Abstract
In Alzheimer’s disease (AD), nonverbal aspects of communication become increasingly important in caregiver–patient interactions when the ability to communicate verbally is fading with progression of the disease. We therefore investigated the impact of cognitive deficits and neuropsychiatric symptoms, particularly apathy, on facial expression in AD. While overall neuropsychiatric symptoms were not associated with facial expression, apathy exhibited substantial correlations, even after controlling for cognitive deficits. Moreover, apathy appeared to moderate the influence of cognitive deficits: without considering apathy, cognitive deficits were associated with less specific facial expressions. After controlling for apathy, cognitive decline was related to increased facial expressiveness. In conclusion, apathetic symptoms appear to be specifically associated with facial expression in AD and thus could contribute to a disregard for patients’ needs in everyday life.
Keywords: facial expression, apathy, Alzheimer’s disease, nursing home residents
Introduction
Perception and interpretation of nonverbal communication components are crucial to perceive and understand feelings and volition of patients with Alzheimer’s disease (AD), for the ability to communicate verbally is gradually fading with the progression of the disease. 1 –4 In this regard, extra attention has to be drawn to patient–caregiver interactions that may largely depend upon nonverbally mediated communication skills, in particular facial expression. Several studies focus on the capacity of emotional perception in dementia. 5 –7 However, little is known about changes in facial expressions in this patient group. In mild AD, a relative increase in negative emotional facial expressions has been described. 8 Patients with AD viewing emotion-eliciting images demonstrate an inverted zygomatic activity pattern compared to healthy controls, although emotional experiences are rated similarly. 9 In severely demented patients, complex patterns of emotional expressions are no longer detectable, while merely fragmentary expressions are observed. 10 While those studies suggest a significant change in emotional facial expression in dementia, the potential impact of cognitive deficits could not thoroughly be examined due to small sample sizes.
Neuropsychiatric symptoms such as depression, apathy, delusional ideation, or hallucinations are frequent among demented nursing home residents and aggravate the clinical picture. 11 Apathy, as one of the most frequent symptoms, is associated with a number of adverse outcomes such as a decreased level of overall functioning, 12 cognitive impairment, 13 faster rate of cognitive decline, 14 and increased caregiver distress. 15 Apathy is generally defined as the absence of emotion, interest, concern, and motivation resulting in a lack of drive and self-initiated behavior. 16,17 In a recent conceptualization presented by Robert et al, 18 apathy is primarily defined as a persisting disorder of motivation, indicated by the above-mentioned cognitive, behavioral, and emotional aspects, respectively, thus leading to functional impairment. However, despite its great relevance as a frequently observed behavioral syndrome, particularly in neurodegenerative disorders, apathy yet awaits a generally accepted operationalization. 17,19
Apathy, but not depression, is significantly associated with severe frontal lobe–related neuropsychological deficits in AD. 20 Studies on patients with brain damage demonstrate that pronounced apathetic symptoms are associated with reduced locomotor activity. 21 However, regarding facial expressions as one core component of psychomotor activity, the potential impact of neuropsychiatric symptoms in general—and apathy in particular—remains unconsidered yet. The present study was performed to explore the determinants of emotional facial expression in AD and to address the impact of cognitive and neuropsychiatric symptoms, which were considered as potential moderating variables.
Methods
Population and Sample Selection
The study population consisted of nursing home residents participating in a survey on quality of life, which is described in detail elsewhere. 22 For the study, a convenience sample of 47 residents with probable AD according to National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria 23 was recruited. Particular care was taken to exclude the participants with other forms of dementia, coexisting severe psychiatric disorders, such as schizophrenia or substance abuse, delirium, or other severe medical diseases, which may impair facial expression. As part of a comprehensive diagnostic procedure, the Hachinski Ischaemic Score 24 was utilized in addition to exclude participants with multi-infarct dementia. Basic perceptual skills were ensured by means of a thorough clinical examination. The ethics committee of the University of Heidelberg, Germany, approved the study. Written informed consent of the residents and/or their legal caregivers was obtained prior to inclusion in the study.
