Abstract
The attachment of Vibrio alginolyticus to glass surfaces was investigated with special reference to the swimming speed due to the polar flagellum. This bacterium has two types of flagella, i.e., one polar flagellum and numerous lateral flagella. The mutant YM4, which possesses only the polar flagellum, showed much faster attachment than the mutant YM18, which does not possess flagella, indicating that the polar flagellum plays an important role. The attachment of YM4 was dependent on Na+ concentration and was specifically inhibited by amiloride, an inhibitor of polar flagellum rotation. These results are quite similar to those for swimming speed obtained under the same conditions. Observations with other mutants showed that chemotaxis is not critical and that the flagellum does not act as an appendage for attachment. From these results, it is concluded that the attachment of V. alginolyticus to glass surfaces is dependent on swimming speed.
The marine bacteria Vibrio alginolyticus and Vibrio parahaemolyticus have two flagellar systems, i.e., a polar flagellum and lateral flagella. These two systems are different in that although the polar flagellum is always present, lateral flagella are synthesized only when cells are grown on surfaces (5, 21, 25) or in a high-viscosity environment (4). Also, the energy sources of polar and lateral flagella are Na+ and H+ motive forces, respectively (2, 12). In other words, cells in liquid phase swim by using the polar flagellum, and their speed is dependent on Na+ concentration.
Belas and Colwell (6) investigated the adsorption of marine Vibrio to chitin particles and showed that the kinetics differ between the polar and lateral flagellar systems. Although they suggested that the lateral flagella serve as bridging materials, experimental confirmation for this is still lacking. On the other hand, motility and chemotaxis have been reported to be involved in the attachment of Vibrio cholerae to animal intestinal mucosal surfaces (11). It has also been reported that the main role of flagellar rotation or motility is to transport the cells toward these surfaces (7).
In the present work, we tried to clarify the role of the polar flagellum in attachment by using several types of mutants. The results show that the attachment of V. alginolyticus to glass surfaces is dependent on swimming speed. The application of DLVO theory, in which bacteria are considered as colloidal particles (1, 15, 16, 20), seems to be problematic. To our knowledge, this is the first report on the relation between bacterial swimming speed and attachment.
MATERIALS AND METHODS
Bacterial strains.
The marine bacterium V. alginolyticus 138-2 was a generous gift from H. Tokuda, University of Tokyo, Tokyo, Japan. The occurrence of the respiratory-driven primary Na+ pump (24) and Na+-driven flagellar motors (12) in this strain have been previously described. M. Homma, Nagoya University, kindly supplied us with the following mutant strains of 138-2: YM4 (Pof+ Laf−) (12), YM18 (Pof− Laf−) (12), NMB94 (Pof+ Laf− Mot−) (9), and NMB86 (Pof+ Laf− Che−) (9). Unless otherwise stated, cells were precultured in 0.25× ZoBell 2216E culture medium prepared with synthetic seawater (SSW), which contains the following (per liter of distilled water [pH 7.5]): NaCl, 23.4 g (400 mM); KCl, 0.8 g; MgSO4 · 7H2O, 4 g; CaCl2, 0.2 g; KBr, 100 mg; SrCl2 · 6H2O, 26 mg; and H3BO3, 20 mg. The full-strength ZoBell 2216E medium contains Polypepton (5 g/liter; Japan Pharmaceuticals) and yeast extract (1 g/liter; Difco) as organic substrates. Cells of an overnight culture grown at 25°C were harvested by centrifugation at 5,000 × g for 5 min at 10°C. After being washed twice with SSW, cells were inoculated into medium for attachment or swimming speed measurements. Measurements were started 30 min after inoculation to ensure physiological adaptation to each medium of different chemical composition. Although precise measurements were not done, the loss of cellular motility during these steps seems to have been negligible. However, this procedure seemed to cause a considerable loss of lateral flagella. Therefore, a comparative study between strains with only lateral flagella and strains with only a polar flagellum was not performed.
Attachment measurement.
