Abstract
To evaluate the value of 50% reduced-dose cerebral computed tomography (CT)perfusion imaging (CTPI) to show the perfusion abnormalities in Alzheimer’s disease (AD), as an attempt to develop a new imaging protocol with lower radiation dose to track the correlation of AD with regional blood flow abnormalities. A total of 52 patients with AD were assigned to the AD group and 28 healthy volunteers served as the control group. All participants were given a 50% reduced-dose cerebral CTPI (current was reduced from 160 to 80 mA) test by a multislice spiral CT scanner. Perfusion parameters of the bilateral frontal cortex, temporal cortex, hippocampus, and basal ganglia were measured, including the cerebral blood volume (CBV), cerebral blood flow (CBF), mean transit time (MTT), and time to peak (TTP). Both the CBV and CBF values of the measured regions were significantly higher in the healthy control group than in the AD group (P < .05), while the MTT and TTP values of these cerebral areas were significantly lower in the healthy control group than in the AD group (P < .05). Four perfusion parameters, namely the MTT of the left frontal cortex, right temporal cortex, right basal ganglia, and right hippocampus, had the greatest sensitivity and a striking correlation with the incidence of AD. The blood flow per unit of time in the regions of interest was significantly lower in the AD group, which provides new evidence for the existence of microcirculation disturbance and ischemia in AD. The 50% reduced-dose cerebral CTPI scan is valuable to show the regional perfusion abnormalities in the patients with AD.
Keywords: dementia, CBV, CBF, cognition, blood perfusion, logistic regression analysis
Introduction
With the world’s increasingly elderly population, dementia has become a common disease with a high prevalence. As of 2005, there were 24.2 million people with dementia worldwide, and the figure is predicted to double every 20 years through 2040. 1
Alzheimer’s disease (AD) and vascular dementia (VD) are the main types of dementia. In the United States alone, 100 000 people die from AD every year. Distinguishing accurately between different types of dementia, for example, AD and VD, is not always possible on purely clinical grounds but such differentiation is necessary for choosing the best therapeutic approach. 2 The clinical grounds, cognitive assessments, only reflect the general cognitive condition, rather than the structural and functional changes of the affected cerebral areas. As a result, it is difficult to determine the cause, duration, and prognosis of AD.
Thanks to the development of imaging technology, functional imaging techniques, such as functional magnetic resonance imaging (MRI), 3 single photon emission computed tomography (SPECT), and position emission tomography (PET), 4,5 are playing increasingly important roles in the diagnosis of dementia. Several studies have shown that there is a reduction in blood flow, oxygen, and glucose consumption in the brains of patients with AD and that these conditions become more severe as the disease progresses. However, there are certain limitations for the clinical application of these new functional imaging techniques. The perfusion model of the functional MRI provides only semiquantitative evaluation of blood perfusion and is less mature than that of the functional computed tomography (CT), while the SPECT and PET are difficult to undertake as a result of high examination fees and lack of equipment. Fortunately, the development of volume rendering with multislice spiral CT has offered an additional option. Now, with 3-dimensional imaging technology, cerebral CT perfusion imaging (CTPI) can measure the TTP, time–density curve (TDC), cerebral blood flow (CBF), and cerebral blood volume (CBV) of abnormal cerebral regions. As cerebral CTPI can detect changes in the blood perfusion of specific cerebral areas, this technique can provide evidence for the functional diagnosis of AD. In fact, cerebral CTPI has been shown to have diagnostic value for ischemic cerebrovascular disease (CVD) in the hyperacute phase. Also, there are a few reports about the diagnostic value of cerebral CTPI in patients with AD. There is a study by Zimny et al that does compare these groups using CT that finds mixed evidence for specificity for diagnostic abilities for CTPI. On the basis of their preliminary report, CTPI seems to be a valuable method of distinguishing AD and VD on the basis of CBF and CBV values. However, it seems to be of little significance in differentiating VD from mixed dementia (MixD) and of no usefulness in distinguishing MixD from AD. 2 A major concern of the cerebral CTPI scan is its high radiation dose. In order to make it possible to introduce CTPI in clinical practice, the radiation dose must be cut dramatically. The purpose of this study was to investigate a new imaging protocol with lower radiation dose to track the correlation of AD with regional perfusion abnormalities and make it as a possible tool for diagnosis.
