombe induced by novel septation mutations. J Cell Sci. 1993;104:485–493. doi: 10.1242/jcs.104.2.485. [DOI] [PubMed] [Google Scholar]
- 41.Snell V, Nurse P. Genetic analysis of cell morphogenesis in fission yeast—a role for casein kinase II in the establishment of polarized growth. EMBO J. 1994;13:2066–2074. doi: 10.1002/j.1460-2075.1994.tb06481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern B, Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- 43.Thuriax P, Nurse P, Carter B. Mutants altered in the control coordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1978;161:215–220. doi: 10.1007/BF00274190. [DOI] [PubMed] [Google Scholar]
- 44.Walker G, McWilliams P G. Induction of a heat shock-type response in fission yeast following nitrogen starvation. Yeast. 1989;5:477–486. [Google Scholar]