Abstract
Background:
Pre-eclampsia is a multi-systemic disease with its attendant increased maternal and perinatal morbidities and mortality. It has been hypothesized that leptin contributes immensely to the natural history of pre-eclampsia. However, there is considerable disagreement in the reports of existing research work on the link between fetomaternal serum leptin levels and pre-eclampsia.
Objective:
To determine and compare the maternal and umbilical cord sera levels of leptin in women with pre-eclampsia and healthy pregnant women.
Study design:
This is an analytical cross-sectional study.
Methods:
The study involved consenting 120 pregnant participants (60 on each arm). Pregnant women diagnosed with pre-eclampsia constituted the investigation group, while the controls were normotensive pregnant women. They were matched for maternal age and body mass index. Venous blood specimens were obtained from the participants for assessment of the serum leptin concentration while umbilical cord blood samples were obtained following delivery of the neonate in advance of the removal of the placenta. The collected blood samples were analysed for the levels of leptin in a blinded pattern. The primary outcome measures were maternal serum leptin levels and umbilical cord serum leptin levels.
Results:
Mean maternal serum leptin concentration in the pre-eclampsia group was significantly higher than that in the control group (24.88 ± 3.92 vs. 15.03 ± 2.98ng/mL, p < 0.001). Similarly, maternal serum leptin concentration was significantly higher in participants with severe pre-eclampsia compared with those with mild pre-eclampsia (25.91 ± 3.5 vs. 22.83 ± 4.02ng/mL, p = 0.003). However, the mean umbilical cord serum leptin level in the pre-eclampsia group was significantly lower than in the control group (6.43 ± 2.08 vs. 7.27 ± 2.24; p = 0.034). There was a weak positive correlation between maternal serum leptin level and neonatal umbilical serum leptin level in the pre-eclamptic group (r = 0.21, p = 0.04).
Conclusion:
Maternal serum leptin concentration is significantly increased in women with pre-eclampsia, compared with their normotensive counterparts. This increase becomes even more pronounced as the severity of the disease progresses. Maternal serum leptin assessment has the potential to become a veritable tool in the diagnosis and monitoring of pregnancies complicated by pre-eclampsia.
Keywords: APGAR score, birthweight, healthy pregnant women, pre-eclampsia, serum leptin, umbilical blood level
Introduction
Pre-eclampsia is a multi-system disease consisting of new onset hypertension and proteinuria after 20 weeks of gestation.1,2 The clinical presentation of pre-eclampsia occurs as the result of a spectrum of microangiopathy of vessels in affected organs, especially the brain, liver, kidney, and placenta. The risk of adverse maternal or foetal outcome is significantly increased in pregnancies complicated by pre-eclampsia. Although there is considerable evidence that most of the clinical manifestations of the disease are due to angiogenic imbalance and widespread endothelial malfunction, the exact aetiology of this syndrome remains largely unknown.1,3 Some studies have advocated that endothelial impairment, vasoconstriction, placental ischaemia, and increased coagulation, which are seen in pre-eclampsia, are attributable to a hitherto unknown serum factor that is produced by trophoblastic tissue. 4 This factor known as leptin may be a component of the immune system.
Multiple data from previous works in this area have demonstrated some links between leptin, inflammatory cytokines, and pre-eclampsia.5,6 The structure of leptin is closely related to that of cytokines. 7 Leptin may, therefore, have an important role in human diseases like pre-eclampsia in which the underlying pathophysiology is a sterile inflammatory response. 6 In addition, pre-eclampsia has been associated with increased maternal plasma leptin concentration,3,8 giving rise to the hypothesis that leptin may be implicated in the pathophysiology of the disorder.5,9 The above gives grounds to the suspicion of a possible connection between serum concentration of leptin and umbilical cord blood levels in women who have pre-eclampsia and their newborn or infants.
Leptin, the first adipokine to be discovered, is a 16-kDa polypeptide hormone made up of 167 amino acids. In human beings, leptin is encoded by the LEP gene (obesity gene), which is situated on the long arm of chromosome 7 (7q32). 10 Umbilical cord blood leptin originates from tissues of the developing foetus. In the serum, leptin is detectable at about 18 weeks gestation and increases to significant levels after 34 weeks of gestation, a period during which the foetus begins to develop adipose tissue. 11
Apart from its role in pregnancy, leptin has been implicated in the development of hypertensive disease by some researchers such as previously documented by Narkiewicz et al. 4 and Villarreal et al. 12 Evidence has indicated that pregnant women with pre-existing hypertension possess an increased chance of developing pre-eclampsia, but the exact contribution of leptin, however, is yet to be comprehensively investigated. 13 While some previous studies by Villarreal et al. 12 and Sattar et al. 14 concluded that pre-eclampsia did not affect serum leptin concentration, contrastingly, Laml et al., 13 Teppa et al., 15 Briana and Malamitsi-Puchner 16 have demonstrated significantly elevated leptin levels in pre-eclamptic women. With many of these works showing conflicting results in the relationship between leptin and pre-eclampsia, there is a need for further research on this relationship, as leptin is presumed to play a significant role in the prediction, prevention, and treatment of pre-eclampsia in the near future. Most of the research done on leptin in pregnancy and pre-eclampsia was carried out on Caucasians, while there exists a paucity of similar works among pregnant Black African women who have a significant burden of the disease prevalence, morbidity, and mortality.
The primary site of leptin synthesis is the adipocytes and in adult life, leptin concentration is directly related to body fat content and to the level of nutrient intake. In infancy, there is also a positive relationship between concentrations of leptin in umbilical cord blood at birth and neonatal birthweight or neonatal adiposity. 17 Furthermore, several works have demonstrated that the placenta secretes the hormone in significant quantities.18,19 Since placental leptin makes significant contributions to intrauterine growth of the foetus 14 and pre-eclampsia remains the commonest aetiology for intrauterine growth restriction, 20 it will be of great interest to assess the concentration of leptin in babies born to pre-eclamptic mothers. Thus, the purpose of this comparative cross-sectional study was to determine and compare the maternal and umbilical cord sera blood levels of leptin in women diagnosed with pre-eclampsia and controls (normal healthy pregnant women).