A cover glass (24 by 32 mm, NEO No. 1; Matsunami LTD., Osaka, Japan) was used as the substratum of attachment. This product was washed by ultrasonication and dried before being packed and shipped by the manufacturer. We compared several methods of precleaning (two kinds of detergent and HCl-ethanol treatment), but there was virtually no difference in the degree of attachment. Therefore we used the cover glass without any pretreatment. One piece of cover glass was soaked in a cell suspension (10 ml) in a glass test tube (30 by 60 mm). Unless otherwise stated, these tubes were incubated on a rotary shaker (100 rpm) at 25°C for 1 h. Without shaking, attached cells did not distribute evenly on the glass, especially at the air-liquid interface. After 1 h, the glass was taken out and washed twice with SSW of the same composition. One drop of DAPI (4′,6-diamidino-2-phenylindole) solution (ca. 1 μg/ml) was placed on the surface of the glass, and the cover glass was placed on a glass slide, followed by observation with a fluorescence microscope (Olympus BH-2) within 30 min. Cells in at least 10 randomly chosen fields were counted at a magnification of ×1,000. Areas near the air-liquid interface, however, were omitted because of nonuniform distribution. Results shown are average values. Assays for each experimental condition were repeated at least three times to confirm the reproducibility of the results.
Swimming speed measurement.
Prior to the measurement of swimming speed, cell suspensions from at least three subsamples were preincubated for 30 min in SSW of the particular composition to be used. Twenty microliters of the suspension was transferred onto a glass slide, and cell movements were immediately recorded with an inverted microscope (Diaphot 300; Nikon, Tokyo, Japan) at a magnification of ×400 and a high-speed video camera (HSV500 HR; Nac, Tokyo, Japan) for 2 min. The focus was set at the top of the surface layer. The images obtained were analyzed by using Movias 2D software (Nac). From one frame, 20 cells were randomly chosen, regardless of their motility. The distance traveled in 40 ms was consecutively measured for up to 1 s. If a cell moved out of the layer being focused on measurement was stopped and the data that had been collected until this time point were used. We did not specifically count the relative numbers of nonswimming cells, which numbers seemed to depend on cellular physiological condition and Na+ concentration. The measurement of swimming speed was repeated for each subsample. Results are shown as averages of the values after subtracting the blank value that was obtained for YM18, the nonmotile mutant.
RESULTS
Time courses with different substrate concentrations.
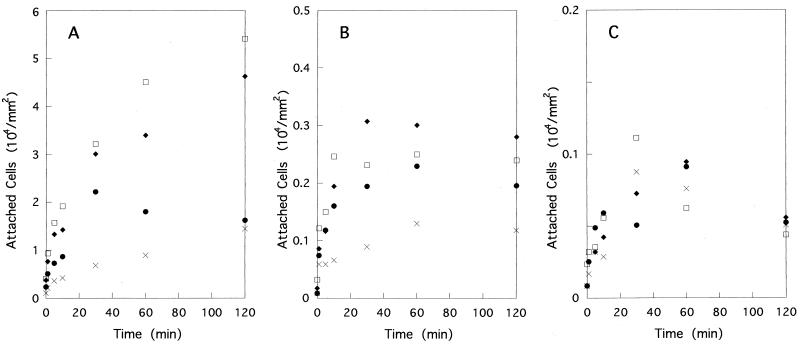
Time courses of bacterial attachment to the glass surface were obtained with different substrate concentrations. For YM4, the number of attached cells increased rapidly for the first 30 min, followed by a slower or no increase up to 2 h (Fig. 1A). A higher concentration of organic matter induced more attachment. A quite similar pattern was observed for NMB86 (Pof+ Laf− Che−), which possesses only a polar flagellum and lacks chemotactic ability (data not shown). The strain without a polar flagellum, YM18, showed a much slower rate of attachment than did YM4 (Fig. 1B). The number of attached cells did not increase after 30 min, and the dependence on organic concentration was less clear. This difference between these strains indicates that the polar flagellum plays an important role in attachment, probably by increasing swimming speed or by acting as an apparatus for attachment. The latter explanation, however, was ruled out because YM94 (Pof+ Laf− Mot−), which possesses a nonrotating polar flagellum, showed a very slow rate of attachment which was comparable to that of YM18 (Fig. 1C).
