Abstract
Objectives:
This investigation sought to characterize risk factors associated with acquisition of traditional and emerging agents of STI in a cohort of young MSM and transgender women.
Methods:
917 participants provided urine and rectal swab submissions assessed by transcription-mediated amplification (TMA)-based assays for Chlamydia trachomatis and Neisseria gonorrhoeae and by off-label TMA-based Trichomonas vaginalis and Mycoplasma genitalium testing. A subset provided specimens at six-month and twelve-month follow-up visits.
Results:
Prevalence of M. genitalium from rectal and urine specimens (21.7% and 8.9%, respectively) exceeded that of C. trachomatis (8.8% and 1.6%) and other STI agents. Black participants yielded higher prevalence of M. genitalium (30.6%) than non-black participants (17.0%; X2=22.39; P<0.0001). M. genitalium prevalence from rectal specimens was 41.5% in HIV-positive participants versus 16.3% in HIV-negative participants (X2=55.72; P<0.0001). Participant age, gender identity, condomless insertive anal/vaginal sexual practice, and condomless receptive anal sexual practice were not associated with rectal C. trachomatis (P≥0.10), N. gonorrhoeae (P≥0.29), T. vaginalis (P≥0.18) or M. genitalium (P≥0.20) detection. While prevalence of T. vaginalis was calculated at ≤ 1.0%, baseline rectal and urine screening status was predictive of detection/non-detection at follow-up. A non-reactive M. genitalium baseline rectal or urine screening result was less predictive of non-reactive follow-up versus C. trachomatis, N. gonorrhoeae, and T. vaginalis.
Conclusions:
Rectal M. genitalium detection is associated with black race and HIV seropositivity. Baseline M. genitalium infection influences subsequent detection of the organism.
Keywords: RADAR, MSM, Rectal swab, Mycoplasma genitalium, Longitudinal survey
SHORT SUMMARY
Rectal swab Mycoplasma genitalium RNA detection is associated with positive HIV serostatus and black race. Baseline urine and rectal swab detection is highly predictive of reactive STI status at follow-up.
INTRODUCTION
Men who have sex with men (MSM) are disproportionately affected by sexually-transmitted infection (STI) compared to other demographics.1 While significant associations have been observed between rectal STI infection and subsequent HIV acquisition in previously HIV- seronegative MSM,2,3 the frequency of screening for non-ulcerative STI agents in these populations is thought to be suboptimal. From an audit of HIV clinics in six United States cities encompassing nearly 15,000 patient encounters, Hoover et al.4 reported rectal Chlamydia trachomatis and Neisseria gonorrhoeae screening rates approximating 2–8% on an annual basis. In contrast, annual screening rates for syphilis ranged from 66% to 75%. The authors further noted that significant rates of C. trachomatis and N. gonorrhoeae detection emanated from asymptomatic patients. Moreover, Frankis et al.5 reported that MSM <25 years of age were less likely to be regularly screened for agents of STI.
Studies have elucidated the significance of STI among young MSM (YMSM). Garofalo et al.6 followed a metropolitan YMSM cohort (ages 16–20 years) for two years, reporting an STI incidence of 6.22 per 100 person-years. Increased risk of STI was associated with non-white race, but was not associated with other demographic and risk behaviors such as age, educational level, substance use, and unprotected anal sex. However, laboratory testing was relegated to urine specimens, utilizing PCR assays for C. trachomatis and N. gonorrhoeae detection. Another study of YMSM aged 16–29 years7 assessed baseline C. trachomatis and N. gonorrhoeae data prior to enrolling participants into an HIV/STI prevention randomized control trial with additional self-collected rectal swab procurement implemented into the baseline screen. At baseline, 15.1% of participants were positive for either agent of STI, with only 4.6% of these participants positive for a urethral STI. More recent data have suggested that programmed intervention measures can reduce the incidence of STI when YMSM cohorts are followed on a longitudinal basis. Mustanski et al.8 randomized 901 participants to an on-line health intervention and demonstrated that STI detection rates at a twelve-month follow-up screening were reduced by 40% in the intervention versus control arm.
Trichomonas vaginalis and Mycoplasma genitalium are emerging agents of STI. These agents have been detected from rectal swab specimens9,10 and laboratories possessing technical expertise now have the capability of testing for these agents via commercial nucleic acid amplification testing.11 To date, studies have not characterized the significance of these organisms in YMSM or young transgender women cohorts in the context of HIV association, sexual behavioral practices, and predictive value of a baseline screening result for detection of the organism at follow-up. Moreover, professional and societal guidelines, such as those published by the United States Centers for Disease Control and Prevention12 have not advocated extraurogenital detection of pathogens such as T. vaginalis. Such guidance may have emanated from diagnostic systems that have not utilized advanced technologies such as endogenous inhibitor removal and RNA amplification. 13 The large RADAR longitudinal cohort14,15 provides a basis for executing such investigations using contemporary and highly-sensitive molecular diagnostic assays; initial findings are presented.
MATERIALS AND METHODS
Cohort eligibility.
RADAR is a cohort study examining HIV risk factors, substance use, and relationship patterns among young transgender women and YMSM in the Chicago, Illinois region. This cohort and methods of recruitment have been described previously and were selected to achieve a multiple cohort, accelerated longitudinal study design.14 Eligibility requirements at time of enrollment include: ages 16–29 years; male assignment at birth; English speaking; and, report of a sexual encounter with a man in the previous year or identification as gay, bisexual, or transgender. The overall sample is augmented by recruitment of serious partners of RADAR cohort members who meet eligibility criteria. Participant interviews included both self-reported and interviewer-administered sections. This investigation received Institutional Review Board approval through Northwestern University. Informed consent was obtained from all study participants.
