Abstract
Background
Physical activity (PA) is important for cardiovascular health as well as social and emotional well-being of children. Patients with long QT syndrome (LQTS) often face PA restrictions and are often prescribed beta-blockers for disease management. The aim of this study was to determine if PA levels were lower in patients with LQTS compared with healthy controls.
Methods
Participants with LQTS from an inherited arrhythmia clinic completed the Physical Activity Questionnaire for Children and Adolescents (PAQ-C/A) and an exercise stress test. PAQ score (a general measure of PA for youth, unitless) and endurance time were compared with healthy controls.
Results
Twenty-three patients with LQTS completed the PAQ and had an exercise stress test within a year of having completed the PAQ. No difference was observed in PAQ scores between LQTS and control groups (LQTS: 2.3 ± 0.15 vs controls: 2.3 ± 0.18; P = 0.78). There was no effect of age on PA in patients with LQTS (P > 0.05), whereas PA significantly decreased in controls with age (eg, 11-12 vs 17-20 years: 3.2 ± 0.07 vs 1.5 ± 0.08, P = 0.005). Endurance time and heart rate at peak exercise were significantly lower in patients with LQTS compared with controls (11 ± 0.5 vs 15 ± 0.5 minutes, P < 0.0001; 169 ± 5 vs 198 ± 2 beats per minute, P < 0.0001).
Conclusions
Despite guideline recommendations restricting PA, risk of sudden cardiac death, and use of beta-blockers, our cohort of patients with LQTS reported similar PA levels as healthy controls.
Résumé
Contexte
L’activité physique est importante pour la santé cardiovasculaire ainsi que le bien-être social et émotionnel des enfants. Chez les patients qui présentent un syndrome du QT long (SQTL), l’activité physique est souvent restreinte, et des bêta-bloquants sont fréquemment prescrits pour la maîtrise de la maladie. L’objectif de cette étude était de déterminer si le degré d’activité physique était inférieur chez les patients atteints du SQTL à celui de témoins en bonne santé.
Méthodologie
Des patients atteints du SQTL d’une clinique d’arythmie héréditaire ont rempli le questionnaire sur l’activité physique pour les enfants et les adolescents (PAQ-C/A, pour Physical Activity Questionnaire for Children and Adolescents) et subi une épreuve d’effort. Le score du PAQ (mesure générale de l’activité physique pour les jeunes, sans unité) et le temps d’endurance ont été comparés à ceux obtenus chez des témoins en bonne santé.
Résultats
Vingt-trois patients atteints du SQTL ont rempli le PAQ et, dans l’année suivante, subi une épreuve d’effort. Pour ce qui est du score du PAQ, aucune différence n’a été observée entre le groupe atteint du SQTL et le groupe témoin (2,3 ± 0,15 chez les patients atteints du SQTL vs 2,3 ± 0,18 chez les témoins; p = 0,78). L’âge était sans effet sur l’activité physique chez les patients atteints du SQTL (p > 0,05), tandis que le degré d’activité physique diminuait significativement avec l’âge chez les témoins (p. ex. 3,2 ± 0,07 chez les témoins de 11 à 12 ans vs 1,5 ± 0,08 chez les témoins de 17 à 20 ans, p = 0,005). Pendant l’effort maximal, le temps d’endurance était significativement plus court et la fréquence cardiaque, significativement plus basse chez les patients atteints du SQTL que chez les témoins (11 ± 0,5 vs 15 ± 0,5 minutes, p < 0,0001; 169 ± 5 vs 198 ± 2 battements par minute, p < 0,0001).
Conclusions
Malgré la restriction de l’activité physique recommandée par les lignes directrices, le risque de mort subite d’origine cardiaque et l’utilisation de bêta-bloquants, dans notre cohorte de patients atteints du SQTL, le degré d’activité physique a été semblable à celui des témoins en bonne santé.
