Abstract
By using a low-resolution macrorestriction map as the foundation (R. Majumder et al., J. Bacteriol. 176:1105–1112, 1996), an ordered cloned DNA map of the 3.2-Mb chromosome of the hypertoxinogenic strain 569B of Vibrio cholerae has been constructed. A cosmid library the size of about 4,000 clones containing more than 120 Mb of V. cholerae genomic DNA (40-genome equivalent) was generated. By combining landmark analysis and chromosome walking, the cosmid clones were assembled into 13 contigs covering about 90% of the V. cholerae genome. A total of 92 cosmid clones were assigned to the genome and to regions defined by NotI, SfiI, and CeuI macrorestriction maps. Twenty-seven cloned genes, 9 rrn operons, and 10 copies of a repetitive DNA sequence (IS1004) have been positioned on the ordered cloned DNA map.
Vibrio cholerae, a noninvasive gram-negative bacterium and the causative agent of the diarrheal disease cholera, is serologically classified as belonging to the O antigenic group. Strains belonging to O group 1 (O1) are responsible for cholera. Strains other than O1 are called non-O1; they can cause only sporadic infections and do not have the potential to cause epidemics (31). Strains of serovar O1 consist of two biotypes, classical and El Tor. Only recently, an outbreak of cholera in India and Bangladesh which subsequently spread into several parts of the subcontinent was caused by a novel non-O1 strain, O139 Bengal (36). However, several pieces of evidence suggested that strain O139 Bengal closely resembles biotype El Tor of the serovar O1 (5, 43).
Construction of genetic maps is restricted to organisms for which genetic tools are available and experimental genetic transfers are feasible. Although a great deal is known about the biochemistry, physiology, and clinical microbiology of V. cholerae (23), the genetic analysis of this organism has been hindered, primarily because of the lack of demonstrable genetic exchange systems. There is no transducing phage of V. cholerae, and transformation of these cells by plasmid DNA only has been demonstrated (34). Conjugation is mediated by a factor, P (6), which unlike the F factor of Escherichia coli cannot integrate into the chromosome and hence cannot induce Hfr donors. Thus, the mobilization of chromosomal DNA is limited in this organism. The alternative to examining the organization of genomes in organisms for which a genetic map is not available is to construct a physical map which will allow the examination of the phylogenetic relationship between organisms and the variations of genome structure between different serovars and biotypes. Even for organisms with well-defined genetic maps, physical methods can provide additional details like the orientation of genes, rearrangements within a genome, acquisition of DNA from other organisms, and mapping of any sequence which can be used as a probe. A combined genetic and physical map of the 3.2-Mb genome of the classical O1 hypertoxigenic strain 569B (38) has recently been constructed by using the enzymes NotI (29), CeuI (32), and SfiI (unpublished observation). The availability of the macrorestriction map enabled examination of the organization of the genomes of V. cholerae strains belonging to different serovars and biotypes. One of the unique observations was intraspecies variation in the number of rrn operons in vibrios. Strains belonging to serovars O1 and O139 have 9 rrn operons, and those belonging to non-O1/non-O139 have 10 rrn operons (32). Genomes of V. cholerae strains belonging to different serovars and biovars, and particularly those of the pathogenic strains, are undergoing rapid rearrangements and exhibit extensive restriction fragment length polymorphism in the CTX genetic element locus (5). While the linkage maps are conserved within biovars, they vary substantially between biovars (32).
The macrorestriction maps are of relatively low resolution and permit detection of gross chromosomal aberrations, and they allow qualitative evaluation of intraspecies genetic variations and identification of individual isolates of a species by comparison of their macrorestriction patterns. The ordered cloned DNA map of the genome generated from a set of overlapping phage or cosmid clones that cover the whole genome, on the other hand, has much greater potential as a tool to study genome structure and reshuffling of genes (14, 20). The phage or cosmid libraries provide a readily renewable source of DNA, which is important particularly for pathogenic microbes like V. cholerae. The ordered cloned DNA map also provides direct access to a given chromosomal locus, permitting surrogate genetics (14) to be conducted, leading to the identification of virulence determinant genes and protective antigens. The ordered cloned DNA library can be used to examine the modulation of transcription of sets of genes that are specifically expressed following exposure to environmental fluctuations (13, 41). A functional description of the bacterial genome can be extended to the protein level by cloning the DNA insert from each cosmid clone into a suitable vector from which controlled expression can be achieved (40). Ordered cloned DNA maps have been constructed for the genomes of relatively few organisms, such as E. coli (26), Mycoplasma pneumonia (44), Desulfovibrio vulgaris (15) Haloferax volcanii (11), Mycobacterium leprae (16), Bacillus subtilis (1), Helicobacter pylori (9), Myxococcus xanthus (21), and Rhodobacter capsulatus (19). The present report describes the construction of an overlapping cloned DNA map of the genome of V. cholerae 569B, done by using the low-resolution macrorestriction map as the foundation. Twenty-seven homologous and heterologous genes, 9 rrn operons, and 10 copies of a repetitive DNA sequence, IS1004, have been positioned on the map.
MATERIALS AND METHODS
Construction of cosmid library.
