Abstract
Objectives:
The aim was to examine the gene environment (GxE) interaction with reference to APO E genotypes, serum lipids and organochlorine pesticides (OCPs) as one of the factors in the etiology of Alzheimer’s disease (AD).
Methods:
A case control study was used to examine, APOE HhaI polymorphism by polymerase chain reaction (PCR)/PCRrestriction fragment length polymorphism method, serum lipids by autoanalyser and OCPs by gas chromatography (GC).
Results:
APOE ∊4 allele frequency was significantly high (p=0.000, OR=5.73, CI=2.68-12.50) in AD as compared to controls. The serum cholesterol, β- hexachlorocyclohexane and dieldrin are risk factors for AD independent of the APOE ∊4 risk allele, recording an odds ratio of 1.16, 11.38 and 10.45 respectively.
Conclusion:
GxE interactions exist with APOE ∊4 allele status that need to be considered for the study design and analysis of such data in future studies of AD.
Keywords: Alzheimer’s disease, gene–environment interaction, organochlorine pesticides, APOE polymorphism, β-HCH, dieldrin
Introduction
Alzheimer’s disease (AD) is the most common and progressive neurological disease that results in the irreversible loss of neurons, particularly in the cortex and hippocampus. 1 The clinical hallmarks are progressive impairment in memory, judgement, decision making, orientation to physical surroundings, and language. Pathologically, AD is characterized by deposition of amyloid-β protein (Aβ) and neurofibrillary tangles (tau protein) in the brain. The etiology of AD has not yet been resolved, it could result from multifactorial etiopathogenesis. Alzheimer’s disease has been linked to at least 5 chromosomes. However, only a portion of patients with AD account for known genetic association. 2
The apolipoprotein E gene (APOE) polymorphism has been recognized as a risk factor for late onset of AD, the frequency of the APOE ∊4 allele being increased in patients with AD as compared to control participants. 3 ,4 Apolipoprotein E (apoE) plays an important role in the metabolism of cholesterol and triglycerides (TGs) by binding to receptors on the liver and help in clearance of chylomicrons and very-low-density lipoproteins (VLDLs) from the bloodstream. 5 It has been shown that increased serum cholesterol levels affect the Aβ production and are associated with risk of AD. 6 Although APOE genotyping increases the specificity of the clinical diagnosis of AD but this association is not absolute. It has been suggested that AD could result from a multifactorial process involving both a genetic predisposition and an exposure to environmental factors. 7,8
The gene–environment interaction (G × E) research shows potential for the psychiatry and neuroscience, although till date only a few studies have been investigated in AD. 9 In fact, evidence for G × E in AD has recently been started to build up. For example, an association between the presence of APOE ∊4 allele and consumption of alcohol and smoking has been shown to increase the risk of AD. 10,11 These epidemiological studies indicate that it makes sense to focus on the future clinical AD studies on measuring both genes and environment and analyzing possible interactions. We are looking for G × E interaction with reference to presence or absence of the ∊4 allele as one of the risk factors for AD.
Pesticides are environmental chemicals used extensively throughout the world including India. In India, 40% of all pesticides used belong to the organochlorine class of chemicals. 12 Organochlorine pesticides (OCPs) are chemically stable, strongly lipophilic as well as very slow in degradation. 12 These compounds are concentrated up in the food chain and can be detected in the diet including drinking water. Thus, the combination of their persistence in the environment and their potential to neurotoxicity suggest that the risk of AD may be associated with OCPs. This study has been designed to examine the G × E interaction with reference to APOE genotypes and OCPs as one of the factors in the etiology of AD.
Materials and Methods
Study Population and Design
In this case–control study, patients with AD and controls belonged to the state of Delhi and other surrounding states of North India. They were examined by neurologist in the outpatients department of neurology, Institute of Human Behaviour and Allied Science, Delhi, during February 2010 to January 2012. The sample size was calculated (α = 0.05, β = 0.20, and power = 0.80) by using PS software, version 3.0.14. The study objectives were explained to 70 patients diagnosed with AD and 75 control participants. Patients who were 50 to 85 years old with complaints of memory or other cognitive impairment were subjected to define by the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association 13 for AD and a magnetic resonance imaging/computed tomography/positron emission tomography brain scan supporting the clinical diagnosis of AD were eligible. Additional inclusion criteria were a score of <23 on the Mini-Mental State Examination and a Clinical Dementia Rating score of ≥0.5. Control group comprised of age- and sex-matched healthy volunteers who came to the hospital for routine health checkup.
