Abstract
Apolipoprotein E (Apoe)- or low-density lipoprotein receptor (Ldlr)- deficient hyperlipidemic mice are the two most commonly used mouse models for atherosclerosis research. These models are used to study the impact of a various genetic factors and different cell types on atherosclerotic lesion formation and to test the development of new therapies. Isolation, excision of the whole aorta and quantification of the Oil Red O-stained atherosclerotic lesions are basic morphometric methods used to evaluate the atherosclerotic burden. The goal of this protocol is to describe a simple, optimized, step-by-step surgical method to dissect, perfuse-fix, isolate, stain, image and analyze atherosclerotic lesions in mouse aortas with Oil Red O. Because atherosclerotic lesions can form anywhere in the entire aortic tree, our whole aorta Oil Red O staining method has the advantages of evaluating lipid-laden plaques in the entire aorta and all of its branches in a single mouse. In addition to the Oil Red O staining, fresh isolated whole aortas can be used for variety of in vitro and in vivo experiments and cell isolations.
Keywords: Ascending aortic aneurysm, abdominal aortic aneurysm, atherosclerosis, apolipoprotein E deficient hyperlipidemic mice, low-density lipoprotein receptor deficient hyperlipidemic mice, Oil Red O staining, en face aorta
Summary:
The goal of this protocol is to provide a step-by-step procedure to analyze the atherosclerotic burden. Investigators can use this protocol to compare the abundance, location, and size of atherosclerotic lesions in different animals.
Introduction:
Coronary artery disease, a leading cause of mortality in the US, is usually caused by atherosclerosis, a process that leads to a buildup of plaques inside arterial walls1. Hyperlipidemia-prone Apoe- and Ldlr-deficient mice are central to investigations of atherosclerosis and its complications and development of therapies2–5.
Quantification of atherosclerotic lesions from an en face aorta is an important end-point analysis for evaluation the impact of a genetic manipulations in different cell types and novel therapies designed to affect atherosclerotic disease initiation, progression, and regression. Atherosclerotic lesions can form anywhere in the aorta and its branches including brachiocephalic, carotid and subclavian arteries in the chest as well as renal, common iliac and femoral arteries below the diaphragm6. A comprehensive evaluation of atherosclerosis burden and its therapy requires assessment of disease burden in different locations, a challenge that is often overlooked.
In this protocol, we describe how to perform a comprehensive analysis of atherosclerotic lesions starting with an unopened whole aorta and then proceeding to an en face preparation in a single mouse. Unopened whole aorta Oil Red O staining allows rapid qualitative assessment of lipid-laden plaques in the entire aorta and all of its branches while the en face preparation provides for quantitative assessment of atherosclerotic lesion distribution in mouse aorta.
We used 8-week-old mice with a smooth muscle cell-specific TGFβR2 deletion on the Apoe−/− hyperlipidemic background (MYH11-CreERT2;Tgfbr2f/f;mT/mGf/f;Apoe−/−, hereafter referred to as TGFβR2iSMC-Apoe mice) and their littermate Apoe−/− controls (MYH11-CreERT2;mT/mGf/f;Apoe−/−, hereafter referred to as Apoe−/− mice) kept for 16 weeks on a high cholesterol, high fat diet (HCHFD) as our study materials7. At study termination, we stained and imaged the unopened whole aortas, including all major branches, with the Oil Red O for qualitative assessment of lipid-laden plaques. We then cut aortas open in an en face preparation, imaged and quantified all atherosclerotic lesions. This protocol can be used to study atherosclerotic lesion development in Apoe−/− or Ldlr−/− hyperlipidemia mice model and can also be used for general aorta-related vascular biology applications.
Discussion:
Apolipoprotein E (Apoe) and low density lipoprotein receptor (Ldlr) deficient mice are very useful for studying development and treatment of atherosclerosis. Investigators can evaluate the impact of genetics and therapeutic manipulations on atherosclerosis-related diseases initiation, progression, and regression using Oil Red O staining of the whole aorta9.
