Abstract
The development of accelerated methods for pathogen identification (ID) and antimicrobial susceptibility testing (AST) for infectious diseases is necessary to facilitate evidence-based antibiotic therapy and reduce clinical overreliance on broad-spectrum antibiotics. Towards this end, droplet-based microfluidics has unlocked remarkably rapid diagnostic assays with single-cell and single-molecule resolution. Yet, droplet platforms invariably rely on testing purified bacterial samples that have been clinically isolated after lengthy (>16 h) plating. While plating-based clinical isolation is important for enriching and separating out bacteria from background in clinical samples and also facilitating buffer exchange, it creates a diagnostic bottleneck that ultimately precludes droplet-based methods from achieving significantly accelerated times-to-result. To alleviate this bottleneck, we have developed facile syringe filter-enabled strategies for bacterial separation, enrichment, and buffer exchange from urine samples. By selecting appropriately sized filter membranes, we separated bacterial cells from background particulates in urine samples and achieve up to 91% bacterial recovery after such 1-step filtration. When interfaced with droplet-based detection of bacterial cells, 1-step filtration improved the limit of detection for bacterial ID and quantification by over an order of magnitude. We also developed a facile buffer exchange strategy to prepare bacteria in urine samples for droplet-based AST that achieved up to 10-fold bacterial enrichment during buffer exchange. Our filtration strategies, can be easily integrated into droplet workflows, enable clinical isolation-free sample-to-answer ID and AST, and significantly accelerate the turnaround of standard infectious disease diagnostic workflows.
Graphical Abstract

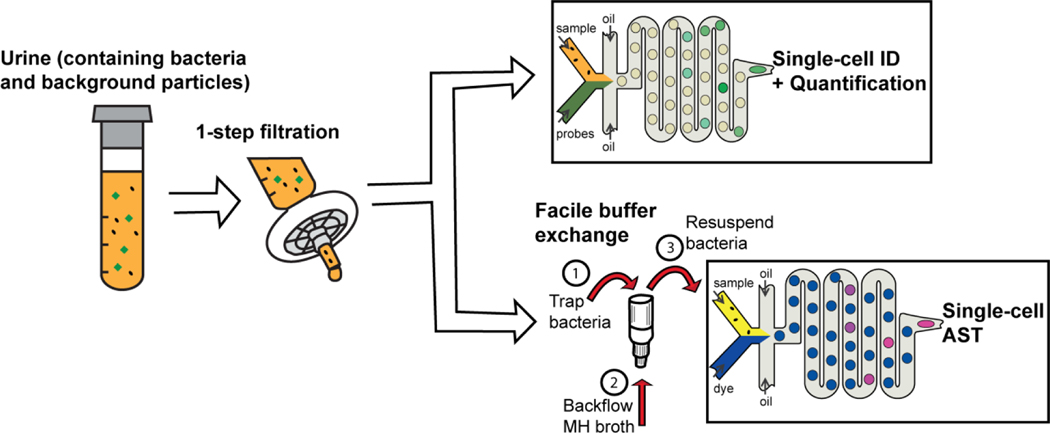
We devised and interfaced a facile filtration-based bacteria recovery and buffer exchange process with droplet microfluidics for clinical isolation-free digital detection and characterization of bacteria pathogens in urine.
Introduction
In order to combat the rising global threat of antimicrobial resistant pathogens and improve patient care for infectious diseases, there is an increasing need for new technological approaches that can deliver prompt diagnosis of bacterial infections1,2. Urinary tract infections (UTIs) in particular present a clinically important case, where slow culture-based diagnostic methods have led to the extensive use of first-line broad spectrum antibiotics, which have been linked to increasing antimicrobial resistance and poor patient outcomes3. As a means to accelerate diagnosis and facilitate pathogen-specific treatment, researchers have been exploring microfluidics-enabled single-cell and single-molecule detection platforms4,5. One of the most promising of these approaches has been droplet-based microfluidics, in which bacterial samples are discretized into thousands to millions of isolated reaction chambers, some of which contain single copies of the biomarker of interest6,7. The small droplet volumes facilitate an equivalent reduction in assay background and a significant enhancement in the local concentration of the target, resulting in improved detection sensitivities and substantially faster times to result.
Researchers have leveraged these attributes of droplets in developing rapid digital methods (<2 h) for detecting the presence of bacteria in prepared or processed biological samples via digital nucleic acid amplification methods such as PCR8, loop-mediated isothermal amplification (LAMP)9,10, and recombinase polymerase amplification (RPA)11. Our group previously devised a droplet-based amplification-free approach to pathogen detection based on sequence-specific hybridization detection of 16S rRNA from single bacterial cells within as little as 15 min12–14. Droplets have also been used to significantly accelerate antimicrobial susceptibility testing, down to 1 h and below with single-cell sensitivity14,15, and across multiple antibiotics with high-throughput16. Having significantly accelerated bacterial detection, quantification, and antimicrobial susceptibility testing, droplet microfluidic platforms represent a promising approach for rapid diagnostic processing of infectious disease samples in clinical microbiology laboratories.