Participants were excluded in both case and control groups if there was no consent for participation in the study, history of cerebral stroke, epilepsy, a history of head trauma, and other concomitant disease potentially associated with dementia, chronic intake of drugs affecting cognitive processes, moderate-to-severe depressive episode, and familial history of any kind of cognitive/behavioral abnormality. Nutritional deficiency, metabolic abnormalities, and infections in the central nervous system were ruled out.
The study protocol and the informed consent form were reviewed and approved by institutional ethics committee of the institute. Before any study-specific procedures were performed, written consent was obtained from each participant.
APOE Genotyping
Genomic DNA was isolated from peripheral blood by salting-out method 14 and amplified for the region of the fourth exon of APOE gene, which encodes amino acid residues, 112 and 158. The specific primers 5′-ACAGAATTCGCCCCGGCCTGGTACAC-3′ and 5′-TAAGCTTGGCACGGCTGTCCAAGGA-3′ were used for amplification as described by Pandey et al. 15 Polymerase chain reaction (PCR) assay was performed on Biorad iQ5 cycler, USA, in a 20 µL volume of 1× high-fidelity master mix (Biorad, United States) containing high-fidelity buffer, 0.04 U/µl DNA polymerase, 1.5 mol/L MgCl2, 200 µmol/L dNTPs. 7.5% dimethyl sulfoxide, 10 ng of genomic DNA, and 0.5 µmol/L of each primer were used in final concentration. The PCR protocol consisted of a 1-minute hold at 98°C followed by 35 cycles of 98°C (30 seconds), 65°C (30 seconds) and 72°C (30 seconds), and final extention at 72°C for 3 minutes. The PCR product of 244 bp was digested by 10 units of Hha1. The digested product was run on 12% polyacrylamide gel followed by the ethidium bromide staining. Allelic sizes were compared with known size molecular weight marker. Approximately 10% samples were repeated for quality control.
Estimation of OCP Levels
Blood samples were collected using standard venepuncture techniques and stored at −80°C until analysis. The OCPs α-hexachlorocyclohexane (α-HCH), β-HCH, γ-HCH, aldrin, dieldrin, endosulfan, pp′-dichlorodiphenyldichloroethylene (pp′-DDE), op′-DDE, pp′-dichlorodiphenyltrichloroethane (pp′-DDT), op′-DDT, pp′-dichlorodiphenyldichloroethane (pp′-DDD), and op′-DDD were assayed. The compounds selected for assay were based on previous findings 16 that indicated the pesticides most commonly found in human samples and based on the compounds assayed by the Environmental Protection Agency. Samples were analyzed on Perkin Elmer gas chromatograph equipped with 63Ni electron capture detector under standard operating procedure. 16
The OCPs were extracted from blood according to the method described by Bush et al. 17 Quantitative analysis of OCPs in each sample was analyzed by comparing the peak area with those obtained from a chromatogram of a mixed organochlorine standard (Supelco, Sigma-Aldrich, United States) of known concentration. The detection limit of the detector was <0.05 pg per chloroethylene with nitrogen as a carrier gas. The detection limit of the method was 4 pg/mL for each OCP. For quality control process, 5 blood samples in triplicate were spiked with a mixed standard of OCPs at 5 and 25 ng/mL. The average recoveries of prepared samples exceeded 95%. The case and control samples were run in the same analytical batches and moreover, a quality check sample was always run with each set of samples for pesticide analysis to maintain accuracy.
Estimation of Lipids
Serum lipids (cholesterol, TGs, high-density lipoprotein [HDL], and LDL) were estimated by commercially available kit manufactured by Centronic GmbH, Germany, on autoanalyzer (Random Access Discrete Autoanalyser, XL-300 from Transasia, Mumbai, India).
Statistical Analysis
The statistical analysis was carried out by SPSS version 14.0. Mean ± standard deviations were calculated to describe the quantitative data, whereas percentages were calculated to describe qualitative data. The χ 2 test was applied for the analysis of genotypic and allelic distribution. Odds ratio (OR) and their 95% confidential intervals (CIs) were calculated by 2 × 2 contingency tables. Independent sample t test was used for comparing mean of normally distributed continuous variables. Mann-Whitney U test was used for comparing continuous variables that did not follow normal distribution. Logistic regression analysis was done with serum cholesterol, HDL cholesterol, LDL cholesterol, β-HCH, dieldrin, and pp′-DDE as predictor variables for AD versus controls status.
