Abstract
Vacuolar processing enzyme (VPE) is a Cys proteinase responsible for the maturation of vacuolar proteins. Arabidopsis thaliana δVPE, which was recently found in the database, was specifically and transiently expressed in two cell layers of the seed coat (ii2 and ii3) at an early stage of seed development. At this stage, cell death accompanying cell shrinkage occurs in the ii2 layer followed by cell death in the ii3 layer. In a δVPE-deficient mutant, cell death of the two layers of the seed coat was delayed. Immunocytochemical analysis localized δVPE to electron-dense structures inside and outside the walls of seed coat cells that undergo cell death. Interestingly, δVPE in the precipitate fraction from young siliques exhibits caspase-1–like activity, which has been detected in various types of plant cell death. Our results suggest that, at the early stage of seed development, δVPE is involved in cell death of limited cell layers, the purpose of which is to form a seed coat.
INTRODUCTION
Most vacuolar proteins are synthesized on rough endoplasmic reticulum as proprotein precursors and then transported to the vacuoles, where they are converted into the respective mature forms. Previously, we identified an enzyme responsible for the maturation of various vacuolar proteins and designated it vacuolar processing enzyme (VPE) (Hara-Nishimura et al., 1991, 1993; Hiraiwa et al., 1993). VPEs belong to a novel family of Cys proteinases (C13; EC 3.4.22.34) (Hara-Nishimura, 1998). VPE homologs are found in various organisms, including plants (Hara-Nishimura et al., 1998; Hara-Nishimura and Maeshima, 2000), mammals (Chen et al., 1998; Shirahama-Noda et al., 2003), and a protozoan, Schistosoma mansoni (Caffrey et al., 2000). We reported that mouse VPE, which is also referred to as legumain, plays a critical role in the endosomal/lysosomal degradation of kidney cells (Shirahama-Noda et al., 2003).
VPE is synthesized as an inactive proprotein precursor. The C-terminal and N-terminal propeptides are sequentially removed autocatalytically to produce the active mature forms (Hiraiwa et al., 1997, 1999). The removal of the C-terminal propeptide is necessary for activation of VPE (Hara-Nishimura et al., 1993). The C-terminal propeptide acts as an autoinhibitory domain that masks the catalytic site (Kuroyanagi et al., 2002). No other factor is necessary for activating VPE molecules. Therefore, VPE is a key enzyme in the vacuolar-processing system, which is responsible for maturation and/or activation of various vacuolar proteins.
Plant VPE homologs are separated into two types: vegetative-type VPEs and seed-type VPEs (Hara-Nishimura, 1998; Hara-Nishimura et al., 1998). Three VPE genes, αVPE, βVPE, and γVPE, were found in Arabidopsis thaliana (Kinoshita et al., 1995a, 1995b). An RNA gel blot analysis and a histochemical analysis with β-glucuronidase reporter constructs showed that αVPE and γVPE are expressed in vegetative organs, whereas βVPE is expressed in seeds (Kinoshita et al., 1999). By analyzing VPE-deficient Arabidopsis mutants, we demonstrated that seed-type βVPE is essential for the proper processing of storage proteins (Shimada et al., 2003). On the other hand, vegetative-type αVPE and γVPE are upregulated in association with various types of cell death and under stressed conditions (Kinoshita et al., 1999; Hara-Nishimura and Maeshima, 2000). VPEs are also induced by wound treatment, and the slow wound response of VPE is regulated by endogenous salicylic acid (Yamada et al., 2001). Theses results implied that vegetative-type VPEs are involved in cell death of plants. Recently, we found that tobacco (Nicotiana tabacum) VPEs, which exhibit caspase-1–like activity, are involved in Tobacco mosaic virus–induced hypersensitive cell death (Hatsugai et al., 2004).
A fourth VPE, designated δVPE, was found in the Arabidopsis genome database. Gruis et al. (2002) (2004) observed the expression of δVPE mRNA in young developing seeds and germinating seeds. However, the physiological function of δVPE is unknown. Here, we report that δVPE is specifically expressed in the seed coat and is associated with cell death. In angiosperm seeds, the embryo and endosperm are surrounded by the seed coat, which protects the embryo from mechanical damage, pathogen attack, and UV damage and maintains the dehydrated dormant stage until proper germination conditions exist. The seed coat also functions as the maternal conduit to the embryo and some metabolites flow from the seed coat to the embryo (Weber et al., 1995; King et al., 1997; Sheen et al., 1999; Wobus and Weber, 1999). The seed coat of cruciferous plants consists of two integuments (the outer and inner integuments) of the ovule. The generation of the integuments has been described in Arabidopsis (Gasser and Robinson-Beers, 1993; Gaiser et al., 1995; Schneitz et al., 1995).
During seed development, each integument goes through a dramatic differentiation process to form the mature seed coat: some of the tissues that are generated in the early and middle stages of seed development disappear at the later stages in Brassicaceae seeds (Beeckman et al., 2000; Western et al., 2000; Windsor et al., 2000). As a major part of the differentiation process, the inner integument becomes compressed and impregnated with pigments imparting the characteristic brown color. However, the molecular mechanisms underlying the process are poorly understood. In this report, we show that δVPE functions in the cell death of the integument of seeds to form the seed coat.
RESULTS
A Novel Type of VPE and an Arabidopsis Mutant Lacking δVPE
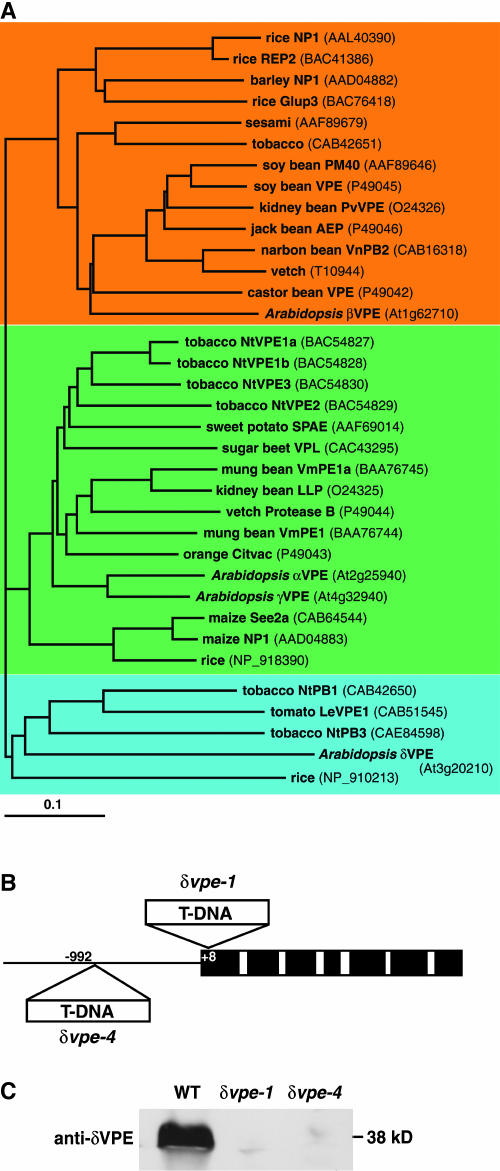
VPEs of higher plants have previously been divided into two types: seed-type VPEs and vegetative-type VPEs. A phylogenetic tree of plant VPEs (Figure 1A) shows the existence of a third type of VPE, which includes Arabidopsis δVPE (At3g20210), tomato (Lycopersicon esculentum) VPE (CAB51545), tobacco NtPB1 (CAB42650), tobacco NtPB3 (CAE84598), and rice (Oryza sativa) VPE (NP_910213). We propose that these VPEs constitute a novel type of VPE (discussed below).
Figure 1.
Plant VPEs and Arabidopsis δVPE Knockout Mutants.
(A) A dendrogram of plant VPEs. VPEs are separated into three types: a vegetative type (green), an embryo type (orange), and a novel type (blue). The dendrogram was drawn with the ClustalW and TreeView programs. The horizontal scale represents the evolutionary distance expressed as the number of substitutions per amino acid.
(B) Schematic representation of the Arabidopsis δVPE gene and the positions of the T-DNA insertions in the δvpe-1 and δvpe-4 alleles. Black boxes indicate exons, open boxes indicate introns, and a solid line indicates the 5′-nonconding region.
(C) Immunoblot analysis of a young silique from the wild-type and δvpe knockout mutants (δvpe-1 and δvpe-4) with antibodies directed against δVPE.
