The mechanism underlying the pathogenesis of endometrial polyps (EP) remains unclear. Endocrinopathy can be corrected by counteracting the effect of oestrogenic stimulation with progesterone, and systemic administration of pregestational agents was shown to block the effect of estrogenic stimulation and reverse the process of excessive endometrial growth [1]. Progesterone is an essential factor involved in the development of endometrial receptivity, and it maintains the homeostasis of the female endocrine system by blocking the synthesis of gonadotropin in the hypothalamus [2]. Meanwhile, the expression of miR-320 is down-regulated in patients with breast cancer [3]. By increasing the expression of miR-320, progesterone may block the infiltration of cells from the trophectoderm and uterus.
Progesterone has been shown to affect the expression of multiple miRNAs [4]. In addition, MCL1 was found to be involved in the control of proliferation of endometrial cells [5]. In this study, we probed the effect of progesterone on the expression of miR-320b and its target gene, MCL1, during the development of EP.
In this study, we enrolled EP patients as the case group (n = 20), and normal healthy participants as the control group (n = 20). EP samples were collected from the case group, while 20 samples of normal endometrium were collected from the control group. The protocols of the study were approved by the Institutional Ethics Committee, and all protocols strictly followed the latest version of the Declaration of Helsinki. Written informed consent was obtained from each participant before this study.
MicroRNA microarray assays were performed to identify potential miRNAs involved in the development of EP. In addition, real-time PCR, Western-blot analysis, and IHC assay were performed to compare the expressions of miR-320b, progesterone receptors, and MCL1 between the EP and control groups. Subsequently, MTT assay and flow cytometry analysis were performed to determine the effect of progesterone on cell proliferation and apoptosis. Computational analysis and luciferase assays were performed to further clarify the potential molecular mechanism of miR-320b in EP.
