Abstract
The aim of this study was to retrospectively determine clinical features, treatment outcomes, and overall survival in four patients with metastatic retinoblastoma at presentation. The mean age at diagnosis was 63 months (range: 24–108 months). Three patients had overt orbital disease of at least one eye and one patient had microscopic orbital disease with scleral infiltration on histopathology. Metastatic sites included regional lymph nodes (RLN) (n = 4), bone marrow (BM) (n = 2), and cerebrospinal fluid (CSF) (n = 1). The most common sites of RLN were ipsilateral preauricular nodes (two patients) and contralateral parotid gland involvement (one patient). The treatment administered included primary enucleation (n = 1), high-dose intravenous chemotherapy (n = 4), secondary enucleation (n = 2), orbital external beam radiotherapy (n = 3), and intrathecal chemotherapy (n = 1). High-risk features included massive choroidal and microscopic scleral infiltration in the eye that underwent primary enucleation. At a mean follow-up of 33 months (range, 4–68 months), one patient with CSF involvement deceased in 4 months. The remaining three patients were alive and disease-free at the last mean follow-up period of 43 months (range, 18–68 months). The results of our study showed that RLN and BM metastasis respond well to treatment while CSF metastasis is associated with poor prognosis.
Keywords: Bone marrow, chemotherapy, metastatic, orbital retinoblastoma, retinoblastoma, survival
Introduction
Retinoblastoma (RB) is the most common intraocular tumor in children accounting for approximately 11% of cancers occurring in the 1st year of life, with 95% diagnosed before 5 years of age.[1] In developed countries, the goal of treatment has shifted from globe salvage to vision preservation; whereas in underdeveloped countries which account for more than 90% of RB children, the intraocular tumor often goes undiagnosed and presents with advanced disease threatening the globe.[2,3] The key to globe salvage and vision preservation in RB depends on early diagnosis and appropriate treatment while untreated RB has a high rate of mortality due to systemic metastasis with disease progression.[4]
The observed worldwide survival outcomes of RB range from 40% in low-income countries to 79% in upper-middle-income nations.[5] In developing countries, delayed diagnosis, poor socioeconomic factors, and late presentation can lead to children presenting with metastatic features, the frequency ranging from 9% to 11%.[6] The presenting features of metastasis are variable and frequently involve the lymph nodes, bone and bone marrow (BM), central nervous system (CNS), and the liver.[6] Factors predicting increased risk for metastatic RB include invasion of the uvea, optic nerve, and orbit.[7] Other risk factors predisposing to metastasis are genetics, age of the patient, gender, laterality, treatment, and the histopathology of the tumor.[7] Primary treatment modalities for intraocular RB range from enucleation, intravenous chemotherapy (IVC), intra-arterial chemotherapy, and focal therapy depending on the stage of tumor, while advanced cases with extraocular extension may require neoadjuvant high dose IVC, extended enucleation, orbital external beam radiotherapy (EBRT), and rarely intrathecal chemotherapy for CNS involvement.[8] However, the survival of children with metastatic RB is still dismal, even with the advent of newer therapies such as high-dose chemotherapy (HDC) and supplemented with stem cell rescue.[9] Metastatic RB can be present at initial presentation or can develop during the course of treatment or sometimes rarely after completion of RB treatment. In our study, we aim to analyze the clinical and histopathological features, treatment outcomes, and survival of children diagnosed with metastatic RB at presentation at a referral RB center in India.
Case Series
The Institutional Review Board approval was obtained for the study. The study was conducted at The Operation Eyesight Universal Institute for Eye Cancer, L V Prasad Eye Institute, Hyderabad, India. All records of RB patients who underwent cerebrospinal fluid (CSF) cytology and BM aspiration and/or fine-needle aspiration cytology (FNAC) from regional lymph nodes (RLN) between 2016 and 2020 were reviewed. As a protocol at our institute, all RB patients with Group E RB and those with overt orbital tumors undergo routine CSF cytology and BM aspiration. Lymph node examination is done in every patient. If any patient has enlarged RLN, FNAC is done from the RLN to confirm inflammatory versus metastatic etiology. Those patients with RLN or CSF or BM metastasis at presentation were included in this study. Those who developed metastasis during treatment or after completion of RB treatment were excluded from this study.
Demographic, clinical, histopathologic, and treatment details were noted of these patients. Tumors were classified based on the International Classification of Intraocular RB[10] and International Retinoblastoma Staging System.[11] The treatment protocol for metastatic RB included six cycles of HDC (with vincristine, etoposide, and carboplatin) followed by enucleation, orbital EBRT, and six more cycles of HDC. If the child had RLN metastasis, EBRT was given to the RLN as well and if the child had CSF metastasis, intrathecal chemotherapy (with methotrexate, cytarabine, and hydrocortisone) was given along with HDC. The outcome (alive or dead) at the last follow-up was also noted.
