Abstract
As we are aging, a number of cutaneous and extracutaneous disorders will be developed. Although the pathogenesis of these aging-associated disorders is not clear yet, abnormalities in the skin are linked to some aging-associated disorders at least to some extent. Inflammatory dermatoses such as psoriasis and atopic dermatitis predispose to the development of cardiovascular diseases, obesity and type 2 diabetes. In addition, both chronologically aged skin and individuals with some aging-associated systemic conditions display altered epidermal function, such as reduced stratum corneum hydration levels, which can provoke cutaneous inflammation. Because aged skin exhibits higher expression levels of inflammatory cytokines, which play a pathogenic role in a variety of aging-associated health condition, the association of the skin with some aging-associated disorders is likely mediated by inflammation. This postulation is supported by the evidence that improvement in either epidermal function or inflammatory dermatoses can mitigate some aging-associated disorders such as mild cognitive impairment and insulin sensitivity. This perspective discusses the association of the skin with aging-associated disorders and highlights the potential of improvement in cutaneous conditions in the management of some health conditions in the elderly.
Keywords: aging, stratum corneum hydration, inflammaging, epidermis, diabetes, obesity, cardiovascular diseases
Introduction
Chronologically aged humans often suffer from multiple cutaneous and extracutaneous disorders, including xerosis, pruritus, type 2 diabetes, atherosclerotic cardiovascular disease, etc. Over 20% of individuals aged ≥60 years suffer from pruritus and eczematous dermatitis.1,2 Xerosis was found in 96% of individuals aged >65 years,3 and 60% of individuals aged >50 years.4 Similarly, prevalence of type 2 diabetes is over 20% in individuals aged ≥60 years.5 The prevalence of cardiovascular diseases is over 70% in individuals aged 65–74 years, with an increase with age.6 Etiologies of these disorders have been widely speculated, including oxidative stress, gut microbiome, air pollution, diets and lifestyle.7–13 These speculations hold some truth. For example, gut microbiota with high capability to obtain energy from diet increase body fat in mice.14 While microbial dysbiosis induces obesity and inflammation,15 administration of probiotic attenuates high-fat diet-induced gain in body weight.16 Correspondingly, reductions in gut microbiota by antibiotic treatment prevent the development of insulin tolerance and body weight gain induced by high-fat diet in mice.17 Oxidative stress is another factor, which is assumed to be associated with aging and aging- associated diseases. Plasma oxidized low-density lipoprotein levels correlate positively with arterial stiffness.18 Moreover, reactive oxygen species can negatively regulate insulin signaling pathway, leading to the development of insulin resistance and obesity.19 Collectively, this line of evidence suggests the contribution of a variety of factors to the pathogenesis of aging-associated disorders. However, a wealth of evidence also indicates a link of aging-associated disorders to inflammatory dermatoses and altered epidermal function. For instance, the prevalence of aging-associated disorders such cardiovascular and metabolic diseases is higher in individuals with inflammatory dermatoses, including psoriasis, atopic dermatitis, hidradenitis suppurativa and seborrheic dermatitis.20–23 Additionally, recent studies suggest a link of epidermal dysfunction to aging-associated disorders.25 We briefly review here the link between the skin and aging-associated disorders and highlight the potential of improvements in cutaneous conditions in the management of some aging-associated disorders.
Association of the Skin with Aging-Associated Conditions
Although the pathogenesis of aging-associated disorders is not fully elucidated yet, the crucial role of chronic, low-grade inflammation, often termed “inflammaging”, in the development of these chronic disorders is hypothesized. Indeed, aged humans display higher circulating levels of proinflammatory cytokines and C-reactive protein.26,27 Inflammation has been linked to the pathogenesis of aging-associated diseases, such as type 2 diabetes, obesity, and Alzheimer’s disease.28–30 Correspondingly, inflammatory dermatoses are the risk factors of aging-associated health conditions. Here, we brief the link of the skin conditions to aging-associated disorders in the elderly.
Psoriasis
The common aging-associated disorders include cardiovascular diseases, type 2 diabetes and cognitive impairment.31,32 Prevalence of all of these disorders is higher in individuals with psoriasis than in those without psoriasis. For example, the prevalence of type 2 diabetes is 4% higher in individuals with psoriasis than in those without psoriasis (10.3% vs 6.2%).33 The odds ratio of psoriasis for diabetes mellitus is 1.61 (95% CI 1.53–1.70, p < 0.001).34 The hazard ratio for type 2 diabetes correlates positively with the involvement of surface area in individuals with psoriasis.35 Similarly, the prevalence of metabolic syndrome is higher in individuals with psoriasis than in the controls (30.3% vs 21.7%), with an odds ratio of 2.077 (95% CI 1.84–2.34).36 Likewise, the adjusted hazard ratio of psoriasis for cancers is 1.66 (95% CI 1.38–2.00).37 Individuals with psoriasis also exhibit higher incidence of cardiovascular events than the controls.38 Interestingly, risk for ischemic heart disease in males with moderate-to-severe psoriasis is higher than that in females (adjusted hazard ratio = 1.67, 95% CI 1.30–2.15 for males; adjusted hazard ratio = 0.99, 95% CI 0.53–1.83 for females).39 The risk for myocardial infarction is 2.24-time greater in patients with moderate-to-severe psoriasis than in the controls.39 However, one study showed no association of psoriasis and cardiovascular events.32 Moreover, psoriasis increases the risk of Alzheimer’s disease, particularly in those without systemic treatment, with an adjusted hazard ratio of 1.09 (95% CI 1.07–112, p < 0.0001).40 Psoriasis also increases the risk of both vascular (hazard ratio = 1.41, 95% CI = 1.09–1.82, p < 0.01) and non-vascular dementia (hazard ratio = 1.13, 95% CI = 1.11–1.15, p < 0.01).41 Furthermore, the link between obesity and psoriasis has also been demonstrated by several studies. Either obesity or metabolic syndrome increases risk of psoriasis,42–44 while psoriasis increases risk of obesity, with an odds ratio of 1.66.45 Thus, obesity and psoriasis negatively affect each other. Collectively, this bulk of evidence demonstrates a link between psoriasis and some aging-associated disorders.