Despite their demonstrated advantages in speed, droplet platforms have traditionally relied on purified bacterial cultures as input, generally acquired after time-consuming clinical isolation. The clinical isolation process, which typically requires plating of infectious clinical samples to allow the infection-causing bacteria to grow into visible colonies, can necessitate at least 16 hours for most UTI pathogens17. Such clinical isolation serves to (1) separate the target bacteria from the clinical sample matrix, such that background materials from the biological sample may be sufficiently diluted and discarded from further analysis, (2) enable buffer exchange, wherein enriched pathogen colonies may be suspended in culture broths or appropriate assay-compatible media for subsequent assay steps, and (3) enrich and amplify the quantity of target pathogen to be well within the dynamic range of typical detection platforms. When considering clinical diagnosis of UTIs in particular, plating-based clinical isolation achieves separation of bacteria from background material in urine (e.g., urea, white blood cells, epithelial cells, etc.), buffer exchange into culture broth for growth-based antimicrobial susceptibility testing, and target enrichment for detection with conventional bulk-based methods (e.g., MALDI-ToF mass spectrometry). Unfortunately, even in emerging microfluidic1,18 and digital microfluidic platforms19, plating-based clinical isolation has remained a mainstay, creating time-consuming, labor-intensive, and resource-consuming bottleneck that precludes these platforms from achieving a significant benefit in turnaround and hence, limiting clinical translatability. Therefore, a simple, culture-free method for bacterial separation, enrichment, and buffer exchange is desirable to fully leverage the speed of droplet platforms and achieve clinical translatability.
Size-based physical separation of bacteria via filtration offers a faster and simpler alternative to bacterial separation, enrichment, and buffer exchange. Indeed, filtration-based bacteria separation and enrichment, including nanofiltration, reverse osmosis, microfiltration and ultrafiltration, has been widely used in foodborne pathogen detection20–22, water contamination monitoring23 and metagenomics studies24. For example, tangential-flow ultrafiltration using hollow fibers or ceramic membranes was used for bacteria recovery from water25,26 and aqueous chicken homogenates27. To further separate large contaminants during bacteria recovery, a 2-stage filtration device with two sequential filters was designed and demonstrated rapid bacteria recovery for a mock sample using bacteria/tungsten powder mixture in water28. However, most of these methods require complicated control systems20,21,25–27 and have not been applied to biofluids20–23,25–28. Conversely, commercial syringe filters are a simple and direct alternative to recover bacteria from urine due to their wide availability and familiarity to clinical microbiologists.
Herein, we describe filtration-enabled sample preparation steps, that leverage widely-available syringe filters and membranes and can be easily integrated into droplet microfluidic workflows (Figure 1). Separation of pathogens from background particulates in urine is accomplished by a 1-step filtration process, wherein a filter membrane is used to trap non-bacterial particles, which are typically larger than bacterial cells, and allow target bacteria to flow through into the filtrate. This 1-step filtration process is particularly useful for avoiding large particles from flowing into microfluidic devices with small physical features, as this can create undesirable clogs or spurious signals. After 1-step filtration, bacterial cells remain suspended in the urine filtrate, and can be readily detected if coupled with a sensitive bacterial detection method that can function in the presence of urine filtrate. Towards this end, we demonstrate the utility of 1-step filtration in detecting single bacterial cells using a robust molecular probe-based assay. Notably, by eliminating spurious signals from background particulates, our 1-step filtration methodology extends the achievable dynamic range for bacterial quantification in urine. In addition to bacterial separation, buffer exchange of pathogens into culture media can be accomplished by filter membranes of pore sizes that are significantly smaller than bacterial cells, by trapping target bacteria onto the filter membrane during flow through and then resuspending these bacteria into culture media via back flow. Such a facile buffer exchange method can not only help prepare urine specimens for growth-based antimicrobial susceptibility testing, but also facilitate desired enrichment of bacterial pathogens during resuspension. Furthermore, when coupled with well-established growth-based fluorescent assays15,29, such buffer exchange helps mitigate the effect of growth-inhibitors like boric acid and sodium formate that are often added as preservatives in urine specimens. To highlight the utility of our facile buffer exchange protocol, we demonstrate single-cell antimicrobial susceptibility assessment of bacteria directly from urine samples. Together, our 1-step filtration and facile buffer exchange steps help avoid the bottlenecks posed by plating-based clinical isolation, and in this endeavor, we are able to fully leverage the speed of droplet platforms to significantly accelerate the clinical diagnostic turnaround for UTIs.
Figure 1: Syringe filter-enabled sample preparation for droplet based diagnostic assays.
We employ easily adaptable syringe filters for 1-step separation of bacterial samples from background particulates in urine. The urine filtrate can be directly used for single-cell pathogen identification and quantification in droplets using a robust molecular probe-based hybridization assay. To remove the urine sample matrix, bacterial cells in the filtrate can be captured on a syringe filter with smaller pores. Buffer exchange into Mueller-Hinton II cation-adjusted (MH) broth and bacteria enrichment can be then be initiated by releasing bacteria trapped in the filter membrane via backflow. The resulting suspension can be used for fluorescence-based single-cell antimicrobial susceptibility assessment in droplets using a viability dye. Together, the 1-step filtration and facile buffer exchange obviate plating-based clinical isolation and can significantly accelerate the diagnostic turnaround of droplet workflows.
