Abstract
Plant photoreceptors that regulate photomorphogenic development include red/far-red-light–absorbing phytochromes and blue/UV-A-light–absorbing cryptochromes. We have undertaken a genetic screen to identify additional components downstream of the photoreceptors in Arabidopsis thaliana. We identified a short hypocotyl mutant under red and blue light, hypersensitive to red and blue 1 (hrb1). Mutation in HRB1 also enhances the end-of-day far-red light response, inhibits leaf expansion and petiole elongation, and attenuates the expression of CAB3 and CHS. Double mutant analysis indicates that phyB is epistatic to hrb1 under red light, and cry1 cry2 is epistatic to hrb1 under blue light for both hypocotyl growth and light-regulated gene expression responses. HRB1 localizes to the nucleus and belongs to a protein family of Drought induced 19 (Di19). HRB1 and all other family members contain a ZZ-type zinc finger domain, which in other organisms is implicated in protein–protein interactions between dystrophin and calmodulin and between transcriptional adaptors and activators. HRB1 activity is also required for red and blue light–induced expression of PHYTOCHROME INTERACTING FACTOR 4 (PIF4). pif4 shows a very similar hypersensitive response as hrb1 to both red light and blue light and is epistatic to hrb1 in control of light-regulated gene expression responses. Thus, the roles of HRB1 and PIF4 together in regulating both red and blue light responses may represent points where red light signaling and blue light signaling intersect.
INTRODUCTION
Plants have evolved several classes of photoreceptors to monitor their environmental light signals. The photoreceptors include red and far-red-light–absorbing phytochromes and UV-A/blue light–absorbing cryptochromes and phototropins (Deng and Quail, 1999; Neff et al., 2000). Among the photoreceptors, phytochromes and cryptochromes regulate seedling deetiolation responses, photoperiodic flowering, and circadian rhythm (Guo et al., 1998; Mas et al., 2000). In Arabidopsis thaliana, the phytochrome gene family has five members, PHYA through PHYE (Clack et al., 1994). Phytochrome A (phyA) is the photoreceptor for far-red light and phytochrome B (phyB) is the major photoreceptor sensing red light (Deng and Quail, 1999; Neff et al., 2000). phyA is able to autophosphorylate and to phosphorylate phytochrome kinase substrate 1 (PKS1) in vitro (Yeh and Lagarias, 1998; Fankhauser et al., 1999). Both phyA and phyB translocate to the nucleus in response to red or far-red light signals (Kircher et al., 1999; Yamaguchi et al., 1999). phyA or phyB have also been demonstrated to interact with PHYTOCHROME INTERACTING FACTOR 3 (PIF3), PKS1, NPDK2, PIF4, ARR4, and ELF3 in vitro (Choi et al., 1999; Fankhauser et al., 1999; Ni et al., 1999; Liu et al., 2001; Sweere et al., 2001; Huq and Quail, 2002; Kim et al., 2003).
Loss of cry1 function impairs seedling deetiolation responses under blue/UV-A light (Ahmad and Cashmore, 1993). CRY1 encodes a flavoprotein with sequence similarity to photolyases, a family of flavoproteins that mediate repair of DNA damage by UV light. However, cry1 lacks photolyase activity and has a C-terminal extension not found in the photolyases (Yang et al., 2000). cry2 is involved in the control of photoperiodic flowering in addition to its role in regulating seedling deetiolation responses (Guo et al., 1998). cry1 is localized to the nucleus under dark conditions and is depleted from the nucleus under continuous white light conditions (Guo et al., 1999; Yang et al., 2000). By contrast, cry2 is predominantly localized to the nucleus under both dark and light conditions (Guo et al., 1999; Yang et al., 2000). cry1 and cry2 also autophosphorylate in a blue light–dependent manner (Shalitin et al., 2002, 2003). The cry2 protein is unstable, and this instability may be mediated by its interaction with COP1 (Wang et al., 2001).
Genetic screens have identified several mutants with a defect either in red light response or in far-red light response (Deng and Quail, 1999; Neff et al., 2000). Many of the signaling components identified so far through the genetic screens reside in the nucleus, but some, such as FIN219 (Hsieh et al., 2000) and PAT1 (Bolle et al., 2000), are cytosolic proteins. FHY1 and SRR1 exist in both the nucleus and the cytoplasm (Desnos et al., 2001; Staiger et al., 2003). Three additional mutants, pef1, psi2, and prr7, were identified by their defects in both red and far-red light responses (Ahmad and Cashmore, 1996; Genoud et al., 1998; Kaczorowski and Quail, 2003). Genetic screens under blue light have identified sub1, a mutant with a hypersensitive response to both blue and far-red light (Guo et al., 2001). SUB1, a cytoplasmic calcium binding protein, functions downstream of crys and negatively modulates phyA-mediated far-red light responses. Recently, a positively acting component in blue light signaling, PP7, has been identified (Møller et al., 2003). The light signals are further modulated by proteosome-mediated degradation of HY5 and LAF1 through the constitutive repressors of photomorphogenesis or COP/DET/FUS components (Osterlund et al., 2000; Seo et al., 2003). A heterotrimeric G-protein α-subunit, a small G protein, the well-known second messengers cyclic GMP and calcium/calmodulin, and protein phosphatase have also been implicated in light signaling (Bowler et al., 1994; Neuhaus et al., 1997; Kang et al., 2001; Okamoto et al., 2001; Jones et al., 2003).
Despite the existence of distinct red, far-red, or blue light signaling pathways, the optimal performance of a plant depends on coordination among the different light signaling pathways. It has been realized that a minimal level of active phytochrome seems to be necessary for full activity of cryptochromes or phototropins (Ahmad et al., 1998). One classic example is the enhancement by red light of phototropic bending toward unilateral blue light (Casal, 2000). The dependence of the blue responses on active phytochromes may result from a direct interaction of both photoreceptors, and early studies indicated that cry1 and cry2 can be phosphorylated by a phyA-associated kinase activity (Ahmad et al., 1998). The enzymatic interaction of phyA with cry1 was further confirmed in targeted yeast two-hybrid assays. Recently, a functional interaction of phyB with cry2 has been implicated in control of hypocotyl elongation, flowering time, and circadian rhythm (Mas et al., 2000).
Equally possible, the dependence of blue light responses on active phytochromes may occur at a common intermediate step of their signaling pathways. We have isolated a light signaling mutant, hypersensitive to red and blue 1 (hrb1), with a hypersensitive hypocotyl growth response to both red and blue light. The hrb1 mutation is caused by a T-DNA knockout event, and a 3.8-kb genomic fragment, spanning the coding sequence of the HRB1 gene, fully rescued the hrb1 phenotype to the wild type. Double mutant analysis indicates that phyB is epistatic to hrb1 under red light, whereas cry1 cry2 is epistatic to hrb1 under blue light. HRB1 localizes to the nucleus and belongs to a novel protein family found in several plant species. All members of this family contain a ZZ-type zinc finger domain, which is implicated in protein–protein interactions in other organisms. Thus, HRB1 may achieve its regulation on red and blue light responses by interacting with other light signaling components through its ZZ-type zinc finger domain. HRB1 activity is also required for the proper expression of PIF4 under red and blue light. Loss-of-function pif4 contains a semidominant mutation and has a hypersensitive hypocotyl growth response to red light (Huq and Quail, 2002). In this study, we demonstrate that pif4 has a similar hypersensitive response to both red and blue light as hrb1, and HRB1 together with PIF4 may represent points where red light signaling and blue light signaling intersect.
