Abstract
During toxicology studies, fasting animals prior to clinical pathology blood collection is believed to reduce variability in some clinical chemistry analytes. However, fasting adds stress to animals that are already stressed from the administration of potentially toxic doses of the test article. The purpose of this study was to assess the impacts of different fasting durations on cynomolgus monkeys’ welfare during toxicology studies. To this end, we assessed the cynomolgus monkeys traditional and ancillary clinical pathology endpoints at different fasting times. We showed that most clinical pathology endpoints were largely comparable between different fasting times suggesting that cynomolgus monkeys could be fasted for as little as 4 hours for toxicology studies, as longer fasting times (up to 20 hours) resulted in stress, dehydration, and significant decreases in blood glucose- changes that impacts animal welfare. Shorter fasting times were associated with higher triglycerides variability among individual animals. Therefore, we propose that shorter fasting time (i.e., 4 hours) should be adequate for most toxicology studies except when: (1) parameters that could be affected by non-fasting conditions are important for safety and pharmacodynamic assessments (i.e., glucose and lipids) and (2) fasting would be needed for the bioavailability of an orally administered test article.
Keywords: fasting, cynomolgus monkeys, clinical pathology, toxicology, animal welfare
Introduction
Clinical pathology is an important tool for monitoring test article activity and toxicity in nonclinical safety studies. Several procedural factors are known to affect the results and interpretation of clinical pathology data. One of these factors includes the fed status of the animals. Some of the most used animal species for nonclinical safety studies include rats, mice, beagle dogs, and non-human primates (NHP), with cynomolgus monkeys being the most used NHP for biomedical research and nonclinical safety assessments. Fasting animals prior to clinical pathology blood collection is believed to reduce variability in some of the clinical chemistry analytes, notably glucose, triglycerides, and cholesterol.1-3 However, fasting is also considered an additional stress factor on animals that are already being subjected to stress from the administration of and exposure to potentially toxic doses of the test articles, as well as, additional study-related procedures. Accumulation of stress factors would negatively impact the welfare of the study animals, which in turn affects the quality of the data generated from these studies, along with interpretations of toxicological relevance. Moreover, different facilities have different feeding regimens (i.e., 1 meal/day or 2 meals/day) for cynomolgus monkeys during the acclimatization period prior to the initiation of toxicology studies.
Therefore, to improve the welfare of the animals prior to and during toxicology studies, we have conducted a study to assess the impact of (1) different feeding regimens during a 14-day acclimatization period and (2) different fasting durations during toxicology studies on the welfare of cynomolgus monkeys. The results showed that different feeding practices during the acclimatization period had no impacts on the body weight, blood fructosamine, and other clinical pathology parameters. While fasting did not cause oxidative stress (as measured by reduced and oxidized glutathione) in cynomolgus monkeys, longer fasting times resulted in glucocorticoid-induced stress (manifested as increased cortisol and decreased lymphocyte [LYMPH] concentrations), dehydration (manifested as increased inorganic phosphate), and significant decreases in glucose-all reflecting changes that could negatively impact animal welfare.
We hope the results from this study will help drug developers make informed decisions on selecting the appropriate fasting duration for cynomolgus monkeys with the intent of improving their welfare while preserving data quality in the conduct of toxicology studies.
Materials and Method
Ethical Statement
Animals were commingled in accordance with Labcorp standard operating procedures. Labcorp is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All procedures in the protocol were in compliance with applicable animal welfare acts and were approved by the local Institutional Animal Care and Use Committee.
Animal Selection and Management
Fifteen female (35-51 months old; body weight: 2.6-3.6 kg) and twenty male cynomolgus monkeys (35-51 months old; body weight: 2.6-4.4 kg) were transferred from the stock colony. Socially housed animals were assigned to the study with the intent of keeping previously established partners together. Animals were acclimated to the study room for 23 days prior to the initiation of the study. Animals were group-housed by sex in European guideline (ETS 123)–compliant pens (up to three animals/pen) and enriched with bedding material. Animals were individually housed during room cleaning and study procedures. For the purpose of this study, there were two feeding regimens. Animals were offered equal amounts of Certified Primate Diet #5048 (PMI Nutrition International Certified LabDiet®) either once (feeding regimen 1) or twice (feeding regimen 2) per day (e.g., 12-16 biscuits once or 6-8 biscuits twice per day depending on body weight) for 14 days prior to the day of the study (fasting day) to mimic the different acclimatization methods utilized prior to the commencement of a toxicology study. Water was provided ad libitum. Environmental controls were set to maintain a temperature range of 20°C to 26°C, a relative humidity range of 30% to 70%, eight or greater air changes/hour, and a 12-hour light/12-hour dark cycle. The light/dark cycle was interrupted for study-related activities. Prior to the study start and at the end of the study, animals were given various cage-enrichment devices (including toys) and fruit, vegetable, or dietary enrichment (that did not require analyses). Toys were rotated at least once a week in order to maintain the interest of the animals and increase usage.
Study Design and Blood Collection
On the day of fasting and blood collection, food was provided to the monkeys within a frame of 2 hours after which food was removed and this marked the beginning of fasting. Blood samples were collected via the femoral vein approximately 4, 8, 12, and 20 hours after the start of fasting.
Body Weights
Body weights were recorded for all animals on days 1, 4, 8, 11, 13, and 15 of the acclimatization periods.
Hematology and Clinical Chemistry
Blood samples were collected from fasted animals via the femoral vein. For hematology, approximately 1.0 mL of whole blood was collected into tubes containing potassium ethylenediaminetetraacetic acid (EDTA) as an anticoagulant, and the tubes were gently inverted six to eight times. Hematological analysis was performed using the Siemens Advia 2120i automated hematology analyzer.
For clinical chemistry, approximately, 1.0 mL of whole blood were collected into serum separator tubes (without anticoagulant), allowed to clot at room temperature, and centrifuged (10-15 minutes at 1500-2000 × g [force], room temperature) within 1 hour of collection. Serum was harvested within 45 minutes of centrifugation. Serum chemistry parameters were measured on the Roche Modular P Automated chemistry analyzer. Cortisol concentrations were measured on the Siemens Immulite 2000.
Insulin, Glucagon, and Fructosamine Analyses
A whole blood sample of 1.5 ml was collected into serum separator tubes (without anticoagulant), allowed to clot at room temperature, and centrifuged (10-15 minutes at 1500-2000 × g [force], room temperature) within 1 hour of collection. Serum was harvested within 45 minutes of centrifugation. Serum was harvested into three aliquots and transferred into conical bottom, polypropylene, screw-capped tubes. Tube 1 was designated for 250 µL of serum for insulin analysis, tube 2 was designated for 50 µL of serum for glucagon analysis, and tube 3 was designated for 150 µL of serum for fructosamine analysis. Serum samples were held on dry ice unless stored immediately in a freezer, set to maintain −60°C to −80°C until analyzed. Insulin was quantified using a chemiluminescence immunoassay on a Beckman Coulter Access II Instrument that uses a Beckman Coulter Access Ultrasensitive Insulin Reagent. Glucagon was measured on the BioTek Synergy 2 microplate reader using an enzyme-linked immunosorbent assay method (Merdocia Glucagon kit, catalog number 10-1281-01, AB, Uppsala, Sweden). Fructosamine was analyzed using the Diazyme Glycated Serum Protein (GSP) Assay which uses proteinase K to digest GSP into low molecular weight glycated protein fragments (GPF) followed by Diazyme specific fructosaminase™ (a microorganism originated amadoriase) to catalyze the oxidative degradation of Amadori product GPF to yield protein fragments (PF) or amino acids, glucosone, and H2O2. The H2O2 released is measured by a colorimetric Trinder end-point reaction. The absorbance at 546-600 nm is proportional to the concentration of glycated serum proteins. The absorbance was measured on the chemistry analyzer, the Beckman Coulter AU640e.
Reduced Glutathione, Oxidized Glutathione, and Cysteine Analysis
Reduced glutathione (GSH), oxidized glutathione (GSSG), and cysteine (Cys) were measured with a derivatization-based liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay as described subsequently. 4 Whole blood was collected into tubes containing tri-potassium EDTA (4.5 µL) as an anticoagulant and 310 mM N-ethylmaleimide (NEM, 20 µL in water) as a derivatization reagent. Approximately 200 µL of whole blood was gently inverted six to eight times and stored on dry ice unless immediately stored in a freezer set to maintain at −60°C to −80°C. Whole blood was stored in conical-bottom, polypropylene, screw-capped tubes. Sample tubes needed to be weighed before and after the addition of blood to account for collected blood volume and dilution. The whole blood tube was designated for GSH, GSSG, and Cys analysis. Standard stock solutions of GSH (2 mg/mL), GSSG (1 mg/mL), and Cys (2mg/mL) were prepared in 1.15% potassium chloride solution. Stable isotope internal standard solutions (1 mg/mL) included 13C4,15N2-GSSG, D2-Cysteine, and 13C2-15N1-GSH prepared in 0.5 N perchloric acid. Surrogate matrix (1.15% potassium chloride solution) calibration curve ranges for GSH and GSSG were 0.03-500 µg/mL and 0.006-100 µg/mL for Cysteine. Calibration standards, quality controls and unknown samples (25 µL) were subjected to sample preparation by the addition of 10 µL of IS mix (50 µg/ml GSH, GSSG, and 5 µg/mL for cysteine) and 4.9 µL of EDTA (75 mg/mL) and NEM (310mM). All samples were mixed for 10 min and centrifuged at 4000 rpm for 1 min followed by addition of 100 µL of 5-sulfosalicylic acid (20% w/v). Samples were then vortex mixed and centrifuged at 4000 rpm at 4°C for 10 minutes. 80 µL of supernatant was transferred into a new sample plate and 2 µL of the supernatant was injected for LC-MS/MS analysis as described below. GSH and Cys were quantified as NEM product derivatives (GSH-NEM & Cys-NEM).