Experimental
Bacteria, antibiotics, and urine samples
A reference strain of E. coli (ATCC 25922) was purchased from ATCC (Manassas, VA). The bacterial strain was plated, and an isolated colony was grown into log phase in tryptic soy broth (TSB). The bacteria were then counted via plating in tryptic soy agar (TSA), and stocks were aliquoted and frozen with 20% glycerol (v/v) at −80 °C. Prior to each experimental run, a fresh aliquot of bacteria was thawed and washed twice with Mueller-Hinton II cation-adjusted broth (MH) (Sigma-Aldrich, St. Loius, MO, USA). During experiments, bacteria were suspended in either 100% blank urine or a 4:1 mixture of MH and blank urine. Blank urine samples were acquired from the Johns Hopkins Medical Microbiology Laboratory. All blank urine samples used in experiments were confirmed culture-negative by plating.
Syringe filters and experimental apparatus
Seven distinct syringe filters were tested in this work. GE Whatman Nuclepore, GE Whatman GMF 150, and GE Whatman FP 30 filters were purchased from GE Life Sciences (Pittsburgh, PA). Primecare’s hydrophilic iPOC-DX Primecare S/G membrane was acquired from International Point of Care, Inc. (Toronto, Canada). GVS’s 13 mm Sterile Abluo Syringe filter (FJ13ASCCA050PL01) was purchased from Amazon (Seattle, WA). The buffer exchange filter Millipore Millex GV was purchased from Sigma-Aldrich (St. Louis, MO), and the Sterlitech PCTE membrane was purchased directly from Sterlitech (Kent, WA). Some tested filters were purchased as a membrane without a housing and syringe adapter. For these filters, a GE Pop-Top (GE Life Sciences, Pittsburgh, PA) syringe filter adapter was used to interface the membrane with standard syringes.
Facile filtration protocols
Prior to droplet generation, stock E. coli (ATCC 25922) was thawed, washed twice, and suspended in blank urine samples at either 2 × 108 CFU mL−1 or 1.5 × 107 CFU mL−1. For the probe-based hybridization assay, the urine samples, now containing bacteria, were drawn into a 1 mL plastic syringe (BD, Franklin Lakes, NJ). The syringe was attached to a GE Pop-Top syringe filter adapter containing an iPOC-DX Primecare membrane, and the urine was pushed through the membrane in a smooth, controlled motion, while collecting the filtrate into a fresh tube. Urine filtrates were then diluted in MH broth to yield a final bacterial concentration of ~5 × 107 CFU mL−1 prior to droplet generation. For the resazurin-based growth assay, urine filtrate was passed through either a Millipore Millex GV filter or a Sterlitech PCTE Membrane filter in order to trap all the bacteria onto the filter membrane. Next, MH was infused through the outlet end of the filter, and the resulting suspension of bacteria in MH was collected into a fresh tube.
Design and operation of microfluidic platform
For the probe-based hybridization assay as well as the resazurin-based growth assay, a simple droplet device was fabricated to enable droplet generation and detection. The device consists of a 10-μm-wide flow-focusing nozzle for droplet generation and an equidimensional constriction channel for droplet detection. In this design, the droplet generation and droplet detection regions are distinct and disconnected, which allowed for incubation flexibility. For the probe-based hybridization assay, the two regions were connected by Tygon tubing which served as an intermediate integrated incubation channel. For the resazurin assay, generated droplets were collected off-chip for incubation and reinjected into the detection region for detection. All devices were fabricated by pouring 50 g of 10:1 (base to curing agent) polydimethylsiloxane (PDMS, Sylgard 184 Silicone Elastomer Kit, Dow Corning, Midland, MI) onto an SU-8 mold prepared by standard photolithography.
In order to initiate the probe-based hybridization assay, we prepared a 200 nM solution of E. coli specific double stranded peptide nucleic acid (dsPNA) probes and 600 nM DNA quencher in a custom hybridization buffer consisting of 50 mM NaCl, 25 mM Tris-HCl pH 8.0, and 0.5% polyvinylpyrrolidone (PVP). The urine filtrate containing bacteria and probes solution were then introduced into separate sections of Tygon tubing. Both sections of Tygon tubing were individually connected to Hamilton 1000 glass syringes (Sigma-Aldrich) containing FC-40 oil (Sigma-Aldrich). FC-40 served as dispensing oil, used to push the aqueous samples from Tygon tubing into the device using a syringe pump (Harvard Apparatus). BioRad QX200 Droplet Generation Oil (BioRad, Hercules, CA) was introduced into the oil inlet of the device by a separate syringe pump. A flow rate of 20 μL h−1 was used for both aqueous phases, while 60 μL h−1 was used for the oil phase. Lysis and hybridization were conducted as the droplets flowed through the Tygon tube connecting the droplet generation and droplet detection regions of the microfluidic device. Importantly, separate lengths of the Tygon tube were clamped onto a 95 °C Peltier heater and a 60 °C Peltier heater, such that all droplets were subject to 2 min at 95 °C followed by 30 min at 60 °C.
Similarly, for the resazurin-based growth assay, a 400 μM solution of resazurin was prepared in MH broth. The buffer-exchanged suspension of bacteria in MH broth and the resazurin solution (with or without an antibiotic) were then introduced into separate sections of Tygon tubing. As with the probe-based experiments, the two solutions were entered into our droplet device along with droplet generation oil (2% 008-FluoroSurfactant, RAN Biotechnologies, Beverly, MA) to generate picoliter droplets. The resulting droplets were then collected and incubated off-chip at 37 °C for approximately 120 min before detection on-chip.