RL95-2 and HEC-1-A cells were cultured and seeded into 96-well plates at a density of 1 × 104 cells/well and incubated overnight for preparation. To test the effect of progesterone at different concentrations, the cells were treated with 0.5, 1, or 2 μM of progesterone for 24 h before evaluating the cellular expressions of miR-320b, progesterone receptor, and MCL1. All experiments were repeated at least 3 times.
The differences in the results from 2 groups were compared by Student’s t tests. The measurement data were presented as mean ± standard deviation. All statistical analysis was performed using GraphPad Prism 7.00 (GraphPad Software, La Jolla, CA). A p-value of < 0.05 was considered statistically significant.
The demographic and clinicopathological characteristics of all participants were collected, and they are summarized in Table I. The results showed that the 2 groups had no obvious differences in terms of the above characteristics.
Table I.
Demographic and clinical data of the study participants
| Parameter | Endometrial polyp (n = 20) | Normal endometrium (n = 20) | P-value |
|---|---|---|---|
| Menopausal status: | 0.847 | ||
| Menopause | 12 (60%) | 11 (55%) | |
| Premenopause | 8 (40% | 9 (45%) | |
| Hypertension: | 0.625 | ||
| Yes | 11 (55%) | 12 (60%) | |
| No | 9 (45%) | 8 (40% | |
| Diabetes mellitus: | 0.715 | ||
| Yes | 10 (50%) | 11 (55%) | |
| No | 10 (50%) | 9 (45%) | |
| BMI | 23.21 ±3.5 | 24.32 ±5.43 | 0.785 |
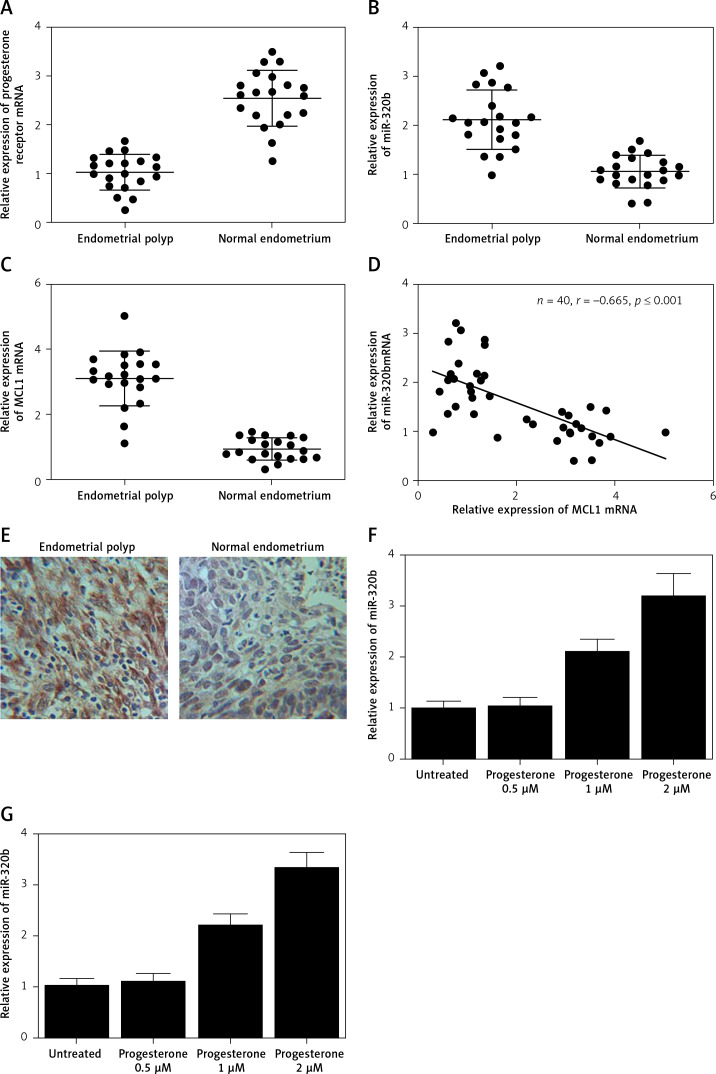
As shown in Supplementary Figure S1, only miR-320b among other miRNAs was evidently down-regulated in EP patients compared to that in the control group. We also found that the EP group showed lower levels of progesterone receptor mRNA (Figure 1 A) and miR-320b (Figure 1 B) along with higher levels of MCL1 mRNA (Figure 1 C) and protein (Figure 1 E). Also, a negative regulatory relationship with a correlation coefficient of –0.5000 (r = –0.5000) was confirmed between the expression of miR-320b and MCL1 (Figure 1 F). These results suggested that progesterone receptors and miR-320b are involved in the development of EP by negatively regulating the expression of MCL1.
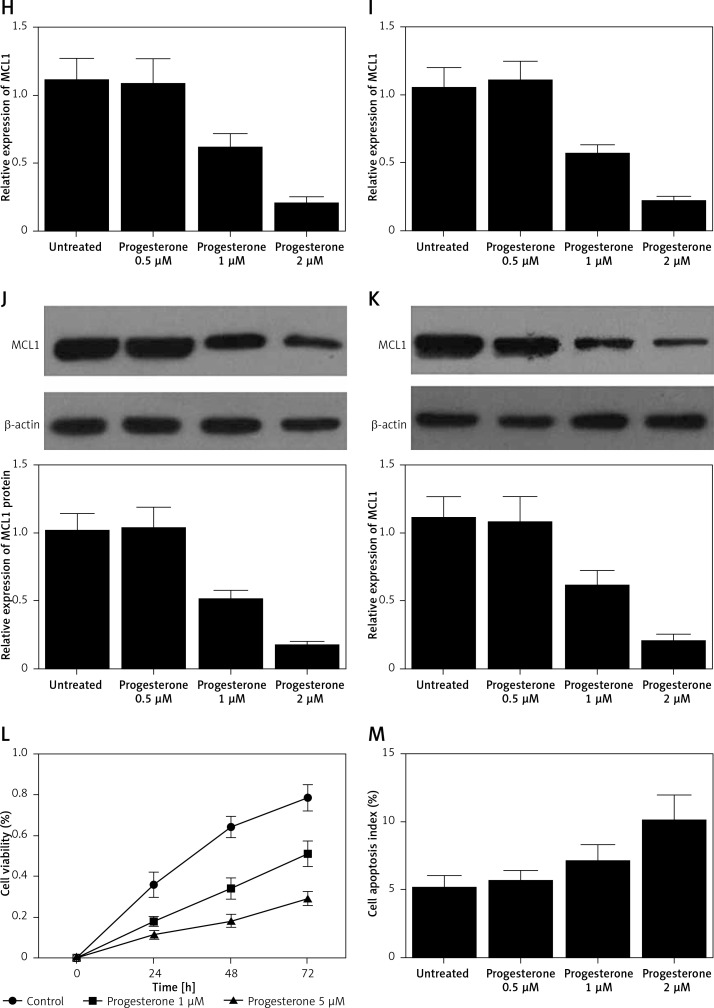
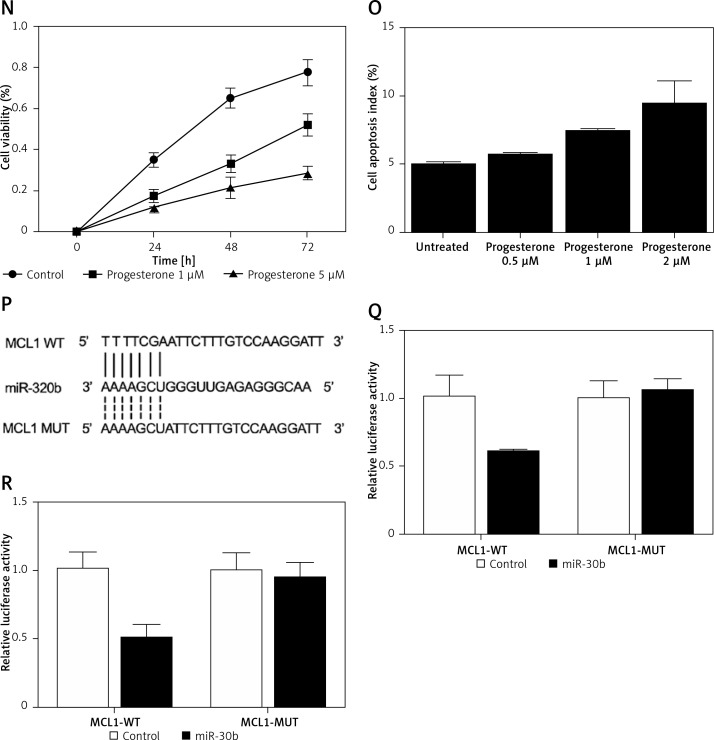
Figure 1.
