Abstract
There is evidence that exercise may reduce the progressive cognitive dysfunction of Alzheimer's disease (AD). However, no previous investigation has studiethe acute effects of adapted games (AG) on patients with AD. The aim of this study was to examine the acute effects of AG on the agitated behavior (rating scale Agitated Behavior Rating Scale [ABRS]) and cognitive performance (Test for Severe Impairment [TSI]) of patients with advanced dementia. Twenty patients (83±4 yrs) participated in AG and placebo activities (PL). Agitated behavior and cognitive performance were compared before and after 30 minutes of AG and PL. In the hour after the AG, agitated behavior decreased by ∼4 ABRS points and cognitive performance increased by ∼5 TSI points. On the contrary, after PL we found no change in agitated behavior or cognitive performance. Our data indicate that AG can momentarily reduce agitated behavior and increase the cognitive performance in participants with AD.
Keywords: adapted games, agitated behavior, cognitive performance, Alzheimer’s
Introduction
Alzheimer's disease (AD) is characterized by a progressive sequence of clinically observable decreases in memory, independence, cognitive function, and mobility. Despite clear and substantial differences between older participants with dementia and children, several studies have shown similarities between the clinical sequences of cognitive and functional decline in subjects with AD and the sequence of acquisition during normal human development. 1 Reisberg et al clearly described this phenomenon called retrogenesis, 2 and they developed the Functional Assessment Staging (FAST) method that includes 16 progressive stages of capacity loss during progressive cognitive degeneration typical of AD. Significant validity and repeatability of this process of capacity impairment in AD have been extensively documented. 3
Agitated behavior is frequent in patients with AD having advanced dementia, and the management of related problems in these patients is crucial to maintaining their quality of life. 4 The neuroleptics and physical restraints are traditional methods used to handle behavior disorders in participants with AD. 5 -7 This approach seems to reduce symptoms like aggressiveness, agitation, delusion, wandering, among others, but a frequent side effect is worsening cognitive function. 8 While many advances in AD care have been made, clearly more needs to be done and there are particular needs for the development of new nonpharmaceutical interventions that can potentially help in the management of patients with AD. 9 Interestingly, nonpharmaceutical passive care actions, for example foot baths, seem to be suitable methods to help bedridden palliative care patients relax. 10 Yamamoto et al 11 reported that warm water foot baths led to relaxation with an increase in parasympathetic response and a decrease in sympathetic response; however, the effect of this nonpharmaceutical passive approach on the agitated behavior of patients with AD is not clear. In this scenario, other studies have reported that nonpharmaceutical passive care strategies such as therapeutic touching 12,13 or more active interventions like light exercise can mitigate agitation behaviors and reduce progressive cognitive dysfunction of participants with dementia. 14 -16 Studies on people with moderate dementia have described the numerous positive effects of physical exercise: improvement in walking capacity, better urinary continence, enhanced communication, reduced depression, and increased activities of daily life (ADLs). 17 Although most of the physical exercise activities used in previous studies had positive effects on cognitive function, none of the authors considered the retrogenesis phenomenon of AD as a possible proposal to evaluate a new therapeutic approach. Emerging literature in healthy adults and subjects with Parkinson’s disease suggests that acute bouts of exercise, or games, selectively facilitate multiple cognitive processes, and under certain conditions exercise can enhance response speed and response accuracy, facilitate cognitive processes that are fundamental to problem-solving and goal-oriented actions. 18 -20 In addition, recent studies have pointed out the positive effects of games on executive function and working memory in children 4 to 12 years old. 21 In fact, O'Malley showed that games like jumping rope, basketball, and soccer improved executive function and mathematical reasoning in a group of children aged 7 to -11 years. 22 Consequently, considering the great differences, but also some of the similarities, between the cognitive functional decline in subjects with AD and the sequence of acquisition during normal human development, a potential successful exercise program in patients with AD should be similar to the natural exercises done by children.
Therefore, the aims of this study were to evaluate the feasibility and the acute effects of an exercise program, with active physical participation in adapted games (AG), on a group of patients with severe dementia. We hypothesized that the effects of ball games could be more effective in reducing the agitated behavior of patients with AD and should have a positive influence on their cognitive performance compared to a placebo program (PL) based on passive activities like foot baths and foot massage.
