Abstract
BACKGROUND:
Retinopathy of prematurity (ROP) is a vasculoproliferative disorder of immature retina, seen in preterm babies. Multiple risk factors attribute to this condition. Our aim was to correlate the role of any early neonatal surgeries with low gestational age (GA) and birth weight (BW) on preterm babies as a risk factor on the progression of ROP.
METHODS:
A prospective, cohort study conducted in a hospital in South India over 3 years, including 600 babies with GA <35 weeks and BW <2500 g. Babies were divided into Group A (ROP) and Group B (no ROP). Group A included A1 (severe ROP) and A2 (nonsevere ROP) based on early treatment of ROP classification. We compared various risk factors of ROP, specifically the association of any early neonatal surgery undergone by these babies, on progression of ROP. The Chi-square test, unpaired t-test, and one-way analysis of variance tests were used for the comparisons and considered statistically significant if P < 0.05.
RESULTS:
28.7% babies developed ROP, with 37.6% requiring treatment (Aggressive ROP in 2.1% of babies). Low GA and BW, longer duration on ventilator, respiratory distress syndrome, apnea of prematurity, Patent Ductus Arteriosus, sepsis, anemia, thrombocytopenia, history of blood transfusion, and history of early neonatal surgery under GA were associated with babies with ROP (P < 0.005), strongly with severe ROP. The most common surgeries undergone by the babies developing ROP were inguinal herniotomy under general anesthesia.
CONCLUSION:
The study predicts preterm babies undergoing early surgical interventions are at risk of progressing to severe ROP, hence warranting frequent follow-ups.
Keywords: Inguinal hernia, newborn, respiration artificial, retinopathy of prematurity
Introduction
Retinopathy of prematurity (ROP) is a vasculoproliferative disease of the retina seen exclusively in immature retina of preterm babies. The sequel of ROP ranges from no visual morbidity to complete blindness in advanced stages. Indian studies have shown that 0.6–1.06/1000 children and 2.05–13.6/1000 children develop blindness and visual impairment, respectively, out of which 30%–72% is avoidable by taking proper prophylaxis.[1,2] Studies from the last decade have shown retinopathies contribute 3.3%–44.4% of childhood blindness, from which ROP contributes approximately 10% of this list.[3,4,5,6,7,8]
The incidence of ROP varies in different countries as each country adapts different cutoff criteria for screening. The incidence of ROP in developed countries has been estimated to be around 12.8%–31.9%.[9,10] In various regions of India, the incidence of ROP has been reported between 38% and 47%.[11] It has been estimated that around 10% of babies screened for ROP require treatment.[12]
ROP is believed to occur in two phases: phase of vasculogenesis due to hyperoxia, where there is down regulation of vascular endothelial growth factor (VEGF) and phase of angiogenesis due to hypoxia and ischemia, where there is up regulation of VEGF which leads to abnormal new vessel formation. We have studied the retinal vascular changes till retina completely matures or ROP regresses and tried to correlate the effect of inflammatory changes induced due to any early neonatal surgical intervention under general anesthesia, during the transition of the two phases of retinal development, which could attribute toward progression of ROP. After an extensive search in the literature, we have not found any studies correlating the effect of early neonatal surgeries under general anesthesia (at postconceptional age [PCA] <36 weeks) on ROP progression, thereby making it an interesting study. We have compared the various risk factors between babies developing severe, nonsevere ROP and no ROP, and analyzed the PCA at which ROP develop, progress and regress in babies with severe and nonsevere ROP.
Methods
The study was conducted after obtaining the Institutional Ethical committee approval (CSP-MED/18/AUG/45/110), which abides by the tenets of Declaration of Helsinki on research on human subjects and in concurrence with the neonatology department. Informed consent was taken from the parents of the babies.
This prospective study was conducted on a cohort of preterm infants in a single tertiary care center in South India over 3 years (December 2018–December 2021). This was a longitudinal, descriptive, observational, nonrandomized, population-based, single hospital-based study.
We studied 600 preterm babies admitted in our hospital's neonatal intensive care unit (NICU), screened for ROP and followed them till the retina matured or ROP regressed.
Babies with a gestational age (GA) <35 weeks and birth weight (BW) <2500 g were included in the study. The babies were screened for ROP by three senior ophthalmologists from day 21 of life or PCA of 31 weeks, whichever was earlier. The follow-up visits were determined based on the vascular status of the retina at the current visit.
Retinopathy of prematurity screening
One hour before the examination, 0.5% tropicamide and 2.5% phenylephrine eye drops were instilled thrice in each eye at an interval of 15 min to ensure complete pupillary dilatation. Just prior to examination with lid speculum and wire vectis, topical anesthesia with 0.5% proparacaine hydrochloride eye drop was applied. Anterior segment examination followed by fundus examination was done with binocular indirect ophthalmoscope and 20 D aspheric lens. The fundus photographs were taken using iPhone XR, MII Retcam and 20 D aspheric lens.