Atopic Dermatitis
Atopic dermatitis is another common inflammatory dermatosis linked to aging-associated disorders such as obesity and mild cognitive impairment.31,46 The association of atopic dermatitis with central obesity is stronger in females than in males.47 Atopic dermatitis is also a risk factor for other aging-associated disorders. Studies showed that atopic dermatitis increases risk for multiple cardiovascular events, including myocardial infarction, stroke, heart failure, ischemic stroke and angina.48,49 The prevalence of cardiovascular diseases is more than 1% higher in individuals with atopic dermatitis than in the controls (5.5% vs 4.1%), with hazard ratio of 1.42 (95% CI 1.33–1.52).50 Atopic dermatitis also increases the risk for hyperlipidemia and hypertension with hazard ratio of 33.02 (95% CI 32.37–33.69, p < 0.0001) and 4.86 (95% CI 4.65–5.09, p < 0.0001), respectively.51 Atopic dermatitis is associated with coronary heart diseases with an adjusted odds ratio of 1.96 (95% CI 1.02–3.77, p = 0.04).52 In contrast, one study showed the atopic dermatitis decreases the risk for myocardial infarction and hypertension.53 No association between atopic dermatitis and cardiovascular events has also been reported.54,55 The link between atopic dermatitis and diabetes mellitus is inconclusive. One study demonstrated that genetically predicted atopic dermatitis is a risk factor for type 2 diabetes, with an odds ratio of 1.07 (95% CI 1.02–1.11, p = 0.003),56 while another study showed that the prevalence of atopic dermatitis is lower in individuals with type 2 diabetes than in those without type 2 diabetes.53 However, a study in a large cohort showed that individuals with type 2 diabetes are more likely to have atopic dermatitis, with an adjusted odds ratio of 5.62 (95% CI 2.15–14.6, p < 0.001).57 Thus, whether atopic dermatitis predisposes to type 2 diabetes or vice versa remains to be determined. Recent studies demonstrated that atopic dermatitis also increases the risk of dementia and Alzheimer’s diseases, with hazard ratio of as high as 3.74.58–60 The link between atopic dermatitis and aging-associated disorders is also evidenced by a positive correlation of the prevalence of these disorders with the severity of atopic dermatitis.61 Hence, atopic dermatitis is associated with some aging-associated disorders.
Other Inflammatory Dermatoses
Numerous studies have demonstrated that other inflammatory dermatoses are also linked to aging-associated disorders. For example, the prevalence of hypertension is higher in individuals with hidradenitis suppurativa than in the controls (34.3% vs 3.0%, p < 0.0001), while the prevalence of diabetes mellitus in patients with hidradenitis suppurativa is over 10 times higher than that of controls (20.4% vs 1.5%, p < 0.0001).62 Hidradenitis suppurativa is also strongly associated with metabolic syndrome (odds ratio = 2.08, 95% CI: 1.61–2.69) and obesity (odds ratio = 2.58, 95% CI: 2.00–3.23).63 Moreover, fasting insulin levels are negatively correlated with the severity of hidradenitis suppurativa (p = 0.04).64 One study showed that weight loss can mitigate hidradenitis suppurativa.65 Thus, whether hidradenitis suppurativa predisposes to obesity or vice versa is not clear. Rosacea is another common inflammatory skin disorder. Individuals with rosacea have a higher risk for cardiovascular diseases and hyperlipidemia.66–68 The prevalence of hyperlipidemia, hypertension and metabolic diseases is positively correlated with the disease severity in individuals with rosacea,69 while the age of the patients with rosacea is positively correlated with the prevalence of cardiovascular (p = 0.11) and metabolic disorders (p < 0.001).70 An over 14-year follow-up study showed that body mass index positively correlates with the risk for rosacea at least in females (p < 0.0001).71 Because of the pathogenic role of inflammation in both obesity and rosacea,29,72,73 management of one condition can benefit the other one. The links of seborrheic dermatitis and bullous pemphigoid to neurological, metabolic and cardiovascular diseases have also been well documented.74–77 Taken together, the bulk of evidence indicates a link between dermatoses and aging-associated disorders. Since the association of cutaneous inflammation with obesity and cognitive impairment has been fairly well summarized in previous publications,31,46 here we only summarize the evidence of the link between dermatoses and other aging-associated disorders (Supplementary Table 1).
Epidermal Dysfunction
In addition to dermatoses, several studies have demonstrated alterations in epidermal function in some aging-associated disorders. Coronary artery disease is common in the elderly. Transepidermal water loss rates, an indicator of epidermal permeability, are 23% higher in individuals with coronary artery disease than in those without coronary artery disease.78 Elevated transepidermal water loss rates and reduced stratum corneum hydration levels have been observed in individuals with type 2 diabetes.79 Individuals with obesity also exhibit higher transepidermal water loss rates than normal controls (14.27 ± 4.4 vs 11.3 ± 2.7, p < 0.05).80 Interestingly, the severity of obesity is positively correlated with epidermal permeability. For example, basal transepidermal water loss rates on the forearm are highest in individuals with obesity, followed by those with overweight and normal/underweight (11.5, 8.8 and 6.9 g/h/m2, respectively).81 Our recent study showed a positive correlation between body mass index and transepidermal water loss rates on the shin of females (Pearson r = 0.07197, p < 0.05).82 Body mass index also correlates positively with skin surface pH while negatively correlating with stratum corneum hydration levels. This line of evidence also indicates a link between some aging-associated health conditions and epidermal dysfunction.
