Abstract
Introduction:
There is considerable interest in utilizing cannabis-based products as adjuvants to opioid agonist therapies as phytocannabinoids like Δ9-tetrahydrocannabinol (THC) or synthetic cannabinoid receptor agonists enhance the pain-relieving effects of opioids without enhancing problematic effects of opioids. Cannabis is a pharmacologically complex plant with hundreds of compounds, some of which may have interactive effects. Therefore, studying compounds like THC in isolation does not accurately reflect the clinical use of cannabis.
Methods:
This study examined the effects of THC and cannabidiol (CBD), the two most prominent compounds in cannabis, on the reinforcing effects of fentanyl in rhesus monkeys in a food versus drug choice procedure. Responding on one lever was reinforced by delivery of a sucrose pellet and responding on another lever by delivery of an i.v. infusion of fentanyl. In each monkey, the largest dose of fentanyl that produced less than 20% drug choice, and the smallest dose of fentanyl that produced more than 80% drug choice was determined. Effects of pretreatment with THC and CBD, alone and in mixtures, were then examined.
Results:
THC, CBD, and THC:CBD mixtures did not reliably enhance or diminish choice for fentanyl up to doses that suppressed responding in most monkeys, though some individual differences were observed, with THC and THC:CBD mixtures decreasing choice for large doses of fentanyl in one monkey and increasing choice for small doses of fentanyl in another.
Conclusions:
Phytocannabinoids like THC and CBD, administered alone or in mixtures, do not appear to reliably alter the reinforcing effects of opioids.
Keywords: Fentanyl, cannabidiol, Δ9-tetrahydrocannabinol, food versus drug choice, rhesus monkey
1. Introduction
Despite tremendous research efforts, opioid use disorder (OUD) remains a significant public health issue in the United States and the escalating nature of this problem in the wake of the COVID-19 pandemic indicates novel strategies to treat OUD are desperately needed. Means to alleviate the burden of OUD could include enhancing their therapeutic (e.g. antinociceptive) effects, minimizing their reinforcing effects, alleviating opioid withdrawal, or preventing individuals from relapsing once opioid use has ceased. Cannabis and cannabinoid receptor agonists represent one potential means to achieve these goals (Wiese and Wilson-Poe, 2018).
Cannabinoid receptor agonists such as the phytocannabinoid THC as well as synthetic agonists enhance the antinociceptive effects of opioids in nonhuman primates (Li et al., 2008; Maguire and France, 2014; Maguire et al., 2013; Nilges et al., 2019). In rodents cannabis-derived, and synthetic cannabinoid receptor agonists, cannabinoid type 1 (CB1) receptor positive allosteric modulators, and inhibitors of endocannabinoid degradation enhance the antinociceptive effects of opioids (Cichewicz et al., 1999; Cichewicz and McCarthy, 2003; Cichewicz et al., 2005; Cox et al., 2007; Finn et al., 2004; Maguire and France, 2018a; Pugh et al., 1996; Slivicki et al., 2020; Slivicki et al., 2018; Smith et al., 1998; Smith et al., 2007; Welch and Stevens, 1992; Williams et al., 2006; Wilson-Poe et al., 2013; Wilson et al., 2008). The analgesic effects of opioids in humans are also enhanced in some studies by smoked or vaporized whole cannabis (Abrams et al., 2011; Cooper et al., 2018). Although these results are promising, combinations of opioid and cannabinoid receptor agonists would be of limited value in clinical scenarios were they to simultaneously enhance problematic effects of opioids as well. Fortunately, while cannabinoid receptor agonists enhance the antinociceptive effects of opioids they do not appear to enhance other effects including ventilatory depression (Weed et al., 2018), discriminative-stimulus effects (Li et al., 2008; Maguire and France, 2016b; Maguire et al., 2013), effects on cognition and impulsivity (Minervini and France, 2018, 2020), or physical dependence (Gerak and France, 2016). Moreover, cannabinoids do not appear to markedly alter the reinforcing effects of opioids. Under single lever self-administration procedures, cannabinoid receptor agonists decrease opioid intake (Li et al., 2012; Maguire and France, 2016a; Maguire et al., 2013), and do not enhance the reinforcing potency of opioids when self-administered as a mixture (Gerak et al., 2019; Maguire and France, 2018b, 2020). These results indicate that opioid/cannabinoid mixtures might not have greater (and perhaps lesser) abuse potential compared with an opioid alone. Choice procedures offer advantages over single lever self-administration paradigms, especially when investigating the effects of drugs like THC, well known for producing sedative/rate decreasing effects. Concurrent access to a non-drug reinforcer (food) in the present studies allows determination of whether phytocannabinoids alter the reinforcing properties of fentanyl (indicated by a reallocation of responding from drug to food reinforcers) or generally disrupt responding (indicated by decreased response rates and trials completed).