FIG. 1.
Time courses of V. alginolyticus attachment at different organic supplement concentrations. Zobell 2216E culture medium was used at 0.25× (squares), 0.05× (diamonds), and 0.005× (circles) concentrations (crosses indicate no organic supplement used). (A) YM4 (Pof+ Laf−), (B) YM18 (Pof− Laf−), (C) NMB94 (Pof+ Laf− Mot−). Please note the differences in the vertical scales among the strains.
Although an assay of cells in medium without a supplement of organic substrates was performed, the number of attached cells measured was small, which made it difficult to get reliable numbers. This especially caused practical problems when many samples were treated in parallel. Considering the results shown in Fig. 1 and other observations, we decided to use 0.05× ZoBell 2216E medium and a 1-h incubation for this assay. This medium contains about 100 mg of organic carbon/liter. We repeated the experiments shown in Fig. 1 without the addition of organic substrates and obtained essentially the same results (data not shown).
Effect of cell concentration on attachment.
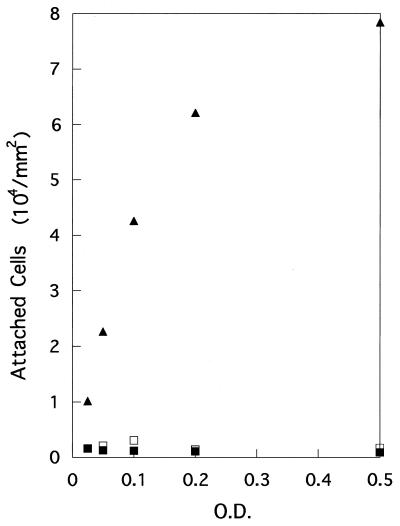
The attachment rate is expected to be dependent on the frequency of bacterial contact with the surface. Under fixed physicochemical conditions, bacterial concentration and swimming speed are the two major factors which determine this frequency. Therefore, attachment rates were first measured with different bacterial concentrations (optical density [OD] of 0.025 to 0.5). As shown in Fig. 2, the number of attached cells of YM4, the polarly flagellated strain, increased linearly with an increase in OD up to 0.1 and then showed a saturation pattern. On the other hand, such a pattern was not observed for strains YM-18 and NMB94. In addition, the numbers of attached cells for the latter two strains were considerably smaller. This may suggest that below a certain swimming speed, bacterial attachment is controlled by physicochemical factors. For the following experiments, we decided to choose an OD of 0.05, a density at which the attachment rate was proportional to the cell concentration.
FIG. 2.
Effects of cell concentration on attachment rates for YM4 (Pof+ Laf−) (triangles), YM18 (Pof− Laf−) (open squares), and NMB94 (Pof+ Laf− Mot−) (solid squares).
Dependence of attachment on monovalent cation concentration.