Aorta Oil Red O staining and lesion quantification is the gold standard endpoint for atherosclerosis research. This technique is inexpensive and doesn’t require special equipment10. Yet it is not easy to obtain high quality Oil Red O stained tissues. Based on our experience, there are three critical steps in this protocol and the whole procedure requires practice and patients. The first critical step is the ability to dissect, remove, and clean all the perivascular adipose tissue around the aorta and its branches before and after Oil Red O staining (Figure 2D & Figure 3C-D). The second is the preparation of freshly made and filtered Oil Red O solution. Finally, it is important to make the en face aorta lay flat on wax dish before mounting on the glass microscope slides (Figure 4C & Figure 5B-C).
Figure 2.
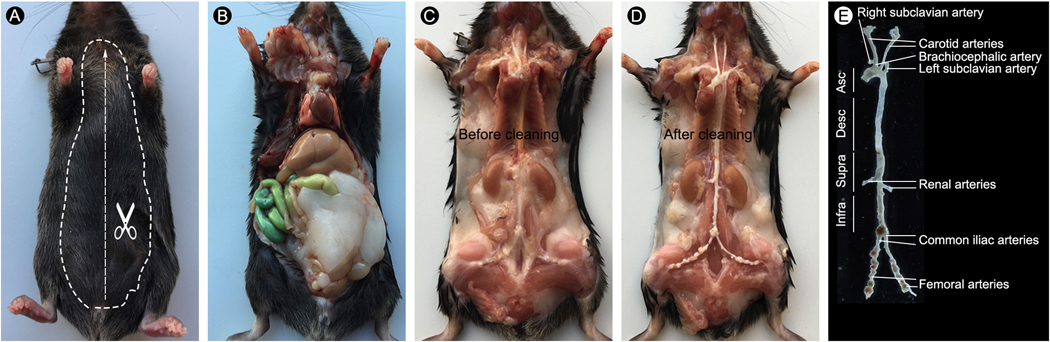
Step-by-step protocol for excision of a 24-week old TGFβR2iSMC-Apoe mouse fed with 4 months of HCHF diet mouse aorta. (A) Mouse under ketamine/xylene anesthesia. Dashed lines indicate where to cut the skin. (B) Dissect the mouse to expose the thoracic and abdominal cavities. (C) Carefully remove the internal organs including lung, liver, spleen, gastrointestinal and reproductive organs and expose the mouse aorta under a dissection microscope. (D) Carefully remove the connective tissues along the aorta as clean as possible. (E) Image of isolated whole aorta with branches
Figure 3.
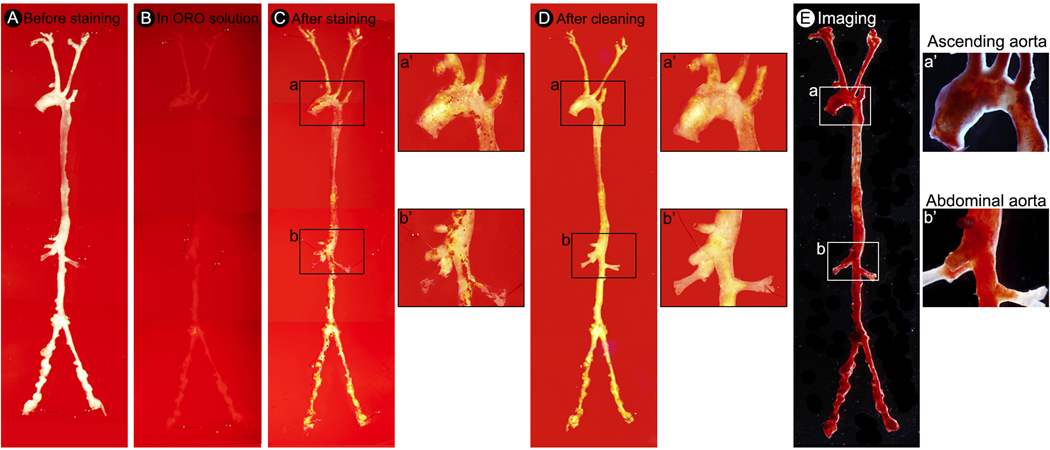
Step-by-step protocol for unopened aorta Oil Red O staining and imaging. (A) Pin the whole aorta with branches on a wax petri dish. (B) Cover the aorta with Oil Red O staining solution. (C) Image illustrate the whole aorta after Oil Red O staining. (D) Image illustrate Oil Red O stained whole aorta after cleaning. (E) Representative photomicrographs of Oil Red O stained whole aorta of TGFβR2iSMC-Apoe mice after 4 months of HCHF diet. (a’) A high-magnification of ascending aorta from a. (b’) A high-magnification of abdominal aorta from b.