LC-MS/MS analysis was conducted using a Sciex QTRAP ® 6500 LC-MS/MS System (AB Sciex Instruments, Redwood City, CA) coupled to Shimadzu Nexera ultra-high-performance liquid chromatography (LC; Shimadzu, Kyoto, Japan). LC mobile phases included (MP-A) 0.1% (v/v) formic acid in water and (MP-B) 0.1% (v/v) formic acid in acetonitrile at a flow rate of 0.45 mL/min. BEH C18 (100 x 2.1 mm, 5µm; Waters Corporation, Milford, MA) was used as the LC column. Gradient LC flow started with 1% MP-B to 40% MP-B in 2.2 minutes; followed by a linear increase to 75% MP-B in 0.3 minutes, 95% MP-B in 0.2 minutes and held at 95% MP-B for 0.8 minute, and to 1% MP-B in 0.5 minutes and equilibrated at 1% MP-B for 1 minute. Total run time was 5 minutes. The column oven temperature was maintained at 40 °C. GSSG, GSH-NEH, and Cys-NEM eluted at 1.8, 1.9, and 2.0 minutes, respectively, under these gradient LC conditions.
Tandem MS analysis was performed on a linear quadrupole ion trap mass spectrometer (QTRAP 6500®; AB Sciex Instruments, Redwood City, CA) equipped with a Turbo V™ ion source operated under standard electrospray ionization (ESI) conditions. Multiple reaction monitoring (MRM) scan mode was used for the mass spectrometric quantification of the GSSG, GSH-NEM, and Cys-NEM in positive mode. Monitored quantitative MRM transitions and corresponding collision energies included: GSSG: 613.0 → 355.0 (CE: 33V), 13C4,15N2-GSSG: 619.2 → 361.2 (CE: 33V), Cys-NEM: 247 → 200.9 (CE: 20V), D2-Cys-NEM: 249.1 → 202.9 (CE: 20V), GSH-NEM: 433.1 → 358.1(CE: 15V), and 13C2-15N1-GSH: 436.1 → 358.1(CE: 15V). Declustering potential and entrance potential were 120 and 10 V, respectively. ESI source parameters included ion spray voltage of 5000 V, temperature of 500°C, curtain gas pressure at 30 psi, nebulizer gas (GS1) at 60 psi, and Heater gas (GS2) 60 psi. Quantitative results for all analytes were obtained using an analyte calibration curve (MS pear area vs concentration). LC-MS/MS data were acquired in Analyst software (Version 1.6.3; AB Sciex Instruments, Redwood City, CA) and processed using Multi Quant software (Version 3.0.2; AB Sciex Instruments, Redwood City, CA).
Data Analysis
All data were analyzed by comparing average values and standard deviations, within the different sexes, across the different fasting periods as compared to baseline values (4-hour time point). These comparisons were made with graphical checks, using statistical tests, or both. When using a statistical test, a two-sample t-test was performed to identify any differences that were deemed statistically meaningful. Irrespective of the statistical significance, all of the effect size estimates were investigated by the subject matter expert to determine if they were practically meaningful. All data analyses were carried out in R (v4.0.5). 5
Results
General Overview
This study was conducted in two phases. In phase 1 (or feeding regimen 1), all animals were fed once a day at 7:00 am for 14 days prior to the day of fasting to mirror one of the acclimatization feeding practices of cynomolgus monkeys prior to the onset of a toxicology study. In phase 2 (or feeding regimen 2), all animals were fed twice a day at 7:00 am and 1:00 pm for 14 days prior to the day of fasting to mirror another acclimatization feeding practice. Therefore, the results will be presented to reflect these two phases or feeding regimens.
Different Acclimatization Feeding Practices Did Not Affect Body Weights
During the acclimatization periods, the body weights of the animals were measured on days 1, 4, 8, 11, 13, and 15. The body weights of the animals were stable regardless of the sex and feeding regimen suggesting that once versus twice-daily feeding regimens during the acclimatization periods did not affect body weight (Supplementary Table 1).
Minimal to No Effects on Hematology Parameters in Short Versus Long Fasting Times
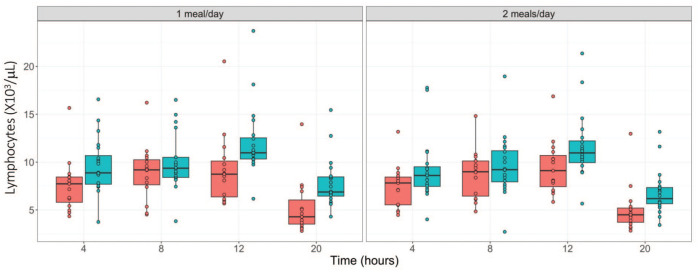
Regardless of the feeding regimen, fasting up to 20 hours did not cause any major changes in the hematology parameters evaluated. As shown in Tables 1 and 2, relative to the 4-hour time point, time-dependent minimal decreases in red cell parameters (hematocrit, hemoglobin, and red blood cell [RBC] counts, down by 7 %) were observed during the two phases in both sexes. These changes were considered to be procedure related as a result of the frequent blood sampling within a 24-hour frame. Other changes included minimal increases in total white blood cell counts (WBC) at 8 hours of fasting in phase one, and at 8 and 12 hours of fasting in phase 2 (up to 1.35-fold increase); minimal increases in neutrophil counts (NEUT; up to 1.5-fold increase) at 8 hours of fasting only in females in both phases; and minimal decreases in lymphocyte counts (LYMPH; up to 1.5-fold decrease) at 20 hours of fasting in both sexes and phases. These changes were considered to be due to normal biological variation. However, despite its minimal nature, the LYMPH decreases may have also been due to fast-related stress given the consistent pattern of decreases at the 20-hour time point in both study phases and both sexes (Figure 1), along with other supportive evidence described below (i.e., increased cortisol).
Table 1.
Hematology results from cynomolgus monkeys fasted for different time periods after a 14-day acclimatization period of one meal/day (feeding regimen 1).
| Analytes | Sex | Fasting, hours | |||
|---|---|---|---|---|---|
| 4 | 8 | 12 | 20 | ||
| WBC, ×103/µL | Males | 14.53 ± 2.8 | 16.71 ± 3.0* | 13.43 ± 3.1 | 11.89 ± 2.9** |
| Females | 15.34 ± 4.6 | 20.28 ± 5.8* | 15.61 ± 3.8** | 13.29 ± 3.9 | |
| NEUT, ×103/µL | Males | 3.96 ± 1.2 | 5.81 ± 1.1** | 4.61 ± 1.5 | 3.26 ± 1.4 |
| Females | 6.72 ± 2.7 | 10.28 ± 4.1** | 8.02 ± 4.4 | 7.33 ± 3.3 | |
| LYMPH, ×103/µL | Males | 9.59 ± 2.9 | 9.96 ± 2.9 | 12.06 ± 3.6* | 7.81 ± 2.6* |
| Females | 7.61 ± 2.8 | 8.93 ± 2.93 | 9.25 ± 3.8 | 5.27 ± 2.9* | |
| MONO, ×103/µL | Males | 0.43 ± 0.1 | 0.50 ± 0.14 | 0.63 ± 0.2** | 0.43 ± 0.1 |
| Females | 0.52 ± 0.2 | 0.65 ± 0.25 | 0.80 ± 0.3** | 0.42 ± 0.2 | |
| EOS, ×103/µL | Males | 0.34 ± 0.4 | 0.25 ± 0.23 | 0.36 ± 0.3 | 0.24 ± 0.2 |
| Females | 0.29 ± 0.4 | 0.21 ± 0.3 | 0.30 ± 0.4 | 0.15 ± 0.3 | |
| BASO, ×103/µL | Males | 0.11 ± 0.07 | 0.08 ± 0.03 | 0.09 ± 0.04 | 0.05 ± 0.02** |
| Females | 0.10 ± 0.08 | 0.09 ± 0.05 | 0.08 ± 0.04 | 0.04 ± 0.02* | |
| LUC, ×103/µL | Males | 0.10 ± 0.04 | 0.11 ± 0.04 | 0.15 ± 0.07** | 0.09 ± 0.04 |
| Females | 0.10 ± 0.06 | 0.13 ± 0.06 | 0.15 ± 0.08 | 0.07 ± 0.04 | |
| HCT, % | Males | 45.27 ± 2.1 | 43.80 ± 1.8* | 43.40 ± 2.1** | 42.90 ± 2.2** |
| Females | 43.43 ± 2.4 | 43.17 ± 3.1 | 41.72 ± 2.7 | 40.45 ± 2.8** | |
| RBC, ×106/µL | Males | 5.85 ± 0.2 | 5.72 ± 0.3 | 5.68 ± 0.3* | 5.64 ± 0.3* |
| Females | 5.59 ± 0.3 | 5.50 ± 0.3 | 5.42 ± 0.3 | 5.26 ± 0.3* | |
| HGB, ×103/µL | Males | 13.54 ± 0.6 | 13.17 ± 0.6 | 13.03 ± 0.6* | 12.96 ± 0.7** |
| Females | 12.68 ± 0.5 | 12.44 ± 0.6 | 12.23 ± 06* | 11.91 ± 0.6** | |
| RETIC, ×103/µL | Males | 52.96 ± 10.6 | 53.42 ± 14.5 | 54.43 ± 13.7 | 52.01 ± 15.9 |
| Females | 55.01 ± 24.2 | 58.37 ± 23.3 | 57.47 ± 21.8 | 58.43 ± 22.2 | |
| RDW, % | Males | 13.10 ± 0.9 | 13.14 ± 1.0 | 13.23 ± 1.0 | 13.30 ± 1.0 |
| Females | 12.55 ± 1.1 | 12.62 ± 1.3 | 12.65 ± 1.3 | 12.77 ± 1.3 | |
| MCV, fL | Males | 77.40 ± 3.5 | 76.69 ± 3.5 | 76.51 ± 3.6 | 76.18 ± 3.7 |
| Females | 78.05 ± 6.7 | 78.88 ± 7.8 | 77.21 ± 7.0 | 77.14 ± 7.2 | |
| MCH, pg | Males | 23.15 ± 1.0 | 23.03 ± 0.9 | 22.95 ± 0.9 | 23.00 ± 1.0 |
| Females | 22.81 ± 1.9 | 22.74 ± 2.0 | 22.67 ± 1.9 | 22.75 ± 2.1 | |
| MCHC, g/dL | Males | 29.95 ± 1.6 | 30.08 ± 1.3 | 30.02 ± 1.4 | 30.23 ± 1.5 |
| Females | 29.27 ± 1.7 | 28.90 ± 1.9 | 29.42 ± 1.8 | 29.53 ± 1.8 | |
| PLT, ×103/µL | Males | 416.15 ± 89.7 | 399.05 ± 87.3 | 392.85 ± 89.9 | 408.35 ± 83.6 |
| Females | 441.53 ± 89.6 | 428.33 ± 91.0 | 429.33 ± 93.2 | 446.33 ± 91.3 | |
| MPV, fL | Males | 9.00 ± 1.3 | 8.86 ± 1.3 | 8.70 ± 1.2 | 8.91 ± 1.2 |
| Females | 8.55 ± 0.7 | 8.64 ± 0.8 | 8.55 ± 0.8 | 8.39 ± 0.7 | |
Abbreviations: BASO, absolute basophil counts; EOS, absolute eosinophil counts; HCT, hematocrit; HGB, hemoglobin; LUC, large unclassified cell counts; LYMPH, absolute lymphocyte counts; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; MCV, mean corpuscular volume; MONO, absolute monocyte counts; MPV, mean platelet volume; NEUT, absolute neutrophil counts; PLT, platelet; RBC, red blood cell count; RDW, red cell width; RETIC, absolute reticulocyte counts; WBC, white blood cell count.