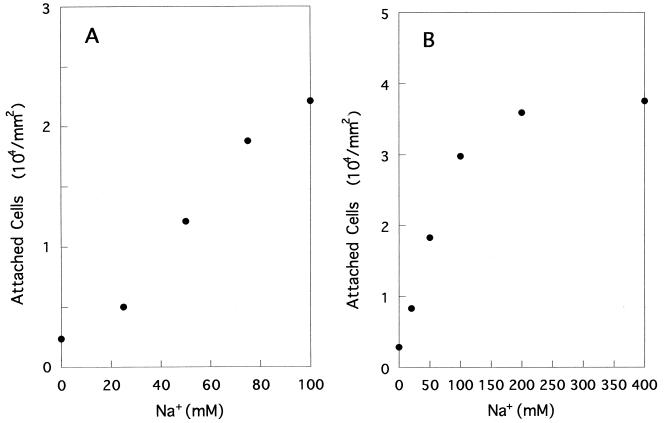
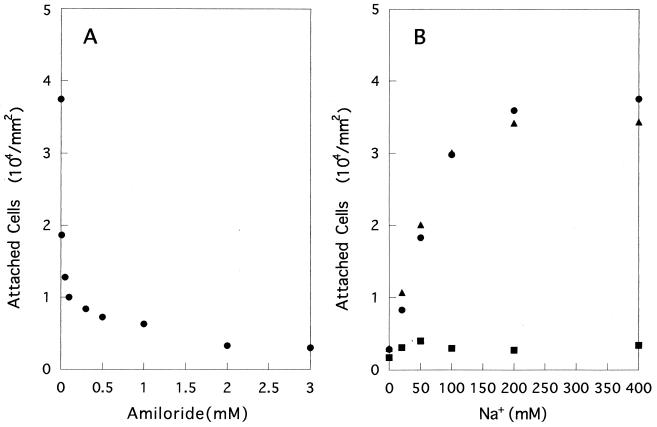
The effects of increasing concentrations of monovalent cations on the attachment of YM4 were observed under two different conditions. First, in order to keep the ionic strength constant, the total concentration of Na+ and K+ was fixed (100 or 400 mM) and the relative concentrations were changed. At a 100 mM total concentration (Fig. 3A), the rate of attachment increased linearly with Na+ concentration, whereas at 400 mM (Fig. 3B), the rate showed a saturation pattern with an increasing concentration of Na+. NMB86, which lacks chemotactic response, showed a quite similar attachment pattern (data not shown).
FIG. 3.
Effects of increasing concentrations of Na+ on the attachment of YM4. Total amount of Na+ and K+ was kept at 100 (A) or 400 (B) mM.
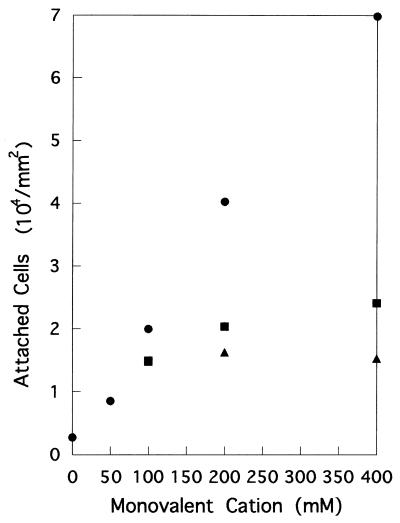
Second, an increasing amount of either Na+, K+, or Li+ was added to basal medium containing 50 mM Na+. Preliminary experiments clarified that the presence of 50 mM Na+ was sufficient to elicit growth of the wild-type strain. A marked effect of Na+ on the attachment of YM4 was noticed (Fig. 4). Addition of K+ or Li+ induced a slight increase in the attachment rate. Two other strains (YM18 and NMB94), however, did not show any appreciable attachment or an effect of Na+ under these conditions (data not shown).
FIG. 4.
Effects of increasing concentrations of monovalent cations on the attachment of YM4. Na+ (circles), K+ (squares), or Li+ (triangles) was added to basal medium containing 50 mM Na+. Total amounts of monovalent cations are indicated on the x axis.
These results indicate that the attachment rates of polarly flagellated strains were dependent on Na+ concentration and that K+ and Li+ can only partially substitute for Na+.
Effect of amiloride.
The Na+-dependent swimming speed of V. alginolyticus 138-2 has been well established (12, 23). However, the possibility of physiological stimulation by Na+ has not been ruled out. In order to evaluate swimming speed dependency, the effect of amiloride, a specific inhibitor of Na+ motive flagellar rotation, was observed. Figure 5A shows the effects of increasing concentrations of amiloride on the attachment of YM4 in the presence of 400 mM NaCl. As little as 0.01 mM amiloride sharply suppressed attachment. Figure 5B shows the Na+-concentration-dependent attachment of YM4 with and without addition of amiloride (3 mM). The attachments were almost completely suppressed regardless of Na+ concentration. Addition of dimethyl sulfoxide (DMSO), a solvent of amiloride, had no effect on attachment. It is noteworthy that NMB86, which lacks chemotaxis, showed essentially the same results (data not shown). These results strongly suggest that the attachment of V. alginolyticus to glass surfaces is dependent on swimming speed.