To determine its function, we used a reverse genetic approach to identify an Arabidopsis mutant that is defective in the gene. We isolated two mutants, δvpe-1 and δvpe-4, in which T-DNA insertions disrupt the δVPE gene in the first exon and in the promoter region, respectively (Figure 1B). In an immunoblot analysis, specific antibodies against δVPE reacted with a 38-kD protein from wild-type siliques (Figure 1C). Siliques of the δvpe-1 and δvpe-4 mutants lacked the band (Figure 1C), indicating that the δVPE protein is absent in these mutants.
δVPE Is Specifically Expressed in the Outer Two Cell Layers of the Inner Integument of Developing Arabidopsis Seeds
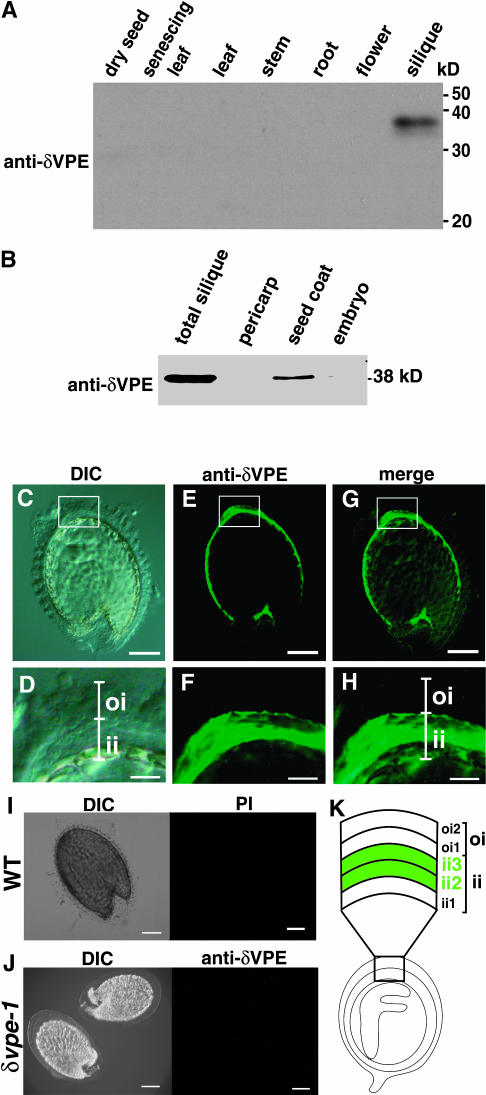
An immunoblot analysis with anti-δVPE antibodies revealed that the 38-kD δVPE is specifically expressed in siliques (Figure 2A). In siliques at the walking-stick-shaped-embryo stage, δVPE was expressed in the seed coat, but not in the embryo or the pericarp (Figure 2B). The localization of δVPE is quite different from that of βVPE, which is found in the embryo (Kinoshita et al., 1999; Shimada et al., 2003).
Figure 2.
δVPE Is Specifically Localized in Two Cell Layers of the Inner Integuments of the Seed Coats in Developing Arabidopsis Seeds.
(A) Immunoblot analysis showing the organ-specific expression of δVPE. Total proteins were extracted from each organ (2 mg fresh weight) of Arabidopsis plants and were subjected to immunoblotting with anti-δVPE antibodies. The molecular masses are given on the right in kilodaltons.
(B) Immunoblot analysis showing the tissue-specific expression of δVPE. Total proteins were extracted from each tissue of one young silique and were subjected to immunoblotting with anti-δVPE antibodies.
(C) to (H) Immunofluorescence analysis of developing seeds at walking-stick-shaped-embryo stage with anti-δVPE antibodies. Green fluorescent images (anti-δVPE), differential interference contrast (DIC) images, and merged images (merge) are shown. (D), (F), and (H) are enlarged images of boxed regions in (C), (E), and (G), respectively. oi, outer integument; ii, inner integument. Bars = 100 μm in (C), (E), and (G) and 25 μm in (D), (F), and (H).
(I) A control experiment with developing wild-type seeds and preimmune serum (PI). Bars = 100 μm.
(J) A control experiment with developing δvpe-1 seeds and anti-δVPE antibodies. Bars = 100 μm.
(K) An illustration of the organization of the cell layers in the seed coat of a developing seed having a walking-stick-shaped embryo. The seed coat is composed of two kinds of integuments: outer integument (oi) of two cell layers (oi1 and oi2) and inner integument (ii) of three cell layers (ii1, ii2, and ii3). Green indicates the cell layers (ii2 and ii3) that express δVPE.
The Arabidopsis seed coat is derived from the maternal parent and is composed of two integuments (the outer and inner integuments) (Leon-Kloosterziel et al., 1994; Beeckman et al., 2000; Western et al., 2000; Windsor et al., 2000). The outer integument consists of two cell layers and the inner integument consists of mostly three cell layers (ii1, ii2, and ii3) (Figure 2K). δVPE was detected in the inner integument, but not in the outer integument of early developing seeds (Figures 2C to 2H). δVPE was localized in the ii2 and ii3 layers of the inner integument, but not in the ii1 layer (Figure 2H). No immunopositive green fluorescence was detected in wild-type seeds with preimmune serum (Figure 2I) or in δvpe-1 seeds with anti-δVPE antibodies (Figure 2J). This indicates the localization of δVPE is limited to the outer two cell layers (ii2 and ii3) of the inner integument of the seed coat of developing Arabidopsis seeds.
δVPE Is Transiently Expressed at the Early Stage of Seed Development
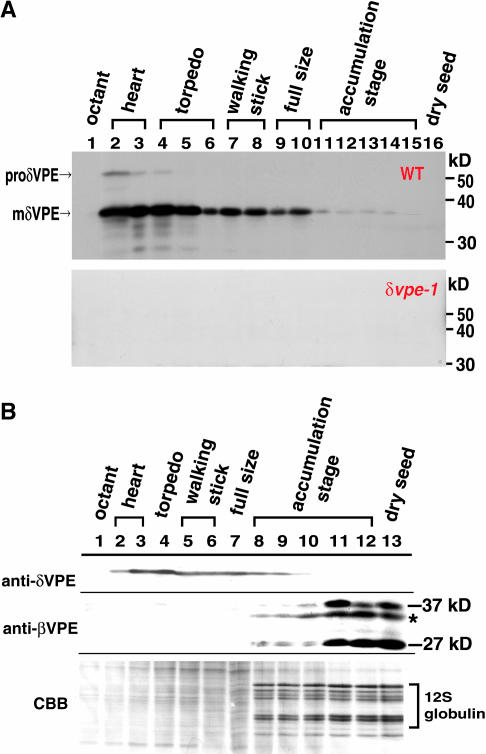
An additional 52-kD band was detected on the immunoblot of the developing seeds in the very early stage (Figure 3A, top). This band was not detected on the blot of the developing δvpe-1 seeds (Figure 3A, bottom). This indicates that the 52-kD band corresponds to a precursor of δVPE, which we have named proδVPE, and that the 38-kD band corresponds to the mature form. This result is consistent with our previous result that VPE is synthesized as an inactive proprotein, which is then processed to produce the active form (Hiraiwa et al., 1997; Kuroyanagi et al., 2002).
Figure 3.
δVPE Is Expressed at an Early Stage in Developing Arabidopsis Seeds, whereas βVPE Is Expressed in the Late Stage in Association with the Accumulation of Seed Storage Proteins.
(A) Developmental changes in the levels of the precursor and mature forms of δVPE during seed development. The wild-type and δvpe-1 siliques were harvested at various stages, and one-tenth of total proteins from developing seeds in each one silique were subjected to immunoblotting with anti-δVPE antibodies. Developmental stages are indicated by shapes of embryo in the seeds: octant, four- to eight-celled embryo (lane 1); heart, heart-shaped embryo (lanes 2 and 3); torpedo, torpedo-shaped embryo (lanes 4 to 6); walking stick, walking-stick-shaped embryo (lanes 7 and 8), full size, almost full-sized embryo (lanes 9 and 10), accumulation stage, embryo accumulating seed storage proteins (lanes 11 To 15); dry seed (lane 16). The molecular masses are given at the right in kilodaltons.