Method
This analytical cross-sectional study was carried out among pregnant women at Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi, Nigeria from 20 March 2020 to 31 September 2020. Ethical approval for the study protocol was sought and approved by the NAUTH Nnewi Ethics committee on 19 March 2020 (Ref no. NAUTH/CS/66/VOL.13/013/2019/100). All participants of the study gave written informed consent. The authors adhered to STROBE Guidelines.
The inclusion criteria were pregnant women aged 18–45 years with pre-eclampsia. Matched healthy pregnant women without pre-eclampsia formed the control group. Those excluded from the study were participants with the following medical disorders in pregnancy: chronic hypertension, diabetes in pregnancy, multiple pregnancy, retroviral disease and renal disease, and history of smoking or malignancy in the past 6 months.
The lowest acceptable sample size was calculated by applying the formula for comparison of means between two groups for quantitative data by Charan and Biswas. 21 N = 2 SD2 (Zα/2 + Zβ)2/d2, where STD is the standard deviation = from Asnafi et al., 3 Zα/2 = Z0.05/2 = 1.96 (from Z table) at type 1 error of 5%, Zβ = Z0.20 = 0.842 (from Z table) at 80% power, d = effect size = difference between mean umbilical cord leptin concentration in both groups. At 10% attrition rate, 60 patients were recruited in each group (60 pre-eclamptic women and 60 normotensive pregnant women).
Subjects recruitment
Participants were enlisted consecutively from consenting gravid women attending the antenatal clinic or labour ward of NAUTH, Nnewi. Cases were women with pre-eclampsia, while the controls were other healthy pregnant women accessing antenatal care or labour ward services without a diagnosis of pre-eclampsia. The cases and controls were matched for age (±2 years), and body mass index (BMI; ±5 kg/m2). Eligible subjects were counselled and their consent was obtained prior to recruitment. The enlistment of cases was sustained until the required sample size had been attained.
Study procedure
The participants rested for a minimum of 5 min, adhering strictly to the standard procedure; their blood pressure was measured using a standard mercury-in-glass sphygmomanometer (Accosson, UK) with an appropriate adult cuff size for each subject and recorded.
For venous blood sample collection, the subject was seated in a comfortable position or lying comfortably in bed. A tourniquet was fastened above the vein of interest on the limb. The skin over the identified vein was cleaned with methylated spirit swab. Thereafter, about 4 mL of blood was withdrawn from the vein and transferred to a previously code-labelled plain sample bottle (to ensure that the senior medical laboratory scientist was blinded to the study and control samples). The sample was allowed to clot for about 2 h and then spun in the centrifuge at 2500 rpm for 10 min. The serum obtained was refrigerated until analysis was carried out by the collaborating senior medical laboratory scientist in a blinded pattern at the Chemical pathology laboratory of NAUTH, Nnewi. Maternal blood sample was collected at the point of diagnosis of labour or once the decision to undertake caesarean section was taken.
Following delivery, 10 mL of blood was collected aseptically from the umbilical vein just after securing the cord and before cleavage of the placenta. The sample was transferred to a plain bottle previously labelled with the subject’s code. The sample was allowed to clot, the serum was extracted by centrifugation at 2500 rpm for 10 min and stored at −20°C for subsequent analysis as for the maternal serum. In addition, the neonatal birthweight, head circumference, Apgar score, length, and ponderal index were recorded.
The quantitative detection of leptin concentration was executed using Leptin (human) ELISA (enzyme-linked immunosorbent assay) Kit (manufactured by BioVision, Inc. USA). The kit is an in vitro enzyme-linked immunosorbent test for the empirical assessment of human leptin. This assay makes use of a human leptin-specific antibody coated on a 96-well plate. Following reactions and subsequent colour change, the intensity of the colour was assessed at 450 nm by spectrophotometry to determine the concentration of leptin. The lowest detectable level of leptin was 2 pg/mL, while the detection range was 2–400 pg/mL. The intra-assay reproducibility was CV < 10% and inter-assay was CV < 12%.
Dipstick urinalysis was done using a clean-catch midstream urine sample having cleaned the vulva with clean water before micturition and subsequently, collection of the urine sample from the middle of a continuous urine stream, into a clean specimen bottle. For subjects already on urethral catheterization, urine sample from the urinary catheter were assessed. Dipstick urinalysis was carried out on the urine sample by the researcher or a trained research assistant using the Medi Test Combi 2 (Neumann-Neander Str.6-8 52355 Düren Germany) dipstick urinalysis strip in accordance with the manufacturer’s directives. The resulting colour transformation on the test strip was compared with the reference ranges on the strip canister.
Proteinuria was classified as significant when the concentration of protein in a 24-h urine sample equals or exceeds 300 mg, or the detection of two plusses (++) on dipstick in at least two urine samples assessed at intervals not less than 4–6 h apart. 22 Pre-eclampsia was defined as the presence of hypertension and significant proteinuria ‘arising de novo after the 20th week of pregnancy in a previously normotensive and nonproteinuric woman’. 2
The primary outcome measures were maternal serum leptin levels and umbilical cord serum leptin levels in both groups, while the secondary outcome measures were APGAR score and mean birthweight in both groups.
Statistical analysis
Following collection, entry, and checking of the data, the participants’ codes were broken. The data collected were analysed using Stata version 14 (StataCorp USA, 2015). Descriptive statistics were computed for all relevant data and shown as frequency tables and charts. Normality testing was done. The association between maternal serum leptin level and neonatal umbilical cord serum leptin level was tested using Pearson’s correlation coefficient. The mean serum leptin levels of pre-eclamptics and normotensive cases were compared using the Student’s t-test. Univariable and multivariable logistic backward linear regression models were used to control for covariates and confounders for pre-eclampsia. Statistical significance levels were set at p value < 0.05.