Collection of participant demographic data and provision of specimens.
From March 2018 through November 2019, the following variables were collected from study participants by self- report surveys administered on computers in private interview rooms: participant age; gender identity; race and ethnicity; condomless anal/vaginal sex in terms of both insertive and receptive positioning. Participants were also asked to provide 10–15 mL of first-void urine [aliquoted to an Aptima Urine Specimen Collection Kit (Hologic, Incorporated; San Diego, CA) at the study site] and self-collect a rectal swab specimen using the Aptima Multitest Swab Specimen Collection Kit (Hologic). All specimens were maintained at 2–30ºC and tested within 30 days of collection. Participants were invited to provide analogous self-reporting data and additional primary specimens during six- and twelve-month follow-up encounters. Study procedures and protocols were identical at each study visit. The fourth-generation Determine™ HIV-1/2 Ag/Ab Combo (Alere Ltd.; Stockport, UK) rapid test assayed the presence of HIV-1 antibodies, HIV-2 antibodies, and free HIV-1 p24 antigen at every visit. Laboratory confirmation followed the Centers for Disease Control and Prevention guidelines for HIV testing.16
Molecular assays.
Detection of C. trachomatis-specific 23S rRNA and N. gonorrhoeae-specific 16S rRNA from first-void urine and rectal swab specimens occurred by FDA-indicated and off- label utilizations, respectively, of Aptima Combo 2 (Hologic). Detection of T. vaginalis 18S rRNA occurred by off-label utilization of Aptima Trichomonas vaginalis (Hologic). Detection of M. genitalium 16S rRNA was facilitated by an analyte-specific reagent provided by Hologic. Accuracy of the aforementioned transcription-mediated amplification-based assays for off-label detection of T. vaginalis rRNA and for detection of M. genitalium rRNA has previously been demonstrated.17–19 All testing was performed on the Panther automated system (Hologic). Relative light unit values ≥ 50,000 generated from M. genitalium TMA were interpreted reactive for M. genitalium rRNA detection.20,21
Data analysis.
For each STI, independent samples t-tests were conducted to test for mean age differences between those who tested reactive and those who tested non-reactive. A series of Chi-square tests were also conducted to investigate associations between each STI result and the following factors: gender identity (cisgender vs. transgender and gender diverse), race/ethnicity (Black/African American vs. other), HIV status, any insertive condomless anal or vaginal sex in the past six months, and any receptive condomless anal sex in the past six months. In cases in which expected cell counts were less than 5, Fisher’s exact P values are reported. Next, for each STI, odds ratios were calculated to determine the likelihood of a participant testing reactive at a follow-up visit given they had tested reactive at a prior visit. Lastly, for participants who tested non-reactive at their first visit, Cox proportional hazards modeling was conducted to calculate the survivor function estimates of a participant testing non-reactive at six- and twelve-month follow-up intervals for each STI. The timescale used for proportional hazards modeling was measured in months. Events with the same survival time were modeled using Breslow’s approximation. The log-rank test was utilized to test differences in survival curves between the four sexually-transmitted agents.
RESULTS
Inclusion and exclusion data.
Of 1235 participants enrolled in RADAR, 950 (76.9%) individuals were screened at least once for the four STI agents during the aforementioned collection period. Of this sample, fifteen individuals were assigned female at birth (sexual partners of RADAR participants) and eighteen were assigned male at birth but reported being older than 29 years of age at baseline. For purposes of these analyses, both of these groups were excluded. Of the remaining 917 study participants, 776 (84.6%) and 678 (73.9%) returned for a six-month and 12-month follow-up visit, respectively, to provide an additional rectal swab and/or first-void urine specimen.
Prevalence data.
The prevalence of N. gonorrhoeae and C. trachomatis infection within this cohort was 6.8% and 8.8% (rectal specimens), 0.8% and 1.6% (urine specimens), and 0.8% and 0.9% (co-occurring specimens; Fig. 1), respectively. In contrast to a very low prevalence of T. vaginalis infection in this cohort (1.0% rectal; 0.1% urine; 0.1% co-occurring), prevalence of M. genitalium infection was high (21.7% rectal; 8.9% urine; 4.3% co-occurring).
Figure 1.