Global Impairment, Cognitive Deficits, and Neuropsychiatric Symptoms
Global impairment and severity of cognitive deficits were assessed with the Global Deterioration Scale (GDS) 25 and the Mini-Mental State Examination (MMSE), 26 respectively. The Neuropsychiatric Inventory (NPI) 27 and an authorized German translation of the Apathy Evaluation Scale (AES) 28 were used to evaluate neuropsychiatric symptoms as part of an interview with a professional caregiver who was familiar with the respective patient. Assessing the severity of apathetic symptoms with the AES, we followed a dimensional approach according to the correlational design of our study. The AES has been developed as a syndrome-independent scale to assess apathetic symptoms in different diagnostic groups, considering motivation as well as cognitive, emotional, and overt behavioral aspects. 29 We had decided for an external assessment of neuropsychiatric symptoms, for patients with AD, particularly suffering from apathy, only have partial awareness of their symptoms, 30 while caregivers are considered to be reliable informants for behavioral changes. 31
Facial Expression
Procedure
Each participant was twice presented with a series of 10 either emotion-eliciting or neutral images sampled from the international affective picture system (IAPS). 32 The pictures were selected with respect to their emotional valence (i.e., 2 positive, 2 negative, and 6 neutral) as rated by a group of healthy control participants prior to the study. 22 In the order of presentation, each emotional picture was placed after a neutral picture. To avoid any daytime-dependent bias, pictures were presented in the morning and the presentation was performed in a quiet, pleasant, and familiar place, preferably in the residents’ apartments. The participants were encouraged to focus their attention on each presented picture (‘Let’s have a look at this one’); simultaneously, facial expression was videotaped. The videos were cut digitally into small sequences, each related to the presentation of 1 picture at a time and according to a standard protocol: The sequence started when the participant began to gaze at the picture and ended once he or she turned away, closed his or her eyes or showed other signs of withdrawing attention, or after a maximum time of 15 seconds, respectively.
Rating and parameterization
The resulting video sequences were analyzed with the Emotional Facial Action Coding System (EMFACS) basing on the Facial Action Coding System (FACS). 33 The FACS was demonstrated to have an excellent reliability for the assessment of individual emotion-specified combinations. 34,35 The EMFACS provides an assessment of facial expression by detecting basic components; each so-called action unit (AU) describes a singular facial movement pattern. In total, the EMFACS defines 27 AUs of which only 7 are indicative of a specific emotion (i.e., joy, surprise joy, surprise, anger, fear, disgust, sadness, and contempt). Experienced psychologists who had received extensive training and supervision on the EMFACS by senior scientists rated the frequency of every single AU in each video sequence.
Subsequently, we generated specific indicators for emotional facial expression by aggregating the data attributed to each picture. To assess the total amount of facial expression (total AU), we calculated mean frequencies of all 27 AUs per picture and averaged over all pictures. Similarly, basing on the 7 specific AUs, we determined the mean frequency of specific facial expression only (specific AU). Finally, we evaluated the congruence or incongruence between facial expression and emotion-eliciting pictures by calculating the mean frequency of facial expression indicating joy (as the most frequent pattern) when presented with the positive stimuli (congruent AU) or the negative stimuli (incongruent AU), respectively.
Statistical analysis
To determine the association between AU indicators and sociodemographic characteristics as well as clinical variables, we calculated the Pearson correlation coefficients. Kruskal-Wallis tests were performed to analyze the association between global impairment and specific facial expression. We addressed the potential overlap between cognitive decline and apathy by calculating partial correlations for the association of AU indicators and both factors, controlling apathy for cognitive decline, and vice versa. All statistical analyses were performed with the Statistical Package for Social Sciences (SPSS) software (version 15.0 for Windows; SPSS Inc, Chicago, Illinois), a P value of less than .05 was considered to indicate the statistical significance.