Results
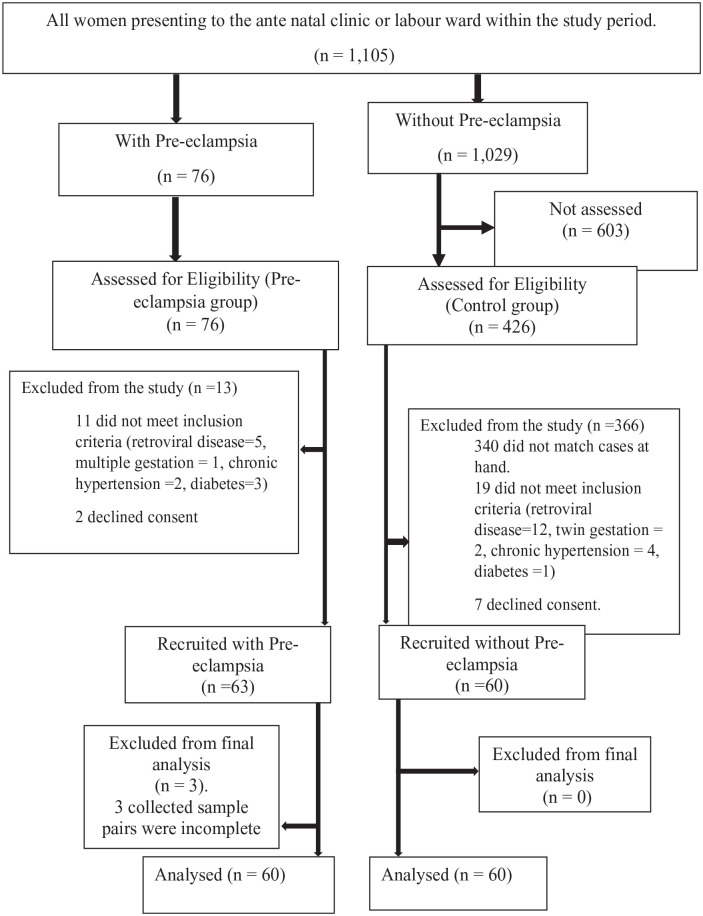
During the study period, out of the 76 and 426 participants in the pre-eclamptic and control groups, respectively, that were assessed for eligibility, 63 participants with pre-eclampsia and 60 participants in the control group were recruited into the study. Three participants with pre-eclampsia had incomplete results and were excluded from the study. Thus the results of 60 participants with pre-eclampsia and that of 60 controls were used for the analysis. This is shown in Figure 1.
Figure 1.
Flow chart of the study.
The baseline characteristics including age, GA, parity, and BMI were similar in both groups as shown in Table 1.
Table 1.
Socio-demographic characteristics of the study participants.
| Variable | Pre-eclamptic group (n = 60) | Control group (n = 60) | t value | p value |
|---|---|---|---|---|
| Age | 29.58 ± 5.21 | 29.26 ± 3.46 | 0.39 | 0.69 |
| Parity | 1.15 ± 1.28 | 1.38 ± 1.34 | −0.97 | 0.33 |
| Maternal weight | 89.03 ± 85.42 | 85.42 ± 12.73 | 1.07 | 0.09 |
| Maternal height | 169.91 ± 6.09 | 168.78 ± 6.58 | 0.97 | 0.33 |
| Maternal BMI | 30.83 ± 3.10 | 29.99 ± 4.14 | 1.24 | 0.21 |
| Pre-eclamptic group (n = 60) | Control group (n = 60) | Total | X2(p) | |
| Educational status | ||||
| Primary | 4 (6.67) | 8 (13.30) | 12 (10.00) | 2.66 (0.26) |
| Secondary | 39 (65.00) | 31 (51.67) | 70 (58.33) | |
| Tertiary | 17 (28.30) | 21 (35.0) | 38 (31.67) | |
| Occupation | ||||
| Artisan | 6 (10.00) | 10 (16.70) | 16 (13.31) | 6.73 (0.24) |
| Civil Servant | 5 (8.3) | 10 (16.7) | 15 (12.50) | |
| Housewife | 5 (8.30) | 8 (13.30) | 13 (10.83) | |
| Others | 4 (6.67) | 6 (10.00) | 10 (8.33) | |
| Student | 9 (15.00) | 6 (10.00) | 15 (12.53) | |
| Trader | 31 (51.67) | 20 (33.3) | 51 (43.50) | |
The differences in the mean levels of the measured clinical variables between the pre-eclamptic and control groups are shown in Table 2. The maternal serum leptin levels in the entire participants ranged from 10.4 to 32.1 ng/mL. This level was significantly higher in the pre-eclamptic group than in the control group (24.88 ± 3.92 vs. 15.03 ± 2.98 ng/mL; p =< 0.001). In addition, there was a significant difference in the mean umbilical serum leptin level in both groups (6.43 ± 2.08 vs. 7.27 ± 2.24 ng/mL; p = 0.034). There was a positive correlation between the maternal serum leptin concentration with umbilical cord serum concentration (r = 0.21, p = 0.04) in the pre-eclamptic group as shown in Figure 2. However, there was no correlation between maternal serum leptin and umbilical cord serum leptin in the control group (r = 0.23, p = 0.07). This is shown in Figure 3. There was a statistically significant difference in the neonatal APGAR scores at the 1st (7.33 ± 1.99 vs. 8.65 ± 1.28, p < 0.001) and 5th min (8.60 ± 1.04 vs. 9.40 ± 0.76, p < 0.001) between the pre-eclamptic and control groups. Also, the mean foetal birthweight was statistically lower in babies delivered by pre-eclamptic women when compared with babies of the control group (2131.18 ± 709.59 vs. 3171 ± 553.72 g; p < 0.001). The details are shown in Table 2.
Table 2.