A – mRNA level of progesterone receptor in the EP group was much lower than that in the control group. B – MiR-320b expression in the EP group was much lower than that in the control group. C – mRNA level of MCL1 in the EP group was much lower than that in the control group. D – A negative regulatory relationship with a correlation coefficient of –0.5000 was confirmed between miR-320b and MCL1 expression. E – The protein level of MCL1 in the EP group was much higher than that in the control group. F – 0.5 μM of progesterone exerted no effect on the expression of miR-320b, while 1 and 2 μM of progesterone increased miR-320b expression in RL95-2 cells in a dose-dependent manner. G – 0.5 μM of progesterone exerted no effect on the expression of miR-320b, while 1 and 2 μM of progesterone increased miR-320b expression in HEC-1-A cells in a dose-dependent manner. H – 0.5 μM of progesterone exerted no effect on the expression of MCL1 mRNA, while 1 and 2 μM of progesterone decreased MCL1 mRNA expression in RL95-2 cells in a dose-dependent manner. I – 0.5 μM of progesterone exerted no effect on the expression of MCL1 mRNA, while 1 and 2 μM of progesterone decreased MCL1 mRNA expression in HEC-1-A cells in a dose-dependent manner. J – 0.5 μM of progesterone exerted no effect on the expression of MCL1 protein, while 1 and 2 μM of progesterone decreased MCL1 protein expression in RL95-2 cells in a dose-dependent manner. K – 0.5 μM of progesterone exerted no effect on the expression of MCL1 protein, while 1 and 2 μM of progesterone decreased MCL1 protein expression in HEC-1-A cells in a dose-dependent manner. L – Progesterone (1, 2 μM) dose-dependently inhibited the proliferation of RL95-2 cells. M – Progesterone (1, 2 μM) dose-dependently inhibited the proliferation of HEC-1-A cells. N – Progesterone (1, 2 μM) dose-dependently promoted the apoptosis of RL95-2 cells. O – Progesterone (1, 2 μM) dose-dependently promoted the apoptosis of HEC-1-A cells. P – MCL1 was identified as a target gene of miR-320b with a ‘seed sequence’ located in the 3’UTR of MCL1. Q – Luciferase activity of RL95-2 cells co-transfected with miR-320b mimics and wild-type MCL1 3’UTR was much lower than that of the cells co-transfected with miR-320b mimics and a scramble control. R – Luciferase activity of HEC-1-A cells co-transfected with miR-320b mimics and wild-type MCL1 3’UTR was much lower than that of the cells co-transfected with miR-320b mimics and a scramble control
Progesterone at different doses (0.5 μM, 1 μM, 2 μM) were used to treat RL95-2 and HEC-1-A cells before the levels of miR-320b and MCL1 expression in treated cells were measured using real-time PCR and Western-blot analysis. Moreover, among the various progesterone doses (0.5 μM, 1 μM, 2 μM) used for cell treatment, only 1 μM and 2 μM progesterone evidently increased the expression of miR-320b (Figures 1 F and 1 G) and suppressed the mRNA (Figures 1 H and 1 I) and protein (Figures 1 J and 1 K) levels of MCL1 in RL95-2 (Figures 1 H and 1 J) and HEC-1-A (Figures 1 I and 1 K) in an accordingly stepwise manner. Also, both 1 μM and 2 μM progesterone dose-dependently inhibited the proliferation of RL95-2 (Figure 1 L) and HEC-1-A (Figure 1 N) cells while promoting their apoptosis (Figure 1 M and 1 O).