Materials and Methods
Participants of the Study
In accordance with the inclusion and exclusion criteria below, 52 patients with AD who visited our clinics between 2008 and 2010 were assigned to the AD group. All patients with AD underwent a cerebral CTPI scan at the first visit and were then given a cognitive assessment 1 year later. There were 28 participants without AD in the healthy control group. Between AD group and healthy group, the χ 2 of gender, educational levels, and age were 2.77 (P = .096), 7.21 (P = .125), and 3.01 (P = .083), respectively, there were no statistical differences. The study was conducted in accordance with the guidelines of the Regional Ethics Committee for conducting research involving humans. Each participant or his or her relative/caregiver provided signed consent to participate in the examination.
Group Information, Inclusion Criteria, and Exclusion Criteria
The AD group
There were 52 patients with AD in the AD group. Among the participants, 32 (61.5%) were men, and 20 (38.5%) were women, with an age range of 50 to 89 years.
The diagnostic criteria for AD were in line with the fourth version of the US Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).
The Hachinski Ischemic Scores (HISs) of the participants were less than or equal to 4.
Specific abnormalities in the participants, such as white matter degeneration and infarction, were excluded by conventional MRI scans.
The healthy control group
There were 28 participants, who were randomly selected from a local community, without AD in the age-matched healthy control group.
Among the control participants, 16 were men and 12 were women, with an age range of 50 to 80 years. Their Clinical Dementia Rating Scale (CDR) scores were 0. Their activities of daily living (ADL) scores were less than or equal to 20.
The participants had no histories of brain surgery, disease of the central nerves system, brain trauma, cardiovascular disease, or endocrinal disease.
There were no positive findings during neurologists’ examinations of the control participants. There was no recent memory impairment. The scores of various neuropsychological scales were at normal levels.
The HIS of the participants were less than or equal to 4.
No abnormalities were detected by conventional MRI scans.
Cognitive Function Tests
The Mini-Mental State Examination (MMSE) and ADL were used to measure cognitive function. The MMSE is a standardized, 30-point scale that encompasses the following items: orientation to time (5 points), orientation to place (5 points), registration (3 points), attention and calculation (5 points), recall (3 points), language (2 points), repetition (1 point), and complex commands (6 points). In the original description of the MMSE, the subtest score for attention was determined by the patient’s ability to calculate serial 7’s or to spell the word WORLD backwards. 6,7 Data from the individual’s initial MMSE scores were used in the analysis. Patients with AD were divided into mild, moderate, and severe dementia, according to the severity of the CDR Scale. The CDR Scale scores were more than 0.5 (0.5—doubtful AD, 1.0—mild AD, 2.0—moderate AD, and 3.0—severe AD).
Fifty-Percent Reduced-Dose CTPI
Scanning method and parameters
First, all participants were given a noncontrast-enhanced CT scan using a Philips Brilliance 16-Slice CT scanner. Next, a CTPI scan was given on specific Field of views (FOVs) (350 mm × 350 mm, a 1024 × 1024 matrix), including the temporal horn, and on the levels of the frontal cortex, temporal cortex, basal ganglia, and hippocampus. The details of the scan included a baseline of the orbitomeatal line, transverse CT slices, a slice width of 6 mm, a conventional head holder, low radiation dose, 80 kV, and 80 mA (the Dose-length product (DLP) of a CTPI scan in each participant was 1280.95 mGy/cm). The contrast medium (Ultravist 370 mg/mL) was given rapidly (4 mL/s) through an elbow intravenous bolus injection with an automatic injector (the dose was 2 mL/kg). At 7 seconds after the contrast medium was injected, a single-level dynamic CT scan was performed once per second, 80 times in total (scan width of 24 mm). The 320 images of the 4 scan levels were then transmitted to the GE ADW 4.4 Workstation. The reorganized dynamic images transmitted to the workstation were postprocessed with a graphical slope method using Perfusion CT, special software for the GE ADW 4.4 Workstation.