Physical activity (PA) is important for health and well-being and is defined as “any bodily movement produced by skeletal muscles that results in energy expenditure.1” In children, PA promotes long-term cardiovascular health and reduces the frequency of adverse cardiac events in later life.2 Each hour of PA is associated with an 8%-11% decrease in self-reported depression,3 and PA interventions have been shown to improve self-reported cognitive, motor, and social function.4 As such, current paediatric guidelines recommend participating in at least 60 minutes of moderate-to-vigorous PA a day.5 However, there is a documented decline in PA in children and adolescents with age that may be further impacted by sex.6,7
Patients with inherited arrhythmia (IA) face additional challenges with restrictions to their PA. In these children, PA has been associated with worsening disease progression,8 complications,9 and even death.10, 11, 12 For example, James et al.8 found that those with arrhythmogenic right ventricular cardiomyopathy who participated in more PA reported experiencing ventricular tachycardia/fibrillation, syncope, palpitations, and chest pain at a younger age compared with their less active counterparts. Although these studies are based on high-level competitive athletes and not the general population, current expert consensus guidelines have erred on the side of over-restriction when it comes to PA in children with IA.13 Children with long QT syndrome (LQTS) are to be restricted to low-intensity sports with reduced cardiovascular demand, such as billiards and golf,10 whereas those with arrhythmogenic right ventricular cardiomyopathy and catecholaminergic polymorphic ventricular tachycardia are often advised to avoid activity altogether.14,15 Many patients with IA require beta-blockers to reduce the risk of sudden death16 and arrhythmic events;17, 18, 19 however, these medications are associated with fatigue and reduced performance,20,21 which could negatively influence PA levels. Such barriers to PA in patients with IA may have negative consequences for cardiovascular health, so a balance between restriction for safety and promotion of activity for health and well-being is needed when managing these patients. In addition, there is increasing evidence that activity is safe in patients with IA.15,22
Understanding the activity levels of patients with LQTS and their ability to perform exercise provides practitioners with a baseline assessment of how to direct care for these patients. Self-report PA questionnaires in children and adolescents such as the Physical Activity Questionnaire for Children and Adolescents (PAQ-C/A) have good correlation with objective measures of PA23,24 and been used to assess PA in healthy children and other chronic disease populations such as those with congenital heart disease.25 Graded exercise tests are commonly used in clinical practice to monitor for arrhythmia and guide medical management, and provide an objective measure of exercise capacity. We sought to understand the PA habits and exercise capacity of a cohort of adolescent patients with LQTS compared with healthy controls.
Materials and Methods
Recruitment
Participants were recruited from the IA clinic at a tertiary paediatric hospital with a multidisciplinary IA clinic. Those who were between the ages of 11 and 20 and diagnosed with LQTS were eligible for the study. Patients were not asked to restrict and/or alter their PA in any way.
Previously reported healthy cohorts were used as a comparison.25,26 Controls were age- and sex-matched.
Physical activity questionnaires
The standardized self-report PAQ-C and -A were used to assess PA. These 7-day recall questionnaires measure moderate-to-vigorous PA for those between grades 4 and 12 (approximately ages 8-20) derived from 8 (PAQ-A) or 9 items (PAQ-C) each scored between 1 (low) and 5 (high) with a mean of the 8 or 9 items as the overall PAQ score out of 5.25,27 The score is unitless. These validated questionnaire scores correlate with activity as measured by monitors23 as well as with body mass index and maximal exercise capacity.24
Exercise testing
A graded exercise stress test was performed on a treadmill using an institutional protocol as a part of the clinical work-up of patients with LQTS.26 Only patients with LQTS who had an exercise stress test within 12 months of completing the PAQ were included in the exercise testing analysis. Treadmill speed started at 2 miles per hour and increased by 0.5 miles per hour every minute.26 Grade stayed constant at 1%. Patients were verbally encouraged to exercise to maximal effort, and the treadmill was stopped once participants reached volitional fatigue or haemodynamically unstable arrhythmias developed, prohibitive symptoms occurred, such as presyncope or exhaustion, or no further diagnostic information could be obtained. Cardiopulmonary exercise testing was not performed. Endurance time was the primary indicator of exercise capacity.
Statistical analysis
Participants in each group were assigned to subgroups based on age (11-12, 13-14, 15-16, and 17-20 years) and sex. The LQTS group was also divided into 2 groups based on whether they were taking a beta-blocker. Frequency tables were generated for categorical variables. Overall mean ± standard error of the mean was calculated for each of the LQTS and control groups. Nonparametric analyses were conducted, with a Mann-Whitney test and Wilcoxon rank sum test being used to determine differences between groups with a significance of P < 0.05. When we compared age and sex between the 2 groups, a 2-way analysis of variance with Sidak’s post hoc test was used with a significance of P < 0.05.
Results
Demographics
Demographic data are summarized in Table 1. The age of study subjects at the time of PAQ completion was similar between patients with LQTS and sex-matched controls (LQTS: 14.7 ± 0.5, n = 23; controls: 14.5 ± 0.4, n = 23; P = 0.31). Patients with LQTS were followed for an average of 3.9 ± 0.4 years with the majority of patients being asymptomatic gene carriers (61%). The most common symptom experienced was syncope (30%) followed by seizures (9%), palpitations (4%), polymorphic ventricular tachycardia, and subsequent implantable cardioverter defibrillator implant (4%). None of the patients with LQTS experienced a cardiac event during PA over the follow-up period. Twenty-one of 23 (91%) patients with LQTS were on beta-blockers at the time of PAQ completion.