Figure 4.
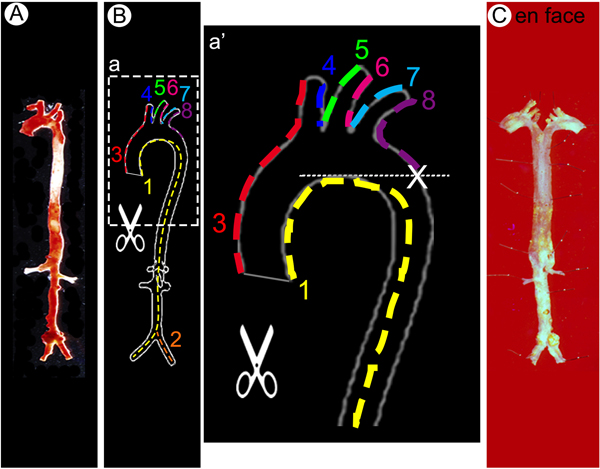
Step-by-step protocol for en face aorta preparation. (A-B) The arterial tree stained with Oil Red O was opened longitudinally in order to flatten the aorta for imaging. Dotted lines along the vessel wall and numbers indicate sequential cuts to be made to open up the vessels. (C) Longitudinally split and pinned whole aorta on a wax petri dish in Y shape.
Figure 5.
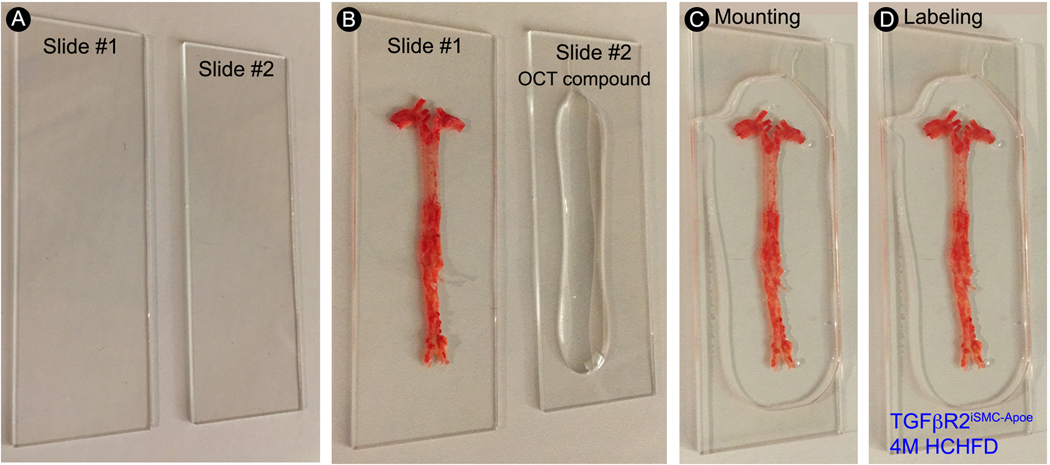
Step-by-step protocol for en face aorta mounting. (A) Gently clean the glass microscope slides with 70% ethanol and dry with clean laboratory wipes. (B) Apply a small amount of OCT compound to the surface of one glass microscope slide and spread the en face aorta flat on the other glass microscope slide. (C) Gently place the glass microscope slide with OCT compound on the top of the en face aorta sample. (D) Label the slide with sample name.
