Abstract
Background: Prevalence of cognitive impairment and dementia has not been studied in Bulgaria up to date. A 2-phase cross-sectional study was designed in order to determine the prevalence of dementia, its subtypes, and mild cognitive impairment in a Bulgarian population. Methods: The study sample consisted of 605 participants over the age of 65, residents of the city of Varna. A total of 540 participants (89%) completed the screening phase of the study. All positive screens and a control group were included in the diagnostic phase of the study, where comprehensive neuropsychological, clinical, and imaging assessments were performed. Results: Dementia was diagnosed in 39 persons (7.2%) and 36 had mild cognitive impairment (6.7%). Alzheimer’s disease was the most frequent type of dementia (3.1%), followed by vascular dementia (2.0%). Discussion: Our results support the hypothesis that prevalence of vascular cognitive impairment may be higher in Bulgaria than in most European countries.
Keywords: Alzheimer’s disease, dementia, mild cognitive impairment, prevalence, Bulgaria
Introduction
Crude prevalence rates of dementia reported in recent studies 1 of European populations over the age of 65 years vary between 5.9% and 9.4%. Age standardized prevalence of dementia in the elderly individuals in Europe, as reported by the EURODEM group, 2 is 6.4%. Alzheimer’s disease (AD) is the most common cause for dementia in the European Union, accounting for 65% to 70% of the cases. 1 Nevertheless, in other countries, such as Japan, vascular dementia (VaD) is the leading subtype. 3 Prevalence of dementia increases significantly with age and doubles every 5 years after the age of 60. 2
No epidemiological studies for dementia have been performed in Bulgaria up to date; therefore no precise data exist for the prevalence of this disorder and its subtypes. Only estimations are available, obtained by extrapolation of the results from European prevalence studies. 2 According to these data, the number of people with dementia and AD in Bulgaria would be, respectively, 90 000 to 100 000 and 60 000 to 70 000. Though these values are widely accepted, there is a strong need for a dedicated epidemiological study that would document the actual prevalence in Bulgaria. The availability of accurate data would contribute to the optimization of treatment, medical, and social care for patients with dementia, which are at present neither well developed nor reimbursed by the National health insurance fund. This would also allow comparisons to be made with the results of similar studies in other countries. An important reason suggesting the need for a study of prevalence not only of dementia in general but also of its subtypes would be that vascular risk factors such as arterial hypertension and cerebrovascular disease (CVD) in Bulgaria are known to be among the highest in Europe. According to a report of the Bulgarian National statistical institute, mortality of CVD (including stroke) in the country was 265.7 per 100 000 in the year 2000 and has reached 301.6 per 100 000 in 2007. 4 The World Health Organization (WHO) data affirm that crude death rate from CVD per 100’000 in Bulgaria is the second highest in Europe, higher values being reported only for the Russian Federation. 5 These facts have raised the question in the Bulgarian neurological community of whether vascular and mixed dementia would be more frequent in Bulgaria than in other European countries. If true, this would imply a different approach to dementia on a national level, including the elaboration of specific preventive and therapeutic strategies.
Our study aimed at determining the prevalence of dementia and of its subtypes and the prevalence of mild cognitive impairment (MCI) in a community-based population over the age of 65 years in the city of Varna, Bulgaria.
Methods
A 2-phase, cross-sectional study was performed in Varna, the third largest city in Bulgaria. A community-based random sample of participants over the age of 65 years was drawn from the patient lists of 13 family physicians whose offices were located in the city’s 2 biggest hospitals and who had enlisted patients from different parts of the town. Of all the 4235 participants aged 65 years or older, being on the lists, every seventh person was selected for participation. Letters explaining the study design and goals, as well as sample informed consent forms were sent to all participants. Phone calls were then made in order to arrange the screening interviews.