Table 1.
Demographics of study subjects
| Patients with LQTS (n = 23) | PAQ controls (n = 23) | Exercise test controls (n = 23) | |
|---|---|---|---|
| Mean age ± SEM | 14.7 ± 0.5 | 14.5 ± 0.4 | 14.5 ± 0.5 |
| 11-12 (y) (n) | 6 | 6 | 5 |
| 13-14 (y) (n) | 9 | 8 | 9 |
| 15-16 (y) (n) | 4 | 7 | 5 |
| 17-20 (y) (n) | 4 | 2 | 4 |
| n, % Male | 10, 43 | 10, 43 | 10, 43 |
| n, % on beta-blocker medication | 21, 91 | – | – |
LQTS, long QT syndrome; n, number of participants; PAQ, Physical Activity Questionnaire; SEM, standard error of the mean.
PAQ scores
PAQ scores in the LQTS and control groups are shown in Table 2. Overall, PAQ scores did not differ between LQTS and controls (LQTS: 2.31 ± 0.15 vs controls: 2.27 ± 0.18; P = 0.78). There was no difference in PAQ score between patients with LQTS on beta-blockers and those not receiving a beta-blocker (2.31 ± 0.17 vs 2.30 ± 0.25; P = 0.86).
Table 2.
PAQ scores of study subjects grouped by age and sex
| LQTS PAQ score | Control PAQ score | P value | |
|---|---|---|---|
| 2.31 ± 0.15 | 2.27 ± 0.18 | 0.78 | |
| Age (y) | |||
| 11-12 | 2.71 ± 0.23 | 3.16 ± 0.073 | 0.31 |
| 13-14 | 2.06 ± 0.29 | 2.38 ± 0.040 | 0.73 |
| 15-16 | 2.56 ± 0.32 | 1.59 ± 0.027 | 0.13 |
| 17-20 | 2.00 ± 0.20 | 1.53 ± 0.081 | 0.63 |
| Sex | |||
| Male | 2.27 ± 0.26 | 2.51 ± 0.34 | 0.49 |
| Female | 2.34 ± 0.19 | 2.08 ± 0.19 | 0.33 |
LQTS, long QT syndrome; PAQ, Physical Activity Questionnaire.
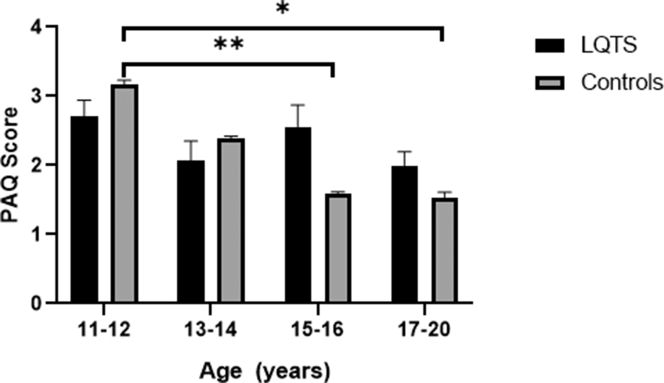
We also examined the relationship between age and PAQ scores. When we compared PAQ scores between patients with LQTS vs controls based on age (11-12, 13-14, 15-16, and 17-20 years), there was no difference in PAQ scores for each of the age ranges (P > 0.05; Table 2). However, as shown in Figure 1 and Table 3, we observed a decrease in PAQ scores with increasing age in controls, in particular between ages 11-12 vs 15-16 years (P = 0.015) and 11-12 vs 17-20 years (P = 0.005). This age-dependent decrease in PAQ scores was not observed in patients with LQTS (P > 0.05).
Figure 1.
PAQ scores of LQTS vs controls based on age. PAQ scores did not differ between LQTS vs controls based on age. PAQ scores decreased with increasing age in controls (11-12 vs 15-16, P < 0.05; 11-12 vs 17-20, P < 0.01). This age-dependent decrease in PAQ scores with increasing age was not observed in patients with LQTS (P > 0.05). ∗P < 0.05; ∗∗P < 0.01. LQTS, long QT syndrome; PAQ, Physical Activity Questionnaire.
Table 3.
Age-dependent comparisons of PAQ scores in patients with LQTS and PAQ controls
| Comparisons | Patients with LQTS P value |
PAQ controls P value |
|---|---|---|
| 11-12 vs 13-14 y | >0.05 | 0.12 |
| 11-12 vs 15-16 y | >0.05 | 0.015∗ |
| 11-12 vs 17-20 y | >0.05 | 0.0050∗ |
| 13-14 vs 15-16 y | >0.05 | 0.60 |
| 13-14 vs 17-20 y | >0.05 | 0.29 |
| 15-16 vs 17-20 y | >0.05 | >0.99 |
LQTS, long QT syndrome; PAQ, Physical Activity Questionnaire.
