Abstract
In this study, the importance of plasma viscosity (PV) as a biomarker in differential diagnosis of dementia subtypes especially Alzheimer’s disease (AD) and vascular dementia (VaD) was investigated. Our study recruited 45 patients with AD, 35 patients with VaD, and control participants. Individuals with inflammatory disease, infection, heart, liver, renal failure, and with high erythrocyte sedimentation rate and C-reactive protein levels were excluded from the study. The cases underwent comprehensive geriatric assessment. The PV measurements were performed with Brookfield DV-II viscometer. The PV measurements of AD, VaD, and control groups were 1.61 ± 0.08, 1.70 ± 0.06, and 1.48 ± 0.06 mPa S, respectively. The PV levels of the dementia group were significantly higher than the control group (P < .001). When the dementia group was analyzed by itself, patients with VaD had higher PV levels than the patients with AD (P < .001). The PV is a biomarker to be used in diagnosis as well as in differentiating between the 2 most common forms of dementia which are AD and VaD.
Keywords: elderly, dementia, Alzheimer’s disease, vascular dementia, differential diagnosis
Introduction
Dementia characterized by the impairment of cognitive functions, such as memory, learning, orientation, language functions, perception, decision making, and planning, is the loss of intellectual and social abilities to a level that would hinder daily functioning of the individual. Alzheimer’s disease (AD) is the most common cause of all dementia cases affecting 50% to 70% of this population. 1 Vascular dementia (VaD) is accepted as the second most common cause according to several authors. The VaD is a form of cognitive impairment developing due to ischemic brain damage caused by cerebrovascular disease, infarction, and leukoaerosis. 2 The VaD constitutes 10% to 25% of all dementia cases. 3 Interestingly, AD and VaD have common risk factors such as hypertension, hypercholesterolemia, hyperhomocysteinemia diabetes mellitus, and apolipoprotein E. 4,5
As the treatment and follow-up procedures for AD and VaD are different in clinical practice, various guidelines and tests have been developed for differential diagnosis. Despite such efforts, as these 2 types of dementia have overlapping etiopathogenesis and findings, imaging techniques such as magnetic resonance imaging (MRI) can even be insufficient for differential diagnosis at times. 6 Techniques such as cerebrospinal fluid studies, single photon emission computed tomography, and positron emission tomography are invasive and expensive. 7,8 Hence, the need for noninvasive, practical, and cheap biochemical markers for AD and VaD is obvious.
Certain studies stated that the pathophysiologies of AD and VaD could be ethiologically linked. Inflammation of the neurons and the endothelium is the most important ethiological factor. In population-based studies, increased serum high-sensitivity C-reactive protein (hs-CRP) concentrations have been associated with poor memory, poor global cognitive performance, and VaD and AD. 9 –12
Changes in the rheological system expressing the blood and plasma viscosity (PV) are known to play important roles in the pathogenesis of atherosclerosis and several other diseases. Among the hemorheological parameters, PV was demonstrated as an independent risk factor for several diseases and primarily for atherosclerosis. 13,14 In a study that was performed, in the long-term follow-up of patients having basal PV elevations, the loss of cognitive function was higher when compared to those with normal PV. 15 There are studies showing a positive correlation between PV, cerebrovascular disease, and VaD. 16
With this study, we aimed to demonstrate whether PV can be used as a biomarker in clinical practice for the differential diagnosis of dementia subtypes especially AD and VaD.
Methods
Participants of the Study
Our cross-sectional study recruited 45 patients with AD and 35 patients with VaD over the age of 65 years admitting to geriatrics outpatient unit together with 62 age- and sex-matched individuals without dementia as a control group. Those with dementia had recently been diagnosed and had not received any treatment for dementia. For all the patients covered in the study, comprehensive geriatric assessment was performed in addition to having a detailed history and physical examination.
