Introduction
The study pessary (SP) is an investigational device designed with collapsible geometry to simplify insertion and removal for women with pelvic organ prolapse (POP) (Fig. 1a and b). The SP comes in three sizes and is made of medical-grade silicone. In its resting state, its configuration is similar to a Gellhorn pessary, with a cap and a stem, and a “space-occupying” design for prolapse support. An open-label, prospective, non-randomized early feasibility trial was conducted in 15 participants, 18 years and older with Stage II-IV POP [1] who were current pessary (CP) users. All participants were maintaining their pessaries with 3–6-month interval provider checks, and were not providing self-care. The goals of the study were to gain preliminary information on the functionality and short-term comfort of the SP, with future plans to study the utility of the pessary for longer term wear and opportunities for self-management. Research approval, including an abbreviated investigational device exemption, was acquired through the Western Institutional Review Board (WIRB, Olympia, WA, USA).
Fig. 1.
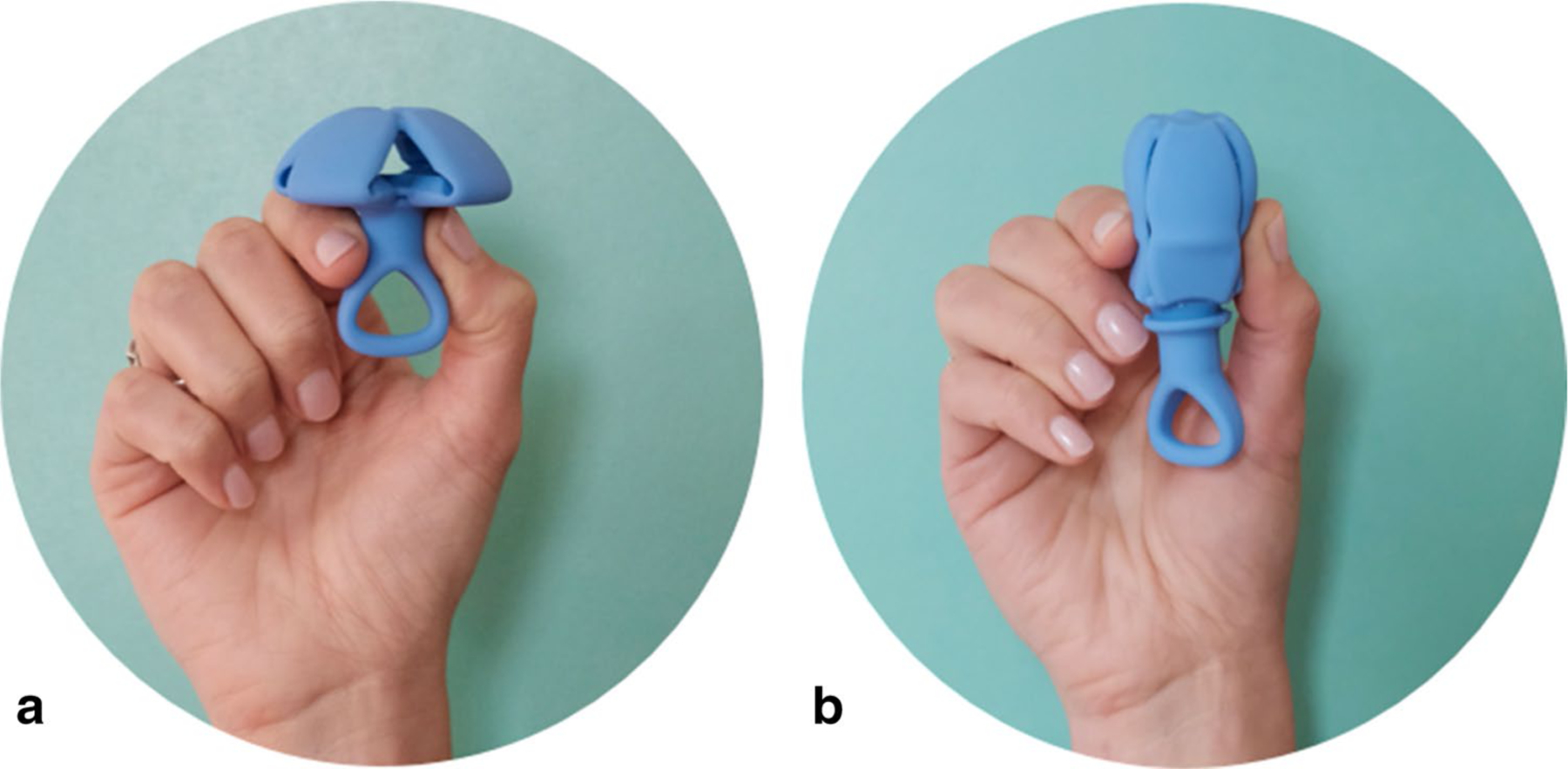
Study pessary. a Natural resting state when providing support. Once inserted, abdominal forces from above keep the pessary in position, similar to a Gellhorn pessary. b Temporary collapsed state during insertion and removal (with 50% reduction in cross-sectional diameter compared to resting state). Collapsed state is achieved with traction on the loop of the stem. Description of SP use: for insertion, the pessary is held in the “collapsed” state with one hand. Once inserted beyond the vestibule, the pessary is released and deploys into the “resting” state for prolapse support, with the stem oriented distally in the vagina. After deployment, abdominal forces maintains the pessary’s “resting” state and prevent expulsion. For removal, the pessary is pulled by a loop on its distal end, causing elongation as it is withdrawn from the vagina
Experience
During a 15-minute office trial, 14 of 15 participants were able to be fit with a SP. Of the 14 fitted, all maintained correct orientation, and none were expelled. One ring pessary participant could not tolerate the SP due to discomfort with insertion. Average insertion and removal pain scores were higher with their CP than with the SP (Fig. 2a and b), though insertion pain scores were lower with the CP in the ring subgroup.