Perspectives
Cutaneous Inflammation Contribution to Aging-Associated Disorders
As aforementioned above, some chronic inflammatory dermatoses are linked to aging-associated disorders, which are likely mediated by inflammation. This assumption is supported by several clinical observations. Individuals with either psoriasis or atopic dermatitis exhibit higher circulating levels of proinflammatory cytokines.83–86 The adjusted odds ratios for obesity and overweight are 1.47 (95% CI 1.31–1.63) and 1.19 (95% CI 1.09–1.30), respectively, in individuals with severe psoriasis.87 In individuals with psoriasis, the risk for either type 2 diabetes or cardiovascular disorders correlates positively with the severity of psoriasis.35,88,89 Accordingly, treatments of psoriasis with either TNF-α inhibitor or IL-17a inhibitor or methotrexate improve psoriasis, accompanied by decrease in cardiovascular events.90,91 Treatments of psoriasis with TNF-α inhibitor for each 6 months can significantly reduce the risk for cardiovascular events (adjusted hazard ratio = 0.73, p < 0.01).92 Another study showed that treatments of psoriasis with etanercept for 24 weeks significantly decrease Psoriasis Area and Severity Index (PASI) (50%), circulating levels of insulin (25%) and IL-6 (55%).93 Similarly, treatments of psoriasis with adalimumab for 6 months significantly improve both PASI and insulin sensitivity.94 This evidence indicates the contribution of inflammation in psoriasis to the pathogenesis of some aging-associated disorders.
Regarding atopic dermatitis, topical treatments of atopic dermatitis with either glucocorticoids or tacrolimus improve atopic dermatitis, insulin sensitivity (12.6%) and inflammation markers in the circulation (30% reduction in C-reactive protein, 27% reduction in IL-6),95 strongly suggesting the influence of cutaneous inflammation on systemic inflammation and its sequelae such as insulin resistance. Obesity and atopic dermatitis can negatively impact each other. For example, weight loss alone can improve atopic dermatitis.96,97 Notably, association of obesity with atopic dermatitis is only observed in individuals with previously diagnosed atopic dermatitis (>1-year history of atopic dermatitis) but not newly diagnosed atopic dermatitis,98 suggesting the pathogenic role of atopic dermatitis-associated chronic inflammation in obesity.
Taken together, this bulk of evidence suggests that chronic cutaneous inflammation can predispose to the development of some aging-associated disorders. Treatments of these inflammatory dermatoses can alleviate and/or prevent the development of some of these disorders, including obesity, diabetes mellitus, cardiovascular disorders and dementia, which all are aging-associated and most likely inflammation-driven disorders.
Chronologically aged humans will eventually suffer from one or more aging-associated disorders, which are linked to inflammaging. However, the worldwide prevalence of psoriasis is less than 2% in individuals aged over 55 years,99,100 while the prevalence of eczematous dermatitis (including atopic dermatitis) is 30–37.7% in individuals aged ≥65 years.101,102 Thus, large portion of chronologically aged humans do not have inflammatory cutaneous diseases. Then, the question is what is/are the sources of inflammaging. One theory is the gut microbiota dysbiosis, in part, attributing to aging-associated inflammaging, in addition to the changes in lifestyle, diet and oxidative stress.103,104 Aged humans exhibit gut microbiota dysbiosis.103 Prolonged gut microbiota dysbiosis can cause sustained increase in intestinal permeability, inducing chronic inflammation, and consequently leading to the development of inflammaging-related diseases.105,106 However, the contribution of the skin to inflammaging and its associated disorders cannot be underestimated, although the surface area of the skin is much smaller (≈2 m2 for males and 1.9 m2 for females) than that of gut (≈32 m2).107,108 The skin is sitting at the interface between the body and the harsh environment, making the skin more vulnerable to a variety of biological, chemical and physical insults, including UV irradiation, air pollution, allergens, irritants, temperature, microorganisms, humidity, etc. in comparison to the gut. Clinically, chronologically aged human skin presents signs and symptoms of inflammation. Up to 78% of aged humans experience pruritus, a sign of cutaneous inflammation.109 The prevalence of dry skin (xerosis) can be as high as 58% in the elderly.110 Dry skin can increase expression levels of proinflammatory cytokines and mast cells in the skin, in addition to increases in sensitivity of the skin to hapten challenge.111–114 Expression levels of proinflammatory cytokines are higher in both the skin and the circulation of aged mice than that of young mice.115 In aged humans, stratum corneum hydration levels correlate negatively with circulating levels of proinflammatory cytokines.116 Both proinflammatory cytokines and histamine are the key mediators in pruritus.117–119 Correspondingly, over 20% of aged individuals with dry skin complain of pruritus,120 while 69% of aged humans with pruritus have dry skin.1 Moreover, pruritus can provoke scratches, causing the disruption of epidermal permeability barrier. Disruption of epidermal permeability barrier alone can increase production and release of proinflammatory cytokines in the epidermis, and the density of mast cells in the dermis, leading exacerbation of pruritus.121,122 Thus, aged skin undergoes a vicious cycle of pruritus and inflammation. Furthermore, another remarkable alteration in the aged epidermis is the elevation in skin surface pH.82,123,124 Elevation in skin surface pH not only delays the recovery of epidermal permeability barrier125 but also induces cutaneous inflammation via protease-activated receptor signaling.126,127 Sustained cutaneous inflammation can induce inflammation in extracutaneous tissues. In support of this assumption, disruption of epidermal permeability barrier increases levels of proinflammatory cytokines in both the skin and circulation of either normal or athymic mice, suggesting the contribution of the skin to inflammation in both the skin and the circulation.115 Hence, epidermal dysfunction can contribute, in part, to inflammaging and its associated disorders.
Improvement in Cutaneous Conditions Can Mitigate Some Aging-Associated Health Conditions
Individuals with chronic cutaneous inflammatory dermatoses, such as psoriasis and atopic dermatitis, have high prevalence of aging-associated disorders, including atherosclerotic cardiovascular disease, obesity and type 2 diabetes.128 Conversely, treatment of psoriasis or atopic dermatitis improves insulin sensitivity and reduces the risk for cardiovascular events.92,94,95 Recent studies also demonstrate the benefits of topical emollients in alleviation of aging-associated disorders. For example, topical applications of emollient for 10 days lower expression levels of proinflammatory cytokines, including IL-1α, IL-1β, IL-6 and TNF-α, in both the skin and the circulation of aged mice.115 Likewise, circulating levels of IL-1β, IL-6 and TNF-α are also markedly decreased in aged humans following topical applications of an emollient for one month.129 Importantly, improvement in epidermal function with topical emollient mitigates the progression of mild cognitive impairment in the elderly.25 In addition, constipation is common in the elderly, with a prevalence of 26% for women and 16% for men aged ≥65 years.130 The prevalence of constipation is associated with dementia (odds ratio = 1.18, p = 0.0032) and non-amnestic mild cognitive impairment (odds ratio = 1.30, p = 0.003).131 Our recent 3-year clinical trial showed that topical applications of emollient to the skin decrease the prevalence of constipation from 27% to 2% in the elderly, while in the untreated control group, the prevalence increases from 33% to 49% (unpublished data). Taken together, this evidence indicates that improvement in cutaneous conditions, including epidermal function, can mitigate at least some aging-associated disorders in the elderly.