Of 875 RB patients seen during the study period, a total of 653 (75%) patients underwent CSF cytology and BM aspiration in view of advanced RB and 15 (2%) patients underwent additional FNAC from RLN due to enlarged lymph nodes. Of these, five (0.6%) patients had RLN metastasis, three (0.3%) had BM metastasis, and two (0.2%) had CSF metastasis. In total, six (0.7%) patients had metastasis due to RB. Of these six patients, one patient had undergone primary treatment of RB elsewhere before presenting to us and one patient developed metastasis after completion of RB treatment. Both these patients were excluded from the study and the remaining four patients who had metastatic RB at presentation were included for further analysis [Table 1].
Table 1.
Our experience with metastatic retinoblastoma in four patients
| Case | Age/gender | Duration of symptoms (months) | Tumor laterality | Worst eye tumor extent | Enlarged RLN | FNAC from RLN | BM aspirate | CSF cytology | Systemic HDC | EBRT | Enucleation | Follow-up duration (months) | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6/female | 72 | B/L | Optic nerve infiltration | Ipsilateral PAN | Positive | Negative | Negative | 12 | Yes | Secondary | 18 | Alive |
| 2 | 2/male | 18 | U/L | Extrascleral tumor extension | Ipsilateral PAN | Positive | Positive | Negative | 12 | Yes | Secondary | 68 | Alive |
| 3 | 4/male | 6 | U/L | Optic nerve infiltration | Bilateral SMN | Negative | Positive | Positive | 4 | - | - | 4 | Dead |
| 4 | 9/female | 24 | U/L | Massive choroidal and scleral infiltration | Contralateral parotid gland and PAN | Positive | Negative | Negative | 12 | Yes | Primary | 42 | Alive |
U/L: Unilateral, B/L: Bilateral, FNAC: Fine-needle aspiration cytology, RLN: Regional lymph nodes, PAN: Preauricular lymph nodes, SMN: Submandibular lymph nodes, BM: Bone marrow, CSF: Cerebrospinal fluid, HDC: High-dose chemotherapy, EBRT: External beam radiotherapy
Case 1
A 6-year-old girl presented with a history of leukocoria in the right eye (RE) since birth. On examination, the child had bilateral RB with optic nerve tumor infiltration in the RE and tumor filling the globe in the left eye (LE). The child also had right-sided preauricular lymph node (PAN) enlargement. FNAC from PAN revealed metastatic deposits in the RLN. BM and CSF were negative for any atypical cells. Based on these, the child was diagnosed to have Stage IIIB tumor RE and Group E tumor LE. The child received six cycles of HDC followed by RE enucleation, EBRT to the right orbit and PAN region, and six more cycles of HDC. Histopathology of the enucleated eye revealed no evidence of any tumor infiltration in the choroid, optic nerve, or sclera. The child did well and is alive at 18 months of follow-up.
Case 2
A 2-year-old boy presented with a history of leukocoria in RE for 18 months. On examination, the child had fungating mass with extraocular tumor extension of RB RE and a normal LE. The child also had right-sided PAN enlargement and FNAC from the PAN revealed metastatic tumor deposits. BM aspirate also revealed metastatic deposits while CSF was negative for any atypical cells. Based on these, the child was diagnosed to have Stage IVA RB. The child received six cycles of HDC followed by enucleation of RE enucleation, EBRT to the right orbit and PAN region, and six more cycles of HDC. Histopathology of the enucleated eye revealed no evidence of any tumor infiltration in the choroid, optic nerve, or sclera. The child did well and is alive at 68 months of follow-up.
Case 3
A 4-year-old old boy presented with a history of leukocoria in RE for 6 months. On examination, the child had RB RE with optic nerve tumor infiltration and a normal LE. The child also had bilateral submandibular lymph node (SMN) enlargement but FNAC from the SMN revealed only inflammatory cells. BM aspirate and CSF cytology revealed metastatic deposits. Based on these, the child was diagnosed to have Stage IVB RB. The child was started on HDC along with intrathecal chemotherapy, but the child succumbed to the disease after four cycles of chemotherapy due to disease progression.