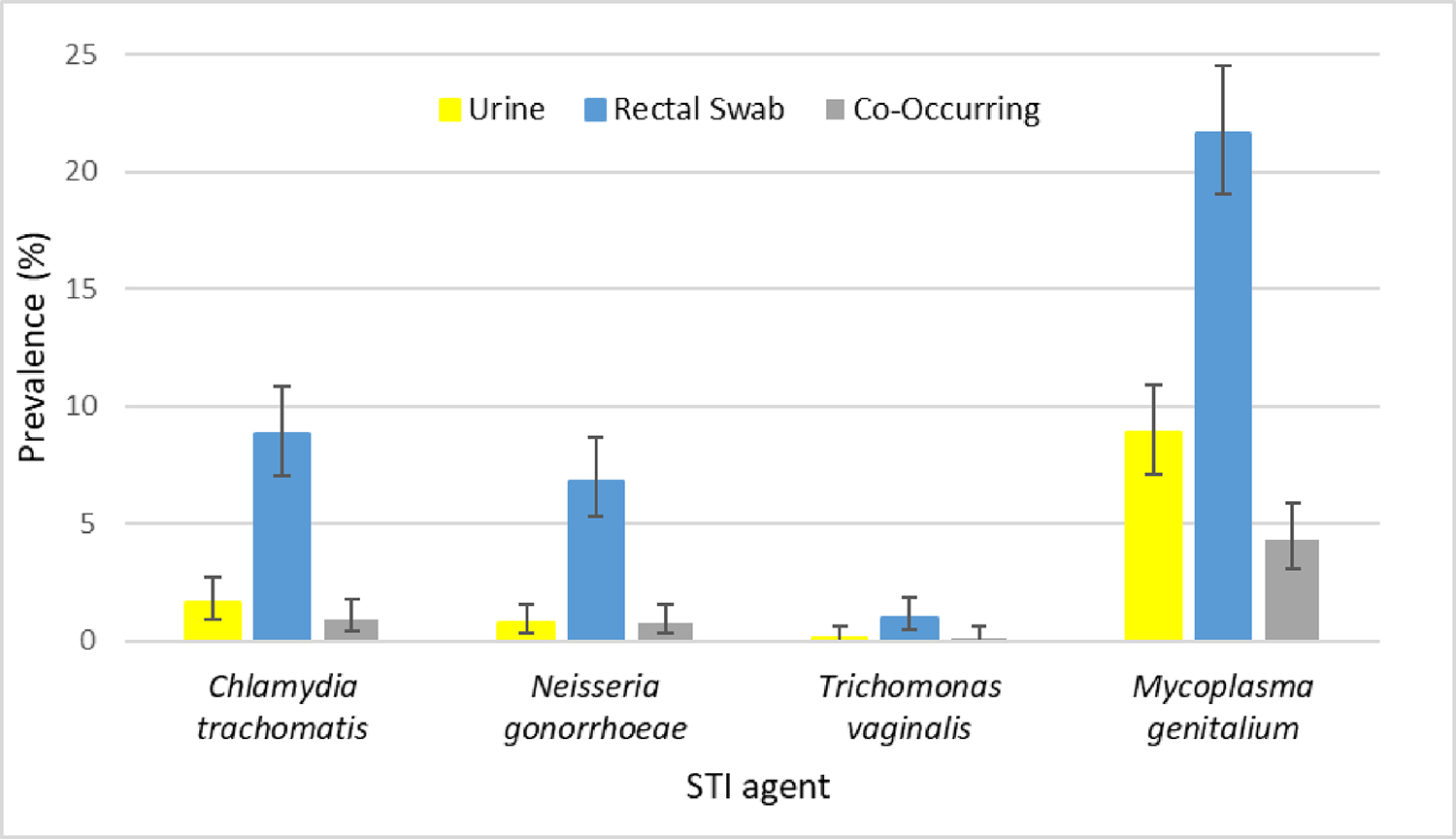
Baseline prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium infection, as detected by transcription-mediated amplification, from primary first-void urine (yellow bars), rectal swab (blue bars), and co-occurring (gray bars) specimens from 917 participants enrolled in the RADAR longitudinal cohort study.
Note: Co-occurring refers to the simultaneous detection of an infection in both the urine and rectal swab specimens. The thin black vertical lines overlaid on each bar represent the 95% confidence interval.
Six-month follow-up screening.
776 of the enrolled participants (84.6%) had evaluable C. trachomatis, N. gonorrhoeae, T. vaginalis, and M. genitalium urine results at six-month follow- up. 772 participants yielded evaluable rectal results for C. trachomatis (Web Reference 1), 770 participants had evaluable N. gonorrhoeae rectal results (Web Reference 2), while 773 participants had evaluable T. vaginalis and M. genitalium rectal results (Web Reference 3 and 4) at six-month follow-up.
Of 66 participants with a reactive C. trachomatis rRNA result from baseline rectal screening who subsequently presented for six-month follow-up, 13 (19.7%) maintained reactive status (Web Reference 1). In contrast, of 167 participants with a reactive M. genitalium rectal screen at baseline visit who presented for six-month follow-up, 103 (61.7%) retained reactive status (Web Reference 4). While urine screening demonstrated less prevalence than rectal screening for most STI agents, 20.0% of the C. trachomatis-reactive participants from baseline urine screening retained reactive status at six-month urine screening. 78.3% (54 of 69) of the participants reactive for M. genitalium rRNA at baseline urine screening who presented for six-month follow- up maintained reactive status.
Twelve-month follow-up screening.
677 of the enrolled participants (73.8%) had evaluable C. trachomatis, N. gonorrhoeae, and T. vaginalis urine results at twelve-month follow-up while 675 participants had evaluable M. genitalium urine results at twelve-month follow-up. 673 participants yielded evaluable rectal results for C. trachomatis (Web Reference 1), 674 participants had evaluable N. gonorrhoeae rectal results (Web Reference 2), while 676 of enrolled participants (73.7%) had evaluable T. vaginalis and M. genitalium rectal results (Web Reference 3 and 4) at twelve-month follow-up.