Results
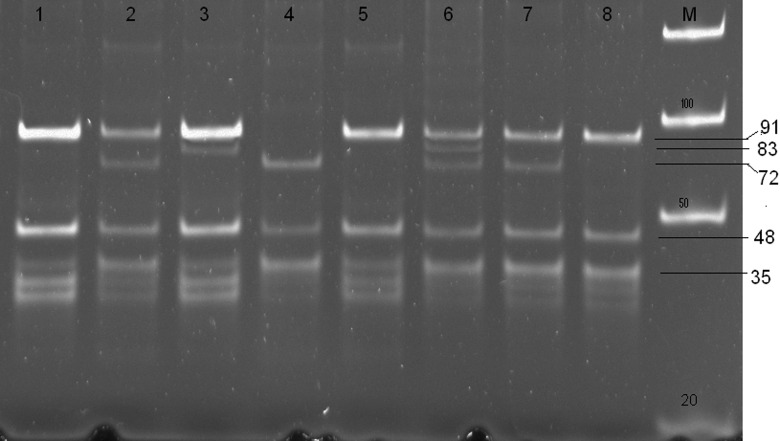
No significant difference was found between cases and controls in terms of demographic characteristics. Cases and controls were homogenous in terms of sex, habitat, dietary habit, smoking habit, and alcohol intake habits (data not shown). Restriction analysis of the 244 bp expected PCR product yielded the specific combination of fragments which is shown in Figure 1. The fragment sizes 38, 18, and 16 bp were common in all genotypes except 38 bp other 2 were too small to detect. The allele frequencies and prevalence of APOE genotypes results are shown in Table 1. The APOE ∊4 allele frequency was significantly high (P = .000, OR = 5.73, CI = 2.68-12.50) in AD as compared to controls. Among the APOE genotypes, ∊3∊4 showed a strong significant association (P = .000, OR = 9.56, CI = 3.81-24.55) with the patients with AD.
Figure 1.
Picture of polyacrylamide gel showing ApoE polymorphism ∊3∊ genotype in lanes 2 and 7. ∊3∊2 genotype in lane 4 and ∊4∊2 genotype in lane 6. Molecular weight marker in lane M.
Table 1.
The APOE Allele and Genotype Frequencies, P Value, OR, and 95% CI for Alzheimer’s Disease (AD) and Control Populationsa
| Alleles/Genotypes | Controls (n = 75) | AD (n = 70) | PValue | OR | CI |
|---|---|---|---|---|---|
| ∊3 | 129 (0.860) | 90 (0.643) | |||
| ∊4 | 11 (0.073) | 44 (0.314)b | .000 | 5.73 | 2.68-12.50 |
| ∊2 | 10 (0.066) | 6 (0.043) | NS | ||
| ∊3∊3 | 55 (0.733) | 23 (0.328) | |||
| ∊3∊4 | 10 (0.133) | 40 (0.571)b | .000 | 9.56 | 3.81-24.55 |
| ∊3∊2 | 9 (0.120) | 4 (0.057) | NS | ||
| ∊4∊4 | – | 1 (0.014) | NS | ||
| ∊4∊2 | 1 (0.013) | 2 (0.028) | NS | ||
| ∊2∊2 | – | – | – |
Abbreviations: CI, confidence interval; NS, not significant; OR, odds ratio; -, not present.
aFigures within parentheses indicate the frequency.
b P < .001.
The independent sample t test results (Table 2) showed that in all participants the mean levels of serum cholesterol and LDL cholesterol were significantly higher (P < .001 in both) and HDL cholesterol levels were significantly lower (P < .01) in patients with AD as compared to the controls. In participants with ∊3∊3 genotypes, the mean level of serum cholesterol was significantly higher (P < .001) and HDL cholesterol level was significantly lower (P < .05) in patients with AD as compared to the controls. The mean level of serum cholesterol and LDL cholesterol were significantly higher (P < .01 and P < .05, respectively) in patients with AD as compared to control participants which have ∊3∊4 genotypes. No significant difference was found in serum lipid levels in participant with other genotypes (∊3∊2, ∊4∊4, and ∊4∊2).
Table 2.