P < .05. **P < .01 relative to the 4-hour time point.
Table 2.
Hematology results from cynomolgus monkeys fasted for different time periods after a 14-day acclimatization period of two meals/day (feeding regimen 2).
| Analytes | Sex | Fasting, hours | |||
|---|---|---|---|---|---|
| 4 | 8 | 12 | 20 | ||
| WBC, ×103/µL | Males | 14.96 ± 3.0 | 16.96 ± 3.8 | 18.60 ± 4.0** | 11.32 ± 3.1** |
| Females | 14.42 ± 3.2 | 19.02 ± 3.5** | 18.01 ± 3.2 | 12.75 ± 3.9 | |
| NEUT, ×103/µL | Males | 4.82 ± 1.9 | 6.42 ± 1.8** | 5.73 ± 1.7 | 3.83 ± 1.5 |
| Females | 5.90 ± 1.6 | 9.16 ± 2.2** | 7.14 ± 1.9 | 6.99 ± 3.2 | |
| LYMPH, ×103/µL | Males | 9.24 ± 3.3 | 9.61 ± 3.2 | 11.61 ± 3.4* | 6.79 ± 2.3** |
| Females | 7.43 ± 2.3 | 8.76 ± 2.6 | 9.46 ± 2.8* | 5.08 ± 2.5* | |
| MONO, ×103/µL | Males | 0.43 ± 0.1 | 0.50 ± 0.2 | 0.68 ± 0.2** | 0.38 ± 0.1 |
| Females | 0.51 ± 0.2 | 0.62 ± 0.3 | 0.78 ± 0.2** | 0.42 ± 0.2 | |
| EOS, ×103/µL | Males | 0.26 ± 0.2 | 0.23 ± 0.2 | 0.34 ± 0.2 | 0.20 ± 0.2 |
| Females | 0.35 ± 0.3 | 0.25 ± 0.2 | 0.38 ± 0.4 | 0.15 ± 0.2 | |
| BASO, ×103/µL | Males | 0.10 ± 0.05 | 0.08 ± 0.04 | 0.10 ± 0.05 | 0.05 ± 0.03** |
| Females | 0.10 ± 0.05 | 0.09 ± 0.07 | 0.09 ± 0.07 | 0.05 ± 0.06* | |
| LUC, ×103/µL | Males | 0.10 ± 0.05 | 0.11 ± 0.05 | 0.14 ± 0.06* | 0.08 ± 0.03 |
| Females | 0.07 ± 0.02 | 0.09 ± 0.03 | 0.09 ± 0.02 | 0.05 ± 0.01* | |
| HCT, % | Males | 44.76 ± 2.0 | 43.01 ± 2.3* | 42.47 ± 2.0** | 42.19 ± 2.1** |
| Females | 43.55 ± 2.2 | 42.85 ± 2.5 | 41.56 ± 2.5* | 40.71 ± 2.7** | |
| RBC, ×106/µL | Males | 5.78 ± 0.3 | 5.60 ± 0.3 | 5.55 ± 0.3* | 5.53 ± 0.3* |
| Females | 5.60 ± 0.4 | 5.50 ± 0.3 | 5.42 ± 0.3 | 5.31 ± 0.3* | |
| HGB, ×103/µL | Males | 13.38 ± 0.8 | 12.97 ± 0.8 | 12.87 ± 0.7* | 12.68 ± 0.7** |
| Females | 12.75 ± 0.7 | 12.42 ± 0.5 | 12.24 ± 0.6* | 11.97 ± 0.6** | |
| RETIC, ×103/µL | Males | 62.50 ± 29.3 | 58.55 ± 31.1 | 58.46 ± 28.0 | 59.29 ± 30.9 |
| Females | 53.02 ± 21.0 | 52.73 ± 17.3 | 53.69 ± 17.9 | 53.73 ± 18.9 | |
| RDW, % | Males | 13.07 ± 0.9 | 13.17 ± 0.9 | 13.24 ± 0.9 | 13.28 ± 0.9 |
| Females | 12.73 ± 1.1 | 12.73 ± 1.0 | 12.77 ± 1.0 | 12.90 ± 1.0 | |
| MCV, fL | Males | 77.50 ± 2.9 | 76.89 ± 3.8 | 76.63 ± 3.5 | 76.35 ± 3.8 |
| Females | 78.00 ± 5.3 | 78.08 ± 5.9 | 76.91 ± 5.1 | 76.81 ± 5.5 | |
| MCH, pg | Males | 23.13 ± 0.9 | 23.18 ± 1.0 | 23.20 ± 1.0 | 22.88 ± 1.0 |
| Females | 22.86 ± 2.0 | 22.71 ± 2.0 | 22.69 ± 2.0 | 22.61 ± 1.9 | |
| MCHC, g/dL | Males | 29.88 ± 1.2 | 30.14 ± 1.1 | 30.30 ± 1.1 | 30.02 ± 1.4 |
| Females | 29.29 ± 1.5 | 29.07 ± 1.8 | 29.48 ± 1.6 | 29.47 ± 1.7 | |
| PLT, ×103/µL | Males | 432.42 ± 110.5 | 415.65 ± 103.5 | 415.30 ± 109.7 | 430.85 ± 107.5 |
| Females | 444.07 ± 110.3 | 436.27 ± 107.7 | 434.67 ± 113.0 | 455.33 ± 122.7 | |
| MPV, fL | Males | 8.81 ± 1.1 | 8.76 ± 1.1 | 8.85 ± 1.4 | 8.58 ± 1.0 |
| Females | 8.68 ± 0.8 | 8.58 ± 0.7 | 8.47 ± 0.8 | 8.55 ± 0.6 | |
Abbreviations: BASO, absolute basophil counts; EOS, absolute eosinophil counts; HCT, hematocrit; HGB, hemoglobin; LUC, large unclassified cell counts; LYMPH, absolute lymphocyte counts; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; MCV, mean corpuscular volume; MONO, absolute monocyte counts; MPV, mean platelet volume; NEUT, absolute neutrophil counts; PLT, platelet; RBC, red blood cell count; RDW, red cell width; RETIC, absolute reticulocyte counts; WBC, white blood cell count.
P < .05. **P < .01 relative to the 4-hour time point.
Figure 1.
Absolute lymphocyte counts (×103/µL) over fasting times in males ( ) and females (
) and females ( ) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization period.
) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization period.
The different acclimatization feeding regimens did not yield any meaningful differences in hematology parameters between the two groups throughout the fasting periods.