Case 4
A 9-year-old girl presented with complaints of decreased vision in the RE for 2 years. On examination, the child had an enlarged right globe with tumor filling the globe and LE was normal. There was no evidence of optic nerve or extraocular tumor extension on orbital imaging. The child also had enlarged contralateral parotid gland along with enlarged PAN. FNAC from the parotid gland revealed metastatic deposits. BM and CSF analyses were normal with no evidence of any atypical cells. Based on these, the child was diagnosed to have Stage IIIB RB. The child underwent RE primary enucleation in view of painful blind eye. Histopathology revealed tumor necrosis with necrosed tumor involving the cornea, iris, ciliary body, choroid, prelaminar optic nerve, and sclera. The child received six cycles of HDC followed by EBRT to the right orbit and contralateral parotid and PAN region, and six more cycles of HDC. The child did well and is alive at 42 months of follow-up. Figure 1a-d highlights the illustrative case example of metastatic RB to RLN, CSF, and BM.
Figure 1.
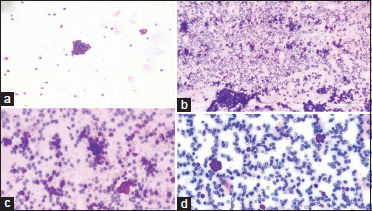
(a) Photomicrograph of cerebrospinal fluid cytology showed cluster of atypical round cells; Giemsa satin, ×100, (b and c) photomicrograph of lymph node aspirates showed cellular smear with cluster of atypical round cells against the background of lymphoid cells and hemorrhage; Giemsa stain, ×100 and ×400, (d) photomicrograph of bone marrow aspirate showed cluster of atypical round cells; Giemsa stain, ×400
Discussion
Metastatic RB, although rare in developed countries, is a leading cause of RB-associated deaths in developing nations, seen in 9%–11% of cases.[6,12] In our study, the metastatic rate was <1%. Based on the pattern of spread, Jubran et al. put forward four divisions of RB metastasis: regional metastasis, such as to lymph nodes, distant metastasis, such as to the bone, and BM, extension into CNS, and trilateral RB.[13] In our study, three patients had RLN, two had BM, and one had CSF metastasis. Risk factors for CNS metastasis include optic nerve involvement, while distant metastasis is often preceded by extrascleral extension, which aids tumor invasion of lymphovascular channels.[7] In our study, patients with CSF metastasis had optic nerve tumor extension and one patient with BM metastasis had extrascleral tumor extension of RB. In addition, involvement of the postlaminar optic nerve has been found to be associated with a greater risk of metastasis and death.[14]
Factors influencing the development of metastatic RB include delay in diagnosis or misdiagnosis, late presentation, and low socioeconomic conditions.[6] A study by Cozza et al. showed that patients who presented with metastatic spread at the time of diagnosis were older than the whole RB population.[15] Age at presentation has also been implicated as an important factor influencing the low survival rates in low-income countries, with studies demonstrating the disparity to be almost a year later in these countries when compared to Western populations.[16] The median age of detection was 36 months according to an Indian study, 36 months and 24 months for unilateral and bilateral RB, respectively, in a study conducted in sub-Saharan Africa, and below 2 and 1 years for unilateral and bilateral disease, respectively, in most data from developed countries.[16,17,18] This study similarly shows an older presentation pattern of RB with an overall median of 60 months. The intraocular disease is seen in 90%–95% of patients presenting in developed countries, while the proportion falls to only 60%–70% in developing countries.[19] In this study, all four patients had advanced disease at presentation with either Stage III or IV on presentation.
Investigations such as MRI, BM, and CSF analysis were done for all patients presenting with Group E tumors and those with orbital disease. CSF studies have been considered gold standard for diagnosing CNS metastasis. A study by Hu et al. revealed CSF cytology to be positive in 44.4% of patients with CNS metastasis and advocated the need for repeat CSF cytology tests for improved detection.[20] This study further proves the sensitivity of this testing in one of our patients who had optic nerve extension on MRI and subsequently tested positive for round tumor cells in CSF cytology. In addition, BM positivity was seen in two patients (40%) and three (60%) patients had RLN involvement. In the largest study on metastatic workup for RB by Bakhshi et al., the use of routine CSF evaluation and BM testing was not recommended for IRSS Stages 0 and I, while data are conflicting for Stage II.[21] In this study too, both BM and CSF positivity were only seen in patients with Stage III RB or beyond.
In our study, RLN metastasis was noted in three patients. RB has been shown to spread to the parotid and RLN, through lymphatic invasion.[22] Overall, all four patients had enlarged RLN at presentation, of which three had FNAC-proven metastatic deposits. Ipsilateral PAN was involved in two patients while contralateral parotid gland and PAN were involved in one patient.