RESULTS
hrb1 Has a Hypersensitive Hypocotyl Growth Response to Red and Blue Light
To isolate new light signaling mutants, we screened a collection of 40,000 Wisconsin lines and a collection of 30,000 SALK lines containing T-DNA insertions (Arabidopsis Biological Resource Center) for a short hypocotyl phenotype under red light. Of six short mutants isolated, two had a phenotype under red light, three had a phenotype under both red and blue light, and one had a phenotype under red, far-red, and blue light. One of the red light mutants is a pif3 allele. The three red/blue light mutants fall into different complementation groups, one of which is characterized here both at the genetic and molecular levels. Based on a hypersensitive hypocotyl phenotype under red and blue light, we named the newly identified mutant hrb1 (for hypersensitive to red and blue 1) (Figure 1). Examined over a range of red light intensities, hrb1 exhibited a hypersensitive response to very low intensity of red light, and the response approached saturation at a much lower intensity of red light than that of the Wassilewskija (Ws) wild type (Figure 1B, left). By comparison, hrb1 responded in a hypersensitive manner to both weak and strong blue light. The responses of both Ws and hrb1 were not saturated over the range of the blue light intensities tested (Figure 1B, right).
Figure 1.
hrb1 Has a Hypersensitive Hypocotyl Response to Red and Blue Light.
(A) Hypocotyl growth responses of 4-d-old Ws and hrb1 seedlings to red light (15 μmol/m2/s), far-red light (0.2 μmol/m2/s), and blue light (6 μmol/m2/s). Data presented are means ± se.
(B) Hypocotyl growth responses of 4-d-old Ws and hrb1 seedlings to different fluence of red light (left), far-red light (middle), and blue light (right).
We also examined if hrb1 had other aberrant light responses (Figure 2). In an end-of-day far-red light (EODFR) experiment, a pulse of saturating far-red light was given to seedlings grown under 8-h red light before being returned to a 16-h period of darkness. A pulse of saturating far-red light converts a large proportion of phyB molecule from its active Pfr form to its inactive Pr form and thus relieves the strong inhibition of hypocotyl elongation by red light (Deng and Quail, 1999; Neff et al., 2000). As a result, red light–grown Ws wild-type seedlings have a longer hypocotyl after receiving an EODFR treatment (Figure 2A). Each individual measurement for hrb1 was paired with another individual measurement for Ws in either R-EODFR or R+EODFR group, and their ratios were calculated. Compared with R-EODFR, hrb1 exhibits a much longer hypocotyl relative to the Ws wild type after an EODFR treatment under such a red light/dark cycle (Figure 2A). These data suggest a role of HRB1 in regulating phyB-mediated red light responses.
Figure 2.
hrb1 Has an Enhanced End-of-Day Far-Red Light Response, Small Cotyledons or Leaves, and a Short Petiole.
(A) End-of-day far-red light responses (EODFRs) of 4-d-old Ws and hrb1 seedlings. The seedlings were grown under 8 h of red light, treated with a 10-min far-red light pulse (R+EODFR), and returned to a 16-h dark period. Controls received no far-red light pulse (R-EODFR). Red and far-red light intensities were as indicated in Figure 1A. Data presented are means ± se. The average hypocotyl lengths of hrb1 relative to Ws was 0.70 (standard deviation 0.059, n = 49) in R-EODFR group and 0.86 (standard deviation 0.067, n = 47) in R+EODFR group. Student's two-tailed heteroscedastic t tests show that the R-EODFR group is significantly different from R+EODFR group (P < 0.05).
(B) Cotyledon area of Ws and hrb1 seedlings measured 5 d after germination and grown under white light in soil or under red, far-red, or blue light on an agar plate.
(C) Leaf area and petiole length of Ws and hrb1 plants measured 17 d after germination and grown under white light in soil.
(D) Ws and hrb1 grown in white light in soil for 17 d after germination. The plants were transferred to an agar plate and photographed. Bar = 4 mm. The intensity of white light was 30 μmol/m2/s.
phyB-9, a loss-of-function PHYB mutant, has smaller cotyledons and leaves but longer petioles under white light. Similar to phyB-9, hrb1 developed smaller cotyledons or leaves than the Ws wild type, measured 5 and 17 d, respectively, after germination, under white light in soil (Figures 2B to 2D). When seedling growth was further examined under red, far-red, and blue light on agar plates, hrb1 showed a reduced cotyledon size only under red and blue light but not under far-red light (Figure 2B). In contrast with the long petiole phenotype of phyB-9, hrb1 had a shorter petiole than the Ws wild type when measured 17 d after germination under white light in soil (Figures 2C and 2D).
Expression of CAB3, CHS, and PIF4 in hrb1
To further examine the effects of hrb1 mutation on red and blue light responses, we studied the expression of CAB3, a gene encoding a chlorophyll a/b binding protein, and CHS, a gene encoding an enzyme involved in anthocyanin biosynthesis (Figure 3). On our autoradiogram, the expression of CAB3 and CHS in Ws wild type was induced by a 4-h treatment with red, far-red, and blue light (Figure 3A). Compared with the Ws wild type, the expression of CAB3 in hrb1 showed a 2.5-fold reduction under blue light, and the expression of CHS in hrb1 showed 2.0- and 3.0-fold reduction, respectively, under red and blue light (Figure 3B). An insignificant change of CAB3 and CHS expression in hrb1 under far-red light further indicates a specific involvement of HRB1 in regulating red and blue light responses (Figures 3A and 3B). Experiments using a 2- or 6-h light induction over a range of light intensities failed to reveal any other significant changes compared with the studies using the current experimental regime (data not shown).
Figure 3.
HRB1 Is Required for the Proper Expression of CAB3, CHS, and PIF4 under Red Light and Blue Light.
(A) RNA gel blot analysis of CAB3, CHS, and PIF4 expression on total RNA isolated from 4-d-old dark-grown Ws or hrb1 seedlings that received no light treatment (D) or were treated for 4 h with red light (R), far-red light (FR), or blue light (B) under intensities as specified in Figure 1A. Ten micrograms of total RNA was loaded on each lane. Experiments were repeated three times, and a representative RNA gel blot is shown in this figure and subsequent figures involving gene expression analysis.
(B) Normalization of CAB3, CHS, and PIF4 messages to rRNA signal. The same blot was exposed to a phosphor image screen, and the intensity of each band was quantified using an ImageQuant program.
We also examined the expression of various light signaling genes in Ws and hrb1 using RT-PCR and detected a change in the expression of PIF4, a gene encoding a phyB interacting protein and a negative regulator of phyB signaling. We further verified the expression of PIF4 in Ws and hrb1 using RNA gel blot analysis (Figure 3A). As shown previously, the expression of PIF4 in Ws was induced by red and far-red light (Huq and Quail, 2002) and was also induced by blue light (Figure 3A). Compared with that of Ws, the red light–induced expression of PIF4 in hrb1 was reduced 2.6-fold, and the blue light–induced expression of PIF4 in hrb1 was reduced 2.1-fold (Figure 3B). Thus, HRB1 activity is required for the proper expression of PIF4 under both red and blue light.