(B) Comparison of the expression pattern of δVPE with that of βVPE during seed development. The wild-type siliques were harvested at the stages indicated in (A), and one-tenth of total proteins from developing wild-type seeds in each silique was subjected to immunoblotting with anti-δVPE antibodies (anti-δVPE) or anti-βVPE antibodies (anti-βVPE) and to Coomassie blue staining (CBB). The 27- and 37-kD bands are two forms of βVPE. 12S globulin is a major storage protein. Asterisk indicates a nonspecific signal.
δVPE levels rapidly and transiently increased in the early stage of embryogenesis, reaching a maximum in the heart-shaped-embryo stage, and then slowly decreased until the full-sized-embryo stage, when storage proteins started to accumulate (Figure 3B). This suggests that δVPE functions in the early stage of seed development. In contrast with δVPE, which was expressed early and transiently, another Arabidopsis VPE, βVPE, increased during the late stage of seed development (Figure 3B). The increase of 37- and 27-kD forms of βVPE was accompanied by the accumulation of seed storage proteins, such as 12S globulins. βVPE expression was consistent with our previous result that βVPE is responsible for the maturation of seed storage proteins (Shimada et al., 2003). The finding that δVPE and βVPE function at different stages suggests the functional differentiation of VPEs in developing seeds: δVPE functions in the shrinkage of the seed coat, whereas βVPE functions in storage protein processing in developing cotyledons.
δVPE Deficiency Prevents Shrinkage of the Inner Integument and the Degradation of Nuclei of the ii2 and ii3 Cell Layers
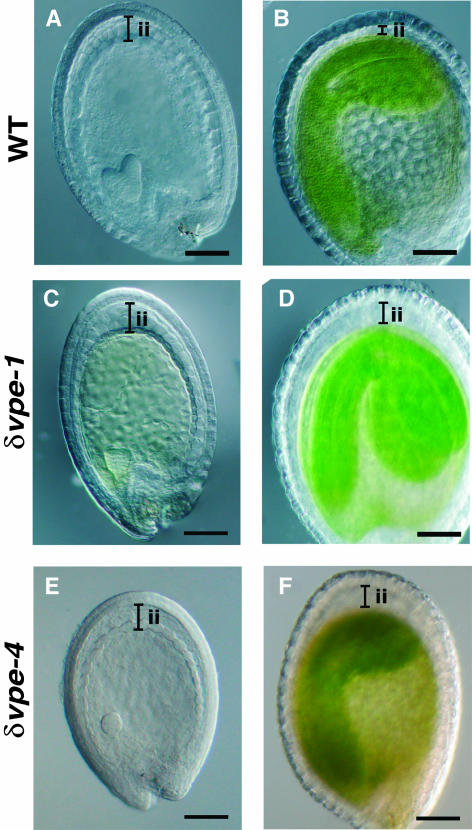
The above results imply that δVPE functions in the formation of seed coats. Therefore, we attempted to clarify the effect of δVPE deficiency on the development of the inner integument of the seed coat. In wild-type plants, the thickness of the inner integument, in which δVPE is localized, decreased from 60 to 70 μm at the early-embryo stage (Figure 4A) to less than half this value at the walking-stick-shaped-embryo stage (Figure 4B). No reduction in the thickness of the outer integument was observed in this period (Figures 4A and 4B). Interestingly, unlike the inner integument of the wild type, the inner integument of the δVPE-deficient mutants δvpe-1 and δvpe-4 remained thick throughout embryogenesis (Figures 4C to 4F). At the early-embryo stage, the inner integument of δvpe seeds, like that of wild-type seeds, had three cell layers (Figures 4A, 4C, and 4E). In the walking-stick-shaped-embryo stage, the inner integument was compressed in the wild type (Figure 4B), but not in δvpe (Figures 4D and 4F).
Figure 4.
Thickness of Inner Integuments of the Seed Coats Is Reduced in the Wild-Type Seeds at the Early Stages, whereas It Is Not Reduced in the δvpe Seeds.
Differential interference contrast images of developing seeds at an early-embryo stage ([A], [C], and [E]) and walking-stick-shaped-embryo stage ([B], [D], and [F]) from the wild type ([A] and [B]), δvpe-1 ([C] and [D]), and δvpe-4 ([E] and [F]). ii, inner integument. Bars = 100 μm.
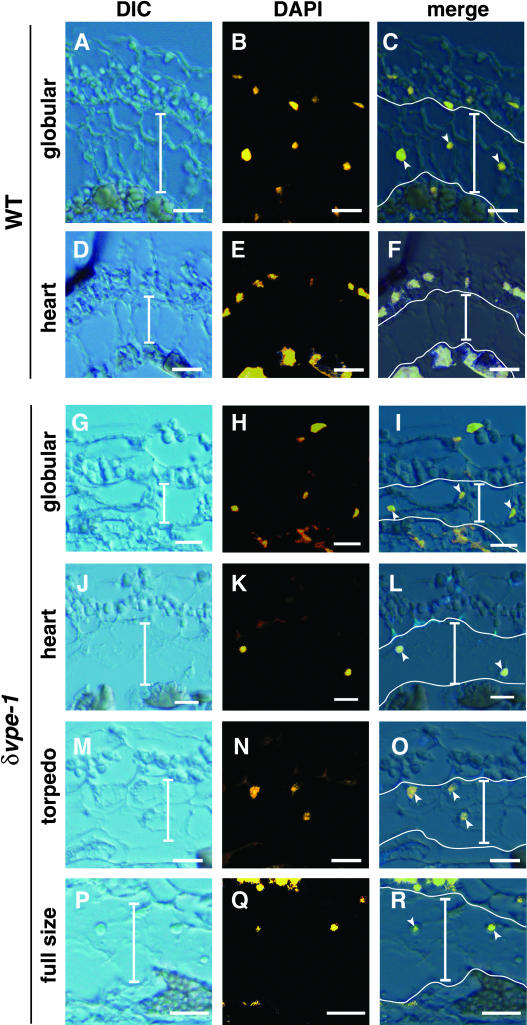
We examined the possibility that the decrease of the wild-type inner integument during development is associated with programmed cell death (Figure 5). In wild-type plants, nuclei in the ii2 and ii3 cell layers, as revealed by DAPI (4′-6-diamidino-2-phenylindole) staining, are clearly visible at the globular embryo stage (Figures 5A to 5C) but disappear at the heart-shaped embryo stage (Figures 5D to 5F). This result is consistent with the developmental aspects of wild-type seeds as shown in Figures 4A and 4B and indicates that the cells of the ii2 and ii3 layers of developing wild-type seeds are programmed to die. On the contrary, the nuclei were detected in developing δvpe-1 seeds with heart-shaped, torpedo-shaped, and even full-sized embryos (Figures 5G to 5R). This result is consistent with the developmental aspects of δvpe seeds as shown in Figures 4C to 4F and shows that the cells of ii2 and ii3 layers in δvpe-1 seeds are still alive. These results suggest that δVPE is involved in cell death. Therefore, δVPE deficiency prevents the degradation of nuclei of the ii2 and ii3 cell layers.
Figure 5.
Degradation of Nuclei in ii2 and ii3 Cell Layers of the Inner Integuments Occurs in the Wild-Type Seeds at the Heart-Shaped-Embryo Stage, whereas It Does Not Occur in the δvpe-1 Seeds at the Early Stages.
Differential interference contrast (DIC) images, DAPI images, and merged images (merge) of developing seeds from the wild type ([A] to [F]) and δvpe-1 ([G] to [R]) are shown. DAPI images were visualized using 365/12-nm-wavelength excitation and a long-pass 397-nm-wavelength emission filter and then were converted in yellow to highlight the nuclei. Developmental stages are indicated at the left. Vertical bars indicate both ii2 and ii3 cell layers. Arrowheads indicate nuclei stainable with DAPI in the ii2 and ii3 cell layers. The cells of ii2 and ii3 layers, which are highly vacuolated, are larger than the cells of the other layers. The cells of outer integument layers contain a lot of starch granules, and the cells of ii1 layer accumulate pigments, which generated autofluorescence. Horizontal bars = 25 μm.