The V. cholerae 569B used in this study was obtained from the National Institute of Cholera and Enteric Diseases, Calcutta, India. V. cholerae cells were grown in a gyratory shaker at 37°C in nutrient broth (NB) containing 0.1 M NaCl (pH 8.0) and maintained as described previously (12, 28, 37). Genomic DNA was prepared by the method of Wilson (45). Five micrograms of genomic DNA was partially digested with MluI and size fractionated in 0.9% low-melting-point (GTG) agarose (FMC, Rockland, Maine). DNA from the 30- to 45-kb region was eluted from the gel, extracted with phenol-chloroform, and ethanol precipitated. The precipitate was dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8]) and preserved at a final concentration of 200 ng/ml at 4°C.
The cosmid Lorist M, having the phage λ origin of replication (obtained from R. L. Charlebois, University of Ottawa, Ottawa, Ontario, Canada) was used for the construction of the library. About 5 μg of the cosmid DNA was digested with MluI, dephosphorylated by using calf intestinal phosphatase (New England Biolabs, Beverly, Mass.), ethanol precipitated, washed with 70% ethanol, and dissolved in 5 μl of TE. Two micrograms of size-fractionated genomic DNA was ligated to 5 μg of vector DNA by using 1 U of T4 DNA ligase (Boehringer Mannheim, Indianapolis, Ind.) in a final volume of 20 μl at 16°C for 16 h. The ligation mixture was diluted to 100 μl with SM buffer (0.58% NaCl, 0.2% MgSO4, 100 mM Tris-HCl, 2% gelatin [pH 7.5]) and packaged with phage λ packaging extract prepared from E. coli BHB 2688 and BHB 2690 cells (22). The packaged phage particles were absorbed for 30 min at 37°C to E. coli ED8767 cells grown to logarithmic phase in terrific broth (TB) containing 1.2% tryptone, 2.4% yeast extract, and 0.4% glycerol and spread on TB agar plates containing 30 μg of kanamycin sulfate per ml. About 4,000 recombinant clones were picked and grown overnight at 37°C in 96-well microtiter plates containing 200 μl of TB containing kanamycin sulfate. Ninety microliters of 50% glycerol was added, and the mixture was stored at −70°C. Cosmid clones were divided into three batches (A, B, and C) each having about 1,350 clones and were numbered A1 to A1350 for batch A, and so on.
Grouping of cosmid clones.
Restriction fragment-specific cosmid clones were identified by hybridizing clones with different NotI, CeuI, and SfiI fragments of V. cholerae genomic DNA. Batches of 500 cosmid clones were grown on Hybond nylon membrane (Amersham, Amersham, England), and colony blot hybridizations were performed by using restriction fragments, labelled by random priming (18), as probes. Hybridization was carried out at 60°C for 12 h, and filters were washed at the desired stringency, dried, and autoradiographed.
Landmark analysis and chromosome walking.
The enzymes BamHI, SalI, StuI, and NcoI, having on average one site per 50 kb of V. cholerae genomic DNA, were chosen as rare-cutter enzymes for landmark analysis. Ten microliters of DNA digested with 2.5 U of MluI and 2.5 U of one of the rare-cutter enzymes in a 20-μl volume at 37°C for 4 to 5 h was loaded on a 45-well 0.9% agarose gel (23 by 25 cm) and electrophoresed at 4°C for 18 h. The overlapping clones were identified manually by analyzing the restriction digestion profiles of cosmid clones. For chromosome walking, RNA probes of the terminal clones of the desired contig were prepared from T7 and SP6 promoters by using a Promega kit (Promega Corp., Southampton, United Kingdom) and hybridized with DNA by dot blotting or colony blotting to obtain candidate extenders.
RESULTS
Construction of cosmid library.
A cosmid library of the genome of the hypertoxinogenic strain 569B of V. cholerae was constructed by cloning genomic DNA partially digested with the enzyme MluI into the cosmid vector Lorist M. Among the enzymes tested to generate genomic DNA fragments, MluI was chosen as the cloning enzyme because it did not produce fragments larger than 25 kb. The optimal conditions for partial digestion of the genomic DNA with MluI were established by digesting DNA with various amounts of enzyme and for different times to generate DNA fragments between 30 and 45 kb. The size of the library was about 4,000 clones carrying inserts of >35 kb, which contained more than 120 Mb of V. cholerae DNA (40-genome equivalent).
Grouping of cosmids into subsets.
By taking advantage of the macrorestriction maps of the V. cholerae 569B genome, the clones of the cosmid library were grouped into subsets. Batches of about 500 clones from the library were transferred onto nylon filters and hybridized with labelled NotI, SfiI, or CeuI fragments of V. cholerae genome separated by pulsed-field gel electrophoresis (PFGE). The fragments that are clearly resolved in PFGE and can be eluted from the gel without contamination by adjacent fragments were used for grouping the clones (Table 1). The number of clones belonging to any particular restriction fragment was sufficient to cover at least five times the size of the fragment. Of 37 NotI (29) and 9 CeuI (32) fragments of the V. cholerae genome, the NotI fragments N1, N2, N4, N7, N8, N12, and N13, covering about 43% of the genome, and the CeuI fragments C3 to C8, covering another 42% of the genome, were used for grouping the clones. Another 8% of the genome was covered by SfiI fragments S2 and S8. The ambiguities arising from clones hybridizing with more than one restriction fragment due to the presence of internal repeat sequences were resolved by hybridizing NotI-digested genomic DNA with riboprobes prepared from the ends of inserts of these cosmid clones. Altogether, 1,065 of 4,000 cosmid clones were used in subsequent analysis. In each group, identical clones were eliminated by digestion with three restriction enzymes and one representative clone was used for further studies. This allowed the reduction of the number of clones for contig assembly to 665.