Results
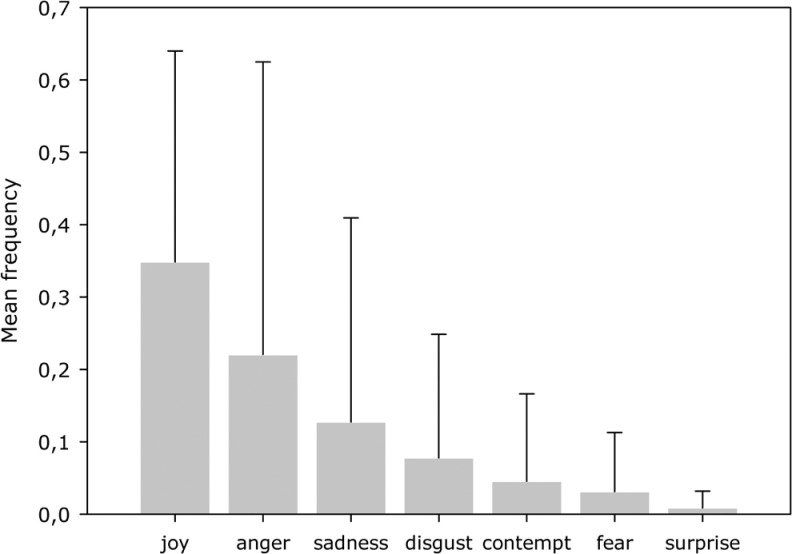
Clinical and sociodemographic characteristics of the sample are displayed in Table 1. Facial expression indicating joy was most frequently assessed followed by anger, sadness, disgust, contempt, fear, and surprise (Figure 1). This pattern of facial expression did not differ significantly in subgroups of patients with varying degrees of global impairment (total AU: χ2 = 4.22, df = 4, P = .389; specific AU: χ2 = 7.791, df = 4, P = .096; congruent AU: χ2 = 5.51, df = 4, P = .243; incongruent AU: χ2 = 2.24, df = 4, P = .711). Table 2 demonstrates the frequency of emotional expression per picture with or without certain emotional valence for the total sample.
Table 1.
Clinical and Sociodemographic Characteristics of the Sample (N = 47)
| Mean (SD) | |
|---|---|
| Female gender, N (%) | 37 (78.7) |
| Age, years | 85.3 (7.3) |
| Length of stay in nursing home, months a | 37.3 (39.2) |
| GDS | 4.5 (1.7) |
| MMSE | 11.9 (8.5) |
| NPI total b | 11.7 (10.3) |
| NPI apathy b | 2.3 (3.5) |
| NPI depression b | 2.3 (3.4) |
| AES b | 26.5 (14.5) |
Abbreviations: SD, standard deviation; GDS, Global Deterioration Scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; AES, Apathy Evaluation Scale.
a Available for N = 43 participants.
b Available for N = 44 participants.
Figure 1.
Mean frequency of emotional expression of the total sample as coded with the Emotional Facial Action Coding System ([EMFACS] error bars indicate standard deviation).
Table 2.
Frequency of Emotional Expression in the Total Sample (N = 47)
| Facial expression per picture | ||||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
| Emotional valence | M | SD | Min | Max | M | SD | Min | Max |
| Positive | 0.73 | 0.61 | 0 | 3.25 | 0.08 | 0.10 | 0 | 0.54 |
| Neutral | 0.23 | 0.28 | 0 | 1.33 | 0.08 | 0.12 | 0 | 0.60 |
| Negative | 0.31 | 0.41 | 0 | 2.00 | 0.10 | 0.15 | 0 | 0.71 |
Abbreviations: M, mean; SD, standard deviation; min, minimum count; max, maximum count.
The correlation coefficients between sample characteristics and different indicators of facial expression are displayed in Table 3. Higher degrees of global impairment and lower cognitive status as indicated by GDS and MMSE, respectively, were significantly associated with decrease in specific AUs. Neither length of stay in the nursing home nor age had a substantial impact on facial expression.
Table 3.
Correlations Between Facial Expression and Clinical and Sociodemographic Characteristics (N = 47)
| MMSE | AES b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Action units | Nursing home (months) a | Age (years) | GDS | r AES | NPI total b | NPI subscale apathy b | NPI subscale depression b | r MMSE | ||
| Total | .13 | .11 | −.22 | .19 | −.33 c | −.24 | −.35 c | −.22 | −.55 d | −.60 e |
| Specific | −.12 | .18 | −.34 c | .33 c | −.04 | −.21 | −.16 | −.11 | −.51 d | −.42 d |
| Congruent | .01 | .09 | −.27 | .21 | −.10 | −.11 | −.19 | .10 | −.38 c | −.35 c |
| Incongruent | .04 | −.15 | −.26 | .25 | −.02 | −.15 | −.22 | −.14 | −.38 c | −.30 c |
Abbreviations: GDS, Global Deterioration Scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; AES, Apathy Evaluation Scale; r AES; Pearson partial correlations corrected for AES; r MMSE, Pearson partial correlations corrected for MMSE.
a Available for N = 43 participants.
b Available for N = 44 participants.
c P < .05.
d P < .01.
e P < .001.