Patients with collagen tissue diseases, vasculitis, diabetes mellitus, infectious diseases, malignancies, hematological diseases associated with hyperviscosity, liver, kidney (those with glomerular filtration rates of 60 mL/min per 1.73 m2 or below), and heart failure, patients with erythrocyte sedimentation rate (ESR) 40 mm/h and above, CRP and fibrinogen elevations, and those using anticoagulants such as coumadin or agents such as pentoxifylline and/or trimetazidine were excluded from the study.
Information about the cases was obtained by face-to-face interviews with the patients and their close ones as well as using the hospital files. Data pertaining to age, sex, height, body weight, education level, personal history, existing treatment, and comorbidities of the patients were recorded.
Comprehensive Geriatric Assessment and Cognitive Assessment
A medical history was obtained and a physical examination was performed; a comprehensive geriatric assessment including activities of daily living ([ADLs] 17 lower scores indicate less ability), instrumental activities of daily living ([IADLs] 18 lower scores indicate poorer ability), Mini-Mental State Examination (MMSE), 19 clock drawing test, 20 and Yesavage Geriatric Depression Scale (GDS) 21 was performed. We made the initial diagnosis of dementia based on Diagnostic and statistical Manual of Mental Disorder (Third Edition Revised) criteria, with the diagnosis of AD being based on the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria 22 and VaD on the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement (NINDS-AIREN) criteria. 23 After cognitive assessment, neuroimaging was performed with MRI. The Clinical Dementia Rating (CDR) Scale 24 was used to determine the severity of dementia (1: early stage, 2: intermediate stage, and 3: advanced stage). Hachinski ischemic scores 25 were calculated for all the patients. A skilled team consisting of geriatricians and a psychologist performed the cognitive assessment. This team arrived at diagnoses of AD and VaD after performing the cognitive tests and applying the criteria explained above. All patients in the dementia group underwent MRI to rule out other intracranial pathologies and support the diagnosis.
Laboratory Tests
Blood samples were obtained using venipuncture of the antecubital vein after an overnight fast and analyzed immediately. For both the groups, complete blood count, liver and renal function tests, serum electrolytes, lipid profile, thyroid function tests, ESR, CRP, vitamin B12, folic acid, fibrinogen, lipoprotein a, and homocysteine levels were measured. The CRP levels were measured with rate nephelometry and plasma proteins were measured by the Biuret method. Fibrinogen measurement was performed by radial immunodiffusion. Turbidimetric immunoassay was used to determine the lipoprotein a levels. Quantitative measurements of homocysteine levels were performed using a fluorescence polarization immunoassay. Serum folic acid and vitamin B12 were measured using electrochemiluminescence immunoassay.
Plasma Viscosity Sampling
A blood sample was placed into a tube containing EDTA anticoagulant for viscosity analysis. Measurement of PV was performed using Brookfield DV-II viscometer (Brookfield, Stoughton, Massachusetts). One milliliter of plasma sample was separated by centrifugation at 3000 rpm for 10 minutes and used for viscosity measurement. Viscosity was measured at shear rates of 100, 20, and 5/s at 37°C. The sample was analyzed in duplicates and the mean viscosity was determined. The intraassay coefficient of variation for the viscometer was 0.33%. In the method that we used, PV was expressed in millipascal second (mPa S).
The hospital ethics committee approved the study protocol and all patients gave informed consents. Informed consent was taken from relatives when the patient with dementia could not give an informed consent.
Statistical Analysis
SPSS (version 15.0; SPSS Inc, Chicago, Illinois) statistical package program was used for the evaluation of data. The results were expressed as mean ± standard deviation (mean ± SD) and median (minimum–maximum). T test was used in the variance analysis of intergroup continuous variables. Levene test was used for post hoc analysis; the variables with P >.05 were further analyzed with HSD, and those having P < .05 with Tamhane’s T2 test. In the correlation of geriatric assessment test scores (MMSE, ADLs, IADLs, and GDS) with PV, Spearman correlation coefficient (r) was calculated. Receiver–operator characteristic (ROC) analysis was used to determine the sensitivity, and specificity of PV in defining dementia and dementia types. Cutoff values, sensitivity and specificity ratios, and area under ROC curve were all calculated. A P value of .05 or less was considered to be statistically significant.