TABLE 1.
Grouping of clones
| Fragment name | Size (kb) | No. of colonies screened | No. of colonies hybridized |
|---|---|---|---|
| N1 | 364 | 2,400 | 140 |
| N2 | 324 | 1,000 | 240 |
| N4 | 189 | 500 | 80 |
| N7 | 166 | 500 | 80 |
| N12, N13 | 112, 106 | 500 | 60 |
| S2 | 296 | 500 | 60 |
| C3 | 325 | 500 | 45 |
| C4 | 275 | 500 | 65 |
| C5 | 180 | 500 | 60 |
| C6 | 120 | 400 | 40 |
| C7 | 78 | 300 | 35 |
| C8 | 72 | 300 | 40 |
| N8, S8 | 150, 175 | 300 | 120 |
Contig assembly.
To generate contigs, overlapping cosmid clones were identified primarily by landmark analysis (10). This involves comparison of gel patterns of different clones digested with the cloning enzyme and the double digest of the cloning enzyme and a rare-cutting enzyme. Restriction enzymes having on average one site per 5 kb in the genome are normally used as the cloning enzymes so that the complete digestion of the cloned DNA yields about six to eight fragments. The second enzyme selected for landmark analysis should have on average one site per 50 kb. Thus, among the several fragments produced following complete digestion of the cloned DNA by the cloning enzyme, at least one will have a site for the second enzyme. This fragment will disappear following digestion with the second enzyme, producing new fragments. If two cosmid clones are overlapping, the common bands produced on complete digestion with the cloning enzyme will disappear upon digestion with the second enzyme and reappear as equal-sized fragments in both the clones.
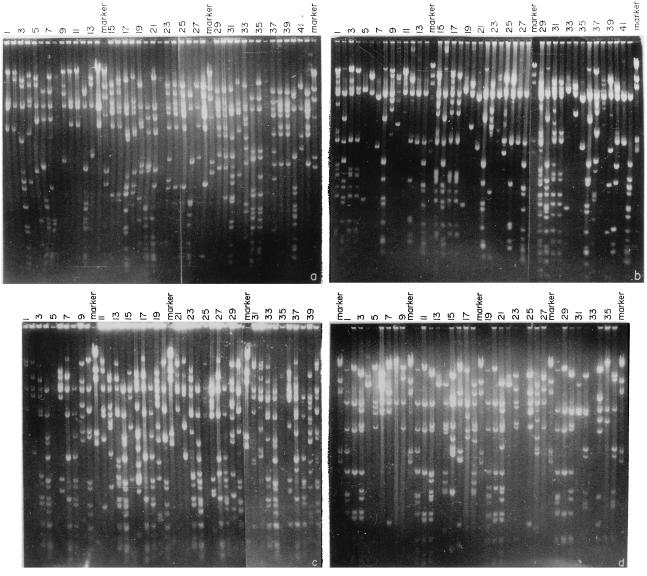
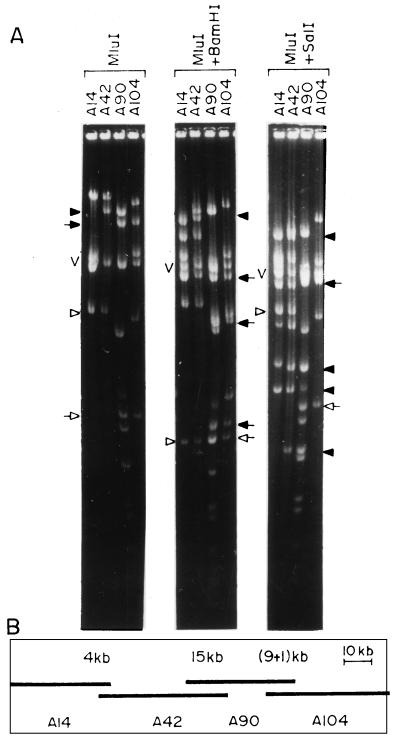
In the present study, MluI was chosen as the cloning enzyme and BamHI, SalI, StuI, and NcoI were chosen as rare-cutting enzymes. Cosmid clones from different groups, selected randomly, were subjected to landmark analysis to generate contigs. About 50 cosmid clones from any particular group were digested with MluI and with MluI and one of the rare-cutting enzymes, and fragments were separated in agarose gels (Fig. 1). Any two clones having at least one MluI fragment in common which disappears following digestion with any of the second enzymes are overlapping clones, and the disappearing common fragment is the landmark and is a measure of the extent of the overlap. For example, the clones A42 and A90 have a 15-kb common fragment following MluI digestion (Fig. 2A). When these clones were digested with MluI and SalI, the common 15-kb fragment was cleaved, producing four fragments of 9.3, 2.4, 2.1, and 1.2 kb (Fig. 2A). Thus, the 15-kb fragment is a landmark and the clones A42 and A90 have an overlap of 15 kb. Similarly, a comparison of the MluI, MluI-plus-BamHI and MluI, MluI-plus-SalI digestion profiles of the clones A14 and A42 (Fig. 2A) showed that these two clones have a 4-kb overlap. Cosmid clones A90 and A104 (Fig. 2A) have two landmarks of 9 and 1 kb and hence have a 10-kb overlap. Thus, from the landmark analysis of four clones, a contig of A14, A42, A90, and A104 was assembled (Fig. 2B). More than 80% of the overlaps were determined by using the landmark strategy alone.
FIG. 1.