Babies were followed up till retina matured or ROP regressed (till at least 50 weeks PCA). ROP was diagnosed and classified based on the International Classification of ROP[13] and guidelines given by early treatment of ROP (ETROP).[12] Babies were grouped into two-babies with ROP (Group A) and babies without ROP (Group B). The worst stage of ROP in either eye of each baby was considered. We excluded babies who expired or were lost to follow-up before the retina matured or ROP regressed. Babies in Group A were further subdivided as-babies with ROP requiring treatment (Group A1) and babies with ROP not requiring treatment (Group A2). Babies with type 1 ROP and Aggressive ROP (AROP)[14] were considered as progression of ROP and included in Group A1 (severe ROP, requiring treatment) and babies with type 2 and mild ROP were included in Group A2 (nonsevere ROP, not requiring treatment). The involution of ROP was noted as down staging of ROP to a peripheral zone or reduction in dilatation and tortuosity of retinal vessels,[14] whereas regressed ROP was considered when severe ROP involuted to a relatively stable stage, not requiring further active treatment.[15]
We studied 23 perinatal and postnatal parameters between babies from both groups and subgroups-GA, BW, gender, low APGAR score (≤6) at 1 min and 5 min, presence of small for GA (SGA), intrauterine growth restriction (IUGR), presence of respiratory distress syndrome (RDS), presence of apnea of prematurity, history of surfactant use, presence of sepsis, presence of acyanotic heart disease (AHD) which included patent ductus arteriosus (PDA), atrial septal defect and ventricular septal defect, presence of combined AHD, presence of intraventricular hemorrhage (IVH), presence of Ileus of prematurity, presence of bronchopulmonary dysplasia (BPD), duration of oxygen support given, history of prolonged mechanical ventilation, presence of anemia, thrombocytopenia, history of blood transfusion, and any surgical intervention undergone.
Definition of each risk factor taken into account:
Low GA: We have considered all babies born at a GA <35 weeks as low GA
Low BW: Babies born with a BW <2500 g were considered as low BW, whereas babies with BW <1500 g were considered as very low BW and babies with BW <1000 g were considered as extremely low BW
Low APGAR: 5 parameters examined and scored at 1 and 5 min of life-heart rate, muscle tone, respiratory effort, skin color, and response to stimulation (each parameter scored as 0, 1, or 2). A score <6 was considered low
SGA: SGA are those babies whose BW falls below the tenth percentile for babies of the same GA
IUGR: IUGR is a condition where the baby does not grow as expected in the mother's womb and is detected prior to delivery on ultrasonography
RDS: RDS was considered when the neonate required more work to breathe in the form of tachypnea (respiratory rate >60 breaths/minute), nasal flaring, grunting or chest retractions, seen within 24 h of birth
Apnea of prematurity: Episodes of apnea are defined either as cessation of breathing for 20 s or shorter periods of breath holding associated with bradycardia (heart rate <100 beats/minute), pallor or cyanosis
Sepsis: Neonatal sepsis has been defined as systemic infection in babies within 28 days of life. Early-onset sepsis in preterm babies is defined as systemic infection within first 72 h of life, whereas late-onset sepsis in preterm babies is considered as systemic infection occurring in babies after 72 h of life up to day 90 or 120 of life. Early onset fungal infections are included within the first 7 days of life. Sepsis is confirmed only on microbial growth on blood culture
AHDs: It includes congenital heart defect, whereby oxygenated blood is circulated abnormally throughout the body
PDA: It is a type of AHD, where the duct connecting aorta and pulmonary artery remains patent even after birth, thereby reducing the blood circulation to rest of the body. It is detected clinically by murmurs and by echocardiography
IVH: IVH is a condition detected on cranial ultrasonography as bleeding into the brain ventricles due to the leakage of fragile blood vessels, commonly seen in premature babies.
Ileus of prematurity: The condition is defined as failure to evacuate meconium from the baby's gut and is diagnosed clinically as abdominal distension and as distended bowel loops without air-fluid levels on abdominal X-ray
BPD: Babies were diagnosed with broncho-pulmonary dysplasia if they needed any supplementary oxygen support beyond 28 days of birth or around their original term date
Oxygen support: Babies with breathing difficulty were given supplementary support in the form of ventilatory support, nasal continuous positive airway pressure, or high flow nasal cannula
Prolonged mechanical ventilation: This is defined as ventilatory support given to the babies with fractionated oxygen (FiO2) >21% for more than 2 weeks
Anemia of prematurity: Hemoglobin level <8 gms/dl
Thrombocytopenia in preterm babies: Thrombocytopenia is defined as platelet level <150,000/µl. Platelet transfusion was given if platelet level was <100,000/µl
Blood transfusion: Blood transfusions in the form of packed red blood cells or platelets to correct anemia and thrombocytopenia, respectively
Surgical interventions: Any surgical interventions undergone by the baby under general anesthesia, that required additional blood transfusion and oxygen support as postoperative care, before 40 weeks of PCA
We noted the PCA at which babies underwent any surgical procedure and correlated it with the age and stage at which they developed ROP.