Hypocotyl Growth and Light-Regulated Gene Expression Responses in hrb1 pif4
PIF4 has been previously demonstrated to negatively regulate phyB signaling through a direct interaction with phyB (Huq and Quail, 2002). Interestingly, the loss-of-function pif4 has a very similar hypocotyl growth response as hrb1 to red light but not to far-red light, and the response of pif4 to blue light remains to be determined (Huq and Quail, 2002). We therefore examined the hypocotyl growth responses of Ws, hrb1, pif4, and hrb1 pif4 to blue light in addition to red and far-red light at a relatively broad range of light intensities. Similar to hrb1, pif4 showed a hypersensitive response to both red and blue light but not to far-red light (Figure 4A). The hrb1 pif4 double mutant exhibited a similar, but not an additive, phenotype under red and blue light as hrb1 or pif4 single mutant, suggesting that HRB1 and PIF4 may function closely in the same signaling branch.
Figure 4.
pif4 Is Epistatic to hrb1 in Control of Light-Regulated Gene Expression Responses.
(A) Hypocotyl growth responses of Ws, hrb1, pif4, and hrb1 pif4 to 1 or 30 μmol/m2/s red light (R-1 or R-30), 2 or 10 μmol/m2/s far-red light (FR-2 or FR-10), and 0.2 or 6 μmol/m2/s blue light (B-0.2 or B-6).
(B) RNA gel blot analysis of CAB3, CHS, and XTR7 expression on total RNA isolated from 4-d-old dark-grown Ws, hrb1, pif4, and hrb1 pif4 seedlings that received no light treatment (D) or were treated for 4 h with red light (R) or blue light (B) under intensities as specified in Figure 1A.
(C) Normalization of CAB3, CHS, and XTR7 messages to rRNA signal. The same blot was exposed to a phosphor image screen, and the intensity of each band was quantified using an ImageQuant program.
We also examined the expression of CAB3 and CHS in hrb1, pif4, and hrb1 pif4 double mutant (Figures 4B and 4C). Compared with the Ws wild type, hrb1 showed a 2.4-fold reduction in the expression of CAB3 only under blue light, but pif4 showed a 3.1-fold reduction in the expression of CAB3 under both red and blue light (Figure 4B). The expression of CHS was greatly affected in pif4 than in hrb1 under both red and blue light (Figure 4B). We also examined the expression of XTR7, which encodes a xyloglucan endotransglycosylase and is likely involved in cell wall polymer hydrolysis and cell wall extension. The expression of XTR7 was downregulated by red and far-red light, as shown previously (Kuno et al., 2000), and also by blue light in this study (Figure 4B). No dramatic change was detected for the light downregulation of XTR7 expression in either hrb1 or pif4 (Figure 4C). However, the expression levels of XTR7 in darkness were reduced by mutation in either HRB1 or PIF4. In all cases examined, the hrb1 pif4 double mutant behaved similarly to the pif4 single mutant; therefore, pif4 is epistatic to hrb1 in the control of light-regulated gene expression responses.
The T-DNA Insertion Cosegregates with the Short Hypocotyl Phenotype of hrb1
To determine if the T-DNA insertion is linked to the hrb1 short hypocotyl phenotype, we crossed hrb1 to Ws wild type and scored the segregation of the kanamycin maker in the F2 progeny. The kanamycin gene on the T-DNA segregated at a ratio of three resistant siblings versus one sensitive sibling (64:19) in the F2 population. This segregation ratio indicates that hrb1 has a single T-DNA locus. When grown on nonselective medium, 25% of the progeny exhibited a short hypocotyl phenotype, suggesting that hrb1 is recessive to the wild-type allele. The seedlings scored for short or long hypocotyl were then transferred to kanamycin medium. All seedlings with a short hypocotyl, but not all of the ones with a long hypocotyl (30 resistant versus 12 sensitive), were able to survive on kanamycin medium. Data thus suggest a tight linkage of T-DNA insertion with the short hypocotyl phenotype of hrb1.
The T-DNA Is Inserted in the First Exon of the HRB1 Gene
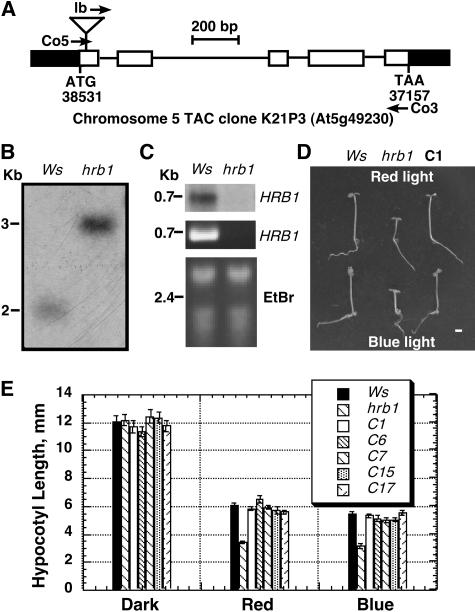
We used a GenomeWalker kit (Clontech, Palo Alto, CA) to clone the mutated HRB1 gene. With primers derived from the T-DNA left and right borders, we identified the plant sequences adjacent to the T-DNA borders in chromosome V. The sequences are included in TAC clone K21P3 (Figure 5A). Hybridization of a DNA gel blot with a probe derived from the plant sequences further revealed a size polymorphism associated with the T-DNA insertion (Figure 5B). A full-length cDNA (accession number At5g49230 or AF372963) was subsequently identified as the putative HRB1 cDNA. Comparison of the cDNA sequence with the corresponding genomic sequence revealed five exons and four introns in the putative HRB1 gene. The T-DNA is inserted in the first exon of HRB1 gene. The nucleotide position for the ATG start codon of HRB1 gene is 38,531 in TAC clone K21P3. The T-DNA insertion caused an 81-bp deletion of the plant sequence between nucleotide 38,580 (adjacent to the right border) and 38,499 (adjacent to the left border). As a result, the HRB1 message was undetectable by both RNA gel blot and RT-PCR analysis (Figure 5C, top and middle). We then cloned a 3.8-kb genomic fragment, spanning from 1.6 kb upstream to 0.6 kb downstream of the HRB1 coding sequence, into binary vector pKF111 (Ni et al., 1998) and transformed the construct into hrb1. The introduced genomic fragment fully complemented hrb1, conferring a phenotype resembling that of the wild type under both red and blue light (Figures 5D and 5E).
Figure 5.
HRB1 Is Expressed in HRB1 but Not hrb1 Plants.
(A) HRB1 gene structure (accession number AT5g49230 or AF372963). Black boxes denote the 5′- and 3′-untranslated regions. White boxes represent exons, and lines represent introns. Co5 and Co3 primers were used for RT-PCR analysis in (C).
(B) Genomic DNA gel blot analysis showing a DNA band shift as a result of a T-DNA insertion in the HRB1 gene. DNA was digested with EcoRI and probed with a radiolabeled HRB1 genomic sequence.