Comparison of mean maternal leptin level, mean neonatal umbilical cord leptin level, and certain feto-maternal outcomes between pre-eclamptic women and healthy normotensive pregnant women.
| Variable | Pre-eclamptic group (n = 60) |
Control group (n = 60) |
t value | p value |
|---|---|---|---|---|
| (Mean ± STD) | (Mean ± STD) | |||
| Gestational age (weeks) | 33.83 ± 3.35 | 37.65 ± 2.47 | 7.10 | <0.001* |
| Maternal serum leptin level (ng/mL) | 24.88 ± 3.92 | 15.03 ± 2.98 | 15.47 | <0.001* |
| MAP | 125.71 ± 10.95 | 82.99 ± 4.98 | 27.48 | <0.001* |
| Birthweight (g) | 2131.18 ± 709.59 | 3171 ± 553.72 | −8.94 | <0.001* |
| Length of neonate (cm) | 44.45 ± 3.20 | 48.01 ± 3.68 | −5.65 | <0.001* |
| Apgar score 1 | 7.33 ± 1.99 | 8.65 ± 1.28 | −4.29 | <0.001* |
| Apgar score 5 | 8.60 ± 1.04 | 9.40 ± 0.76 | −4.78 | <0.001* |
| Ponderal index (g/cm3) | 2.68 ± 0.54 | 2.92 ± 0.72 | −2.08 | <0.001* |
| Umbilical cord serum leptin (ng/mL) | 6.43 ± 2.08 | 7.27 ± 2.24 | −2.14 | 0.034* |
Abbreviations: BMI = body mass index; STD = standard deviation; MAP = mean arterial blood pressure.
Significant p value < 0.05.
Figure 2.
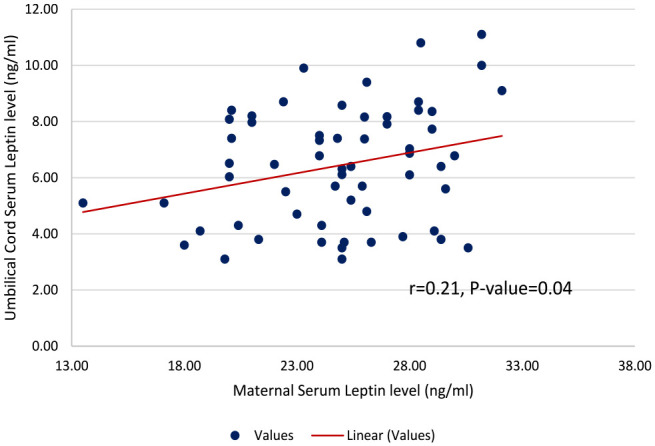
Scatterplot showing the level of linear correlation between maternal serum leptin and umbilical cord serum leptin in the pre-eclamptic group (r = 0.217, p = 0.04).
Figure 3.
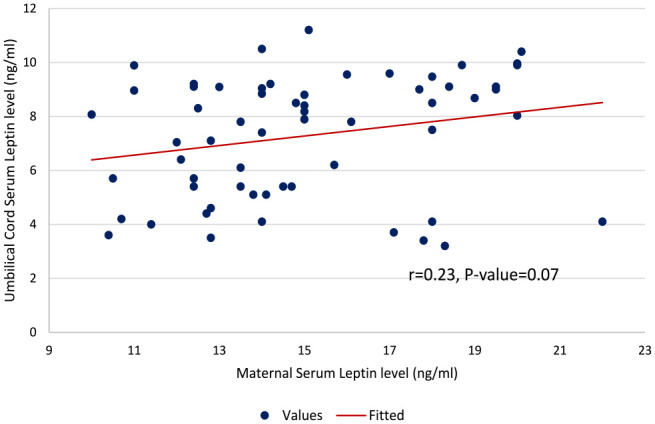
Scatterplot showing the level of linear correlation between maternal serum leptin and umbilical cord serum leptin in the control group (r = 0.235, p = 0.07).
The differences in the mean levels of the measured clinical variables between the degrees of pre-eclampsia within the case group are shown in Table 3. The BMI, neonatal birthweight, neonatal ponderal index, and umbilical cord serum leptin level were similar in both groups (p > 0.05). However, the mean arterial pressure (MAP; 129.11 ± 10.5 mmHg vs. 118.93 ± 8.6; p < 0.001) and the maternal serum leptin level (25.91 ± 3.5 vs. 22.83 ± 4.02 ng/mL; p = 0.003) were significantly higher in cases with severe pre-eclampsia than in those with mild pre-eclampsia. This is shown in Table 3.
Table 3.
Comparison of mean maternal leptin level, mean neonatal umbilical cord leptin level and certain feto-maternal outcomes between severe pre-eclamptic and mild pre-eclamptic women.
| Variable | Degree of pre-eclampsia | t value | p value | |
|---|---|---|---|---|
| Mild (n = 20) (Mean ± SD) |
Severe (n = 40) (Mean ± SD) |
|||
| BMI (kg/m2) | 29.85 ± 2.36 | 31.32 ± 3.33 | −1.768 | 0.053 |
| MAP (mmHg) | 118.93 ± 8.6 | 129.11 ± 10.5 | −3.745 | <0.001 |
| Maternal serum leptin level (ng/mL) | 22.83 ± 4.02 | 25.91 ± 3.5 | −3.062 | 0.003 |
| Birthweight (g) | 1986.8 ± 476.47 | 2203.38 ± 796.94 | −1.117 | 0.269 |
| Length of neonate (cm) | 44.2 ± 3.22 | 44.58 ± 3.23 | −0.424 | 0.673 |
| Ponderal index (g/cm3) | 2.53 ± 0.4 | 2.76 ± 0.6 | −1.59 | 0.117 |
| Umbilical cord serum leptin level (ng/mL) | 6.29 ± 1.58 | 6.51 ± 2.3 | −0.39 | 0.698 |
Severe pre-eclampsia = severe hypertension (BP ⩾ 160/110) with significant proteinuria.
Mild pre-eclampsia severe = blood pressure (< 160/110) with significant proteinuria.
Significant p value < 0.05.