Droplet fluorescence detection and data analysis
Each droplet device was imaged using a 4× objective lens and a CCD camera during droplet generation and after droplet incubation in order to confirm stable and uniform droplet generation. For both types of droplet experiments in this work, detection was conducted on-chip by passing droplets sequentially through a 10 μm-wide detection constriction. The detection constriction was aligned to a custom optical stage consisting of a 488 nm laser excitation source which was used for probe excitation, and a 552 nm laser which was used for resorufin excitation (OBIS, Coherent, Inc., Santa Clara, CA). Avalanche photodiode detectors (APD) (SPCM-AQRH13, ThorLabs, Newton, NJ) were used to measure fluorescence emission from droplets. Lasers were operated at 4 mW (for probe excitation) or 1 mW (for resorufin excitation) power and were focused into the detection constriction of the device using a 40× objective (Thorlabs RMS40X-PF, NA 0.75, focal depth approximately 0.6 μm). Droplet fluorescence data was continuously acquired and recorded using the APD with 0.1 ms sampling time. A custom-built LabVIEW program was used for fluorescence data acquisition.
Droplet data was analyzed using a custom MATLAB program. The program looks for individual droplet peaks in fluorescence time traces and then plots the droplet intensities as a histogram. Resulting histograms typically follow a bimodal distribution. In order to classify the relevant subpopulations (empty droplets and “positive” droplets), we fit a Gaussian mixture model using the expectation maximization algorithm in MATLAB (‘fitgmdist’) to the droplet data. We inspect the fitted envelope against the plotted data to ensure a snug histogram fit to the empty droplet population. The envelope fit to the positive droplet population is usually ignored due to poorer fit characteristics, often due to relatively sparse number of datapoints compared to the much more abundant empty droplet population. We then calculate the mean intensity and standard deviation of the empty droplet population. All droplets with intensities over 4.5 standard deviations (for probe-based hybridization assay) or 6 standard deviations (for resazurin-based growth assay) above the empty droplet mean intensity are classified as bacteria-containing “positive droplets” (Supplementary Figure 1). To quantify bacteria, we determine the mean occupancy per droplet and then calculate the bacteria concentration using the droplet size measured by microscope imaging.
Results and Discussion
Syringe filter for separating uropathogens from background particulates in urine samples
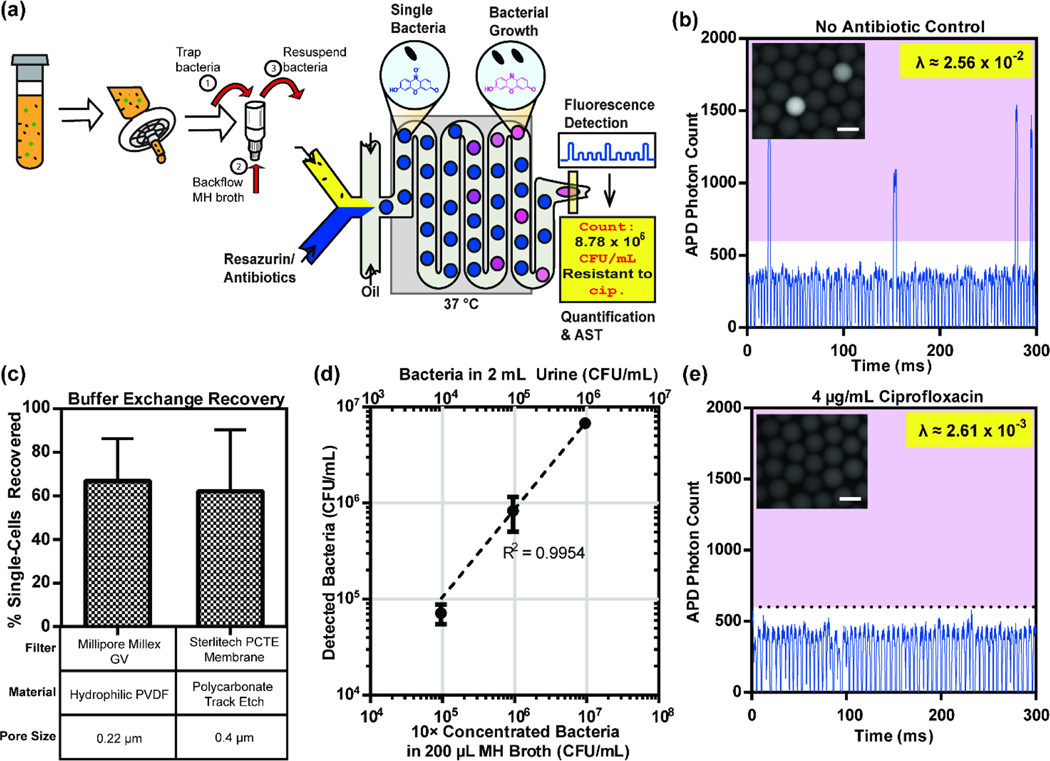
We first identified commercially available syringe filters that can achieve the desired separation of target bacteria from clinical urine specimens. By passing urine samples through the filter, we aimed to trap large particulates (>2 μm) that are commonly found in urine (e.g., white blood cells, epithelial cells, proteins, fats, salt crystals, casts, etc.) in the membrane, while allowing uropathogenic bacteria (characteristic length ~1 μm) to flow through and be collected in the filtrate. To achieve this, we tested 5 syringe filters (GE Whatman Nuclepore, iPOC-DX Primecare S/G, GE Whatman GMF 150, GVS FJ13ASCCA05–0PL01, and GE Whatman FP 30), of varying pore sizes (1.5 μm to 5 μm), for bacterial recovery after 1-step filtration. We plated clinical urine specimens that were found to be positive for uropathogenic E. coli before and after one passage through each of the syringe filters and compared the colony counts to assess recovery. Notably, the Whatman Nuclepore, iPOC-DX S/G (micrographs, Figure 2ai and 2aii) and Whatman GMF 150 filters demonstrated maximum recovery of bacterial colonies (88.41%, 65.49%, and 58.82% respectively), while successfully eliminating larger particulates (“white blood cells” in Figure 2ai). On the other hand, 2 of the filters tested that were made up of cellulose acetate (GVS FJ13ASCCA05–0PL01 and Whatman FP 30) retained a significant amount of bacteria in the filtration process (Figure 2b), likely due to stronger adhesion of bacteria onto the