(C) RNA gel blot hybridization of 10 μg of total RNA from Ws and hrb1 with radiolabeled HRB1 coding sequence (top panel). RT-PCR analysis of HRB1 transcripts with 1 μg of total RNA from Ws and hrb1 using Co5 and Co3 primer pairs (middle panel). UV fluorescence of 1 μg of total RNA from Ws and hrb1 (bottom panel).
(D) Complementation of the hrb1 mutant phenotype with a 3.8-kb genomic fragment containing the HRB1 gene. Four-day-old seedlings of Ws, hrb1, and hrb1 carrying the HRB1-containing transgene (C1) grown under continuous red light (top) or continuous blue light (bottom). Bar = 1 mm.
(E) Complementation of the hrb1 mutant phenotype under red and blue light in multiple lines carrying an HRB1 transgene.
HRB1 Belongs to the Drought Induced 19 Protein Family and Contains a ZZ-Type Zinc Finger Motif
HRB1 encodes a polypeptide of 211 amino acids and belongs to the Drought induced 19 (Di19) protein family (Figure 6B). This protein family has no obvious similarity to any protein with a known function in the database and includes six Arabidopsis proteins (gi 13937216, gi 2191171, gi 671440, gi 18377875, gi 469110, and gi 3912926), two rice proteins (gi 14719312 and gi 15289975), and one cotton protein (gi 25992529). Di19 has been found to be strongly expressed in both roots and leaves of Arabidopsis during sustained drought conditions (Gosti et al., 1995). After searching the database further, we identified a ZZ-type zinc finger motif homologous to proteins from mouse and human within HRB1 protein sequence (Figure 6A; Yu et al., 1997; Tanaka et al., 2000). The Cys-x2-Cys motifs in ZZ domains are reminiscent of the Cys-x2-Cys knuckles that occur in zinc fingers (Davies et al., 1996). Unlike most other zinc fingers, the ZZ-type zinc finger identified in HRB1, its mouse and human orthologs, and dystrophin contain only four of the six conserved residues. The remaining two Cys residues are replaced by His residues (Figure 6A). The ZZ-type zinc finger motif also appears to be conserved in all Di19-like proteins (Figure 6B).
Figure 6.
HRB1 Encodes a ZZ-Type Zinc Finger Protein.
(A) Alignment of the ZZ-type zinc finger motif of HRB1 with the ZZ-type zinc finger protein 313 from mouse and human. Cys-x2-Cys motifs and conserved His residues are underlined. The IVGL or QEGL motif as a variant of ZZ conserved DYDL motif is indicated with a double underline.
(B) Amino acid sequence alignment of HRB1 (gi 13937216) with the Di19 protein family from Arabidopsis (gi 2191171 to gi 3912926), rice (Oryza sativa) (gi 14719312 and gi 15289975), and cotton (Gossypium hirsutum) (gi 25992529). Highly conserved residues are indicated by shaded reverse contrast. Asterisks indicate similar residues. Putative nuclear localization signal, similar to the common SV40 large antigen with a pattern of four basic amino acid residues, is labeled with a single line above the residues. Double lines above the residues represent the Robbins and Dingwall nuclear targeting consensus sequence. The conserved ZZ-type zinc finger domain is also indicated as a dotted line above the amino acid residues for the Di19 protein family.
HRB1 Localizes to the Nucleus, and HRB1 Expression Is Enhanced by Red, Far-Red, and Blue Light
Examination of the HRB1 protein sequence with the PSORT program also identified a putative nuclear localization sequence and a Robbins and Dingwall nuclear targeting consensus sequence (Figure 6B; Robbins et al., 1991). The putative nuclear localization sequence is of the SV40 large antigen type and contains four basic amino acid residues. The Robbins and Dingwall sequence consists of two basic residues, a 10-residue spacer, and another basic region in which at least three residues out of five residues are basic. Using an in vivo transient expression system, we found that HRB1:green fluorescent protein (GFP) fusion protein was localized to the nucleus in onion (Allium cepa) epidermal cells under either dark or light conditions (Figure 7A, top). Staining with 4′,6-diamidino-2-phenylindole verified that the spots with the strong green fluorescence correspond to the locations of the nuclei (Figure 7A, bottom). By contrast, cells transformed with GFP alone showed green fluorescence in both the cytoplasm and the nucleus.
Figure 7.
HRB1 Localizes to the Nucleus, and HRB1 Expression Is Enhanced by Red, Far-Red, and Blue Light.
(A) Subcellular localization of GFP or HRB1:GFP fusion proteins in onion epidermal cells. The image shown was taken for cells exposed to white light (30 μmol/m2/s). DAPI, 4′,6-diamidino-2-phenylindole. Bar = 100 μm.
(B) Autoradiogram of RNA gel blots showing enhanced expression of HRB1 by red, far-red, and blue light (left) and normalization of relative HRB1 message levels to rRNA signal (right). Total RNA was isolated from 4-d-old dark-grown Ws seedlings that received no light treatment (D) or were treated for 4 h with red light (R), far-red light (FR), or blue light (B) under intensities as specified in Figure 1A. Ten micrograms of total RNA was loaded on each lane and probed with a radiolabeled HRB1 coding sequence. The same blot was exposed to a phosphor image screen, and the signals were quantified using an ImageQuant program.
(C) Light-induced expression of HRB1 in various photoreceptor mutants. Total RNA was isolated from 4-d-old dark-grown seedlings that received no light treatment (D) or were treated for 4 h with red light (R), far-red light (FR), or blue light (B) under intensities as specified in Figure 1A. phyB-9, phyA-211, and cry1 cry2 were abbreviated as phyB, phyA, and cry1/2.
(D) Normalization of relative HRB1 message levels to rRNA signal. The same blot was exposed to a phosphor image screen, and the signals were quantified using an ImageQuant program.
We next examined HRB1 expression under various monochromatic light conditions through RNA gel blot analysis (Figure 7B). HRB1 mRNA abundance is detected by an HRB1 coding sequence probe, and the same blot was also probed with ribosome RNA. After a normalization of HRB1 mRNA abundance to rRNA signal, we found that the expression of HRB1 was induced 3.1-fold by red light, 2.6-fold by far-red light, and 2.3-fold by blue light over the dark expression level (Figure 7B). The red and far-red-light–induced expression of HRB1 was largely diminished in the phyB-9 or phyA-211 mutant (Figures 7C and 7D). Apparently, phyB is the major photoreceptor mediating the red light–induced expression of HRB1 among all red light–sensing phytochromes, phyB to phyE. However, the blue light–induced expression of HRB1 was only partially reduced in each single cry mutant, cry1 or cry2, but largely diminished in the cry1 cry2 double mutant. Therefore, cry1 and cry2 may have redundant function in mediating the blue light–induced expression of HRB1. Although HRB1 negatively regulates red and blue light signaling for the inhibition of hypocotyl elongation response, red and blue light enhance the expression of HRB1. A similar pattern of function and expression regulation has also been observed for PIF4 (Huq and Quail, 2002).