Indicates significance.
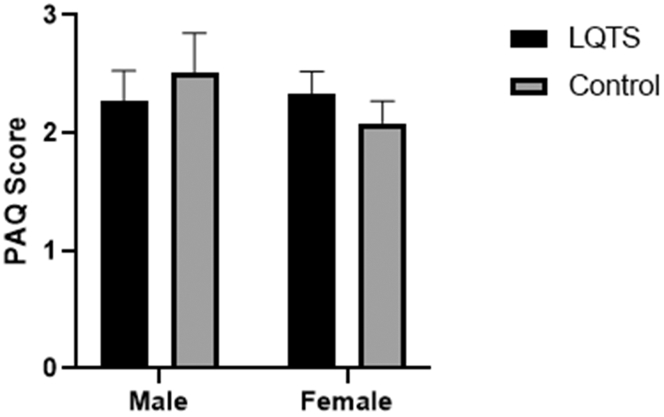
Sex (male vs female) also had no effect on PAQ scores in either the patients with LQTS (P = 0.68) or control groups (P = 0.41; Fig. 2). Similarly, as presented in Table 2, no effect of diagnosis (LQTS vs control) was observed in male PAQ scores (P = 0.49) or female PAQ scores (P = 0.33).
Figure 2.
Sex differences in PAQ scores. PAQ scores did not differ between males and females in either the LQTS or control groups (P > 0.05). LQTS, long QT syndrome; PAQ, Physical Activity Questionnaire.
Exercise testing characteristics
The 23 patients with LQTS whose PAQ scores were included in analysis also completed exercise testing within 12 months of completing the PAQ (average 2.3 ± 0.9 months lapsed between the exercise stress test and PAQ completion). Exercise characteristics of patients with LQTS and controls are summarized in Table 4. Mean ages at the time of exercise testing did not differ between the 2 groups (LQTS: 14.8 ± 0.5, exercise test controls: 14.5 ± 0.5, P = 0.31; see Table 4). There were no arrhythmias or adverse events reported with any of the exercise stress tests.
Table 4.
Exercise characteristics of study subjects
| Variable | Patients with LQTS (n = 23) | Exercise test controls (n = 23) | P value |
|---|---|---|---|
| Age (y) | 14.8 ± 0.5 | 14.5 ± 0.5 | 0.31 |
| Exercise duration (min) | 10.9 ± 0.5 | 15.1 ± 0.5 | <0.0001 |
| HR at rest (bpm) | 79.8 ± 2.5 | 79.2 ± 2.9 | 0.79 |
| HR at peak exercise (bpm) | 169 ± 4.6 | 198.3 ± 1.7 | <0.0001 |
| Blood pressure (mm Hg) | |||
| Systolic blood pressure at rest | 110.4 ± 3.0 | 114.8 ± 2.4 | 0.24 |
| Diastolic blood pressure at rest | 70.3 ± 2.2 | 71.2 ± 1.5 | 0.77 |
| Peak systolic blood pressure | 160.3 ± 4.8 | 157.2 ± 4.9 | 0.75 |
| Peak diastolic blood pressure | 73.9 ± 2.0 | 71.6 ± 1.9 | 0.33 |
bpm, beats per minute; HR, heart rate; LQTS, long QT syndrome; mm Hg, millimetre of mercury; n, number of participants.
When we compared supine blood pressure and heart rate (see Table 4), the patients with LQTS did not differ from exercise test controls in supine systolic blood pressure (LQTS: 110 ± 3 mm Hg, controls: 115 ± 2 mm Hg; P = 0.24), supine diastolic blood pressure (LQTS: 70 ± 2 mm Hg, exercise test controls: 71 ± 2 mm Hg; P = 0.77), or supine heart rate (LQTS: 80 ± 3 beats per minute [bpm], exercise test controls: 79 ± 3 bpm; P = 0.79). At peak exercise, patients with LQTS reached systolic and diastolic blood pressures that did not differ significanly from controls (peak systolic blood pressure for patients with LQTS: 160 ± 5 mm Hg vs exercise test controls: 157 ± 5 mm Hg, P = 0.75; peak diastolic blood pressure for patients with LQTS: 74 ± 2 mm Hg, exercise test controls: 72 ± 2 mm Hg, P = 0.33). However, heart rate at peak exercise was significantly decreased in patients with LQTS compared with exercise test controls (patients with LQTS: 169 ± 5 bpm, controls: 198 ± 2 bpm; P < 0.0001). Patients with LQTS on beta-blockers had a lower heart rate at peak exercise vs untreated patients with LQTS (LQTS on beta-blockers: 166.3 ± 4.5, LQTS no medications: 198.0 ± 2.0; P = 0.012). Endurance time was also significantly lower in patients with LQTS compared with exercise test controls (LQTS: 11 ± 0.5 vs controls: 15 ± 0.5 minutes, P < 0.0001). PAQ score and treadmill time were not correlated in patients with LQTS (r = 0.34, P = 0.10).