Methods
Eligible individuals were selected from among nursing home residents of an Alzheimer’s care unit (ACU) at the Mons Mazzali Geriatric Institute in Mantua, Italy. Inclusion criteria 23 were 65 years of age or older and a Mini-Mental State Examination (MMSE) score between 5 and 15.
Participants
Based on the inclusion criteria, 28 residents of the ACU (6 men and 22 women) were selected for the study. Relatives of the selected residents received written and oral information about the study and all of them agreed to participate and gave their written, informed consent. The Institutional Review Board of the Department of Neurological, Neuropsychological, Morphological and Motor Sciences of the University of Verona and the Mons Mazzali Institute Ethical Committee approved the study.
The medical staff of the ACU reviewed the clinical files of the 28 selected residents and excluded 4 (3 men and 1 women) because of severe heart and pulmonary disease. The medical staff also made a record of the anthropometrics, current medication. and clinical characteristics of the participants, as well as gait and mobility limitations (Performance-Orinted Mobility Assesment [POMA], test validity 0.64-0.70; test reliability .88-.97; Table 1). 24
Table 1.
Participant Characteristics
| Participant characteristics Mean ± SD (range) | ||
|---|---|---|
| Age (years) | 83 ± 4 (76-87) | |
| Body mass (kg) | 63 ± 4 (71-58) | |
| Height (m) | 1.55 ± 0.07 (1.50-1.71) | |
| POMA (0-28) | 15 ± 9 (0-26) | |
| BANSS (7-28) | 17 ± 2 (14-20) | |
| Barthel (0-100) | 52 ± 11 (30-80) | |
| MMSE (0-30) | 11 ± 3 (8-15) | |
| FAST (1-7f) | 6e (6d-7a) | |
| Comorbidity (N cases/total) | ||
| Cardiopathy | 7/20 | |
| Hypertension | 4/20 | |
| Depression | 4/20 | |
| Presbyopia | 4/20 | |
| Medications (N cases/total) | ||
| Quetiapine | 11/20 | |
| Acetylsalicylic acid | 8/20 | |
| Delorazepam | 8/20 | |
| Citalopram | 6/20 | |
Abbreviations: POMA, Performance -Oriented Assesment; gait/balance test 24 ; BANSS, Bedford Alzheimer nursing severity scale 25 ; Barthel, activities of daily living 26 ; MMSE, Mini-Mental State Examination 23 ; FAST, functional assessment staging.3 Note. The FAST test range from 1 to 7, with some sub-scales on the stage 6 (a - e), and on the stage 7 (a - f).
Baseline Measures
The medical staff of the ACU recorded anthropometric measures (height and weight) using a professional mechanical scale fitted with a stadiometer (Seca mod. 713; IIIM; Seca Medical Scales and Measuring Systems, Birmingham, UK). A physiotherapist of the ACU evaluated the nursing home residents’ gait/balance performance using the POMA test (Table 1). 24
The ACU’s neuropsychologist and head nurse analyzed the global cognitive functions of participants through the MMSE. 23 The residents' functional impairments were determined using the Bedford Alzheimer Nursing Severity Scale (BANSS; test validity 0.62-0.79) 25 and the Barthel index (test validity α = .935; test reliability = .989). 26 According to the neurological retrogenesis of AD, functional stages of capacity loss of the selected patients were determined through the FAST (test validity 0.59-0.73; test reliability .86-.87; Table 1). 27
Study Overview