Results
The mean ages of AD, VaD, and control groups were calculated as 76.4 ± 7.8, 77.2 ± 7.7, and 75.5 ± 4.8 years, respectively (Table 1). The groups had similar age and sex features. Other demographical features are expressed in Table 1.
Table 1.
Demographical Features of the Patients.
| AD (n = 45) | VaD (n = 35) | Control (n = 62) | P Value a | |
|---|---|---|---|---|
| Age, mean ± SD | 76.4 ± 7.8 | 77.2 ± 7.7 | 75.5 ± 4.8 | .7 .6 |
| Female/Male, n (%) | 24/21 (53/47%) | 24/11 (69/31%) | 33/29 (53/47%) | .6 .3 |
| BMI, mean ± SD | 24.5 ± 4.4 | 24.4 ± 4.3 | 23.5 ± 2.6 | .2 .9 |
| HT, n (%) | 35 (78%) | 32 (91%) | 49 (79%) | .8 .1 |
| Hyperlipidemia, n (%) | 16 (36%) | 15 (43%) | 24 (39%) | .9 .6 |
| History of CVI, n (%) | 3 (7%) | 12 (34%) | 0 | <.001 .003 |
| History of depression, n (%) | 4 (9%) | 8 (23%) | 4 (7%) | .2 .1 |
| Illiterate, n (%) | 11 (24%) | 9 (26%) | 10 (17%) | .07 .2 |
| Family history of dementia, n (%) | 11 (24%) | 10 (29%) | 5 (8%) | .008 .8 |
Abbreviations: AD, Alzheimer’s disease; BMI, body mass index (kg/m2); CVI, cerebrovascular incident; HT, hypertension; SD, standard deviation; VaD, vascular dementia.
a The first line is the P value when patients with dementia (AD + VaD) are compared to the controls; the second line is the P value when dementia patients are compared among themselves (AD and VaD).
For the AD, VaD, and control groups included in the study, mean ± SD, median, and P values of the biochemical parameters as well as MMSE, ADLs, IADLs, GDS, and Hachinski scores are given in Table 2.
Table 2.
Geriatric Evaluation Tests of the Cases and Laboratory Results.
| Geriatric Evaluation Tests | AD (n = 45) | VaD (n = 35) | Control (n = 62) | P Value a |
|---|---|---|---|---|
| MMSEs, mean ± SD | 15.6 ± 8.5 | 17.1 ± 6.9 | 25.6 ± 4.1 | <.001 .7 |
| ADLs median (range) | 16 (12-18) | 17 (13-18) | 18 (16-18) | .1 .5 |
| IADLs median (range) | 12 (8-14) | 14 (10-17) | 19 (17-24) | <.001 .9 |
| GDS median (range) | 7 (0-10) | 2 (0-6) | 3 (0-8) | .7 .1 |
| Hachinski score (median) | 3 (2-5) | 8 (6-17) | 1 (1-3) | <.001 <.001 |
| Laboratory results, mean ± SD | ||||
| Hematocrit % | 40.8 ± 3.6 | 39.5 ± 3.2 | 40.5 ± 3.0 | .7 .09 |
| Platelet, 103/µL | 267.7 ± 84.5 | 285.5 ± 87.2 | 256.7 ± 65.2 | .1 .4 |
| Total cholesterol, mg/dL | 184.6 ± 47.1 | 192.9 ± 43.9 | 196.1 ± 18.4 | .2 .4 |
| Low-density lipoprotein cholesterol, mg/dL | 109.3 ± 35.9 | 115.00 ± 32.6 | 118.9 ± 13.7 | .1 .5 |
| High-density lipoprotein cholesterol, mg/dL | 48.4 ± 16.6 | 49.8 ± 15.9 | 45.6 ± 12.2 | .2 .7 |
| Triglyceride, mg/dL | 130.5 ± 56.7 | 137.5 ± 63.9 | 153.9 ± 34.1 | .02 .6 |