We also examined if there were any sex differences in exercise testing characteristics between patients with LQTS and exercise test controls (see Table 5). Both exercise duration and peak heart rates were significantly lower in both male and female patients with LQTS as compared with male and female controls (P < 0.01). As expected, endurance time and peak heart rate during exercise were also significantly lower in LQTS females vs control males (P < 0.0002) and in LQTS males vs female controls (P < 0.01).
Table 5.
Exercise test characteristics of patients with LQTS and controls based on sex
| Variable | Patients with LQTS | Exercise test controls | P value |
|---|---|---|---|
| N | |||
| Male | 10 | 10 | |
| Female | 13 | 13 | |
| Age (y) | |||
| Male | 14.1 ± 0.8 | 13.8 ± 0.7 | >0.99 |
| Female | 15.3 ± 0.6 | 15.0 ± 0.6 | >0.99 |
| Exercise duration (min) | |||
| Male | 11.0 ± 0.9 | 15.8 ± 0.9 | 0.0001∗ |
| Female | 10.8 ± 0.5† | 14.4 ± 0.4‡ | 0.0011∗ |
| HR at rest (bpm) | |||
| Male | 85.3 ± 3.6 | 79.2 ± 4.9 | 0.83 |
| Female | 75.6 ± 3.0 | 79.0 ± 2.7 | 0.98 |
| HR at peak exercise (bpm) | |||
| Male | 172.8 ± 7.1 | 198.9 ± 2.5 | 0.0073∗ |
| Female | 166.2 ± 6.1† | 197.0 ± 2.5‡ | 0.0002∗ |
| Blood pressure (mm Hg) | |||
| Systolic blood pressure at rest | |||
| Male | 118.3 ± 5.4 | 113 ± 4.3 | 0.92 |
| Female | 104.4 ± 2.4 | 115.8 ±2.8 | 0.13 |
| Diastolic blood pressure at rest | |||
| Male | 68.5 ± 4.5 | 69.4 ± 1.9 | >0.99 |
| Female | 71.7 ± 1.8 | 72 ± 2.2 | >0.99 |
| Peak systolic blood pressure | |||
| Male | 163.8 ± 9.2 | 157.6 ± 9.8 | 0.99 |
| Female | 157.8 ± 5.2 | 157.2 ± 4.8 | >0.99 |
| Peak diastolic blood pressure | |||
| Male | 73.1 ± 4.3 | 68.2 ± 3.3 | 0.82 |
| Female | 74.5 ± 1.8 | 73.5 ± 1.9 | >0.99 |
bpm, beats per minute; HR, heart rate; LQTS, long QT syndrome; mm Hg, millimetre of mercury.
Significance <0.05.
Significance <0.05 compared with male controls.
Significance <0.05 compared with male LQTS.
Discussion
Despite stringent guidelines, risk of sudden cardiac death, and the use of beta-blockers, our cohort of patients with LQTS report similar levels of PA as healthy controls. Our patients with LQTS demonstrated reduced exercise capacity reflected by a lower exercise duration and peak heart rate compared with our healthy controls (see Table 2). Despite this reduction in exercise capacity, it is reassuring that our LQTS patients are no different from our healthy population in terms of level of PA (see Fig. 1).
This is a novel finding that is reassuring. Bratt and Östman-Smith28 reported that children with hypertrophic cardiomyopathy reported decreased PA 1 year after diagnosis, whereas Christian et al.29 found that children with IA were involved in less moderate-to-vigorous PA compared with normative data when measured with accelerometers. As current PA recommendations in the IA population are tailored towards competitive athletes,14,30,31 there is variability in the way cardiologists approach activity restriction for the average IA child,32 which could explain the difference between these studies. For example, there are differences existing in restriction for those who are phenotypically positive vs negative.33 Despite the risk associated with competitive sport, cardiologists recognize the importance of PA for overall well-being in their patients.