membrane material30. In addition to E. coli, we also tested the recovery of the common uropathogenic families Enterococcus and Klebsiella using Whatman Nuclepore and iPOC-DX S/G. We found that both filters offer sufficient recovery of the two UTI pathogens, even though these recoveries are slightly inferior to that of E. coli, the most common uropathogen (74.71% and 40.96% for Enterococcus, 67.43% and 30.46% for Klebsiella, see Supplementary Figure 2). Despite its superior bacterial recovery, we observed large debris leaking through the Nuclepore filter (Supplementary Figure 3), and therefore decided to continue with the iPOC-DX S/G filter for subsequent experiments.
Figure 2: 1-step filtration of background particulates from urine samples.
(a) Culture-positive urine samples were imaged (i) before filtration and (ii) after passing once through the iPOC-DX S/G filter, showing that the large particulates including white blood cells are removed, but the bacteria remain in the filtrate. Scale bars are ~20 μm (b) Culture-positive urine samples were used for comparison of bacterial recovery after filtration from 5 different syringe filters with distinct materials and pore sizes. A majority of bacterial cells are recovered with the GE Whatman Nuclepore, iPOC-DX S/G, and GE Whatman GMF 150 filters. The error bars are the standard deviations of replicated experiments (n= 6, 6, 7, 3 and 3 for Whatman Nuclepore, iPOC-DX S/G, Whatman GMF 150, GVS FJ13ASCCA05-0PL01 and Whatman FP 30, respectively.
1-step filtration to facilitate pathogen detection and quantification directly from urine
Our 1-step filtration protocol can eliminate large background particulates from urine samples, and is therefore instrumental in preparing urine samples for droplet-based detection. To demonstrate the benefit of this approach, we used 1-step filtered samples as input into a droplet-based bacteria detection platform that makes use of a robust molecular-probe-based assay that works in the presence of urine filtrate13,14. Briefly, our platform functions by co-encapsulating urine and molecular peptide nucleic acid (PNA) probes that are specific to bacterial 16S rRNA into 4 pL droplets. The chemically stable PNA probes consist of a target-complementary oligonucleotide tagged with a fluorophore label and a shorter complimentary DNA sequence with a quencher. In the presence of bacterial 16S rRNA target, hybridization of the PNA probe to the target sequence displaces the DNA quencher through competitive binding, allowing the probe to fluoresce. Droplets containing single bacterial cells and PNA probes are passed through temperature regions to facilitate lysis of bacteria and release of rRNA and hybridization of PNA probes to their 16S rRNA target. Finally, droplets are sequentially interrogated using a custom laser-induced fluorescence (LIF) detector. The color of the resulting time trace of droplet fluorescence can be used to determine the identity of the uropathogen, and the relative frequency of highly fluorescent droplets can be used to determine the bacterial load (Figure 3a).
Figure 3: Rapid and accurate digital quantification of bacteria from urine samples.
(a) We employed a single-cell molecular probe-based hybridization assay for pathogen identification and quantification. Our platform functions by inputting urine filtrate along with fluorophore-labeled PNA probes. Generated droplets pass a 95 °C heater to lyse bacteria and release rRNA followed by a 60 °C heater to facilitate probe hybridization to target 16S rRNA. (b) Droplets containing digitized bacteria in urine filtrate emit a strong fluorescence signal, higher than that of empty droplets. The frequency of these positive droplets, λ, was quantified by histogram analysis. (c) Particulates in urine can result in spurious signals and therefore impose a high limit of detection for bacteria quantification. One-step filtration of urine reduced limit of detection by more than an order of magnitude. The droplet fluorescent signals are representative results of replicated experiments (n= 2 for both before and after filtration in b; n =4, 2 for before filtration and after filtration, respectively, in c).