In comparison with other Oil Red O staining protocols, our protocol provides for qualitative and quantitative assessment of lipid-laden plaques in the unopened aorta and the en face aorta in a single mouse. The initial qualitative assessment of the unopened Oil Red O staining gives us a general idea about the plaque distribution and plaque size in aorta and all the branches before quantification of the en face aorta. The limitations of the study are that (1) 2-dimensional comparison and analysis of 3-dimensional atherosclerotic plaques does not reflect the true extent of atherosclerotic plaque volumes; (2) atherosclerotic lesion quantification is very time-consuming; and (3) requires animal sacrifice.
After the aorta is successfully isolated, it can be used for a wide variety of assays for molecular studies. For example, it can be used for biomechanic studies and histologic analysis to characterize reginal aorta morphology11. Additionally, one can isolate endothelial cells and smooth muscle cells from the freshly isolated whole aorta for cell culture, FACS analysis, and single cell RNA sequencing analysis.
In summary, this protocol provides step-by-step procedures to analyze the atherosclerotic burden. Investigators can use this protocol to compare the atherosclerotic lesion abundance, location, and size between animals.
Protocol:
All animal procedures were performed under protocols approved by the Yale University Institutional Animal Care and Use Committee.
- Mice.
- 1.1. mT/mG (stock no. 007676), and Apoe−/− (stock no. 002052) mice were purchased from the the Jackson Laboratory. Myh11-CreERT2 were a gift from Stefan Offermanns (available from the Jackson Laboratory as stock no. 019079). Tgfbr2fl/fl mice were obtained from Harold L. Moses (Vanderbilt University).
- 1.2. MYH11-CreERT2;mT/mGf/f;Apoe−/− and MYH11-CreERT2;Tgfbr2f/f;mT/mGf/f;Apoe−/− mice have been previously described7. Mutant strains were bred to the C57BL/6J background for more than 6 generations.
- 1.3. Myh11-CreERT2 Cre mouse line provides a powerful tool for studying the role of smooth muscle cells in vascular homeostasis and vascular pathology. The Cre allele was inserted into Y chromosome and female mice do not express this construct.
- Mouse genotyping, Tamoxifen induction, and high cholesterol high fat diet feeding.
- 2.1. Perform mouse genotyping using mouse ear DNA and PCR analysis. Mouse ear DNA was isolated using the DNeasy Blood & Tissue kit, according to the manufacturer’s instructions. PCR primers are listed in Table of Materials.
- 2.2. Induce Cre-Lox recombination by tamoxifen injection at 1 mg/day i.p. for 5 days in 6-week-old MYH11-CreERT2;mT/mGf/f;Apoe−/− and MYH11-CreERT2;Tgfbr2f/f;mT/mGf/f;Apoe−/− male mice.
- 2.3. Induce atherosclerosis: 8-week-old male mice (2 weeks after tamoxifen treatment) were placed on a HCHFD (40 kcal % Fat, 1.25 % Cholesterol, 0 % Cholic Acid) for 16 weeks.
- Reagent and dissection tool preparation.
- 3.1. Stock Oil Red O solution preparation: Dissolve 1 g of Oil Red O with 100 ml isopropyl alcohol.
- 3.2. Working Oil Red O solution preparation: Mix 24 ml of stock Oil Red O solution with 16 ml dH2O. Filter the diluted Oil Red O with 0.45 μm Sterile Syringe Filters (solution only good for 1–2 hours).
- 3.3. 60% isopropyl alcohol preparation: Mix 60 ml of isopropyl alcohol with 40 ml dH2O.