Formation of Seed Coat Is Accompanied by the Sequential Shrinkage of the Outer Two Cell Layers of the Inner Integument
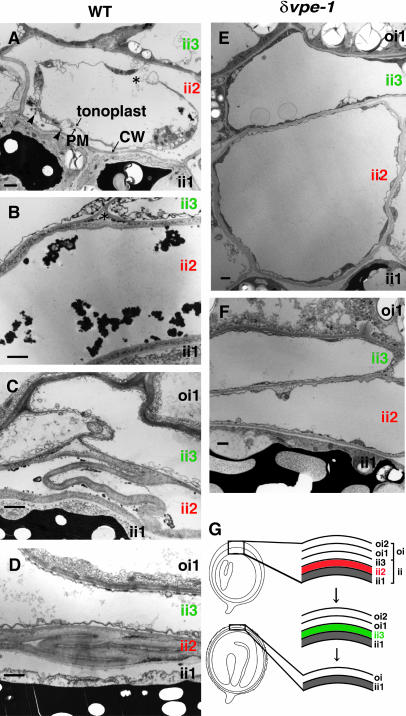
Figures 6A to 6D show the cytological changes in the inner integuments of developing wild-type seeds at the torpedo-shaped-embryo stages. At first, cells in the ii2 layer started shrinkage and plasmolysis (Figure 6A). The plasma membrane and tonoplast are partially disrupted (indicated by an asterisk in Figure 6A). At this stage, cells in the ii1 and ii3 layers remained in their normal shape. Then, the plasma membrane and tonoplast of the cells in the ii2 layer disappeared (Figure 6B). At this stage, disrupted organelles and cellular materials from the dead cells formed large aggregates. Cell layers that had lost their contents were compressed, resulting in folded cell walls (Figures 6C and 6D). Subsequently, cells in the ii3 layer started to shrink, so that the ii3 layer became compressed like the ii2 layer (data not shown). The final seed coat consisted of a one-cell-layer inner integument (ii1) and a one-cell-layer outer integument (data not shown). The cells of the ii1 layer were filled with large, electron-dense compartments (Figures 6A, 6C, and 6D). The cells of the ii2 and ii3 layers had no such subcellular feature, although they each had a developed central vacuole. The sequential shrinkage of two cell layers in the inner integument is summarized in Figure 6G.
Figure 6.
Morphological Changes of ii2 and ii3 Cell Layers of the Inner Integuments in the Developing Wild-Type and δvpe-1 Seeds at the Torpedo-Shaped-Embryo Stage.
(A) Cell shrinkage and plasmolysis occurs first in the ii2 cell layers of developing wild-type seeds. Plasma membrane and tonoplast are partially disrupted (asterisk). At this stage, cells in the ii1 and ii3 layers are intact. Electron-dense structures (arrowheads) appear in the space between the plasma membrane (PM) and cell wall (CW).
(B) The plasma membranes and cellular organelles are disrupted in cells of the ii2 layers in developing wild-type seeds.
(C) and (D) Dead cells of the ii2 layers in developing wild-type seeds are finally compressed.
(E) and (F) Cells of the ii2 and ii3 layers in developing δvpe-1 seeds remain intact.
(G) An illustration of the disappearance of the ii2 cell layer (red) followed by that of the ii3 cell layers (green) in developing wild-type seeds.
Bars = 1 μm (A) to (F).
On the other hand, cell shrinkage and plasmolysis did not occur in ii2 and ii3 cell layers of developing δvpe-1 seeds at the torpedo-shaped-embryo stages, and the plasma membrane and tonoplast were intact (Figures 6E and 6F). This result is consistent with the results in Figures 4 and 5 and indicates that δVPE-deficiency prevents the sequential shrinkage of the ii2 and ii3 layers.
δVPE Is Localized to Novel Electron-Dense Structures Inside and Outside the Cells in the Inner Integument
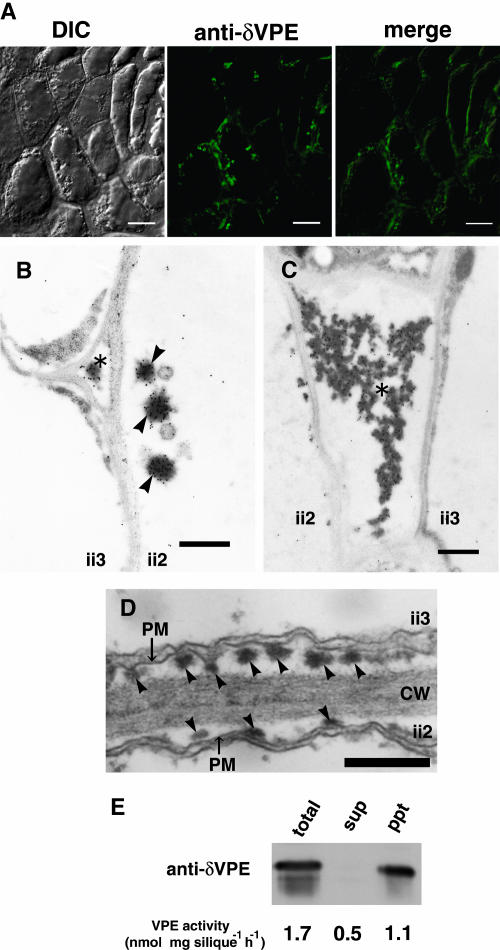
After cell shrinkage, small electron-dense structures appeared in the space between the plasma membrane and the cell wall (Figure 6A, indicated by arrowheads) and in the extracellular space (Figure 6B, indicated by an asterisk) in the ii2 and ii3 cell layers of the inner integument. Immunofluorescence indicating the localization of δVPE was detected in granules that are distributed in the peripheral region of the cells (Figure 7A). Immunogold particles were detected on electron-dense structures in the space between the plasma membrane and the cell wall (Figure 7B, indicated by arrowheads) and in the extracellular space (Figures 7B and 7C, indicated by asterisks). A control experiment with preimmune serum showed no gold particles in the electron-dense structures (data not shown). Therefore, the electron-dense structures correspond to the granules detected on the immunofluorescence micrograph. These results indicate that δVPE is localized in the electron-dense structures, which appear after cell shrinkage (Figures 6A and 6B), in the ii2 and ii3 cell layers. The electron-dense structures were also observed in δvpe-1 seeds (Figure 7D). This suggests that the structures are aggregates composed of δVPE and other proteins.
Figure 7.
Localization of δVPE in Electron-Dense Structures of Shrinking Cells in the Inner Integuments of Developing Seeds at the Torpedo-Shaped-Embryo Stage.
(A) Immunofluorescence analysis of surfaces of the inner integuments with anti-δVPE antibodies. A green fluorescent image (anti-δVPE), a differential interference contrast (DIC) image, and a merged image (merge) are shown. Bars = 10 μm.
(B) and (C) Immunoelectron micrographs of the inner integuments with anti-δVPE antibodies showing the localization of δVPE in electron-dense structures outside (asterisks) and inside (arrowheads) cell walls. Bars = 500 nm.
(D) Electron micrograph showing the existence of electron-dense structures (arrowheads) in the space between the plasma membrane (PM) and cell wall (CW) of developing seeds of δvpe-1. Bars = 500 nm.
(E) Accumulation of δVPE protein and VPE activity in the pellet fraction of young siliques. Total protein from young siliques was centrifuged to obtain the supernatant (sup) and precipitate (ppt) fractions. Each fraction was subjected to immunoblot analysis with anti-δVPE antibodies and to an assay of VPE activity.
δVPE in Insoluble Aggregates Has Proteolytic Activity
Our observations implied that δVPE plays some role in the disappearance of the ii2 and ii3 cell layers of the inner integuments. This raises the question whether δVPE exhibits proteolytic activity in the electron-dense structures. We attempted to isolate the electron-dense structures. Homogenized young siliques were separated into two fractions by centrifugation, the supernatant and pellet fractions. An immunoblot analysis of each fraction with anti-δVPE antibodies showed that δVPE was present in the pellet fraction but not in the supernatant fraction (Figure 7E). This suggests that δVPE aggregates to form the electron-dense structures in the inner integument.
No activity toward Z-AAN-MCA was detected in the siliques of the VPE-null mutant in which all four VPE genes are disrupted (αvpe-1 βvpe-3 γvpe-1 δvpe-1), indicating that VPEs are responsible for the activity detected in the wild-type siliques. δvpe-1 siliques had ∼24% of the VPE activity in wild-type siliques, suggesting that δVPE is responsible for ∼76% of the activity, and the other VPEs are responsible for ∼24% of the activity. When homogenized siliques were separated into the pellet fraction and supernatant fraction, ∼70% of the activity was in the pellet, and ∼30% of the activity was in the supernatant (Figure 7E). Considering no δVPE in the supernatant (Figure 7E, immunoblot), the VPE activity in the pellet might be derived from δVPE, and the activity in the supernatant might be derived from other VPEs in siliques. These findings suggest that the proteinase activity that was observed in the electron-dense aggregates was due to δVPE.