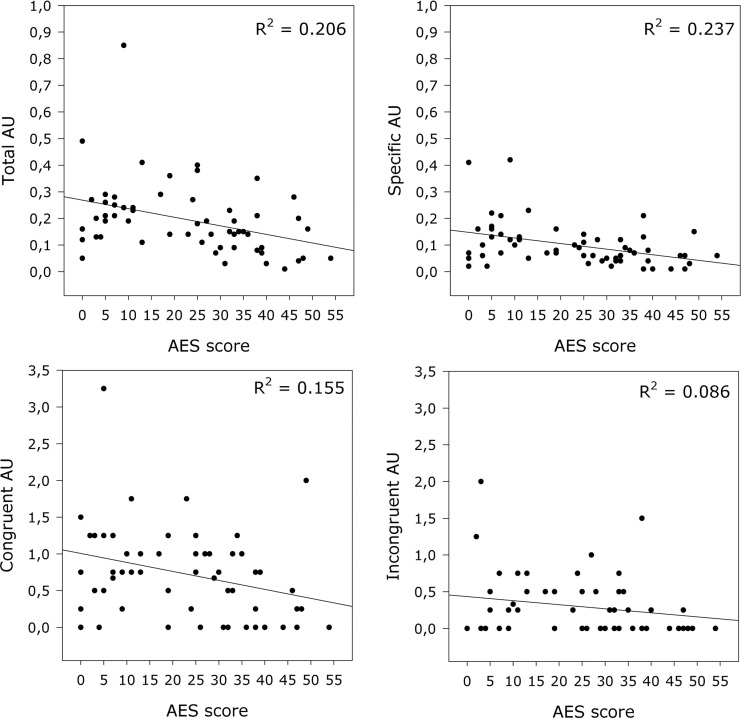
While apathy as assessed with the respective NPI subscale demonstrated a significant correlation only with total AUs, the AES score showed significant negative correlations with all 4 indicators (Figure 2). Overall neuropsychiatric symptom burden indicated by NPI total score, as well as depression according to the respective NPI subscale, were not significantly associated with the facial expression.
Figure 2.
Association of Apathy Evaluation Scale (AES) scores and frequencies of facial expression. Regression lines and the amount of explained variance (R 2) are depicted.
Measures of cognitive deficits and neuropsychiatric symptoms were significantly intercorrelated; lower MMSE scores were associated with higher overall neuropsychiatric symptoms and apathy but not depression (Table 4). We therefore adjusted the association of facial expressions and cognitive deficits (as indicated by MMSE score) on one hand, and facial expressions and apathy (as indicated by AES score) on the other hand, by controlling for the respective confounding influence. After controlling for apathy, cognitive decline was associated with an increase in total facial expression, while the association with specificity disappeared. Regarding apathy, controlling for cognitive decline corroborated the pronounced relationship of apathy and total, specific, congruent, and incongruent facial expression (Table 3).
Table 4.
Correlations Between Clinical and Sociodemographic Characteristics (N = 47)
| Nursing home (months) a | Age (years) | GDS | MMSE | NPI total b | NPI subscale apathy b | NPI subscale depression b | AES b | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | .06 | 1 | ||||||
| GDS | .10 | .05 | 1 | |||||
| MMSE | −.11 | .06 | −.94 c | 1 | ||||
| NPI Total | .02 | −.18 | .45 d | −.40 d | 1 | |||
| NPI Apathy | −.01 | −.16 | .40 d | −.44 d | .38 e | 1 | ||
| NPI Depression | .03 | .17 | .22 | −.11 | .59 c | .14 | 1 | |
| AES | .06 | −.07 | .68 c | −.67 c | .36 e | .62 c | .23 | 1 |
Abbreviations: GDS, Global Deterioration Scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; AES, Apathy Evaluation Scale.
a Available for N = 43 participants.
b Available for N = 44 participants.
c P < .001.
d P < .01.
e P < .05.
Discussion
Our study yielded 3 major findings: (i) apathy moderates the influence of cognitive deficits on facial expression, (ii) cognitive deficits are associated with increased rate of total facial expression after controlling for apathy, and (iii) apathy is significantly correlated with decreased overall and specific facial expression. Neither frequency of emotional pattern nor congruence of facial expression differed significantly between participants with varying degrees of global impairment. Likewise, the overall burden of neuropsychiatric symptoms was not related to facial expression. Our results indicate that apathy has a specific influence on facial expressions as a core component of nonverbal communication in AD.