Distribution of Serum Lipid Levels According to the APOE Genotypes in Patients With Alzheimer’s Disease (AD) and Control Participantsa
| Variables (ng/mL) | All Participant | ∊3∊3 | ∊3∊4 | ∊3∊2 | ∊4∊4 | ∊4∊2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 75) | AD (n = 70) | Control (n = 55) | AD (n = 23) | Control (n = 10) | AD (n = 40) | Control (n = 09) | AD (n = 04) | Control (n = 00) | AD (n = 01) | Control (n = 01) | AD (n = 02) | |
| Cholesterol | 122.03 ± 30.37 | 169.89 ± 38.84b | 114.45 ± 24.67 | 134.48 ± 23.97b | 161.70 ± 26.17 | 190.60 ± 24.68c | 121.33 ± 34.73 | 145.0 ± 19.69 | – | 300.0 | 147.0 | 147.50 ± 10.61 |
| TG | 158.19 ± 53.02 | 157.96 ± 76.81 | 150.36 ± 50.41 | 162.17 ± 90.05 | 176.40 ± 29.61 | 167.05 ± 69.58 | 165.56 ± 53.25 | 118.0 ± 40.03 | – | 109.0 | 340.0 | 101.50 ± 21.92 |
| HDL | 46.29 ± 9.09 | 41.54 ± 8.40c | 46.75 ± 8.93 | 41.35 ± 7.04d | 46.12 ± 5.97 | 41.38 ± 9.56 | 44.78 ± 13.10 | 44.50 ± 4.36 | – | 36.0 | 37.0 | 44.0 ± 8.48 |
| LDL | 71.47 ± 24.61 | 106.13 ± 45.63b | 62.07 ± 16.33 | 55.52 ± 13.86 | 113.80 ± 11.83 | 137.80 ± 27.92d | 79.22 ± 26.06 | 74.50 ± 4.43 | – | 176.0 | 100.0 | 83.0 ± 1.41 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; -, not detected.
a The values are presented as mean ± SD.
b P < .001.
c P < .01.
d P < .05.
Mann-Whitney U test (Table 3) revealed a significant difference in β-HCH levels (U = 1237.00, W = 4087.00, z = −6.29, P = .000), dieldrin levels (U = 1449.00, W = 4299.00, z = −5.81, P = .000), and pp′-DDE levels (U = 2062.00, W = 4912.00, z = −2.69, P = .007) in all participants of cases and controls. These 3 OCP levels β-HCH (U = 193.00, W = 1733.00, z = −5.74, P = .000), dieldrin (U = 360.00, W = 1900.00, z = −4.23, P = .000), and pp′-DDE (U = 449.50, W = 1989.50, z = −2.53, P = .012) were also significant in participants which have ∊3∊3 genotypes, whereas participants which have ∊3∊4 genotype, only the mean level of dieldrin was found to be significantly higher in patients with AD as compared to controls (U = 85.00, W = 140.00, z = −3.03, P = .002). The OCP levels were not significant in other APOE genotypes (∊3∊2, ∊4∊4, and ∊4∊2). The OCPs, op′-DDE, op′-DDT, pp′-DDD, and op′-DDD were not detected in both the groups.
Table 3.
Distribution of Organochlorine Pesticides (OCPs) According to the APOE Genotypes in Alzheimer’s Disease (AD) and Control Participantsa
| Variables (ng/mL) | All Participants | ∊3∊3 | ∊3∊4 | ∊3∊2 | ∊4∊4 | ∊4∊2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 75) | AD (n = 70) | Control (n = 55) | AD (n = 23) | Control (n = 10) | AD (n = 40) | Control (n = 09) | AD (n = 04) | Control (n = 00) | AD (n = 01) | Control (n = 01) | AD (n = 02) | |
| α-HCH | 0.56 ± 0.27 (4.0) | 0.25 ± 0.95 (8.57) | 0.08 ± 0.32 (5.45) | 0.10 ± 0.50 (4.35) | – | 0.30 ± 1.08 (10.0) | – | – | – | 3.40 | – | – |
| β-HCH | 0.25 ± 0.59 (17.33) | 4.16 ± 4.61b (60.0) | 0.27 ± 0.64 (16.36) | 5.85 ± 5.75b (73.91) | 0.28 ± 0.49 (30.0) | 3.07 ± 3.75 (50.0) | 0.11 ± 0.33 (11.11) | 2.84 ± 3.42 (50.0) | – | 7.12 | – | 7.66±1.78 |
| γ-HCH | 0.07 ± 0.35 (5.33) | 0.32 ± 1.35 (10.0) | 0.04 ± 0.22 (3.36) | 0.51 ± 2.14 (8.69) | 0.04 ± 0.13 (10.0) | 0.20 ± 0.69 (10.0) | 0.29 ± 0.89 (11.11) | – | – | 2.61 | – | – |
| Dieldrin | 0.16 ± 0.61 (9.33) | 4.82 ± 6.65b (50.0) | 0.12 ± 0.44 (9.09) | 3.70 ± 4.94b (47.82) | – | 6.27 ± 7.59c (57.5) | 0.15 ± 0.46 (11.11) | 0.30 ± 0.6 (25.0) | – | – | 4.02 | – |