δVPE Exhibits Caspase-1–Like Activity
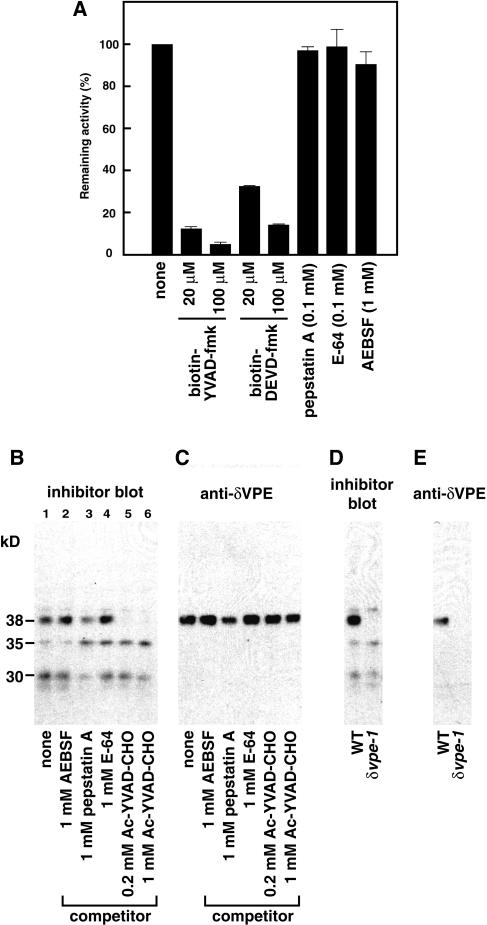
Evidence from extensive studies (discussed below) indicates that caspase activity is also involved in various types of plant programmed cell death. Our recent data show that vegetative-type VPEs of tobacco exhibit caspase-1–like activity and are involved in hypersensitive cell death (Hatsugai et al., 2004). We examined the effects of various proteinase inhibitors on the activity of VPE. Figure 8A shows that the VPE activity was strongly inhibited by biotin-YVAD-fmk (a caspase-1 inhibitor) and also by Ac-DEVD-fmk, as is mammalian caspase-1 (Ekert et al., 1999). None of pepstatin A (an aspartic proteinase inhibitor), E-64 (a papain-type Cys proteinase inhibitor), or AEBSF (a Ser proteinase inhibitor) had any effect on VPE activity. The result implied that δVPE has caspase-1–like activity.
Figure 8.
δVPE Exhibits Caspase-1–Like Activity.
(A) Effects of various proteinase inhibitors on the activity of VPE. Extracts from the developing seeds at the torpedo-shaped-embryo stage were preincubated with each proteinase inhibitor (as indicated) and then subjected to a VPE assay.
(B) and (C) Extracts from the developing seeds at the torpedo-shaped-embryo stage were preincubated with each proteinase inhibitor (as indicated) before incubation with biotin-YVAD-fmk and then subjected to a biotinylated inhibitor blot (B) and to an immunoblot with anti-δVPE antibodies (C). Proteinase inhibitors used as a competitor: AEBSF, Ser protease inhibitor; pepstatin A, aspartic protease inhibitor; E-64, papain-type Cys protease inhibitor; Ac-YVAD-CHO, caspase-1 inhibitor.
(D) and (E) Extracts from developing seeds of the wild type and δvpe-1 were subjected to a biotinylated inhibitor blot (D) and to an immunoblot with anti-δVPE antibodies (E).
To detect the protein responsible for the caspase-1–like activity, we developed a biotinylated inhibitor blot analysis with an irreversible caspase-1 inhibitor, biotin-YVAD-fmk (Figure 8B). In this analysis, an enzyme that conjugates with the inhibitor becomes visible on the blot with streptavidin-conjugated horseradish peroxidase. Three major bands of 38, 35, and 30 kD were detected on the blot of the extract from developing wild-type seeds with a torpedo-shaped embryo (Figure 8B, lane 1). We examined the competitive effects of various inhibitors on in vitro formation of the enzyme inhibitor complex with extracts from seeds (Figure 8B, lanes 2 to 6). When 0.2 or 1.0 mM Ac-YVAD-CHO was added as a competitor with biotin-YVAD-fmk, the 38-kD band completely disappeared on the blot, but the 35- and 30-kD bands did not disappear (Figure 8B, lanes 5 and 6). This result indicates that the protein that has caspase-1–like activity is a 38-kD protein and that the other two bands are nonspecific. The 38-kD band is consistent with the molecular mass of δVPE (Figure 8C). This suggests that the caspase-1–like activity is due to δVPE. To examine this possibility, an extract from wild-type or δvpe-1 developing seeds with a torpedo-shaped embryo was subjected to a biotinylated inhibitor blot analysis (Figure 8D) and an immunoblot analysis (Figure 8E). In the biotinylated inhibitor blot analysis, as in the immunoblot analysis, a 38-kD band was detected in the extract from wild-type seeds, but not in the extract from δvpe-1 seeds. These results indicate that the 38-kD protein is δVPE and that δVPE is an authentic proteinase exhibiting caspase-1–like activity that appears in association with the cell death of the inner integument.
DISCUSSION
δVPE Is Involved in Cell Death during Seed Coat Formation
The Arabidopsis seed coat just after fertilization consists of an outer integument made up of two cell layers and an inner integument made up of three layers. After desiccation of the matured seeds, the seed coat consists of one-cell-layered outer integument and one-cell-layered inner integument (ii1). The ii1 layer accumulates pigments (Devic et al., 1999). The elimination of the cell layers (ii2 and ii3) of the inner integument starts at the heart-shaped-embryo stage in Arabidopsis (Figure 4) as reported in Brassica napus (Wan et al., 2002). Cell shrinkage and vacuolar disruption occur in these layers at the early stage of seed development (Figure 6). DNA fragmentation, a typical feature of animal apoptosis, was detected in the inner integument of B. napus at an early stage of seed development with DAPI stain and a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay (Wan et al., 2002). We found that degradation of the nuclei of the ii2 and ii3 cell layers of developing Arabidopsis wild-type seeds occurred during an early seed development (Figure 5). These results show that the cell death to eliminate the inner integument cell layers is programmed during seed development. Our findings clearly show that δVPE is specifically and transiently expressed in the cell layers of the inner integument that are eliminated (Figure 2) and that δVPE deficiency delays the targeted cell death and nuclear degradation in the ii2 and ii3 cell layers of the inner integument (Figures 4 to 656). We obtained two δvpe mutants, δvpe-1 and δvpe-4, neither of which expressed the δVPE protein in developing seeds (Figure 1C). Both mutants exhibited the identical phenotype of disintegration of the ii2 and ii3 cell layers. Therefore, δVPE is a key player in the developmental cell death of the outer two cell layers, which occurs during seed coat formation.
We observed that the inner integuments of the δvpe single mutant and VPE-null mutant were finally diminished at the full-sized-embryo stage, resulting in formation of thin seed coats in the dry seeds (data not shown). It is possible that the compression of the seed coats at the final stage is caused by necrotic cell death as a result of mechanical pressure by growing embryo and desiccation of seeds, rather than programmed cell death. There is no difference in the physical appearances of the dry seeds between δvpe mutants and the wild type. δVPE deficiency did not affect seed dormancy or germination (data not shown).
Caspase-1–Like Activity of δVPE
Some regulatory mechanisms that underlie programmed cell death are thought to be conserved in plants and animals, and many studies have provided evidence that programmed cell death in both kingdoms shares components that include activities of caspases. Caspase-1–like activity has been detected in Arabidopsis suspension cultured cells during nitric oxide–induced cell death (Clarke et al., 2000) and in tobacco BY-2 cells during isopentenyladenosine-induced apoptosis (Mlejnek and Prochazka, 2002). Caspase-3–like activity has been detected in tobacco suspension cells during heat shock–induced apoptosis (Tian et al., 2000) and in embryonic suspension cells of barley (Hordeum vulgare) (Korthout et al., 2000). Both caspase-1– and caspase-3–like activities were observed in tomato suspension cells during chemically induced apoptosis (De Jong et al., 2000). Both caspase-1 and caspase-3 inhibitors abolish cell death in various plants (del Pozo and Lam, 1998; Clarke et al., 2000; De Jong et al., 2000; Korthout et al., 2000). A caspase-1 inhibitor abolished the DNA laddering induced by carrot (Daucus carota) cell extracts (Zhao et al., 1999), and a caspase-3 inhibitor abolished the DNA laddering during cell death induced by menadione in tobacco protoplasts (Sun et al., 1999).Together, these results indicate that caspase activity is also involved in programmed cell death of plants.