We noted the PCA at which ROP was first detected, progressed, started regressing, and completely regressed in babies from group A (ROP). Babies developing severe ROP were treated with peripheral laser ablation or intravitreal anti-VEGF (Bevacizumab) injection.
Statistical analysis
The data were analyzed with IBM SPSS Statistics for Windows, Version 23.0 (Armonk, NY, USA: IBM Corp). For categorical variables, frequency analysis and percentage analysis were used. For continuous variables, mean and standard deviation were used. To find the significant difference between the bivariate samples for independent groups, unpaired sample t-test was used. To find the significance in continuous variables, one-way analysis of variance (ANOVA) with Tukey's Post hoc test was used and for repeated measures, the repeated measures of ANOVA was used with Bonferroni correction to control the type I error on multiple comparison. To find the significance in categorical data, Chi-square test or Fisher's exact was used. Multivariate logistic regression analysis was used between groups and subgroups based on risk factors that were significant on univariate analysis. In all the above, statistical tools the probability value <0.05 is considered statistically significant.
Results
Out of the 600 preterm babies we screened, only 574 babies had complete medical records, followed up regularly and were included in the study [Figure 1]. One hundred and sixty-five babies (28.7%) developed ROP. Type 1 ROP was seen in 37.6%, type 2 in 45.5% and mild ROP in 17%. None of the babies included in our study had advanced stage ROP. 1.8% babies developed AROP and were included under severe ROP (Group A1), requiring urgent attention. All 62 babies with severe ROP [Figure 2] underwent peripheral laser ablation [Figure 3]. Only 2 babies with AROP, received intravitreal anti-VEGF (Bevacizumab) injection additionally.
Figure 1.
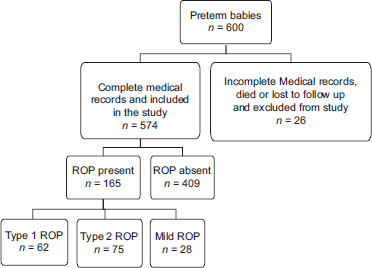
Diagram showing flow of participants through the study
Figure 2.
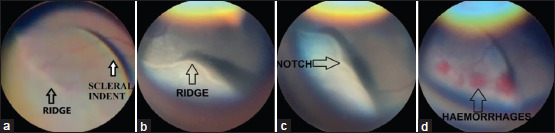
Fundus photograph showing (a) Stage 2 Zone 2 ROP without plus disease (nonsevere ROP), (b) and (c) Stage 3 Zone 2 ROP with plus (severe ROP), (d) Stage 3 Zone 2 ROP with plus and hemorrhages on the ridge (severe ROP). ROP: Retinopathy of prematurity
Figure 3.

Fundus photograph of right eye and left eye of baby with type 1 ROP after receiving peripheral laser ablation. ROP: Retinopathy of prematurity
The study group comprised of 315 (54.9%) male babies and 259 (45.1%) female babies. 32.1% male babies and 24.3% female babies developed ROP (P = 0.053). SGA and IUGR had no significant difference between babies who developed ROP and those who did not (P = 0.204 and P = 0.144, respectively).
The mean GA for the study group was 30.8 ± 2.3 weeks; mean BW was 1425 ± 437.9 g. The mean duration of oxygen support required by the babies was 8.2 ± 14.9 days and the mean duration of days on ventilator (with FiO2 >21%) was 1.5 ± 5.0 days.
Among the babies developing ROP, the mean GA was 28.7 ± 2.12 weeks, the mean BW was 1095.3 ± 339.5 g, the mean duration of oxygen support required by these babies was 18.3 ± 22.3 days, and mean duration of days on ventilator (with FiO2 >21%) was 4.1 ± 7.8 days.
On comparing the various risk factors between babies who developed severe ROP (Group A1), nonsevere ROP (Group A2) and no ROP (Group B), low GA, low BW, prolonged duration of oxygen support, especially on ventilator, low APGAR score (≤6) at 1 min, presence of RDS, apnea of prematurity, history of surfactant use, history of combined AHD, presence of any AHD, history of PDA, presence of IVH, presence of sepsis, anemia, thrombocytopenia, history of blood transfusion, history of prolonged oxygen support, BPD, ileus of prematurity, and history of any surgery undergone by the baby during the NICU stay were strongly associated with severe ROP on univariate analysis (P < 0.05) [Table 1].
Table 1.