Besides the influence of cognitive deficits, our results emphasize the impact of apathy on facial expressions in varying degrees of AD. With increasing apathy severity, that is higher AES score, the participants showed less overall and less specific facial expression. As demonstrated, this seems to be not simply a result of cognitive decline or dementia severity; on the contrary, after controlling for apathy, progression of dementia was associated with increased but less specific facial expression. Our data suggest that either apathy itself has an influence on facial expression, or both are subserved by related cerebral networks. Even though diminished motivation is one core symptom, 36,37 the syndrome of apathy also comprises loss of spontaneous emotions or emotional responsiveness. 18,19,29 The latter may be reflected in the loss of specific facial expression observed in our study. Apathy is a frequent symptom in AD that increases with progression of the disease, 38 although there is a subgroup of patients with only minor or without any signs of apathy, respectively. 11 Increased apathy, as indicated by higher AES scores, is associated with reduced overall locomotor activity in patients with brain damage, 21 thus additionally contributing to a loss of facial expression. Yet, the cerebral correlates of apathy in AD are poorly understood, but it is assumed that apathy is mediated by mesial–frontal neural networks. 39 One may assume that facial expression is also related to those networks, thus alterations of the latter in the course of AD contributing to both, apathy and diminished facial expression. A unified definition of apathy will allow a group comparison of apathetic and nonapathetic participants, 17 –19 facilitating an in-depth analysis. Hence, future studies may replicate these findings in samples that are diagnosed as apathetic.
Our result that cognitive deficits are associated with loss of specific facial expression point toward a progressive loss of control of facial expressions, corroborating previous findings. 8 Although the latter study did not control for apathy as a confounding variable, it is plausible to assume that a patient group with mild dementia was only moderately affected by apathetic symptoms, since apathy increases with progression of the disease. 38 Evidence arises from anatomical studies that neural substrates of voluntary and spontaneous emotional expression are mediated by distinct pathways. 40,41 While the former are associated with motor and premotor cortex activity, limbic structures are also relevant for the generation of spontaneous facial expressions. It is hypothesized that these 2 pathways become increasingly disrupted in the progress of dementia, thus resulting in a relative loss of voluntary facial control. 9 Alterations of limbic structures that might be associated with changes in spontaneous facial expression are not only characteristic for AD but also found in other dementia subtypes, such as vascular dementia. 42 Future studies might investigate the specific impact of those subtypes on aspects of nonverbal communication.
In the present study, the MMSE was used to evaluate the association between cognitive status and facial expression. However, results should be interpreted with caution since the MMSE as a broad screening instrument for cognitive decline does not allow an in-depth analysis of cognition. Nevertheless, our results demonstrate that both cognitive and noncognitive symptoms may distinctively influence nonverbal facial expressions even though apathy appears to moderate the influence of cognitive decline.
Although adverse consequences of apathy for patients, relatives, and professional caregivers are well known, 15 the precise mechanisms by which these effects are mediated yet await further clarification. Facial expression is a core component of social interaction and communication. From this perspective, effective communication may largely depend on accurate perception but also on expression of distinctive emotional states. Indeed, it is suggested that accuracy of judgments about the emotional content of facial expressions largely depends on the concordance of age, with best results being achieved within similar age groups. 43 Hence, in a clinical context, age differences between caregivers and nursing home residents may additionally compromise nonverbally mediated communication skills. Implementation of a training program for staff caregivers to foster the sensitivity toward nonverbal communication increased positive affect in demented patients and reduced caregiver-related symptomatology when compared to a behavioral placebo and a waiting list group. 44 In this context, apathetic symptoms appear to be an important source of influence on facial expressiveness and could thus additionally contribute to a relative disregard of patients’ needs in everyday life.
Our study is limited by the fact that we included solely nursing home residents. Thus, a potential impact of nursing home environment on facial expression in AD cannot be excluded. Furthermore, alterations in facial expression may influence the external estimation of apathy; on the other hand, apathy was assessed with the AES, which does not consider merely behavioral but rather cognitive and intrinsic aspects of apathy.
In conclusion, our results demonstrate a key influence of apathy, rather than cognitive decline, on facial expression in AD. These findings are in accordance with the concept that apathy, besides from a primary lack of motivation, also comprises a loss of spontaneous emotions or emotional responsiveness. Facing the relevance of nonverbal communication in this patient group, future studies should further address the potential impact of apathy on caregiver–patient interactions. This may lead to an improved sensitivity of staff and family members toward the needs of this potentially neglected patient group.