Fig. 2.
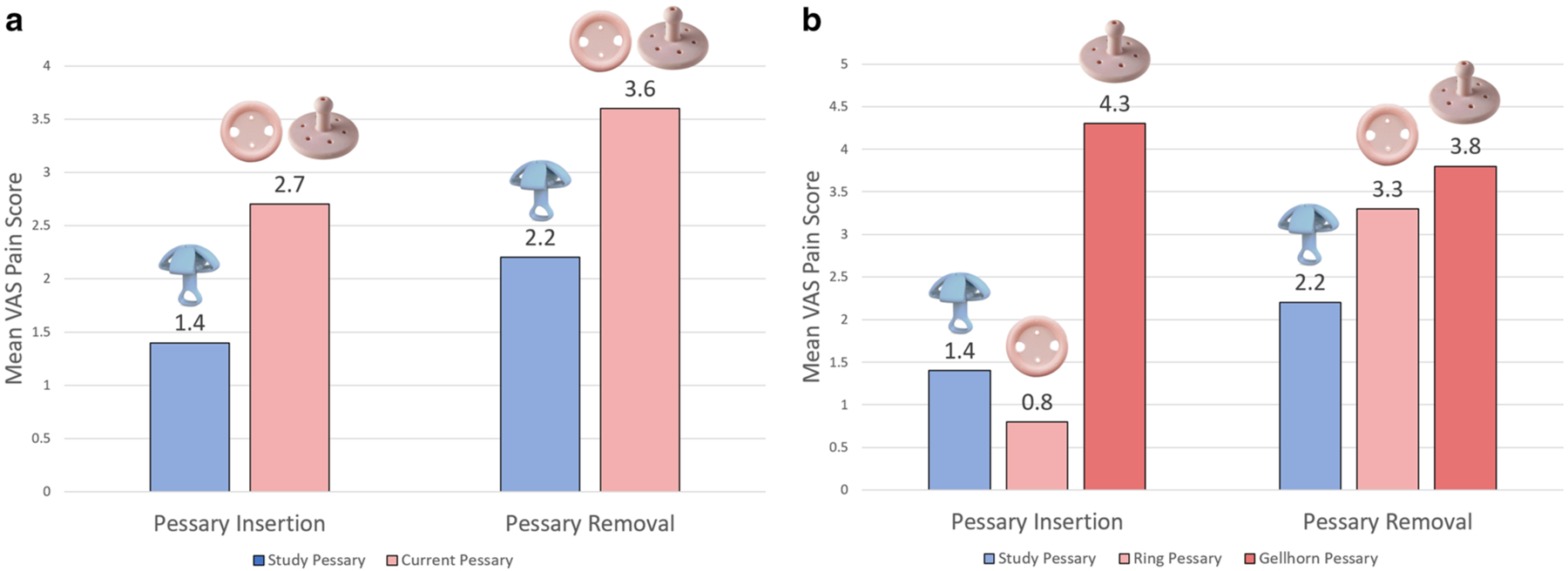
Participant-reported mean visual analogue scale (VAS) discomfort scores with pessary insertion and removal. a VAS scores with insertion and removal of study pessary versus current pessary. b VAS discomfort scores with insertion and removal of study pessary versus ring and Gellhorn pessary. Composite participant demographics: mean age 77.7 years (SD ± 8.2), predominantly White race (93.3%), median parity 3 (range: 1–4), with a median of three vaginal deliveries (range: 0–4), median prolapse stage of II (range: II–IV), and body mass index (BMI) primarily in the range of 20–30 kg/m2 (60%). Of the 15 total participants enrolled, eight were Gellhorn users and seven were Ring users. Comparing the two groups, the Gellhorn users were older (mean age 78.1 years vs 77.3 years) and had a higher median prolapse stage (III vs II). Both groups had equivalent median parity (3) and median number of vaginal deliveries (2). Additionally, 37.5% of Gellhorn users and 28.6% of Ring users had a BMI > 30 kg/m2
Conclusion
Our results endorse preliminary mechanical feasibility of this novel pessary for prolapse support, and suggest less discomfort with insertion and removal for Gellhorn users. Further investigation into long-term safety and efficacy is warranted.
Funding
NICHD Grant: 2R44HD097809-02.
Conflicts of interest
Kris Strohbehn (M.D.) is the Chief Editor for the Female Pelvic Medicine and Reconstructive Surgery section of WebMD eMedicine and receives institutional grant support from Reia, LLC; he is also on the Executive Board for the Society of Gynecologic Surgeons. Paul Hanissian (M.D.) is a co-founder of Reia, LLC. The remaining authors report no disclosures.
Footnotes
Consent Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Reference
- 1.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–7. 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]