Conclusions
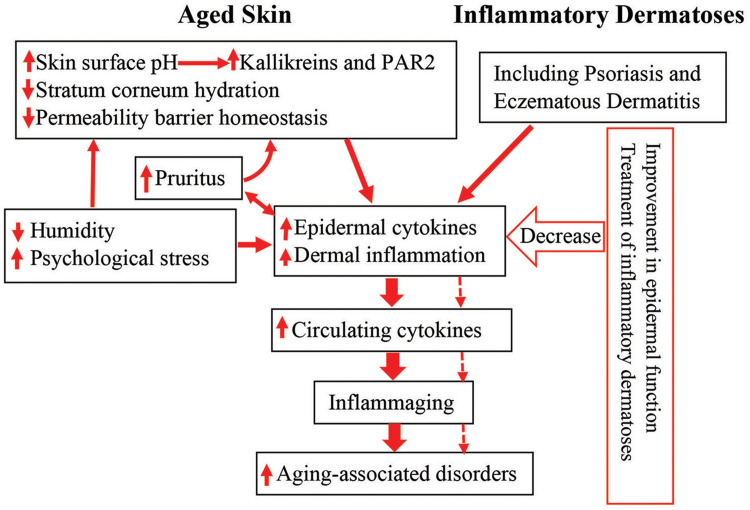
The pathogenesis of aging-associated disorders is attributable, at least in part, to inflammaging. In addition to gut microbiota dysbiosis, cutaneous inflammation can be one of the origins of inflammaging, contributing to the development of aging-associated disorders. In addition to inflammatory dermatoses, epidermal dysfunction can also contribute to inflammaging. Aged humans display multiple functional abnormalities in the epidermis. Either increased epidermal permeability or elevated skin surface pH or reduced stratum corneum hydration can induce cutaneous inflammation. Excessive exogenous insults such as in low humidity environment can exacerbate cutaneous inflammation, provoking pruritus. Pruritus-induced scratching can disrupt the epidermal permeability barrier, in turn, inducing and/or worsening downstream inflammation. Hence, either chronic inflammatory dermatoses or epidermal dysfunction can cause inflammaging. Accordingly, treatment of inflammatory dermatoses and correction of epidermal functional abnormalities can decrease inflammation (Figure 1). Therefore, improvement in cutaneous conditions can benefit at least some of aging-associated health conditions in the elderly.
Figure 1.
Schematic diagram: link between cutaneous conditions and aging-associated disorders in the elderly. Aged humans exhibit epidermal dysfunction. Both epidermal dysfunction and inflammatory dermatoses can provoke cutaneous inflammation. Prolonged cutaneous inflammation can result in inflammaging, leading to the development of inflammaging-associated disorders in the elderly (indicated in solid arrows). Conversely, either appropriate treatment of inflammatory dermatoses or improvement in epidermal function can decrease cutaneous inflammation, preventing the development and progression of inflammaging, consequently alleviating inflammaging-associated disorders in the elderly (indicated in dotted arrows).
Abbreviation: PAR2, protease-activated receptor 2.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Valdes-Rodriguez R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95(4):417–421. doi: 10.2340/00015555-1968 [DOI] [PubMed] [Google Scholar]
- 2.Yalçin B, Tamer E, Toy GG, Oztaş P, Hayran M, Alli N. The prevalence of skin diseases in the elderly: analysis of 4099 geriatric patients. Int J Dermatol. 2006;45(6):672–676. doi: 10.1111/j.1365-4632.2005.02607.x [DOI] [PubMed] [Google Scholar]
- 3.Völzer B, El Genedy-Kalyoncu M, Fastner A, et al. Prevalence and associations of xerosis cutis, incontinence-associated dermatitis, skin tears, pressure ulcers, and intertrigo in aged nursing home residents: a representative prevalence study. Int J Nurs Stud. 2023;141:104472. doi: 10.1016/j.ijnurstu.2023.104472 [DOI] [PubMed] [Google Scholar]
- 4.Mekić S, Jacobs LC, Gunn DA, et al. Prevalence and determinants for xerosis cutis in the middle-aged and elderly population: a cross-sectional study. J Am Acad Dermatol. 2019;81(4):963–969.e2. doi: 10.1016/j.jaad.2018.12.038 [DOI] [PubMed] [Google Scholar]
- 5.Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17(9):534–548. doi: 10.1038/s41574-021-00512-2 [DOI] [PubMed] [Google Scholar]
- 6.Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain A, Paranjape S. Prevalence of type 2 diabetes mellitus in elderly in a primary care facility: an ideal facility. Indian J Endocrinol Metab. 2013;17(Suppl 1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahad O, Frenis K, Kuntic M, Daiber A, Münzel T. Accelerated aging and age-related diseases (CVD and neurological) due to air pollution and traffic noise exposure. Int J Mol Sci. 2021;22(5):2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell TC, Parpia B, Chen J. Diet, lifestyle, and the etiology of coronary artery disease: the Cornell China study. Am J Cardiol. 1998;82(10B):18T–21T. doi: 10.1016/S0002-9149(98)00718-8 [DOI] [PubMed] [Google Scholar]
- 12.Campbell TC, Junshi C. Diet and chronic degenerative diseases: perspectives from China. Am J Clin Nutr. 1994;59(5 Suppl):1153S–1161S. doi: 10.1093/ajcn/59.5.1153S [DOI] [PubMed] [Google Scholar]
- 13.Everitt AV, Hilmer SN, Brand-Miller JC, et al. Dietary approaches that delay age-related diseases. Clin Interv Aging. 2006;1(1):11–31. doi: 10.2147/ciia.2006.1.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 15.Amabebe E, Robert FO, Agbalalah T, Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. 2020;123(10):1127–1137. doi: 10.1017/S0007114520000380 [DOI] [PubMed] [Google Scholar]
- 16.Kong C, Gao R, Yan X, Huang L, Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175–184. doi: 10.1016/j.nut.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 17.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- 18.Brinkley TE, Nicklas BJ, Kanaya AM, et al. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension. 2009;53(5):846–852. doi: 10.1161/HYPERTENSIONAHA.108.127043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008 [DOI] [PubMed] [Google Scholar]
- 20.Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33(1):41–55. doi: 10.1016/j.det.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 21.Yuan M, Cao WF, Xie XF, Zhou HY, Wu XM. Relationship of atopic dermatitis with stroke and myocardial infarction: a meta-analysis. Medicine. 2018;97(49):e13512. doi: 10.1097/MD.0000000000013512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan K, Charlton O, Smith SD. Hidradenitis suppurativa and metabolic syndrome - systematic review and adjusted meta-analysis. Int J Dermatol. 2019;58(10):1112–1117. doi: 10.1111/ijd.14500 [DOI] [PubMed] [Google Scholar]