- 3.4. 4% formaldehyde in 1X DPBS preparation: Dilute 10 ml of 16 % formaldehyde with 30 ml 1X DPBS.
- 3.5. Clean all the dissection tools with 70 % ethanol (Figure 1).
- Euthanasia (Figure 2A).
- 4.1. Measure the mouse weight prior to euthanasia.
- 4.2. Euthanize the mouse by intraperitoneal injection of Ketamine and Xylazine (Ketamine and Xylazine solution; each milliliter contains 10 mg/mL Ketamine and 2 mg/mL Xylazine).
- 4.3. Place the mouse in a supine position (belly side up).
- Open the chest and abdominal cavity and perfuse the heart (Figure 2B).
- 5.1. Prepare a 10 ml syringe with 10 ml of 1X DPBS. Cap with a 25G needle. The syringe will be used to flush the heart.
- 5.2. Hold up the mouse skin with Tweezer (Style 5) and cut the skin with fine scissors from the base of the abdomen to the top of the neck.
- 5.3. Open the abdominal wall below the ribcage.
- 5.4. Lift the sternum with Tweezer (Style 5) and cut the diaphragm; then cut away the ribcage to expose the thoracic cavity.
- 5.5. Make a small incision in the right atrium of the heart.
- 5.6. Perfuse the vascular through apical left ventricular puncture by slowly injecting 10 ml 1X DPBS.
- 5.7. Clean the chest cavity of extraneous blood and fluid by using NonWoven Sponge to absorb material.
- 5.8. Once thoroughly perfused, the liver and kidney become a light brown color.
- Isolation of the aorta and branches (Figure 2C).
-
6.1. Remove organs including lung, liver, spleen, gastrointestinal and reproductive organs and cut the clavicle using Tweezer (Style 5) and fine scissors while leaving the heart, kidney and aorta intact in situ.CRITICAL STEP: Make sure not to lacerate the heart or any major blood vessels.
- 6.2. Place the mouse under a stereomicroscope.
-
6.3. Dissect aorta and aorta branches including brachiocephalic artery, carotid arteries, subclavian arteries, renal arteries, common iliac arteries, and femoral arteries using Tweezer (Style 4) and Spring Scissors.CRITICAL STEP: Cover the aorta with a wet NonWoven Sponge to avoid its dehydration while dissecting the aorta branches.
-
6.4. Carefully dissect and remove adventitial adipose and connective tissue around the aorta and aorta branches using Tweezer (Style 4) and Spring Scissors.CRITICAL STEP: Since inflammation is very prominent in this aneurysm hyperlipidemia mouse, it is very difficult to remove adventitia. Be careful not to tear or nick the aorta and aorta branches. This step requires lots of practice and patient.
-
- Fix the heart and aorta (Figure 2D-E).
-
7.1. Prepare a 10 ml syringe with 10 ml of 4 % formaldehyde in 1X DPBS. Cap with a 25G needle.CAUTION: Formaldehyde is hazards. Please read the MSDS before working with this chemical. Wear gloves and safety glasses and make dilution solutions inside a fume hood.NOTE: 4 % formaldehyde solution degrades over time. It is important to use freshly made 4 % formaldehyde for fixation.
-
7.2. Fix the vascular tree through apical left ventricular puncture by slowly injecting 10 ml of 4 % formaldehyde.NOTE: Formaldehyde fixation interferes with several downstream applications, such as cell culture, FACS analysis, and single cell RNA sequencing analysis. Please skip this step if aorta will be used for any of these applications.
- 7.3. Clean the chest cavity of extraneous fluid with a NonWoven Sponge to absorb the material.
-
7.4. Separate the heart from the aorta by holding the heart with the Tweezer (Style 4) and using McPherson-Vannas Micro Dissecting Spring Scissors.NOTE: To perform en face Oil Red O staining from this step, we recommend cutting the aorta open in situ instead of ex vivo and proceed to step 8. This makes it easy to make the en face aorta lay flat.