| Total protein, g/L | 7.2 ± 0.4 | 7.3 ± 0.3 | 7.5 ± 0.3 | .007 .4 |
| Albumin, g/L | 3.81 ± 0.2 | 3.86 ± 0.3 | 3.9 ± 0.3 | .05 .4 |
| ESR, mm/h | 22.8 ± 10.5 | 24.8 ± 9.5 | 20.5 ± 6.8 | .007 .2 |
| CRP, mg/dL | 0.44 ± 0.35 | 0.48 ± 0.32 | 0.43 ± 0.33 | .1 .6 |
| Homocysteine, μmol/L | 12.7 ± 5.7 | 14.6 ± 5.3 | 12.2 ± 4.6 | .2 .09 |
| Fibrinogen, mg/dL | 3.0 ± 0.5 | 3.4 ± 0.6 | 2.8 ± 0.4 | <.001 .006 |
| Lipoprotein a, mg/dL | 17.8 ± 3.1 | 20.3 ± 3.4 | 16.3 ± 5.1 | .06 .08 |
| Thyroid-stimulating hormone, µIU/mL | 2.5 ± 1.4 | 2.1 ± 1.5 | 2.6 ± 1.3 | .2 .1 |
| Vitamin B12, pg/mL | 319.5 ± 230.5 | 406.9 ± 383.1 | 326.6 ± 97.3 | .2 .8 |
| Folic acid, ng/mL | 12.1 ± 4.4 | 12.9 ± 3.4 | 10.0 ± 4.7 | .1 .1 |
| PV, mPa s | 1.61 ± 0.08 | 1.70 ± 0.06 | 1.48 ± 0.06 | <.001 <.001 |
Abbreviations: AD, Alzheimer’s disease; MMSEs, Mini-Mental State Examination score; ADLs, activities of daily living score; IADLs, instrumental activities of daily living score; GDSs, Geriatric Depression Scale score; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PV, plasma viscosity; SD, standard deviation; VaD, vascular dementia.
a The first line is the P value when patients with dementia (AD + VaD) are compared to the controls; the second line is the P value when dementia patients are compared among themselves (AD and VaD).
The PV values for AD, VaD, and control groups were 1.61 ± 0.08, 1.70 ± 0.06, and 1.48 ± 0.06 mPa S, respectively. The PV levels of patients with dementia were significantly higher than the controls (P < .001).When the dementia group was evaluated within itself, patients with VaD had higher PV values than those with AD (P < .001; Table 2, Figure 1).
Figure 1.
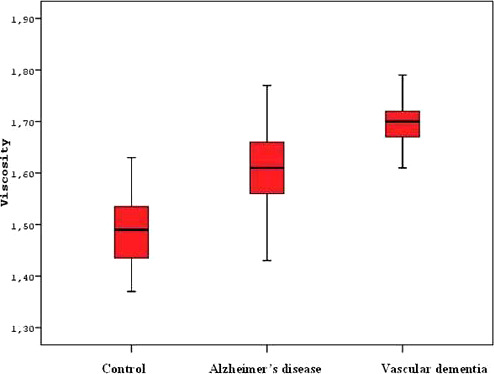
Comparison of PV in AD, VaD, and controls
The PV levels were in negative correlation with MMSE, ADLs, and IADLs scores statistically (r = −.62, r = −.39, and r = −.41, respectively, P < .001 for all). There was a positive correlation with Hachinski score and PV (r = .40, P < .001). There was no significant correlation between GDS score and PV (r = .05, P = .5).
Those variables with significant P values that could influence PV in univariate analysis were analyzed using multivariate analysis. The high levels of PV in dementia was shown to be independent of all other variables (odds ratio [OR]: 2420 [1.012-3.651], P < .001; Table 3). In multivariate analysis, PV was identified as the factor increasing the dementia risk. For each 0.001 unit increase in PV, OR for dementia risk was calculated as 1.40 (1.23-1.60; P < .0001).
Table 3.