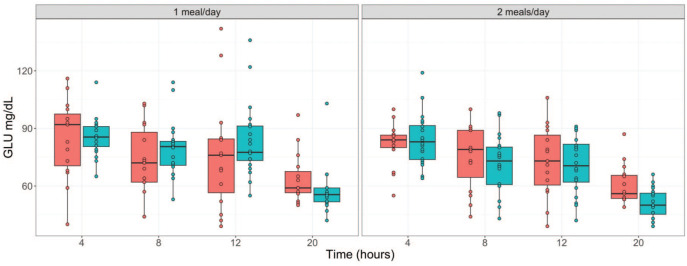
Glucose and Inorganic Phosphate Serum Concentrations Were the Serum Chemistry Parameters Most Impacted in Long Versus Short Fasting Times
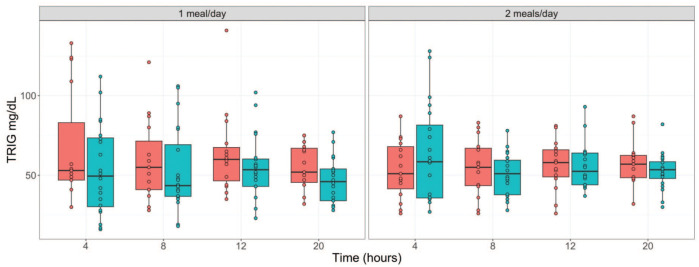
While there were generally minimal to no changes in most of the serum chemistry parameters regardless of the study phase/feeding regimen, serum glucose (GLU) and inorganic phosphate (IP) concentrations were mild to moderately impacted at the longer fasting time points. In both feeding regimens and sexes, relative to the 4-hour time point, moderate decreases in GLU (down by 40 %) were observed after 20 hours of fasting, while mild increases in IP (up to 40 % increase) were observed at ≥ 12 hours of fasting (Tables 3 and 4; Figure 2). Other parameters that were minimally affected relative to the 4-hour time point regardless of the study phase included triglyceride (TRIG), cholesterol (CHOL), and total bilirubin (tBIL). Minimal decreases in TRIG (down by 17 %) were noted at the 20-hour time point in both sexes in phase one and at the ≥12-hour fasting time points in only males in phase 2 (Tables 3 and 4; Figure 3). CHOL concentrations were minimally decreased (down by ~ 8 %) at the ≥ 12-hour fasting time points in phase 1 females and in both sexes at phase 2. tBIL concentrations were also minimally increased (up to 2.7-fold increase) at the 20-hour fasting time point in both phases and sexes. Despite this pattern of changes, they were still considered to be well within the normal biological variation for these parameters.
Table 3.
Serum chemistry results from cynomolgus monkeys fasted for different time periods after a 14-day acclimatization period of one meal/day (feeding regimen 1).
| Analyte | Sex | Fasting, hours | |||
|---|---|---|---|---|---|
| 4 | 8 | 12 | 20 | ||
| TRIG, mg/dL | Males | 53.55 ± 28.5 | 53.25 ± 26.3 | 55.50 ± 19.6 | 46.55 ± 14.1 |
| Females | 66.80 ± 35.8 | 58.87 ± 25.6 | 63.87 ± 26.2 | 55.40 ± 13.5 | |
| CHOL, mg/dL | Males | 144.80 ± 19.5 | 145.90 ± 19.6 | 143.00 ± 20.0 | 141.45 ± 21.6 |
| Females | 150.47 ± 19.8 | 148.13 ± 20.6 | 142.93 ± 21.7 | 141.27 ± 20.8 | |
| GLU, mg/dL | Males | 85.55 ± 10.0 | 79.30 ± 14.4 | 83.25 ± 19.4 | 57.45 ± 12.4** |
| Females | 84.87 ± 20.6 | 73.00 ± 18.7 | 76.33 ± 29.2 | 63.80 ± 13.1** | |
| TP, g/dL | Males | 7.18 ± 0.3 | 7.16 ± 0.3 | 6.93 ± 0.4* | 6.92 ± 0.4* |
| Females | 7.28 ± 0.4 | 7.23 ± 0.4 | 6.99 ± 0.4 | 6.97 ± 0.4* | |
| ALB, g/dL | Males | 4.57 ± 0.2 | 4.60 ± 0.2 | 4.44 ± 0.2 | 4.42 ± 0.2* |
| Females | 4.23 ± 0.3 | 4.30 ± 0.2 | 4.11 ± 0.2 | 4.13 ± 0.2 | |
| GLOB, g/dL | Males | 2.61 ± 0.3 | 2.56 ± 0.3 | 2.49 ± 0.3 | 2.50 ± 0.3 |
| Females | 3.05 ± 0.2 | 2.93 ± 0.2 | 2.87 ± 0.3 | 2.85 ± 0.2* | |
| A: G Ratio | Males | 1.78 ± 0.3 | 1.84 ± 0.3 | 1.82 ± 0.3 | 1.80 ± 0.3 |
| Females | 1.39 ± 0.1 | 1.46 ± 0.1 | 1.43 ± 0.1 | 1.47 ± 0.1 | |
| CRP, mg/dL | Males | 0.47 ± 0.2 | 0.48 ± 0.2 | 0.57 ± 0.2 | 0.66 ± 0.3* |
| Females | 0.62 ± 0.3 | 0.56 ± 0.3 | 0.71 ± 0.3 | 0.85 ± 0.5 | |
| UN, mg/dL | Males | 26.60 ± 5.5 | 25.95 ± 4.8 | 26.50 ± 4.9 | 26.95 ± 4.9 |
| Females | 23.00 ± 4.5 | 23.67 ± 5.1 | 24.67 ± 5.3 | 25.47 ± 4.6 | |
| CREA, mg/dL | Males | 1.03 ± 0.1 | 1.03 ± 0.1 | 1.07 ± 0.1 | 1.05 ± 0.1 |
| Females | 1.03 ± 0.1 | 1.03 ± 0.1 | 1.03 ± 0.2 | 1.09 ± 0.2 | |
| PHOS, mg/dL | Males | 5.97 ± 1.0 | 6.43 ± 0.7 | 8.16 ± 0.7** | 8.25 ± 0.7** |
| Females | 5.08 ± 0.7 | 5.95 ± 1.0** | 7.08 ± 1.0** | 6.31 ± 1.0** | |
| Ca, mg/dL | Males | 11.22 ± 0.5 | 11.07 ± 0.4 | 11.20 ± 0.5 | 10.75 ± 0.4** |
| Females | 10.99 ± 0.8 | 11.23 ± 0.8 | 11.00 ± 0.7 | 10.43 ± 0.4* | |
| TBIL, mg/dL | Males | 0.12 ± 0.04 | 0.11 ± 0.03 | 0.12 ± 0.04* | 0.21 ± 0.09** |
| Females | 0.10 ± 0.00 | 0.13 ± 0.05 | 0.16 ± 0.06 | 0.27 ± 0.12** | |
| ALP, U/L | Males | 629.55 ± 140.2 | 628.40 ± 141.9 | 621.40 ± 139.6 | 625.80 ± 145.4 |
| Females | 344.87 ± 104.3 | 351.00 ± 103.7 | 343.47 ± 105.6 | 345.87 ± 104.5 | |
| GGT, U/L | Males | 87.80 ± 30.4 | 88.00 ± 30.0 | 84.90 ± 29.1 | 84.50 ± 29.0 |
| Females | 49.67 ± 15.0 | 50.14 ± 14.4 | 48.20 ± 15.0 | 47.33 ± 14.1 | |
| ALT, U/L | Males | 45.95 ± 14.6 | 47.30 ± 13.2 | 47.15 ± 11.9 | 47.40 ± 12.2 |
| Females | 40.40 ± 15.7 | 41.87 ± 16.5 | 41.13 ± 15.5 | 42.53 ± 16.5 | |
| AST, U/L | Males | 44.50 ± 13.6 | 55.65 ± 14.3* | 56.90 ± 14.8** | 52.05 ± 14.5 |
| Females | 35.40 ± 15.2 | 40.53 ± 9.9 | 44.27 ± 14.1 | 42.60 ± 9.9 | |
| K, mmol/L | Males | 5.18 ± 0.4 | 5.08 ± 0.5 | 5.34 ± 0.5 | 5.31 ± 0.5 |
| Females | 4.79 ± 0.5 | 5.03 ± 0.6 | 5.29 ± 0.6* | 4.92 ± 0.4 | |
| Na, mmol/L | Males | 148.20 ± 2.1 | 146.40 ± 3.0* | 146.75 ± 2.5 | 147.65 ± 2.1 |
| Females | 147.53 ± 3.3 | 147.47 ± 3.9 | 145.47 ± 2.9 | 146.47 ± 1.8 | |
| Cl, mmol/L | Males | 104.00 ± 2.0 | 101.75 ± 2.1** | 101.85 ± 2.2** | 103.55 ± 2.3 |
| Females | 105.07 ± 2.8 | 103.00 ± 2.3* | 102.60 ± 2.1* | 104.33 ± 1.7 | |
| HCO3, mmol/L | Males | 21.10 ± 2.8 | 20.15 ± 3.4 | 20.25 ± 2.4 | 22.00 ± 1.8 |
| Females | 19.87 ± 2.8 | 16.80 ± 3.5* | 18.53 ± 2.3 | 20.13 ± 2.0 | |
Abbreviations: A: G ratio, Albumin to globulin ratio; ALB, Albumin; ALP, Alkaline phosphatase; ALT, Alanine transaminase; AST, Aspartate aminotransferase; Ca, Calcium; CHOL, Cholesterol; Cl, Chlorine; CREA, Creatinine; CRP, C-reactive protein; GGT, Gamma-glutamyl transferase; GLOB, Globulin; GLU, Glucose; HCO3, Bicarbonate; K, Potassium; Na, Sodium; PHOS, Phosphorus; TBIL, Total bilirubin; TP, Total protein; TRIG, Triglyceride; UN, Urea nitrogen.