All participants who had signed the informed consent were administered a screening questionnaire, requiring approximately 30 minutes for completion. Information was gathered about demographics, level of education, professional activities, current health state and medical history, subjective cognitive complaints, motor complaints, dietary habits, past and current alcohol consumption and smoking, and current use of medical drugs. The questionnaire comprised 2 neuropsychological screening tests, the Mini-Mental State Examination (MMSE), 6 and the Memory Impairment Screen (MIS), 7 as well as an instrumental activities of daily living assessment scale (the 4-item IADLs score [4-IADL]). 8
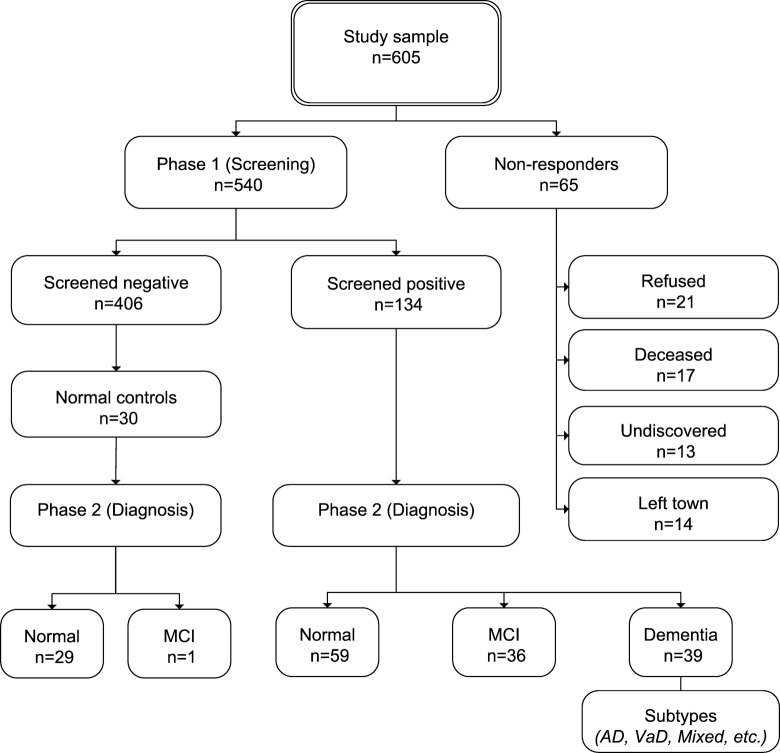
All participants who scored less than 25 points on MMSE or less than 6 points on MIS or more than 1 point on 4-IADL were regarded as positively screened for cognitive impairment. Together with a random sample of 30 negatively screened participants, they were invited to visit the First Neurological Clinic of Sveta Marina University hospital in Varna for the second phase of the study. It consisted of a comprehensive neuropsychological battery: Consortium to Establish a Registry for Alzheimer's Disease (CERAD) cognitive battery, 9 MIS (alternative form), CERAD depression scale, followed by physical examination, neurological examination, brain computed tomography (CT), and laboratory assessments including hematology, hemocoagulation, and biochemistry for all patients diagnosed with dementia. All patients’ records were finally reviewed by a committee of neurologists with expertise in dementias. The committee established a consensus diagnosis of dementia, MCI, or no cognitive impairment according to respective criteria (Table 1). Study design is shown on Figure 1. The study was approved by the Ethics committee of the Varna Medical University.
Table 1.
Diagnostic Criteria Used in the Study
| Diagnosis | Criteria |
|---|---|
| Dementia | DSM-IV 10 |
| MCI | Petersen criteria, modified by Portet et al 11 |
| Alzheimer’s disease | NINCDS-ADRDA 12 |
| Vascular dementia | NINDS-AIREN 13 |
| Dementia with Lewy bodies | Consensus clinical and pathological diagnostic criteria for DLBs 14 |
| Frontotemporal dementia | Revised Manchester/Lund criteria 15 |
| “Mixed” dementia | NINCDS-ADRDA + presence of CVD |
Abbreviations: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); MCI, mild cognitive impairment; DLBs, dementia with Lewy bodies; CVD, cerebrovascular disease; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke, Alzheimer's Disease and Related Disorders Association; NINDS-AIREN, National Institute of Neurological Disorders and Stroke, Association Internationale pour la Recherche et l'Enseignement en Neurosciences.