- 7.5. Isolate and excise the aorta and its major from 1 mm above the carotid artery to the end of femoral artery using Tweezer (Style 4) and Spring Scissors.
-
7.6. Transfer the vessel into a wax petri dish or 1.5 ml eppendorf tube and fill with 1X DPBS until it covers the aorta.NOTE: The protocol can be paused here.
-
- Oil Red O staining and imaging of an unopened whole aorta (Figure 3).
- 8.1. Pin the vessel onto a wax petri dish using minutien pins (Figure 3A).
- 8.2. Rinse the vessel 1 time with 1X DPBS.
-
8.3. Pour 25 ml of fresh Oil Red O solution into the petri dish (Figure 3B).CRITICAL STEP: (1) Isopropanol is hazards. It is a flammable liquid. Use proper personal protective equipment. (2) Oil Red O solution can easily precipitate. The precipitated particles could interfere with the subsequent staining procedure. It is important to remove the precipitate by filtering the Oil Red O solution through a 0.45 μm filter before use. (3) It is best to prepare a fresh Oil Red O solution and discard any unused portion. (4) In addition to Oil Red O, Sudan IV is another chemical compound used for staining of lipids, triglycerides and lipoproteins. However, Oil Red O has gradually replaced Sudan IV because the red color produced by Oil Red O is much more intense and therefore can make fat much easier to see.
- 8.4. Stain the aorta for 60 min at room temperature. Oil Red O will stain lipid-rich plaque red leaving other non-plaque containing areas pale.
-
8.5. Wash once for 20 min with 60 % isopropanol at room temperature.CRITICAL STEP: Over-rinsing can destain the plaque.
- 8.6. Rinse the aorta 3 times with dH2O for 5 min to remove isopropanol.
-
8.7. Under a stereomicroscope, gently clean all perivascular adipose tissue around the aorta using Tweezer (Style 4) and Spring Scissors (Figure 3C-D).CRITICAL STEP: It is important to clean all perivascular adipose tissue around aorta and aorta branches after staining because Oil Red O stained perivascular adipose tissue can give false background and interfere with plaque morphometry and plaque area quantification. Make sure not to remove a portion of the aortic wall. Fill the wax dish with dH2O until it covers the stained aorta during cleaning. NOTE: This step requires lots of practice and patient.
- 8.8. Transfer the vessel to a clean glass microscope slide.
-
8.9. Acquire digital micrographs using a camera connected to a light microscope. Save high resolution images, preferably in tagged image file format (TIFF) (Figure 3E).NOTE: The protocol can be paused here. To prevent the aorta from drying, transfer the vessel into a 1.5 ml eppendorf tube and fill with 1X DPBS until it covers the aorta and store it at 4 °C.
- En face aorta mounting (Figure 4–5).
- 9.1. Transfer the vessel to a wax petri dish and fill with 1X DPBS until it covers the aorta.
- 9.2. Sever carotid, subclavian arteries of aortic arch and iliac arteries in abdominal aorta 1–2 mm after bifurcations. Sever renal arteries. (Figure 4A)
- 9.3. Cut open the aorta preparation longitudinally, along the inner curvature (Figure 4B1) and alone iliac arteries (Figure 4B-2) with McPherson-Vannas Micro Dissecting Spring Scissors.
- 9.4. Cut open the three branches of the aortic arch, including the innominate, left common carotid, the left subclavian artery, along the greater curvature until the base level of inner curvature (X mark) (Figure 4B-3-8) with McPherson-Vannas Micro Dissecting Spring Scissors.
-
9.5. Pin the aorta flat (lumen site up) in wax dish with minutien pins and apply 1X DPBS until it covers the aorta to prevent it from drying (Figure 4C).CRITICAL STEP: It is important to make the rolled-up aorta flat and pin it en face without stretching it. This step will take a few days depending on the severity of the atherosclerosis.NOTE: (1) For aortas from Apoe−/− or Ldlr−/− animals, we recommend the investigators to pin the aorta flat for 24 hrs. (2) The protocol can be paused here.