HRB1 Regulates Both phyB Signaling and cry Signaling
To examine how HRB1 functions with respect to the photoreceptors phyB and crys, we generated hrb1 phyB-9, hrb1 cry1, and hrb1 cry2 double mutants and an hrb1 cry1 cry2 triple mutant. The loss-of-function phyB-9 mutant has a long hypocotyl phenotype under red light, whereas hrb1 exhibits a short hypocotyl phenotype under red light. Under two different intensities of red light, different lines of the hrb1 phyB-9 double mutant showed a similar long hypocotyl phenotype (Figures 8A and 8C). Thus, phyB-9 is epistatic to hrb1 based on their genetic interaction, and the observed epistasis likely reflects a dependence of hrb1 phenotype on phyB. The slightly longer hypocotyl of the hrb1 phyB-9 double mutant than the phyB-9 single mutant under red light may be due to a hybrid vigor. We therefore included Ws × Landsberg erecta (Ler) wild type and Ws × Columbia (Col) wild types to control the difference in hypocotyl growth between crosses of different ecotypes. Under blue light, phyB-9 has a hypocotyl elongation response indistinguishable from that of the Col wild type, and the hrb1 phyB-9 double mutant exhibits a similar hypocotyl phenotype as the hrb1 single mutant (Figure 8B).
Figure 8.
HRB1 Regulates phyB Signaling and cry Signaling.
(A) Hypocotyl growth responses of Ws, hrb1, Col, phyB-9, Ws Col, hrb1 phyB-9, Ler, cry1, Ws Ler, hrb1 cry1, cry2, and hrb1 cry2 to 6 or 15 μmol/m2/s red light (Red-6 or Red-15).
(B) Hypocotyl growth responses of Ws, hrb1, Col, phyB-9, Ws Col, hrb1 phyB-9, Ler, cry1, Ws Ler, hrb1 cry1, cry2, and hrb1 cry2 to 4 or 10 μmol/m2/s blue light (Blue-4 or Blue-10) or 30 μmol/m2/s white light (White-30).
(C) Hypocotyl growth responses of multiple hrb1 phyB-9 lines to 6 μmol/m2/s red light (Red-6) and multiple hrb1 cry1 or hrb1 cry2 lines to 4 μmol/m2/s blue light (Blue-4).
(D) Hypocotyl growth responses of cry1 cry2 and multiple hrb1 cry1 cry2 lines to 10 μmol/m2/s blue light (Blue-10). Ws/Col′ indicates a further cross of Ws Col to Col to control the difference between different ecotypes. Data presented are means ± se. Each measurement includes 40 to 50 seedlings.
Under blue light, both cry1 and cry2 exhibit a long hypocotyl phenotype, although cry2 has a less severe phenotype. By contrast, hrb1 has a short hypocotyl phenotype under blue light. In both the hrb1 cry1 and the hrb1 cry2 double mutants, cry1 or cry2 is partially epistatic to hrb1 because the long hypocotyl phenotype of cry1 or cry2 is partially suppressed by the hrb1 mutation under blue light (Figure 8B). However, the cry1 cry2 double mutant is fully epistatic to hrb1, and such epistasis may suggest that the expression of the hrb1 phenotype under blue light is dependent on both cry1 and cry2 molecules (Figure 8D). Under red light, we noticed that cry1 had a slightly longer hypocotyl than its wild type Ler, but cry1 does not function to perceive red light (Figure 8A). The hrb1 cry1 double mutant had a hypocotyl phenotype resembling that of the hrb1 single mutant under red light, indicating that the red light phenotype of the hrb1 cry1 double mutant is unlinked to the cry1 mutation. Under red light, cry2 had a similar hypocotyl elongation response compared with the Col wild type, whereas the hrb1 cry2 double mutant exhibited a short hypocotyl phenotype as observed in the hrb1 single mutant (Figure 8A).
The long hypocotyl phenotype of phyB-9 was also dominant under white light in the hrb1 phyB-9 double mutant, and the hrb1 mutation also strongly suppressed the long hypocotyl phenotype of cry1 under white light (Figure 8B). Both phyB and cry1 do have a long hypocotyl phenotype under white light, and the blue component in white light is apparently unable to suppress the long hypocotyl phenotype of phyB-9 through the action of wild type crys. Similarly, the red component in white light also fails to suppress the long hypocotyl phenotype of cry1 through the action of wild-type phyB molecules. Therefore, the detected epistasis of hrb1 with phyB or cry1 under white light is similar to the one-on-one interaction of hrb1 with phyB or cry1 under respective monochromatic red or blue light.
Genetic Interactions of HRB1 with phyB or cry in Control of Light-Regulated Gene Expression
The genetic interaction between HRB1 and phyB or cry was also studied by examining the expression of CAB3, CHS, and PIF4 in the phyB or cry single mutant and the phyB-9 hrb1 and cry hrb1 double or triple mutants (Figure 9). The red light–induced expression of CAB3, CHS, and PIF4 was greatly affected by the phyB-9 mutation and was also affected in the phyB-9 hrb1 double mutant in a way very similar to the phyB-9 single mutant (Figure 9). Therefore, phyB-9 is epistatic to hrb1 not only for hypocotyl elongation response but also for light-regulated gene expression response (Figures 8 and 9). A strong argument can be made for the expression of CAB3 under red light, which was affected in both the phyB-9 single mutant and the phyB-9 hrb1 double mutant, but was not affected in the hrb1 single mutant (Figures 3 and 9). By contrast, the blue light–induced expression of CAB3, CHS, and PIF4 was only partially impaired in either cry1 or cry2 single mutant, much affected in cry1 hrb1 and cry2 hrb1 double mutants, and greatly diminished in the cry1 cry2 double mutant (Figure 9). The expression of CAB3, CHS, and PIF4 in the hrb1 cry1 cry2 triple mutant was very similar to that in the cry1 cry2 double mutant, indicating an epistasis of cry1 cry2 to hrb1 (Figures 10A and 10B).
Figure 9.
hrb1 Interacts Genetically with phyB-9 or cry in Control of Light-Induced Expression of CAB3, CHS, and PIF4.
(A) RNA gel blot analysis of CAB3, CHS, and PIF4 expression on total RNA isolated from 4-d-old dark-grown seedlings that received no light treatment (D) or were treated for 4 h with red light (R) or blue light (B) under intensities as specified in Figure 1A. phyB-9 and cry1 cry2 were abbreviated as phyB and cry1/2, respectively. Ten micrograms of total RNA was loaded on each lane.
(B) Normalization of CAB3, CHS, and PIF4 messages to rRNA signal. The same blot was exposed to a phosphor image screen, and the intensity of each band was quantified using an ImageQuant program.
Figure 10.
cry1 cry2 Is Epistatic to hrb1, and a Model Predicts the Function of HRB1 in Light Signaling.
(A) RNA gel blot analysis of CAB3, CHS, and PIF4 expression on total RNA isolated from 4-d-old dark-grown seedlings that received no light treatment (D) or were treated for 4 h with blue light (B) under intensities as specified in Figure 1A. cry1 cry2 and hrb1 cry1 cry2 were abbreviated as cry1/2 and hrb1/cry1/2, respectively. Ten micrograms of total RNA was loaded on each lane.
(B) Normalization of CAB3, CHS, and PIF4 messages to rRNA signal. The same blot was exposed to a phosphor image screen, and the intensity of each band was quantified using an ImageQuant program.