Measurement of cerebral blood perfusion parameters
Three radiologists who were experienced in imaging diagnosis of neuropathic diseases were responsible for measuring and interpreting of the CTPI images that were transmitted to the GE ADW 4.4 Workstation (at least 2 associate chief radiologists worked together on the processing). The same radiologist (F.C.) manually sketched out the region of interest (ROI) and then made multipoint measurements (keeping the big vessels out of the way) in the bilateral temporal cortex, hippocampus (the tip and body level), frontal cortex, and basal ganglia (lenticular nucleus). Every ROI contained 50 to 100 pixels. In the temporal and frontal cortex, the ROIs were irregular in order to delineate cortex accurately and more oval in the regions of the basal ganglia and hippocampus. The anterior cerebral arteries and superior sagittal sinus served as reference points for inflow arteries and outflow veins to generate the TDC. The radiologists then analyzed and calculated all the dynamic images to obtain false-color images and perfusion parameters of the ROIs, including the CBF, CBV, mean transit time (MTT), and time to peak (TTP). The parameter values of the ROIs were recorded in a database. Every image was processed by at least 2 associate chief radiologists simultaneously to ensure that a consensus was reached on any perfusion abnormality.
Data Processing and Statistical Analysis
The measurement data were analyzed with SPSS statistic software, version 13.0. All measurement data were first examined with a normality test. A t test and multifactor application logistic regression analysis were used to measure the statistical difference of the CTPI parameters in different functional cerebral areas, as well as CTPI performance and its value in the diagnosis of AD.
Results
Perfusion Parameters of ROIs Between the 2 Groups
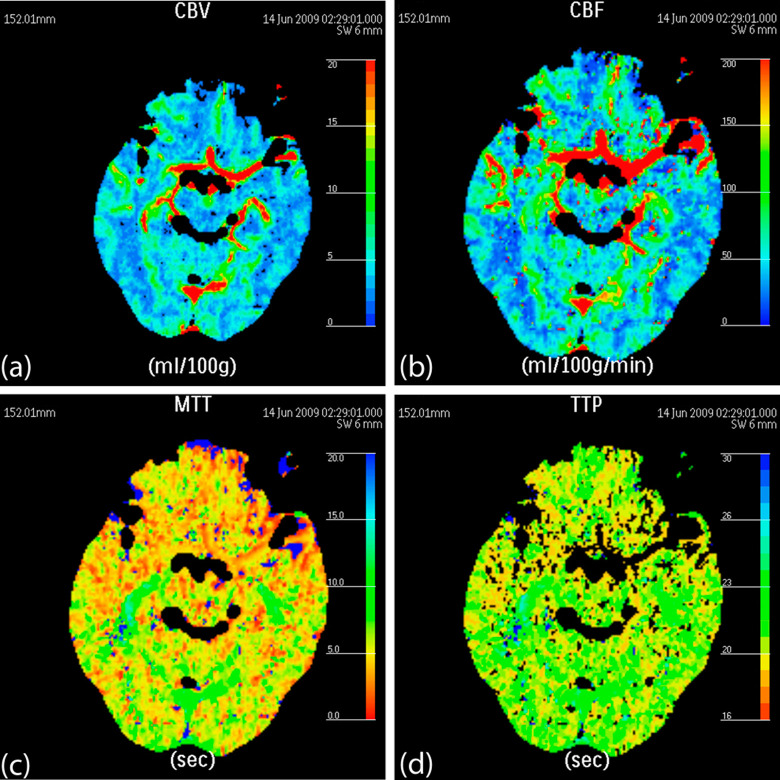
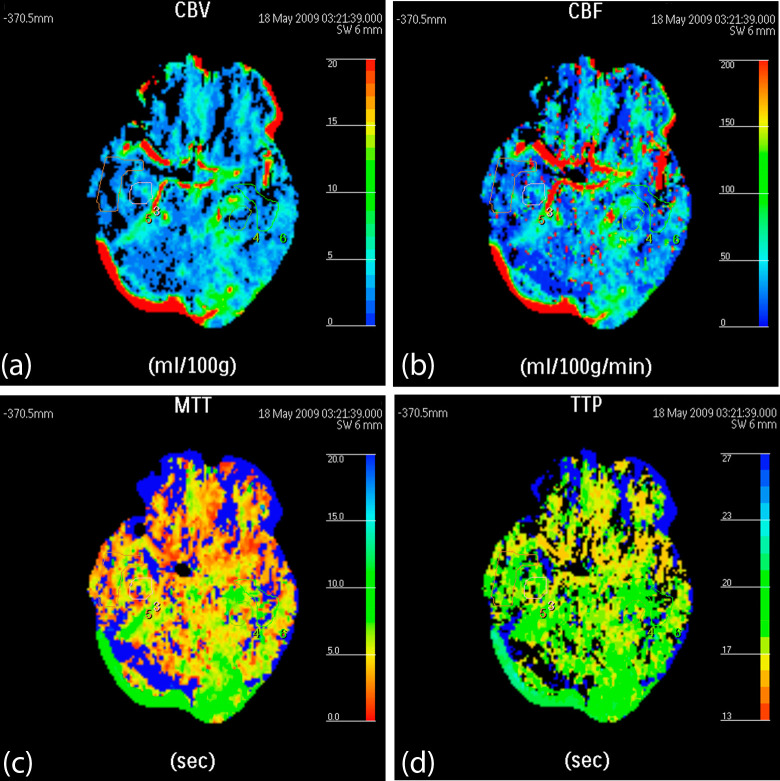
The CBVs and CBFs of the bilateral frontal cortex, temporal cortex, basal ganglia, and hippocampus were significantly higher in the healthy control group (Figure 1) than in the AD group (Figure 2), with a P value less than .05 (Table 1). Meanwhile, the MTTs and TTPs of the aforementioned ROIs were significantly lower in the healthy control group than in the AD group, with a P value less than .05 (Table 1). These results indicated that the participants in the AD group had significantly lower blood flow per minute than the participants in the healthy control group.
Figure 1.
False-color pictures of a 73-year-old healthy female at the hippocampus level (A, the cerebral blood volume [CBV] map of the bilateral temporal cortex, hippocampus, and occipital cortex; B, the cerebral blood flow [CBF] map; C, the mean transit time [MTT] map; and D, the time to peak [TTP] map).