- 23.Sodagar S, Ghane Y, Heidari A, et al. Association between metabolic syndrome and prevalent skin diseases: a systematic review and meta-analysis of case-control studies. Health Sci Rep. 2023;6(9):e1576. doi: 10.1002/hsr2.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal K, Das S. Metabolic syndrome-the underbelly of dermatology. Gulf J Dermatol Venereol. 2019;26(2):1–10. [Google Scholar]
- 25.Ye L, Wang Z, Kim Y, et al. A topical emollient mitigates the progression of cognitive impairment in the elderly: a randomized, open-label pilot trial. J Eur Acad Dermatol Venereol. 2022;36(8):1382–1388. doi: 10.1111/jdv.18162 [DOI] [PubMed] [Google Scholar]
- 26.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132(1):24–31. doi: 10.1046/j.1365-2249.2003.02137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon CJ, Rowsey PJ, Bishop BL, Ward WO, Macphail RC. Serum biomarkers of aging in the Brown Norway rat. Exp Gerontol. 2011;46(11):953–957. doi: 10.1016/j.exger.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 28.Tsalamandris S, Antonopoulos AS, Oikonomou E, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50–59. doi: 10.15420/ecr.2018.33.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–863. doi: 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen S, Elias PM, Wakefield JS, Mauro TM, Man MQ. The link between cutaneous inflammation and cognitive impairment. J Eur Acad Dermatol Venereol. 2022;36(10):1705–1712. doi: 10.1111/jdv.18360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parisi R, Rutter MK, Lunt M, et al. Psoriasis and the risk of major cardiovascular events: cohort study using the clinical practice research datalink. J Invest Dermatol. 2015;135(9):2189–2197. doi: 10.1038/jid.2015.87 [DOI] [PubMed] [Google Scholar]
- 33.Holm JG, Thomsen SF. Type 2 diabetes and psoriasis: links and risks. Psoriasis. 2019;9:1–6. doi: 10.2147/PTT.S159163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735 [DOI] [PubMed] [Google Scholar]
- 35.Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, Gelfand JM. Psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol. 2018;78(2):315–322.e1. doi: 10.1016/j.jaad.2017.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhary S, Pradhan D, Pandey A, et al. The association of metabolic syndrome and psoriasis: a Systematic Review and Meta-Analysis of Observational Study. Endocr Metab Immune Disord Drug Targets. 2020;20(5):703–717. doi: 10.2174/1871530319666191008170409 [DOI] [PubMed] [Google Scholar]
- 37.Chen YJ, Wu CY, Chen TJ, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65(1):84–91. doi: 10.1016/j.jaad.2010.04.046 [DOI] [PubMed] [Google Scholar]
- 38.Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. 2020;37(5):2017–2033. doi: 10.1007/s12325-020-01346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung KJ, Kim TG, Lee JW, et al. Increased risk of atherosclerotic cardiovascular disease among patients with psoriasis in Korea: a 15-year nationwide population-based cohort study. J Dermatol. 2019;46(10):859–866. doi: 10.1111/1346-8138.15052 [DOI] [PubMed] [Google Scholar]
- 40.Kim M, Park HE, Lee SH, Han K, Lee JH. Increased risk of Alzheimer’s disease in patients with psoriasis: a nationwide population-based cohort study. Sci Rep. 2020;10(1):6454. doi: 10.1038/s41598-020-63550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Chen ST, Li HJ, et al. Association between psoriasis and dementia: current evidence. Front Aging Neurosci. 2020;12:570992. doi: 10.3389/fnagi.2020.570992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barros G, Duran P, Vera I, Bermúdez V. Exploring the links between obesity and psoriasis: a comprehensive review. Int J Mol Sci. 2022;23(14):7499. doi: 10.3390/ijms23147499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snekvik I, Nilsen TIL, Romundstad PR, Saunes M. Metabolic syndrome and risk of incident psoriasis: prospective data from the HUNT Study, Norway. Br J Dermatol. 2019;180(1):94–99. doi: 10.1111/bjd.16885 [DOI] [PubMed] [Google Scholar]
- 44.Kim HN, Han K, Park YG, Lee JH. Metabolic syndrome is associated with an increased risk of psoriasis: a nationwide population-based study. Metabolism. 2019;99:19–24. doi: 10.1016/j.metabol.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 45.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54. doi: 10.1038/nutd.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang S, Zhu T, Wakefield JS, Mauro TM, Elias PM, Man MQ. Link between obesity and atopic dermatitis: does obesity predispose to atopic dermatitis, or vice versa? Exp Dermatol. 2023;32(7):975–985. doi: 10.1111/exd.14801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali Z, Ulrik CS, Agner T, Thomsen SF. Association between atopic dermatitis and the metabolic syndrome: a systematic review. Dermatology. 2018;234(3–4):79–85. doi: 10.1159/000491593 [DOI] [PubMed] [Google Scholar]
- 48.Ascott A, Mulick A, Yu AM, et al. Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. 2019;143(5):1821–1829. doi: 10.1016/j.jaci.2018.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivert LU, Johansson EK, Dal H, Lindelöf B, Wahlgren CF, Bradley M. Association between atopic dermatitis and cardiovascular disease: a Nationwide Register-based Case-control Study from Sweden. Acta Derm Venereol. 2019;99(10):865–870. doi: 10.2340/00015555-3235 [DOI] [PubMed] [Google Scholar]