Cannabis is a pharmacologically complex plant containing hundreds of active compounds (e.g. phytocannabinoids, terpenes), the pharmacological actions of the majority of which are poorly understood. Therefore, studying phytocannabinoids such as THC in isolation likely does not accurately represent the effects of cannabis. Therefore, the present studies sought to investigate effects of THC and CBD, the two most prominent phytocannabinoids, on the reinforcing effects of fentanyl in rhesus monkeys working under a food versus drug choice procedure. Unit doses of fentanyl maintaining less than 20% drug choice (small dose) and greater than 80% drug choice (large dose) were determined individually for each monkey and the effects of THC and CBD alone and in mixtures of 1:10 and 1:32 parts THC to CBD on choice of both doses of fentanyl were examined. Blockade of μ-opioid receptors with naltrexone reduced choice selectively for the large dose of fentanyl without altering choice for the small dose of fentanyl. THC and CBD, administered alone or in mixtures, did not reliably enhance choice for a small dose of fentanyl or decrease choice for a large dose of fentanyl. The results of this study indicate THC and CBD, alone or in mixtures, do not appear to reliably impact the abuse potential of opioids.
2. Materials and Methods
2.1. Subjects.
Four adult male rhesus monkeys were housed individually in stainless steel cages with interior space measuring 81 cm tall by 81 cm wide by 72 cm deep; the home cage also served as the experimental chamber (details provided under “Surgery and equipment”). Two monkeys (MO and WI) previously participated in food versus drug choice experiments, including administration of opioids and cannabinoids (Maguire and France, 2018b); the two other monkeys (IP, AS) had no experience with the food versus drug choice procedure and had not participated in any experiment for 3 months prior to training. The colony room was maintained under a 14/10-hr light/dark cycle with lights on at 0600 hr. Chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI, USA), fresh fruit, peanuts, and treats were provided daily after the experimental session in amounts that maintained healthy weights, and water was continuously available. Experiments were conducted in accordance with guidelines set forth by the Guide for the Care and Use of Laboratory Animals (8th edition; 2011) and protocols were approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee.
2.2. Surgery and equipment.
Intravenous (i.v.) catheters were implanted under aseptic conditions as described previously (Maguire and France, 2018b, 2020). Briefly, monkeys were sedated with 10 mg/kg ketamine, and anesthesia was maintained under oxygen and isoflurane. A 5-french polyurethane catheter (Access Technologies, Skokie, IL, USA) was inserted into a vein (e.g., jugular or femoral) and tunneled subcutaneously to an exit point in the back. The exteriorized part of the catheter was passed through a stainless-steel tether and connected to an 18-g stainless-steel fluid channel swivel mounted on the rear wall; monkeys wore a jacket that protected the catheter and secured the tether (Lomir Biomedical, Quebec, Canada). The swivel was attached to a syringe mounted in a pump (PHM-108, Med Associates, Fairfax, VT, USA) that infused at a rate of 3.6 ml/min. A custom-made 20 cm by 28 cm stainless steel instrument panel was mounted on one side wall. The panel contained two horizontally aligned response levers (ENV-610M, Med Associates), and two stimulus lights (ENV-621L, Med Associates), one red and one green, were horizontally aligned above each of the two levers. Directly above the instrument panel was an aperture through which 300-mg raspberry flavored sucrose pellets (5TUT, Test Diet, Richmond, IN, USA) were delivered via activation of a pellet dispenser.
2.3. Food versus drug choice procedure.
Daily sessions comprised 2 forced trials followed by up to 30 choice trials; both forced trials had to be completed before choice trials were presented. The first forced trial began with illumination of one green stimulus light signaling the beginning of a response period; 30 consecutive responses on the lever located directly below the light turned that light off, delivered the reinforcer associated with that lever for that session (1 food pellet or an i.v. infusion), and initiated 5-minute timeout during which all lights were off and responding had no programmed consequence. At the end of the timeout, the green light above the other lever was illuminated, signaling the beginning of the second forced trial; 30 consecutive responses on the lever located directly below that light turned the light off, delivered the reinforcer associated with that lever during that session, and initiated a timeout. The order in which options were presented during forced trials varied randomly across sessions. Choice trials began only after both forced trials were completed. During choice trials, both green lights were illuminated, and 30 consecutive responses on one lever delivered the reinforcer associated with that lever, turned off both lights, and initiated a timeout. Responses on one lever reset the response requirement for the other lever. For all sessions, responding on one lever delivered one food pellet, and responding on the other lever delivered an i.v. infusion. There was no time limit for completing individual trials; sessions ended after 32 trials (2 forced trials plus 30 choice trials) were completed or 3 hours, whichever occurred first.