The relationship between maternal serum leptin and pre-eclampsia is shown in Table 4. After correcting for age, gestational age (GA), parity, and BMI, the result showed that for every unit increase in serum leptin, the odds of diagnosing pre-eclampsia increased by about twofold (adj OR: 2.01, 95% CI = 1.43–2.82, p value < 0.001).
Table 4.
Risk factors for pre-eclampsia in the study population.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Unadjusted odds ratio (OR) | 95% confidence interval | p value | Adjusted odds ratio (adjOR) | 95% confidence interval | p value | |
| Maternal serum leptin (ng/mL) | 2.13 | 1.56–2.91 | <0.001 | 2.01 | 1.43–2.82 | <0.001 |
| Umbilical cord serum leptin (ng/mL) | 0.83 | 0.70–0.98 | 0.03 | 0.76 | 0.50–1.15 | 0.19 |
| Age (years) | 1.02 | 0.94–1.11 | 0.51 | 0.96 | 0.74–1.16 | 0.55 |
| Parity | 0.88 | 0.65–1.19 | 0.88 | 0.98 | 0.39–2.43 | 0.96 |
| Gestational age | 1 | 0.924–1.09 | 0.93 | 1.06 | 0.85–1.32 | 0.57 |
| ^BMI (kg/m2) | 1.06 | 0.96–1.17 | 0.21 | 0.93 | 0.66–0.66 | 0.68 |
In the pre-eclamptic group, there was a weak correlation between the BMI and maternal serum leptin concentration (r = 0.302, p = 0.018), while there was a strong positive correlation between the two variables in the normotensive group (r = 0.722, p < 0.001). However, maternal serum leptin levels correlated positively with the umbilical cord leptin levels in the pre-eclamptic group (r = 0.21, p = 0.04). This is shown in Figure 2.
In terms of mode of delivery, 71.7% (n = 43) of the women in the pre-eclamptic group delivered through caesarean section, while only 28.3% (n = 17) delivered vaginally. Conversely, 16.7% (n = 10) in the control group delivered through caesarean section, while 83.3% (n = 50) delivered vaginally. Also, 50.0% of babies delivered in the pre-eclamptic group, and 47.0% in the control group were females. This is shown in Table 5.
Table 5.
Mode of delivery and neonatal sex in both groups.
| Variable | Pre-eclamptic group (n = 60) |
Control group (n = 60) |
Total Freq (%) (n = 120) |
|---|---|---|---|
| Route of delivery | |||
| Caesarean section | 43 (71.67) | 10 (16.67) | 53 (44.17) |
| Vaginal | 17 (28.30) | 50 (83.30) | 67 (55.83) |
| X2 = 35.94 | p < 0.01 | ||
| Gender of neonates | |||
| Female | 30 (50.00) | 28 (46.67) | 58 (48.33) |
| Male | 30 (50.00) | 32 (53.30) | 62 (51.67) |
Discussion
The principal findings of the study are that the mean maternal serum leptin concentration in the pre-eclamptic group was significantly higher than that in the non-pre-eclamptic group (24.88 ± 3.92 vs. 15.03 ± 2.98 ng/mL, p < 0.001) and maternal serum leptin concentration was significantly higher in severe pre-eclamptic participants compared with those with mild pre-eclampsia (25.91 ± 3.5 vs. 22.83 ± 4.02 ng/mL, p = 0.003). In addition, the mean umbilical cord serum leptin level in the pre-eclamptic group was significantly lower than in the control group (6.43 ± 2.08 vs. 7.27 ± 2.24; p = 0.034). There was a weak positive correlation between the maternal serum leptin concentration with umbilical cord serum concentration (r = 0.21, p = 0.04).
Overall, the results showed the mean maternal serum leptin level among the participants with pre-eclampsia, when compared with the normotensive group, was significantly higher (p < 0.001) in the pre-eclamptic group. In a similar study by Teppa et al. 15 in 2000, they concluded that maternal leptin concentration increases in pre-eclampsia in comparison to normotensive pregnancies (41.8 ± 5.6 vs. 25.9 ± 4.1 ng/mL, respectively, p = 0.01). The Teppa et al. study differs from our current study, because they include three arms of the study group, namely 18 normal and 18 pre-eclamptic patients matched by pre-pregnancy BMI, and 18 never-pregnant women matched by BMI with the pregnant groups. In addition, the Teppa et al. study evaluated free leptin concentration, which was not evaluated by the current study that evaluated the total leptin. In contrast to the findings in our study, Dalamaga et al. 23 in 2011 did not find any statistically significant difference in the maternal serum leptin levels between the pre-eclamptics and normotensive controls (p = 0.15). Although the intra-assay coefficient of variation and inter-assay coefficient of variation of the two tests utilized for leptin determination were similar, serum leptin levels were measured using radioimmunoassay (LINCO Research, St. Charles, MO), while in the present study, it was ELISA Kit (manufactured by BioVision, Inc. USA). The Martínez-Abundis et al. 24 study evaluated the serum leptin levels in pre-eclamptic parturients and in normotensive pregnant women, the inclusion and exclusion criteria were similar to the present study; however, the authors concluded that there were no significant differences in serum leptin concentrations between the participants with mild or severe pre-eclampsia and normotensive pregnant women (p > 0.05). In an extensive systematic, review of which leptin was evaluated in 91 studies in 2020, Daskalakis et al. 25 revealed that there was generally an increased level of leptin in pre-eclampsia across all the trimesters. Unlike our current study, the aim of the systematic review by Daskalakis et al. was to accumulate the current literature evidence in order to examine the pattern of serum adipokine levels among pre-eclamptic women, and not aimed exclusively to compare pre-eclamptic and normal pregnant women. This increase was observed in both mild and severe pre-eclamptic women.