Of the 673 twelve-month follow-up visits involving C. trachomatis rectal screening, 52 of these participants yielded a reactive baseline C. trachomatis rRNA result. 7 of these 52 (13.5%) maintained reactive C. trachomatis rectal status at twelve-month follow-up (Web Reference 1). In contrast, nine participants presenting for a twelve-month follow-up had a baseline urine screen for C. trachomatis. None of these patients yielded a reactive C. trachomatis rRNA result upon additional screening. Of 676 twelve-month follow-up participants who self-collected a rectal specimen, 140 had previously yielded detectable M. genitalium rRNA during baseline screening (Web Reference 4). Of these 140 participants, 62 (44.3%) maintained reactive M. genitalium rectal status. Retention of reactive M. genitalium status was also common in the context of urine screening. From 57 twelve-month follow-up participants who demonstrated detectable M. genitalium rRNA at baseline urine screening, 40 (70.2%) yielded an additional reactive M. genitalium rRNA screening result.
Predictive value of a baseline STI agent first-void urine and rectal swab screen.
Data from web references 1–4 were the basis for calculation of odds ratios for repeat positives and for calculation of probability of survival data (Table 1). Participants that were reactive for C. trachomatis rRNA at baseline rectal and urine screening were 3.48 (95% CI: 1.77,6.84) and 31.46 (95% CI: 5.49,180.20) times more likely, respectively, to be reactive for the same agent at six-month follow-up. Similar findings were noted for N. gonorrhoeae screening. In contrast, detection of T. vaginalis or M. genitalium rRNA from baseline rectal screening presented a 18.85 (95% CI: 1.97,180.14) and 13.05 (95% CI: 8.72,19.53) times greater likelihood of repeat positive results at six-month follow-up. Baseline urine M. genitalium rRNA detection presented a 129.41 (95% CI: 62.27,268.93) times greater likelihood of urine M. genitalium detection at six-month follow-up screening.
TABLE 1:
Predictive value of a baseline reactive or non-reactive rectal swab or first-void urine screening result on subsequent six- and twelve-month follow-up screening results for four sexually-transmitted infection (STI) agents within the RADAR longitudinal cohort investigation.
| Specimen Source | STI Agent | Odds Ratioa | Survival Analysisb (%) | ||
|---|---|---|---|---|---|
| Six-month Follow-up (95% CI) | Twelve-month Follow-up (95% CI) | Six-month Follow-up (95% CI) | Twelve-month Follow-up (95% CI) | ||
| Rectal swab | Chlamydia trachomatis | 3.48 (1.77, 6.84) | 2.07 (0.88, 4.86) | 94.3 (92.2, 95.8) | 87.5 (84.3, 90.1) |
| Neisseria gonorrhoeae | 4.81 (2.29, 10.12) | 6.90 (3.14, 15.15) | 95.5 (93.7, 96.8) | 91.6 (88.9, 93.6) | |
| T richomonas vaginalis | 18.85 (1.97, 180.14) | 73.21 (9.26, 578.67) | 99.1 (98.1, 99.6) | 97.9 (96.4, 98.8) | |
| Mycoplasma genitalium | 13.05 (8.72, 19.53) | 5.33 (3.51, 8.11) | 90.4 (87.7, 92.5) | 81.0 (77.1, 84.3)c | |
| Urine | Chlamydia trachomatis | 31.46 (5.49, 180.20) | 0.00 (Undefined) | 99.2 (98.2, 99.6) | 97.2 (95.2, 98.4) |
| Neisseria gonorrhoeae | 42.28 (3.83, 466.74) | 0.00 (Undefined) | 99.6 (98.8, 99.9) | 98.9 (97.4, 99.5) | |
| Trichomonas vaginalis | Undefined | Undefined | 99.9 (99.1, 100.0) | 99.9 (99.1, 100.0) | |
| Mycoplasma genitalium | 129.41 (62.27, 268.93) | 69.88 (33.97, 143.78) | 97.7 (96.3, 98.6) | 95.8 (93.7, 97.2)d | |
Likelihood that a participant screening reactive at baseline encounter would yield a reactive screening result at six- or twelve-month follow-up
Probability (expressed as percentage) that a participant screening non-reactive at baseline encounter would yield a non-reactive screening result at six- or twelve-month follow-up
Survival rate for M. genitalium is significantly worse when compared to C. trachomatis (P = 0.004), N. gonorrhoeae (P < 0.0001), and T. vaginalis (P < 0.0001)
Survival rate for M. genitalium is significantly worse when compared to C. trachomatis (P = 0.023), N. gonorrhoeae (P = 0.001), and T. vaginalis (P < 0.0001)
With respect to twelve-month follow-up, antecedent reactive urine screening results were less predictive for C. trachomatis, N. gonorrhoeae, and T. vaginalis rRNA detection (Table 1) compared to M. genitalium. Participants with a baseline reactive urine M. genitalium rRNA screen were 69.88 (95% CI: 33.97,143.78) times more likely to yield a reactive urine M. genitalium result at twelve-month follow-up. When compared to six-month follow-up odds ratios, rectal twelve-month follow-up odds ratios were relatively consistent for C. trachomatis and N. gonorrhoeae; however, T. vaginalis saw approximately a four-fold increase. Predictability of a reactive baseline M. genitalium urine result for a subsequent twelve-month follow-up screening result decreased nearly two-fold.