Landmark analysis of cosmids for identifying overlapping clones. (a and b) Digestion patterns of cosmid clones with MluI (lanes 1 to 14), MluI plus BamHI (lanes 15 to 28), and MluI plus SalI (lanes 29 to 42). (c and d) Digestion patterns of cosmid clones with MluI (lanes 1 to 10), MluI plus BamHI (lanes 11 to 20), MluI plus SalI (lanes 21 to 30), and MluI plus StuI (lanes 31 to 40).
FIG. 2.
Identification of overlapping clones and contig assembly by landmark analysis. (A) MluI, MluI-plus-BamHI, and MluI-plus-SalI digestion patterns of four cosmid clones. The closed arrowhead in the MluI digest represents fragments common to cosmid clones A42 and A90, which is not cleaved by BamHI but produced four fragments (closed arrowheads) following SalI digestion. The closed and open arrows represent two fragments common to cosmid clones A90 and A104. In the MluI-BamHI double digest, both the fragments disappeared and identical new fragments appeared (closed and open arrows). In the MluI-SalI double digest, only the fragment identified by the closed arrow disappeared and identical new fragments appeared (closed arrow). The open arrowhead represents a fragment common to A42 and A14 in the MluI digest which disappeared following BamHI digestion, producing identical new fragments (open arrowhead). The MluI fragment common to A42 and A14 did not disappear upon digestion with MluI plus SalI (open arrow). V, vector DNA. (B) Assembled contig comprising four overlapping cosmids, A14, A42, A90, and A104. The extent of overlap between the clones is marked above each overlap.
For some clones, the common MluI fragment(s) did not disappear following digestion with any of the four rare-cutting enzymes used and thereby did not allow the identification of the landmarks. To overcome this problem, one option is to use more rare-cutting enzymes, which is labor intensive. The other option, which was adopted in the present study, is chromosome walking with riboprobes generated from the two ends of the clone to determine overlapping clones. This approach was used for clones with one MluI common fragment. Clones having multiple MluI common fragments were directly taken as overlapping clones, since it is unlikely that two nonoverlapping clones will generate multiple similar-sized fragments. In cases where all the expected reappearing fragments of the landmark following digestion with the second enzyme could not be detected in the gel, the disappearance of the common MluI fragment(s) was taken as evidence that two clones were overlapping.
Map integration.
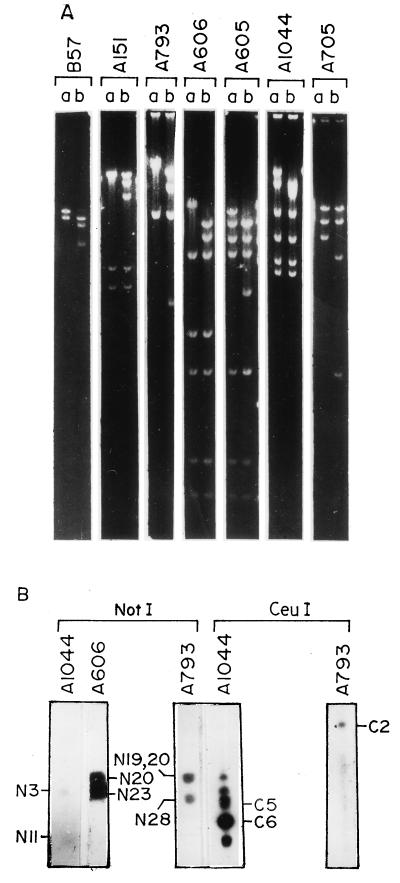
To generate a relational map, the assembled contigs were positioned on the macrorestriction map (29). This involved the following steps. (i) Cosmid clones containing NotI site(s) were identified. V. cholerae 569B genomic DNA was digested with NotI, end labelled, and subsequently digested with HindIII to generate probes specific for ends of a NotI fragment. All the assembled cosmid clones were hybridized with these probes, and the clones that lit up were digested with NotI to confirm the presence of a NotI site (Fig. 3A). (ii) Contigs were positioned in the NotI map. To position the contig with respect to the junction between two NotI fragments, clones having a NotI site(s) in the contig were used as probes in Southern blot hybridization of NotI-digested V. cholerae 569B genomic DNA. For example, the clone A1044 hybridized with NotI fragments N3 and N11 (Fig. 3B), which are linked. Similarly, the clone A606 hybridized with N20 and N23 (Fig. 3B). The clone A793 hybridized with three fragments, N19, N28, and N20 (Fig. 3B), which are linked (29). Whenever required, the positions of the contigs on the macrorestriction map were confirmed by hybridizing NotI site-containing cosmids with CeuI-digested V. cholerae 569B genomic DNA. CeuI has nine sites in the genome, and all the sites are located in the rrn operons (27). The clone A1044, having one NotI site and one CeuI site, strongly hybridized with the CeuI fragments C6 and C5 (Fig. 3B), which span the junction of N3 and N11 (29). Because of the presence of an rrn operon in the clone, all the other CeuI fragments also hybridized with it, though relatively weakly. The clone A793, having no CeuI site, hybridized only with CeuI fragment C2, which spans N19-N28-N20 of the NotI map (Fig. 3B). The positioning of the contigs in the combined NotI-CeuI (Fig. 4) map was further confirmed by identifying the cosmid clones with SfiI sites in conformity with the combined SfiI-NotI-CeuI macrorestriction map.
FIG. 3.