Acknowledgments
The authors thank Rainer Krause and Anke Kirsch, Department of Clinical Psychology, Saarland University, Saarbrücken, Germany, for their contribution to the EMFACS analysis. We are also grateful to Stefanie Becker and Roman Kaspar, Institute of Gerontology, University of Heidelberg, Germany, for statistical advice.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The study was funded in part by the German government (Bundesministerium für Familie, Senioren, Frauen und Jugend).
References
- 1. Hailstone JC, Ridgway GR, Bartlett JW, et al. Voice processing in dementia: a neuropsychological and neuroanatomical analysis. Brain. 2011;134(pt 9):2535–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Müller N, Guendouzi JA. Order and disorder in conversation: encounters with dementia of the Alzheimer’s type. Clin Linguist Phon. 2005;19(5):393–404. [DOI] [PubMed] [Google Scholar]
- 3. Carlomagno S, Santoro A, Menditti A, Pandolfi M, Marini A. Referential communication in Alzheimer’s type dementia. Cortex. 2005;41(4):520–534. [DOI] [PubMed] [Google Scholar]
- 4. Frank EM. Effect of Alzheimer’s disease on communication function. J S C Med Assoc. 1994;90(9):417–423. [PubMed] [Google Scholar]
- 5. Albert MS, Cohen C, Koff E. Perception of affect in patients with dementia of the Alzheimer type. Arch Neurol. 1991;48(8):791–795. [DOI] [PubMed] [Google Scholar]
- 6. Hargrave R, Maddock RJ, Stone V. Impaired recognition of facial expressions of emotion in Alzheimer’s disease. J Neuropsych Clin Neurosci. 2002;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 7. Keane J, Calder AJ, Hodges JR, Young AW. Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia. 2002;40(6):655–665. [DOI] [PubMed] [Google Scholar]
- 8. Smith MC. Facial expression in mild dementia of the Alzheimer type. Behav Neurol. 1995;8(3):149–156. [Google Scholar]
- 9. Burton KW, Kaszniak AW. Emotional experience and facial expression in Alzheimer’s disease. Neuropsychol Dev Cogn. 2006;13(3-4):636–651. [DOI] [PubMed] [Google Scholar]
- 10. Asplund K, Norberg A, Adolfsson R, Waxman HM. Facial expressions in severely demented patients—a stimulus-response study of four patients with dementia of the Alzheimer type. Int J Geriatr Psych. 1991;6(8):599–606. [Google Scholar]
- 11. Seidl U, Lueken U, Völker L, et al. Nicht-kognitive Symptome und psychopharmakologische behandlung bei demenzkranken Heimbewohnern [Non-cognitive symptoms and psychopharmacological treatment in demented nursing home residents]. Fortschr Neurol Psych. 2007;75(12):720–727. [DOI] [PubMed] [Google Scholar]
- 12. Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psych. 2003;11(2):214–221. [PubMed] [Google Scholar]
- 13. Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–135. [DOI] [PubMed] [Google Scholar]
- 14. Doody RS, Massman P, Mahurin R, Law S. Positive and negative neuropsychiatric features in Alzheimer’s disease. J Neuropsych Clin Neurosci. 1995;7(1):54–60. [DOI] [PubMed] [Google Scholar]
- 15. Coen RF, Swanwick GR, O'Boyle CA, Coakley D. Behaviour disturbance and other predictors of carer burden in Alzheimer’s disease. Int J Geriatr Psych. 1997;12(3):331–336. [PubMed] [Google Scholar]
- 16. Marin RS. Differential diagnosis and classification of apathy. Am J Psych. 1990;147(1):22–30. [DOI] [PubMed] [Google Scholar]
- 17. Robert PH, Mulin E, Mallea P, David R. Review: apathy diagnosis, assessment, and treatment in Alzheimer’s disease. CNS Neurosci Ther. 2010;16(5):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psych. 2009;24(2):98–104. [DOI] [PubMed] [Google Scholar]
- 19. Robert PH. For a unified definition of apathy. J Psychosom Res. 2011;71(3):197. [DOI] [PubMed] [Google Scholar]
- 20. Kuzis G, Sabe L, Tiberti C, Dorrego F, Starkstein SE. Neuropsychological correlates of apathy and depression in patients with dementia. Neurology. 1999;52(7):1403–1407. [DOI] [PubMed] [Google Scholar]