Figure 2.
False-color pictures of a 72-year-old male with the diagnosis of moderate Alzheimer’s disease (AD) at the hippocampus level (A, the cerebral blood volume [CBV] map: the CBVs of the bilateral temporal cortex and hippocampus are lower and therefore are presented in blue; B, the cerebral blood flow [CBF] map: the CBFs of the bilateral temporal cortex and hippocampus are lower and therefore are presented in a deeper color than the healthy participants [Figure 1B]; C, the mean transit time [MTT] map: the bilateral temporal cortex and hippocampus are presented in yellow and green, which means the MTT is longer than that of healthy participants [Figure 1C]; D, the time to peak [TTP] map: the bilateral temporal cortex and hippocampus are presented in yellow, green and blue). The red component value of the TTP is higher than that of healthy participants (Figure 1D), reflecting a longer TTP in patients with AD than in healthy participants.
Table 1.
Perfusion Parameters of ROIs Between the 2 Groups a
| ROIs | Perfusion parameters | AD group (n = 50) | Healthy control group (n = 28) | F value | t value | P value |
|---|---|---|---|---|---|---|
| Left frontal cortex | CBV | 1.62 ± 0.86 | 2.29 ± 0.91 | 0.103 | −3.148 | .002 |
| CBF | 18.35 ± 1.31 | 27.04 ± 18.00 | 5.346 | −2.802 | .007 | |
| MTT | 6.34 ± 4.30 | 4.61 ± 1.11 | 1.997 | 2.083 | .041 | |
| TTP | 18.03 ± 6.32 | 14.40 ± 3.80 | 0.950 | 2.767 | .007 | |
| Right frontal cortex | CBV | 1.70 ± 0.83 | 2.10 ± 0.86 | 1.162 | −2.022 | .047 |
| CBF | 18.35 ± 6.93 | 23.27 ± 10.59 | 0.555 | −2.205 | .030 | |
| MTT | 12.41 ± 4.74 | 8.28 ± 3.07 | 2.448 | 3.525 | .001 | |
| TTP | 18.23 ± 6.18 | 14.94 ± 3.24 | 2.064 | 2.621 | .011 | |
| Left temporal cortex | CBV | 1.91 ± 0.80 | 2.42 ± 1.04 | 0.164 | −2.249 | .027 |
| CBF | 15.13 ± 6.52 | 20.11 ± 5.05 | 0.762 | −2.988 | .004 | |
| MTT | 11.13 ± 3.66 | 9.21 ± 2.80 | 1.746 | 2.060 | .044 | |
| TTP | 18.90 ± 6.04 | 15.26 ± 3.90 | 0.462 | 2.881 | .005 | |
| Right temporal cortex | CBV | 1.99 ± 0.89 | 2.56 ± 0.84 | 0.345 | −2.373 | .021 |
| CBF | 2.30 ± 1.18 | 3.21 ± 1.38 | 0.281 | −2.847 | .006 | |
| MTT | 11.70 ± 5.92 | 7.72 ± 1.84 | 5.801 | 3.847 | .000 | |
| TTP | 18.61 ± 7.10 | 15.65 ± 4.08 | 0.546 | 2.029 | .046 | |
| Left basal ganglia | CBV | 2.24 ± 1.06 | 3.04 ± 1.06 | 2.049 | −3.165 | .002 |
| CBF | 31.89 ± 11.37 | 39.86 ± 19.05 | 3.056 | −2.011 | .048 | |
| MTT | 5.67 ± 4.46 | 4.01 ± 0.79 | 4.809 | 2.507 | .015 | |
| TTP | 17.41 ± 6.48 | 13.97 ± 0.75 | 0.833 | 2.561 | .012 | |
| Right basal ganglia | CBV | 2.30 ± 1.18 | 3.11 ± 1.18 | 2.091 | −2.876 | .005 |
| CBF | 15.53 ± 6.31 | 22.08 ± 8.80 | 1.416 | −3.272 | .002 | |
| MTT | 10.96 ± 3.21 | 8.28 ± 3.08 | 0.219 | 3.062 | .003 | |