(C) In the nucleus, HRB1 may interact with a light signaling component (LSC) or a transcription regulator (TR) through its ZZ-type zinc finger domain. The interaction either generates signals to regulate various light responses or leads to a regulated expression of LSCs, including PIF4. Arrows and T-bars represent positive or negative effects, respectively. Solid lines and dotted lines represent the blue or red light signaling pathways, respectively, for deetiolation. The model suggests that both LSC and the transcription regulator, but not HRB1, may act directly downstream of phyB or crys.
DISCUSSION
We have demonstrated that hrb1 contains a loss-of-function mutation, causing both a short hypocotyl and a short petiole phenotype (Figures 1 and 2). HRB1 thus acts negatively to regulate red and blue light–mediated inhibition of hypocotyl and petiole elongation. By contrast, hrb1 shows small leaves and reduced expression of CAB3, CHS, and PIF4 (Figures 2 and 3). HRB1 thus acts positively to regulate red and blue light–mediated leaf expansion and gene expression. Apparently, HRB1 can function either negatively or positively to regulate red and blue light responses. Similarly, PIF3 has also been shown to act either as a positive regulator or a negative regulator of red and far-red light signaling (Kim et al., 2003).
Similar to hrb1, pif4 also shows a hypersensitive hypocotyl growth response to red and blue light (Figure 4A). The hypocotyl growth response of the hrb1 pif4 double mutant to red and blue light is similar to that of the hrb1 or pif4 single mutant, suggesting that HRB1 and PIF4 may function closely in the same signaling branch. By contrast, a severe or additive phenotype of the hrb1 pif4 double mutant compared with that of either single mutant would indicate that HRB1 and PIF4 function distantly on separate signaling branches. Within the same signaling branch, HRB1 may function upstream of PIF4 because HRB1 activity is required for the proper expression of PIF4 under both red and blue light. Thus, the hypersensitive hypocotyl growth response of hrb1 to red and blue light may be attributed to a reduced PIF4 expression in hrb1 under red and blue light. The pif4 mutant contains a semidominant mutation, and a half reduction in PIF4 message can result in a noticeable change in hypocotyl growth response (Huq and Quail, 2002). Our studies of control of light-regulated expression on CAB3 and CHS in the hrb1 pif4 double mutant seem to support the proposal (Figure 4B). Mutation in either HRB1 or PIF4 partially impaired red and blue light–induced expression of CAB3 and CHS, but the effects by PIF4 mutation were much more severe. In all cases examined, the hrb1 pif4 double mutant behaves similarly to the pif4 single mutant; therefore, pif4 is epistatic to hrb1 in the control of light-regulated gene expression responses. The change we detected in the expression of CAB3 and CHS in pif4 under both red and blue light is different from the previous reports that the expression of CAB3 and other light-regulated genes was unaffected in pif4 (Huq and Quail, 2002). The cause of this discrepancy remains unclear.
The HRB1 protein contains a ZZ-type zinc finger motif (Figure 6A). The ZZ-type zinc finger motif has been demonstrated to be involved in protein–protein interactions between cytoskeleton dystrophin and calmodulin and between transcriptional adapters, such as CREB binding proteins or p300, and transcriptional activators (Davies et al., 1996). The motif is also present in many RING domain proteins thought to be involved in protein traffic or protein degradation (Jin et al., 2002; Itoh et al., 2003). The ZZ-type zinc finger motif in dystrophin appears to bind calmodulin, and a missense mutation on one of the conserved Cys residues in dystrophin causes Duchenne muscular dystrophy (Davies et al., 1996). Within the CREB binding and p300 proteins, the binding sites for E1A and TFIIB have been narrowed down to regions that contain the ZZ-type zinc finger and a second TAZ zinc finger (Davies et al., 1996). The motifs in RING domain proteins may function as additional sites of protein–protein interactions (Jin et al., 2002; Itoh et al., 2003). We infer that ZZ-type zinc finger domain in HRB1 is likely involved in interactions with other light signaling components in either the red or blue light signaling pathway.
Genetic interaction studies on hypocotyl growth and light-regulated gene expression responses suggest that phyB-9 is epistatic to hrb1, and such epistasis likely reflects a dependence of hrb1 phenotype on phyB (Figures 8 and 9). On the other hand, the epistasis between hrb1 and phyB-9 may indicate that HRB1 functions upstream of phyB. Such an argument can be supported by the fact that HRB1 activity is required for the proper expression of PIF4, a gene encoding a phytochrome interacting protein (Figure 3A). A similar genetic epistasis has been observed for either pif3 or pif4 with phyB (Halliday et al., 1999; Huq and Quail, 2002), suggesting that PIF3 or PIF4 may also function upstream of phyB. Indeed, the nature of the interaction between PIF4 and phyB is probably a negative regulation of phyB-mediated inhibition of hypocotyl elongation.
Genetic interaction studies on hypocotyl growth response suggest that cry1 cry2 is epistatic to hrb1, and such epistasis may indicate a dependence of hrb1 phenotype on both cry1 and cry2 under blue light (Figure 8). Genetic interaction studies on light-regulated gene expression responses in the hrb1 cry1 cry2 triple mutant also suggest a similar epistasis (Figures 9 and 10). However, an epistatic relationship may not be easily deduced from these studies on light-regulated gene expression responses in the hrb1 cry1 or the hrb1 cry2 double mutant. It is largely because the expression of CAB3, CHS, and PIF4 expression is impaired in the same direction in hrb1 and cry1 or cry2. The blue light–induced expression of CAB3, CHS, and PIF4 is impaired weakly in cry1 or cry2 single mutants, intermediately in cry1 hrb1 or cry2 hrb1 double mutants, and strongly in the cry1 cry2 double mutant (Figure 9). Such attenuation by the cry1 hrb1 (or cry2 hrb1) double mutation on the expression of those genes may be due to the cry1 (or cry2) mutation and a reduced cry2 (or cry1) signaling through the hrb1 mutation. The genetic interaction of HRB1 with crys is different from that of SUB1 with crys. Another striking difference is the opposite way in regulating the expression of CHS. SUB1 appears to regulate the expression of CHS negatively (Guo et al., 2001), whereas HRB1 appears to regulate the expression of CHS positively (Figure 3).
A model is proposed for the roles of HRB1 in red and blue light signaling (Figure 10C). Based on the function of its ZZ-type zinc finger domain, HRB1 may interact with a light signaling component (LSC) in the nucleus. Through the interaction, either HRB1 can regulate the activity of the LSC or the activity of HRB1 can be regulated by the LSC, therefore generating positive or negative signals to regulate various light responses. Alternatively, HRB1 may function as a transcription adapter that interacts with a transcription regulator. The interaction may instead result in a regulated expression of a few LSCs, including PIF4. The model suggests that LSC or the transcription regulator, but not HRB1, may act directly downstream of crys or phyB. In the proposed model, HRB1 together with PIF4 may define points where red light signaling and blue light signaling intersect.