2.4. Experimental design.
Baseline measures for food versus fentanyl choice were examined in individual monkeys to determine the largest dose of fentanyl producing no more than 20% drug choice (small dose; 0.0001 mg/kg/infusion for all 4 subjects), and the smallest dose of fentanyl producing at least 80% drug choice (large dose; 0.001 mg/kg/infusion for subjects MO and IP, 0.0032 mg/kg/infusion for subjects WI and AS). Percent drug choice for each dose was deemed stable when values did not differ by more than 20% for 3 consecutive days. Pretreatment tests were separated by 5 days of daily choice training sessions. Test days were conducted only if percent drug choice for the 3 preceding choice sessions did not differ by more than 20% of the average of baseline levels for each respective dose of fentanyl, and percent drug choice. Under choice procedures, protracted periods of responding on one lever can occasion a bias towards responding on that lever. Therefore, saline substitutions were conducted occasionally (2-5 instances across monkeys) when the large dose of fentanyl was otherwise available during the first part of the study to ensure sensitivity of responding to reinforcing effects of the infusion; saline remained available until percent infusion choice for a single session was not more than 20%. Likewise, in the second part of the study, when the small dose of fentanyl was available, choice sessions were conducted with the large dose of fentanyl substituted occasionally between tests (4-9 instances across monkeys) until percent drug choice for a single session was at least 80%. The order of testing was as follows: large dose fentanyl + naltrexone vehicle, naltrexone (0.032 mg/kg), CBD vehicle, CBD (10, 17.8 mg/kg), THC vehicle, THC (0.32, 1, 0.1, 0.032 mg/kg), naltrexone (0.032 mg/kg), 1:10 THC:CBD (0.32:3.2, 0.032:0.32, 0.1:1 mg/kg), naltrexone (0.032 mg/kg), 1:32 THC:CBD (0.32:10.24, 0.1:3.2, 0.032:1.02 mg/kg), naltrexone (0.032 mg/kg). Small dose fentanyl + naltrexone (0.032 mg/kg), CBD (10 mg/kg), THC vehicle, THC (0.32, 1, 0.032, 0.1 mg/kg), 1:10 THC:CBD (0.32:3.2 mg/kg), 1:32 THC:CBD (0.32:10.24 mg/kg).
2.5. Data analyses.
Percent drug choice was calculated by dividing the number of trials completed on the drug lever during choice trials by the total number of choice trials completed and multiplying by 100. Total trials completed indicate the total number of trials completed on both levers during each session. The effects of pretreatments on percent drug choice or total trials completed were analyzed by paired samples t-test or a repeated measures one-way analysis of variance, as appropriate, with the effects of pretreatments on small and large doses of fentanyl being analyzed separately. Analyses were conducted and figures were generated using GraphPad Prism 9 software (San Diego, CA).
2.6. Drugs.
THC base, CBD base, naltrexone hydrochloride, and fentanyl hydrochloride were generously provided by the National Institute on Drug Abuse Drug Supply Program. Naltrexone and fentanyl were dissolved in saline. THC and CBD were initially dissolved in 100% ethanol, then were diluted further in a vehicle of ethanol, emulphor and saline (1:1:9 respectively). All pretreatments were filtered with sterile 0.2 μm syringe filters, then administered i.v. 15 minutes prior to choice sessions, a timepoint corresponding to maximum plasma levels in non-human primates for naltrexone (Reuning et al., 1979), THC (Ginsburg et al., 2014) and CBD (Gray et al., 2022).
3. Results
Under control conditions (baseline), the small dose of fentanyl occasioned 5% drug choice with an average of 32 trials completed, whereas the large dose of fentanyl occasioned approximately 97% drug choice with an average of 22 trials completed. The average % infusion choice on the first session of saline substitution, when the large dose of fentanyl was available otherwise, ranged from 19.3-47.2% across monkeys, with a group mean of 32.6. Mean drug choice on the first day of substitution with the large dose of fentanyl, when the small dose of fentanyl was otherwise available, ranged from 64.5-100% across individual monkeys, with a group mean of 86.3%. Naltrexone (0.032 mg/kg, i.v.) reduced choice for the large dose of fentanyl (t3=7.862, p=0.004, Figures 1A, 2A), and increased the number of trials completed, though effects on trials completed failed to reach statistical significance (p=0.07, Figures 1B, 2B). When administered in combination with the small dose of fentanyl, naltrexone did not alter percent drug choice (p=0.39, Figures 1A, 2A) or the number of trials completed (p=0.18, Figures 1B, 2B).
Figure 1.
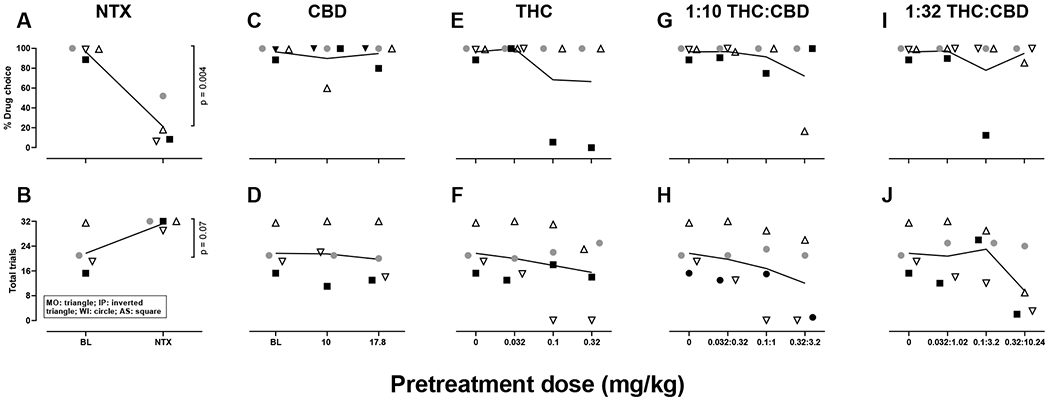
Individual subjects data for the large dose fentanyl alone and in combination with naltrexone, CBD, THC, and THC:CBD mixtures. Triangles represent subject MO, inverted triangles subject IP, circles subject WI, squares subject AS, and horizontal lines the group mean. Open symbols represent animals choosing between 0.001 mg/kg/infusion of fentanyl and food, closed symbols represent animals choosing between 0.0032 mg/kg/infusion of fentanyl and food. N=4. NTX: naltrexone, CBD: cannabidiol, THC: Δ9-tetrahydrocannabinol.