The cure rates of RB are significantly higher in developed countries (>90%) compared to underdeveloped or developing countries.[23] Poor prognosis is due to advanced disease at presentation.[24,25] However, intensified therapy consisting of HDC and autologous hematopoietic stem cell rescue is being touted as a viable option to increase treatment efficacy in advanced cases.[25] The pattern of spread is an important prognostic indicator. CNS involvement has been reported to have a dismal prospect by several studies, including Gündüz et al. who found that patients with distal organ metastasis favored better than those with concurrent distal and CNS, or solely CNS metastasis.[12] Several studies have shown that patients with distant metastasis have survival rates of around 50% at 5 years.[12,26] However, a study conducted by Rodriguez-Galindo et al., showed complete response to intensive chemotherapy, radiation therapy, and autologous hematopoietic stem cell rescue in patients with BM metastases.[26] In our study, a total of 12 cycles of HDC in combination with enucleation and external beam radiation to the orbit and metastatic RLN were given to all patients. Patients with RLN and BM metastasis responded well to treatment with good disease-free survival in all three affected cases. However, in case of CSF involvement, intrathecal chemotherapy along with HDC and EBRT is the preferred or palliative care considering poor treatment response. However, the prognosis is poor with this treatment as seen in our case.
Another contributor to metastatic risk is the presence of histopathological high-risk features, such as optic nerve involvement (postlaminar or optic nerve transection), scleral invasion, massive choroidal infiltration, and extrascleral extension.[22] Studies have shown high-risk features to be associated with children presenting at an older age, low-income countries, and associated with a higher risk of relapse and decreased chances of recovering vision.[17,23] A study conducted by Gupta et al. in India found that the rate of histopathologic risk factors is to be 54.2% among all patients with RB and 0.06% of those with high-risk features associated with metastasis.[17] In our study, one child underwent primary enucleation and was detected to have high-risk features including massive choroidal infiltration and scleral infiltration. This patient had RLN metastasis and recovered well with treatment.
Conclusion
Metastatic RB at presentation is rare and is seen in <1% of cases. None of the cases with intraocular RB with no high-risk histopathology features are associated with metastasis. RLN or BM or CSF metastasis can occur in patients presenting with overt orbital disease or those with high-risk features on histopathology. We recommend routine lymph node examination, CSF cytology, and BM aspirate in all cases presenting with overt orbital disease or those with high-risk features on histopathology. Patients with RLN or BM metastasis recover well with multimodal intensive treatment while those with CSF metastasis have poor prognosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This work was supported by The Operation Eyesight Universal Institute for Eye Cancer and Hyderabad Eye Research Foundation, Hyderabad, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Murphree AL, Benedict WF. Retinoblastoma: Clues to human oncogenesis. Science. 1984;223:1028–33. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Galindo C, Wilson MW, Chantada G, Fu L, Qaddoumi I, Antoneli C, et al. Retinoblastoma: One world, one vision. Pediatrics. 2008;122:e763–70. doi: 10.1542/peds.2008-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93:21–3. doi: 10.1136/bjo.2008.138750. [DOI] [PubMed] [Google Scholar]
- 4.Abramson DH, Beaverson K, Sangani P, Vora RA, Lee TC, Hochberg HM, et al. Screening for retinoblastoma: Presenting signs as prognosticators of patient and ocular survival. Pediatrics. 2003;112:1248–55. doi: 10.1542/peds.112.6.1248. [DOI] [PubMed] [Google Scholar]
- 5.Naseripour M. “Retinoblastoma survival disparity”: The expanding horizon in developing countries. Saudi J Ophthalmol. 2012;26:157–61. doi: 10.1016/j.sjopt.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali MJ, Honavar SG, Reddy VA. Distant metastatic retinoblastoma without central nervous system involvement. Indian J Ophthalmol. 2013;61:357–9. doi: 10.4103/0301-4738.97077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finger PT, Harbour JW, Karcioglu ZA. Risk factors for metastasis in retinoblastoma. Surv Ophthalmol. 2002;47:1–16. doi: 10.1016/s0039-6257(01)00279-x. [DOI] [PubMed] [Google Scholar]