- 50.Lee SW, Kim H, Byun Y, et al. Incidence of cardiovascular disease after atopic dermatitis development: a Nationwide, Population-Based Study. Allergy Asthma Immunol Res. 2023;15(2):231–245. doi: 10.4168/aair.2023.15.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung HJ, Lee DH, Park MY, Ahn J. Cardiovascular comorbidities of atopic dermatitis: using National Health Insurance data in Korea. Allergy Asthma Clin Immunol. 2021;17(1):94. doi: 10.1186/s13223-021-00590-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy. 2015;70(10):1300–1308. doi: 10.1111/all.12685 [DOI] [PubMed] [Google Scholar]
- 53.Drucker AM, Qureshi AA, Dummer TJB, Parker L, Li WQ. Atopic dermatitis and risk of hypertension, type 2 diabetes, myocardial infarction and stroke in a cross-sectional analysis from the Canadian partnership for tomorrow project. Br J Dermatol. 2017;177(4):1043–1051. doi: 10.1111/bjd.15727 [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Zhuo C, Zheng L. Assessing causal associations of atopic dermatitis with heart failure and other cardiovascular outcomes: a Mendelian Randomization Study. Front Cardiovasc Med. 2022;9:868850. doi: 10.3389/fcvm.2022.868850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hewitt N, Fang W, Kolodney MS. A cross-sectional comparison of eczema and psoriasis patients with regard to cardiovascular disease and risk factors. J Eur Acad Dermatol Venereol. 2023;37(7):e939–e940. doi: 10.1111/jdv.19035 [DOI] [PubMed] [Google Scholar]
- 56.Lu F, Wu B, Wang Y. Mendelian randomization indicates that atopic dermatitis contributes to the occurrence of diabetes. BMC Med Genomics. 2023;16(1):132. doi: 10.1186/s12920-023-01575-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kok WL, Yew YW, Thng TG. Comorbidities associated with severity of atopic dermatitis in young adult males: a National Cohort Study. Acta Derm Venereol. 2019;99(7):652–656. doi: 10.2340/00015555-3175 [DOI] [PubMed] [Google Scholar]
- 58.Woo YR, Cho M, Han KD, Cho SH, Lee JH. Increased risk of dementia in patients with atopic dermatitis: a Nationwide Population-Based Cohort Study. Acta Derm Venereol. 2023;103:adv4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan TL, Bai YM, Cheng CM, et al. Atopic dermatitis and dementia risk: a nationwide longitudinal study. Ann Allergy Asthma Immunol. 2021;127(2):200–205. doi: 10.1016/j.anai.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 60.Magyari A, Ye M, Margolis DJ, et al. Adult atopic eczema and the risk of dementia: a population-based cohort study. J Am Acad Dermatol. 2022;87(2):314–322. doi: 10.1016/j.jaad.2022.03.049 [DOI] [PubMed] [Google Scholar]
- 61.Sicras-Mainar A, Navarro-Artieda R, Carrascosa Carrillo JM. Economic impact of atopic dermatitis in adults: a Population-Based Study (IDEA Study). Actas Dermosifiliogr. 2018;109(1):35–46. doi: 10.1016/j.ad.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 62.Shlyankevich J, Chen AJ, Kim GE, Kimball AB. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71(6):1144–1150. doi: 10.1016/j.jaad.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 63.Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150(12):1273–1280. doi: 10.1001/jamadermatol.2014.1165 [DOI] [PubMed] [Google Scholar]
- 64.Mendiratta V, Yadav V, Bhardwaj AV, Pangti R. Clinical and metabolic characteristics in hidradenitis suppurativa - an Indian perspective. Indian J Dermatol. 2023;68(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kromann CB, Ibler KS, Kristiansen VB, Jemec GB. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94(5):553–557. doi: 10.2340/00015555-1800 [DOI] [PubMed] [Google Scholar]
- 66.Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73(2):249–254. doi: 10.1016/j.jaad.2015.04.028 [DOI] [PubMed] [Google Scholar]
- 67.Duman N, Ersoy Evans S, Atakan N. Rosacea and cardiovascular risk factors: a case control study. J Eur Acad Dermatol Venereol. 2014;28(9):1165–1169. doi: 10.1111/jdv.12234 [DOI] [PubMed] [Google Scholar]
- 68.Akin Belli A, Ozbas Gok S, Akbaba G, Etgu F, Dogan G. The relationship between rosacea and insulin resistance and metabolic syndrome. Eur J Dermatol. 2016;26(3):260–264. doi: 10.1684/ejd.2016.2748 [DOI] [PubMed] [Google Scholar]
- 69.Rainer BM, Fischer AH, Luz Felipe da Silva D, Kang S, Chien AL. Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol. 2015;73(4):604–608. doi: 10.1016/j.jaad.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 70.Yang F, Wang L, Shucheng H, Jiang X. Differences in clinical characteristics of rosacea across age groups: a retrospective study of 840 female patients. J Cosmet Dermatol. 2023;22(3):949–957. doi: 10.1111/jocd.15470 [DOI] [PubMed] [Google Scholar]
- 71.Li S, Cho E, Drucker AM, Qureshi AA, Li WQ. Obesity and risk for incident rosacea in US women. J Am Acad Dermatol. 2017;77(6):1083–1087.e5. doi: 10.1016/j.jaad.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 72.Gerber PA, Buhren BA, Steinhoff M, Homey B. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15(1):40–47. doi: 10.1038/jidsymp.2011.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen C, Wang P, Zhang L, et al. Exploring the pathogenesis and mechanism-targeted treatments of rosacea: previous understanding and updates. Biomedicines. 2023;11(8):2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu YY, Lu CC, Tsai CY, et al. Impact of seborrheic dermatitis on osteoporosis risk: a population-based cohort study. J Dermatol. 2022;49(12):1291–1298. doi: 10.1111/1346-8138.16578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imamoglu B, Hayta SB, Guner R, Akyol M, Ozcelik S. Metabolic syndrome may be an important comorbidity in patients with seborrheic dermatitis. Arch Med Sci Atheroscler Dis. 2016;1(1):e158–e161. doi: 10.5114/amsad.2016.65075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang B, Chen X, Liu Y, Chen F, Yang N, Li L. Relationship between bullous pemphigoid and metabolic syndrome: a 12-year case-control study conducted in China. Ther Adv Chronic Dis. 2022;13:20406223221130707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalińska-Bienias A, Kowalczyk E, Jagielski P, Bienias P, Kowalewski C, Woźniak K. The association between neurological diseases, malignancies and cardiovascular comorbidities among patients with bullous pemphigoid: case-control study in a specialized Polish center. Adv Clin Exp Med. 2019;28(5):637–642. doi: 10.17219/acem/90922 [DOI] [PubMed] [Google Scholar]