Figure 2.
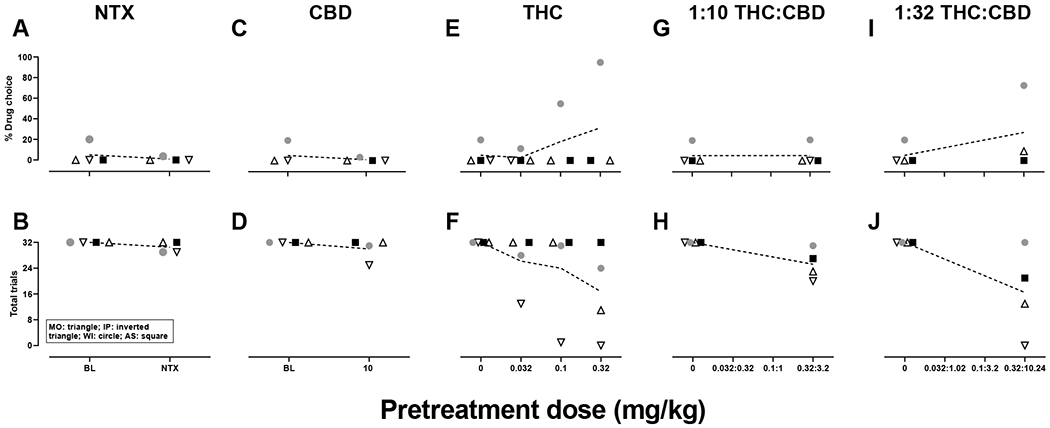
Individual subjects data for the small dose of fentanyl alone and in combination with naltrexone, CBD, THC and THC:CBD mixtures. Triangles represent subject MO, inverted triangles subject IP, circles subject WI, squares subject AS and horizontal lines the group mean. Small dose fentanyl was 0.0001 mg/kg/infusion for all subjects. N=4. NTX: naltrexone, CBD: cannabidiol, THC: Δ9-tetrahydrocannabinol.
Administered alone, CBD did not alter choice for the large (p=0.66, Figures 1C, 2C) or small dose of fentanyl (p=0.39, Figures 1C, 2C). CBD similarly did not alter the number of trials completed when given with the large (p=0.45, Figure 1B, 2B) or small (p=0.32, Figure 1B, 2B) dose of fentanyl. Some individual differences were observed in the effects of CBD with the 10 mg/kg dose decreasing choice for the large dose of fentanyl by approximately 40% in one monkey (MO, Figure 2C).
Administered alone, THC did not reliably alter choice for the large (p=0.36, Figures 1E, 2E) or small (p= 0.36, Figures 1E, 2E) dose of fentanyl. THC also did not alter the number of trials completed in combination with the large (p=0.14, Figures 1F, 2F) or small (p=0.17, Figures 1F, 2F) dose of fentanyl. Individual differences in effects of THC were observed; however, 0.1 and 0.32 mg/kg decreased choice for the large dose of fentanyl by approximately 83% and 88%, respectively, in monkey AS (Figure 2E) and increased choice for the small dose of fentanyl by 35% and 75%, respectively, in monkey WI (Figure 2E). In monkey IP, 0.1 and 0.32 mg/kg THC in combination with the large dose of fentanyl eliminated responding (Figure 2F). In combination with the small dose of fentanyl, 0.032 mg/kg THC reduced the number of trials completed by approximately 60%, 0.1 and 0.32 mg/kg THC eliminated responding in monkey IP, and 0.32 mg/kg THC reduced the number of trials completed by approximately 66% in monkey MO (Figure 2F).
The 1:10 THC:CBD mixtures did not alter choice for the large (p=0.42, Figures 1G, 2G) or small (p=0.39, Figures 1G, 2G) dose of fentanyl. The 1:10 THC:CBD mixtures, on average, decreased the number of trials completed in combination with the large (p=0.06, Figures 1H, 2H) and small (p=0.067, Figures 1H, 2H) doses of fentanyl, though these differences failed to reach statistical significance. Individual differences were observed in the effects of the 1:10 THC:CBD mixtures. For example, the 0.32:3.2 mg/kg mixture reduced choice for the large dose of fentanyl by approximately 82% in monkey MO (Figure 2G). When administered in combination with the large dose of fentanyl, 0.1:1 and 0.32:3.2 mg/kg mixtures eliminated responding in monkey IP whereas the 0.32:3.2 mg/kg mixture decreased responding by 93% in monkey AS (Figure 2H). In combination with the small dose of fentanyl, the 0.32:3.2 mg/kg mixture decreased the number of trials completed by 28% or 37% in monkeys MO and IP, respectively (Figure 2H).