Survival estimates (Table 1) were generated as probabilities (and expressed as percentages) that a participant screening non-reactive at baseline encounter would yield a non-reactive screening result at six- or twelve-month follow-up visits. Participants remaining non-reactive at follow-up visits demonstrated a consistent rank order of T. vaginalis, N. gonorrhoeae, C. trachomatis, and M. genitalium rRNA screening from highest to lowest (Table 1). In general, survival data derived from urine screening were increased over those relative to rectal analysis. For both specimen sources, the survival rates for M. genitalium rRNA detection were significantly worse than those for C. trachomatis (P(rectal)=0.004; P(urine)=0.023), N. gonorrhoeae (P(rectal)<0.0001; P(urine)=0.001), and T. vaginalis (P(rectal)<0.0001; P(urine)<0.0001).
Demographic and behavioral associations with STI agents.
Participant age, participant cisgender status, and the practices of condomless insertive anal/vaginal sex and condomless receptive anal sex demonstrated no association with rectal detection of C. trachomatis- (P≥0.10), N. gonorrhoeae- (P≥0.29), T. vaginalis- (P≥0.18), and M. genitalium-specific rRNA (P≥0.20; Table 2). However, M. genitalium rRNA was more commonly detected in rectal specimens of black/African American participants compared to non-black/African American participants (30.6% vs 17.0%; X2=22.39; P<0.0001). Similar findings were observed with rectal detection of N. gonorrhoeae rRNA more common among black/African Americans (9.6% vs 5.4%; X2=5.67; P=0.02). Regarding HIV serostatus, rectal M. genitalium rRNA detection was more common among HIV-seropositive compared to HIV-seronegative individuals (41.5% vs 16.3%; X2=57.72; P<0.0001). Similar HIV seropositivity associations, but to a lesser degree, were observed for rectal detection of C. trachomatis rRNA (X2=5.05; P=0.02) and N. gonorrhoeae rRNA (X2=32.03; P<0.0001; Table 2).
TABLE 2:
Demographic factors and sexual practices associated with transcription-mediated amplification-based rectal detection of Chlamydia trachomatis-, Neisseria gonorrhoeae-, Trichomonas vaginalis-, and Mycoplasma genitalium-specific rRNA collected at baseline within the RADAR longitudinal cohort study.
| Demographic/practice | Rectal C. trachomatis rRNA Detection | Rectal N. gonorrhoeae rRNA Detection | Rectal T. vaginalis rRNA Detection | Rectal M. genitalium rRNA Detection |
|---|---|---|---|---|
|
| ||||
| Mean age (± SD)a | ||||
| Agent detected | 23.9 ± 3.2 | 24.1 ± 3.2 | 25.0 ± 3.1 | 23.8 ± 3.1 |
| Agent not detected | 23.6 ± 3.1 [P = 0.43] | 23.6 ± 3.1 [P = 0.29] | 23.7 ± 3.1 [P = 0.18] | 23.6 ± 3.1 [P = 0.37] |
|
| ||||
| Gender (%)b | ||||
| Cisgender | 8.8 | 6.6 | 1.0 | 21.5 |
| Non-cisgender | 8.9 [X2=0.01; P=0.98] | 8.9 [X2=0.77; P=0.38] | 1.0 [X2=0.00; P=1.00] | 22.8 [X2=0.08; P=0.78] |
|
| ||||
| Race (%)b | ||||
| Black/African American | 9.3 | 9.6 | 1.9 | 30.6 |
| Non-black/African American | 8.6 [X2=0.13; P=0.72] | 5.4 [X2=5.67; P=0.02] | 0.5 [X2=4.13; P=0.07] | 17.0 [X2=22.39; P<.0001] |
|
| ||||
| HIV serostatus (%)b | ||||
| Positive | 12.9 | 15.9 | 2.0 | 41.5 |
| Negative | 7.7 [X2=5.05; P=0.02] | 4.4 [X2=32.03; P<.0001] | 0.7 [X2=2.82; P=0.11] | 16.3 [X2=57.72; P<.0001] |
|
| ||||
| Condomless insertive anal/vaginal sex (%)b | ||||
| Reported | 8.4 | 6.4 | 1.0 | 22.9 |
| Not reported | 9.2 [X2=0.22; P=0.64] | 7.2 [X2=0.19; P=0.66] | 1.0 [X2=0.01; P=1.00] | 20.7 [X2=0.65; P=0.42] |
|
| ||||
| Condomless receptive anal sex (%)b | ||||
| Reported | 10.5 | 7.2 | 1.0 | 23.6 |
| Not reported | 7.4 [X2=2.64; P=0.10] | 6.6 [X2=0.13; P=0.72] | 1.0 [X2=0.01; P=1.00] | 20.0 [X2=1.66; P=0.20] |
|
| ||||
Associations assessed using independent sample t-tests
Associations assessed using Chi-square tests. In cases in which expected cell counts were less than 5, Fisher’s exact P values are reported.
Similar associations were derived from urine screening for the four STI agents (Table 3), yet many of these associations were calculated as less significant than those observed with rectal screening. 15.7% of HIV-seropositive individuals provided a urine specimen reactive for M. genitalium rRNA, while 7.0% of HIV-seronegative participants were reactive for M. genitalium rRNA (X2=14.59; P<0.001). Detection of N. gonorrhoeae rRNA was more common in HIV-seropositive participants (X2=10.41; P<0.01). However, in contrast to significant C. trachomatis rRNA detection from rectal swab specimens of HIV-seropositive participants, detection of C. trachomatis rRNA from urine was not associated with HIV serostatus (P=1.00). Analogous to detection from rectal swab specimens, M. genitalium rRNA was detected from urine provided by 13.5% of black/African American participants, while this detection rate was 6.4% from non- black/African American participants (X2=12.99; P<0.001). C. trachomatis rRNA was also more frequently detected from urine of black/African American participants (X2=4.24; P=0.04), though significant detection of this agent from rectal swab specimens of black/African American participants was not observed. The converse was true for N. gonorrhoeae rRNA detection. M. genitalium rRNA detection in urine was associated with condomless insertive anal/vaginal sex (X2=3.79; P=0.05). Urine detection of M. genitalium rRNA within this longitudinal cohort was additionally associated with increased age (P<0.01; Table 3).