(A) Identification of NotI linking clones. Cosmid clones hybridizing with probes generated from the ends of NotI-digested V. cholerae genomic DNA were digested with BamHI (lanes a) and BamHI and NotI (lanes b). (B) Southern blot hybridization of PFGE-separated NotI- and CeuI-digested V. cholerae 569B genomic DNA with NotI linking cosmid clones A1044, A606, and A793 as probes. The linked NotI and CeuI fragments are marked. The clone A793, having no CeuI site, hybridized only with CeuI fragment C2.
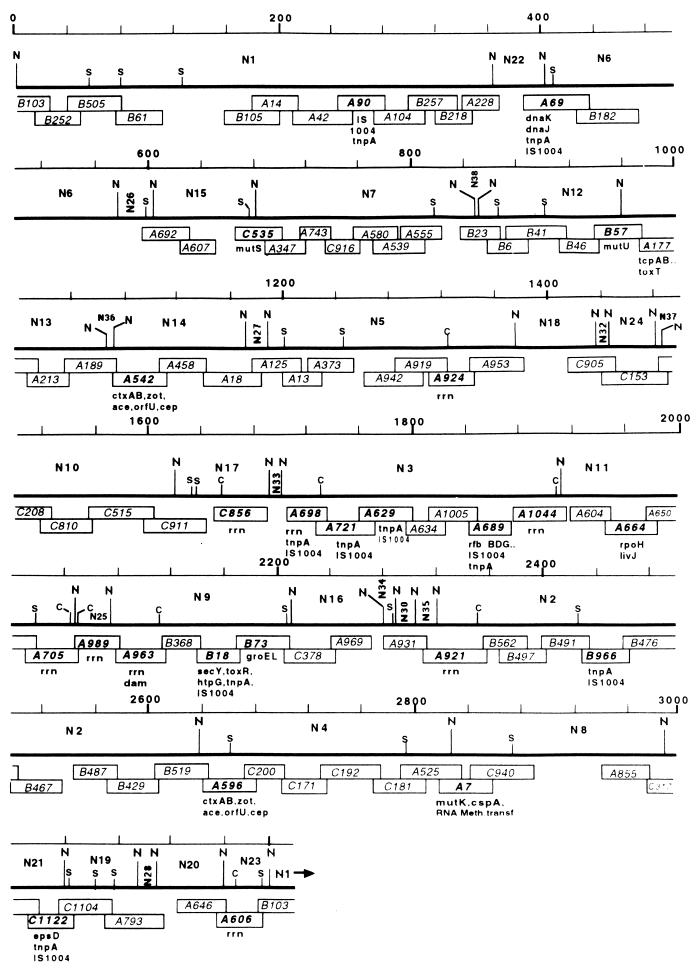
FIG. 4.
Linearized ordered cloned DNA map of the 3.2-Mb circular chromosome of V. cholerae 569B and positioning of genetic markers on the map. The thick lines represent a composite macrorestriction map consisting of the NotI (N), SfiI (S), and CeuI (C) restriction sites. The linkages between different NotI fragments were taken from the published physical map (29). The rightmost end of each thick line is contiguous with the leftmost end of the following line. Since the genome is circular, NotI sites in the far upper left and far lower right are the same. Each cosmid is represented by an open rectangular box with an identification number in the center. The lengths of the boxes reflect their sizes in kilobases and also the extents of overlap between any two overlapping cosmids. Positions of the genetic markers are shown below the cosmids they belong to. The thin line represents the scale in kilobases, where the first NotI site is taken as zero.
Closing of gaps in the map.
To close or reduce the gaps between the contigs generated by landmark analysis, chromosome walking was performed. Riboprobes generated by using T7 or SP6 promoters of the cosmid Lorist M from the ends of the terminal clones of each contig were hybridized to clones belonging to a particular group. Chromosome walking allowed identification of about 20% of the overlaps in the contig assembled. While chromosome walking allowed identification of overlapping clones, it could not provide information about the extent of the overlap. The overlapping clones identified by chromosome walking were thus subjected to landmark analysis to estimate the length of overlap. By combining landmark analysis and chromosome walking, 92 cosmid clones in 13 contigs covering about 90% of the V. cholerae genome have been positioned in the overlapping cloned DNA map (Fig. 4). One 120-kb gap and 14 small gaps (ranging from 10 to 50 kb) are yet to be filled.
Positioning of V. cholerae genes on the cloned DNA map.
Twenty-seven cloned genes and 10 copies of one IS element have been positioned on the ordered cloned DNA map of the V. cholerae 569B genome (Fig. 4) by hybridization using homologous and heterologous genes as probes (Table 2). The gene probes used comprised virulence determinant genes, DNA mismatch repair genes, stress response genes, and genes involved in protein translocation. The genes were positioned on the macrorestriction map (29) rather arbitrarily on fragments to which they hybridized, not reflecting their true order in the genome. It will be possible to determine the order of genes in the chromosome and the approximate distances between them from the ordered cloned DNA map. For example, in the low-resolution macrorestriction map, tcp and one of the ctx genetic elements were positioned in NotI fragment N14 (29). The high-resolution map showed that the tcp and ctx genes are located in two cosmids, A177 and A542, respectively, falling within NotI fragments N13 and N14, and that the distance between the two genes is about 50 to 80 kb. The dam, secY, and groEL genes, positioned in NotI fragment N9 in the macrorestriction map, are located in the cosmids A963, B18, and B73, respectively, and the order in which these genes are present in the chromosome is dam-secY-groEL (Fig. 4). Nine rrn operons were positioned in the map on cosmids having CeuI sites. The CeuI sites in the V. cholerae genome were taken as the positions of the rrn operons.