- 21. Müller U, Czymmek J, Thone-Otto A, Von Cramon DY. Reduced daytime activity in patients with acquired brain damage and apathy: a study with ambulatory actigraphy. Brain Inj. 2006;20(2):157–160. [DOI] [PubMed] [Google Scholar]
- 22. Becker S, Kruse A, Schröder J, Seidl U. Das Heidelberger Instrument zur Erfassung von Lebensqualität bei Demenz (H.I.L.DE.). Dimensionen von Lebensqualität und deren Operationalisierung [The Heidelberg instrument for the assessment of quality of life in people suffering from dementia (H. I. L. DE.)-dimensions of quality of life and methods of operationalization]. Z Gerontol Geriatr. 2005;38(2):108–121. [DOI] [PubMed] [Google Scholar]
- 23. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 24. Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632–637. [DOI] [PubMed] [Google Scholar]
- 25. Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psych. 1982;139(9):1136–1139. [DOI] [PubMed] [Google Scholar]
- 26. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 27. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 28. Lueken U, Seidl U, Schwarz M, et al. Die Apathy Evaluation Scale: erste ergebnisse zu den psychometrischen eigenschaften einer deutschsprachigen Übersetzung der Skala [Psychometric properties of a German version of the Apathy Evaluation Scale]. Fortsch Neurol Psych. 2006;74(12):714–722. [DOI] [PubMed] [Google Scholar]
- 29. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiat Res. 1991;38(2):143–162. [DOI] [PubMed] [Google Scholar]
- 30. Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer’s disease. Am J Psych. 2001;158(6):872–877. [DOI] [PubMed] [Google Scholar]
- 31. Strauss ME, Pasupathi M, Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer’s disease. Psychol Aging. 1993;8(4):475–480. [DOI] [PubMed] [Google Scholar]
- 32. Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville: University of Florida, Center for Research in Psychophysiology; 1999. [Google Scholar]
- 33. Ekman P, Friesen WV. Facial Action Coding System (FACS): Manual. Palo Alto: Consulting Psychologist Press; 1978. [Google Scholar]
- 34. Ekman P. Methodes for measuring facial action. In: Scherer KR, Ekman P, eds. Handbook of Methods in Nonverbal Behavior Research. Cambridge: Cambridge University Press; 1982:44–90. [Google Scholar]
- 35. Sayette MA, Cohn JF, Wertz JM, Perrott MA, Parrott DJ. A psychometric evaluation of the facial action coding system for assessing spontaneous expression. J Nonverbal Behav. 2001;25(3):167–185. [Google Scholar]
- 36. Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiat Clin Neurosci. 1991;3(3):243–254. [DOI] [PubMed] [Google Scholar]
- 37. Marin RS, Wilkosz PA. Disorders of diminished motivation. J Head Trauma Rehabil. 2005;20(4):377–388. [DOI] [PubMed] [Google Scholar]
- 38. Lueken U, Seidl U, Völker L, Schweiger E, Kruse A, Schröder J. Development of a short version of the Apathy Evaluation Scale specifically adapted for demented nursing home residents. Am J Geriatr Psych. 2007;15(5):376–385. [DOI] [PubMed] [Google Scholar]
- 39. Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiat Clin Neurosci. 1998;10(3):314–319. [DOI] [PubMed] [Google Scholar]
- 40. Rinn WE. The neuropsychology of facial expression: a review of the neurological and psychological mechanisms for producing facial expressions. Psychol Bull. 1984;95(1):52–77. [PubMed] [Google Scholar]
- 41. Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124(pt 1):176–208. [DOI] [PubMed] [Google Scholar]
- 42. Pantel J, Schröder J, Essig M, et al. In vivo quantification of brain volumes in subcortical vascular dementia and Alzheimer’s disease. An MRI-based study. Dement Geriatr Cogn Disord. 1998;9(6):309–316. [DOI] [PubMed] [Google Scholar]
- 43. Malatesta CZ, Izard CE, Culver C, Nicolich M. Emotion communication skills in young, middle-aged, and older women. Psychol Aging. 1987;2(2):193–203. [DOI] [PubMed] [Google Scholar]
- 44. Magai C, Cohen CI, Gomberg D. Impact of training dementia caregivers in sensitivity to nonverbal emotion signals. Int Psychogeriatr. 2002;14(1):25–38. [DOI] [PubMed] [Google Scholar]