The vast majority of bacterial cells contained in urine samples that passed through the 1-step filtration protocol can be detected with single-cell sensitivity in droplets. We demonstrated our workflow by spiking in ~5 × 107 CFU mL−1 of E. coli (ATCC 25922) into culture-negative (“blank”) urine. After 1-step filtration using the iPOC-DX S/G filter, the filtrate was entered into our droplet device along with 200 nM of E. coli-specific PNA probes. In this work, we choose to demonstrate our platform with E. coli cells and an E. coli-specific PNA probe, but we note from our previous work that PNA probes can be used to target a wide array of uropathogens13. Following on-chip lysis and hybridization, the resulting time trace of droplet peaks (5 s of data or approximately 4000 droplets) was plotted and compared to the unfiltered control (Figure 3b). Both droplet traces contained 2 populations of droplets – dimly fluorescent empty droplets and strongly fluorescent “positive” droplets which encapsulate a single bacterial cell. We performed histogram-based quantification15,31 of >70000 droplets from each condition, and as expected, before (mean occupancy, λ = 3.21×10−2) and after 1-step filtration ( λ = 2.83×10−2), we measure a similar frequency of positive droplets, indicating that the 1-step filtration process results in minimal loss of bacterial cells. Finally, we analyzed a total of >1000000 droplets across multiple urine samples and noted an average bacterial recovery of 91% with the iPOC-DX S/G filter.
One-step filtration can extend the dynamic range for bacterial quantification in droplets. The dynamic range of bacterial detection in droplet platforms is set by the limit of detection (LoD) (or limit of blank) which is the frequency of false-positive peaks in the negative control. Many traditional droplet platforms suffer from suboptimal LoD32, which can be attributed to insufficient assay optimization to account for background sources of false-positive peaks. Urine samples in particular may contain a variety of such background particulates that can lead to false-positive droplet signals and subsequently affect the accuracy and LoD of bacterial detection. Passing urine through syringe filters can reduce LoD for bacteria quantification. Optically turbid blank urine samples were entered, without bacteria, into our platform for discretization, followed by hybridization, and droplet detection. Without filtration, we observed several false-positive peaks in the droplet time trace, occurring at a frequency of λ = 5.48×10-4. One-step filtration of the same urine samples resulted in more than an order of magnitude reduction in LoD (λ = 5.22×10−5), effectively extending the dynamic range of quantification by over an order of magnitude for challenging urine samples.
Facile filtration-based buffer exchange and enrichment enables urine-to- antimicrobial susceptibility assessment
In urine samples collected at healthcare settings, it is common to use additives like boric acid and sodium formate in order to prevent bacterial growth during specimen storage and transportation. However, in order to perform growth-based phenotypic AST, these uropathogenic bacteria must be resuspended in nutrient-rich culture broth. To achieve this, we devised a facile syringe filter-enabled buffer exchange protocol to prepare bacteria for growth-based AST. Briefly, we use a syringe filter that allows for reversable flow and consists of a pore size smaller than the characteristic length of common uropathogenic bacteria. We first enter the urine filtrate through the inlet of the filter, trapping bacteria onto the filter membrane and discarding the liquid phase urine matrix (step “1” in Figure 4a). Next, we back flow MH broth from the outlet of the filter and collect the output from the inlet of the filter (step “2” in Figure 4a). As the broth travels through the filter membrane, it releases the trapped bacterial cells, which then become resuspended in the collected MH broth (step “3” in Figure 4a). Compared to time-consuming plating-based clinical isolation, our facile buffer exchange can be performed within a few minutes and effectively prepare uropathogenic bacteria for AST.
Figure 4: Single-cell antimicrobial susceptibility assessment from urine samples.
We employed a single-cell resazurin-based growth assay for pathogen quantification and antimicrobial susceptibility assessment. Our platform functions by inputting urine filtrate that has passed through our buffer exchange methodology along with resazurin and antibiotics. Generated droplets are incubated at 37 °C to stimulate bacterial replication. (b) Droplets containing growing bacteria emit a strong fluorescence signal, higher than that of empty droplets. The frequency of these positive droplets, λ, is quantified by histogram analysis. (c) Comparison of bacterial recovery is assessed for 2 syringe filters used in the buffer exchange methodology. (d) Bacterial concentration can be amplified 10-fold across typical bacterial concentrations in UTIs during buffer exchange. (e) A significant loss of growing bacteria is observed in the presence of the antibiotic ciprofloxacin. Scale bars are ~30 μm in both (b) and (e). Millipore Millex GV was used in (b) and (e) while Sterlitech PCTE Membrane was used for (d). The error bars are the standard deviations of replicated experiments (n=3 and 2 for Millipore Millex GV and Sterlitech PCTE membrane, respectively, in c; n =3 in d). Moreover, b and e are the representative result of duplicated experiments.
In order to demonstrate the utility of our facile buffer exchange, we use it for sample preparation for in-droplet bacterial quantification and single-cell AST15 directly from urine samples. Briefly, we encapsulated buffer-exchanged bacterial cells and a cell viability indicator, resazurin, into 8 pL droplets. Resazurin is a widely used viability indicator that can be reduced to highly-fluorescent resorufin in the presence of bacterial metabolism. Droplets containing single bacterial cells and resazurin were then incubated at 37 °C in order to facilitate bacterial replication and production of resorufin. Finally, the droplets were sequentially interrogated via our LIF detector. The relative frequency of highly fluorescent droplets was used to determine the bacterial load as well as the effect of antibiotics on bacterial growth (Figure 4a).