P < .05. **P < .01 relative to the 4-hour time point.
Table 4.
Serum chemistry results from cynomolgus monkeys fasted for different time periods after a 14-day acclimatization period of two meals/day (feeding regimen 2).
| Analyte | Sex | Fasting, hours | |||
|---|---|---|---|---|---|
| 4 | 8 | 12 | 20 | ||
| TRIG, mg/dL | Males | 63.50 ± 30.7 | 50.85 ± 13.6 | 55.85 ± 13.9 | 52.70 ± 11.4 |
| Females | 53.53 ± 18.3 | 54.47 ± 18.0 | 56.47 ± 15.8 | 57.87 ± 13.8 | |
| CHOL, mg/dL | Males | 148.80 ± 23.7 | 148.75 ± 25.5 | 143.85 ± 24.0 | 141.30 ± 25.4 |
| Females | 155.60 ± 14.4 | 154.33 ± 15.9 | 145.53 ± 16.1 | 143.87 ± 14.9* | |
| GLU, mg/dL | Males | 84.35 ± 13.6 | 71.85 ± 14.7** | 70.75 ± 14.8** | 51.05 ± 7.7** |
| Females | 81.73 ± 11.5 | 75.07 ± 16.7 | 72.93 ± 18.4 | 60.53 ± 10.2** | |
| TP, g/dL | Males | 7.17 ± 0.3 | 7.14 ± 0.4 | 6.94 ± 0.4 | 6.85 ± 0.3** |
| Females | 7.33 ± 0.4 | 7.24 ± 0.4 | 6.97 ± 0.5* | 7.03 ± 0.4 | |
| ALB, g/dL | Males | 4.46 ± 0.3 | 4.48 ± 0.3 | 4.36 ± 0.3 | 4.32 ± 0.3 |
| Females | 4.25 ± 0.3 | 4.24 ± 0.2 | 4.07 ± 0.3 | 4.11 ± 0.2 | |
| GLOB, g/dL | Males | 2.71 ± 0.4 | 2.66 ± 0.4 | 2.59 ± 0.4 | 2.53 ± 0.4 |
| Females | 3.08 ± 0.3 | 3.00 ± 0.3 | 2.90 ± 0.4 | 2.91 ± 0.3 | |
| A: G Ratio | Males | 1.68 ± 0.3 | 1.73 ± 0.3 | 1.73 ± 0.3 | 1.74 ± 0.3 |
| Females | 1.39 ± 0.1 | 1.41 ± 0.1 | 1.41 ± 0.2 | 1.44 ± 0.1 | |
| CRP, mg/dL | Males | 0.57 ± 0.3 | 0.59 ± 0.3 | 0.67 ± 0.4 | 0.87 ± 0.5* |
| Females | 0.79 ± 1.0 | 0.78 ± 0.9 | 0.81 ± 0.6 | 0.86 ± 0.5 | |
| UN, mg/dL | Males | 25.05 ± 4.2 | 25.50 ± 4.8 | 24.40 ± 4.9 | 27.80 ± 4.2* |
| Females | 23.60 ± 4.4 | 24.00 ± 5.3 | 26.40 ± 4.6 | 26.07 ± 5.3 | |
| CREA, mg/dL | Males | 1.01 ± 0.1 | 1.05 ± 0.1 | 1.06 ± 0.1 | 1.05 ± 0.1 |
| Females | 1.03 ± 0.1 | 1.02 ± 0.1 | 1.04 ± 0.2 | 1.06 ± 0.1 | |
| PHOS, mg/dL | Males | 6.35 ± 0.8 | 6.79 ± 0.8 | 8.70 ± 0.9** | 8.52 ± 0.8** |
| Females | 5.27 ± 1.0 | 5.78 ± 0.7 | 7.07 ± 0.9** | 6.57 ± 0.8** | |
| Ca, mg/dL | Males | 10.91 ± 0.4 | 10.90 ± 0.4 | 11.04 ± 0.4 | 10.60 ± 0.3** |
| Females | 10.89 ± 0.5 | 10.91 ± 0.6 | 10.83 ± 0.4 | 10.44 ± 0.3** | |
| TBIL, mg/dL | Males | 0.11 ± 0.03 | 0.13 ± 0.04 | 0.14 ± 0.06 | 0.24 ± 0.09** |
| Females | 0.13 ± 0.05 | 0.11 ± 0.03 | 0.17 ± 0.07 | 0.31 ± 0.10** | |
| ALP, U/L | Males | 612.55 ± 135.2 | 621.30 ± 142.0 | 619.10 ± 133.8 | 618.80 ± 127.8 |
| Females | 443.67 ± 363.6 | 457.60 ± 383.8 | 439.07 ± 338.8 | 441.27 ± 327.7 | |
| GGT, U/L | Males | 85.95 ± 30.1 | 85.35 ± 30.7 | 83.10 ± 29.1 | 81.75 ± 29.2 |
| Females | 55.47 ± 21.1 | 56.40 ± 21.9 | 53.67 ± 20.2 | 53.47 ± 19.7 | |
| ALT, U/L | Males | 44.40 ± 10.0 | 47.75 ± 10.2 | 47.85 ± 10.7 | 48.15 ± 10.7 |
| Females | 42.87 ± 21.9 | 45.67 ± 21.2 | 44.60 ± 17.5 | 45.73 ± 17.1 | |
| AST, U/L | Males | 45.15 ± 10.4 | 60.55 ± 17.9** | 59.50 ± 16.1** | 53.15 ± 15.1 |
| Females | 35.60 ± 16.4 | 45.13 ± 10.6 | 45.40 ± 11.6 | 43.93 ± 12.2 | |
| K, mmol/L | Males | 5.10 ± 0.5 | 4.87 ± 0.5 | 5.23 ± 0.4 | 5.26 ± 0.4 |
| Females | 4.85 ± 0.4 | 4.85 ± 0.4 | 4.99 ± 0.4 | 4.87 ± 0.3 | |
| Na, mmol/L | Males | 148.15 ± 2.0 | 146.25 ± 2.2** | 147.30 ± 2.4 | 147.15 ± 1.8 |
| Females | 147.87 ± 2.1 | 146.07 ± 3.0 | 145.80 ± 2.3* | 146.53 ± 2.4 | |
| Cl, mmol/L | Males | 104.15 ± 1.6 | 101.45 ± 1.8** | 102.15 ± 2.0** | 103.05 ± 2.1 |
| Females | 104.80 ± 1.9 | 102.00 ± 2.2** | 102.53 ± 2.5** | 103.93 ± 1.7 | |
| HCO3, mmol/L | Males | 21.75 ± 2.7 | 19.55 ± 2.6* | 19.70 ± 3.1* | 21.50 ± 1.5 |
| Females | 20.20 ± 2.5 | 18.47 ± 1.8* | 19.47 ± 1.6 | 19.20 ± 2.5 | |
Abbreviations: A: G ratio, Albumin to globulin ratio; ALB, Albumin; ALP, Alkaline phosphatase; ALT, Alanine transaminase; AST, Aspartate aminotransferase; Ca, Calcium; CHOL, Cholesterol; Cl, Chlorine; CREA, Creatinine; CRP, C-reactive protein; GGT, Gamma-glutamyl transferase; GLOB, Globulin; GLU, Glucose; HCO3, Bicarbonate; K, Potassium; Na, Sodium; PHOS, Phosphorus; TBIL, Total bilirubin; TP, Total protein; TRIG, Triglyceride; UN, Urea nitrogen.
P < .05. **P < .01 relative to the 4-hour time point.
Figure 2.
Serum glucose concentration (mg/dL) over fasting times in males ( ) and females (
) and females ( ) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods. GLU indicates Glucose.
) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods. GLU indicates Glucose.
Figure 3.
Serum triglyceride concentration (mg/dL) over fasting times in males ( ) and females (
) and females ( ) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods. TRIG indicates Triglyceride.
) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods. TRIG indicates Triglyceride.
Increased Triglyceride Concentrations Variability Associated With Shorter Fasting Times
Although the decreases in triglyceride concentrations at the 20-hour time point were considered minimal and well within the normal biological variation, the triglyceride concentrations at the earlier time points indicated higher variability between individual animals as supported by higher standard deviations (Tables 3 and 4) and wider confidence intervals (Figure 3). In phase 1 (feeding regimen 1), the variability was much higher in both sexes at the 4- to 12-hour time points relative to the 20-hour time point. In phase 2 (feeding regimen 2), the variability was only higher at the 4-hour time point in males, and at the 4- and 8-hour time points in females. These findings were suggestive of the possibility that it could take up to 12 hours for gastrointestinal food digestion and absorption to be completed in cynomolgus monkeys.