TABLE 3:
Demographic factors and sexual practices associated with transcription-mediated amplification-based first-void urine detection of Chlamydia trachomatis-, Neisseria gonorrhoeae-, Trichomonas vaginalis-, and Mycoplasma genitalium- specific rRNA within the RADAR longitudinal cohort study.
| Demographic/practice | Urine C. trachomatis rRNA Detection | Urine N. gonorrhoeae rRNA Detection | Urine T. vaginalis rRNA Detection | Urine M. genitalium rRNA Detection |
|---|---|---|---|---|
|
| ||||
| Mean age (± SD)a | ||||
| Agent detected | 23.4 ± 3.0 | 22.7 ± 3.7 | 24.8 | 24.6 ± 3.3 |
| Agent not detected | 23.7 ± 3.1 [P = 0.78] | 23.7 ± 3.1 [P = 0.41] | 23.7 ± 3.1 [P = 0.70] | 23.6 ± 3.0 [P < 0.01] |
|
| ||||
| Gender (%)b | ||||
| Cisgender | 1.7 | 0.7 | 0.1 | 9.5 |
| Non-cisgender | 1.0 [X2=0.31; P=1.00] | 1.0 [X2=0.07; P=0.57] | 0.0 [X2=0.13; P=1.00] | 3.9 [X2=3.49; P=0.06] |
|
| ||||
| Race (%)b | ||||
| Black/African American | 2.8 | 1.3 | 0.3 | 13.5 |
| Non-black/African American | 1.0 [X2=4.24; P=0.04] | 0.5 [X2=1.55; P=0.24] | 0.0 [X2=1.87; P=0.35] | 6.4 [X2=12.99; P<0.001] |
|
| ||||
| HIV serostatus (%)b | ||||
| Positive | 1.5 | 2.6 | 0.0 | 15.7 |
| Negative | 1.7 [X2=0.02; P=1.00] | 0.3 [X2=10.41; P<0.01] | 0.1 [X2=0.28; P=1.00] | 7.0 [X2=14.59; P<0.001] |
|
| ||||
| Condomless insertive anal/vaginal sex (%)b | 1.7 | 0.7 | 0.2 | 10.9 |
| Reported | 1.6 [X2=0.00; P=0.99] | 0.8 [X2=0.04; P=1.00] | 0.0 [X2=1.15; P=0.46] | 7.2 [X2=3.79; P=0.05] |
| Not reported | ||||
|
| ||||
| Condomless receptive anal sex (%)b | ||||
| Reported | 1.7 | 0.2 | 0.0 | 8.0 |
| Not reported | 1.6 [X2=0.00; P=0.99] | 1.2 [X2=2.95; P=0.13] | 0.2 [X2=0.87; P=1.00] | 9.6 [X2=0.73; P=0.39] |
Associations assessed using independent sample t-tests
Associations assessed using Chi-square tests. In cases in which expected cell counts were less than 5, Fisher’s exact P values are reported.
DISCUSSION
In this study among a diverse sample of YMSM and young transgender women in Chicago, we observed lower prevalence than other major metropolitan cities for rectal and urogenital N. gonorrhoeae and C. trachomatis infection.22 In contrast, a lower prevalence of T. vaginalis and a high prevalence of M. genitalium, either urogenital or rectal, was observed among the cohort. We also observed high continued reactive status for M. genitalium at 6- and 12-month follow- ups compared to baseline rectal and urine screenings. Participants reactive for any agent of STI at baseline were more likely to be reactive at subsequent 6-month follow-up; meanwhile, rectal swab follow-up ratios comparing 12-month to 6-month follow-up were relatively consistent for all agents except M. genitalium. M. genitalium was subsequently more common among black participants and those living with HIV compared to white and HIV-negative participants. C. trachomatis was also more common among black participants in urine, but not rectal samples. Similar, but more muted, results were observed among participants living with HIV when examining rates of C. trachomatis and N. gonorrhoeae. No significant differences in rates were noted when examining participant age, gender identity, sexual positioning, or condomless anal sex.
M. genitalium has been shown to be a common cause of undiagnosed nongonococcal urethritis23 with initial findings from Australia revealing that the agent primarily infected bisexual and heterosexual men.24 Recent work comparing M. genitalium infection rates in MSM to men who have sex with women (MSW) reported an overall increase from 5.2% to 12.8%, with rates now comparable between both risk groups.25 Moreover, the study documented higher rates of macrolide-resistant M. genitalium among MSM (89.7%) compared to MSW (50%). These findings are particularly salient as M. genitalium has been shown to have reduced macrolide susceptibility,25–27 and has been associated with high rates of persistent infection,28 although single-dose macrolides have demonstrated more efficacy than multi-dose doxycycline.29 Meanwhile among HIV-infected MSM in Alabama, high rates of macrolide resistance continued to be observed in addition to moderate rates of fluoroquinolone resistance,30 suggesting antibiotic-resistant M. genitalium is common to MSM in many countries.