TABLE 2.
Positioning of cloned genes in the contigs of the ordered cloned DNA map
| Gene(s) | Gene product(s) or function | Source | Cosmid(s) | Reference |
|---|---|---|---|---|
| mutS | DNA mismatch repair | V. cholerae | C535 | 4 |
| mutL | DNA mismatch repair | V. cholerae | B57 | 4 |
| mutK | DNA mismatch repair | V. cholerae | A7 | Unpublished data |
| dam | Adenine methyltransferase | V. cholerae | A963 | 2 |
| cspA | Cold shock protein | V. cholerae | A7 | Unpublished data |
| RNA methyltransferase gene | RNA methyltransferase | V. cholerae | A7 | Unpublished data |
| rpoH | ς32 | V. cholerae | A664 | 39 |
| udhA | Unknown dehydrogenase | V. cholerae | A664 | 39 |
| groEL | Hsp60 | E. coli | B73 | 17 |
| dnaK | Hsp70 | V. cholerae | A69 | Unpublished data |
| grpE | DNA synthesis | V. cholerae | A69 | Unpublished data |
| dnaJ | Hsp40 | V. cholerae | A69 | Unpublished data |
| L15 L36 | Ribosomal large-subunit proteins | V. cholerae | B18 | 7 |
| secY | Inner membrane protein translocator | V. cholerae | B18 | 7 |
| epsD | Protein secretion | V. cholerae | C1122 | Unpublished data |
| ctxAB | Cholera toxin | V. cholerae | A542, A596 | 24 |
| zot | Zonula occludens toxin | V. cholerae | A542, A596 | 3 |
| ace | Accessory cholera enterotoxin | V. cholerae | A542, A596 | 42 |
| cep | Core-encoded pilus | V. cholerae | A542, A596 | 35 |
| orfU | Unknown open reading frame | V. cholerae | A542, A596 | 42 |
| toxR | Virulence gene activator | V. cholerae | B18 | 43 |
| htpG | Stress response protein | V. cholerae | B18 | 43 |
| tcpAB | Toxin-coregulated pilus | V. cholerae | A177 | 25 |
| toxT | Transcriptional activator | V. cholerae | A177 | 25 |
| tnpA (IS1004) | Transposase | V. cholerae | A90, A69, A458, A698, A721, A629, B18, B966, A855, C1122 | 8 |
| rfbBDEG | O antigen | V. cholerae | A689 | 30 |
DISCUSSION
The present report describes the construction of a high-resolution overlapping cloned DNA map of the genome of hypertoxinogenic strain 569B of V. cholerae. Thirteen contigs covering 2.85 Mb (about 90% of the whole genome) have been assembled. The availability of the macrorestriction map of the V. cholerae genome was extremely useful in grouping the cosmid clones into defined subsets and reducing the number of clones to be analyzed. Besides, the knowledge of NotI, SfiI, and CeuI sites in the physical map helped in accurately positioning and orienting contigs containing clones having sites for one of these enzymes.
The success of generating an ordered cloned DNA map depends primarily on the efficiency of detecting overlaps. Several different approaches have been adopted by different investigators to identify overlapping clones. These include (i) restriction mapping of randomly selected clones (26), (ii) fingerprinting (33), (iii) chromosome walking, and (iv) identification of overlapping clones from shared landmarks (10). Each of these approaches has its own limitations, and to construct high-resolution maps of genomes of prokaryotic organisms it is always necessary to combine results obtained from two or more of these approaches. Although the landmark analysis was tested only with one organism, H. volcanii, to identify overlapping clones (11), this was preferred over the other strategies, in the present study, for several reasons. This approach allowed detection of small overlaps, and from a relatively small number of clones, an ordered cloned DNA map can be constructed. A minimal set of 92 overlapping clones was sufficient to generate contigs covering 90% of the V. cholerae genome by this approach. A total of 72% of the overlaps were less than 10 kb, and the length of none of the overlaps was more than 20 kb. Except in a few cases where chromosome walking was necessary, four rare-cutting enzymes were adequate to identify landmarks. Furthermore, this method does not require extensive use of radioisotopes, which makes it less hazardous.
One of the problems encountered during the construction of the map was instability of cosmid clones. When maintained in E. coli, some of the clones were spontaneously deleted. The deletion of some of these clones could be due to the presence of toxic genes. This might be one of the reasons for the presence of the small gaps in the cloned DNA map. The other possibility is that the DNA segments in these regions are not represented in the library. A lambda clone library of V. cholerae genomic DNA is under construction, and this will be used to bridge the gaps in the ordered cosmid map and to get complete coverage.
It has been possible to refine and more accurately position genetic loci in the high-resolution map; in the macrorestriction map, in comparison, the genes were arbitrarily positioned on the restriction fragments to which they hybridized. Some more genes in addition to those placed in the macrorestriction map, viz., grpE, dnaJ, mutK, cspA, epsD, tnpA, rfb, and genes encoding RNA methyltransferase and ribosomal large-subunit proteins L15 and L36, have been positioned on the ordered cloned DNA map. A 628-bp repeat sequence, IS1004, has been reported to be present in the V. cholerae genome (8). The present study showed that there are 10 copies of this repeat sequence in the genome of strain 569B of V. cholerae, and their locations in the genome have been determined. Several clones other than those containing IS1004 in the cosmid library hybridized with more than one NotI restriction fragment, suggesting the presence of yet-unidentified repeat sequences in those clones. With the addition of more genes, the utility of the map is expanding and its resolution is improving. This will lead to more insight into chromosome organization and help to identify new virulence determinant factors and to understand the molecular basis of pathogenicity of this important human pathogen.