Univariate and Multivariate Analysis Results of Biochemical Parameters
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| P Value | OR | P Value | OR | |
| Triglyceride | .02 | 1.00 (0.992-1.021) | .07 | 1.011 (0.999-1.021) |
| Total protein | .002 | 2.012 (1.657-2.618) | .8 | 1.272 (0.938-1.696) |
| Albumin | .007 | 1.223 (0.927-1.512) | .3 | 1.156 (0.913-1.352) |
| ESR | .002 | 0.936 (0.897-0.976) | .08 | 0.922 (0.843-1.003) |
| Fibrinogen | <.001 | 1.154 (0.964-1.367) | .7 | 1.312 (1.156-1.487) |
| PV | <.001 | 3.120 (1.371-5.427) | <.001 | 2.420 (1.012-3.651) |
Abbreviations: ESR, erythrocyte sedimentation rate; PV, plasma viscosity; OR, odds ratio.
Based on the CDR scores, 56% of the patients were at early stage (n = 45), 25% were at intermediate stage (n = 20), and 19% (n = 15) were at advanced stage. Means of PV and its SDs were calculated based on CDR stages (1.65 ± 0.08, 1.64 ± 0.08, and 1.68±0.11, respectively). When P values of early stage patient were compared to those of intermediate and advanced stages, they were found as 0.9 and 0.6, respectively. When the P value of PV for intermediate stage patients was compared to that of advanced stage patients, the figure was 0.5. In conclusion, there was no statistically significant correlation between the severity of the dementia and the PV.
In ROC analysis, PV values higher than 1.54 have 91% sensitivity and 82% specificity for differentiating cases with dementia from nondementia, and the value of area under curve is 0.944 (Figure 2). The PV values higher than 1.66 have a 77% sensitivity and 76% specificity for differentiating AD from VaD, and the value of area under curve is 0.805 (P < .001).
Figure 2.
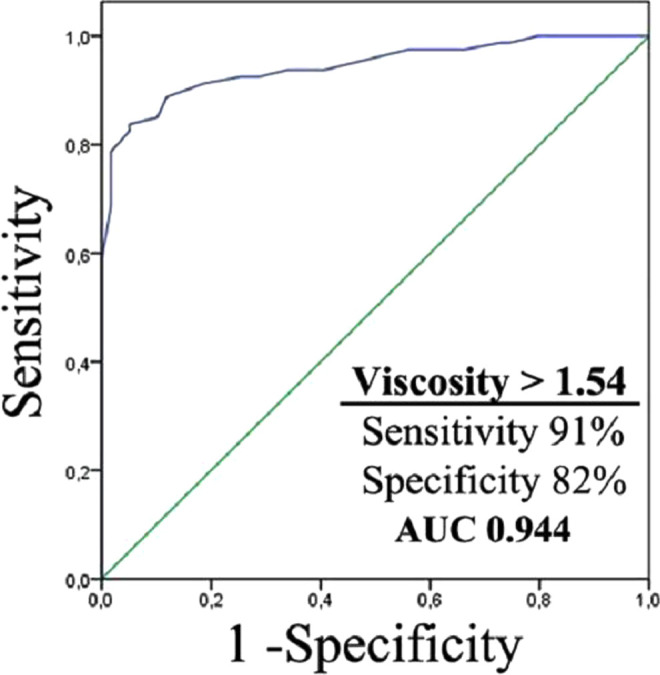
ROC curve of PV in patients with dementia
Discussion
In the elderly population, AD is the most common cause of dementia with a frequency of 50% to 70%. The VaD is responsible for 10% to 25% of all dementia cases. 3 We definitely need noninvasive, practical, and cheap biochemical markers for the diagnosis and differential diagnosis of dementia.
In this study, we tested the PV measurement for the patients with dementia and for controls without dementia. We identified that elevations of PV was an independent risk factor for dementia (Table 3). In patients with dementia, PV was higher than controls without dementia. Furthermore, PV of patients with VaD were higher than the patients with AD (Table 2, Figure 1). In our study, there was no correlation with the clinical severity of dementia and PV.
The sensitivity and specificity of the cutoff values found for differentiating cases with dementia from nondementia was higher than the values found for differentiating VaD from AD, but both the results were statistically significant. These results showed that measurement of PV can be useful in diagnosis and differential diagnosis of dementia in clinical practice.