FIG. 5.
Effects of amiloride on the attachment of YM4. (A) Effect of an increasing concentration (0.01 to 3 mM) of amiloride on YM4 attachment. Amiloride was first dissolved in DMSO and then added to assay tubes. (B) Effect of amiloride (3 mM) on the attachment of YM4 with different concentrations of Na+. Circles, without DMSO or amiloride; triangles, with DMSO; squares, with DMSO and amiloride. The total amount of Na+ and K+ was kept at 400 mM.
Swimming speed.
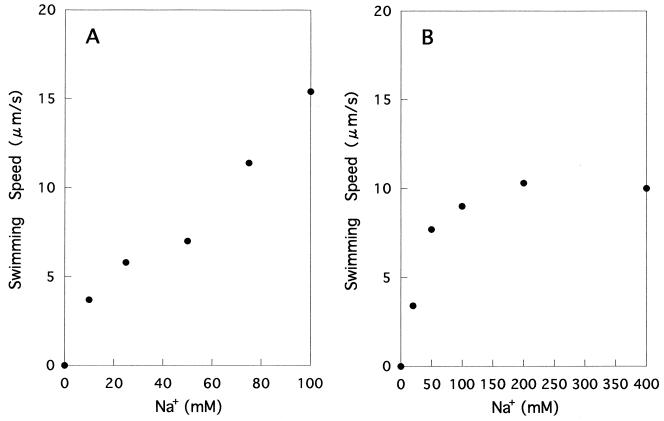
The swimming speed of YM4 was measured under the same conditions used for the measurement of attachment. In the medium with 100 mM Na+ and K+, swimming speed increased linearly with an increasing concentration of Na+ (Fig. 6A). When the total concentration was 400 mM, a saturation curve was obtained (Fig. 6B). These patterns were quite similar to those of the attachment rates (Fig. 3).
FIG. 6.
Effects of increasing concentrations of Na+ on the swimming speed of YM4. The total amount of Na+ and K+ was kept at 100 (A) or 400 (B) mM.
Swimming speed was measured for randomly chosen cells regardless of their motility. Therefore, the values shown here are less than those reported by other workers (see Fig. 2 in reference 12). Although we repeated this experiment several times, at each Na+ concentration a slightly higher speed was always observed at the 100 than at the 400 mM total concentration (Fig. 6). If only actively swimming cells were chosen for the calculation, the mean speeds at 400 mM were just comparable to those at 100 mM. This is not due to a technical inconsistency, because precautions were taken to keep the cellular conditions constant throughout the experiments. We interpret this phenomenon as follows. For the measurement, 20 μl of cell suspension was placed on the glass slide. Cells moving rapidly might immediately start to attach to the glass, leaving slower cells in the liquid phase. Because the swimming speed was expected to increase with Na+ concentration, this effect might become more prominent at 400 mM. A similar process might also occur during the preincubation just prior to the swimming speed measurement. Actually, the latter effect was microscopically confirmed by the time course observation. This technical problem and the slight inconsistency in the data, however, are of minor importance for the essential interpretation of the present data; i.e., the similarity of the patterns for attachment and swimming speed indicates that bacterial attachment is dependent on swimming speed.
DISCUSSION
It has been known that bacterial attachment to surfaces is affected by various physicochemical and biological factors including bacterial surface hydrophobicity (8, 14), surface appendages, extracellular polymeric substances (7), bacterial physiological state, electrolyte concentration in the medium (16), and surface charge (17). The present results indicate that the attachment or irreversible adsorption of V. alginolyticus to glass surfaces is dependent on time, bacterial concentration, organic concentration, Na+ concentration, type of flagella, and an inhibitor of Na+ motive flagellar rotation. On the basis of these results and the data on the dependence of swimming speed on Na+ concentration, we conclude that the attachment of V. alginolyticus to glass surfaces is swimming speed dependent.