Figure 1.
Study design and participants.
Results
A total of 605 participants whose general health and cognitive status were unknown to the authors were included in the study sample. Informed consent was signed by 540 participants who were included in the study, while nonresponders were 65, response rate equaling 89.3% (Table 2). Twenty-one of the nonresponders refused to sign the informed consent, 17 had died, 13 could not be found at the available addresses and telephone numbers, and 14 had moved out of town (Figure 1). Of all 540 evaluated participants during phase 1, 134 (24.8%) screened positive for cognitive impairment and 406 (75.2%) were negative screens.
Table 2.
Study Sample by Age and Sex
| Number (M/F) | Age (years) | |
|---|---|---|
| Sample total | 605 (260/345) | 73.2 + 5.7 |
| Responders | 540 (223/317) | 73.0 + 5.5 |
| Nonresponders | 65 (37/28) | 74.8 + 6.9 |
Phase 2 was completed successfully by all intended participants. Dementia was diagnosed in 39 of the positively screened persons, 36 had MCI, and 59 were classified as having no cognitive impairment. Two cases of pseudodementia due to depression and 1 due to illiteracy were observed. As these 3 participants had neither dementia nor MCI, they were included in the normal group. Among the 30 negatively screened control participants who underwent comprehensive neuropsychological assessment, there were 1 additional case of MCI and no cases of dementia.
Prevalence for each diagnosis is represented as the number of cases conforming to respective diagnostic criteria per 100 participants of the study sample (n = 540). The overall prevalence of dementia (excluding nonresponders) was 7.2% (95% confidence interval [CI] = 5.0-9.4), while MCI was diagnosed in 6.7% of the participants (95% CI = 4.6-8.8). Alzheimer’s disease was the most frequent type of dementia, accounting for 3.1% (95% CI = 2.3-3.9) of the sample, followed by VaD (2.0%, 95% CI = 0.8-3.2), mixed dementia (1.3%, 95% CI = 0.3-2.3), and dementia with Lewy bodies ([DLBs] 0.4%, 95% CI = 0.1-1.2; Table 2). Though the criteria for frontotemporal dementia were included in the study protocol, no cases of the disorder were found. Two cases of dementia according to Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition [DSM-IV]) criteria were determined, which did not comply with any specific criteria for dementia subtypes. As etiological factors capable of explaining dementia were found in both cases (head trauma and surgical intervention for temporal meningioma, respectively), they were classified as “other dementias” (Table 3).
Table 3.
Frequency, Prevalence, and 95% CI of Dementia Types and MCI in the Study Sample a
| Dementia type | Frequency | Prevalence | Prevalence (% of all dementias) | 95% CI |
|---|---|---|---|---|
| Alzheimer’s disease | 17 | 3.1% | 43.7% | 2.3-3.9 |
| Vascular dementia | 11 | 2.0% | 28.2% | 0.8-3.2 |
| Mixed dementia | 7 | 1.3% | 17.9% | 0.3-2.3 |
| Lewy body disease | 2 | 0.4% | 5.1% | 0.1-1.2 |
| Other dementias | 2 | 0.4% | 5.1% | 0.1-0.2 |
| Dementia total | 39 | 7.2% | 100.0% | 5.0-9.4 |
| MCI | 36 | 6.7% | 4.6-8.8 |
Abbreviations: CI, confidence interval; MCI, mild cognitive impairment.
a n = 540.