- 9.6. Clean the glass microscope slides with 70% ethanol and delicate task wipers (Figure 5A).
- 9.7. Transfer the aorta into a clean glass microscope slide, and put 15 drops of Optimal Cutting Temperature (OCT) compound in another clean glass microscope slide (Figure 5B).
- 9.8. Carefully place the glass microscope slide with OCT compound over the aorta, avoid trapping air bubbles on the slide (Figure 5C).
-
9.9. Label the slides with sample names (Figure 5D).NOTE: The mounted en face aorta slides can be stored in the moisture chamber at 4 °C for several months.
- Imaging and lesion quantification of en face aorta (Figure 6).
- 10.1. Acquire digital micrographs using a camera connected to a light microscope. Save high resolution images, preferably in tagged image file format (TIFF) (Figure 6A).
- 10.2. Transfer the images of en face stained whole aorta to a computer equipped with Image J software.
-
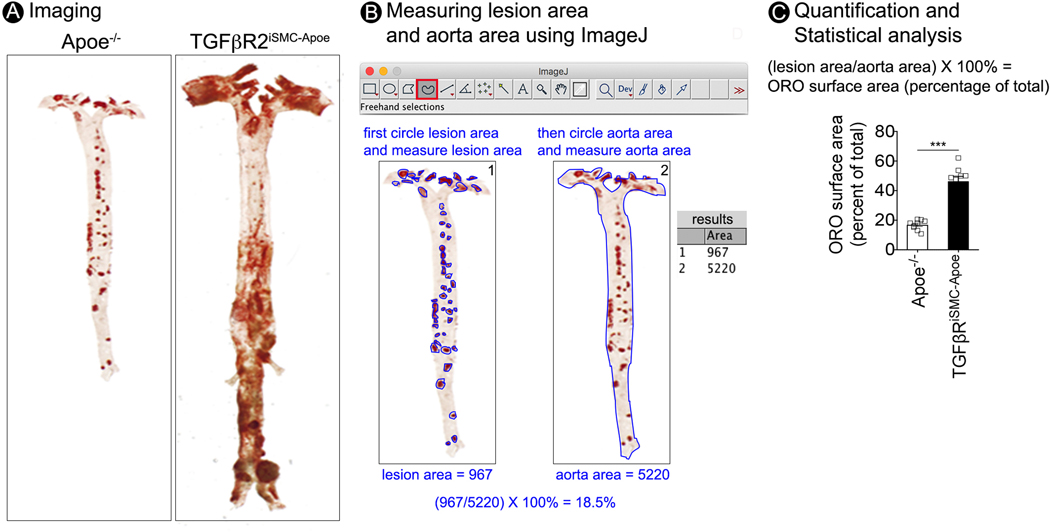
10.3. In ImageJ, select the freehand selection tool and circle all Oil Red O stained plaque manually (intense red spots) while pressing the Alt key (for PC) or shift key (for Mac). Then click measure in the analyze menu to display lesion area in the result window (Figure 6B left).NOTE: There are several pitfalls in the quantification of atherosclerotic lesions: (1) Any small pieces of stained adventitial fat that remained attached to the aorta from step 8.7 can give false background and interfere with plaque quantification; (2) Removing a portion of the aortic wall or damaging the aorta from steps 6.4 and 8.7 can interfere with plaque quantification; (3) Bubbles and folds formed in the aorta after mounting (step 9.8) can interfere with plaque quantification; (4) Atherosclerotic plaque is a 3-dimensional phenomenon and measurements performed in a 2-dimensional plane may miss the true extent of the plaque. In addition to analyze en face aorta plaque area, we recommend the investigators to analyze the plaque size in aortic root, brachiocephalic artery, ascending aorta, and abdominal aorta separately8.