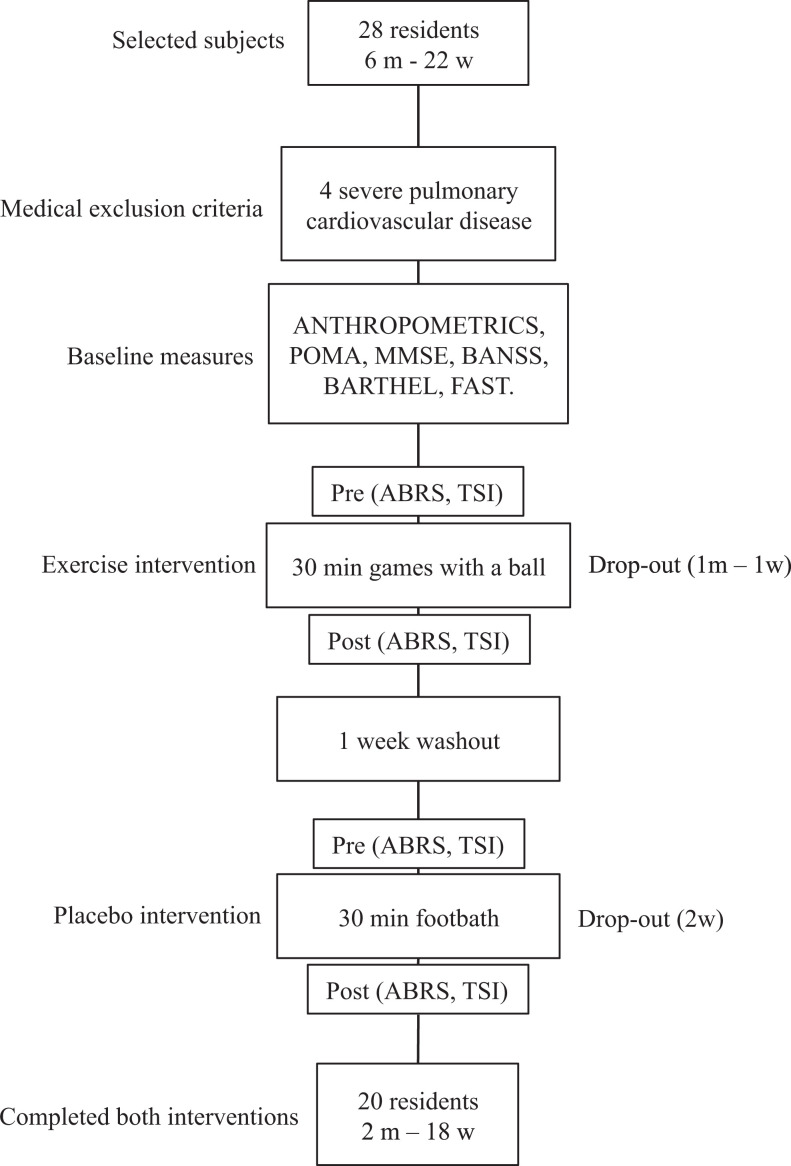
Following the baseline assessments, the 24 selected residents were randomly assigned to an exercise group that would do active activities with a soft ball (AG) or to a placebo group (PL) with relaxing passive activities like foot baths and foot massage, with 1 week of washout in between. Each participant completed 2 sessions in both the AG and PL groups (Figure 1).
Figure 1.
Study overview.
m, man; w, women; POMA, Performance -Oriented Assesment; gait/balance test 24 ; BANSS, Bedford Alzheimer nursing severity scale 25 ; Barthel, activities of daily living 26 ; MMSE, Mini-Mental State Examination 23 ; FAST, functional assessment staging 3 ; ABRS, agitated behavior rating scale 28 ; TSI, test for severe impairment. 29
Acute Measures
In order to evaluate the acute effects of the ball games or the foot baths and foot massages (placebo), 1 hour before and 1 hour after the AG and PL sessions, the residents' agitated behaviors were recorded by the head nurse of the ACU using the Agitated Behavior Rating Scale (ABRS; coefficients of variation in the present study = 7.1). 28 The ABRS consists of 5 major behaviors: manual manipulation, restraint escape, searching or wandering, tapping or banging, and vocalization. Scores could range from 0 to 15 points for each observation period. To evaluate the acute effect on cognitive function associated with the activity, the neuropsychologist of the ACU collected the 24 items of the Test for Severe Impairment (TSI; coefficients of variation in the present study = 7.7). 29 The TSI is a psychometric performance-based scale and consists of 24 items divided into 6 different cognitive domains. Maximum credit for each section is 4 points, for a maximum score of 24, which indicates no cognitive limitation. The test uses simple common objects and responses are not always language dependent but may be based on the observation of a patient’s response such as pointing, gesturing, or manipulation of objects. Each patient immediately before and after the AG and PL sessions was accompanied to an appropriate room of the ACU and the TSI was carried out in 10 minutes. As suggested by Gardner et al, 30 the number of errors during a standardized TSI is used to evaluate a possible learning effect associated with the AG and PL; thus the neuropsychologist recorded the sum of errors related to the 24 items in the TSI and grouped them into 6 different cognitive domains: immediate and delayed memory (4 questions), general knowledge (4 questions), conceptualization (4 questions), language comprehension (4 questions), language production (4 questions), and motor performance (4 questions). The average of errors for each cognitive domain was calculated before and after the AG and PL sessions; the intervening time between the 2 TSI tests was 30 minutes (Figure 2). The kinesiologist and nurses at the ACU registered the patient’s adherence for regular participation in each session in a log. If the patient had anxiety, tried to escape from the experimental setting, or felt pain, panic, or discomfort during the AG or PL sessions, the activity was immediately stopped and the impeding cause was recorded in the log.