Comparison of risk factors between babies with severe retinopathy of prematurity, nonsevere retinopathy of prematurity and no retinopathy of prematurity
| Group A1 (severe ROP), n (%) | Group A2 (nonsevere ROP), n (%) | Group B (no ROP), n (%) | P | |
|---|---|---|---|---|
| Gender† | ||||
| Male | 40 (64.5) | 61 (59.8) | 214 (52.2) | 0.104 |
| Female | 22 (35.5) | 41 (40.2) | 196 (47.8) | |
| Gestational age‡ (weeks) | 27.8±1.9 | 29.4±2 | 31.7±1.9 | 0.005* |
| Birth weight‡ (g) | 940.6±218.1 | 1185.5±366 | 1557.9±401.1 | 0.005* |
| Presence of SGA† | 12 (19.4) | 14 (13.7) | 78 (19) | 0.401 |
| Presence of IUGR† | 14 (22.6) | 17 (16.7) | 57 (13.9) | 0.193 |
| Apgar (≤6) at 1 min† | 33 (53.2) | 48 (47.1) | 104 (25.4) | 0.0005* |
| Apgar (≤6) at 5 min† | 6 (9.7) | 5 (4.9) | 19 (4.6) | 0.248 |
| RDS† | 61 (98.4) | 84 (82.4) | 289 (70.5) | 0.0005* |
| Apnoea of prematurity† | 52 (83.9) | 60 (58.8) | 113 (27.6) | 0.0005* |
| Need for surfactant† | 27 (43.5) | 33 (32.4) | 56 (13.7) | 0.0005* |
| BPD† | 30 (48.4) | 11 (10.8) | 12 (2.9) | 0.0005* |
| Acyanotic heart disease† | 34 (54.8) | 33 (32.4) | 50 (12.2) | 0.0005* |
| PDA† | 27 (43.5) | 27 (26.5) | 40 (9.8) | 0.0005* |
| Combined acyanotic heart disease† | 8 (12.9) | 13 (12.7) | 14 (3.4) | 0.0005* |
| IVH† | 15 (24.2) | 12 (11.8) | 29 (7.1) | 0.0005* |
| Ileus of prematurity† | 6 (9.7) | 8 (7.8) | 11 (2.7) | 0.007* |
| Anaemia† | 42 (67.7) | 30 (29.4) | 38 (9.3) | 0.0005* |
| Thrombocytopenia† | 13 (21) | 9 (8.8) | 17 (4.1) | 0.0005* |
| History of blood transfusion† | 39 (62.9) | 27 (26.5) | 32 (7.8) | 0.0005* |
| Sepsis† | 26 (41.9) | 16 (15.7) | 30 (7.3) | 0.0005* |
| History of any surgery undergone by the baby during their stay in NICU† | 14 (22.6) | 9 (8.8) | 15 (3.7) | 0.0005* |
| Oxygen support required† | 61 (98.4) | 87 (85.3) | 300 (73.2) | 0.0005* |
| Number of days of oxygen support required‡ | 30.3±25.6 | 11±16.4 | 4.2±7.4 | 0.0005* |
| Number of days on ventilator‡ | 7±9.4 | 2.3±6 | 0.5±2.6 | 0.0005* |
| PCA at which babies underwent surgery‡ | 35.2±9 | 37.1±2.7 | 37.1±5.3 | 0.703 |
*P<0.05 was considered significant, †Calculated as number, percentage, Chi-square test used, ‡Calculated as mean±SD, one-way ANOVA test used. SD: Standard deviation, ROP: Retinopathy of prematurity, SGA: Small for gestational age, IUGR: Intrauterine growth restriction, RDS: Respiratory distress syndrome, BPD: Bronchopulmonary dysplasia, PDA: Patent ductus arteriosus, IVH: Intraventricular hemorrhage, NICU: Neonatal intensive care unit, PCA: Postconceptional age
Thirty-eight babies from our study group underwent surgeries under general anesthesia during their stay in NICU. The babies who underwent surgeries and developed severe ROP were observed to have surgeries at a younger PCA (mean PCA 35.2 weeks) compared to babies developing nonsevere ROP and no ROP groups (mean PCA 37.1 weeks), but this difference in PCA was not statistically significant (P = 0.703) [Figure 4]. On multivariate logistic regression analysis between Group A and B, low BW, history of AHD, and sepsis were significantly associated with ROP (P < 0.05).
Figure 4.