Discussion
In this population-based sample of elderly individuals from Varna, Bulgaria, we found a prevalence of dementia equal to 7.2% (95% CI = 5.0-9.4). Our results are comparable to those reported for samples of similar age and race in Europe (6.2%-7.6%), 2,16,17 in America (2%-10% in the United States 18 and 8% in Canada 19 ), in Australia (5.3%-7.6% 20 ), though being closer to the higher values. Studies of dementia prevalence in countries from Eastern and Central Europe are fewer, presenting a considerable heterogeneity of assessed populations, sample sizes, diagnostic standards, and conventions used to report findings. 21 Nevertheless, results for dementia prevalence comparable to ours are reported, for example by Gabryelewicz in Poland (7.8%) 22 and by Stefanova et al in Serbia (6.7%). 23
A study conducted in Taiwan 24 using a design very similar to ours (2 phases, screening with MMSE and diagnostic phase with the CERAD battery) shows a lower prevalence of dementia, equal to 3.7%, that the authors attribute mostly to some demographic characteristics of the sample.
Prevalence of dementia subtypes in the present study demonstrates some differences when compared with results commonly reported in the literature. We found a lower rate of AD (43%), while in most studies conducted in Europe, United States, and Australia it exceeds 50%. 2,18,20 On the other hand, vascular and mixed dementias are more frequent in our sample. This could be explained by the high prevalence of vascular disorders in Bulgaria and their role in the pathogenesis of these dementia subtypes. The term mixed dementia is considered not precise enough by some authors, being supported only by scarce epidemiological evidence 25 and referring to a condition which is difficult to diagnose using currently available clinical and morphologic criteria. 26 However, the prevalence of mixed dementia in our study, equal to 17.9% of all dementias, further emphasizes the importance of vascular lesions. If combined, VaD and mixed dementia would account for more cases than pure AD, representing 46.1% versus 43.7% of all dementias in the sample.
The small number of DLBs cases found in our study and the lack of cases with frontotemporal dementia could be due to the generally low prevalence of these disorders, whose precise determination would require a larger study sample.
Similarly to dementia, prevalence of MCI in our study, equaling to 6.7% (95% CI = 4.59-8.81), is comparable to that found in other studies. 17,27,28 Our results also conform to the mathematical model developed by Yesavage et al, stating that the prevalence of MCI is commonly twice as high as that of AD. 29 Among the control group of 30 negatively screened persons, we found 1 case of MCI. This suggests that the prevalence of MCI in the sample could be higher than what we obtained and could be explained by the relatively low cut-off scores of the neuropsychological tests we used for screening in order to achieve better specificity for the diagnosis of dementia, though lowering sensitivity.
Our study has certain limitations, such as the relatively small sample size and the inclusion of an urban region only. Nevertheless, being the first study to assess the prevalence of dementia in Bulgaria, it provides data that have not been available before and gives an overall impression of a problem largely underestimated so far, not only by medical professionals but also by health authorities in the country. Moreover, it could be used as a baseline for future studies of dementia incidence and mortality, which have not been assessed in Bulgaria up to now either.
The results we obtained support the hypothesis that prevalence of vascular cognitive impairment may be higher in Bulgaria than in other European countries, especially those in Western Europe. If future studies confirm this finding, there would be an excellent possibility to reduce the overall burden of dementia in Bulgaria by putting additional efforts in vascular prevention and prophylaxis. In this regard, studies of larger populations need to be performed in Bulgaria. They would not only provide more precise data on the prevalence of dementia and MCI representative for the whole country but would determine the prevalence of rarer subtypes of dementia as well.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
For this research project Dr. Dimitrov received a grant from the French government (Bourse du gouvernement francais) and visited the Neuroepidemiology unit of INSERM in Paris, France.
References
- 1. Berr C, Wancata J, Ritchie K. Prevalence of dementia in the elderly in Europe. Eur.Neuropsychopharmacol. 2005;15(4):463–471. [DOI] [PubMed] [Google Scholar]
- 2. Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 suppl 5):S4–S9. [PubMed] [Google Scholar]
- 3. Ikeda M, Hokoishi K, Maki N, et al. Increased prevalence of vascular dementia in Japan: a community-based epidemiological study. Neurology. 2001;57(5):839–844. [DOI] [PubMed] [Google Scholar]
- 4. National statistical Institute. Population and demographic processes 2007 [in Bulgarian]. Sofia, 2008.