Figure 2.
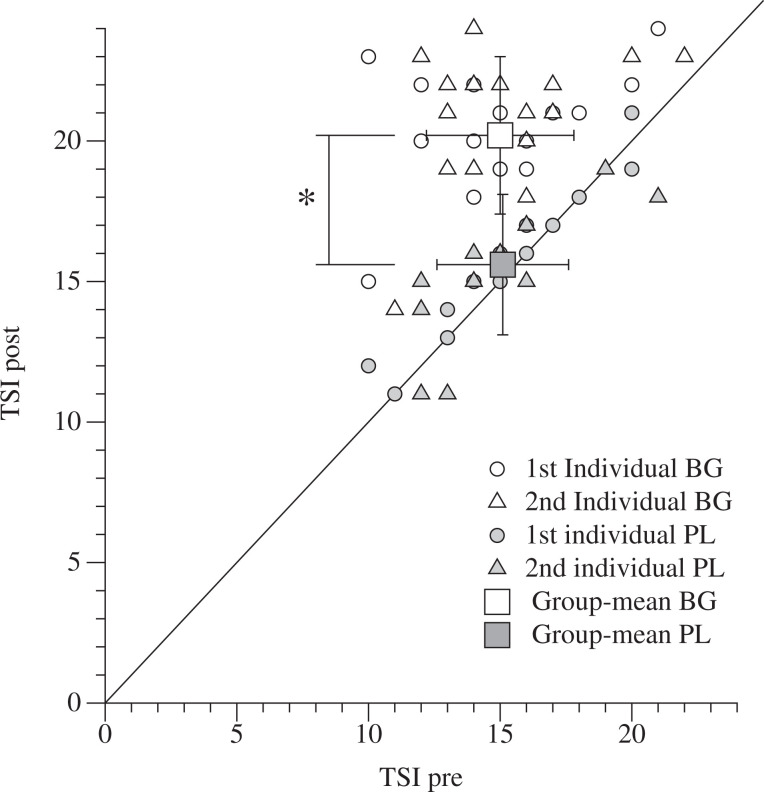
Individual and Group mean Test for Severe Impairment. TSI indicates test for severe impairment. 29 Open circles represent individual TSI score before and after the first ball games (adapted games [AG]), open triangles represent individual TSI score before and after the second AG session. Open squares represent group mean of TSI before and after AG. Filled circles represent individual TSI score before and after the first placebo exercise (PL), filled triangles represent individual TSI score before and after the second PL activity. Filled squares represent group mean of TSI before and after PL. Group means are mean ± standard deviation (SD). *between-groups p < .05.
Adapted Ball Games
The nursing home residents selected for the study where randomly grouped for the AG in groups of 5 patients. We adopted this small group setting because the goal of the AG was to involve all the patients in the active games for the entire duration of the session (∼30 minutes). All the participants remained sitting in standard armchairs or wheelchairs for the duration of the games, with a distance of 1 m between the chairs and forming a circle. A kinesiologist, specialized in adapted physical activity, controlled the AG from the center of the circle. The AG session consisted of 2 AG with a ball (a) tossing and catching a ball with both hands from the kinesiologist or to/from other patients in the group; the goal of this game was not to drop the ball on the floor; (b) kicking and stopping the ball with one foot, rolled to them by the kinesiologist or other patients in the group; the goal of the game was not to miss the ball and keep it inside the circle. Each game was played for 15 minutes, in random order, using a soft sponge ball. The AG activity was done 2 times on different days for each nursing home participant; the sessions were held in the morning from 9 to 10 in an all-purpose room of the ACU.
Placebo Activity Sessions
To determine the acute effects of a passive activity (used as placebo) on the AD patients' agitated behavior and their TSI scores, a foot bath was given to all the nursing home residents selected for the study. The patients were accompanied by a nurse one at a time to the adapted bathroom of the ACU. They sat on a standard chair or in their own wheelchair while their feet soaked in warm water in a standard tub. The water was mixed with natural soap and aromatic oils. Every 10 minutes the nurse gave the patient’s feet a gentle massage as suggested in previous studies on therapeutic massage in patients with AD. 12,13 The aim of the foot bath was to get the patients to relax through a passive activity. The PL session was repeated 2 times in the morning from 9 to 10 on different days for each participant, and the duration of the foot baths was the same as for the ball games (∼30 minutes).