The 1:32 THC:CBD mixtures did not alter choice for the large (p=0.45, Figures 1I, 2I) or small (p=0.33, Figures 1I, 2I) dose of fentanyl. Though failing to reach statistical significance, the 1:32 THC:CBD mixtures produced a modest decrease in the number of trials completed in combination with the large dose of fentanyl (p=0.072, Figure 1J, 2J), but failed to alter the number of trials completed when administered in combination with the small dose of fentanyl (p=0.11, Figures 1J, 2J). Again, individual differences in the effects of these mixtures were apparent. In combination with the large dose of fentanyl, the 0.1:3.2 mg/kg mixture decreased percent drug choice by approximately 76% (Figure 2I), whereas the 0.32:10.24 mg/kg mixture eliminated responding in monkey AS (Figure 2J). Rate suppressing effects were also observed in monkey IP following the 0.032:1.02, 0.1:3.2 and 0.32:10.24 mg/kg mixtures in combination with the large dose of fentanyl, decreasing the number of trials completed by approximately 26, 37, and 85%, respectively (Figure 2J). The 0.32:10.24 mg/kg mixture also suppressed responding in monkey MO, reducing the number of trials completed by approximately 69% (Figure 2J). In combination with the small dose of fentanyl, the 0.32:10.24 mg/kg mixture increased percent drug choice by 53% in monkey WI (Figure 2I). The 0.32:10.24 mg/kg mixture also decreased the number of trials completed by 60% in monkey MO, 35% in monkey AS, and eliminated responding in monkey IP (Figure 2J).
4. Discussion
Mounting evidence suggests phytocannabinoids might be a safe and effective means for managing pain alone and as adjuvants to opioid agonist therapies for the treatment of pain. Besides representing a non-opioid strategy for pain management, phytocannabinoids like THC and synthetic cannabinoid receptor agonists enhance the antinociceptive potency of opioids in rodents (Cichewicz et al., 1999; Cichewicz and McCarthy, 2003; Cichewicz et al., 2005; Cox et al., 2007; Finn et al., 2004; Maguire and France, 2018a; Pugh et al., 1996; Smith et al., 1998; Smith et al., 2007; Welch and Stevens, 1992; Williams et al., 2006; Wilson-Poe et al., 2013; Wilson et al., 2008), nonhuman primates (Li et al., 2008; Maguire and France, 2014; Maguire et al., 2013; Nilges et al., 2019), and possibly humans (Abrams et al., 2011; Cooper and Haney, 2010), raising the possibility of treating pain with combinations of an opioid and a cannabinoid. Critically, several studies have demonstrated phytocannabinoids and synthetic agonists do not appear to enhance other effects of opioids including ventilatory depression (Weed et al., 2018), physical dependence and withdrawal (Gerak and France, 2016), discriminative stimulus effects (Li et al., 2008; Maguire and France, 2016b; Maguire et al., 2013) or positive reinforcing effects (Gerak et al., 2019; Li et al., 2012; Maguire and France, 2016a, 2018b, 2020; Maguire et al., 2013). However, previous studies examined the effects THC or synthetic cannabinoid receptor agonists in isolation. As THC is one of several hundred compounds present in the cannabis plant, some of which might have interactive effects, studying the effects of individual compounds in isolation likely does not accurately reflect the effects of cannabis. Given this complexity, recapitulating the pharmacological effects of cannabis in a laboratory setting is challenging. The present studies examined the effects of THC and CBD, the two most prominent phytocannabinoids present in cannabis, alone and in combination, on the reinforcing effects of fentanyl in rhesus monkeys performing a food versus drug choice procedure. While these efforts do not completely recapitulate the pharmacological effects of cannabis, they are an important step forward in investigating potential interactions between phytocannabinoids in regard to their potential modulation of the abuse-related effects of opioids. Over a range of doses, THC, CBD, and THC:CBD mixtures did not reliably alter choice for large or small doses of fentanyl, although some individual differences were apparent. THC and CBD failed to reliably alter fentanyl choice up to (and exceeding) doses used in human clinical studies. . For example, doses of THC tested in the present studies (0.032-0.32 mg/kg, corresponding to approximately 2.5-25 mg for an average adult male human) encompass subeffective (de Vries et al., 2017), to analgesic doses (Cooper et al., 2013), as well as doses that enhance the pain relieving effects of opioids in human clinical trials (Narang et al., 2008). The doses of CBD examined in these studies (approximately 800-1,400 mg for an average adult male human) also encompass (and surpass) doses of CBD effective at reducing cue-induced opioid craving and anxiety in humans with heroin use disorder (Hurd et al., 2019). Moreover, the suppression of responding observed following THC administration indicates behaviorally active doses were tested, while CBD was studied up to maximally tolerable doses.