| Aldrin | 0.09 ± 0.04 (5.33) | 0.22 ± 0.81 (8.57) | 0.12 ± 0.47 (7.27) | 0.37 ± 1.21 (8.69) | – | 0.07 ± 0.33 (5.0) | – | 0.30 ± 0.60 (25.0) | – | 2.80 | – | – |
| α-Endosulfan | 0.07 ± 0.30 (6.66) | 0.26 ± 0.85 (10.0) | 0.06 ± 0.23 (7.27) | 0.19 ± 0.64 (8.69) | – | 0.25 ± 0.81 (10.0) | 0.22 ± 0.66 (11.11) | 1.05 ± 2.10 (25.0) | – | 0.33 | – | – |
| β-Endosulfan | 0.05 ± 0.27 (4.0) | 0.10 ± 0.50 (4.28) | 0.06 ± 0.32 (5.45) | – | – | 0.11 ± 0.49 (5.0) | – | 0.70 ± 1.40 (25.0) | – | – | – | – |
| pp′-DDT | 0.14 ± 0.64 (6.66) | 0.55 ± 1.86 (10.0) | 0.17 ± 0.73 (7.27) | 0.30 ± 1.44 (4.35) | 0.14 ± 0.44 (10.0) | 0.67 ± 2.22 (12.5) | – | 1.20 ± 2.40 (25.0) | – | – | – | – |
| pp′-DDE | 0.82 ± 1.93 (22.66) | 2.52 ± 5.69d (41.42) | 0.66 ± 1.44 (21.82) | 4.38 ± 9.15d (43.48) | 0.91 ± 2.11 (30.0) | 1.90 ± 2.55 (47.5) | 1.78 ± 3.78 (22.22) | – | – | – | – | – |
Abbreviations: DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; HCH, hexachlorocyclohexane; -, not detected.
a The values are presented as mean ± SD, figures within parentheses indicate percentage of sample in which OCP was detected.
b P< .001.
c P < .01.
d P < .05.
Direct logistic regression (Table 4) was performed to assess the impact of a number of factors on the likelihood of developing AD. The full model containing all predictors was not statistically significant. Only serum cholesterol (P < .05), β-HCH (P = .01), and dieldrin (P < .05) levels were statistically significant.
Table 4.
Risk Factors for Alzheimer’s Disease
| B | SE | Wald | df | Sig | OR | 95.0% CI for OR | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Step 1a | Cholesterol | 0.145 | 0.067 | 4.724 | 1 | 0.030 | 1.157 | 1.014 | 1.319 |
| HDL | 0.015 | 0.082 | 0.033 | 1 | 0.856 | 1.015 | 0.864 | 1.192 | |
| LDL | −0.075 | 0.057 | 1.696 | 1 | 0.193 | 0.928 | 0.829 | 1.038 | |
| β-HCH | 2.432 | 0.942 | 6.669 | 1 | 0.010 | 11.380 | 1.797 | 72.058 | |
| Dieldrin | 2.347 | 1.093 | 4.613 | 1 | 0.032 | 10.454 | 1.228 | 89.012 | |
| pp′-DDE | 0.189 | 0.274 | 0.479 | 1 | 0.489 | 1.209 | 0.707 | 2.067 | |
Abbreviations: CI, confidence interval; df, degrees of freedom; HCH, hexachlorocyclohexane; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio; pp′-DDE, pp′-dichlorodiphenyldichloroethylene; SE, standard error; Sig, significance.
a Variable(s) entered on step 1: cholesterol, HDL, LDL, β-HCH, dieldrin, pp′-DDE.
Discussion
This is one of the first studies in which the role of APOE genotypes along with OCP levels was seen in patients with AD and healthy participants. No differences were found between demographical profiles such as age, sex, habitat, dietary habit, smoking, and alcoholic status. The human glycoprotein (apoE), one of the least abundant plasma apoproteins, is coded by APOE. The APOE gene is polymorphic and is located on chromosome 19q13 with 3 common allele, ∊2, ∊3, and ∊4 (coding for 3 isoforms E2, E3, and E4) which produce 3 homozygous (∊2∊2, ∊3∊3, and ∊4∊4) and 3 heterozygous (∊2∊3, ∊3∊4, and ∊4∊2) phenotypes in the human population. 18 These isoforms differ by single amino acid substitutions (missense mutation) at codons 112 and/or 158. The ∊3 allele, considered to be the parental allele, has cystine at codon 112 and arginine at codon 158. 19 The ∊2 allele has cystine at both positions, while the ∊4 allele contains arginine at both positions. 19 The APOE ∊3 allele and homozygous ∊3∊3 genotype were most prevalent in controls, whereas APOE ∊4 allele and heterozygous ∊3∊4 genotype in AD.