In our study, the mean umbilical cord serum leptin in neonates of pre-eclamptics was significantly lower than that among babies of normotensive participants. Although this difference was statistically significant, it contrasts with the findings of Ødegård et al., 26 who noted a higher concentration of umbilical cord plasma leptin in infants of pre-eclamptic mothers in comparison to infants of normotensive participants (p < 0.01). In a recent study by Madny in Egypt, umbilical cord leptin concentrations were found to be significantly higher in women with pre-eclampsia than in the non-pre-eclamptic controls (6.65 ± 1.11 vs. 4.04 ± 1.10 ng/mL respectively, p < 0.01).
The mean maternal serum leptin level in our study was significantly higher in severe pre-eclampsia in comparison to mild pre-eclampsia (25.91 ± 3.5 vs. 22.83 ± 4.02 ng/mL; p = 0.003), whereas there was a non-statistically significant increase in the mean serum concentration of umbilical cord leptin in severe pre-eclamptics compared with mild pre-eclamptics (6.51 ± 2.3 vs. 6.29 ± 1.58 ng/mL, p = 0.698). This is generally in keeping with the findings in the work by Madny, 27 Narkiewicz et al. 4 and Acromite et al., 28 all of which showed that umbilical cord leptin levels increase with increasing severity of maternal disease.
The results of this study showed a weak positive correlation between the maternal serum leptin concentration with umbilical cord serum concentration (r = 0.21, p = 0.04). This is similar to the work by Madny 27 in Egypt, in which there was a positive linear correlation between maternal and umbilical cord leptin (r = 0.602, p < 0.01). It is also similar to the findings of the work by Panjeta et al., 18 in which there were positive correlations between maternal and umbilical cord leptin concentrations among pre-eclamptic subjects (r = 0.687, p < 0.001) and among normotensive pregnant controls (r = 0.407, p = 0.044). The possible reason for the weak positive correlation was that the majority of the women with pre-eclampsia had a severe form of the disease, necessitating pre-term delivery since the average GA at delivery was 33.83 ± 3.35 weeks.
The data in the field of association between maternal and neonatal leptin levels in women with pre-eclampsia were heterogeneous for the following reasons. First, most likely, the level of maternal serum leptin is correlated with the severity of pre-eclampsia. According to several studies, women with severe pre-eclampsia had higher levels of maternal serum leptin.29,30 However, others reported the opposite 31 or no association between leptin level and severity. Second, in the vast majority of investigations, late second and third trimester levels of serum leptin were observed to be increased in women with clinical pre-eclampsia. When compared with pregnant women who had straightforward pregnancies, pre-eclamptic leptin levels do not peak between weeks 28 and 32, but instead continue to rise significantly, only to drop again after delivery. Furthermore, variations in leptin levels did not account for variations in disease severity resulting from ethnic differences. 32 Finally, early- and late-onset pre-eclampsia may have distinct leptin profiles, 33 with higher levels discovered in several investigations in women with early onset pre-eclampsia.29,34 This is in contrast to other research findings, which showed that term pre-eclamptic women had greater maternal serum leptin levels than pre-term pre-eclamptic women.
To correct for any confounding variables of the relationship between maternal serum leptin and the evolution of pre-eclampsia, univariable and multivariable logistic regressions were conducted using the stepwise backward elimination method. The outcome variable was the pre-eclamptic status of the participants (pre-eclampsia/normal), while the maternal serum leptin level was the primary explanatory variable. After correcting for age, GA, parity, and BMI, we found that for every unit increase in serum leptin, the odds of diagnosing pre-eclampsia increased by about twofold (adj OR: 2.01, 95% CI = 1.43–2,89, p value < 0.01). In the West African sub-region, hypertensive disorders, of which pre-eclampsia and eclampsia are very important subclasses, are among the major causes of maternal mortality. In most settings, it is difficult to predict this condition or prognosticate its severity. With the findings in this study, it is possible to infer that maternal serum leptin is an independent predictor of pre-eclampsia. In addition to other tools, it will make the management of these patients easier.
As much as was possible, attempt was made to match the participants, especially with respect to BMI, which had been unarguably delineated as the most significant determinant of maternal serum level of leptin. Again, the senior laboratory scientist who analysed the samples was blinded to which samples belonged to which group to avoid bias. However, we could not match the study participants on both arms with respect to the mode of delivery as this may have an effect on the umbilical cord leptin levels. There are conflicting results with regard to the effect of route of delivery on the serum leptin level. Some have demonstrated that vaginal delivery increases umbilical cord leptin levels due to stressful events of labour when compared with caesarean section, while some studies showed no association between umbilical cord leptin concentration and mode of delivery. Kirel et al. 35 and Mahmoud et al. 36 studies demonstrated that the mode of delivery has no effect on the cord leptin levels.
The strength of the study is that two groups of participants were matched and the collaborating senior laboratory scientist who analysed the samples was blinded to which samples belonged to the investigative and control groups. Nevertheless, the study limitations were that the study was a hospital-based study, and thus inferences made cannot be used for generalizations about the general population. Also, there could be a potential impact from unmatched variables, including the mode of delivery and GA at delivery on serum leptin levels. In addition, participants in the investigative arm were analysed at a point when they had already developed the condition, and this makes it difficult to draw conclusions on causality. The study did not assess the maternal serum leptin concentration of the pre-eclamptic participants before the inception of the disease. This would have yielded a better insight into the pathogenesis of the disease and the role of leptin in its development. It would have also thrown more light on the use of leptin as a biomarker for the prediction of pre-eclampsia. However, this is subject for further research and possibly in a multi-centred setting.
Conclusion
Maternal serum leptin concentration is significantly increased in pregnant women who have pre-eclampsia, compared with their normotensive counterparts. This increase becomes even more pronounced as the severity of the disease progresses. Although the umbilical cord serum leptin levels in the pre-eclamptic group were significantly lower than in the control group, there was a positive correlation between the maternal serum leptin concentrations with umbilical cord serum leptin concentration. Increased BMI also correlates positively with increased levels of maternal serum leptin.