Statistical Analysis
Statistical analysis was done using StatPlus for Macintosh, version 2009 (AnalystSoft Inc). Analysis of variance (2-way ANOVA) and Tukey all pairwise multiple comparison procedures were used to test the effects of AG and PL; a P value below .05 was considered statistically significant. Data are presented as mean ± standard deviation (SD).
Results
Participant Characteristics
The clinical characteristics of the participants—anthropometrics, medication, and mobility—are shown in Table 1. Although 7 patients selected for this study showed severe mobility limitations and were in wheelchairs, none of them had difficulty in performing the ball activities or foot baths and foot massage.
General cognitive capacity, functional impairments in performing ADLs and the FAST stages of participants are shown in Table 1. The mean MMSE was 11 ± 2 confirming the severe cognitive impairment of the selected residents. Both ADL indexes, BANSS 17 ± 2 and Barthel 52 ± 17, indicated substantial functional impairment in performing ADLs. The range of functional capacity loss determined by the FAST method was between the 6d and 7a stages, which corresponds to a moderately severe to severe Alzheimer stage.
Compliance to the Experiment Activities
Two residents, 1 man and 1 woman, experienced anxiety and discomfort during the 2 AG sessions; 1 woman refused to do the foot bath; and 1 additional patient interrupted the PL activity because she felt discomfort in the feet. Twenty patients, 2 men and 18 women, completed both session requirements; thus equal dropouts of 2 patients were recorded during the AG or PL sessions (Figure 2).
Agitated Behavior
Five major agitated behaviors and total agitated behaviors recorded 1 hour before and 1 hour after the AG and PL sessions are shown in Table 2. The ABRS coefficients of variation between the repetition of the 2 AG sessions was 6.5% (before) and after 7.8% (after). None of the agitated behaviors were different before the AG and PL. The ball games significantly decreased agitated behaviors: manual manipulation (−47%; P < .05), searching or wandering (−54%; P < .05), tapping or banging (−78%; P <.05), and vocalization (−70%; P < .05); whereas escaping restraints had no significant change (−40%; P =.7). After the AG, the average of total agitated behaviors had drastically decreased by 60% (P < .05). The placebo activity significantly reduced only the manual manipulation agitated behavior (−41%; P < .05); and despite a trend in the reduction of ABRS, total agitated behaviors recorded 1 hour after PL did not decline (−23%; P = .8).
Table 2.
Agitated Behaviora
| AG (N = 20) | PL (N = 20) | |||
|---|---|---|---|---|
| ABRS | Pre | Post | Pre | Post |
| Total | 6.5 ± 2.2 | 2.6 ± 1.6b,c | 6.3 ± 2.3 | 4.9 ± 2.3 |
| Manual | 1.5 ± 0.9 | 0.8 ± 0.6b | 1.4 ± 0.8 | 0.9 ± 0.9b |
| Escape | 0.8 ± 0.8 | 0.6 ± 0.6 | 0.9 ± 0.9 | 0.8 ± 0.8 |
| Search | 1.3 ± 0.8 | 0.6 ± 0.6b | 1.0 ± 0.8 | 0.9 ± 0.8 |
| Tapping | 1.6 ± 0.9 | 0.5 ± 0.5b,c | 1.4 ± 1.0 | 1.2 ± 1.0 |
| Vocal | 1.4 ± 0.7 | 0.5 ± 0.5b,c | 1.5 ± 1.1 | 1.2 ± 1.2 |
Abbreviations: ABRS agitated behavior rating scale 28 ; SD, standard deviation; AG, adapted games.
aData expressed as mean ± SD. AG exercise games with a ball, PL foot baths, and foot massage.
bIn-group P < .05.
cBetween groups P < .05.
Acute Effects on Cognitive Performance
The individual and group mean values of the test for severe impairment recorded before and after the 2 sessions of AG and PL are shown in Figure 3. The TSI coefficients of variation between the repetition of the 2 AGs was 7.2% (before) and 8.3% (after). Before the experiment sessions TSI was similar in both conditions (AG = 15.1 ± 2.8, and PL = 15.2 ± 2.3; P= .9). After the games with a ball, the mean TSI increased significantly (20.2 ± 3.1; P < .05) but had not changed after the placebo sessions (15.6 ± 2.5; P = .5). Both individual and mean data of TSI immediately after the AGs were 23% greater compared to the PL (P < .05).