- 78.Öksüm Solak E, Gökçek GE, Kartal D, et al. The relationship between the severity of coronary artery disease and skin measurement parameters. Skin Res Technol. 2021;27(1):101–107. doi: 10.1111/srt.12917 [DOI] [PubMed] [Google Scholar]
- 79.Man MQ, Wakefield JS, Mauro TM, Elias PM. Alterations in epidermal function in type 2 diabetes: implications for the management of this disease. J Diabetes. 2022;14(9):586–595. doi: 10.1111/1753-0407.13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ibuki A, Kuriyama S, Toyosaki Y, et al. Aging-like physiological changes in the skin of Japanese obese diabetic patients. SAGE Open Med. 2018;6:2050312118756662. doi: 10.1177/2050312118756662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Löffler H, Aramaki JU, Effendy I. The influence of body mass index on skin susceptibility to sodium lauryl sulphate. Skin Res Technol. 2002;8(1):19–22. doi: 10.1046/j.0909-752x [DOI] [PubMed] [Google Scholar]
- 82.Ye L, Lai Q, Wen S, Wang X, Yang B, Man MQ. Correlation of body mass index with epidermal biophysical properties varies with gender in Chinese. Skin Pharmacol Physiol. 2022;35(4):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Renert-Yuval Y, Thyssen JP, Bissonnette R, et al. Biomarkers in atopic dermatitis-a review on behalf of the international eczema council. J Allergy Clin Immunol. 2021;147(4):1174–1190.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mastraftsi S, Vrioni G, Bakakis M, et al. Atopic dermatitis: striving for reliable biomarkers. J Clin Med. 2022;11(16):4639. doi: 10.3390/jcm11164639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bai F, Zheng W, Dong Y, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta-analysis. Oncotarget. 2017;9(1):1266–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169(2):266–282. doi: 10.1111/bjd.12355 [DOI] [PubMed] [Google Scholar]
- 87.Fleming P, Kraft J, Gulliver WP, Lynde C. The relationship of obesity with the severity of psoriasis: a systematic review. J Cutan Med Surg. 2015;19(5):450–456. [DOI] [PubMed] [Google Scholar]
- 88.Lee MS, Lin RY, Lai MS. Increased risk of diabetes mellitus in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population-based cohort study. J Am Acad Dermatol. 2014;70(4):691–698. doi: 10.1016/j.jaad.2013.11.023 [DOI] [PubMed] [Google Scholar]
- 89.Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(13):1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi LQ, Lian N, Sun JT, Liu LH, Chen M. Association between the systemic treatment of psoriasis and cardiovascular risk. Chin Med J. 2021;134(5):518–520. doi: 10.1097/CM9.0000000000001249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Zang J, Liu C, Yan Z, Shi D. Interleukin-17 links inflammatory cross-talks between comorbid psoriasis and atherosclerosis. Front Immunol. 2022;13:835671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79(1):60–68. doi: 10.1016/j.jaad.2018.02.050 [DOI] [PubMed] [Google Scholar]
- 93.Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20(4):731–736. [DOI] [PubMed] [Google Scholar]
- 94.Pina T, Armesto S, Lopez-Mejias R, et al. Anti-TNF-α therapy improves insulin sensitivity in non-diabetic patients with psoriasis: a 6-month prospective study. J Eur Acad Dermatol Venereol. 2015;29(7):1325–1330. doi: 10.1111/jdv.12814 [DOI] [PubMed] [Google Scholar]
- 95.Gether L, Storgaard H, Kezic S, et al. Effects of topical corticosteroid versus tacrolimus on insulin sensitivity and bone homeostasis in adults with atopic dermatitis-a randomized controlled study. Allergy. 2023;78(7):1964–1979. doi: 10.1111/all.15690 [DOI] [PubMed] [Google Scholar]
- 96.Jung MJ, Kim HR, Kang SY, Kim HO, Chung BY, Park CW. Effect of weight reduction on treatment outcomes for patients with atopic dermatitis. Ann Dermatol. 2020;32(4):319–326. doi: 10.5021/ad.2020.32.4.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Son JH, Chung BY, Jung MJ, Choi YW, Kim HO, Park CW. Influence of weight loss on severity of atopic dermatitis in a 20-year-old female with atopic dermatitis. Ann Dermatol. 2018;30(5):626–628. doi: 10.5021/ad.2018.30.5.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie B, Wang Z, Wang Y, Liu M, Wang Y. Associations of obesity with newly diagnosed and previously known atopic diseases in Chinese adults: a case-control study. Sci Rep. 2017;7:43672. doi: 10.1038/srep43672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mehrmal S, Uppal P, Nedley N, Giesey RL, Delost GR. The global, regional, and national burden of psoriasis in 195 countries and territories, 1990 to 2017: a systematic analysis from the Global Burden of Disease Study 2017. J Am Acad Dermatol. 2021;84(1):46–52. doi: 10.1016/j.jaad.2020.04.139 [DOI] [PubMed] [Google Scholar]
- 100.Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol. 2009;60(3):394–401. doi: 10.1016/j.jaad.2008.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yew YW, Kuan AHY, George PP, Zhao X, Tan SH. Prevalence and burden of skin diseases among the elderly in Singapore: a 15-year clinical cohort study. J Eur Acad Dermatol Venereol. 2022;36(9):1648–1659. doi: 10.1111/jdv.18205 [DOI] [PubMed] [Google Scholar]