Glucose Homeostasis Regulatory Hormones Largely Changed as Expected in Response to Decreased Glucose in Long Versus Short Fasting Times
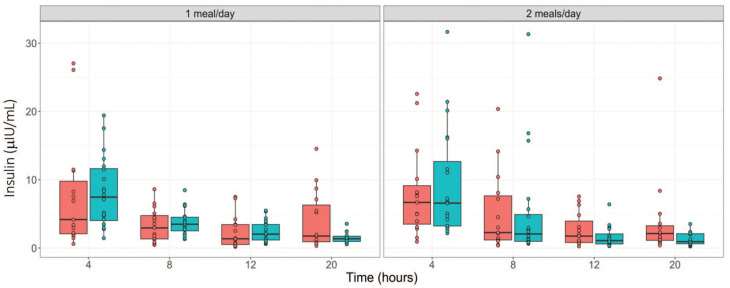
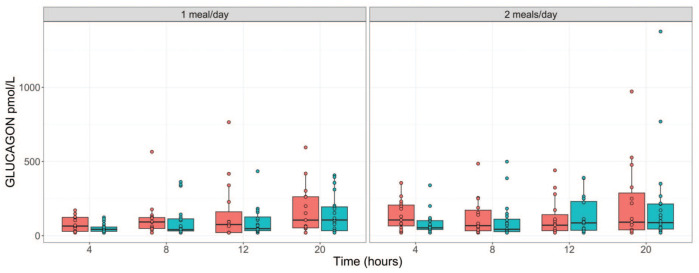
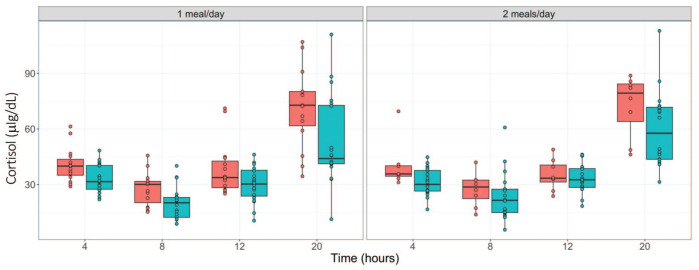
During fasting, the amount of glucose generated from dietary carbohydrates reduces over time. To maintain glucose homeostasis and reduce glucose cellular uptake, insulin secretion is suppressed, and the secretions of cortisol and glucagon are elevated. 6 To confirm these changes, we assessed the kinetics of these hormones. As shown in Tables 5 and 6 (and Figure 4), time-dependent decreases in insulin concentrations were observed in males regardless of the study phase with the highest decreases observed at the 20-hour time point (down by ~83%). In females, for both study phases, time-dependent decreases in insulin concentrations were also observed until the 12-hour time point, with a trend toward slight reversibility at the 20-hour time point but still lower than the 4-hour (phase 1) or ≤ 8-hour (phase 2) time points. For glucagon, time-dependent increases (up to 2.8-fold increase) were observed in both sexes in phase 1 and in males in phase 2. In the phase 2 females, increases in glucagon concentrations were only observed at the 20-hour time point (Tables 5 and 6; Figure 5). For cortisol, up to ~ 2-fold increases were observed at the 20-hour time point in both sexes regardless of the study phase. Overall, other than the trend toward reversibility for insulin in females at the 20-hour time point, the hormonal changes aligned with the conventional knowledge and expected changes related to fasting (Tables 5 and 6; Figure 6).
Table 5.
Results for glucose homeostasis-regulatory proteins from serum of cynomolgus monkeys fasted for different time periods after a 14-day acclimatization period of one meal/day (feeding regimen 1).
| Analyte | Sex | Fasting, hours | |||
|---|---|---|---|---|---|
| 4 | 8 | 12 | 20 | ||
| Insulin, µIU/mL | Males | 8.15 ± 5.1 | 3.76 ± 1.9** | 2.38 ± 1.5 | 1.54 ± 0.9** |
| Females | 7.80 ± 8.4 | 3.31 ± 2.5 | 2.32 ± 2.4* | 4.05 ± 4.3 | |
| Glucagon, pmol/L | Males | 51.16 ± 30.3 | 98.63 ± 11.38 | 93.78 ± 98.1 | 145.53 ± 128.6** |
| Females | 78.00 ± 50.6 | 114.95 ± 133.0 | 155.48 ± 208.0 | 174.57 ± 167.4* | |
| Cortisol, µg/dL | Males | 32.73 ± 7.6 | 19.67 ± 8.7** | 30.04 ± 9.6 | 53.15 ± 23.6** |
| Females | 40.66 ± 9.2 | 27.75 ± 8.9** | 38.52 ± 14.4 | 71.61 ± 21.1** | |
| Fructosamine, µmol/L | Males | 65.55 ± 13.4 | 66.85 ± 14.2 | 58.05 ± 13.1 | 52.95 ± 13.2** |
| Females | 44.27 ± 16.2 | 50.80 ± 17.2 | 43.33 ± 15.4 | 36.33 ± 14.4 | |
P < .05. **P < .01 relative to the 4-hour time point.
Table 6.
Results for glucose homeostasis-regulatory proteins from serum of cynomolgus monkeys fasted for different time periods after a 14-day acclimatization period of two meals/day (feeding regimen 2).
| Analyte | Sex | Fasting, hours | |||
|---|---|---|---|---|---|
| 4 | 8 | 12 | 20 | ||
| Insulin, µIU/mL | Males | 9.30 ± 8.0 | 5.11 ± 7.7 | 1.59 ± 1.5** | 1.28 ± 0.9** |
| Females | 8.06 ± 6.6 | 5.18 ± 5.9 | 2.69 ± 2.5** | 3.91 ± 6.1 | |
| Glucagon, pmol/L | Males | 81.50 ± 74.6 | 100.44 ± 127.7 | 127.56 ± 121.4 | 208.52 ± 324.5 |
| Females | 137.93 ± 99.2 | 125.27 ± 128.3 | 120.13 ± 128.9 | 218.51 ± 265.7 | |
| Cortisol, µg/dL | Males | 31.34 ± 7.3 | 23.48 ± 12.7* | 33.31 ± 7.6 | 59.98 ± 19.8** |
| Females | 40.15 ± 12.3 | 27.44 ± 9* | 35.24 ± 8.3 | 72.63 ± 16.7** | |
| Fructosamine, µmol/L | Males | 55.00 ± 19.8 | 60.45 ± 19.0 | 53.45 ± 18.8 | 42.55 ± 15.9* |
| Females | 44.93 ± 14.9 | 47.40 ± 13.8 | 42.93 ± 11.9 | 34.00 ± 10.6* | |
P < .05. **P < .01 relative to the 4-hour time point.
Figure 4.
Serum insulin concentration (µIU/mL) over fasting times in males ( ) and females (
) and females ( ) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods.
) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods.
Figure 5.
Serum glucagon concentration (pmol/L) over fasting times in males ( ) and females (
) and females ( ) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods.
) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods.
Figure 6.
Serum cortisol concentration (µg/dL) over fasting times in males ( ) and females (
) and females ( ) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods.
) following phase 1 (1 meal/day) and phase 2 (2 meals/day) acclimatization periods.
Changes in Fructosamine Correlated With Decreases in Glucose
Fructosamine is a glycated protein that is formed by the post-translational irreversible nonenzymatic linking of glucose to plasma proteins (primarily albumin). Inherently, the half-life of fructosamine is about 2-3 weeks aligning with that of albumin, as its removal is more dependent on the degradation or loss of albumin7-10 rather than that of glucose. As such, we hypothesized that the type of feeding regimen during the acclimatization period should have more effect on fructosamine concentration than 20 hours of fasting. As shown in Tables 5 and 6, at the 4-hour time point (closest time point to the end of the acclimatization period), fructosamine concentrations were higher in phase 1 (feeding regimen 1) males than the phase 2 (feeding regimen 2) males, while the concentration in females was comparable between the two study phases. In theory, as described earlier, higher frequency of feeding should result in higher glucose and albumin concentrations with subsequent fructosamine concentrations. However, the glucose and albumin concentrations at the 4-hour time points were comparable between the two feeding regimens in both sexes. Therefore, we considered the higher fructosamine concentrations in the phase 1 (or feeding regimen 1) males relative to the phase 2 (feeding regimen) males to be of no biological significance. In females, the results suggested that serum albumin and consequently serum fructosamine concentrations were not affected by the number of meals/day during the 14-day acclimatization period.
Surprisingly, decreases in fructosamine were observed at the 20-hour time point (up to 1.3-fold decrease) in both sexes regardless of the study phase, correlating with the concurrent decreases in glucose. This indicated that fructosamine concentrations were more affected by glucose concentrations than albumin concentrations during the fasting period.
Fasting Did Not Cause Oxidative Stress
Certain xenobiotics and/or their metabolites (e.g., quinones) can directly or indirectly cause oxidative stress through excessive production of reactive oxygen species (ROS) leading to depletion of antioxidants such as GSH.10-12 To determine whether the type of diet regimen during the 14-day acclimatization period and fasting up to 20 hours can contribute to oxidative stress during a toxicology study, we assessed the serum concentrations of GSH, GSSG, GSH: GSSG ratio, and Cys. In phase 1, the results showed minimal decreases in GSH at the ≥ 8-hour time points in both sexes, minimal increase in GSSG only at the 12-hour time point in males, minimal decreases in the GSG: GSSG ratio at the ≥ 12-hour time points in males, and no change in cysteine (Supplementary Table 2). In phase 2, minimal decreases in GSH were observed at the ≥ 8-hour time points in males and/or females with no change in GSSG, leading to decreases in the GSG: GSSG ratio in males at the 12-hour time point, and in females at the 20-hour time point. No changes in cysteine were observed (Supplementary Table 3). Despite these minor shifts in the GSG: GSSG ratios in the two study phases, these changes were considered biologically irrelevant as there were still overwhelming serum GSH concentrations relative to that of GSSG, indicating fasting did not cause oxidative stress.