Figure 3.
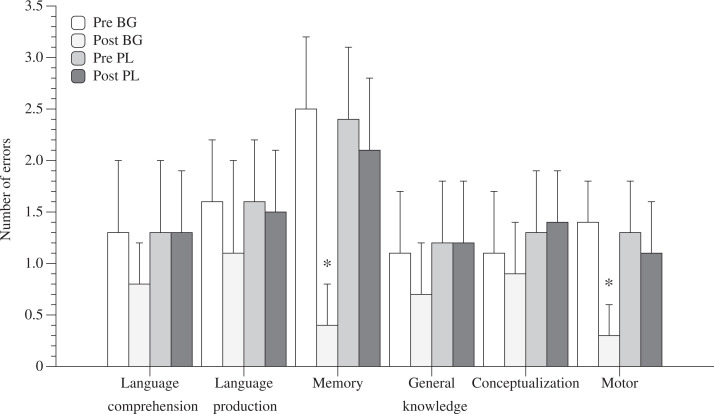
Number of errors on the 6 subscale cognitive domains of TSI. Data expressed as mean ± standard deviation (SD). * in-group and between groups p < .05.
Learning Effect
The average number of errors for 6 different cognitive domains—memory, general knowledge, conceptualization, language comprehension, language production, and motor performance—recorded during the TSI before and after the AG and PL sessions are shown in Figure 3. None of the 6 different cognitive domains were different before the AG and PL activities. After the ball games, the mean number of errors on memory and motor performance had significantly decreased (−84%; P < .05) and (−70%; P < .05), respectively. No difference was found in the mean number of errors for the remaining 4 cognitive domains.
Discussion
The care of patients with AD having advanced dementia is a major problem in Western countries. 31 Certain nonpharmaceutical passive care approaches like foot bathing and therapeutic touching are promising methods to reduce the agitated behavior of participants with AD. 10 -13 On the other hand, active exercise-based activities like walking programs are feasible approaches to reduce progressive cognitive dysfunction. 14 Indeed, subjects with AD and children are extremely different in most of the aspects of their lives, but similar in certain ways nevertheless, as suggested by the neurological retrogenesis of AD. 1 In this scenario, it appears that a successful exercise program to reduce cognitive impairment in the AD population needs to mimic the games typically played by children. To demonstrate the feasibility and acute effects on agitated behavior, cognitive performance and the learning effects related to these active or passive programs, we organized simple ball games specifically designed for patients with AD, and passive relaxing foot baths and foot massage activities. Our data indicate that active small-group games like tossing, catching, and kicking a lightweight ball can momentarily reduce agitated behavior and increase cognitive performance in people with advanced dementia. Similarly, a significant learning effect seems to be associated with these AG by enhancing memory and the motor cognitive domains.
Despite acknowledgment of their disadvantages, physical and pharmaceutical restraints are frequently used as standard methods to manage patients' agitated behavior. 5 Moreover, the AIFA (Italian Drug Agency) recommends the use of drugs only to control severe behavioral disorders that do not respond to nonpharmacological intervention (such as changes in environment, counseling, and so on). Accordingly, other studies have reported the positive effects of nonpharmaceutical or physical restraints but rather therapeutic passive interventions to control agitation in participants with dementia. In this scenario, the results of the present study clearly indicate that for people with advanced dementia, activities that require active physical participation for 30 minutes can reduce agitated behavior in the hour immediately following the exercise. Possible explanations for these results are likely the acute post-exercise-enhancing serotonin release that could decrease agitation in patients with AD.