As for the effects of organic substrates on the rate of attachment, several explanations are possible; i.e., these substrates are involved in stimulation of bacterial metabolic activity and subsequent growth, change in surface characteristics, increase in swimming speed, and change in physicochemical condition at the glass surface. The fact that YM4 showed clear concentration dependency, whereas NMB94, the nonmotile mutant, did not may indicate that the first three explanations cannot be critical ones. Preliminary observations indicated that under the above-described conditions, the doubling time of the wild-type strain was slightly more than 2 h. Therefore, growth and subsequent increase in cellular concentration could contribute little under the experimental conditions used here. The most probable explanation is a stimulation of swimming speed. We have confirmed that swimming speed is dependent on organic concentration within the range of concentrations used in the present experimental conditions (data not shown). In any case, the attachment patterns shown in Fig. 3 to 5 remained the same regardless of the presence of an organic supplement. Therefore, the effects of organic supplements should not lead to a misinterpretation of our results.
The attachment rates specifically depend on sodium concentration (Fig. 3 and 4). By comparing the data in Fig. 3 and 4 with the data in Fig. 6, we interpret this relation to be due to the effects of Na+ concentration on swimming speed resulting from polar flagellum rotation. The following facts have been well established for V. alginolyticus: (i) polar flagellum rotation is energized by Na+ motive force and is dependent on Na+ concentration (12, 23), (ii) Li+ can only partially substitute for Na+ (13), and (iii) polar flagellum rotation is specifically inhibited by amiloride (12, 22). The present results with YM4 all showed good agreement with these characteristics. Furthermore, observations with YM18 (Pof− Laf−), NMB94 (Pof+ Laf− Mot−), and NMB86 (Pof+ Laf− Che−) clearly indicate that the rotation of the polar flagellum is essential, the role of the polar flagellum as an attachment appendage (21) is of negligible importance, and chemotactic response was not critical. In other words, even if there was an accumulation of organic substrates on the glass surface, it should not disturb the basic attachment pattern.
Increasing the concentration of Na+ also might have other effects. Its effects on physicochemical factors on the glass, however, can be ruled out, because the concentration of cations (Na+ and K+) was kept constant (Fig. 3). The electrophoretic mobilities of YM4 were measured under the conditions shown in Fig. 3B. The fact that the values were almost constant, i.e., −1.8 × 10−8 to −1.9 ×10−8 m2 V−1 s−1, means that sodium concentration does not affect the surface electrostatic characteristics of the cells (16a). From these facts, we conclude that the major factor that elicited the present attachment patterns was Na+-dependent swimming speed.
Belas and Colwell (6) observed the adsorption of laterally and polarly flagellated Vibrio to chitin particles. They clarified that among the 49 bacterial strains tested, the adsorption of all the laterally flagellated strains followed the Langmuir adsorption isotherm or surface saturation kinetics, whereas only 35% of polarly flagellated strains followed this kinetics. They put emphasis on the role of lateral flagella as bridging materials (see Fig. 3 in reference 6). Our preliminary test with a mutant (YM19) that constitutively synthesized lateral flagella, however, showed much slower attachment rates than those of YM4, and involvement of lateral flagella as bridging materials was not confirmed. The experimental conditions used by Belas and Colwell (6) were considerably different from ours. First, they observed attachment rates to chitin particles by measuring radioactivity of labeled cells. Second, they estimated the number of adsorbed bacteria after 210 min, during which time period an equilibrium between attached and free-living bacteria had been achieved. (Our observations were made for the initial attachment phase that was before the equilibrium.) Third, they used wild-type strains of V. alginolyticus and V. parahaemolyticus. Those bacteria synthesized lateral flagella only after the cells adsorbed on the chitin particle. Therefore, they were looking at a different stage of attachment. We suggest that for the initial attachment stage, the polar flagellum should play an important role by increasing swimming speed or frequency of collision with the surface. Once the cells adsorb, lateral flagella may influence the subsequent attachment, as was shown by the data of Belas and Colwell (6). However, it is still not clear if lateral flagella act as an attachment apparatus. The production and importance of extracellular polymers as bridging materials remain to be clarified.