Maternal serum leptin assessment has the potential to become a veritable tool in the diagnosis and monitoring of pregnancies complicated by pre-eclampsia, which is one of the major contributors to maternal morbidity and mortality in sub-Saharan Africa. This calls for more research in the area.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057231213272 for Maternal and umbilical cord blood levels of leptin in pre-eclamptic and healthy pregnant women: A comparative cross-sectional study by Lazarus Ugochukwu Okafor, George Uchenna Eleje, Joseph Ifeanyichukwu Ikechebelu, Emmanuel Onyebuchi Ugwu, Obi Betrand Nwosu, Okechukwu Christian Ikpeze, Gerald Okanandu Udigwe, Ahizechukwu Chigoziem Eke, Samuel A Okoli, Charlotte Blanche Oguejiofor, Chukwudi Anthony Ogabido, Divinefavour Echezona Malachy, Ifeanyichukwu Jude Ofor and Chigozie Geoffrey Okafor in Women’s Health
Acknowledgments
The authors express their gratitude to the subjects who participated in the study, NAUTH hospital management and the antenatal/labour/delivery unit staff of the hospital.
Footnotes
ORCID iDs: George Uchenna Eleje  https://orcid.org/0000-0002-0390-2152
https://orcid.org/0000-0002-0390-2152
Okechukwu Christian Ikpeze  https://orcid.org/0000-0002-7433-8755
https://orcid.org/0000-0002-7433-8755
Chigozie Geoffrey Okafor  https://orcid.org/0000-0003-4458-8216
https://orcid.org/0000-0003-4458-8216
Declarations
Ethical approval and consent to participate: The ethical approval for the study was obtained from the NAUTH Nnewi, Nigeria Ethics Review Committee on 19 March 2020 (Ref no. NAUTH/CS/66/VOL.13/013/2019/100). The study was conducted according to the ethical principles for human subjects according to the Helsinki Declaration. Written informed consent was obtained from all the participants prior to recruitment into the study.
Consent for publication: Not applicable.
Author contribution(s): Lazarus Ugochukwu Okafor: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
George Uchenna Eleje: Data curation; Formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Joseph Ifeanyichukwu Ikechebelu: Formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Emmanuel Onyebuchi Ugwu: Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Obi Betrand Nwosu: Methodology; Writing – original draft; Writing – review & editing.
Okechukwu Christian Ikpeze: Methodology; Writing – original draft; Writing – review & editing.
Gerald Okanandu Udigwe: Methodology; Writing – original draft; Writing – review & editing.
Ahizechukwu Chigoziem Eke: Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Samuel A Okoli: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Charlotte Blanche Oguejiofor: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Chukwudi Anthony Ogabido: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Divinefavour Echezona Malachy: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Ifeanyichukwu Jude Ofor: Methodology; Writing – original draft; Writing – review & editing.
Chigozie Geoffrey Okafor: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Availability of data and materials: The data for this study are available upon formal request from the authors.
References
- 1. Waugh JJS, Smith MC. Hypertensive disorders. In: Edmonds DK, Lees C, Bourne TH. (eds) Dewhurst’s textbook of obstetrics & gynaecology. Hoboken, NJ: John Wiley & Sons, 2018, pp. 71–84. [Google Scholar]
- 2. Kenny L. Hypertensive disorders of pregnancy. In: Kenny L, Myers J. (eds) Obstetrics by ten teachers. London: Taylor & Francis Group, 2017, pp. 272–297. [Google Scholar]
- 3. Asnafi N, Sharbatdaran M, Hajian K. Comparison of maternal and neonatal serum leptin levels in preeclampsia and normal pregnancy. Iran J Reprod Med 2011; 9(2): 131–134. [PMC free article] [PubMed] [Google Scholar]
- 4. Narkiewicz K, Somers VK, Mos L, et al. An independent relationship between plasma leptin and heart rate in untreated patients with essential hypertension. J Hypertens 1999; 17(2): 245–249. [DOI] [PubMed] [Google Scholar]
- 5. Koçyigit Y, Atamer Y, Atamer A, et al. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol 2004; 19(5): 267–273. [DOI] [PubMed] [Google Scholar]
- 6. Geldenhuys J, Rossouw TM, Lombaard HA, et al. Disruption in the regulation of immune responses in the placental subtype of preeclampsia. Front Immunol 2018; 9: 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adali E, Yildizhan R, Kolusari A, et al. Increased visfatin and leptin in pregnancies complicated by pre-eclampsia. J Matern Fetal Neonatal Med 2009; 22(10): 873–879. [DOI] [PubMed] [Google Scholar]
- 8. Taylor BD, Ness RB, Olsen J, et al. Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women. Hypertension 2015; 65(3): 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams MA, Havel PJ, Schwartz MW, et al. Pre-eclampsia disrupts the normal relationship between serum leptin concentrations and adiposity in pregnant women. Paediatr Perinat Epidemiol 1999; 13(2): 190–204. [DOI] [PubMed] [Google Scholar]
- 10. Henson MC, Castracane VD. Leptin in pregnancy: an update. Biol Reprod 2006; 74(2): 218–229. [DOI] [PubMed] [Google Scholar]
- 11. Mellati AA, Mazloomzadeh S, Anjomshoaa A, et al. Multiple correlations between cord blood leptin concentration and indices of neonatal growth. Arch Med Res 2010; 41(1): 26–32. [DOI] [PubMed] [Google Scholar]
- 12. Villarreal D, Reams G, Freeman RH, et al. Renal effects of leptin in normotensive, hypertensive, and obese rats. Am J Physiol 1998; 275(6): R2056–R2060. [DOI] [PubMed] [Google Scholar]