| TTP | 17.60 ± 7.00 | 13.92 ± 3.80 | 0.915 | 2.565 | .012 | |
| Left hippocampus | CBV | 1.99 ± 0.89 | 2.56 ± 0.84 | 0.345 | −2.373 | .021 |
| CBF | 18.35 ± 1.31 | 27.04 ± 18.00 | 5.346 | −2.802 | .007 | |
| MTT | 11.56 ± 4.83 | 9.57 ± 1.10 | 13.778 | 2.462 | .018 | |
| TTP | 19.94 ± 7.83 | 15.76 ± 2.80 | 5.430 | 2.985 | .004 | |
| Right hippocampus | CBV | 1.91 ± 0.80 | 2.54 ± 1.43 | 0.298 | −2.131 | .037 |
| CBF | 17.32 ± 7.23 | 22.08 ± 8,80 | 0.236 | −2.223 | .030 | |
| MTT | 11.82 ± 3.60 | 9.57 ± 1.10 | 17.299 | 2.319 | .023 | |
| TTP | 19.93 ± 7.31 | 15.00 ± 2.59 | 6.180 | 3.774 | .000 |
Abbreviations: AD, Alzheimer’s disease; CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time; ROI, region of interest; TTP, time to peak.
a Unit of CBF: mL/min per 100 g; unit of CBV: mL/100 g; unit of MMT: s; unit of TTP: s. For all statistical tests, α = .05 or P < .05 was considered statistically significant.
Logistic Regression Analysis of the CTPI Perfusion Parameters of ROIs Between the 2 Groups
The health condition was set as the dependent variable, and the CTPI perfusion parameters of the ROIs were set as independent variables. The correlations between the health condition and the CTPI perfusion parameters of ROIs were then examined.
Perfusion parameters with the highest sensitivity for identifying AD
The 4 parameters with the highest sensitivity for identifying AD were the MTTs of the left frontal cortex, right temporal cortex, right basal ganglia, and right hippocampus.
Variables and coefficients of the regression equation
As the first step, the MTT of the left frontal cortex was included as the first independent variable in the equation. Then, the MTTs of the right hippocampus, right basal ganglia, and right temporal cortex were included (as 3 independent variables), in sequential order from the second time to the fourth time. Finally, these 4 independent variables were included in the equation with their regression coefficients of 41.342, −9.170, 25.197, and −32.710, respectively (Table 2). The ultimate regression equation was as follows:
Table 2.
Variables and Coefficients of the Regression Equation
| B | SE | Wald | df | Sig. | Exp (B) | |
|---|---|---|---|---|---|---|
| Left frontal cortex MTT | 41.342 | 4798.002 | .000 | 1 | .893 | 9E + 017 |
| Right temporal cortex MTT | −9.170 | 4337.928 | .000 | 1 | .998 | .000 |
| Right basal ganglia MTT | 25.197 | 5588.697 | .000 | 1 | .996 | 9E + 010 |
| Right hippocampus MTT | −32.710 | 3721.851 | .000 | 1 | .993 | .000 |
| Constant | −51.421 | 11574.277 | .000 | 1 | .996 | .000 |
Abbreviation: TTP, time to peak; SE, standard error.
where x 1 indicates the MTT of the left frontal cortex; x 2, the MTT of the right temporal cortex; x 3, the MTT of the right basal ganglia; and x 4, the MTT of the right hippocampus.