In addition, there is an increasing body of evidence that activity is safe in patients with IA. In a cohort of 322 athletes with LQTS, catecholaminergic polymorphic ventricular tachycardia, hypertrophic cardiomyopathy, left ventricular noncompaction, idiopathic ventricular fibrillation, or catecholamine sensitive premature ventricular contractions, Ackerman et al.22 found that only 9 developed a nonlethal cardiac event, with 4 of them not occurring in an athletic scenario. Furthermore, in a population of 130 athletes with LQTS, Johnson and Ackerman34 report that only 1 individual experienced a cardiac event secondary to exercise. Finally, in a group of 129 athletes under the age of 21 with implantable cardioverter defibrillators, Saarel et al.35 report only 1 incidence of ventricular tachycardia/fibrillation with no events of death or arrest secondary to arrhythmia. As a result, there has been a shift in activity prescription in the past 5 years,10 with emphasis being placed on shared decision-making to promote patient autonomy.34,36 It is important to provide each patient with the available evidence while attempting to understand how each patient values his or her exercise.36
At the same time, it is well known that over half of the paediatric population worldwide is not meeting exercise recommendations and that compliance in general is low.37 Paediatric PA declines with age.6,7,37 Although the age-associated decline in PA was noted in the control group, this was not seen in the LQTS group (see Fig. 2). This is likely due to our relatively small LQTS sample size. The recommended cutoffs denoting sufficient PA for cardiovascular health are 2.9 and 2.7 for boys and girls, respectively,6 and neither of the groups met these numbers. This further highlights the need for better exercise prescription in our paediatric population, irrespective of diagnosis.
The findings in studies of beta-blockers and exercise capacity have shown mixed results.20,28 Studies by Anderson et al.20 and Beloka et al.21 have found reduced exercise capacity in those on the medications, with evidence suggesting that the effects are secondary to decreased cardiac output and oxygen delivery to working muscles.21 However, Bratt and Östman-Smith28 report no impact of beta-blockers on exercise. Our patients with LQTS demonstrated reduced exercise capacity (reduced exercise duration and heart rate at peak exercise) compared with our healthy controls (see Table 2). Previous studies in children and adolescents have reported a lack of association between PA and cardiovascular endurance particularly with light-to-moderate activity.38, 39, 40 It is possible that beta-blockers played a role in our finding, as the majority of our patients with LQTS (91%) were on beta-blockade. At the same time, their reduced exercise capacity could simply be secondary to a lack of exposure to PA. Nonetheless, despite reduced exercise capacity, our LQTS population has kept pace with our healthy controls in their level of PA.
Limitations
Although the PAQ-A/C is a validated questionnaire for the determination of PA in youth, the use of these questionnaires has significant methodological limitations. A different cohort of exercise testing controls was used rather than comparing with the exercise testing of the PAQ controls. Direct measures of PA levels and exercise capacity including accelerometry and maximal cardiopulmonary exercise testing could be used to validate our findings in the future. Recall is subjective and limited, especially in the paediatric population.6 Furthermore, PA can vary depending on the time of year.27 As it was not possible to gather all the data during the same season, there may be some seasonal variation in data. Despite this, we believe that the PAQ provides a simple time- and cost-efficient way to assess PA.27 Although our sample size is small, this is a large cohort for paediatric IA activity studies. Most of our patients with LQTS were on beta-blockade at the time of exercise testing with the exception of 2 patients. Therefore, because of the small sample size, the results of patients with LQTS on no beta-blockade should be interpreted with caution. Finally, as this was a single-centre study, the views on PA restriction by cardiologists at this centre may not reflect that of other paediatric cardiologists,32 and as such, the results may not generalize well to other populations.
Conclusions
This study is an important first step in understanding the PA and exercise capacity of the LQTS population. Despite guideline recommendations restricting PA, our patients with LQTS have similar PA levels but lower exercise capacity compared with healthy controls. Objective measurements of PA using activity trackers and of exercise capacity such as cardiopulmonary exercise testing in this population are warranted. Further work is needed to determine whether the use of the mainstay therapy of beta-blockers is responsible for the lower exercise capacity of these patients.
Acknowledgements
We would like to thank the participants for their cooperation and contribution to this study.
Ethics Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and ethical approval was obtained from the University of British Columbia Children's and Women's Health Centre of British Columbia Research Ethics Board (H16-02011).