METHODS
Plant Growth Conditions
The hrb1 mutant was isolated from a T-DNA insertion population in the Ws background (40,000 lines donated by University of Wisconsin, Arabidopsis Biological Resource Center). Genetic screens and subsequent characterization of hrb1 were conducted in GM growth medium minus sucrose. Monochromatic red, far-red, or blue light were generated with an LED SNAP-LITE (Quantum Devices, Barnereld, WI). Light intensity and peak wavelength were measured with a SPEC-UV/PAR spectroradiometer (Apogee Instruments, Logan, UT). Hypocotyl, cotyledon, and leaf images were taken using an Olympus digital Camedia C-700 (Tokyo, Japan), and their length and area were measured using NIH image (public domain; Bethesda, MD). Petiole length and leaf area were measured for the first fully expanded true leaf.
Molecular Cloning of HRB1
Genomic DNA was isolated from white light–grown plants using the DNeasy plant mini kit (Qiagen, Valencia, CA). Plant DNA sequence flanking the T-DNA left border sequence was obtained with a GenomeWalker kit (Clontech, Palo Alto, CA). The left T-DNA border primers used were JL202 (5′-CATTTTATAATAACGCTGCGGACATCTAC-3′) and JL270 (5′-TTTCTCCATATTGACCATCATACTCATTG-3′). Total RNA was isolated from plants grown under various light conditions using the SV total RNA isolation system (Promega, Madison, WI). DNA gel blot analysis, RNA gel blot analysis, and RT-PCR analysis were performed as described (Ni et al., 1998).
Complementation and Subcellular Localization
A 3.8-kb genomic fragment, spanning 1.6 kb upstream to 0.6 kb downstream of the HRB1 coding sequence, was cloned into binary vector pKF111 (Ni et al., 1998). The fragment was generated using PCR from TAC clone K21P3 (Arabidopsis Biological Resource Center) and contains end-incorporated ClaI and XhoI restriction sites. Transformation of the construct into hrb1 and selection of the transgenic plants were performed as described previously (Ni et al., 1998).
The putative nuclear localization sequence and Robbins and Dingwall nuclear targeting consensus sequence were identified using the PSORT program (http://psort.nibb.ac.jp). For the transient assay on HRB1 subcellular localization, the HRB1 coding sequence containing XbaI and XmaI restriction sites was PCR-generated and cloned into a modified PBI121 vector, in which the β-glucuronidase coding sequence was replaced with a GFP coding sequence. Transformation of HRB1:GFP into onion (Allium cepa) epidermal cells and 4′,6-diamidino-2-phenylindole staining were performed as described previously (Ni et al., 1998). GFP fluorescence and nuclear images were acquired using a Nikon Eclipse E800 microscope (Tokyo, Japan) with a Cool Cam color CCD camera (Cool Camera, Decatur, GA) and Imago Pro Plus version 3.0 software (Media Cybermetics, Silver Spring, MD).
Double Mutant Analysis
The hrb1 pif4 double mutant was generated by genetic cross. Both hrb1 and pif4 are in the Ws background and contain a T-DNA insertion in their coding regions (Huq and Quail, 2002). Homozygous hrb1 or pif4 mutation was genotyped using PCR at F2 or F3 generation. The phyB-9 (Col), hy4-1 (cry1, Ler), and cry2-1 (Col) mutants were obtained from the Arabidopsis Stock Center and were used to generate hrb1 phyB-9, hrb1 cry1, and hrb1 cry2 double mutants. phyB-9 is an ethyl methanesulfonate mutant, whereas both hy4-1 and cry2-1 contain large deletions. The homozygous phyB-9 mutation was selected at the F2 generation based on a striking long hypocotyl phenotype under red light. The homozygous cry1 or cry2 mutation was selected at the F2 generation based on a long hypocotyl phenotype under blue light but verified by PCR genotyping at the F3 generation. The homozygous hrb1 mutation was selected by a linked resistance to kanamycin from the F3 individuals in a homozygous phyB-9 or cry1 or cry2 background. Ws × Col wild type and Ws x Ler wild type were also generated to control the difference in hypocotyl growth between crosses of different ecotypes. Both phyA-211 (Arabidopsis Stock Center) and the cry1 cry2 double mutant used for gene expression analysis are in Col ecotype background. To generate hrb1 cry1 cry2 triple mutants, hrb1 cry2 (Ws Col) was crossed to cry1 cry2 (Col). The cry1 cry2 double mutant contains large deletions in both loci. The homozygous cry1 mutation was verified by PCR genotyping at the F3 generation, and homozygous hrb1 mutation was selected by a linked resistance to kanamycin from the F3 individuals in a homozygous cry1 and cry2 background. A cross of Ws Col to Col was also generated to control the difference between different ecotypes.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AF372963.
Acknowledgments
We thank Neil Olszewski and William Gray for comments on the manuscript, David Marks for help and use of the Nikon Eclipse E800 microscope, and David Somers and Kimberly Torbert for help and use of the PDS-1000/He biolistic particle delivery system. We also thank Enamul Huq and Peter Quail for pif4 mutant lines, Chentao Lin for the cry1 cry2 double mutant, and the Ohio State Stock Center for Arabidopsis T-DNA insertion collections and BAC clones. This work was supported in part by University of Minnesota start-up and Grant-in-Aid funds (to M.N.) and by grants from the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service (2002-35304-12357 and 2004-35304-14939 to M.N.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Min Ni (nixxx008@tc.umn.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.029165.