Together with aging, we see a decrease in cognitive functions; AD, VaD, and other degenerative dementia are influenced by different ethiopatogenetic factors.
Inflammatory mechanisms can be responsible for cognitive impairment and dementia. 26 The CRP has been found in and around β amyloid plaques in the brains of the patients with dementia. 27 Systemic low-grade inflammation and increased serum concentrations of hs-CRP have been associated with impaired cognition and an increased risk of VaD and AD in some follow-up studies. 9,11,28,29
Inflammation-related decreases in cerebral vascular perfusion and neuronal degeneration that results from this seem to be a common pathophysiological process for AD and VaD. It is obvious that several factors are responsible for this pathophysiology. Changes seen in rheological system is one of these. Hemorheological disturbances result in several changes both in the vascular wall and in the blood that circulates within this structure. As a result, microcirculation decreases in these tissues. 30 Decreases in the microcirculation lead to hypoxia. Hypoxia influences a wide range of mitogenic and fibrogenic effects and modifies the expression of genes involved in angiogenesis and capillary permeability, vasomotor response, glycolysis, matrix metabolism, and cell survival. 31 Lowe identified that in determining the blood flow in cerebral microcirculation, PV had a more important role than hematocrit and related blood viscosity. 32
Marioni et al conducted a study with 5-year follow-up with 2312 participants at the age of 50 to 80. They investigated the effects of CRP, fibrinogen, hematocrit, and PV on cognitive functions. Baseline PV was negatively correlated with the follow-up test scores for general cognitive ability. 15 In our study, at the low scores of MMSE which showed cognitive function, PV was identified to be high. This negative correlation was statistically significant.
In a study by Schneider et al, PV was shown to increase significantly in subcortical arteriosclerotic encephalopathy (Binswangers disease) and this increase hampered the microcirculation leading to progressive degeneration of cerebral white matter. 33 Similar to the results of this study, our study had higher PV values in VaD group compared to AD and control groups (Table 2, Figure 1).
Based on Hachinski ischemic staging, having a score of 7 or above favors VaD. 25 In our study, patients with VaD had a median Hachinski staging score of 8. Having a significant correlation between PV and Hachinski ischemic score was in accordance with the significant correlation between PV and VaD.
In our study, there was no significant correlation between stages showing the clinical severity of dementia and PV. This result supported the fact that high levels of PV could play a role in the pathogeneses of AD and especially of VaD; yet after the appearance of dementia symptoms, it does not play an active role in the progression of the disease. In order to identify the importance of PV in the progression of dementia, we need studies with the participation of larger number of patients in a controlled manner with long-term follow-up. These studies will also shed light into understanding the role of treatments geared at decreasing PV in dementia.
Changes in PV are associated with the changes in serum proteins mainly seen as decreases in albumin or decreases and increases in globulins. 34 Individuals having plasma protein abnormalities that might influence PV were not included in the study. Despite this intervention, total protein and albumin levels were low in the dementia group. In multivariate analysis, PV was shown to be high in dementia independent of the total protein and albumin levels (Table 3).
Fibrinogen is the most important protein responsible for PV. 35 Oijen et al demonstrated the association of high fibrinogen levels with AD and VaD. 36 Chang et al conducted a study in 21 patients with AD and 23 control participants; the blood viscosity and fibrinogen were found to be higher in this disease group than the control group. 37 The PV is influenced by fibrinogen to a significant degree, as we know that several other pathologies could influence the fibrinogen levels, we excluded the patients having high fibrinogen measurements from our study. Despite the fact that fibrinogen was within the normal range in our study, it was higher in VaD group than AD and control groups. In multivariate analysis, PV was shown to be high in dementia and VaD independent of fibrinogen (Table 3).
The PV was not affected by the changes in blood cells (anemia and polycythemia), age, or gender like ESR. 38 Although PV is known as acute inflammatory marker, some studies showed that PV can be elevated in some chronic diseases like CRP 39 ,40 The elevation of PV in dementia and especially in VaD can be interpreted, like elevation of CRP in coronary heart disease.