- 5. European detailed mortality database (DMDB). Copenhagen, WHO Regional Office for Europe. http://www.euro.who.int/InformationSources/Data/20070615_2. Electronic Citation. Accessed 29 April, 2009.
- 6. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 7. Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the memory impairment screen. Neurology. 1999;52(2):231–238. [DOI] [PubMed] [Google Scholar]
- 8. Barberger-Gateau P, Commenges D, Gagnon M, Letenneur L, Sauvel C, Dartigues JF. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc. 1992;40(11):1129–1134. [DOI] [PubMed] [Google Scholar]
- 9. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. [DOI] [PubMed] [Google Scholar]
- 10. The American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, 1994. [Google Scholar]
- 11. Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. 2006;77(6):714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 13. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. [DOI] [PubMed] [Google Scholar]
- 14. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers.Dis. 2006;9(3 suppl):417–423. [DOI] [PubMed] [Google Scholar]
- 15. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. [DOI] [PubMed] [Google Scholar]
- 16. Ott A, Breteler MM, van Harskamp F, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ. 1995;310(6985):970–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tognoni G, Ceravolo R, Nucciarone B, et al. From mild cognitive impairment to dementia: a prevalence study in a district of Tuscany, Italy. Acta Neurol Scand. 2005;112(2):65–71. [DOI] [PubMed] [Google Scholar]
- 18. Alloul K, Sauriol A, Kennedy W, et al. Alzheimer's disease: a review of the disease, its epidemiology and economic impact. Arch Gerontol Geriatr. 1998;27(3):189–221. [DOI] [PubMed] [Google Scholar]
- 19. Lindsay J, Sykes E, McDowell I, Verreault R, Laurin D. More than the epidemiology of Alzheimer's disease: contributions of the Canadian Study of Health and Aging. Can J Psychiatry. 2004;49(2):83–91. [DOI] [PubMed] [Google Scholar]
- 20. Dementia in Australia: National data analysis and development. Australian Institute of Health and Welfare (AIHW) AIHW cat. no. AGE 53. Canberra, 2006.
- 21. Alzheimer Europe: prevalence of dementia in Eastern Europe. http://www.alzheimer-europe.org/Our-Research/European-Collaboration-on-Dementia/Prevalence-of-dementia2/Prevalence-of-dementia-in-Eastern-Europe. Electronic Citation. Accessed January 20, 2010.
- 22. Gabryelewicz T. The prevalence of dementia in the population of the Warsaw district of Mokotow from 65 to 84 years of age. Psychiatr Pol. 1999;33(3):353–366. [PubMed] [Google Scholar]
- 23. Stefanova E, Pekmezovic T, Nalic D, Kostic VS. The diagnosis of dementia is unspecified--report of a pilot survey of dementia in belgrade. Gerontology. 2004;50(4):260–261. [DOI] [PubMed] [Google Scholar]
- 24. Lin RT, Lai CL, Tai CT, Liu CK, Yen YY, Howng SL. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. J Neurol Sci. 1998;160(1):67–75. [DOI] [PubMed] [Google Scholar]
- 25. Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138(6):353–364. [DOI] [PubMed] [Google Scholar]
- 26. Jellinger KA. The enigma of mixed dementia. Alzheimers Dement. 2007;3(1):40–53. [DOI] [PubMed] [Google Scholar]
- 27. Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349(9068):1793–1796. [DOI] [PubMed] [Google Scholar]
- 28. Koivisto K, Reinikainen KJ, Hanninen T, et al. Prevalence of age-associated memory impairment in a randomly selected population from eastern Finland. Neurology. 1995;45(4):741–747. [DOI] [PubMed] [Google Scholar]
- 29. Yesavage JA, O'Hara R, Kraemer H, et al. Modeling the prevalence and incidence of Alzheimer's disease and mild cognitive impairment. J Psychiatr Res. 2002;36(5):281–286. [DOI] [PubMed] [Google Scholar]