In the present study, 31.4% of the patients with AD and 7.3% of controls carried at least 1 ∊4 allele and 4.3% of patients with AD and 6.6% of controls carried ∊2 allele, respectively. The patients with AD are 5.7 times more susceptible when having APOE ∊4 allele. Among the genotypes, ∊3∊4 shows a strong significant association with AD (9.56 times elevation). The present findings regarding the APOE ∊4 allele frequency is in the corroboration with other studies of Indian origin. 20,21 Allele frequencies of the APOE polymorphism have been reported to vary among different populations. 20,21 A possible explanation for this might be due to genetic drift by founder effect and/or due to the different prevalence rates of AD and/or the selection bias of samples.
Earlier studies indicated that the higher serum cholesterol and LDL cholesterol levels were associated with the risk of AD. 22,23 The present study showed that the mean levels of serum cholesterol and LDL cholesterol were significantly higher, whereas HDL cholesterol levels were significantly lower in patients with AD as compared to the controls. Experimental studies suggest that serum cholesterol and LDL cholesterol levels are associated with increased deposition of Aβ 1-42 in AD. 24,25 Huaqi Xiong et al, 24 explained the detailed mechanism by which higher cholesterol levels affects this process but the detailed mechanism is still unclear by which higher LDL cholesterol affects this process, whether they increase AD risk directly or through indirect mechanisms in which their metabolites can enter the brain and promote the accumulation of Aβ. Higher levels of HDL cholesterol were associated with a decreased risk of AD as compared with lower HDL cholesterol levels. 26 Amyloid β binds to HDL, maintaining its solubility in cerebrospinal fluid and plasma. This HDL–Aβ interaction prevents the deposition of Aβ in the brain. 27
We have also observed the influence of APOE genotypes on serum lipid levels. Apolipoprotein E is involved in the transportation of cholesterol from peripheral tissues to the liver for catabolism. Overexpression of ∊4 allele increases serum cholesterol and LDL cholesterol levels 28 and it may be involved in the development of cognitive impairment and may also promote extracellular aggregation of Aβ peptide in the aging brain, 29 which is the main pathological feature of AD. The APOE has been shown to bind directly to the Aβ peptide and influence its fibrillogenesis and clearance. However, ∊4 allele is less effective than the ∊3 and ∊2 alleles to mediate neuronal repair, remodeling, and protection. 30,31
The present study showed that the mean levels of serum cholesterol and LDL cholesterol in ∊3∊4 genotypes were significantly higher in patients with AD as compared to controls. The ∊3∊4 genotype codes E4 isoform of apoE. The binding capacity of E4 isoforms to the VLDL receptor is high 32 as a result of which serum cholesterol and LDL cholesterol levels were elevated. In wild type/common ∊3∊3 genotypes, serum cholesterol levels were significantly higher. However, HDL cholesterol levels were significantly lower in patients with AD as compared to controls which follow the observations of previous studies. 26,27
Studies have shown an association of ∊4 allele with AD, which proved that it is a susceptible gene for AD. 20,21 The present study indicates that ∊4 allele is not absolutely contributing to AD genotypes as we have found the presence of ∊4 allele in healthy participants, whereas it was absent in diagnosed patients with AD (n = 27). The APOE genotyping might improve the specificity of the clinical diagnosis of AD. However, the presence of the ∊4 allele alone is neither necessary nor sufficient for the risk of AD. Other factors may participate independently or in concert with APOE to determine the overall risk of AD. The G × E research shows potential for the etiology of several neurological diseases. The OCPs are persistent in the environment and their potential to neurotoxicity suggests that the risk of AD may be associated with OCPs.