The potential limitation of this study should also be addressed. Sectional studies like ours are inadequate in identifying causality relationships when compared to longitudinal studies. In order to understand whether elevated PV is the cause or the result of dementia, we need long-term prospective studies with higher patient participation. Furthermore, the demographical data of our study group were obtained from the patients themselves or their close ones. Hence, the possible error related to demographical data should also be taken into account.
We used accepted guidelines and appropriate laboratory tests for diagnosis of dementia, but the differential diagnosis of dementia is a hard job. The definite diagnosis of dementia can only be made by pathological evaluation of the brain tissue. However, data from the clinical studies showed that autopsy findings in AD cases can confirm clinical diagnosis in a ratio of 80% to 90%. 41,42
Conclusion
This study showed that, PV which can be measured in peripheral blood samples is a simple and cheap biomarker that can support the clinical and imaging findings of dementia. This is true both for diagnosis of dementia and for the differential diagnosis of AD and VaD which are the 2 most commonly observed forms of dementia. However, this finding should be tested and supported in larger case series including patients with other types of dementia in a prospective study design.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15(4):169–173. [DOI] [PubMed] [Google Scholar]
- 2. Román GC. Advances in nosology, diagnosis, treatment and prevention. Panminerva Med. 2004;46(4):207–215. [PubMed] [Google Scholar]
- 3. Fratiglioni L, Launer LJ, Andersen K, et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurology. 2000;54(11 suppl 5):10–15. [PubMed] [Google Scholar]
- 4. Mielke MM, Zandi PP. Hematologic risk factors of vascular disease and their relation to dementia. Dement Geriatr Cogn Disord. 2006;21(5-6):335–352. [DOI] [PubMed] [Google Scholar]
- 5. Nilsson K, Gustafson L, Hultberg B. Plasma homocysteine and cognition in elderly patients with dementia or other psychogeriatric diseases. Dement Geriatr Cogn Disord. 2010;30(3):198–204. [DOI] [PubMed] [Google Scholar]
- 6. Logue MW, Posner H, Green RC, et al. Magnetic resonance imaging-measured atrophy and its relationship to cognitive functioning in vascular dementia and Alzheimer's disease patients. Alzheimers Dement. 2011;7(5):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galasko D. Biomarkers for Alzheimer's disease clinical needs and application. J Alzheimers Dis. 2005;8(4):339–346. [DOI] [PubMed] [Google Scholar]
- 8. Masdeu J. Neuroimaging in Alzheimer's disease: an overview [in Spanish]. Rev Neurol. 2004;38(12):1156–1165. [PubMed] [Google Scholar]
- 9. Teunissen CE, van Boxtel MP, Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134(1-2):142–150. [DOI] [PubMed] [Google Scholar]
- 10. Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. [DOI] [PubMed] [Google Scholar]
- 11. Ravaglia G, Forti P, Maioli F, et al. Blood inflammatory markers and risk of dementia: the conselice study of brain aging. Neurobiol Aging. 2007;28(12):1810–1820. [DOI] [PubMed] [Google Scholar]
- 12. Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61(5):668–672. [DOI] [PubMed] [Google Scholar]
- 13. Lee AJ, Smith WC, Lowe GD, Tunstall-Pedoe H. Plasma fibrinogen and coronary risk factors: the Scottish Heart Health Study. J Clin Epidemiol. 1990;43(9):913–919. [DOI] [PubMed] [Google Scholar]
- 14. Resch KL, Ernst E, Matrai A, Paulsen HF. Fibrinogen and viscosity as risk factors for subsequent cardiovascular events in stroke survivors. Ann Intern Med. 1992;117(5):371–375. [DOI] [PubMed] [Google Scholar]