Despite much effort, however, the plant proteinases responsible for the observed caspase activity have not been identified. Our findings demonstrate that δVPE has caspase-1–like activity and is involved in the developmental cell death of the seed coat. These results are consistent with our recent observation that tobacco VPEs exhibiting caspase-1–like activity are involved in Tobacco mosaic virus–induced hypersensitive cell death (Hatsugai et al., 2004). VPEs might be responsible for the caspase-1–like activity that has been detected in a variety of plant cell death. There is no sequence similarity between VPEs and caspases. However, VPE recognizes a caspase-1 substrate, and VPE activity is inhibited by a caspase-1 inhibitor. VPE cleaves a peptide bond at the C-terminal side of Asp (Becker et al., 1995; Hiraiwa et al., 1999) as does caspase-1, although VPE is known to be an asparaginyl endopeptidase (Hara-Nishimura, 1998; Hara-Nishimura and Maeshima, 2000).
Electron-Dense Structure in Which δVPE Is Localized
Previously, we reported the subcellular localization of seed-type VPE in protein storage vacuoles (Hara-Nishimura et al., 1993; Hiraiwa et al., 1993) and that of vegetative-type VPE in lytic vacuoles (Kinoshita et al., 1999). Unexpectedly, we found that the electron-dense structures in which δVPE is localized occur outside of the cells of the ii2 and ii3 layers during the targeted cell death (Figure 7). A possible explanation for the extracellular localization of δVPE is that δVPE is selectively secreted from the cells because it lacks the C-terminal region of 15 amino acids that are found in other types of VPEs and that probably include a vacuolar targeting signal.
Based on what is known about VPE, δVPE might function in maturation and activation of some hydrolases, which are upregulated during cell death. It is possible that the electron-dense structures are the site at which the dying cells of the seed coat are degraded. In animals, dying cells are packaged into apoptotic bodies and then engulfed by phagocytes, such as macrophages and neutrophils (Franc, 2002; Geske et al., 2002). However, plants do not have phagocytes, and cells surrounded by a rigid cell wall must degrade their own contents. The plant system for degrading dying cells is different from the animal one. Plants appear to have evolved a VPE-mediated system, whereas animals have evolved a caspase-mediated system.
Different Expression of δVPE from Those of the Other VPEs
Our results clearly show that δVPE is specifically localized in two cell layers of the inner integuments of the Arabidopsis seed coat (Figure 2). This is consistent with a histochemical analysis with a β-glucuronidase reporter gene that was driven by a δVPE promoter (S. Nakaune and I. Hara-Nishimura, unpublished data). The highly specific expression of δVPE in particular cell layers of the seed coat is different from the expression of other Arabidopsis VPE homologs: αVPE and γVPE are expressed in the vegetative organs and βVPE is expressed in the developing embryo (Kinoshita et al., 1999). Transcripts of LeVPE1, which is a putative ortholog of δVPE, were found to increase during the early phases of fruit development, reach a maximum at the end of the expansion phase (15 to 20 d after anthesis), and then slowly decrease (Lemaire-Chamley et al., 1999). The expressions of two tobacco VPEs (NtPB1 and NtPB3), which are also putative orthologs of δVPE, were found in early seed development (Zakharov and Muntz, 2004). These features are similar to the expression pattern of δVPE (Figure 3).
In maturing Arabidopsis seeds, two major storage proteins, 12S globulin and 2S albumin, are synthesized as precursors. βVPE is essential for proper processing of the precursor molecules into the mature storage proteins (Shimada et al., 2003; Gruis et al., 2004). Interestingly, vegetative-type VPEs, αVPE and γVPE, partly compensate for βVPE deficiency in the βvpe seeds (Shimada et al., 2003; Gruis et al., 2004). Triple VPE mutant (αvpe-1 βvpe-3 γvpe-1) seeds accumulate no properly processed mature storage proteins (Shimada et al., 2003; Gruis et al., 2004). This result suggests that δVPE is not involved in processing storage proteins. This is consistent with the result that δvpe seeds normally process storage proteins as do wild-type seeds (Gruis et al., 2002, 2004). Even though both δVPE and βVPE are accumulated in developing seeds, the tissues in which they are expressed are completely different: δVPE is expressed in the maternal tissues and βVPE is expressed in tissues of the next generation (embryo). Unlike αVPE or γVPE, δVPE has no ability to compensate for the deficiency of βVPE.
METHODS
Plant Materials and Growth Conditions
Seeds of Arabidopsis thaliana (ecotype Columbia [Col-0]) were surface sterilized with 95% ethanol and then sown onto 0.8% agar in MS medium (Wako, Tokyo, Japan). After a 4-d incubation at 4°C to break seed dormancy, the seeds were germinated and grown at 22°C under continuous light (100 μE s−1 m−2) for 21 d. The seedlings were transferred onto vermiculite for subsequent growth.
Isolation of δvpe Mutants and Generation of a VPE-Null (Quadruple) Mutant of Four VPE Genes
We used two T-DNA insertion mutants of Arabidopsis. δvpe-1 was isolated from a large population of Arabidopsis plants provided by the Kazusa DNA Institute (Chiba, Japan) by PCR-based screening. We used the following primers: δVPE-kFW, 5′-TCATGCAAGGTGCTTATGTGTGA-3′; δVPE-kRV, 5′-CGTCCGTACCGTACAGTTGGTA-3′; P06RB, 5′-TTCCCTTAATTCTCCGCTCATGATC-3′ for the T-DNA right border. δvpe-4 (SALK_009856) was obtained from the Arabidopsis Biological Resource Center (Columbus, OH). Insertion mutant information was obtained from the SALK Institute Genomic Analysis Laboratory Web site (http://signal.salk.edu). These mutants have a Columbia background. We crossed the triple mutant (αvpe-1 βvpe-3 γvpe-1; Shimada et al., 2003) and the single mutant δvpe-1 with each other and isolated a VPE-null mutant (αvpe-1 βvpe-3 γvpe-1 δvpe-1).
Polyclonal Antibodies against δVPE
The cDNA of Arabidopsis δVPE (AV555212) was donated by the Kazusa DNA Research Institute (Asamizu et al., 2000). A PCR-amplified DNA fragment encoding the putative mature region (amino acids 40 to 407) of δVPE was inserted into the pET32a vector (Novagen, Madison, WI). A fusion protein with a His-tag was synthesized in Escherichia coli BL21 (DE3) cells and purified on a Ni2+ column. The purified protein (1 mg) in 1 mL of 50 mM Na-phosphate buffer, pH 7.2, and 6.4 M urea was emulsified with an equal volume of Freund's complete adjuvant. The emulsion was injected subcutaneously into a rabbit. After 3 weeks, three booster injections with incomplete adjuvant were given at intervals of 7 d. One week after the final booster injection, blood was drawn and antibody was prepared. The antibodies exhibited strong specificity for δVPE-related proteins (Figure 4) and were of a titer sufficiently high to detect δVPE-related proteins (data not shown).
Immunoblot Analysis
The siliques and other organs of Arabidopsis were homogenized in 20 mM Tris-HCl, pH 8.0, and 2% (w/v) SDS before centrifugation to collect soluble proteins. The proteins were subjected to SDS-PAGE and were transferred electrophoretically to a polyvinylidene difluoride membrane (0.22 μm; Nihon Millipore, Tokyo, Japan). The membrane was incubated with anti-δVPE antibodies (diluted 5000-fold) in TBS-T (50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 0.05% [v/v] Tween 20) after blocking with 5% (w/v) skim milk in TBS-T. Horseradish peroxidase–conjugated donkey antibodies directed against rabbit IgG (diluted 1:5000; Amersham Pharmacia Biotech, Tokyo, Japan) were used as the secondary antibodies. The δVPE-related proteins were visualized with an enhanced chemiluminescence kit (ECL system; Amersham Pharmacia Biotech). We also used antibodies directed against βVPE (Shimada et al., 2003).