Compared to past work among other samples, we continue to find high rates of M. genitalium infection among our cohort of YMSM as well as very high rates of persistent infection at 6- and 12-month follow-up, particularly when compared to other measured STI. This suggests that we may, in fact, be observing macrolide-resistant bacteria in our sample as well, although phenotypic or genotypic antibiotic resistance profiles were not measured. Taken together, the body of work among MSM suggests a stark increase in both incident13 and persistent M. genitalium infections--findings which may have implications for downstream HIV infection. Prior research has shown that HIV-negative MSM who utilized pre-exposure prophylaxis (PrEP) were twice as likely to be infected with M. genitalium and that consistent condom use was associated with a reduction in infection risk.27 Meanwhile, in another study among African women, M. gentialium was associated with a two-fold increase in HIV infection.31 Future research should aim to develop a better understanding of why M. genitalium infections seem to persist among MSM, whether this may be related to movement of the bacterium through networks of MSM, and why this population is more prone to macrolide-resistant infections.
While clinically-significant T. vaginalis infection in males has been reviewed,32 few reports discuss organism detection from male rectal swab specimens. Cosentino et al.10 documented a nearly 9% TMA detection rate of T. vaginalis nucleic acid from rectal swabs obtained from women and a 0.9% detection rate on those collected from males. The two positive male rectal swab specimen results were duplicated through use of an alternative TMA primer set. One study in MSM9 reported three positive T. vaginalis TMA results from 500 rectal swabs. One of these positive results was duplicated by repeat TMA analysis and all three swabs were negative by PCR. A report from South Africa33 described seven MSM with positive T. vaginalis PCR results from rectal swab specimens. Two of these patients presented with symptoms of proctitis, although one of these individuals had concomitant detection of N. gonorrhoeae and M. genitalium DNA from a urethral swab specimen. In a region of Africa severely affected by the HIV/AIDS epidemic, the detection rate of T. vaginalis by rectal swab PCR was 2.1%.34 All 26 rectal swab specimen detections of T. vaginalis rRNA in our study were confirmed by repeat testing; however, symptomatic status of participants in this investigation was not ascertained. Additional studies of this cohort can assist in investigations of both the clinical significance of rectal T. vaginalis rRNA detection and the potential cost-benefit of site-specific screening for this STI agent. Of particular importance would be the characterization of confirmed T. vaginalis proctitis and its delineation from potential deposit contamination in the context of recent receptive anal sex.
While we observed several important findings with regards to STI, persistent infections and associated risk behaviors, our findings should be viewed in the context of their limitations. First, we did not assess either treatment of STI or resistance mutations and thus were unable to assess whether persistent infection indeed represented repeat infection or acquisition from a different sexual partner. Second, it would have also benefited this analysis to have examined symptomatic status; future studies should incorporate this information into their work. Next, due to very low prevalence of both T. vaginalis and urethral STIs in the sample, confidence intervals for these estimates are extremely broad. Fourth, although self-report information on STI treatment was collected at follow-up visits, we determined this data lacked reliability and would be potentially misleading in the interpretation of our results, thus we have not reported it here.. Additionally, this sample was a community sample, as opposed to a probability sample. Therefore, findings may not be generalizable to the larger population of YMSM.
Even in the context of our limitations we have demonstrated very high infection rates of M. genitalium, low rates of T. vaginalis, and rates of C. trachomatis and N. gonorrhoeae comparable to MSM in other large cities. We also observed increased risk of STI for black participants and those living with HIV compared to non-black and HIV-negative participants, respectively, while noting no significant differences by age, gender identity, sexual positioning, or use of condoms. These findings, particularly those surround M. genitalium, warrant further investigation in order to better understand movement of the STI through the population of MSM as well as to avoid downstream STI-associated HIV infection.
Supplementary Material
Key Messages:
This longitudinal study characterized STI agents in a cohort of young MSM and young transgender women in Chicago (USA).
Rectal Mycoplasma genitalium detection was associated with black race, HIV seropositivity; factors related to age and sexual positioning produced no association.
Initial Trichomonas vaginalis rectal screening results were highly predictive of twelve- month T. vaginalis status.
Initial negative Mycoplasma genitalium screening result was less predictive of a 6- and 12-month screening result when compared to other STI agents.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute on Drug Abuse at the National Institutes of Health (U01DA036939, PI: Mustanski; F32DA046313, PI: Morgan). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The sponsor had no involvement in the conduct of the research or the preparation of the article. The authors would like to thank the entire RADAR research team, particularly Dr. Thomas Remble for overseeing the project. The authors also wish to thank Justin Franz, Peter Cleary, and Michael Pulte for assistance at the study sites and the RADAR participants for sharing their experiences with us.
Conflict of Interest and Source of Funding:
E. Munson has received honoraria and travel grants from Hologic, Incorporated. B. Mustanski has received consulting fees from Hologic, Incorporated. For the remaining authors, none were declared. This work was funded by grants from the National Institute on Drug Abuse (U01DA036939; F32DA046313). Hologic, Incorporated provided multi-test swab and urine collection kits, as well as testing reagents for the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The sponsor had no involvement in the conduct of the research.