To our knowledge the influence of swimming speed on attachment has never been investigated before. This is because, first, it is difficult to control the swimming speed of bacteria for which flagellar rotation is energized by proton influx. Second, according to the DLVO theory (20), there is a large free energy barrier that inhibits a particle from coming into close contact with the surface. Although this barrier decreases with increasing electrolyte concentration, it is generally regarded as being too high to be overcome by bacterial swimming speed or the kinetic energy even at the electrolyte concentration of seawater (16). In the present work, however, we could confirm a simple and clear relationship between swimming speed and attachment. The swimming speed of bacteria in a sodium-driven flagellar system is much easier to manipulate. Another advantage is that some specific inhibitors such as amiloride (10, 22) or phenamil (3) are available for use in this system. The relation between swimming speed and attachment may force us to reconsider the application of DLVO theory to bacterial attachment.
To analyze the kinetics of this bacterial system we are currently measuring the surface charge (electrophoretic mobility) under different conditions. Preliminary results indicate that calculations based on the Ohshima’s recent theory (18, 19) for “soft” particles give a reasonable estimation of the magnitude of the energy barrier in this system (unpublished data). These results make it possible to explain attachment rates simply from the rates of collision of bacterial cells with the surface.
In conclusion, the attachment of V. alginolyticus to glass surfaces is dependent on swimming speed. To our knowledge, these are the first quantitative data on this factor. Kinetic analysis is now being pursued based on Ohshima’s theory for soft particles.
ACKNOWLEDGMENTS
We thank H. Tokuda for providing us with V. alginolyticus 138-2. We also thank I. Kawagishi and M. Homma for the mutant strains and helpful comments on our work.
REFERENCES
- 1.Abbott A, Rutter P R, Berkeley R C W. The influence of ionic strength, pH and a protein layer on the interaction between Streptococcus mutans and glass surfaces. J Gen Microbiol. 1983;129:439–445. doi: 10.1099/00221287-129-2-439. [DOI] [PubMed] [Google Scholar]
- 2.Atsumi T, McCarter L, Imae Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature (London) 1992;355:182–184. doi: 10.1038/355182a0. [DOI] [PubMed] [Google Scholar]
- 3.Atsumi T, Sugiyama S, Gragoe E J, Jr, Imae Y. Specific inhibition of the Na+-driven flagellar motors of alkalophilic Bacillus strains by the amiloride analog phenamil. J Bacteriol. 1990;172:1634–1639. doi: 10.1128/jb.172.3.1634-1639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atsumi T, Maekawa Y, Yamada T, Kawagishi I, Imae Y, Homma M. Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus. J Bacteriol. 1996;178:5024–5026. doi: 10.1128/jb.178.16.5024-5026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belas M R, Colwell R R. Scanning electron microscope observation of the swarming phenomenon of Vibrio parahaemolyticus. J Bacteriol. 1982;150:956–959. doi: 10.1128/jb.150.2.956-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belas M R, Colwell R R. Adsorption kinetics of laterally and polarly flagellated Vibrio. J Bacteriol. 1982;151:1568–1580. doi: 10.1128/jb.151.3.1568-1580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Characklis W G. Biofilm processes. In: Characklis W G, Marshall K C, editors. Biofilms. New York, N.Y: John Wiley & Sons; 1990. pp. 195–231. [Google Scholar]