Immunofluorescence Analysis
The developing seeds of Arabidopsis that had walking-stick-shaped embryos were fixed for 40 min in 7.2% (w/v) formaldehyde, 0.1% (v/v) Nonidet P-40, 10% (v/v) dimethyl sulfoxide, and 50 mM Na-phosphate buffer, pH 7.2, washed twice with TBS-T for 5 min, incubated in TBS-T containing 5% (w/v) Cellulase Onozuka R-10 (Yakult, Tokyo, Japan) and 2% (w/v) Pectolyase Y-23 (kikkoman, Tokyo, Japan) for 20 min at 30°C, washed twice with TBS-T, incubated in blocking buffer (2% [w/v] BSA and TBS-T) for 30 min, incubated with anti-δVPE antibodies (diluted 500-fold) in the blocking buffer for 40 min, washed three times for 5 min each, incubated for 1 h with goat anti-rabbit IgG antibodies conjugated with Alexa Fluor 488 (absorbance, 495 nm; emission, 519 nm; Molecular Probes, Eugene, OR), washed three times for 5 min with TBS-T, and mounted.
Ultrastructural Analysis and Immunogold Labeling
The developing seeds with torpedo-shaped embryos were vacuum infiltrated for 1 h with a fixative that consisted of 4% paraformaldehyde, 1% glutaraldehyde, and 0.06 M sucrose in 0.05 M cacodylate buffer, pH 7.4. The tissues were cut into slices of <1 mm in thickness with a razor blade and treated for another 2 h with freshly prepared fixative. Procedures for ultrastructural studies were essentially the same as described previously (Hara-Nishimura et al., 1993).
Immunogold labeling procedures were essentially the same as described previously (Hara-Nishimura et al., 1993), except for the use of anti-δVPE antibodies. Postfixation was omitted for immunoelectron microscopy. The samples were dehydrated in a graded dimethylformamide series at −20°C and embedded in London Resin White (London Resin Co., Basingstoke, Hampshire, UK). Blocks were polymerized under a UV lamp at a Reichert ultramicrotome (Leica, Heidelberg, Germany). The ultrathin sections were incubated with 1% (w/v) BSA in PBS for 1 h at room temperature and then incubated with anti-δVPE antibodies (diluted 1:5000) in blocking solution overnight at 4°C. The sections were washed with PBS and then incubated for 30 min at room temperature with a solution of protein A-gold (10 or 15 nm; Amersham Pharmacia Biotech) that had been diluted 1:30 in the blocking solution. The sections were washed with distilled water and then were stained with uranyl acetate and lead citrate. After staining, all sections were examined with a transmission electron microscope (model 1200EX; JEOL, Tokyo, Japan) at 80 kV.
DAPI Staining
The young wild-type or δvpe-1 siliques were fixed for 40 min in 7.2% (w/v) formaldehyde, 0.1% (v/v) Nonidet P-40, 10% (v/v) dimethyl sulfoxide, and 50 mM Na-phosphate buffer, pH 7.2. The fixed tissues were dehydrated through a series of ethanol solutions (50, 50, 60, 70, 85, 100, and 100%) for 30 min each, infiltrated with a t-butyl alcohol/Paraplast Plus (Oxford Labware, St. Louis, MO) mixture and then embedded in Paraplast. Thin sections (7-μm thick) were cut on a microtome (RM2155; Leica) and mounted on slides. The samples were dried overnight at 40°C and incubated in xylene to remove Paraplast from the tissue sections. The sections were washed in 100% ethanol twice and stained by 1 μg/mL DAPI in methanol for 30 min.
Enzyme Assays for VPE Activity and Caspase-Like Activity
We used fluorogenic substrates conjugated with α-(4-methyl-coumaryl-7-amide) (MCA; Peptide Institute, Osaka, Japan): Z-AAN-MCA (VPE-specific substrates). Arabidopsis siliques frozen in liquid nitrogen were ground using a mortar and pestle and were homogenized with 50 mM Na-acetate buffer, pH 5.5, containing 50 mM NaCl, 1 mM EDTA, and 100 mM DTT. We used the whole extract as enzyme solution. Alternatively, the soluble and insoluble fractions were obtained by centrifugation at 15,000g for 5 min. The insoluble fraction was resuspended with the same buffer. The reaction was started by adding each fluorogenic substrate (to a final concentration of 500 μM) to the homogenate, and an increase in the fluorescence of the 100-μL reaction mixture was measured at 465 nm with a fluorescence spectrophotometer (GENios; Tecan, Tokyo, Japan). The inhibitors used were as follows: 0.1 mM pepstatin A, 0.1 mM E-64, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 20 to 100 μM biotin-YVAD-fmk, and 20 to 100 μM biotin-DEVD-fmk.
Biotinylated Inhibitor Blot Analysis
We used the extract from developing seeds with torpedo-shaped embryo. For a biotinylated inhibitor blot analysis, the sample solutions were preincubated with or without each inhibitor as described above and incubated in 100 mM Na-acetate, pH 5.5, containing 100 mM DTT with a final concentration of 20 μM biotin-YVAD-fluoromethylketone (biotin-YVAD-fmk; Calbiochem, San Diego, CA) for 60 min. The resulting complex of enzyme and biotin-YVAD-fmk was subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was treated with a blocking solution and then with streptavidin-conjugated horseradish peroxidase (diluted, 2000-fold; Amersham Pharmacia Biotech) for 30 min. Detection was performed with an enhanced chemiluminescence kit (ECL system).
Acknowledgments
This work was supported by Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation, Grants-in-Aid for Scientific Research (16085203 and 16657013) and 21st Century Center of Excellence Research Kyoto University (A14) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by a postdoctoral fellowship to S.N. from the Japan Society for the Promotion of Science (16001229).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ikuko Hara-Nishimura (ihnishi@gr.bot.kyoto-u.ac.jp).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.026872.
References
- Asamizu, E., Nakamura, Y., Sato, S., and Tabata, S. (2000). A large scale analysis of cDNA in Arabidopsis thaliana: Generation of 12,028 non-redundant expressed sequence tags from normalized and size-selected cDNA libraries. DNA Res. 7, 175–180. [DOI] [PubMed] [Google Scholar]
- Becker, C., Shutov, A.D., Nong, V.H., Senyuk, V.I., Jung, R., Horstmann, C., Fischer, J., Nielsen, N.C., and Muntz, K. (1995). Purification, cDNA cloning and characterization of proteinase B, an asparagine-specific endopeptidase from germinating vetch (Vicia sativa L.) seeds. Eur. J. Biochem. 228, 456–462. [PubMed] [Google Scholar]
- Beeckman, T., De Rycke, R., Viane, R., and Inze, D. (2000). Histological study of seed coat development in Arabidopsis thaliana. J. Plant Res. 113, 139–148. [Google Scholar]
- Caffrey, C.R., Mathieu, M.A., Gaffney, A.M., Salter, J.P., Sajid, M., Lucas, K.D., Franklin, C., Bogyo, M., and McKerrow, J.H. (2000). Identification of a cDNA encoding an active asparaginyl endopeptidase of Schistosoma mansoni and its expression in Pichia pastoris. FEBS Lett. 466, 244–248. [DOI] [PubMed] [Google Scholar]
- Chen, J.M., Dando, P.M., Stevens, R.A., Fortunato, M., and Barrett, A.J. (1998). Cloning and expression of mouse legumain, a lysosomal endopeptidase. Biochem. J. 338, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, A., Desikan, R., Hurst, R.D., Hancock, J.T., and Neill, S.J. (2000). NO way back: Nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 24, 667–677. [DOI] [PubMed] [Google Scholar]
- De Jong, A.J., Hoeberichts, F.A., Yakimova, E.T., Maximova, E., and Woltering, E.J. (2000). Chemical-induced apoptotic cell death in tomato cells: Involvement of caspase-like proteases. Planta 211, 656–662. [DOI] [PubMed] [Google Scholar]
- del Pozo, O., and Lam, E. (1998). Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr. Biol. 8, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Devic, M., Guilleminot, J., Debeaujon, I., Bechtold, N., Bensaude, E., Koornneef, M., Pelletier, G., and Delseny, M. (1999). The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J. 19, 387–398. [DOI] [PubMed] [Google Scholar]
- Ekert, P.G., Silke, J., and Vaux, D.L. (1999). Caspase inhibitors. Cell Death Differ. 6, 1081–1086. [DOI] [PubMed] [Google Scholar]
- Franc, N.C. (2002). Phagocytosis of apoptotic cells in mammals, Caenorhabditis elegans and Drosophila melanogaster: Molecular mechanisms and physiological consequences. Front. Biosci. 7, d1298–d1313. [DOI] [PubMed] [Google Scholar]
- Gaiser, J.C., Robinson-Beers, K., and Gasser, S. (1995). The Arabidopsis SUPERMAN gene mediates asymmetric growth of the outer integument of the ovule. Plant Cell 7, 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser, C.S., and Robinson-Beers, K. (1993). Pistil development. Plant Cell 5, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geske, F.J., Monks, J., Lehman, L., and Fadok, V.A. (2002). The role of the macrophage in apoptosis: Hunter, gatherer, and regulator. Int. J. Hematol. 76, 16–26. [DOI] [PubMed] [Google Scholar]
- Gruis, D.F., Schulze, J., and Jung, R. (2004). Storage protein accumulation in the absence of the vacuolar processing enzyme family of cysteine proteases. Plant Cell 16, 270–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruis, D.F., Selinger, D.A., Curran, J.M., and Jung, R. (2002). Redundant proteolytic mechanisms process seed storage proteins in the absence of seed-type members of the vacuolar processing enzyme family of cysteine proteases. Plant Cell 14, 2863–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I. (1998). Asparaginyl endopeptidase. In Handbook of Proteolytic Enzymes, A.J. Barrett, N.D. Rawlings, and J.F. Woessner, eds (London: Academic Press), pp. 746–749.