REFERENCES
- 1.Ramchandani MS, Golden MR. Confronting rising STIs in the era of PrEP and treatment as prevention. Curr HIV/AIDS Rep 2019; 16:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley CF, Vaughan AS, Luisi N, et al. The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia. AIDS Res Hum Retroviruses 2015; 31:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustanski B, Ryan DT, Newcomb ME, et al. Very high HIV incidence and associated risk factors in a longitudinal cohort study of diverse adolescent and young adult men who have sex with men and transgender women. AIDS Behav 2020;24:1966–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis 2010; 37:771–776. [DOI] [PubMed] [Google Scholar]
- 5.Frankis J, Goodall L, Clutterbuck D, et al. Regular STI testing amongst men who have sex with men and use social media is suboptimal--a cross-sectional study. Int J STD AIDS 2017; 28:573–583. [DOI] [PubMed] [Google Scholar]
- 6.Garofalo R, Hotton AL, Kuhns LM. Incidence of HIV infection and sexually-transmitted infections and related risk factors among very young men who have sex with men. J Acquir Immune Defic Syndr 2016; 72:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustanski B, Feinstein BA, Madkins K, et al. Prevalence and risk factors for rectal and urethral sexually transmitted infections from self-collected samples among young men who have sex with men participating in the Keep It Up! 2.0 randomized controlled trial. Sex Transm Dis 2017; 44:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustanski B, Parsons JT, Sullivan PS, et al. Biomedical and behavioral outcomes of Keep It Up!: an eHealth HIV prevention program RCT. Am J Prev Med 55:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis SC, Kent CK, Klausner JD, et al. Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005–2006. Sex Transm Dis 2008; 35:797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosentino LA, Campbell T, Jett A, et al. Use of nucleic acid amplification testing for diagnosis of anorectal sexually transmitted infections. J Clin Microbiol 2012; 50:2005–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burd EM. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev 2010; 23:550–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR- 03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 13.Munson E, Reynoso A, Pass M, et al. Comprehensive molecular screening in a cohort of young men who have sex with men and transgender women: effect of additive rectal specimen source collection and analyte testing. Accepted with revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustanski B, Morgan E, D’Aquila R, et al. Individual and network factors associated with racial disparities in HIV among young men who have sex with men: results from the RADAR cohort study. J Acquir Immune Defic Syndr 2019; 80:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustanski B, Ryan DT, Hayford C, et al. Geographic and individual associations with PrEP stigma: results from the RADAR cohort of diverse young men who have sex with men and transgender women. AIDS Behav 2018; 22:3044–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention and Association of Public Health Laboratories. Technical update on HIV-1/2 differentiation assays. 2016; https://stacks.cdc.gov/view/cdc/40790. Accessed 11 April 2017.
- 17.Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecular assessment: a retrospective analysis. J Clin Microbiol 2017; 55:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napierala M, Munson E, Wenten D, et al. Detection of Mycoplasma genitalium from male primary urine specimens: an epidemiologic dichotomy with Trichomonas vaginalis. Diagn Microbiol Infect Dis 2015; 82:194–198. [DOI] [PubMed] [Google Scholar]
- 19.Munson KL, Napierala M, Munson E, et al. Screening of male patients for Trichomonas vaginalis with transcription-mediated amplification in a community with a high prevalence of sexually transmitted infection. J Clin Microbiol 2013; 51:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wroblewski JK, Manhart LE, Dickey KA, et al. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J Clin Microbiol 2006; 44:3306–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huppert JS, Mortensen JE, Reed JL, et al. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018. Atlanta: US: Centers for Disease Control and Prevention, 2019. [Google Scholar]
- 23.Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2013; 58:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mezzini TM, Waddell RG, Douglas RJ, et al. Mycoplasma genitalium: prevalence in men presenting with urethritis to a South Australian public sexual health clinic. Intern Med J 2013; 43:494–500. [DOI] [PubMed] [Google Scholar]
- 25.McIver R, Jalocon D, McNulty A, et al. Men who have sex with men with Mycoplasma genitalium-positive nongonococcal urethritis are more likely to have macrolide-resistant strains than men with only female partners: a prospective study. Sex Transm Dis 2019; 46:513–517. [DOI] [PubMed] [Google Scholar]
- 26.Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis 2006; 12:1149–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couldwell DL, Jalocon D, Power M, et al. Mycoplasma genitalium: high prevalence of resistance to macrolides and frequent anorectal infection in men who have sex with men in western Sydney. Sex Transm Infect 2018; 94:406–410. [DOI] [PubMed] [Google Scholar]
- 28.Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One 2008; 3:e3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mena LA, Mroczkowski TF, Nsuami M, et al. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium–positive urethritis in men. Clin Infect Dis 2009; 48:1649–1654. [DOI] [PubMed] [Google Scholar]
- 30.Dionne-Odom J, Geisler WM, Aaron KJ, et al. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in Alabama. Clin Infect Dis 2017; 66:796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 23:611–620. [DOI] [PubMed] [Google Scholar]
- 32.Meites E, Gaydos CA, Hobbs MM, et al. A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin Infect Dis 2015; 61(suppl 8):S837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman CM, Fritz L, Radebe O, et al. Rectal Trichomonas vaginalis infection in South African men who have sex with men. Int J STD AIDS 2018; 29:1444–1447. [DOI] [PubMed] [Google Scholar]
- 34.Vuylsteke B, Semde G, Sika L, et al. High prevalence of HIV and sexually transmitted infections among male sex workers in Abidjan, Cote d’Ivoire: need for services tailored to their needs. Sex Transm Infect 2012; 88:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