We searched an online microRNA database (www.mirdb.org) and identified MCL1 as a potential target gene of miR-320b with a binding site located in MCL1 3’UTR (Figure 1 P). Subsequently, as shown in Figure 1Q and 1R, the transfection of miR-320b significantly reduced the luciferase activity of wild-type MCL1 3’UTR but not that of mutant MCL1 3’UTR in RL95-2 (Figure 1 Q) and HEC-1-A (Figure 1 R) cells.
In the glandular tissues of the endometrial epithelium, the presence of oestrogen can prolong the survival and proliferation of stem cells as well as the migration of daughter cells [6]. Also, progesterone was shown to inhibit the effect of oestrogen by promoting the synthesis of inhibitory factors [7]. In this study, we recruited both EP patients and normal healthy subjects to compare their expression of 13 different miRNAs. The results showed that only the expression of miR-320b was lower in the subjects with EP. We also found that the EP group was associated with much lower expression of progesterone receptor and miR-320b, along with a much higher level of MCL1. In addition, a negative regulatory relationship with a correlation coefficient of –0.5000 was confirmed between the expression of miR-320b and MCL1.
Progesterone can inhibit the expression of ER genes while promoting ER protein degradation [8]. Furthermore, the downregulation of cadherin 6 and other adhesion molecules by progesterone was shown to inhibit cancer cell proliferation and invasion. It was also shown that a reduced level of miR-320 can increase the level of Mcl-1 expression in cervical cancer. In this study, we found that progesterone dose-dependently increased the expression of miR-320b while decreasing the expression of MCL1 in RL95-2 and HEC-1-A cells. As a member of the Bcl-2 family, Mcl-1 exerts anti-apoptotic effects by activating many pathways involved in cell survival [9]. Therefore, Mcl-1 is often over-expressed in human cancers and can protect cancer cells against apoptosis. In this study, we demonstrated that progesterone inhibited cell proliferation while promoting their apoptosis.
Our study demonstrates that progesterone can regulate the expression of multiple miRNAs. Our findings shed light on the complex regulatory mechanisms involved in endometrial cell function, thus potentially contributing to the development of novel therapeutic approaches for various endometrial disorders and reproductive health issues. Also, our study identifies MCL1 as being involved in the control of endometrial cell proliferation, which provides new insights into targeted therapies to control aberrant cell growth or treat endometrial pathologies by inhibiting MCL1 expression. However, there are limitations to our study. The sample number of participants in our study was relatively small, which could limit the statistical power and generalizability of our study. In our future study, a broader population will be recruited to increase the representativeness of our future findings. Also, in our future study, we will investigate the association between progesterone usage and the formation of endometrial polyps, as well as the effect of progesterone on the existing polyp size.
In conclusion, the findings of this study demonstrate that progesterone treatment could attenuate proliferation of EP by regulating the expression of multiple genes such as MCL1 and miR-320b.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
References
- 1.Yu K, Huang ZY, Xu XL, Li J, Fu XW, Deng SL. Estrogen receptor function: impact on the human endometrium. Front Endocrinol 2022; 13: 827724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JX, Lin S, Kong SB. Psychological Stress and functional endometrial disorders: update of mechanism insights. Front Endocrinol 2021; 12: 690255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Wang JG, Zhang L, et al. MicroRNA-320a inhibits breast cancer metastasis by targeting metadherin. Oncotarget 2016; 7: 38612-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CY, Hsieh TH, Tsai CF, et al. Synthetic steroid hormones regulated cell proliferation through micro-RNA-34a-5p in human ovarian endometrioma. Biol Reprod 2016; 94: 60. [DOI] [PubMed] [Google Scholar]
- 5.Bolomsky A, Vogler M, Köse MC, et al. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J Hematol Oncol 2020; 13: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousins FL, Filby CE, Gargett CE. Endometrial stem/progenitor cells-their role in endometrial repair and regeneration. Front Reprod Health 2021; 3: 811537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedotcheva TA, Fedotcheva NI, Shimanovsky NL. Progesterone as an anti-inflammatory drug and immunomodulator: new aspects in hormonal regulation of the inflammation. Biomolecules 2022; 12: 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter KR, Balko JM, Hagan CR. Progesterone receptor promotes degradation of STAT2 to inhibit the interferon response in breast cancer. Oncoimmunology 2020; 9: 1758547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian S, Wei Z, Yang W, Huang J, Yang Y, Wang J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol 2022; 12: 985363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.