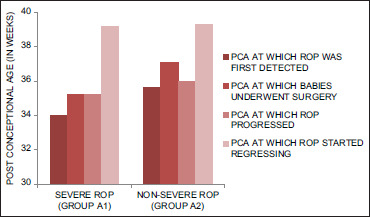
Graph comparing PCA at which ROP developed, surgery undergone, ROP progressed and ROP started regressing between severe ROP (group A1) and nonsevere ROP (group A2). ROP: Retinopathy of prematurity, PCA: Postconceptional age
Consecutively, on comparing babies with severe ROP and nonsevere ROP, low GA (27.8 ± 1.9 days vs. 29.4 ± 2 days), low BW (940.6 ± 218.1 g vs. 1185.5 ± 366 g), RDS (98.4% vs. 82.4%), apnea of prematurity (83.9% vs. 58.8%), BPD (48.4% vs. 10.8%), IVH (24.2% vs. 11.8%), sepsis (41.9% vs. 15.7%), presence of AHDs (54.8% vs. 32.4%), with PDA in particular (43.5% vs. 26.5%), anemia (67.7% vs. 29.4%), thrombocytopenia (21% vs. 8.8%), history of blood transfusions (62.9% vs. 26.5%), history of neonatal surgery (22.6% vs. 8.8%), history of supplemental oxygen (98.4% vs. 85.3%), prolonged duration of oxygen support (30.3 ± 25.6 days vs. 11 ± 16.4 days), particularly on ventilator (7 ± 9.4 days vs. 2.3 ± 6 days) were strongly associated with severe ROP (P < 0.05). On multivariate regression analysis, only the presence of AHD was strongly associated with severe ROP (P = 0.004).
Although babies with severe ROP had a greater requirement of surfactant compared to babies with nonsevere ROP, there was no significant difference between the groups (P = 0.149). Similarly, low APGAR scores and ileus of prematurity had no association with severe ROP (P > 0.05).
Klebsiella pneumoniae (17.1%) was the most common cause of sepsis amongst all the babies with ROP, especially in babies progressing to severe ROP (19.2%).
The most common surgery undergone by babies developing severe ROP was inguinal herniotomy under general anesthesia (n = 7) and resection and anastomosis of bowel for necrotizing enterocolitis under general anesthesia (n = 7). Both the surgeries took approximately an hour. Herniotomy has low surgical risk with minimum physiological effect, whereas resection and anastomosis of bowel have intermediate risk with moderate changes in hemodynamics with risk of blood loss, as given under University of California at Los Angeles (UCLA) Surgery Risk Stratification. One baby who developed type 1 ROP with APROP underwent ventriculo-peritoneal shunt with Omaya reservoir [Table 2].[13]
Table 2.
Surgeries undergone by babies developing retinopathy of prematurity and correlating with gestational age, birth weight and the stage of retinopathy of prematurity based on international classification of retinopathy of prematurity classification[13]
| Baby | GA (weeks) | BW (g) | Surgery undergone | Any additional surgery | Worst stage of ROP | Treatment required for ROP |
|---|---|---|---|---|---|---|
| 1 | 29 | 1150 | Inguinal herniotomy under GA | Nil | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 2 | 29.3 | 715 | Inguinal herniotomy under GA | Yes | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 3 | 27.6 | 605 | Inguinal herniotomy under GA | Yes | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 4 | 26 | 820 | Inguinal herniotomy under GA | Yes | Stage 3 zone 2 (anterior) ROP with AROP and plus disease | Yes |
| 5 | 28.4 | 750 | Inguinal herniotomy under GA | Nil | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 6 | 27.2 | 900 | Inguinal herniotomy under GA | Yes | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 7 | 26.3 | 845 | Inguinal herniotomy under GA | Yes | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 8 | 29.6 | 1200 | Inguinal herniotomy with orchidopexy under GA | Nil | Stage 2 zone 2 (anterior) ROP without plus | No |
| 9 | 27.1 | 1090 | inguinal herniotomy under GA | Nil | Stage 2 zone 2 (anterior) ROP without plus | No |
| 10 | 29.1 | 725 | Unilateral inguinal herniotomy under GA | Nil | Stage 1 zone 2 (intermediate) ROP without plus | No |
| 11 | 30.1 | 1100 | Inguinal herniotomy under GA | Yes | stage 1 zone 3 ROP without plus | No |
| 12 | 28 | 770 | Inguinal herniotomy under GA | Nil | Stage 2 zone 2 (anterior) ROP without plus | No |
| 13 | 26.1 | 735 | Inguinal herniotomy under GA | Nil | Stage 2 zone 2 (anterior) ROP without plus | No |
| 14 | 27 | 1100 | Bilateral inguinal herniotomy under GA | Nil | Stage 2 zone 2 (anterior) ROP without plus | No |
| 15 | 28.2 | 880 | Resection and anastomosis of bowel for NEC stage 3 under GA | Nil | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 16 | 25.2 | 850 | Resection and anastomosis of bowel for NEC stage 2 under GA | Nil | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 17 | 28.2 | 810 | Resection and anastomosis of bowel for NEC stage 2 under GA | Nil | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 18 | 26.5 | 1130 | Resection and anastomosis of bowel for NEC stage 2 under GA | Nil | Stage 3 zone 2 (anterior) ROP with plus | Yes |
| 19 | 28.2 | 810 | Resection and anastomosis of bowel for nonnecrotising enterocolitis under GA | Nil | Stage 3 zone 2 (anterior) ROP with plus | Yes |
| 20 | 28.2 | 630 | Resection and anastomosis of bowel for NEC stage 2 under GA | Nil | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 21 | 26 | 650 | Resection and anastomosis of bowel for NEC under GA | Nil | Stage 3 zone 2 (intermediate) ROP with plus | Yes |
| 22 | 26.1 | 955 | Ventriculo-peritoneal shunt and Omaya reservoir under GA | AROP | Yes | |
| 23 | 32.1 | 1780 | Congenital diaphragmatic hernia repair under GA | Nil | Stage 2 zone 2 (anterior) ROP without plus | No |
NEC: Necrotizing enterocolitis, GA: Gestational age, BW: Birth weight, ROP: Retinopathy of prematurity, AROP: Aggressive ROP
The mean PCA at which ROP was first detected, started regressing and regressed was 35.0 ± 3.1 weeks, 39.3 ± 4.0 weeks and 49.4 ± 6.6 weeks, respectively. The mean PCA at which babies developing ROP underwent any surgery was 35 ± 7 weeks. Babies with severe ROP were detected at an early PCA (mean PCA 34 weeks), progressed at a mean PCA 35.2 weeks and took a longer time to regress (mean PCA 39.2 weeks) compared to babies with nonsevere ROP [Table 3].