ACKNOWLEDGMENTS
We thank R. L. Charlebois, University of Ottawa, Ottawa, Ontario, Canada, for providing the cosmid vector Lorist M and E. coli ED8767 and E. M. Bik, National Institute of Public Health and the Environment, Bithoven, The Netherlands, for providing tnpA and rfbBDEG genes. We also thank all members of the Biophysics Division, Indian Institute of Chemical Biology, for their kind cooperation and encouragement during this study.
S.C. and N.A.B. are grateful to the Council of Scientific & Industrial Research, New Delhi, India, for a predoctoral fellowship and pool officership, respectively. This work was supported by the Department of Biotechnology (grants BT/TF/15/03/91, BT/MB/05/12/94, and BT/R&D/PRO109/15/8/96) of the Government of India.
REFERENCES
- 1.Azevedo V, Alvarez E, Zumstein E, Damiani G, Sgaramella V, Ehrlich S D, Serror P. An ordered collection of Bacillus subtilis DNA segments cloned in yeast artificial chromosome. Proc Natl Acad Sci USA. 1993;90:6047–6051. doi: 10.1073/pnas.90.13.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandyopadhyay R, Das J. DNA adenine methyl-transferase encoding gene (dam) of Vibrio cholerae. Gene. 1994;140:67–71. doi: 10.1016/0378-1119(94)90732-3. [DOI] [PubMed] [Google Scholar]
- 3.Baudry B, Fasano A, Ketley J, Kaper J B. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992;60:428–434. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bera T K, Ghosh S K, Das J. Cloning and characterization of the mutL and mutS genes of Vibrio cholerae: nucleotide sequence of the mutL gene. Nucleic Acids Res. 1989;17:6241–6251. doi: 10.1093/nar/17.15.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhadra R K, Roychoudhury S, Banerjee R K, Kar S, Majumder S, Sengupta S, Chatterjee S, Khetawat G, Das J. Cholera toxin (CTX) genetic element in Vibrio cholerae O139. Microbiology. 1995;141:1977–1983. doi: 10.1099/13500872-141-8-1977. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Sinha V B, Iyer S S. Chromosome mobilization in Vibrio cholerae (biotype El Tor) mediated by sex factor P. J Gen Microbiol. 1973;78:119–124. doi: 10.1099/00221287-78-1-119. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya D, Das J. The secY gene of V. cholerae: identification, cloning and characterization. Gene. 1997;196:261–266. doi: 10.1016/s0378-1119(97)00238-2. [DOI] [PubMed] [Google Scholar]
- 8.Bik E M. DNA fingerprinting of Vibrio cholerae strains with a novel sequence insertion element: a tool to identify epidemic strains. J Clin Microbiol. 1996;34:1453–1461. doi: 10.1128/jcm.34.6.1453-1461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukanov N O, Berg D E. Ordered cosmid library and high-resolution physical-genetic map of Helicobacter pylori strain NCTC11638. Mol Microbiol. 1994;11:509–523. doi: 10.1111/j.1365-2958.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 10.Charlebois R L, Hofman J D, Schalkwyk L C, Lam W L, Doolittle W F. Genome mapping in halobacteria. Can J Microbiol. 1989;15:21–29. doi: 10.1139/m89-004. [DOI] [PubMed] [Google Scholar]
- 11.Charlebois R L. Detailed physical map and set of overlapping clones covering the genome of the archaebacterium Haloferax volcanii DS2. J Mol Biol. 1991;222:509–524. doi: 10.1016/0022-2836(91)90493-p. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri K, Bhadra R K, Das J. Cell surface characteristics of environmental and clinical isolates of Vibrio cholerae non-O1. Appl Environ Microbiol. 1992;58:3567–3573. doi: 10.1128/aem.58.11.3567-3573.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang S, Daniels D L, Blatner F R. Global regulation of gene expression in Escherichia coli. J Bacteriol. 1993;175:2026–2036. doi: 10.1128/jb.175.7.2026-2036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole S T, Girons I S. Bacterial genomics. FEMS Microbiol Rev. 1994;14:139–160. doi: 10.1111/j.1574-6976.1994.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 15.Deckers H M, Voordouw G. Identification of a large family of genes for putative chemoreceptor proteins in an ordered library of the Desulfovibrio vulgaris Hidenborough genome. J Bacteriol. 1994;176:351–358. doi: 10.1128/jb.176.2.351-358.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiglmeirer K, Honore N, Woods S A, Caydron B, Cole S T. Use of an ordered cosmid library to deduce the genomic organization of Mycobacterium leprae. Mol Microbiol. 1993;7:197–206. doi: 10.1111/j.1365-2958.1993.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 17.Fayet O, Ziegelhoffer T, Georgopoulus C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171:1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 19.Fonstein M, Haselkorn R. Chromosomal structure of Rhodobacter capsulatus strain SB1003: cosmid encyclopedia and high-resolution physical and genetic map. Proc Natl Acad Sci USA. 1993;90:2522–2526. doi: 10.1073/pnas.90.6.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonstein M, Haselkorn R. Physical mapping of bacterial genomes. J Bacteriol. 1995;177:3361–3369. doi: 10.1128/jb.177.12.3361-3369.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Q, Chen H, Kuspa A, Cheng Y, Kaiser D, Shimkets L J. A physical map of the Myxococcus xanthus chromosome. Proc Natl Acad Sci USA. 1994;91:9584–9587. doi: 10.1073/pnas.91.20.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hohn B. In vitro packaging of λ and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- 23.Kaper J B, Morris G, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaper J B, Lockman H, Baldini M M, Levine M M. A recombinant live oral cholera vaccine. Bio/Technology. 1984;2:345–349. [Google Scholar]