References
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366, 162–166. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., and Cashmore, A.R. (1996). The pef mutants of Arabidopsis thaliana define lesions early in the phytochrome signaling pathway. Plant J. 10, 1103–1110. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., Jarrilo, J.A., Smirnova, O., and Cashmore, A.R. (1998). The cry1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1, 939–948. [DOI] [PubMed] [Google Scholar]
- Bolle, C., Koncz, C., and Chua, N.-H. (2000). PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 14, 1269–1278. [PMC free article] [PubMed] [Google Scholar]
- Bowler, C., Neuhaus, G., Yamagata, H., and Chua, N.-H. (1994). Cyclic GMP and calcium mediate phytochrome phototransduction. Cell 77, 73–81. [DOI] [PubMed] [Google Scholar]
- Casal, J.J. (2000). Phytochromes, cryptochromes, phototropin: Photoreceptor interactions in plants. Photochem. Photobiol. 71, 1–11. [DOI] [PubMed] [Google Scholar]
- Choi, G., Yi, H., Lee, J., Kwon, Y.K., Soh, M.S., Shin, B., Luka, Z., Hahn, T.R., and Song, P.S. (1999). Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature 401, 610–613. [DOI] [PubMed] [Google Scholar]
- Clack, T., Mathews, S., and Sharrock, R.A. (1994). The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 25, 413–427. [DOI] [PubMed] [Google Scholar]
- Davies, K.E., Blake, D.J., Ponting, C.P., Winder, S.J., and Kendrick-Jones, J. (1996). ZZ and TAZ: New putative zinc fingers in dystrophin and other proteins. Trends Biochem. Sci. 21, 11–13. [PubMed] [Google Scholar]
- Deng, X.W., and Quail, P.H. (1999). Signalling in light-controlled development. Semin. Cell Dev. Biol. 10, 121–129. [DOI] [PubMed] [Google Scholar]
- Desnos, T., Puente, P., Whitelam, G.C., and Harberd, N.P. (2001). FHY1: A phytochrome A-specific signal transducer. Genes Dev. 15, 2980–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., Yeh, A.C., Lagarias, J.C., Zhang, H., Elich, T.D., and Chory, J. (1999). PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284, 1539–1541. [DOI] [PubMed] [Google Scholar]
- Genoud, T., Millar, A.J., Nishizawa, N., Kay, S.K., Schafer, E., Nagatani, A., and Chua, N.-H. (1998). An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10, 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti, F., Bertauche, N., Vartanian, N., and Giraudat, J. (1995). Abscisic acid-dependent and -independent regulation of gene expression by progressive drought in Arabidopsis thaliana. Mol. Gen. Genet. 246, 10–18. [DOI] [PubMed] [Google Scholar]
- Guo, H., Duong, H., Ma, N., and Lin, C. (1999). The Arabidopsis blue light receptor cryptochrome 2 is a nuclear protein regulated by a blue light-dependent post-translational mechanism. Plant J. 19, 279–287. [DOI] [PubMed] [Google Scholar]
- Guo, H., Mockler, T., Duong, H., and Lin, C. (2001). SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science 291, 487–490. [DOI] [PubMed] [Google Scholar]
- Guo, H., Yang, H., Mockler, T., and Lin, C. (1998). Regulation of flowering time by Arabidopsis photoreceptors. Science 279, 1360–1363. [DOI] [PubMed] [Google Scholar]
- Halliday, K.J., Hudson, M., Ni, M., Qin, M.M., and Quail, P.H. (1999). poc1: An Arabidopsis mutant perturbed in phytochrome signaling because of a T-DNA insertion in the promoter of PIF3, a gene encoding a phytochrome-interacting bHLH protein. Proc. Natl. Acad. Sci. USA 96, 5832–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, H.L., Okamoto, H., Wang, M., Ang, L.H., Matsui, M., Goodman, H., and Deng, X.W. (2000). FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev. 14, 1958–1970. [PMC free article] [PubMed] [Google Scholar]
- Huq, E., and Quail, P.H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21, 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, M., et al. (2003). Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4, 67–82. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Blue, E.K., Dixon, S., Shao, Z., and Gallagher, P.J. (2002). A death-associated protein kinase (DAPK)-interacting protein, DIP-1, is an E3 ubiquitin ligase that promotes tumor necrosis factor-induced apoptosis and regulates the cellular levels of DAPK. J. Biol. Chem. 277, 46980–46986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.M., Ecker, J.R., and Chen, I.G. (2003). A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol. 131, 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski, K.A., and Quail, P.H. (2003). Arabidopsis PSEUDO-RESPONSE REGULATOR7 is a signaling intermediate in phytochrome-regulated seedling deetiolation and phasing of the circadian clock. Plant Cell 15, 2654–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.G., Yun, J., Kim, D.H., Chung, K.S., Fujioka, S., Kim, J.I., Dae, H.W., Yoshida, S., Takatsuto, S., Song, P.S., and Park, C.M. (2001). Light and brassinosteroid signals are integrated via a dark-induced small G protein in etiolated seedling growth. Cell 105, 625–636. [DOI] [PubMed] [Google Scholar]
- Kim, J., Yi, H., Choi, G., Shin, B., Song, P.S., and Choi, G. (2003). Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15, 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Kozma-Bognar, L., Kim, L., Adam, E., Harter, K., Schafer, E., and Nagy, F. (1999). Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11, 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno, N., Muramatsu, T., Hamazato, F., and Furuya, M. (2000). Identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis using a fluorescent differential display technique. Plant Physiol. 122, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X.L., Covington, M.F., Fankhauser, C., Chory, J., and Wagner, D.R. (2001). ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis phyB signal transduction pathway. Plant Cell 13, 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas, P., Devlin, P.F., Panda, S., and Kay, S.A. (2000). Functional interaction of phytochrome B and cryptochrome 2. Nature 408, 207–211. [DOI] [PubMed] [Google Scholar]
- Møller, S.G., Kim, Y.S., Kunkel, T., and Chua, N.-H. (2003). PP7 is a positive regulator of blue light signaling in Arabidopsis. Plant Cell 15, 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M.M., Fankhauser, C., and Chory, J. (2000). Light: An indicator of time and place. Genes Dev. 14, 257–271. [PubMed] [Google Scholar]
- Neuhaus, G., Bowler, C., Hiratsuka, K., Yamagata, H., and Chua, N.-H. (1997). Phytochrome-regulated repression of gene expression requires calcium and cGMP. EMBO J. 16, 2554–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 657–667. [DOI] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1999). Binding of phytochrome B to its nuclear signaling partner PIF3 is reversibly induced by light. Nature 400, 781–784. [DOI] [PubMed] [Google Scholar]
- Okamoto, H., Matsui, M., and Deng, X.W. (2001). Overexpression of the heterotrimeric G-protein α-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis. Plant Cell 13, 1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund, M.T., Hardtke, C.S., Wei, N., and Deng, X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466. [DOI] [PubMed] [Google Scholar]
- Robbins, J., Dilworth, S.M., Laskey, R.A., and Dingwall, C. (1991). Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: Identification of a class of bipartite nuclear targeting sequence. Cell 64, 615–623. [DOI] [PubMed] [Google Scholar]
- Shalitin, D., Yang, H., Mockler, T.C., Maymon, M., Guo, H., Whitelam, G.C., and Lin, C. (2002). Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417, 763–767. [DOI] [PubMed] [Google Scholar]
- Shalitin, D., Yu, X., Maymon, M., Mockler, T., and Lin, C. (2003). Blue light–dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell 15, 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, H.S., Yang, J.Y., Ishikawa, M., Bolle, C., Ballesteros, M.L., and Chua, N.-H. (2003). LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423, 995–999. [DOI] [PubMed] [Google Scholar]
- Staiger, D., Allenbach, L., Salathia, N., Fiechter, V., Davis, S.J., Millar, A.J., Chory, J., and Fankhauser, C. (2003). The Arabidopsis SRR1 gene mediates phyB signaling and is required for normal circadian clock function. Genes Dev. 17, 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere, U., Eichenberg, K., Lohrmann, J., Mira-Rodado, V., Baurle, I., Kudla, J., Nagy, F., Schafer, E., and Harter, K. (2001). Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294, 1108–1111. [DOI] [PubMed] [Google Scholar]
- Tanaka, T.S., et al. (2000). Genome-wide expression profiling of mid-gestation placenta and embryo using a 15,000 mouse developmental cDNA microarray. Proc. Natl. Acad. Sci. USA 97, 9127–9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Ma, L.G., Li, J.M., Zhao, H.Y., and Deng, X.W. (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294, 154–158. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagatani, A. (1999). Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., Wu, Y., Tang, R., Liu, D., Liu, Y., and Cashmore, A.R. (2000). The C-termini of Arabidopsis cryptochrome mediate a constitutive light response. Cell 103, 815–827. [DOI] [PubMed] [Google Scholar]
- Yeh, K., and Lagarias, C. (1998). Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc. Natl. Acad. Sci. USA 95, 13976–13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W., Anderson, B., Worley, K.C., Muzny, D.M., Ding, Y., Liu, W., Ricafrente, J.Y., Wentland, M.A., Lennon, G., and Gibbs, R.A. (1997). Large-scale concatenation cDNA sequencing. Genome Res. 7, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]