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.RJ F. Bishop White Kennett's father. Notes Queries. 1902;s9-IX:365–366. [Google Scholar]
- 2.Sharma S., Merghani A., Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J. 2015;36:1445–1453. doi: 10.1093/eurheartj/ehv090. [DOI] [PubMed] [Google Scholar]
- 3.Kandola A., Lewis G., Osborn D.P.J., Stubbs B., Hayes J.F. Depressive symptoms and objectively measured physical activity and sedentary behaviour throughout adolescence: a prospective cohort study. Lancet Psychiatry. 2020;7:262–271. doi: 10.1016/S2215-0366(20)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulfer K., Duppen N., Kuipers I.M., et al. Aerobic exercise influences quality of life of children and youngsters with congenital heart disease: a randomized controlled trial. J Adolesc Health. 2014;55:65–72. doi: 10.1016/j.jadohealth.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Sarquella-Brugada G., Campuzano O., Iglesias A., et al. Genetics of sudden cardiac death in children and young athletes. Cardiol Young. 2013;23:159–173. doi: 10.1017/S1047951112001138. [DOI] [PubMed] [Google Scholar]
- 6.Voss C., Ogunleye A.A., Sandercock G.R. Physical Activity Questionnaire for children and adolescents: English norms and cut-off points. Pediatr Int. 2013;55:498–507. doi: 10.1111/ped.12092. [DOI] [PubMed] [Google Scholar]
- 7.Colley R.C., Garriguet D., Janssen I., et al. Physical activity of Canadian children and youth: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011;22:15–23. [PubMed] [Google Scholar]
- 8.James C.A., Bhonsale A., Tichnell C., et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. doi: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruwald A.C., Marcus F., Estes N.A., 3rd, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36:1735–1743. doi: 10.1093/eurheartj/ehv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung C.C., Laksman Z.W., Mellor G., Sanatani S., Krahn A.D. Exercise and inherited arrhythmias. Can J Cardiol. 2016;32:452–458. doi: 10.1016/j.cjca.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Gimeno J.R., Tome-Esteban M., Lofiego C., et al. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur Heart J. 2009;30:2599–2605. doi: 10.1093/eurheartj/ehp327. [DOI] [PubMed] [Google Scholar]
- 12.Mont L. Arrhythmias and sport practice. Heart. 2010;96:398–405. doi: 10.1136/hrt.2008.160903. [DOI] [PubMed] [Google Scholar]
- 13.Priori S.G., Blomstrom-Lundqvist C., Mazzanti A., et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 14.Maron B.J., Chaitman B.R., Ackerman M.J., et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109:2807–2816. doi: 10.1161/01.CIR.0000128363.85581.E1. [DOI] [PubMed] [Google Scholar]
- 15.Ackerman M.J., Zipes D.P., Kovacs R.J., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 10: The Cardiac Channelopathies: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2424–2428. doi: 10.1016/j.jacc.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Ostman-Smith I. Hypertrophic cardiomyopathy in childhood and adolescence—strategies to prevent sudden death. Fundam Clin Pharmacol. 2010;24:637–652. doi: 10.1111/j.1472-8206.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 17.Viitasalo M., Oikarinen L., Swan H., et al. Effects of beta-blocker therapy on ventricular repolarization documented by 24-h electrocardiography in patients with type 1 long-QT syndrome. J Am Coll Cardiol. 2006;48:747–753. doi: 10.1016/j.jacc.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 18.Rosso R., Kalman J.M., Rogowski O., et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:1149–1154. doi: 10.1016/j.hrthm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert-Barness E., Marshall R.J. Cardiomyopathy in children. W V Med J. 2000;96:352–356. [PubMed] [Google Scholar]
- 20.Anderson R.L., Wilmore J.H., Joyner M.J., et al. Effects of cardioselective and nonselective beta-adrenergic blockade on the performance of highly trained runners. Am J Cardiol. 1985;55:149D. doi: 10.1016/0002-9149(85)91072-0. 54D. [DOI] [PubMed] [Google Scholar]
- 21.Beloka S., Gujic M., Deboeck G., et al. Beta-adrenergic blockade and metabo-chemoreflex contributions to exercise capacity. Med Sci Sports Exerc. 2008;40:1932–1938. doi: 10.1249/MSS.0b013e31817fbe11. [DOI] [PubMed] [Google Scholar]
- 22.Turkowski K.L., Bos J.M., Ackerman N.C., Rohatgi R.K., Ackerman M.J. Return-to-play for athletes with genetic heart diseases. Circulation. 2018;137:1086–1088. doi: 10.1161/CIRCULATIONAHA.117.031306. [DOI] [PubMed] [Google Scholar]