- 102.Sinikumpu SP, Jokelainen J, Haarala AK, Keränen MH, Keinänen-Kiukaanniemi S, Huilaja L. The high prevalence of skin diseases in adults aged 70 and older. J Am Geriatr Soc. 2020;68(11):2565–2571. doi: 10.1111/jgs.16706 [DOI] [PubMed] [Google Scholar]
- 103.Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun Ageing. 2021;18(1):2. doi: 10.1186/s12979-020-00213-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conway J, Duggal A. Ageing of the gut microbiome: potential influences on immune senescence and inflammageing. Ageing Res Rev. 2021;68:101323. [DOI] [PubMed] [Google Scholar]
- 105.Elias-Oliveira J, Leite JA, Pereira ÍS, et al. NLR and intestinal dysbiosis-associated inflammatory illness: drivers or dampers? Front Immunol. 2020;11:1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–466.e4. doi: 10.1016/j.chom.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55(4):515–524. [DOI] [PubMed] [Google Scholar]
- 108.Helander HF, Fändriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol. 2014;49(6):681–689. doi: 10.3109/00365521.2014.898326 [DOI] [PubMed] [Google Scholar]
- 109.Chung BY, Um JY, Kim JC, Kang SY, Park CW, Kim HO. Pathophysiology and treatment of pruritus in elderly. Int J Mol Sci. 2020;22(1):174. doi: 10.3390/ijms22010174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.White-Chu EF, Reddy M. Dry skin in the elderly: complexities of a common problem. Clin Dermatol. 2011;29(1):37–42. [DOI] [PubMed] [Google Scholar]
- 111.Xu W, Jia S, Xie P, et al. The expression of proinflammatory genes in epidermal keratinocytes is regulated by hydration status. J Invest Dermatol. 2014;134(4):1044–1055. doi: 10.1038/jid.2013.425 [DOI] [PubMed] [Google Scholar]
- 112.Doi T, Mizukawa Y, Shimoda Y, Yamazaki Y, Shiohara T. Importance of water content of the stratum corneum in mouse models for contact hypersensitivity. J Invest Dermatol. 2017;137(1):151–158. [DOI] [PubMed] [Google Scholar]
- 113.Ashida Y, Denda M. Dry environment increases mast cell number and histamine content in dermis in hairless mice. Br J Dermatol. 2003;149(2):240–247. doi: 10.1046/j.1365-2133.2003.05408.x [DOI] [PubMed] [Google Scholar]
- 114.Denda M, Sato J, Tsuchiya T, Elias PM, Feingold KR. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998;111(5):873–878. doi: 10.1046/j.1523-1747.1998.00364.x [DOI] [PubMed] [Google Scholar]
- 115.Hu L, Mauro TM, Dang E, et al. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J Invest Dermatol. 2017;137(6):1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang B, Lv C, Ye L, et al. Stratum corneum hydration inversely correlates with certain serum cytokine levels in the elderly, possibly contributing to inflammaging. Immun Ageing. 2023;20(1):7. doi: 10.1186/s12979-023-00331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trier AM, Kim BS. Cytokine modulation of atopic itch. Curr Opin Immunol. 2018;54:7–12. doi: 10.1016/j.coi.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shibuya R, Takimoto-Ito R, Kambe N, Kabashima K. A new era with the development of cytokine-based therapy for pruritus. J Invest Dermatol. 2022;142(1):47–52. [DOI] [PubMed] [Google Scholar]
- 119.Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev. 2018;282(1):168–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jiang Q, Wang Y, Liu Y, et al. Prevalence and associated factors of dry skin among older inpatients in hospitals and nursing homes: a multicenter cross-sectional study. Int J Nurs Stud. 2022;135:104358. doi: 10.1016/j.ijnurstu.2022.104358 [DOI] [PubMed] [Google Scholar]
- 121.Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10(3):119–126. [PubMed] [Google Scholar]
- 122.Lin TK, Man MQ, Santiago JL, et al. Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J Invest Dermatol. 2013;133(2):469–478. doi: 10.1038/jid.2012.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Man MQ, Xin SJ, Song SP, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22(4):190–199. doi: 10.1159/000231524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127(12):2847–2856. doi: 10.1038/sj.jid.5700913 [DOI] [PubMed] [Google Scholar]
- 125.Mauro T, Holleran WM, Grayson S, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290(4):215–222. doi: 10.1007/s004030050293 [DOI] [PubMed] [Google Scholar]
- 126.Jang H, Matsuda A, Jung K, et al. Skin pH is the master switch of kallikrein 5-mediated skin barrier destruction in a murine atopic dermatitis model. J Invest Dermatol. 2016;136(1):127–135. doi: 10.1038/JID.2015.363 [DOI] [PubMed] [Google Scholar]
- 127.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J. 2010;51(6):808–822. doi: 10.3349/ymj.2010.51.6.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stefanadi EC, Dimitrakakis G, Antoniou CK, et al. Metabolic syndrome and the skin: a more than superficial association. Reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9. doi: 10.1186/s13098-018-0311-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ye L, Mauro TM, Dang E, et al. Topical applications of an emollient reduce circulating pro-inflammatory cytokine levels in chronically aged humans: a pilot clinical study. J Eur Acad Dermatol Venereol. 2019;33(11):2197–2201. doi: 10.1111/jdv.15540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61(2):152–158. [PMC free article] [PubMed] [Google Scholar]
- 131.Wang F, Fei M, Hu WZ, et al. Prevalence of constipation in elderly and its association with dementia and mild cognitive impairment: a Cross-Sectional Study. Front Neurosci. 2022;15:821654. doi: 10.3389/fnins.2021.821654 [DOI] [PMC free article] [PubMed] [Google Scholar]