- 15. Marioni RE, Stewart MC, Murray GD, et al. Peripheral levels of fibrinogen, C-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosom Med. 2009;71(8):901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tikhomirova IA, Oslyakova AO, Mikhailova SG. Microcirculation and blood rheology in patients with cerebrovascular disorders. Clin Hemorheol Microcirc. 2011;49(1-4):295–305. [DOI] [PubMed] [Google Scholar]
- 17. Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14(suppl 5):116–118. [DOI] [PubMed] [Google Scholar]
- 18. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 20. Stähelin HB, Monsch AU, Spiegel R. Early diagnosis of dementia via a two-step screening and diagnostic procedure. Int Psychogeriatr. 1997;9(suppl 1):123–130. [DOI] [PubMed] [Google Scholar]
- 21. Yesevage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 22. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 23. Romàn GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. [DOI] [PubMed] [Google Scholar]
- 24. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 25. Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632–637. [DOI] [PubMed] [Google Scholar]
- 26. McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22(6):799–809. [DOI] [PubMed] [Google Scholar]
- 27. Kuo HK, Yen CJ, Chang CH, et al. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4(6):371–380. [DOI] [PubMed] [Google Scholar]
- 28. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 29. Tilvis RS, Kähönen-Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–274. [DOI] [PubMed] [Google Scholar]
- 30. Ernst E, Weihmayr T, Schmid M, Baumann M, Matrai A. Cardiovascular risk factors and hemorheology. Physical fitness, stress and obesity. Atherosclerosis. 1986;59(3):263–269. [DOI] [PubMed] [Google Scholar]
- 31. Irace C, Scarinci F, Scorcia V, et al. Association among low whole blood viscosity, haematocrit, haemoglobin and diabetic retinopathy in subjects with type 2 diabetes. Br J Ophthalmol. 2011;95(1):94–98. [DOI] [PubMed] [Google Scholar]
- 32. Lowe GD. Is sticky blood a treatable determinant of cognitive decline and of dementia? Age Ageing. 2001;30(2):101–103. [DOI] [PubMed] [Google Scholar]
- 33. Schneider R, Ringelstein EB, Zeumer H, Kiesewetter H, Jung F. The role of plasma hyperviscosity in subcortical arteriosclerotic encephalopathy (Binswanger's disease). J Neurol. 1987;234(2):67–73. [DOI] [PubMed] [Google Scholar]
- 34. Lowe GD, Wood DA, Douglas JT, et al. Relationships of plasma viscosity, coagulation and fibrinolysis to coronary risk factors and angina. Thromb Haemost. 1991;65(4):339–343. [PubMed] [Google Scholar]
- 35. Rosenson RS, McCormick A, Uretz EF. Distribution of blood viscosity values and biochemical correlates in healthy adults. Clin Chem. 1991;42(8 pt 1):1189–1195. [PubMed] [Google Scholar]
- 36. van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36(12):2637–2641. [DOI] [PubMed] [Google Scholar]
- 37. Chang CY, Liang HJ, Chow SY, Chen SM, Liu DZ. Hemorheological mechanisms in Alzheimer's disease. Microcirculation. 2007;14(6):627–634. [DOI] [PubMed] [Google Scholar]
- 38. Ng T. Erythrocyte sedimentation rate, plasma viscosity and C-reactive protein in clinical practice. Br J Hosp Med. 1997;58(10):521–523. [PubMed] [Google Scholar]
- 39. Memeh CU. The relationship between body weight and plasma viscosity in hypertensive diabetic Nigerians. J Hypertens. 1990;8(8):711–714. [DOI] [PubMed] [Google Scholar]
- 40. Yetkin O, Tek I, Yetkin F, Numanoglu N. Role of pleural viscosity in the differential diagnosis of exudative pleural effusion. Respirology. 2007;12(2):267–271. [DOI] [PubMed] [Google Scholar]
- 41. Dickson DW. Neuropathological diagnosis of Alzheimer's disease: a perspective from longitudinal clinicopathological studies. Neurobiol Aging. 1997;18(suppl 4):21–26. [DOI] [PubMed] [Google Scholar]
- 42. DeKosky ST, Harbaugh RE, Schmitt FA, et al. Cortical biopsy in Alzheimer's disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations. Intraventricular Bethanecol Study Group. Ann Neurol. 1992;32(5):625–632. [DOI] [PubMed] [Google Scholar]