Validity of the equation
The diagnosis probability of the equation increased after x 4 was included (Table 3). The fitting effect was enhanced as more variables were included in the equation. In brief, the MTTs of the left frontal cortex, right temporal cortex, right basal ganglia, and right hippocampus had significant correlations with the incidence of AD. The combination of MTTs of the 4 aforementioned cerebral areas had the greatest diagnostic value.
Table 3.
The Chi-Square Test of the Logistic Regression Equation a
| Step | Improvement |
Model |
Correct class, % | Variable | ||||
|---|---|---|---|---|---|---|---|---|
| Chi-square | df | Sig. | Chi-square | df | Sig. | |||
| 1 | 14.631 | 1 | .000 | 14.631 | 1 | .000 | 80.0% | IN: left frontal cortex MTT |
| 2 | 36.954 | 1 | .000 | 51.584 | 2 | .000 | 96.4% | IN: Right hippocampus MTT |
| 3 | 20.518 | 1 | .000 | 72.103 | 3 | .000 | 100.0% | IN: Right basal ganglia MTT |
| 4 | .000 | 1 | .983 | 72.103 | 4 | .000 | 100.0% | IN: right temporal cortex MTT |
aNo more variables can be deleted from or added to the current model. End block: 1.
The Relationship of MTTs and the Level of AD
In the pairwise comparisons among moderate and severe AD groups, mild AD group and healthy controls group, the MTTs of left frontal cortex, right temporal cortex, right basal ganglia, and right hippocampus were significantly different, but there was no significant difference between moderate AD and severe AD. The MTTs of these cerebral areas were significantly lower in the healthy control than in the mild AD group, and those in mild group were significantly lower than in the moderate and severe AD group (Table 4), with a P value less than .05. These results indicated that the MTTs of aforementioned cerebral areas can reflect level of regional perfusion abnormalities in the patients with AD.
Table 4.
Comparison of the Mean MTT Parameter from the Different Brain Regions Between the AD and Healthy Control
| Diagnosis | Number of patient | Left frontal cortex | Right temporal cortex | Right basal ganglia | Right hippocampus |
|---|---|---|---|---|---|
| Mild AD | 17 | 5.84 ± 1.28 | 9.87 ± 3.05 | 9.21 ± 1.32 | 10.25 ± 4.23 |
| Moderate AD | 26 | 6.25 ± 3.67 | 11.23 ± 4.78 | 10.67 ± 3.10 | 11.23 ± 3.63 |
| Severe AD | 9 | 6.42 ± 4.23 | 11.83 ± 5.89 | 11.46 ± 4,82 | 11.94 ± 4.20 |
| Healthy control | 28 | 4.61 ± 1.11 | 7.72 ± 1.84 | 8.28 ± 3.08 | 9.57 ± 1.10 |
| F value | 1.977 | 5.801 | 0.219 | 17.299 | |
| P value | .041 | .000 | .003 | .023 |
Abbreviation: AD, Alzheimer’s disease.