- 8.Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, et al. Retinoblastoma. Nat Rev Dis Primers. 2015;1:15021. doi: 10.1038/nrdp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palma J, Sasso DF, Dufort G, Koop K, Sampor C, Diez B, et al. Successful treatment of metastatic retinoblastoma with high-dose chemotherapy and autologous stem cell rescue in South America. Bone Marrow Transplant. 2012;47:522–7. doi: 10.1038/bmt.2011.108. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Chantada G, Doz F, Antoneli CB, Grundy R, Clare Stannard FF, Dunkel IJ, et al. A proposal for an international retinoblastoma staging system. Pediatr Blood Cancer. 2006;47:801–5. doi: 10.1002/pbc.20606. [DOI] [PubMed] [Google Scholar]
- 12.Gündüz K, Müftüoglu O, Günalp I, Unal E, Taçyildiz N. Metastatic retinoblastoma clinical features, treatment, and prognosis. Ophthalmology. 2006;113:1558–66. doi: 10.1016/j.ophtha.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Jubran RF, Erdreich-Epstein A, Butturini A, Murphree AL, Villablanca JG. Approaches to treatment for extraocular retinoblastoma: Children’s hospital Los Angeles experience. J Pediatr Hematol Oncol. 2004;26:31–4. doi: 10.1097/00043426-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Kaliki S, Shields CL, Shah SU, Eagle RC, Jr., Shields JA, Leahey A. Postenucleation adjuvant chemotherapy with vincristine, etoposide, and carboplatin for the treatment of high-risk retinoblastoma. Arch Ophthalmol. 2011;129:1422–7. doi: 10.1001/archophthalmol.2011.289. [DOI] [PubMed] [Google Scholar]
- 15.Cozza R, De Ioris MA, Ilari I, Devito R, Fidani P, De Sio L, et al. Metastatic retinoblastoma: Single institution experience over two decades. Br J Ophthalmol. 2009;93:1163–6. doi: 10.1136/bjo.2008.148932. [DOI] [PubMed] [Google Scholar]
- 16.Lukamba RM, Yao JA, Kabesha TA, Budiongo AN, Monga BB, Mwembo AT, et al. Retinoblastoma in Sub-Saharan Africa: Case studies of the republic of Côte d’Ivoire and the democratic republic of the Congo. J Glob Oncol. 2018;4:1–8. doi: 10.1200/JGO.17.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R, Vemuganti GK, Reddy VA, Honavar SG. Histopathologic risk factors in retinoblastoma in India. Arch Pathol Lab Med. 2009;133:1210–4. doi: 10.5858/133.8.1210. [DOI] [PubMed] [Google Scholar]
- 18.Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet (London, England) 2012;379:1436–46. doi: 10.1016/S0140-6736(11)61137-9. [DOI] [PubMed] [Google Scholar]
- 19.Chantada GL, Qaddoumi I, Canturk S, Khetan V, Ma Z, Kimani K, et al. Strategies to manage retinoblastoma in developing countries. Pediatr Blood Cancer. 2011;56:341–8. doi: 10.1002/pbc.22843. [DOI] [PubMed] [Google Scholar]
- 20.Hu H, Zhang W, Wang Y, Huang D, Shi J, Li B, et al. Characterization, treatment and prognosis of retinoblastoma with central nervous system metastasis. BMC Ophthalmol. 2018;18:107. doi: 10.1186/s12886-018-0772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakhshi S, Meel R, Kashyap S, Sharma S. Bone marrow aspirations and lumbar punctures in retinoblastoma at diagnosis: Correlation with IRSS staging. J Pediatr Hematol Oncol. 2011;33:e182–5. doi: 10.1097/MPH.0b013e3182103f5c. [DOI] [PubMed] [Google Scholar]
- 22.Xiao W, Ye H, Zeng H, Tang L, Chen R, Gao Y, et al. Associations among socioeconomic factors, lag time, and high-risk histopathologic features in eyes primarily enucleated for retinoblastoma. Curr Eye Res. 2019;44:1144–9. doi: 10.1080/02713683.2019.1623898. [DOI] [PubMed] [Google Scholar]
- 23.Padma M, Kumar N, Nesargi PS, Aruna Kumari BS, Appaji L, Viswanathan A. Epidemiology and clinical features of retinoblastoma: A tertiary care center’s experience in India. South Asian J Cancer. 2020;9:56–8. doi: 10.4103/sajc.sajc_89_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang P, Li YJ, Zhang SB, Cheng QL, Zhang Q, He LS. Metastatic retinoblastoma of the parotid and submandibular glands: A rare case report. BMC Ophthalmol. 2017;17:229. doi: 10.1186/s12886-017-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunkel IJ, Khakoo Y, Kernan NA, Gershon T, Gilheeney S, Lyden DC, et al. Intensive multimodality therapy for patients with stage 4a metastatic retinoblastoma. Pediatr Blood Cancer. 2010;55:55–9. doi: 10.1002/pbc.22504. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Galindo C, Wilson MW, Haik BG, Lipson MJ, Cain A, Merchant TE, et al. Treatment of metastatic retinoblastoma. Ophthalmology. 2003;110:1237–40. doi: 10.1016/S0161-6420(03)00258-6. [DOI] [PubMed] [Google Scholar]