Discussion
One of the most important observations in this study was the lack of remarkable changes in triglyceride concentrations between the 4-hour and 20-hour time points. Since one of the primary reasons for fasting laboratory animals for toxicology studies is to ensure a comparable and stable baseline triglyceride concentration among the individual animals in a study, the lack of any significant difference in serum triglycerides between the short and long fasting time points in this study was quite surprising. This may be attributed to the low dietary intake of fat as all monkeys were fed the PMI Nutrition International Certified LabDiet® that contains ~ 5% fat, which is considered a low-fat diet. 13 For these reasons, the authors believe there’s little justification for fasting cynomolgus monkeys on a similar nutritional diet for more than 4 hours for toxicology studies. That said, the triglyceride concentrations at the earlier fasting time points showed higher variability between individual animals suggestive of the possibility that gastrointestinal food digestion and absorption could take up to 12 hours in cynomolgus monkeys. As such, when triglyceride is considered a critical endpoint in a toxicology study and important drug development decisions will be made based on triglyceride assessment, the authors recommend a longer fasting time (>12-20 hours) prior to blood sample collections for clinical pathology assessment.
Another interesting observation in this study was the trend of insulin concentrations toward reversibility at the 20-hour fasting time points in females regardless of the study phase (or feeding regimen). Physiological increases in blood glucose concentration stimulate insulin secretion which subsequently promotes glucose uptake by the liver, skeletal muscle, adipose tissue, and several other organs. 6 When the blood glucose drops due to fasting, glucagon secretion is increased and insulin secretion is suppressed 6 in order to maintain fasting normoglycemia. This pattern was largely observed in this study. During fasting, there must be a balance between the actions of insulin and glucagon in order to maintain normoglycemia. 6 An imbalance of glucagon-predominant activities during fasting severely impacts cellular uptake of glucose such that calorie generation is inadequate14-17 leading to general body fatigue/weakness. Therefore, the slight increases in insulin in females at the 20-hour time point may reflect a physiological response to maintain cellular uptake of glucose, as the glucose and glucagon concentrations in females were slightly higher relative to males in both study phases. This could suggest that female monkeys’ glucose homeostasis machinery may have a lower threshold for hypoglycemia relative to males. Hence, to avoid this type of hormonal imbalance that could impact the welfare of the animals during toxicology studies, outside of any scientific justification, shorter fasting times should be favored over longer fasting times as the blood glucose concentrations were comparable between the 4-, 8-, and 12-hour time points in both sexes and study phases.
GSH is a ubiquitous tripeptide thiol that is composed of Cys, glutamic acid, and glycine. 18 GSH is an important intracellular and extracellular protective antioxidant that engages in various key roles in the control of detoxification of certain xenobiotics and heavy metals, signaling processes, as well as other functions.19-27 It is well known that certain xenobiotics and/or their metabolites (e.g., quinones) can directly or indirectly cause oxidative stress through excessive production of ROS leading to depletion of antioxidants such as GSH. Xenobiotics can directly facilitate the formation of ROS (e.g., superoxide and peroxides) that can cause damage to biomolecules and/or affect signaling pathways or indirectly by altering protective proteins (i.e., antioxidant enzymes and metal transporters/chelators) and their expression (epigenetics).10-12 Previous studies have shown that dietary restriction with a lack of essential amino acids and starvation can cause cysteine deficiency which in turn could lead to GSH depletion in rats after 24 hours of food deprivation.28-31 As such, the administration of xenobiotics to fasted animals provides two perfect dynamics that can increase oxidative stress in toxicology studies and subsequently impact animal welfare and therefore, data quality. To assess whether fasting can cause similar GSH decreases in cynomolgus monkeys as observed in rodents,28-32 we evaluated the GSH, GSSG, and the GSH: GSSG ratio, along with Cys concentrations. The results showed that glutathione parameters were not impacted as reported in rodents suggesting fasting does not cause oxidative stress in cynomolgus monkeys. That said, given the clinical chemistry endpoints were largely comparable between different fasting times, we still maintain our position that cynomolgus monkeys do not need to be fasted for more than 4 hours for toxicology studies except when scientifically justified.
All other clinical pathology changes were largely observed at the 20-hour time point and they included stress-related changes (increased cortisol and decreased LYMPH), changes associated with bilirubin uptake by the liver due to fatty acid mobilization (increased tBIL) 6 and changes associated with decreased glomerular filtration rate (GFR) as a result of dehydration (increased inorganic phosphates). While the frequent handling of the animals for blood collection could have impacted the stress-related changes, we do not believe that was the case in this study. This is because the stress-related changes were not observed during the periods of most frequent handling (4 hours between blood collection times. i.e., 4-, 8- and 12-hour time points) but rather after an extended period between handling procedures (8 hours between blood collection times). While minimal in nature, the presence of these changes altogether has the potential to negatively impact the welfare of animals that are already under stress related to the exposure to high (sometimes toxic) doses of the test articles. As such, cynomolgus monkeys should not be subjected to long fasting times except when scientifically justified. We are currently conducting similar studies on other animals that are commonly used for nonclinical safety assessment including beagle dogs, rats, and mice. The results from these studies will be published in due time.
Since our main objective was to reduce fasting duration, we did not conduct an assessment of clinical pathology parameters under non-fasting conditions (i.e., 0 hours). With this in mind, the authors are open to the possibility that fasting of cynomolgus monkeys fed low-fat based diets may not be necessary for toxicology studies except: (1) when fasting would be needed for the bioavailability of an orally-administered test article33-40 and (2) when parameters that will be affected by non-fasting conditions are needed for test article safety and pharmacodynamic assessments (i.e., glucose, triglyceride, and cholesterol).
While there were several parameters showing statistically significant P-values, we only considered changes that were deemed biologically significant based on the authors’ expertise.
Any statistically significant result only serves as a reference for biological significance and must be rationally interpreted with corresponding biological processes to decide its biological significance. Statistical significance does not mean a real variation or effect in a biological system. 41 For example, the changes in serum chloride concentrations were statistically significant at the 8-hour and 12-hour time points but statistically normal in both sexes in the two study phases (Tables 3 and 4). These changes were considered to be within normal biological variation with no biological relevance as these changes do not impact the health of the animals, and there was no temporal consistency to these changes.
A study was recently published on the age-related changes in clinical pathology parameters in cynomolgus monkeys with differences noted in reticulocyte and lymphocyte counts, creatinine, triglyceride, phosphorus, and globulin concentrations, along with alkaline phosphatase and gamma-glutamyl transferase activities. 42 Although we did not evaluate how fasting can impact clinical pathology parameters among different age groups, we do not have any reasons to believe our conclusions will be impacted by age differences.
In conclusion, this study evaluated the effects of different acclimatization practices and fasting durations on standard and ancillary clinical pathology parameters. The results showed that the different acclimatization practices did not have any remarkable effects on the body weight, blood fructosamine, or other clinical pathology parameters; and fasting up to 20 hours did not cause oxidative stress in cynomolgus monkeys. Additionally, longer fasting times resulted in stress (increased cortisol and decreased LYMPH), decreased GFR (increased inorganic phosphate), and biologically significant decreases in glucose-all changes that could impact animal welfare. While there were no significant differences in triglyceride concentrations between the short and long fasting times, shorter fasting times were associated with higher triglyceride variability among individual animals relative to the longer fasting times. Based on these findings, the authors recommend short fasting times for most toxicology studies except when parameters that will be affected by non-fasting conditions are important for safety and pharmacodynamic assessments (i.e., glucose, triglyceride, and cholesterol).
Supplemental Material
Supplemental material, sj-docx-1-tpx-10.1177_01926233231193395 for Reduced Fasting Duration in Cynomolgus Monkeys Enhances Animal Welfare During Toxicology Studies by Adeyemi O. Adedeji, Tony Pourmohamad, Niraj Tripathi, Shelly Zhong, Kenna R. Degner, Fiona Zhong, Dewakar Sangaraju, Kevin Williams and Noel Dybdal in Toxicologic Pathology
Acknowledgments
The authors acknowledge Paula Katavalos, DVM PhD, from Genentech for study design support; Xiaofeng Zhao from Genentech for the technical support; the technical expertise and support of clinical pathology staff from Labcorp including Brenda Ortiz, Emily Marach, Megan Heidemann, Sue Thao, Laura Wieczorkiewicz, Sarah Krueger, Kara Finco, and Curlena Wilder from Labcorp Madison; Michele Hall and Bruce Beechler from Labcorp Greenfield; and the translational biomarker team from Labcorp Greenfield. The authors also thank Regan Bell, DVM, MS, Diplomate ACVP; and Klaudia Polak VMD, MS, Diplomate, ACVP from Labcorp Early Development Laboratories Inc. and Michelle L. Lepherd, BVSc, PhD, DACVP, DABT from Genentech for their critical review of the manuscript.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All work was supported by Genentech, a member of the Roche group and Labcorp Early Development Laboratories Inc.