Table 3.
Comparison of postconceptional age at which retinopathy of prematurity was first detected, retinopathy of prematurity started regressing and retinopathy of prematurity completely regressed between type 1 retinopathy of prematurity, type 2 retinopathy of prematurity and mild retinopathy of prematurity
| PCA | ETROP classification | n | Mean±SD | 95% CI for mean |
P | |
|---|---|---|---|---|---|---|
| LB | UB | |||||
| PCA at which ROP was detected | Type 1 ROP | 62 | 34.0±2.0 | 33.4 | 34.6 | 0.0005* |
| Type 2 ROP | 75 | 34.4±2.1 | 33.9 | 34.9 | ||
| Mild ROP | 28 | 39.1±4.2 | 37.3 | 41.0 | ||
| PCA at which ROP started regressing | Type 1 ROP | 62 | 39.2±4.9 | 37.8 | 40.5 | 0.002* |
| Type 2 ROP | 75 | 38.5±3.0 | 37.8 | 39.2 | ||
| Mild ROP | 28 | 41.9±3.6 | 40.3 | 43.5 | ||
| PCA at which ROP regressed | Type 1 ROP | 62 | 53.2±6.2 | 51.5 | 54.9 | 0.0005* |
| Type 2 ROP | 75 | 46.6±5.3 | 45.3 | 47.9 | ||
| Mild ROP | 28 | 48.3±6.6 | 45.4 | 51.3 | ||
*P<0.05 was considered significant, one-way ANOVA test. Values are presented as number, mean and SD. ROP: Retinopathy of prematurity, ETROP: Early treatment for ROP classification. PCA: Postconceptional age, SD: Standard deviation, CI: Confidence interval, LB: Lower bound, UB: Upper bound
Discussion
ROP is vasculo-proliferative disease seen in the immature retina of preterm babies, which has multiple factors influencing its pathology, most important being unregulated effect of high vascular oxygen. In our study, 28.7% babies developed ROP, out of which 37.6% developed threshold ROP, requiring intervention. In CRYO-ROP study[15] and Alexandria (ALEX)-ROP study,[16] the incidence of ROP was 66%–68% and 21.4%, respectively. In the ETROP[12] study, 36.9% babies developed severe ROP, which is similar to our study. In various studies conducted in India between 1992 and 2012, 6.3%–44.9% babies developed severe ROP.[17,18,19]
The American Academy of Pediatrics[20] and UK screening guidelines[21] recommend screening for ROP for smaller babies, following which we would have missed 30.9% and 15.8% babies developing ROP, respectively. The latest Indian guidelines[22] recommend screening for preterm babies <34 weeks and BW <2000 g, following which we would have missed 3% babies developing ROP, out of which 1 baby developed threshold ROP requiring treatment.
In our study, we found low GA, AHD, and sepsis were strongly associated with ROP on multivariate analysis, and AHD was particularly associated with severe ROP. Low GA, low BW, low APGAR score (≤6) at 1 min, RDS, apnea of prematurity, use of surfactants, BPD, AHDs, PDA in particular, IVH, history of neonatal surgery, prolonged duration of oxygen support, and prolonged duration on ventilator support were the independent risk factors of ROP, more so with severe ROP. The AHD s probably play a role toward progression to severe ROP due to left-to-right shunting of blood in the heart, which circulates and impairs the oxygen balance in the retina. Between the subgroups, babies developing severe ROP had lower GA and BW, followed by nonsevere ROP group and no ROP group. Our findings are similar to ALEX-ROP study conducted in Egypt, where GA (mean PCA 28 weeks) and BW (mean weight 965.11 g) were lower in babies developing type 1 ROP than type 2 ROP (mean PCA 31.5 weeks, mean BW 1105 g).