Reisberg et al described the phenomenon of retrogenesis in AD, where the clinical course of degenerative dementia replicates children’s typical maturation course of development. 32 During the progressive cognitive and functional deterioration of AD, 16 stages of capacity loss correspond to 16 functional stages of capacity development in children; therefore the range of functional capacity loss recorded in patients with AD recruited in the present study (6d-7a; Table 1) corresponded to the functional stages of children aged 2 to 4.5 years. 32 Recent studies on adult healthy participants, and patients with Parkinson’s disease clearly indicate that acute bouts of exercise facilitate some cognitive processes such as response speed, response accuracy, problem solving, and goal-oriented action. 19,20,33 Other studies provide convincing evidence for the positive influence of exercise on working memory, because exercise likely improves the ability to block irrelevant information and to select and respond to task-relevant information. 34 It is important to note that in the range of children age corresponding to the FAST stages of our participants with AD, the effect of games like jumping rope, basketball, and soccer clearly stimulate executive function and working memory. 21,22 Recent evidence suggests that physical activity is beneficial to cognitive performance during the human lifetime, but the mechanisms that cause this positive relationship might be different in children and healthy adults because adult brains are anatomically and functionally different from those of children. 35,36 However, whereas the brains of children are still developing their functional and anatomical organization, the brains of patients with AD are progressively developing functional and anatomical disorganization. 37 Recent findings by magnetic resonance imaging (MRI) studies support these relationships in brain function anatomy in both participants with AD and children, where the maturation sequence advances in a pattern opposite to the classical neurodegenerative sequence in AD. 37 In agreement with these data, our results show that games usually played by children can momentarily increase the cognitive performance of people with advanced dementia. Specifically, a significant learning effect seems to be associated with the AG because they enhanced memory and motor cognitive domains. Therefore, we can speculate that a similar exercise game stimulus could induce comparable transitory effects in subjects with AD and children via the activation of the prefrontal cortex induced by the games, 21,35,36 and likely improves the ability to block irrelevant information and to select and respond to task-relevant information. 19,34
Study Limitations
The main limitation of our study was the relatively small sample size; only 20 residents in the ACU completed the study. Because of this small sample size an elevated type I error could limit the interpretations and multiple comparisons of results. Moreover, this single group experimental design could potentially influence the observed results of the PL or AG activities. It is also important to underline that the positive effects shown by the simple ball games are transitory, and our conclusions are based on the acute effects of these AG on people with AD.
Conclusions
Our data show that small-group games usually played by children like tossing, catching, and kicking a ball can easily be performed by people with advanced dementia with or without mobility limitations. These AG momentarily reduce agitated behavior and increase cognitive performance in participants with AD. Potential explanations for these results are likely the acute post exercise game increases in dopamine and serotonin combined with a possible higher activation of the prefrontal cortex induced by the games. However, further studies are needed that specifically address the long-term effects of AG on cognitive function in patients with severe dementia.
Acknowledgments
This work was supported by the Mons Mazzali Foundation in Mantua, Italy. The Authors wish to acknowledge the contributions of the kinesiologists in the Alzheimer Care Unit Dr Elena Leggeri, Dr Barbara Barchetti, and the student Cassandra Antrobus.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article
References
- 1. Reisberg B, Franssen EH, Souren LE, Auer SR, Akram I, Kenowsky S. Evidence and mechanisms of retrogenesis in Alzheimer's and other dementias: management and treatment import. Am J Alzheimers Dis Other Demen. 2002;17(4):202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reisberg B, Franssen EH, Souren LE, Auer S, Kenowsky S. Progression of Alzheimer's disease: variability and consistency: ontogenic models, their applicability and relevance. J Neural Transm Suppl. 1998;54:9–20. [DOI] [PubMed] [Google Scholar]
- 3. Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. [DOI] [PubMed] [Google Scholar]
- 4. Taft LB. Conceptual analysis of agitation in the confused elderly. Arch Psychiatr Nurs. 1989;3(2):102–107. [PubMed] [Google Scholar]
- 5. Luo H, Lin M, Castle N. Physical restraint use and falls in nursing homes: a comparison between residents with and without dementia. Am J Alzheimers Dis Other Demen. 2011;26(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castle NG, Wagner LM, Ferguson JC, Handler SM. Nursing home deficiency citations for safety. J Aging Soc Policy. 2011;23(1):34–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller KG. Long-term care: facility quality and safety. Issue brief. Issue Brief Health Policy Track Serv. 2010:1–42. [PubMed] [Google Scholar]