- Hara-Nishimura, I., Inoue, K., and Nishimura, M. (1991). A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 294, 89–93. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Kinoshita, T., Hiraiwa, N., and Nishimura, M. (1998). Vacuolar processing enzymes in protein-storage vacuoles and lytic vacuoles. J. Plant Physiol. 152, 668–674. [Google Scholar]
- Hara-Nishimura, I., and Maeshima, M. (2000). Vacuolar processing enzymes and auqaporins. In Vacuolar Compartments in Plants, A.D.G. Robinson and J.C. Rogers, eds (London: Sheffield Academic Press), pp. 20–42.
- Hara-Nishimura, I., Takeuchi, Y., and Nishimura, M. (1993). Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell 5, 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai, N., Kuroyanagi, M., Yamada, K., Meshi, T., Tsuda, S., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (2004). A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305, 855–858. [DOI] [PubMed] [Google Scholar]
- Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1997). Expression and activation of the vacuolar processing enzyme in Saccharomyces cerevisiae. Plant J. 12, 819–829. [DOI] [PubMed] [Google Scholar]
- Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1999). Vacuolar processing enzyme is self-catalytically activated by sequential removal of the C-terminal and N-terminal propeptides. FEBS Lett. 447, 213–216. [DOI] [PubMed] [Google Scholar]
- Hiraiwa, N., Takeuchi, Y., Nishimura, M., and Hara-Nishimura, I. (1993). A vacuolar processing enzyme in maturing and germinating seeds: Its distribution and associated changes during development. Plant Cell Physiol. 34, 1197–1204. [Google Scholar]
- King, S.P., Lunn, L.E., and Furbank, R.T. (1997). Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiol. 114, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, T., Nishimura, M., and Hara-Nishimura, I. (1995. a). The sequence and expression of the γ-VPE gene, one member of a family of three genes for vacuolar processing enzymes in Arabidopsis thaliana. Plant Cell Physiol. 36, 1555–1562. [PubMed] [Google Scholar]
- Kinoshita, T., Nishimura, M., and Hara-Nishimura, I. (1995. b). Homologues of a vacuolar processing enzyme that are expressed in different organs in Arabidopsis thaliana. Plant Mol. Biol. 29, 81–89. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., Yamada, K., Hiraiwa, N., Nishimura, M., and Hara-Nishimura, I. (1999). Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J. 19, 43–53. [DOI] [PubMed] [Google Scholar]
- Korthout, H., Berecki, G., Bruin, W., van Duijin, B., and Wang, M. (2000). The presence and subcellular localization of caspase 3-like proteinases in plant cells. FEBS Lett. 475, 139–144. [DOI] [PubMed] [Google Scholar]
- Kuroyanagi, M., Nishimura, M., and Hara-Nishimura, I. (2002). Activation of Arabidopsis vacuolar processing enzyme by self-catalytic removal of an auto-inhibitory domain of the C-terminal propeptide. Plant Cell Physiol. 43, 143–151. [DOI] [PubMed] [Google Scholar]
- Lemaire-Chamley, M., Petit, J., Raymond, P., and Chevalier, C. (1999). Isolation and characterization of a fruit specific cDNA clone for vacuolar processing enzyme from tomato. Plant Physiol. 121, 1054. [Google Scholar]
- Leon-Kloosterziel, K.M., Keijzer, C.J., and Koornneef, M. (1994). A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell 6, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlejnek, P., and Prochazka, S. (2002). Activation of caspase-like proteases and induction of apoptosis by isopentenyladenosine in tobacco BY-2 cells. Planta 215, 158–166. [DOI] [PubMed] [Google Scholar]
- Schneitz, K., Hulskamp, M., and Pruitt, R.E. (1995). Wild-type ovule development in Arabidopisis thaliana: A light microscope study of cleared whole-mount tissue. Plant J. 7, 731–749. [Google Scholar]
- Sheen, J., Zhou, L., and Jang, J.C. (1999). Sugars as signaling molecules. Curr. Opin. Plant Biol. 2, 410–418. [DOI] [PubMed] [Google Scholar]
- Shimada, T., et al. (2003). Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J. Biol. Chem. 278, 32292–32299. [DOI] [PubMed] [Google Scholar]
- Shirahama-Noda, K., Yamamoto, A., Sugihara, K., Hashimoto, N., Asano, M., Nishimura, M., and Hara-Nishimura, I. (2003). Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J. Biol. Chem. 278, 33194–33199. [DOI] [PubMed] [Google Scholar]
- Sun, Y.L., Zhao, Y., Hong, X., and Zhai, Z.H. (1999). Cytochrome c release and caspase activation during menadione-induced apoptosis in plants. FEBS Lett. 462, 317–321. [DOI] [PubMed] [Google Scholar]
- Tian, R.H., Zhang, G.Y., Yan, C.H., and Dai, Y.R. (2000). Involvement of poly(ADP-ribose) polymerase and activation of caspase-3-like protease in heat shock-induced apoptosis in tobacco suspension cells. FEBS Lett. 474, 11–15. [DOI] [PubMed] [Google Scholar]
- Wan, L., Xia, O., and Selvaraj, G. (2002). Early stages of seed development in Brassica napus: A seed coat-specific cysteine proteinase associated with programmed cell death of the inner integument. Plant J. 30, 1–10. [DOI] [PubMed] [Google Scholar]
- Weber, H., Borisjuk, L., Heim, U., Buchner, P., and Wobus, U. (1995). Seed coat-associated invertases of fava bean control both unloading and storage functions: Cloning of cDNAs and cell type-specific expression. Plant Cell 7, 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western, T.L., Skinner, D.J., and Haughn, G.W. (2000). Differentiation of mucilage secretory cells of the Arabidposis seed coat. Plant Physiol. 122, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor, J.B., Symonds, V., Mendenhall, J., and Lloyd, A.M. (2000). Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 22, 483–493. [DOI] [PubMed] [Google Scholar]
- Wobus, U., and Weber, H. (1999). Seed maturation: Genetic programmes and control signals. Curr. Opin. Plant Biol. 2, 33–38. [DOI] [PubMed] [Google Scholar]
- Yamada, K., Matsushima, R., Nishimura, M., and Hara-Nishimura, I. (2001). A slow maturation of a cysteine protease with a granulin domain in the vacuoles of senescing Arabidopsis leaves. Plant Physiol. 127, 1626–1634. [PMC free article] [PubMed] [Google Scholar]
- Zakharov, A., and Muntz, K. (2004). Seed legumains are expressed in stamens and vegetative legumains in seeds of Nicotiana tatabacum L. J. Exp. Bot. 55, 1463–1471. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Jiang, Z.F., Sun, Y.L., and Zhai, Z.H. (1999). Apoptosis of mouse liver nuclei induced in the cytosol of carrot cells. FEBS Lett. 448, 197–200. [DOI] [PubMed] [Google Scholar]