In our study, anemia, thrombocytopenia, blood transfusions, IVH and sepsis were independent risk factors of ROP, especially with progression to severe ROP. This is similar to studies by Pai et al.,[23] Lim et al.,[24] Pascal et al.,[25] and Chang.[26] All these factors add to the oxidative stress on the immature retina, thereby up regulating VEGF levels, which eventually leads to neovascularization.
We observed the mean PCA at which babies developed any ROP was 34 weeks, progressed at 35.2 weeks and started regressing by 39 weeks. Literature also states median PCA at which ROP and threshold ROP is detected is 34 weeks and 34–38 weeks, respectively[12,22] and started regressing by 44 weeks.[14] We hypothesized this period between 34 and 39 weeks is crucial for progression or regression of ROP. We studied the role of surgeries under general anesthesia on the preterm babies during this period and found babies developing severe ROP (Group A1) underwent surgeries at an earlier PCA (mean PCA = 35 weeks) compared to nonsevere ROP group (Group A2) (mean PCA = 37 weeks). Even though the difference in the PCA of surgeries between severe and nonsevere ROP groups is not statistically significant, one cannot negate the effect of the stormy period and prolonged ventilation the babies undergo due to surgery, at such a young age, on the partially vascularized retina. Many of these babies undergoing surgery also need additional blood transfusions. These conditions possibly up regulate the VEGF in the hypoxic retina, leading to abnormal vascularization. Studies have stated that the nasal retina is vascularized by approximately 36 weeks, beyond which if ROP develops would be considered in zone 3, and hence, a nonsevere form of ROP.[12] Hence, if these babies with ROP are exposed to any hyperoxic conditions and systemic inflammation due to surgery, prior to 36 weeks, it could progress toward severe ROP.
Babies with severe ROP started regressing late and completely regressed beyond 50 weeks PCA, which could be attributed to the effect of interventions taken during threshold period of the disease. This is similar to the findings by study by Ni et al., where milder forms of ROP started involuting early (mean PCA = 40.4 weeks) and regressed at an earlier age (mean PCA = 50.6 weeks), whereas the severe ROP started involuting at a later age and took a longer duration to regress.[27]
The babies progressing to severe ROP had a stormy and prolonged NICU stay with multiple systemic disorders and a prolonged supplemental oxygen exposure including mechanical ventilation with FiO2 more than 21%. These babies had to be monitored more frequently (twice or once a week) and needed urgent intervention in the form of laser peripheral ablation or intravitreal Bevacizumab or both. Whereas, the nonsevere ROP babies would mostly regress spontaneously and were followed up at a relaxed interval of 2–3 weeks till complete regression of ROP was noted.
The limitation of our study is that we need to analyze the risk of surgical intervention and progression of ROP at multiple hospitals to reflect it as a risk factor on the population of preterm babies undergoing early surgeries.
Conclusion
Based on our work, we concluded that, while screening babies for ROP, one must be mindful of one's nation's demography, as we have seen some heavier preterm babies (BW >1500 g) developing ROP. We observed low BW, AHD and sepsis, are strongly associated ROP, with AHD particularly having a strong correlation with severe ROP. Finally, we recommend the babies undergoing neonatal surgeries at an early PCA (<40 weeks), need frequent follow ups, possibly beginning from 1st postoperative day and continue beyond 50 weeks till there is regression, to prevent any chances of threshold ROP or any other serious complications that may lead to future visual morbidity and alert the parents about the need of follow-up visits and early treatment, even after the baby is discharged from the hospital.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge the Department of Neonatology, SRIHER, for their constant support. We would also like to acknowledge Indian Council of Medical Research (ICMR) for supporting this study.