- 13. Laml T, Preyer O, Hartmann BW, et al. Decreased maternal serum leptin in pregnancies complicated by preeclampsia. J Soc Gynecol Investig 2001; 8(2): 89–93. [PubMed] [Google Scholar]
- 14. Sattar N, Greer IA, Pirwani I, et al. Leptin levels in pregnancy, marker for fat accumulation and mobilization? Acta Obstet Gynecol Scand 1998; 77(3): 278–283. [PubMed] [Google Scholar]
- 15. Teppa RJ, Ness RB, Crombleholme WR, et al. Free leptin is increased in normal pregnancy and further increased in preeclampsia. Metabolism 2000; 49(8): 1043–1048. [DOI] [PubMed] [Google Scholar]
- 16. Briana DD, Malamitsi-Puchner A. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci 2009; 16(10): 921–937. [DOI] [PubMed] [Google Scholar]
- 17. Mühlhäusler BS, Roberts CT, Yuen BS, et al. Determinants of fetal leptin synthesis, fat mass, and circulating leptin concentrations in well-nourished ewes in late pregnancy. Endocrinology 2003; 144(11): 4947–4954. [DOI] [PubMed] [Google Scholar]
- 18. Panjeta P, Ghalaut VS, Bala J, et al. Inverse correlation between insulin-like growth factor-1 and leptin levels in preeclampsia. J Basic Clin Reprod Sci 2016; 5: 153–175. [Google Scholar]
- 19. Youssry MA, Gabreel MA, Patel TA. Changes in maternal serum leptin levels during pregnancy and after labor in preeclampsia, and its correlation to neonatal cord leptin. Open J Obstet Gynecol 2016; 6: 588–600. [Google Scholar]
- 20. Kokot F, Wiecek A, Adamczak M, et al. Pathophysiological role of leptin in patients with chronic renal failure, in kidney transplant patients, in patients with essential hypertension, and in pregnant women with preeclampsia. Artif Organs 1999; 23(1): 70–74. [DOI] [PubMed] [Google Scholar]
- 21. Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med 2013; 35(2): 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller D. Hypertension in pregnancy. In: Decherney A, Laufer N, Nathan L, et al. (eds) Current diagnosis and treatment obstetrics and gynecology. New York: McGraw-Hill, 2013, pp. 459–464. [Google Scholar]
- 23. Dalamaga M, Srinivas SK, Elovitz MA, et al. Serum adiponectin and leptin in relation to risk for preeclampsia: results from a large case-control study. Metabolism 2011; 60(11): 1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martínez-Abundis E, González-Ortiz M, Pascoe-González S. Serum leptin levels and the severity of preeclampsia. Arch Gynecol Obstet 2000; 264(2): 71–73. [DOI] [PubMed] [Google Scholar]
- 25. Daskalakis G, Bellos I, Nikolakea M, et al. The role of serum adipokine levels in preeclampsia: a systematic review. Metabolism 2020; 106: 154172. [DOI] [PubMed] [Google Scholar]
- 26. Ødegård RA, Vatten LJ, Nilsen ST, et al. Umbilical cord plasma leptin is increased in preeclampsia. Am J Obstet Gynecol 2002; 186(3): 427–432. [DOI] [PubMed] [Google Scholar]
- 27. Madny EH. Leptin values in maternal and umbilical cord blood in pregnant women with preeclampsia. Egypt J Fertil Steril 2019; 23: 11–17. [Google Scholar]
- 28. Acromite M, Ziotopoulou M, Orlova C, et al. Increased leptin levels in preeclampsia: associations with BMI, estrogen and SHBG levels. Hormones 2004; 3(1): 46–52. [DOI] [PubMed] [Google Scholar]
- 29. Salimi S, Farajian-Mashhadi F, Naghavi A, et al. Different profile of serum leptin between early onset and late onset preeclampsia. Dis Markers 2014; 2014: 628476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El Shahat AM, Ahmed AB, Ahmed MR, et al. Maternal serum leptin as a marker of preeclampsia. Arch Gynecol Obstet 2013; 288(6): 1317–1322. [DOI] [PubMed] [Google Scholar]
- 31. Anato V, Garmendia JV, Bianco NE, et al. Serum leptin levels in different types of hypertension during pregnancy. Res Commun Mol Pathol Pharmacol 2000; 108(3–4): 147–153. [PubMed] [Google Scholar]
- 32. Jenkins LD, Powers RW, Adotey M, et al. Maternal leptin concentrations are similar in African Americans and Caucasians in normal pregnancy, preeclampsia and small-for-gestational-age infants. Hypertens Pregnancy 2007; 26(1): 101–109. [DOI] [PubMed] [Google Scholar]
- 33. Weedon-Fekjær MS, Sheng Y, Sugulle M, et al. Placental miR-1301 is dysregulated in early-onset preeclampsia and inversely correlated with maternal circulating leptin. Placenta 2014; 35(9): 709–717. [DOI] [PubMed] [Google Scholar]
- 34. Masuyama H, Segawa T, Sumida Y, et al. Different profiles of circulating angiogenic factors and adipocytokines between early- and late-onset pre-eclampsia. BJOG 2010; 117(3): 314–320. [DOI] [PubMed] [Google Scholar]
- 35. Kirel B, Tekin N, Tekin B, et al. Cord blood leptin levels: relationship to body weight, body mass index, sex and insulin and cortisol levels of maternal-newborn pairs at delivery. J Pediatr Endocrinol Metab 2000; 13: 71–77. [DOI] [PubMed] [Google Scholar]
- 36. Mahmoud AT, EL-Rafea Elbassuoni MA, Ellahony DM, et al. A study of serum leptin level in both full-term and preterm newborns. Menoufia Med J 2017; 30: 765–769. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057231213272 for Maternal and umbilical cord blood levels of leptin in pre-eclamptic and healthy pregnant women: A comparative cross-sectional study by Lazarus Ugochukwu Okafor, George Uchenna Eleje, Joseph Ifeanyichukwu Ikechebelu, Emmanuel Onyebuchi Ugwu, Obi Betrand Nwosu, Okechukwu Christian Ikpeze, Gerald Okanandu Udigwe, Ahizechukwu Chigoziem Eke, Samuel A Okoli, Charlotte Blanche Oguejiofor, Chukwudi Anthony Ogabido, Divinefavour Echezona Malachy, Ifeanyichukwu Jude Ofor and Chigozie Geoffrey Okafor in Women’s Health