In the present study, the mean levels of β-HCH, dieldrin, and pp′-DDE were found to be higher in patients with AD as compared to the controls. It has been previously suggested that OCP produce free radicals which lead to oxidative stress. 33 The brain having high lipid content and poor antioxidant defenses makes the brain an ideal target for free radical attack. One theory on the pathogenesis of AD postulates that neurodegeneration is the result of oxidative stress and damage to vulnerable cerebral tissues. 34 Richardson et al, 35 indicated that β-HCH levels were not associated with AD, whereas pp′-DDE levels were associated with AD but this observation was based on only 20 patients with AD and 43 controls and done in different cohorts. It has also been noticed that most of the OCPs that are banned in the developed countries are still being used extensively in the developing nations throughout the world including India. The higher levels of pp′-DDE were also found as per the observation of previous studies. 36,37 Dieldrin is a major metabolite of aldrin and metabolized at a much slower rate to hydrophilic metabolites due to extremely apolar nature. The present study indicates that elevated dieldrin levels are associated with AD. Experimental studies (in vitro and in vivo) using mammalian system suggest that dieldrin induces oxidative stress and apoptosis. 38 These cellular responses are common during the progression and manifestation of human neurological diseases, such as AD and Parkinson’s disease. However, the dieldrin-mediated neurotoxicity mechanisms are not fully characterized.
In this study, the intergenotypic OCP levels were also screened. Of the total number of patient with AD, 32.85% patients had common/wild type of ∊3∊3 genotypes, and in these patients the β-HCH, dieldrin, and pp′-DDE were found to be significantly higher as compared to the controls. However, 57.14% of patients with AD had ∊3∊4 genotype and only dieldrin level was significantly higher. It has also been suggested that the antioxidant property among APOE alleles is followed as ∊2 > ∊3 > ∊4. 34 This study shows the role of β-HCH, dieldrin, and pp′-DDE with the presence of AD in those with the absence of ∊4 allele. Interpretation cannot be made for the rest of the genotypes because of less sample size which is the major limitation of this study.
Alzheimer’s disease is influenced not only by genetic factors but also by environmental components. The study indicates a potential association between OCPs and AD varied by APOE ∊4 allele status. The OCPs showed a positive association with AD in participants without an APOE ∊4 allele. The direct logistic regression result shows that beside ∊4 allele, higher levels of total cholesterol, β-HCH, and dieldrin were also associated with increased risk of AD, recording an OR of 1.16, 11.38, and 10.45, respectively. This indicates that for an additional 1 unit increment of total cholesterol, β-HCH, and dieldrin levels, chances of developing AD in a population will be 11.16, 11.38, and 10.45, respectively. β-Hexachlorocyclohexane and/or dieldrin were not detected in 3 patients diagnosed with AD which were not carrying ∊4 allele which may suggest the multifactorial etiopathology nature of AD.
In conclusion, the results of the study suggest that besides the APOE ∊4 allele, the presence of β-HCH and dieldrin in serum are important determinants of the presence of AD. Among lipids, higher total cholesterol levels significantly increased the odds of the AD presence. Multifactorial genetic and environmental determinants of the AD should be studied in more detail by prospective studies with a larger sample size to further understand the mechanisms involved.
Acknowledgment
Mr Neeraj Kumar Singh is thankful to Indian Council of Medical Research (ICMR), New Delhi, India, for providing Senior Research Fellowship (Reference No. 3/1/2/5/Neuro/2004-NCD-I).
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348(14):1356–1364. [DOI] [PubMed] [Google Scholar]
- 2. Van Broeckhoven CL. Molecular genetics of Alzheimer’s disease: identification of genes and gene mutations. Eur Neurol. 1995;35(1):8–19. [DOI] [PubMed] [Google Scholar]
- 3. Verghese PB, Castellano JM, Holtzman DM. Roles of apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 5. Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155(6):487–495. [DOI] [PubMed] [Google Scholar]
- 6. Hall K, Murrell J, Ogunniyi A, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66(2):223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Environmental pesticide exposure as a risk for Alzheimer’s disease: a case-control study. Environ Res. 2001;86(1):37–45. [DOI] [PubMed] [Google Scholar]
- 8. Chouliaras L, Sierksma AS, Kenis G, et al. Gene–environment interaction research and transgenic mouse models of Alzheimer’s disease. Int J Alzheimers Dis. 2010;2010:859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chouliaras L, Rutten BP, Kenis G, et al. Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Prog Neurobiol. 2010;90(4):498–510. [DOI] [PubMed] [Google Scholar]