ORCID iD: Adeyemi O. Adedeji  https://orcid.org/0000-0002-5153-1990
https://orcid.org/0000-0002-5153-1990
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Pickering RG, Pickering CE. The effects of reduced dietary intake upon the body and organ weights, and some clinical chemistry and haematological variates of the young Wistar rat. Toxicol Lett. 1984;21(3):271-277. doi: 10.1016/0378-4274(84)90083-3 [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Yoshikawa M, Sasaoka K, et al. Effects of diurnal rhythms and fasting in the rat and mouse physiological conditions. Exp Pathol. 1974;68:767-775. [Google Scholar]
- 3. Hall R. Principles of clinical pathology for toxicity studies. In: Wallace HA, ed. Principles and Methods of Toxicology. 4th ed. Philadelphia, PA: Taylor & Francis; 2001:1001-1038. [Google Scholar]
- 4. Lee SG, Yim J, Lim Y, Kim JH. Validation of a liquid chromatography tandem mass spectrometry method to measure oxidized and reduced forms of glutathione in whole blood and verification in a mouse model as an indicator of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1019:45-50. doi: 10.1016/j.jchromb.2015.10.041 [DOI] [PubMed] [Google Scholar]
- 5. R Core Team. R: a language and environment for statistical computing. https://www.r-project.org/
- 6. Stockham SL, Scott M. Fundamentals of Veterinary Clinical Pathology. 2nd ed. Hoboken, NJ: Blackwell Publishing; 2008. [Google Scholar]
- 7. Schleicher ED, Olgemöller B, Wiedenmann E, Gerbitz KD. Specific glycation of albumin depends on its half-life. Clin Chem. 1993;39(4):625-628. [PubMed] [Google Scholar]
- 8. Beck R, Steffes M, Xing D, et al. The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol, and continuous glucose monitoring. Pediatr Diabetes. 2011;12(8):690-695. doi: 10.1111/j.1399-5448.2011.00764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9(2):169-176. doi: 10.1177/1932296814567227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steffensen IL, Dirven H, Couderq S, et al. Bisphenols and oxidative stress biomarkers-associations found in human studies, evaluation of methods used, and strengths and weaknesses of the biomarkers. Int J Environ Res Public Health. 2020;17(10):3609. doi: 10.3390/ijerph17103609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hofer T. Oxidation of 2′-deoxyguanosine by H2O2–ascorbate: evidence against free OH. and thermodynamic support for two-electron reduction of H2O2. J Chem Soc Perkin Trans. 2001;2:210-213. doi: 10.1039/b006394k [DOI] [Google Scholar]
- 12. Möller L, Hofer T, Zeisig M. Methodological considerations and factors affecting 8-hydroxy-2-deoxyguanosine analysis. Free Radic Res. 1998;29(6):511-524. doi: 10.1080/10715769800300561 [DOI] [PubMed] [Google Scholar]
- 13. Mubiru JN, Garcia-Forey M, Higgins PB, et al. A preliminary report on the feeding of cynomolgus monkeys (Macaca fascicularis) with a high-sugar high-fat diet for 33 weeks. J Med Primatol. 2011;40(5):335-341. doi: 10.1111/j.1600-0684.2011.00495.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reeves RE, South DJ, Blytt HJ, Warren LG. Pyrophosphate:D-fructose 6-phosphate 1-phosphotransferase. A new enzyme with the glycolytic function of 6-phosphofructokinase. J Biol Chem. 1974;249(24):7737-7741. [PubMed] [Google Scholar]
- 15. Selig M, Xavier KB, Santos H, Schönheit P. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol. 1997;167(4):217-232. doi: 10.1007/BF03356097 [DOI] [PubMed] [Google Scholar]
- 16. Stryer L. Glycolysis. In: Stryer (ed.) Biochemistry. 4th ed. New York, NY: W. H. Freeman; 1995:483-495. [Google Scholar]
- 17. Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry. 2nd ed. Hoboken, NJ: John Wiley; 2006. [Google Scholar]
- 18. Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30(1-2):1-12. doi: 10.1016/j.mam.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chasseaud LF. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175-274. doi: 10.1016/s0065-230x(08)60848-9 [DOI] [PubMed] [Google Scholar]
- 20. Burk RF. Glutathione-dependent protection by rat liver microsomal protein against lipid peroxidation. Biochim Biophys Acta. 1983;757(1):21-28. doi: 10.1016/0304-4165(83)90148-4 [DOI] [PubMed] [Google Scholar]
- 21. Gorla N, de Ferreyra EC, Villarruel MC, de Fenos OM, Castro JA. Studies on the mechanism of glutathione prevention of carbon tetrachloride-induced liver injury. Br J Exp Pathol. 1983;64(4):388-395. [PMC free article] [PubMed] [Google Scholar]
- 22. Sugimura Y, Yamamoto K. Effect of orally administered reduced- and oxidized-glutathione against acetaminophen-induced liver injury in rats. J Nutr Sci Vitaminol. 1998;44(5):613-624. [DOI] [PubMed] [Google Scholar]
- 23. Ueno Y, Kizaki M, Nakagiri R, Kamiya T, Sumi H, Osawa T. Dietary glutathione protects rats from diabetic nephropathy and neuropathy. J Nutr. 2002;132(5):897-900. doi: 10.1093/jn/132.5.897 [DOI] [PubMed] [Google Scholar]
- 24. Magnani M, Fraternale A, Casabianca A, et al. Antiretroviral effect of combined zidovudine and reduced glutathione therapy in murine AIDS. AIDS Res Hum Retroviruses. 1997;13(13):1093-1099. doi: 10.1089/aid.1997.13.1093 [DOI] [PubMed] [Google Scholar]
- 25. Schwartz JL, Shklar G. Glutathione inhibits experimental oral carcinogenesis, p53 expression, and angiogenesis. Nutr Cancer. 1996;26(2):229-236. doi: 10.1080/01635589609514479 [DOI] [PubMed] [Google Scholar]
- 26. Shklar G, Schwartz J, Trickler D, Cheverie SR. The effectiveness of a mixture of beta-carotene, alpha-tocopherol, glutathione, and ascorbic acid for cancer prevention. Nutr Cancer. 1993;20(2):145-151. doi: 10.1080/01635589309514281 [DOI] [PubMed] [Google Scholar]
- 27. Kern JK, Geier DA, Adams JB, Garver CR, Audhya T, Geier MR. A clinical trial of glutathione supplementation in autism spectrum disorders. Med Sci Monit. 2011;17(12):CR677-82. doi: 10.12659/msm.882125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higashi T, Tateishi N, Sakamoto Y. Liver glutathione as a reservoir of L-cysteine. Prog Clin Biol Res. 1983;125:419-434. [PubMed] [Google Scholar]
- 29. Maruyama E, Kojima K, Higashi T, Sakamoto Y. Effect of diet on liver glutathione and glutathione reductase. J Biochem. 1968;63(3):398-399. [PubMed] [Google Scholar]
- 30. Pessayre D, Dolder A, Artigou JY, et al. Effect of fasting on metabolite-mediated hepatotoxicity in the rat. Gastroenterology. 1979;77(2):264-271. [PubMed] [Google Scholar]
- 31. Schnell RC, Bozigian HP, Davies MH, Merrick BA, Park KS, McMillan DA. Factors influencing circadian rhythms in acetaminophen lethality. Pharmacology. 1984;29(3):149-157. doi: 10.1159/000138005 [DOI] [PubMed] [Google Scholar]
- 32. Vogt BL, Richie JP., Jr. Fasting-induced depletion of glutathione in the aging mouse. Biochem Pharmacol. 1993;46(2):257-263. doi: 10.1016/0006-2952(93)90412-p [DOI] [PubMed] [Google Scholar]
- 33. Welling PG. Influence of food and diet on gastrointestinal drug absorption: a review. J Pharmacokinet Biopharm. 1977;5(4):291-334. doi: 10.1007/BF01061694 [DOI] [PubMed] [Google Scholar]
- 34. Welling PG. Effects of food on drug absorption. Pharmacol Ther. 1989;43(3):425-441. doi: 10.1016/0163-7258(89)90019-3 [DOI] [PubMed] [Google Scholar]
- 35. Devriese LA, Koch KM, Mergui-Roelvink M, et al. Effects of low-fat and high-fat meals on steady-state pharmacokinetics of lapatinib in patients with advanced solid tumours. Invest New Drugs. 2014;32(3):481-488. doi: 10.1007/s10637-013-0055-4 [DOI] [PubMed] [Google Scholar]
- 36. Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413-420. doi: 10.1023/a:1016212804288 [DOI] [PubMed] [Google Scholar]
- 37. Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11-23. doi: 10.1007/s11095-004-9004-4 [DOI] [PubMed] [Google Scholar]
- 38. Gu CH, Li H, Levons J, et al. Predicting effect of food on extent of drug absorption based on physicochemical properties. Pharm Res. 2007;24(6):1118-1130. doi: 10.1007/s11095-007-9236-1 [DOI] [PubMed] [Google Scholar]
- 39. Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet. 1999;36(3):233-254. doi: 10.2165/00003088-199936030-00004 [DOI] [PubMed] [Google Scholar]
- 40. van de Waterbeemd H, Lennemas H, Artursson P. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability. 2nd ed. vol. 40. Weinheim, Germany: Wiley-VCH Verlag GmbH; 2008. [Google Scholar]
- 41. Zhan X, Long Y, Zhan X, Mu Y. Consideration of statistical vs. Biological significances for omics data-based pathway network analysis. Med One. 2017;2(1):e170002. doi: 10.20900/mo.20170002 [DOI] [Google Scholar]
- 42. Li X, Li D, Biddle KE, et al. Age- and sex-related changes in body weights and clinical pathology analytes in cynomolgus monkeys (Macaca Fascicularis) of Mauritius origin. Vet Clin Pathol. 2022;51(3):356-375. doi: 10.1111/vcp.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tpx-10.1177_01926233231193395 for Reduced Fasting Duration in Cynomolgus Monkeys Enhances Animal Welfare During Toxicology Studies by Adeyemi O. Adedeji, Tony Pourmohamad, Niraj Tripathi, Shelly Zhong, Kenna R. Degner, Fiona Zhong, Dewakar Sangaraju, Kevin Williams and Noel Dybdal in Toxicologic Pathology