Discussion
The pathological mechanism of AD is cerebral atrophy and hypoperfusion caused by the loss of neurocytes. The most frequently affected cerebral areas are the entorhinal cortex and hippocampus, followed by the cerebral cortex and white matter. In patients with AD, the loss of neurons and impairment of dendrite interconnection are often coupled with gliosis, 8 which lowers the severity of cerebral atrophy by compensating for the reduction of cerebral volume and affects conventional CT and MRI scans in identifying cerebral atrophy to a certain extent. Although the major pathological change of AD is cerebral neurodegeneration, there is evidence of vascular risk factors and CVD in patients with AD, which indicates that vascular-related risk factors may play important roles in the development and progression of AD. 9 Some reports have shown the coexistence and collaboration of pathological lesions and vascular lesions and have inferred that the combined lesions were the driving forces in the development of AD. 10 For patients with such combined lesions, their diagnosis could be MixD with dominance of AD. It has been pointed out that CVDs may be significant causes and trigger the factors of AD. 11,12
Cerebral CTPI is a functional imaging technique that reflects cerebral microcirculation and reveals changes in cerebral microcirculation and metabolism that cannot be detected by conventional CT or MRI scans. With the advantage of bolus injections of contrast medium and continuous single-level dynamic scanning, CTPI can accurately reflect changes in vessels and blood perfusion; additionally, CTPI is able to detect functional abnormalities of brain tissue prior to the presence of morphological changes. During a CTPI scan, perfusion parameters, such as CBF, CBV, MTT, and TTP, can be measured. The CBF represents the blood flow that passes through vascular structures (includes all sizes of arteries, veins, capillaries, and sinuses) in a certain volume of brain tissue per unit of time, and it is affected by the size of the vessels and the number of open capillaries. The CBV refers to the total volume of the cerebral vascular bed in the ROI, including the capillaries and all sizes of arteries and veins. The MTT reflects the mean transit duration (from blood inflow into the cerebral artery to blood outflow from the cerebral vein). The TTP indicates the duration from the injection of contrast medium to peak of density, representing the speed of blood perfusion in ROIs rather than perfusion features of the brain tissue. A major advantage of CTPI is its ability to report anatomic and hemodynamic information on brain tissue in a short period of time. 13 The operational principle of the cerebral CTPI technique is performing continuous, dynamic, single-level scans on ROIs after the intravenous bolus injection of contrast medium, generating the TDC of ROIs, calculating blood perfusion parameters from the TDC under the mathematic model of deconvolution, transferring the parameters and then analog signals into a digital matrix, and finally displaying the signals with different grayscales and false colors to form a blood perfusion false-color image. 14 By using deconvolution, we can calculate the residual instrumental function of contrast medium in an ROI based on the TDC of the contrast medium in the arteries and brain tissue, measure the arterial inflow and venous outflow of the contrast medium in brain tissue with the residual instrumental function, generate perfusion parameters, and then evaluate the blood perfusion status of tissues and organs. In other words, CTPI can present a vivid picture of the physiologic conditions of brain tissue. 15 Generally speaking, differences in the blood perfusion status of various cerebral areas are attributed to the capillary volume or number of each area. A study showed that the capillary density ratio of gray matter to white matter ranges between 3 to 1 and 2 to 1, a difference that is the basis of the contrast ratio of CT images. 16 In our study, the CBVs and CBFs were significantly higher in the healthy control group than in the AD group, while the MTTs were significantly lower in the healthy control group than in the AD group. The results further demonstrate that the pathologic basis of AD is neuron injury due to impaired microcirculation, and they provide evidence for a new diagnostic method and a new perspective on treatment for AD. The results of our study indicate the unique advantage of CTPI in providing hemodynamic information on brain tissue. As the changes of hemodynamic parameters indirectly reflect physiologic and metabolic conditions of brain tissue, the CTPI scan can provide evidence for the early diagnosis of AD. The results of our study agree with the existing studies on physiologic and pathologic characteristics of AD. In addition, our study offers functional imaging evidence to support the theory that impaired neuron metabolism and blood perfusion play important roles in the development of AD.
In our study, the CBVs and CBFs of the bilateral frontal cortex, temporal cortex, basal ganglia, and hippocampus were significantly higher in the healthy control group than in the AD group, while the MTTs were significantly lower in the healthy control group than in the AD group. The results indicate the reduction of blood perfusion and slowing of the bloodstream in the brain tissue of patients with AD, which allows us to identify AD among all participants with the 50% reduced-dose cerebral CTPI scan. There were 4 perfusion parameters with the highest diagnostic value, namely the MTTs of the left frontal cortex, right temporal cortex, right basal ganglia, and right hippocampus, which can display the difference between the mild AD and the moderate-to-severe AD and reflect the level of AD. Combining the MTTs of the 4 cerebral areas showed greater diagnostic value with higher sensitivity, specificity, and accuracy than any 1 of the 4 alone. Conversely, there was a certain weakness of the 50% reduced-dose cerebral CTPI scan. The scan can detect ischemia of ROIs but cannot tell the difference between various ischemic brain diseases. Therefore, differentiation of VD and other ischemic brain diseases is necessary before a diagnosis of AD can be made.