- 10. Elbaz A, Dufouil C, Alpérovitch A. Interaction between genes and environment in neurodegenerative diseases. C R Biol. 2007;330(4):318–328. [DOI] [PubMed] [Google Scholar]
- 11. Dufouil C, Tzourio C, Brayne C, Berr C, Amouyel P, Alpérovitch A. Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers smokers. Epidemiology. 2000;11(3):280–284. [DOI] [PubMed] [Google Scholar]
- 12. Abhilash PC, Singh N. Pesticide use and application: an Indian scenario. J Hazard Mater. 2009;165(1-3):1–12. [DOI] [PubMed] [Google Scholar]
- 13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 14. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pandey P, Pradhan S, Mittal B. Presenilin gene predisposes to late-onset degenerative but not vascular dementia: a comparative study of PS1 and ApoE genes in a North Indian Cohort. Dement Geriatr Cogn Disord. 2007;24(3):151–161. [DOI] [PubMed] [Google Scholar]
- 16. Pathak R, Suke SG, Ahmed RS, et al. Endosulfan and other organochlorine pesticide residues in maternal and cord blood in North Indian population. Bull Environ Contam Toxicol. 2008;81(2):216–219. [DOI] [PubMed] [Google Scholar]
- 17. Bush B, Snow J, Koblintz R. Polychlorobiphenyl (PCB) congeners, p, p′-DDE and hexachlorobenzene in maternal and fetal cord blood from mothers in upstate NewYork. Arch Environ Contam Toxicol. 1984;13(5):517–527. [DOI] [PubMed] [Google Scholar]
- 18. Das HK, McPherson J, Bruns GA, Karathanasis SK, Breslow JL. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J Biol Chem. 1985;260(10):6240–6247. [PubMed] [Google Scholar]
- 19. Assmann G, Funke H, Jabs HU. Analytical procedures for the detection and characterization of apolipoprotein E mutants. Am Heart J. 1987;113(2 pt 2):598–603. [DOI] [PubMed] [Google Scholar]
- 20. Kapur S, Sharad S, Kapoor M, Bala K. ApoE genotypes: risk factor for Alzheimer’s disease. JIACM. 2006;7(2):118–122. [Google Scholar]
- 21. Thelma BK, Juyal RC, Dodge HH, Pandav R, Chandra V, Ganguli M. APOE polymorphism in a rural older population-based sample in India. Hum Biol. 2001;73(1):135–144. [DOI] [PubMed] [Google Scholar]
- 22. Warren MW, Hynan LS, Weiner MF. Lipids and adipokines as risk factors for Alzheimer’s disease. J Alzheimers Dis. 2012;29(1):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabbagh M, Zahiri HR, Ceimo J, et al. Is there a characteristic lipid profile in Alzheimer’s disease? J Alzheimers Dis. 2004;6(6):585–589. [DOI] [PubMed] [Google Scholar]
- 24. Xiong H, Callaghan D, Jones A, et al. Cholesterol retention in Alzheimer’s brain is responsible for high β- and γ-secretase activities and Aβ production. Neurobiol Dis. 2008;29(3):422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Refolo LM, Pappolla MA, LaFrancois J, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8(5):890–899. [DOI] [PubMed] [Google Scholar]
- 26. Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67(12):1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 2001;15(10):1858–1860. [DOI] [PubMed] [Google Scholar]
- 28. Alvim RO, Freitas SR, Ferreira NE, et al. APOE polymorphism is associated with lipid profile, but not with arterial stiffness in the general population. Lipids Health Dis. 2010;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fryer JD, Simmons K, Parsadanian M, et al. Human apolipoprotein E4 alters the amyloid-β 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25(11):2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994;269(38):23403–23406. [PubMed] [Google Scholar]
- 31. Mahley RW, Huang Y. Apolipoprotein E: from atherosclerosis to Alzheimer’s disease and beyond. Curr Opin Lipidol. 1999;10(3):207–217. [DOI] [PubMed] [Google Scholar]
- 32. Saito H, Dhanasekaran P, Baldwin F, Weisgraber KH, Phillips MC, Lund-Katz S. Effects of polymorphism on the lipid interaction of human apolipoprotein E. J Biol Chem. 2003;278(42):40723–40729. [DOI] [PubMed] [Google Scholar]
- 33. Sharma H, Zhang P, Barber DS, Liu B. Organochlorine pesticides dieldrin and lindane induce cooperative toxicity in dopaminergic neurons: role of oxidative stress. Neurotoxicology. 2010;31(2):215–222. [DOI] [PubMed] [Google Scholar]
- 34. Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60(8):759–767. [DOI] [PubMed] [Google Scholar]
- 35. Richardson JR, Shalat SL, Buckley B, et al. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol. 2009;66(7):870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36(1):100–103. [DOI] [PubMed] [Google Scholar]
- 37. Hatcher JM, Richardson JR, Guillot TS, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204(2):619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanthasamy AG, Kitazawa M, Yang Y, Anantharam V, Kanthasamy A. Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): implications for neurodegeneration in Parkinson’s disease. Mol Brain. 2008;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]