- 8.Fletcher M, Loeb G I. Influence of substratum characteristics on the attachment of a marine Pseudomonad to solid surfaces. Appl Environ Microbiol. 1979;37:67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homma M, Oota H, Kojima S, Kawagishi I, Imae Y. Chemotactic responses to an attractant and a repellent by the polar and lateral flagellar systems of Vibrio alginolyticus. Microbiology. 1996;142:2777–2783. doi: 10.1099/13500872-142-10-2777. [DOI] [PubMed] [Google Scholar]
- 10.Imae Y, Atsumi T. Na+-driven bacterial flagellar motors. J Bioenerg Biomembr. 1989;21:705–716. doi: 10.1007/BF00762688. [DOI] [PubMed] [Google Scholar]
- 11.Jones G W. The adhesive properties of Vibrio cholerae and other Vibrio species. In: Beachey E H, editor. Bacterial adherence. London, United Kingdom: Chapman and Hall; 1980. pp. 219–249. [Google Scholar]
- 12.Kawagishi I, Maekawa Y, Atsumi T, Homma M, Imae Y. Isolation of the polar and lateral flagellum-defective mutants in Vibrio alginolyticus and identification of their flagellar driving energy sources. J Bacteriol. 1995;177:5158–5160. doi: 10.1128/jb.177.17.5158-5160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J Z, Dapice M, Khan S. Ion selectivity of the Vibrio alginolyticus flagellar motor. J Bacteriol. 1990;172:5236–5244. doi: 10.1128/jb.172.9.5236-5244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loosdrecht M C M van, Lyklema J, Norde W, Schraa G, Zehnder A J B. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loosdrecht M C M van, Lyklema J, Norde W, Zehnder A J B. Bacterial adhesion: a physicochemical approach. Microb Ecol. 1989;17:1–15. doi: 10.1007/BF02025589. [DOI] [PubMed] [Google Scholar]
- 16.Marshall K C, Stout R, Mitchell R. Mechanism of the initial events in the sorption of marine bacteria to surfaces. J Gen Microbiol. 1971;68:337–348. [Google Scholar]
- 16a.Morisaki, H. Unpublished data.
- 17.Neihof R A, Loeb G I. The surface charge of particulate matter in seawater. Limnol Oceanogr. 1972;17:7–16. [Google Scholar]
- 18.Ohshima H. Electrophoretic mobility of soft particles. J Colloid Interface Sci. 1994;163:474–483. doi: 10.1006/jcis.2000.6759. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima H. Electrophoresis of soft particles. Adv Colloid Interface Sci. 1995;62:189–235. doi: 10.1016/j.cis.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Rutter P R, Vincent B. Physicochemical interactions of the substratum, microorganisms and the fluid phase. In: Marshall K C, editor. Microbial adhesion and aggregation. Berlin, Germany: Springer-Verlag; 1984. pp. 21–38. [Google Scholar]
- 21.Silverman M, Belas R, Simon M. Genetic control of bacterial adhesion. In: Marshall K C, editor. Microbial adhesion and aggregation. Berlin, Germany: Springer-Verlag; 1984. pp. 95–107. [Google Scholar]
- 22.Sugiyama S, Cragoe E J, Jr, Imae Y. Amiloride, a specific inhibitor for the Na+-driven flagellar motors of alkalophilic Bacillus. J Biol Chem. 1988;263:8215–8219. [PubMed] [Google Scholar]
- 23.Tokuda H, Asano M, Shimamura Y, Unemoto T, Sugiyama S, Imae Y. Roles of the respiratory Na+ pump in bioenergetics of Vibrio alginolyticus. J Biochem. 1988;103:650–655. doi: 10.1093/oxfordjournals.jbchem.a122323. [DOI] [PubMed] [Google Scholar]
- 24.Tokuda H, Unemoto T. A respiration-dependent primary sodium extrusion system functioning at alkaline pH in marine bacterium Vibrio altinolyticus. Biochem Biophys Res Commun. 1981;102:265–271. doi: 10.1016/0006-291x(81)91516-3. [DOI] [PubMed] [Google Scholar]
- 25.Ulitzur S. The mechanism of swarming of Vibrio algionlyticus. Arch Microbiol. 1975;104:67–71. doi: 10.1007/BF00447301. [DOI] [PubMed] [Google Scholar]