In previous studies utilizing a single-response self-administration procedure, cannabinoid receptor agonists including THC reduced opioid intake in rodents (Braida et al., 2001; Nguyen et al., 2019) and nonhuman primates (Li et al., 2012; Maguire and France, 2016a; Maguire et al., 2013), suggesting a possible reduction in the reinforcing effects of opioids. However, this interpretation is complicated by the fact that compounds like THC can have sedative or rate-decreasing effects. For this reason, the present studies utilized a choice procedure wherein the dependent measures of percent drug choice and total trials completed allow the differentiation of whether reductions in drug intake are due to allocation of responding towards non-drug reinforcers (indicating a reduction in the reinforcing effects of fentanyl) or a non-specific suppression of responding (rate-decreasing effects). THC administration in the current study reduced the number of trials completed in 2 of 4 subjects, though substantial individual differences in sensitivity to the rate-decreasing effects of THC were observed. These results are generally consistent with a previous study (Maguire and France, 2018b) indicating reductions in opioid intake observed following THC administration are likely due to a suppression of behavior rather than altered reinforcing effects. However, in one monkey (AS) several doses of THC and a single dose of the 1:32 THC:CBD mixture reduced choice for the large dose of fentanyl without reducing the number of trials completed. This shift in behavioral allocation from drug to food reinforcers might indicate a reduction in the reinforcing effects of fentanyl in this individual. Conversely, in another monkey (WI), THC and some THC:CBD mixtures dose-dependently increased choice for the small dose of fentanyl. Modest increases in the abuse-related effects of opioids following cannabis consumption have been reported in clinical studies (Babalonis et al., 2019; Cooper et al., 2018). Intriguing lines of evidence suggest that prior experience with cannabis might qualitatively alter interactions between the opioid and cannabinoid systems For example, in individuals who use cannabis, naltrexone blunted the intoxicating effects of small doses of THC and enhanced the anxiogenic effects of large doses of THC (Haney, 2007). Conversely, in non-cannabis smokers, naltrexone had the opposite effect where it enhanced the intoxicating effects of small doses of THC and blunted the anxiogenic effects of large doses of THC. Other studies in cannabis users have indicated blockade of opioid receptors enhances the abuse liability of cannabis (Cooper and Haney, 2010; Haney et al., 2003). Therefore, it is conceivable that prior experience with cannabinoids might have contributed to the individual differences observed in the present study (e.g., THC reducing or enhancing the choice of fentanyl). For example, one monkey (AS) had no prior experience with cannabinoids and showed a decrease in choice for the large dose of fentanyl following several doses of THC and one dose of THC:CBD mixtures. whereas another monkey (WI) had prior exposure to THC as well as other cannabinoid receptor agonists (Maguire and France, 2018b), and showed an increase in choice for low doses of fentanyl following several doses of THC and one dose of THC:CBD mixtures.. However, monkey MO also had prior experience with THC, which neither increased nor decreased choice for either dose of fentanyl.
CBD, which was studied up to the maximally tolerable dose in this study, did not reliably alter choice of either dose of fentanyl or the number of trials completed. The lone exception was observed in one monkey (MO), in whom 10 mg/kg CBD reduced choice for the large dose of fentanyl by approximately 40%. The lack of effect of CBD on fentanyl intake is consistent with a prior report in rodents (Ren et al., 2009) indicating that CBD does not alter heroin self-administration even at doses that reduce reinstatement of cue-induced responding previously reinforced by heroin. In humans, CBD alone reduced cue-induced anxiety and craving in abstinent individuals undergoing treatment for heroin use disorder (Hurd et al., 2019).
During these experiments, given the experimental conditions of short access to low doses of fentanyl, none of the animals studied displayed any indication of being physically dependent. As conditions of physical dependence and withdrawal can bidirectionally modulate opioid reinforcement (Carrera et al., 1999; Chen et al., 2006; Cooper et al., 2010; Cooper et al., 2008; Griffiths et al., 1981; Lenoir et al., 2013; McConnell et al., 2021; Negus, 2006; Negus and Rice, 2009; Townsend et al., 2021), future studies may examine whether THC, CBD, and THC:CBD mixtures alter the reinforcing effects of opioids such as fentanyl under conditions of physical dependence and withdrawal. Additionally, all the subjects in these experiments were male. The therapeutic and abuse-related effects of cannabis may differ as a function of sex in both humans and rodents (for review (Cooper and Craft, 2018). However, the individual differences in the effects of THC on response rate and percent drug choice are likely to overshadow any differences based on sex. One final caveat to address is the influence cannabinoids on feeding (for review see (Tarragon and Moreno, 2019). Phytocannabinoids like THC, under some conditions, induce hyperphagia (Farrimond et al., 2010; Jarbe and DiPatrizio, 2005) or increase the palatability of sucrose (De Luca et al., 2012; Jarrett et al., 2005). The effects of CBD on appetitive behaviors are mixed with some reports suggesting CBD enhances sucrose intake (Bi et al., 2020) and others failing to confirm this effect and suggesting CBD attenuates the hyperphagic effects of THC (Scopinho et al., 2011). However, in the present experiment no systematic enhancement in sucrose consumption was evident following THC, CBD, or THC:CBD mixture pretreatments. Under a few limited circumstances, THC or select doses of THC:CBD mixtures decreased choice for larger doses of fentanyl, or in other words enhanced choice for sucrose reinforcers. Whether these reductions in drug choice were due to a decrease of the reinforcing value of fentanyl, or an enhancement of the reinforcing value of sucrose cannot be ascertained using a food versus drug choice procedure.