- 10.4. Circle outer border line of the aorta and click measure in the analyze menu to display aorta area in the result window (Figure 6B right).
- 10.5. Export all the measurements to an excel file.
- 10.6. Calculate the ratio of plaque area from total aorta area and normalize the value as percentage of total Oil Red O surface area.
- 10.7. Calculate the ratio of plaque area in 8–10 Apoe−/− and 8–10 TGFβR2iSMC-Apoe mice. Give data as mean±SEM (Figure 6C).
- 10.8. Perform unpaired Student t test for statistical analysis of ratio of plaque area data comparision to another mouse group. Consider differences in mean values as significant at p0.05.
Figure 1.
All the dissection tools used in this protocol.
Figure 6.
Step-by-step protocol for en face aorta imaging and atherosclerotic lesion quantification. (A) Microphotographs of en face aortas from Apoe−/− and TGFβR2iSMC-Apoe mice after 4 months of HCHF diet stained with Oil Red O. (B) Images illustrating the process for computer-assisted quantification of the atherosclerotic lesions. (C) Lesion area quantification: % lesion area refers to Oil Red O stained as a % of the total aortic surface. All data shown as mean ± SEM ( ***p<0.001; unpaired two-tailed Student’s t test) (for each time point N=9 for Apoe−/− mice; and N=9 for TGFβR2iSMC-Apoe mice).
Representative results:
In this protocol, we analyzed atherosclerotic lesions in TGFβR2iSMC-Apoe mice after 4 months of HCHF diet7. In addition to extensive atherosclerosis, these mice develop both thoracic and abdominal aortic aneurysms as previously reported. Compared with Apoe−/− mice, TGFβR2iSMC-Apoe mice aortic wall has severe atherosclerosis, making it very difficult to dissect the lesions (Figure 2C-E). In addition, the aneurysms are particularly extensive below the suprarenal aorta, highly reminiscent of advanced human aortic aneurysms.
A representative unopened aorta Oil Red O staining image from HCHFD-fed TGFβR2iSMC-Apoe mouse is shown in Figure 3E. The image shows TGFβR2iSMC-Apoe mouse developed both ascending and abdominal aortic aneurysm and have accelerated atherosclerotic lesion formation in aorta branches including brachiocephalic artery, carotid artery, subclavian arteries, iliac arteries, femoral arteries, and renal arteries.
Figure 6A shows the en face Oil Red O staining image of Apoe−/− and TGFβR2iSMC-Apoe mice. Compared to the Apoe−/−, our TGFβR2iSMC-Apoe mouse has exhibited severe aneurysmal enlargement and marked elongation of the entire aorta.
Supplementary Material
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/61277.
REFERENCES:
- 1.Lusis AJ Atherosclerosis. Nature. 407, 233–241 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emini Veseli B. et al. Animal models of atherosclerosis. European Journal of Pharmacology. 816, 3–13 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Plump AS et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 71, 343–353 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 258, 468–471 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi S. et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. Journal of Clinical Investigation. 92, 883–893 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis Thrombosis. 14, 133–140 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Chen P-Y et al. Smooth muscle cell reprogramming in aortic aneurysms. Cell Stem Cell. Accepted (2020). [DOI] [PMC free article] [PubMed]
- 8.Andres-Manzano MJ, Andres V, Dorado B. Oil Red O and Hematoxylin and Eosin Staining for Quantification of Atherosclerosis Burden in Mouse Aorta and Aortic Root. Methods in Molecular Biology. 1339, 85–99 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Chen PY et al. Endothelial TGF-beta signalling drives vascular inflammation and atherosclerosis. Nature Metabolism. 1, 912–926 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nature Protocols. 8, 1149–1154 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Ferruzzi J, Madziva D, Caulk AW, Tellides G, Humphrey JD Compromised mechanical homeostasis in arterial aging and associated cardiovascular consequences. Biomechanics and Modeling Mechanobiology. 17, 1281–1295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.