- 25.Keasler S P, Hall R H. Detecting and biotyping Vibrio cholerae O1 with multiplex polymerase chain reaction. Lancet. 1993;341:1661. doi: 10.1016/0140-6736(93)90792-f. [DOI] [PubMed] [Google Scholar]
- 26.Kohara Y, Akiyama K, Isono K. The physical map of the whole Escherichia coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu S-L, Sanderson K E. A physical map of the Salmonella typhimurium LT2 genome made by using XbaI analysis. J Bacteriol. 1992;174:1662–1672. doi: 10.1128/jb.174.5.1662-1672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohia A, Majumdar S, Chatterjee A N, Das J. Effect of changes in the osmolarity of the growth medium on Vibrio cholerae cells. J Bacteriol. 1985;163:1158–1166. doi: 10.1128/jb.163.3.1158-1166.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majumder R, Sengupta S, Khetawat G, Bhadra R K, Roychoudhury S, Das J. Physical map of the genome of Vibrio cholerae 569B and localization of genetic markers. J Bacteriol. 1996;176:1105–1112. doi: 10.1128/jb.178.4.1105-1112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manning P A, Heuzenroeder M W, Yeadon J, Leabesley D I, Reeves P R, Rowley D. Molecular cloning and expression in Escherichia coli K12 of the O antigens of the Ogawa and Inaba serotypes of the lipopolysaccharides of Vibrio cholerae O1 and their potential for vaccine development. Infect Immun. 1986;53:272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris J G. Non-O group I Vibrio cholerae: a look at the epidemiology of an occasional pathogen. Epidemiol Rev. 1990;12:179–191. doi: 10.1093/oxfordjournals.epirev.a036052. [DOI] [PubMed] [Google Scholar]
- 32.Nandi S, Khetwat G, Sengupta S, Majumder R, Kar S, Bhadra R K, Roychoudhry S, Das J. Rearrangements in the genomes of Vibrio cholerae strains belonging to different serovars and biotypes. Int J Syst Bacteriol. 1997;47:858–862. doi: 10.1099/00207713-47-3-858. [DOI] [PubMed] [Google Scholar]
- 33.Olson V M, Dutchik J E, Graham M Y, BroDeur G M, Helms C, Frank M, MacCollin M, Scheinman R, Frank T. Random clone strategy for genomic restriction mapping in yeast. Proc Natl Acad Sci USA. 1986;83:7826–7830. doi: 10.1073/pnas.83.20.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panda D K, Dasgupta U, Das J. Transformation in Vibrio cholerae by plasmid DNA. Gene. 1991;105:107–111. doi: 10.1016/0378-1119(91)90520-l. [DOI] [PubMed] [Google Scholar]
- 35.Pearson G D N, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a size specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramamurthy T, Garg S, Sharma R, Bhattacharya S K, Nair G B, Shimada T, Takeda T, Karasawa T, Kurazano H, Pal A, Takeda Y. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;341:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 37.Roy N K, Das G, Balganesh T S, Dey S N, Ghosh R K, Das J. Enterotoxin, DNA and alkaline phosphatase of Vibrio cholerae before and after animal passage. J Gen Microbiol. 1982;128:1927–1932. doi: 10.1099/00221287-128-9-1927. [DOI] [PubMed] [Google Scholar]
- 38.Roychoudhury S, Bhadra R K, Das J. Genome size and restriction fragment length polymorphism analysis of Vibrio cholerae strains belonging to different serovars and biotypes. FEMS Microbiol Lett. 1994;115:329–334. doi: 10.1111/j.1574-6968.1994.tb06659.x. [DOI] [PubMed] [Google Scholar]
- 39.Sahu G K, Chowdhury R, Das J. The rpoH gene encoding ς32 homolog of Vibrio cholerae. Gene. 1997;189:203–207. doi: 10.1016/s0378-1119(96)00849-9. [DOI] [PubMed] [Google Scholar]
- 40.Sankar P, Hutton E, VanBogelen R A, Clark R L, Neidhard F C. Expression analysis of cloned chromosomal segments of Escherichia coli. J Bacteriol. 1993;175:5145–5152. doi: 10.1128/jb.175.16.5145-5152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trieselmann B A, Charlebois R L. Transcriptionally active regions in the genome of the archaebacterium Haloferax volcanii. J Bacteriol. 1992;174:30–34. doi: 10.1128/jb.174.1.30-34.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trucksis M, Galen J E, Michalski J, Fasano A, Kaper J B. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldor M, Mekalanos J J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994;62:72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenzel R, Hermann R. Cloning of complete Mycoplasma pneumoniae genome. Nucleic Acids Res. 1989;17:7029–7043. doi: 10.1093/nar/17.17.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current Protocols in Molecular Biology. Vol. 1. New York, N.Y: John Wiley and Sons; 1994. pp. 2.4.3–2.4.5. [DOI] [PubMed] [Google Scholar]