Overall, the results of this study indicate that the phytocannabinoids THC and CBD, administered alone or in mixtures, do not reliably alter fentanyl choice over food in nonhuman primates, adding to the body of literature suggesting cannabis or cannabis constituents might be a safe and effective means to enhance the antinociceptive potency of opioids without enhancing abuse potential. Ongoing studies aim to determine whether phytocannabinoids like THC and CBD are effective at combating other aspects associated with opioid use disorder in humans including physical dependence, withdrawal, and relapse.
Acknowledgements
The authors thank Faith Aguilar and Samuel Womack for excellent technical assistance and Wouter Koek for his helpful discussion on analysis and data presentation strategies.
Funding and Disclosure
This work was supported by USPHS grants R01DA005018 and T32DA031115 and grant AQ-0039 from the Welch Foundation (CPF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no competing interests to declare.
References
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL, 2011. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 90(6), 844–851. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Lofwall MR, Sloan PA, Nuzzo PA, Fanucchi LC, Walsh SL, 2019. Cannabinoid modulation of opioid analgesia and subjective drug effects in healthy humans. Psychopharmacology (Berl) 236(11), 3341–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GH, Galaj E, He Y, Xi ZX, 2020. Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents. Addict Biol 25(4), e12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Parolaro D, Sala M, 2001. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur J Pharmacol 413(2-3), 227–234. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Schulteis G, Koob GF, 1999. Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone. Psychopharmacology (Berl) 144(2), 111–120. [DOI] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF, 2006. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31(12), 2692–2707. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP, 1999. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther 289(2), 859–867. [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA, 2003. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther 304(3), 1010–1015. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP, Smith FL, 2005. Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal delta9-tetrahydrocannabinol. Eur J Pharmacol 525(1-3), 74–82. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M, 2018. Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 43(10), 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Comer SD, Haney M, 2013. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology 38(10), 1984–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Craft RM, 2018. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 43(1), 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2010. Opioid antagonism enhances marijuana’s effects in heavy marijuana smokers. Psychopharmacology (Berl) 211(2), 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Shi YG, Woods JH, 2010. Reinforcer-dependent enhancement of operant responding in opioid-withdrawn rats. Psychopharmacology (Berl) 212(3), 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Truong YN, Shi YG, Woods JH, 2008. Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther 326(3), 920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP, 2007. Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol 567(1-2), 125–130. [DOI] [PubMed] [Google Scholar]
- De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G, 2012. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 63(1), 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H, Pain, Nociception Neuroscience Research, G., 2017. Tetrahydrocannabinol Does Not Reduce Pain in Patients With Chronic Abdominal Pain in a Phase 2 Placebo-controlled Study. Clin Gastroenterol Hepatol 15(7), 1079–1086 e1074. [DOI] [PubMed] [Google Scholar]
- Farrimond JA, Whalley BJ, Williams CM, 2010. A low-Delta9 tetrahydrocannabinol cannabis extract induces hyperphagia in rats. Behav Pharmacol 21(8), 769–772. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V, 2004. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci 19(3), 678–686. [DOI] [PubMed] [Google Scholar]
- Gerak LR, France CP, 2016. Combined Treatment with Morphine and Delta9-Tetrahydrocannabinol in Rhesus Monkeys: Antinociceptive Tolerance and Withdrawal. J Pharmacol Exp Ther 357(2), 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Weed PF, Maguire DR, France CP, 2019. Effects of the synthetic cannabinoid receptor agonist JWH-018 on abuse-related effects of opioids in rhesus monkeys. Drug Alcohol Depend 202, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Hruba L, Zaki A, Javors MA, McMahon LR, 2014. Blood levels do not predict behavioral or physiological effects of Delta(9)-tetrahydrocannabinol in rhesus monkeys with different patterns of exposure. Drug Alcohol Depend 139, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RA, Heal DJ, Maguire DR, Gerak LR, Javors MA, Smith S, France CP, 2022. Preclinical Assessment of the Abuse Potential of Purified Botanical Cannabidiol: Self-Administration, Drug Discrimination, and Physical Dependence. J Pharmacol Exp Ther 382(1), 54–65. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV, 1981. Choice between food and heroin: effects of morphine, naloxone, and secobarbital. J Exp Anal Behav 35(3), 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, 2007. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology 32(6), 1391–1403. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW, 2003. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology (Berl) 166(1), 77–85. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM, Salsitz E, 2019. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am J Psychiatry 176(11), 911–922. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, DiPatrizio NV, 2005. Delta9-THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist delta9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav Pharmacol 16(5-6), 373–380. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Limebeer CL, Parker LA, 2005. Effect of Delta9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav 86(4), 475–479. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH, 2013. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology 38(7), 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Koek W, France CP, 2012. Interactions between Delta(9)-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol 23(8), 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP, 2008. Interactions between Delta(9)-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology (Berl) 199(2), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2014. Impact of efficacy at the mu-opioid receptor on antinociceptive effects of combinations of mu-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther 351(2), 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2016a. Effects of daily delta-9-tetrahydrocannabinol treatment on heroin self-administration in rhesus monkeys. Behav Pharmacol 27(2-3 Spec Issue), 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2016b. Interactions between cannabinoid receptor agonists and mu opioid receptor agonists in rhesus monkeys discriminating fentanyl. Eur J Pharmacol 784, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2018a. Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio. Eur J Pharmacol 819, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2018b. Reinforcing effects of opioid/cannabinoid mixtures in rhesus monkeys responding under a food/drug choice procedure. Psychopharmacology (Berl) 235(8), 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2020. Interactions between opioids and cannabinoids: Economic demand for opioid/cannabinoid mixtures. Drug Alcohol Depend 212, 108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP, 2013. Interactions between mu-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther 345(3), 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SA, Brandner AJ, Blank BA, Kearns DN, Koob GF, Vendruscolo LF, Tunstall BJ, 2021. Demand for fentanyl becomes inelastic following extended access to fentanyl vapor self-administration. Neuropharmacology 182, 108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini V, France CP, 2018. Effects of morphine/CP55940 mixtures on an impulsive choice task in rhesus monkeys. Behav Pharmacol 29(1), 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini V, France CP, 2020. Effects of opioid/cannabinoid mixtures on impulsivity and memory in rhesus monkeys. Behav Pharmacol 31(2&3), 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, Jamison RN, 2008. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain 9(3), 254–264. [DOI] [PubMed] [Google Scholar]
- Negus SS, 2006. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther 317(2), 711–723. [DOI] [PubMed] [Google Scholar]
- Negus SS, Rice KC, 2009. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology 34(4), 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Hwang CS, Vandewater SA, Janda KD, Cole M, Taffe MA, 2019. Delta(9)-tetrahydrocannabinol attenuates oxycodone self-administration under extended access conditions. Neuropharmacology 151, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilges MR, Bondy ZB, Grace JA, Winsauer PJ, 2019. Opioid-enhancing antinociceptive effects of delta-9-tetrahydrocannabinol and amitriptyline in rhesus macaques. Exp Clin Psychopharmacol. [DOI] [PubMed] [Google Scholar]
- Pugh G Jr., Smith PB, Dombrowski DS, Welch SP, 1996. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther 279(2), 608–616. [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL, 2009. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci 29(47), 14764–14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuning RH, Batra VK, Ludden TM, Jao MY, Morrison BE, McCarthy DA, Harrigan SE, Ashcraft SB, Sams RA, Bathala MS, Staubus AE, Malspeis L, 1979. Plasma naltrexone kinetics after intravenous bolus administration in dogs and monkeys. J Pharm Sci 68(4), 411–416. [DOI] [PubMed] [Google Scholar]
- Scopinho AA, Guimaraes FS, Correa FM, Resstel LB, 2011. Cannabidiol inhibits the hyperphagia induced by cannabinoid-1 or serotonin-1A receptor agonists. Pharmacol Biochem Behav 98(2), 268–272. [DOI] [PubMed] [Google Scholar]
- Slivicki RA, Iyer V, Mali SS, Garai S, Thakur GA, Crystal JD, Hohmann AG, 2020. Positive Allosteric Modulation of CB1 Cannabinoid Receptor Signaling Enhances Morphine Antinociception and Attenuates Morphine Tolerance Without Enhancing Morphine-Induced Dependence or Reward. Front Mol Neurosci 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivicki RA, Saberi SA, Iyer V, Vemuri VK, Makriyannis A, Hohmann AG, 2018. Brain-Permeant and -Impermeant Inhibitors of Fatty Acid Amide Hydrolase Synergize with the Opioid Analgesic Morphine to Suppress Chemotherapy-Induced Neuropathic Nociception Without Enhancing Effects of Morphine on Gastrointestinal Transit. J Pharmacol Exp Ther 367(3), 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Cichewicz D, Martin ZL, Welch SP, 1998. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol Biochem Behav 60(2), 559–566. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP, 2007. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol 571(2-3), 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarragon E, Moreno JJ, 2019. Cannabinoids, Chemical Senses, and Regulation of Feeding Behavior. Chem Senses 44(2), 73–89. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Kim RK, Robinson HL, Marsh SA, Banks ML, Hamilton PJ, 2021. Opioid withdrawal produces sex-specific effects on fentanyl-vs.-food choice and mesolimbic transcription. Biol Psychiatry Glob Open Sci 1(2), 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed PF, Gerak LR, France CP, 2018. Ventilatory-depressant effects of opioids alone and in combination with cannabinoids in rhesus monkeys. Eur J Pharmacol 833, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SP, Stevens DL, 1992. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther 262(1), 10–18. [PubMed] [Google Scholar]
- Wiese B, Wilson-Poe AR, 2018. Emerging Evidence for Cannabis’ Role in Opioid Use Disorder. Cannabis Cannabinoid Res 3(1), 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IJ, Edwards S, Rubo A, Haller VL, Stevens DL, Welch SP, 2006. Time course of the enhancement and restoration of the analgesic efficacy of codeine and morphine by delta9-tetrahydrocannabinol. Eur J Pharmacol 539(1-2), 57–63. [DOI] [PubMed] [Google Scholar]
- Wilson-Poe AR, Pocius E, Herschbach M, Morgan MM, 2013. The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacol Biochem Behav 103(3), 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AR, Maher L, Morgan MM, 2008. Repeated cannabinoid injections into the rat periaqueductal gray enhance subsequent morphine antinociception. Neuropharmacology 55(7), 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]