A major concern of the cerebral CTPI scan is its high radiation dose. In our study, the electric current of CT scan was lowered by 50%, from 160 to 80 mA, cutting the radiation dose dramatically. During our cerebral CTPI scan, the radiation dose received by each participant was 1280.95 mGy/cm (measured by DLP), which was 50% lower than the DLP of 2562 mGy/cm of a conventional cerebral CTPI scan. Through this 50% reduced-dose cerebral CTPI scan, images with diagnostic value were obtained, while the participants received a radiation dose that equaled that of a conventional contrast-enhanced cerebral CT scan. This feature makes it possible to introduce this new method in clinical practice.
In short, the 50% reduced-dose cerebral CTPI scan is valuable in displaying regional blood flow abnormalities of patients with AD. The results of our study agree with the existing studies on physiologic and pathologic characteristics of AD. In addition, our study offers functional imaging evidence to support the theory that impaired neuron metabolism and blood perfusion play important roles in the development. The 50% reduced-dose cerebral CTPI scan may be a possible tool for diagnosis of AD.
Acknowledgment
We appreciate the valuable comments from other members of our departments.
Footnotes
Authors’ Notes: Z. Tang conceived of the study and participated in its design. X. L. Pi was guarantor of intergrity of the entire study and participated in data acquisition. H. M. Fu carried out cognitive assessments. Z. W. Qu participated in the design of the study and performed the clinical studies. Chen Feng carried out low-dose CT perfusion and performed the statistical analysis. L. H. Shi and H. T. Gong carried out low-dose CT perfusion. All authors read and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Fengxian district of Shanghai grant (FK20111001) and Pudong new area of Shanghai grant (PWRd2007-10).
References
- 1. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zimny A, Sasiadek M, Leszek J, Czarnecka A, Trypka E, Kiejna A. Does perfusion CT enable differentiating Alzheimer’s disease from vascular dementia and mixed dementia? A preliminary report. J Neurol Sci. 2007;257(6):114–120. [DOI] [PubMed] [Google Scholar]
- 3. Jagust W. Positron emission tomography and magnetic resonance imaging in the diagnosis and prediction of dementia. Alzheimers Dement. 2006;2(1):36–42. [DOI] [PubMed] [Google Scholar]
- 4. Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–2127. [DOI] [PubMed] [Google Scholar]
- 5. Hanyu H, Shimizu S, Hirao K, et al. Differentiation of dementia with lewy bodies from Alzheimer’s disease using Mini-Mental State Examination and brain perfusion SPECT. J Neurol Sci. 2006;250(7):97–102. [DOI] [PubMed] [Google Scholar]
- 6. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 7. Dean PM, Feldman DM, Morere D, Morton D. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753–760. [DOI] [PubMed] [Google Scholar]
- 8. Chui H. Neuropathology lessons in vascular dementia. Alzheimer Dis Assoc Disord. 2005;19(1):45–52. [DOI] [PubMed] [Google Scholar]
- 9. Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Terry RD, Peck A, De Teresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10(2):184–192. [DOI] [PubMed] [Google Scholar]
- 11. Harris RJ, Symon L, Branston NM, Bayhan M. Changes in extracellular calcium activity in cerebral ischaemia. J Cereb Blood Flow Metab. 1981;1(2):203–209. [DOI] [PubMed] [Google Scholar]
- 12. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. [DOI] [PubMed] [Google Scholar]
- 13. Cenic A, Nabavi DG, Craen RA, Gelb AW, Lee TY. Dynamic CT measurement of cerebral blood flow: a validation study. AJNR Am J Neuroradiol. 1999;20(1):63–73. [PubMed] [Google Scholar]
- 14. Yang Y, Engelien W, Xu S, Gu H, Silbersweig DA, Stern E. Transit time, trailing time, and cerebral blood flow during brain activation: measurement using multislice, pulsed spin-labeling perfusion imaging. Magn Reson Med. 2000;44(5):680–685. [DOI] [PubMed] [Google Scholar]
- 15. Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol. 2003;76(904):220–231. [DOI] [PubMed] [Google Scholar]
- 16. Fieselmann A, Kowarschik M, Ganguly A, Hornegger J, Fahrig R. Deconvolution-based CT and MR brain perfusion measurement: theoretical model revisited and practical implementation details. Int J Biomed Imaging. 2011;2011:467–563. [DOI] [PMC free article] [PubMed] [Google Scholar]