References
- 1.Wadhwani M, Vashist P, Singh SS, Gupta V, Gupta N, Saxena R. Prevalence and causes of childhood blindness in India: A systematic review. Indian J Ophthalmol. 2020;68:311–5. doi: 10.4103/ijo.IJO_2076_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titiyal JS, Pal N, Murthy GV, Gupta SK, Tandon R, Vajpayee RB, et al. Causes and temporal trends of blindness and severe visual impairment in children in schools for the blind in North India. Br J Ophthalmol. 2003;87:941–5. doi: 10.1136/bjo.87.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnaiah S, Subba Rao B, Lakshmi Narasamma K, Amit G. A survey of severe visual impairment in children attending schools for the blind in a coastal district of Andhra Pradesh in South India. Eye (Lond) 2012;26:1065–70. doi: 10.1038/eye.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Israfil AT, Gogate PM, Kulkarni V, Shinde M. Improving functional vision in schools for the blind students with low vision aids in Pune. Indian J Clin Ophthalmol Res. 2014;2:99–101. [Google Scholar]
- 5.Bhalerao SA, Tandon M, Singh S, Dwivedi S, Kumar S, Rana J. Visual impairment and blindness among the students of blind schools in Allahabad and its vicinity: A causal assessment. Indian J Ophthalmol. 2015;63:254–8. doi: 10.4103/0301-4738.156930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danayak PM, Patel RB. Avoidable blindness and its correction in schools for the blind in Gujarat. Int J Adv Med. 2015;2:370–4. [Google Scholar]
- 7.Kemmanu V, Hegde K, Giliyar SK, Shetty BK, Kumaramanickavel G, McCarty CA. Prevalence of childhood blindness and ocular morbidity in a rural pediatric population in Southern India: The Pavagada pediatric eye disease study-1. Ophthalmic Epidemiol. 2016;23:185–92. doi: 10.3109/09286586.2015.1090003. [DOI] [PubMed] [Google Scholar]
- 8.Blencowe H, Moxon S, Gilbert C. Update on blindness due to retinopathy of prematurity globally and in India. Indian Pediatr. 2016;53(Suppl 2):S89–92. [PubMed] [Google Scholar]
- 9.Ludwig CA, Chen TA, Hernandez-Boussard T, Moshfeghi AA, Moshfeghi DM. The epidemiology of retinopathy of prematurity in the United States. Ophthalmic Surg Lasers Imaging Retina. 2017;48:553–62. doi: 10.3928/23258160-20170630-06. [DOI] [PubMed] [Google Scholar]
- 10.Holmström G, Tornqvist K, Al-Hawasi A, Nilsson Å, Wallin A, Hellström A. Increased frequency of retinopathy of prematurity over the last decade and significant regional differences. Acta Ophthalmol. 2018;96:142–8. doi: 10.1111/aos.13549. [DOI] [PubMed] [Google Scholar]
- 11.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(Suppl 1):35–49. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–48. [PMC free article] [PubMed] [Google Scholar]
- 13.International Committee for the Classification of Retinopathy of Prematurity The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 14.Repka MX, Palmer EA, Tung B. Involution of retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 2000;118:645–9. doi: 10.1001/archopht.118.5.645. [DOI] [PubMed] [Google Scholar]
- 15.The natural ocular outcome of premature birth and retinopathy. Status at 1 year. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1994;112:903–12. doi: 10.1001/archopht.1994.01090190051021. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed IS, Badeeb AA. The Alexandria retinopathy of prematurity model (Alex-ROP): Postnatal weight gain screening algorithm application in a developing country. Int J Ophthalmol. 2019;12:296–301. doi: 10.18240/ijo.2019.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopal L, Sharma T, Ramachandran S, Shanmugasundaram R, Asha V. Retinopathy of prematurity: A study. Indian J Ophthalmol. 1995;43:59–61. [PubMed] [Google Scholar]
- 18.Jalali S, Matalia J, Hussain A, Anand R. Modification of screening criteria for retinopathy of prematurity in India and other middle-income countries. Am J Ophthalmol. 2006;141:966–8. doi: 10.1016/j.ajo.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Hungi B, Vinekar A, Datti N, Kariyappa P, Braganza S, Chinnaiah S, et al. Retinopathy of prematurity in a rural neonatal intensive care unit in South India – A prospective study. Indian J Pediatr. 2012;79:911–5. doi: 10.1007/s12098-012-0707-y. [DOI] [PubMed] [Google Scholar]
- 20.Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 21.Fielder AR, Haines L, Scrivener R, Wilkinson AR, Pollock JI, Royal Colleges of Ophthalmologists and Paediatrics and Child Health, and the British Association of Perinatal Medicine Retinopathy of prematurity in the UK II: Audit of national guidelines for screening and treatment. Eye (Lond) 2002;16:285–91. doi: 10.1038/sj.eye.6700131. [DOI] [PubMed] [Google Scholar]
- 22.Shukla R, Murthy GV, Gilbert C, Vidyadhar B, Mukpalkar S. Operational guidelines for ROP in India: A summary. Indian J Ophthalmol. 2020;68:S108–14. doi: 10.4103/ijo.IJO_1827_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai HS, Joy R, Cherian V, Peter P. Anemia in relation to severity of retinopathy of prematurity in preterm babies born in tertiary care Centre in South India. Int J Contemp Pediatr. 2020;7:2005. [Google Scholar]
- 24.Lim ZD, Pheng E, Min ET, Van Rostenberghe H, Shatriah I. Comparison of mean platelet counts in preterm infants with and without retinopathy of prematurity. Int J Environ Res Public Health. 2021;18:3783. doi: 10.3390/ijerph18073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascal AJ, Shaikh A, Hajee MY, Lazzaro DR, Shrier EM. Association of intraventricular hemorrhage with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2011;52:3141. [Google Scholar]
- 26.Chang JW. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS One. 2019;14:e0219934. doi: 10.1371/journal.pone.0219934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni YQ, Huang X, Xue K, Yu J, Ruan L, Shan HD, et al. Natural involution of acute retinopathy of prematurity not requiring treatment: Factors associated with the time course of involution. Invest Ophthalmol Vis Sci. 2014;55:3165–70. doi: 10.1167/iovs.13-13744. [DOI] [PubMed] [Google Scholar]