- 8. Vigen CL, Mack WJ, Keefe R, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s Disease: outcomes from CATIE-AD. Am J Psychiatry. 2011;168(8):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lippa CF. Review of Issue: Nonpharmaceutical and Pharmaceutical Interventions for Dementia Patients. Am J Alzheimer's Dis Demen. 2010;25(8):625–626. [Google Scholar]
- 10. Yamamoto K, Nagata S. Physiological and psychological evaluation of the wrapped warm footbath as a complementary nursing therapy to induce relaxation in hospitalized patients with incurable cancer: a pilot study. Cancer Nurs. 2011;34(3):185–192. [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto K, Aso Y, Nagata S, Kasugai K, Maeda S. Autonomic, neuro-immunological and psychological responses to wrapped warm footbaths--a pilot study. Complement Ther Clin Pract. 2008;14(3):195–203. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki M, Tatsumi A, Otsuka T, et al. Physical and psychological effects of 6-week tactile massage on elderly patients with severe dementia. Am J Alzheimers Dis Other Demen. 2010;25(8):680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woods DL, Dimond M. The effect of therapeutic touch on agitated behavior and cortisol in persons with Alzheimer's disease. Biol Res Nurs. 2002;4(2):104–114. [DOI] [PubMed] [Google Scholar]
- 14. Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimer's Dis Other Demen. 2011;26(5):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dechamps A, Diolez P, Thiaudiere E, et al. Effects of exercise programs to prevent decline in health-related quality of life in highly deconditioned institutionalized elderly persons: a randomized controlled trial. Arch Intern Med. 2010;170(2):162–169. [DOI] [PubMed] [Google Scholar]
- 16. Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158–165. [DOI] [PubMed] [Google Scholar]
- 17. MacRae PG, Asplund LA, Schnelle JF, Ouslander JG, Abrahamse A, Morris C. A walking program for nursing home residents: effects on walk endurance, physical activity, mobility, and quality of life. J Am Geriatr Soc. 1996;44(2):175–180. [DOI] [PubMed] [Google Scholar]
- 18. Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol (Amst). 2003;112(3):297–324. [DOI] [PubMed] [Google Scholar]
- 19. Coles K, Tomporowski PD. Effects of acute exercise on executive processing, short-term and long-term memory. J Sports Sci. 2008;26(3):333–344. [DOI] [PubMed] [Google Scholar]
- 20. Araujo Lima AM, Cordeiro Hirata Fde C, Sales de Bruin G, Salani Mota RM, Sales de Bruin VM. The influence of playing a non-reward game on motor ability and executive function in Parkinson's disease. Behav Neurol. 2012;25(2):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Malley G. Aerobic exercise enhances executive function and academic achievement in sedentary, overweight children aged 7-11 years. J physiother. 2011;57(4):255. [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 24. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. [DOI] [PubMed] [Google Scholar]
- 25. Bellelli G, Frisoni GB, Bianchetti A, Trabucchi M. The Bedford Alzheimer Nursing Severity scale for the severely demented: validation study. Alzheimer Dis Assoc Disord. 1997;11(2):71–77. [DOI] [PubMed] [Google Scholar]
- 26. Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 27. Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatr. 1997;(suppl 1):167–171. [DOI] [PubMed] [Google Scholar]
- 28. Bliwise DL, Carroll JS, Lee KA, Nekich JC, Dement WC. Sleep and “sundowning” in nursing home patients with dementia. Psychiatry Res. 1993;48(3):277–292. [DOI] [PubMed] [Google Scholar]
- 29. Albert M, Cohen C. The Test for Severe Impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40(5):449–453. [DOI] [PubMed] [Google Scholar]
- 30. Gardner MK, Hill RD, Was CA. A procedural approach to remembering personal identification numbers among older adults. PLoS One. 2011;6(10): e25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. 2012 Alzheimer's disease facts and figures. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2012;8(2):131–168. [DOI] [PubMed] [Google Scholar]
- 32. Reisberg B, Kenowsky S, Franssen EH, Auer SR, Souren LE. Towards a science of Alzheimer's disease management: a model based upon current knowledge of retrogenesis. Int Psychogeriatr. 1999;11(1):7–23. [DOI] [PubMed] [Google Scholar]
- 33. Audiffren M, Tomporowski PD, Zagrodnik J. Acute aerobic exercise and information processing: energizing motor processes during a choice reaction time task. Acta Psychol (Amst). 2008;129(3):410–419. [DOI] [PubMed] [Google Scholar]
- 34. Lambourne K, Audiffren M, Tomporowski PD. Effects of acute exercise on sensory and executive processing tasks. Med Sci Sports Exerc. 2010;42(7):1396–1402. [DOI] [PubMed] [Google Scholar]
- 35. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. [DOI] [PubMed] [Google Scholar]
- 36. Chaddock L, Erickson KI, Prakash RS, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson PM, Apostolova LG. Computational anatomical methods as applied to ageing and dementia. Br J Radiol. 2007;80 Spec No 2:S78–S91. [DOI] [PubMed] [Google Scholar]