We first identified appropriate syringe filters that may be used for effective bacterial trapping and buffer exchange. In order to assess the effectiveness of each commercially-available filter, we measured the total recovery of bacterial cells, as the bacterial load measured after buffer exchange divided by the bacterial load without filtration. For all our experiments, we began with 1.5 × 107 CFU mL−1 E. coli spiked into blank urine. The bacterial load (λ = 2.56×10−2) was determined by histogram analysis of the droplet fluorescence time trace, wherein droplets with growing bacteria produced a high fluorescence signal (Figure 4b) than empty droplets. Bright-field and fluorescence microscopy (Figure 4b, inset) was used to confirm bacterial replication in droplets. We chose to use filters with pore sizes much smaller than the dimensions of single bacterial cells to allow trapping of bacterial cells (Millipore Millex GV and Sterlitech PCTE membrane). We also ensured that the filters had small membrane diameters in order to minimize cross-sectional surface area during buffer exchange and enhance bacterial release and recovery. In our comparison test, both the Millipore Millex GV as well as the Sterlitech PCTE Membrane filter performed well with recoveries as high as 66% (Figure 4c). Some of the observed loss may be attributed to inefficiencies in releasing all the trapped bacterial cells during backflow as well as any dead volume in the filtration process. Nevertheless, we were able to recover and resuspend the vast majority of bacterial cells from the original urine sample for further analysis. In order to counteract any apparent loss, our methodology allows us to enrich or concentrate bacterial cells from the filtrate. By simply backflowing a smaller volume of MH than the original urine sample that contained bacteria, we could achieve up to 10-fold concentration of bacterial cells (Figure 4d). We demonstrated 10-fold concentration of bacterial cells from 2 mL of urine samples for 3 different concentrations of bacteria (104 to 106 CFU mL−1) within the clinically relevant dynamic range for UTIs. Such enrichment can help further accelerate the turnaround time of both bacteria detection as well as antimicrobial susceptibility assessment.
As a final demonstration of the utility of our facile buffer exchange protocol, we performed rapid single-cell antimicrobial susceptibility assessment using our droplet platform. We spiked in 1.5 × 107 CFU mL−1 E. coli into 200 μL of blank urine and performed 1-step purification and buffer exchange into MH broth using the Millipore Millex GV filter. Next, we infused the bacterial sample along with resazurin and a bactericidal concentration of 4 μg/mL ciprofloxacin into our droplet device and performed the standard droplet AST workflow. In the resulting fluorescence time trace, we observed the inhibitory effect of ciprofloxacin, as reflected by the reduced frequency of highly-fluorescent droplets (Figure 4e). Our results herein with bacteria suspended in urine samples are consistent with our previous work where we used reference bacterial strains directly spiked into MH broth15.
Conclusions
In this work we present simple and fast approaches for preparing urine samples for microfluidic droplet-based bacterial diagnostic tests. Traditional diagnostic assays have relied extensively on plating-based clinical isolation in order to separate bacterial cells from background particulates in the clinical sample matrix and enable enrichment and resuspension of bacterial cells into assay-compatible buffers (or buffer exchange). In order to move away from such time-consuming, labour-intensive, and resource-demanding clinical isolation, we leverage simple size-based filtration of bacterial cells and devise a 1-step filtration protocol for bacterial detection in urine samples and a facile buffer exchange protocol for rapid antimicrobial susceptibility assessment. Our 1-step filtration allows for up to 91% bacterial recovery, while rejecting background particulates, and in doing so, enhances LoD of in-droplet bacterial quantification by over an order of magnitude. Our facile buffer exchange protocol can be used to effectively perform in-droplet, single-cell AST from urine samples, and can achieve up to 10-fold concentration of bacteria. Subsequently, we show that it is possible to significantly curtail the extent of sample preparation necessary and truly take advantage of the time savings long-touted by microfluidic digitization.
Our filtration methodology can be easily implemented for a wide array of bioassays and microfluidic workflows. In addition to UTIs, we foresee our methodology to be directly compatible with other commonly infected clinical biofluids including cerebrospinal fluid and plasma. Furthermore, our methodology offers a rapid alternative to time-consuming clinical isolation and even resource-consuming centrifugation. We therefore also foresee our methodology to serve as the front end to a wide variety of molecular assays – for detecting proteins 33, enzymes 34, or even metabolite markers 35. Finally, we predict easy integration of our methodologies into standard microfluidic flow control instrumentation, based on our reliance on easily adaptable syringe filters. With continued expansion of its applicability, we foresee increased integration and adoption of our demonstrated filtration methods in order to enable direct incorporation of clinical and biological samples into microfluidic workflows.
Supplementary Material
Acknowledgements
The authors thank Professor Pak Kin Wong, Dr. Liben Chen, Dr. Christopher Puleo, and Ms. Christine Surrette for helpful discussions during this project. This work has been supported by the National Institutes of Health (R01AI117032 and R01AI138978).