- 23.Janz K.F., Lutuchy E.M., Wenthe P., Levy S.M. Measuring activity in children and adolescents using self-report: PAQ-C and PAQ-A. Med Sci Sports Exerc. 2008;40:767–772. doi: 10.1249/MSS.0b013e3181620ed1. [DOI] [PubMed] [Google Scholar]
- 24.Gobbi E., Elliot C., Varnier M., Carraro A. Psychometric properties of the Physical Activity Questionnaire for Older Children in Italy: testing the validity among a general and clinical pediatric population. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voss C., Dean P.H., Gardner R.F., Duncombe S.L., Harris K.C. Validity and reliability of the Physical Activity Questionnaire for Children (PAQ-C) and Adolescents (PAQ-A) in individuals with congenital heart disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duff D.K., De Souza A.M., Human D.G., Potts J.E., Harris K.C. A novel treadmill protocol for exercise testing in children: the British Columbia Children's Hospital protocol. BMJ Open Sport Exerc Med. 2017;3 doi: 10.1136/bmjsem-2016-000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalski K.C., Crocker P.R.E., Donen R.M. University of Saskatchewan; Saskatoon, SK, Canada: 2004. The Physical Activity Questionnaire for Older Children (PAQ-C) and Adolescents (PAQ-A) Manual; p. 38. [Google Scholar]
- 28.Bratt E.L., Östman-Smith I. Effects of lifestyle changes and high-dose beta-blocker therapy on exercise capacity in children, adolescents, and young adults with hypertrophic cardiomyopathy. Cardiol Young. 2015;25:501–510. doi: 10.1017/S1047951114000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian S., Somerville M., Taylor S., et al. The impact of physical activity modification on the well-being of a cohort of children with an inherited arrhythmia or cardiomyopathy. Cardiol Young. 2020;30:692–697. doi: 10.1017/S1047951120000803. [DOI] [PubMed] [Google Scholar]
- 30.Budts W., Borjesson M., Chessa M., et al. Physical activity in adolescents and adults with congenital heart defects: individualized exercise prescription. Eur Heart J. 2013;34:3669–3674. doi: 10.1093/eurheartj/eht433. [DOI] [PubMed] [Google Scholar]
- 31.Pelliccia A., Fagard R., Bjornstad H.H., et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–1445. doi: 10.1093/eurheartj/ehi325. [DOI] [PubMed] [Google Scholar]
- 32.Roston T.M., De Souza A.M., Sandor G.G., Sanatani S., Potts J.E. Physical activity recommendations for patients with electrophysiologic and structural congenital heart disease: a survey of Canadian health care providers. Pediatr Cardiol. 2013;34:1374–1381. doi: 10.1007/s00246-013-0654-z. [DOI] [PubMed] [Google Scholar]
- 33.Christian S., Somerville M., Giuffre M., Atallah J. Physical activity restriction for children and adolescents diagnosed with an inherited arrhythmia or cardiomyopathy and its impact on body mass index. J Cardiovasc Electrophysiol. 2018;29:1648–1653. doi: 10.1111/jce.13713. [DOI] [PubMed] [Google Scholar]
- 34.Johnson J.N., Ackerman M.J. Return to play? Athletes with congenital long QT syndrome. Br J Sports Med. 2013;47:28–33. doi: 10.1136/bjsports-2012-091751. [DOI] [PubMed] [Google Scholar]
- 35.Saarel E.V., Law I., Berul C.I., et al. Safety of sports for young patients with implantable cardioverter-defibrillators: long-term results of the multinational ICD sports registry. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006305. [DOI] [PubMed] [Google Scholar]
- 36.Li C.O., Roston T.M., MacEwing C., Sanatani S. To play or not to play? sports participation and shared decision-making in athletes with inherited heart rhythm disorders. Br J Sports Med. 2020;54:1126–1128. doi: 10.1136/bjsports-2019-101236. [DOI] [PubMed] [Google Scholar]
- 37.Grao-Cruces A., Sanchez-Oliva D., Segura-Jimenez V., et al. Changes in compliance with school-based physical activity recommendations in Spanish youth: The UP&DOWN longitudinal study. Scand J Med Sci Sports. 2019;29:554–565. doi: 10.1111/sms.13355. [DOI] [PubMed] [Google Scholar]
- 38.Chen W., Hammond-Bennett A., Hypnar A., Mason S. Health-related physical fitness and physical activity in elementary school students. BMC Public Health. 2018;18:195. doi: 10.1186/s12889-018-5107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aires L., Silva P., Silva G., et al. Intensity of physical activity, cardiorespiratory fitness, and body mass index in youth. J Phys Act Health. 2010;7:54–59. doi: 10.1123/jpah.7.1.54. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen P.L., Moeller N.C., Korsholm L., et al. The association between aerobic fitness and physical activity in children and adolescents: the European youth heart study. Eur J Appl Physiol. 2010;110:267–275. doi: 10.1007/s00421-010-1491-x. [DOI] [PubMed] [Google Scholar]