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Shin DJ, Andini N, Hsieh K, Yang S. and Wang T-H, Annu. Rev. Anal. Chem, 2019, 12, 41–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer FP, Christner M, Hentschke M. and Rohde H, Infect. Dis. Rep,, DOI: 10.4081/idr.2017.6839. [DOI] [PMC free article] [PubMed]
- 3.Gross M, Curr. Biol, 2013, 23, R1063–R1065. [DOI] [PubMed] [Google Scholar]
- 4.Hassan S. and Zhang X, Curr. Anal. Chem,, DOI: 10.2174/1573411015666181224145845. [DOI]
- 5.Scheler O, Postek W. and Garstecki P, Curr. Opin. Biotechnol, 2019, 55, 60–67. [DOI] [PubMed] [Google Scholar]
- 6.Kaushik AM, Hsieh K. and Wang TH, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology, 2018, 10, e1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barea JS, Lee J. and Kang DK, Micromachines, 2019, 10, 1–25. [Google Scholar]
- 8.Bian X, Jing F, Li G, Fan X, Jia C, Zhou H, Jin Q. and Zhao J, Biosens. Bioelectron, 2015, 74, 770–777. [DOI] [PubMed] [Google Scholar]
- 9.Azizi M, Zaferani M, Cheong SH and Abbaspourrad A, ACS Sensors,, DOI: 10.1021/acssensors.8b01206. [DOI] [PubMed]
- 10.Rane T, Chen TD, Zec L, Wang HC, Lab Chip, 2014, 15, 776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler F, Schwemmer F, Trotter M, Wadle S, Zengerle R, von Stetten F. and Paust N, Lab Chip, 2015, 15, 2759–2766. [DOI] [PubMed] [Google Scholar]
- 12.Rane TD, Zec HC, Puleo C, Lee AP and Wang T-H, Lab Chip, 2012, 12, 3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mach KE, Kaushik AM, Hsieh K, Wong PK, Wang T. and Liao JC, Analyst, 2019, 144, 1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushik AM, Hsieh K, Mach KE, Lewis S, Puleo CM, Carroll KC, Liao JC and Wang TH, Adv. Sci,, DOI: 10.1002/advs.202003419. [DOI] [PMC free article] [PubMed]
- 15.Kaushik AM, Hsieh K, Chen L, Shin DJ, Liao JC and Wang TH, Biosens. Bioelectron, 2017, 97, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang W, Sarkar S, Lin ZS, McKenney S. and Konry T, Anal. Chem, 2019, 91, 6242–6249. [DOI] [PubMed] [Google Scholar]
- 17.Davenport M, Mach KE, Shortliffe LMDD, Banaei N, Wang T-HH and Liao JC, Nat. Rev. Urol, 2017, 14, 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Yang X. and Zhao W, SLAS Technol., 2017, 22, 585–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminski TS, Scheler O. and Garstecki P, Lab Chip, 2016, 16, 2168–2187. [DOI] [PubMed] [Google Scholar]
- 20.Ku S, Kreke T, Ximenes E, Foster K, Gilpin CJ and Ladisch MR, Biotechnol. Prog, 2017, 33, 687–695. [DOI] [PubMed] [Google Scholar]
- 21.Zuponcic J, Bomrad C, Ku S, Foster K, Ximenes E. and Ladisch MR, Biotechnol. Prog, 2019, 35, 1–5. [DOI] [PubMed] [Google Scholar]
- 22.Gwak S, Kim J. and Oh S, LWT, 2020, 132, 109840. [Google Scholar]
- 23.Francy DS, Stelzer EA, Brady AMG, Huitger C, Bushon RN, Ip HS, Ware MW, Villegas EN, Gallardo V. and Lindquist HDA, Appl. Environ. Microbiol, 2013, 79, 1342–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nnadozie CF, Lin J. and Govinden R, Biotechnol. Prog, 2015, 31, 853–866. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Hill VR, Hahn D, Johnson TB, Pan Y, Jothikumar N. and Moe CL, J. Microbiol. Methods, 2012, 88, 155–161. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, qing Xu C, Guo T. and Hong L, Sci. Rep, 2018, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Ximenes E, Amalaradjou MAR, Vibbert HB, Foster K, Jones J, Liu X, Bhunia AK and Ladisch MR, Appl. Environ. Microbiol, 2013, 79, 7048–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malenfant DJ, Paulick AE and Rehse SJ, Spectrochim. Acta - Part B At. Spectrosc., 2019, 158, 105629. [Google Scholar]
- 29.Boedicker JQ, Li L, Kline TR and Ismagilov RF, Lab Chip, 2008, 8, 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade PF, de Faria AF, Quites FJ, Oliveira SR, Alves OL, Arruda MAZ and do CM. Gonçalves, Cellulose, 2015, 22, 3895–3906. [Google Scholar]
- 31.Guan W, Chen L, Rane TD and Wang T-H, Sci. Rep, 2015, 5, 13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trypsteen W, Kiselinova M, Vandekerckhove L. and De Spiegelaere W, J. virus Erad, 2016, 2, 162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyu F, Xu M, Cheng Y, Xie J, Rao J. and Tang SKY, Biomicrofluidics, 2015, 9, 044120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang D-K, Ali MM, Zhang K, Huang SS, Peterson E, Digman MA, Gratton E. and Zhao W, Nat. Commun, 2014, 5, 5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neufeld BH, Tapia JB, Lutzke A. and Reynolds MM, Anal. Chem, 2018, 90, 6867–6876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.